Abstract
There has been much interest in the health effects of dietary fat, but few studies have comprehensively compared the acute metabolic fate of specific fatty acids in vivo. We hypothesized that different classes of fatty acids would be variably partitioned in metabolic pathways and that this would become evident over 24 h. We traced the fate of fatty acids using equal amounts of [U-13C]linoleate, [U-13C]oleate, and [U-13C]palmitate given in a test breakfast meal in 12 healthy subjects. There was a tendency for differences in the concentrations of the tracers in plasma chylomicron-triacylglycerol (TG) (oleate > palmitate > linoleate). This pattern remained in plasma nonesterified fatty acid (NEFA) and very low-density lipoprotein (VLDL)-TG (P ≤ 0.01 and P ≤ 0.02 for [U-13C]oleate vs. both [U-13C]palmitate and [U-13C]linoleate for NEFA and VLDL-TG, respectively). There was significantly more [U-13C]linoleate than the other two tracers in plasma cholesteryl ester and phospholipid (PL). Using the values for isotopic enrichment in the different lipid fractions compared with the test meal, we calculated the contribution of meal fatty acids to the respective fractions. At 24 h, 10% of plasma PL-linoleate originated from the breakfast test meal. This was significantly greater than for oleate and palmitate (both 3 ± 0.3%; P < 0.05). This pattern was also true for erythrocyte PL fatty acids. The marked rapid incorporation of linoleate from a single meal into blood PL fractions may have functional consequences such as maintenance of membrane fluidity and may explain why linoleate is a useful biomarker of dietary intake.
Keywords: chylomicrons, nonesterified fatty acids, very low-density lipoprotein, stable isotopes, postprandial metabolism
when fatty acids are ingested, they are incorporated into chylomicrons within the enterocyte. Once in the systemic circulation, they enter metabolic pathways or exchange with endogenous molecules, particularly phospholipids (PLs). Surprisingly, there is limited evidence comparing the metabolic handling of the major dietary fatty acids in vivo in humans. Moreover, the differences in the abundance of fatty acids in specific blood lipid pools may in part be due to differences in the way that dietary fatty acids are handled, both in terms of absorption across the gastrointestinal tract and partitioning toward either esterification or oxidation. Stable isotope tracers offer the possibility to specifically study the metabolic fate of meal fatty acids.
Although the data comparing the in vivo metabolism of saturated and mono- and polyunsaturated fatty acids are limited, there appears to be an acceptance that the majority of fatty acids, with the exception of stearic acid (22), are metabolized in a similar manner. Typically the postprandial metabolism of different fats has been compared, although findings are difficult to interpret, as outcomes such as particle size and triacylglycerol (TG) content have been measured (25, 26, 32, 33, 51) rather than fatty acid composition of different fractions. Others have compared the metabolism of dietary fatty acids, primarily by comparing the fatty acid composition of a test meal with the fatty acid composition of chylomicron-TG. These studies (6, 42, 50, 55, 57, 58) have consistently demonstrated that the fatty acid composition of chylomicron-TG resembles, but is not identical to, that of the test meal. However, the fatty acid composition of other lipid fractions, such as plasma PLs and cholesteryl esters (CEs), appears to be more regulated representing the specificity of fatty acyl-CoA transferases (22). Studies with stable isotopes have more specifically compared postprandial fatty acid metabolism. Emken et al. (10) noted that the incorporation of [2H2]-labeled fatty acids (palmitate, stearate, oleate, elaidate and linoleate) into chylomicron-TG was comparable. However, only two individuals were investigated. Measuring the incorporation of dietary fatty acids into very low-density lipoprotein (VLDL)-TG requires specific techniques. Traditionally “VLDL” has been separated by ultracentrifugation techniques to isolate with the Svedberg flotation rate (Sf) 20-400 fraction, which includes VLDL and chylomicron remnant particles. Two studies (18, 19) have used conventional along with immunoaffinity chromatography techniques to specifically isolate VLDL particles. They reported differences in the postprandial metabolism of [U-13C]palmitate, eicosapentaenoic acid, and docosahexaenoic acid given in a test meal. However, little is known of differences in dietary fatty acid partitioning over a longer time period.
Erythrocyte and plasma fatty acid composition reflects changes in dietary fatty acid intake within 1–3 days (29, 47). This suggests a rapid exchange of fatty acids within the blood. Further, data (11) from a small study (n = 3) using stable isotope labeled fatty acids ([2H2]oleic acid and [2H2]elaidic acid) suggested that the incorporation of dietary fatty acids into erythrocyte PLs occurs at an estimated rate of 2–8% per 24-h period.
The aim of the present study was to investigate the postprandial metabolic partitioning of the three most common fatty acid classes in blood (22) over a 24-h period using isotopically labeled fatty acids, specifically [U-13C]linoleate (18:2 n-6), [U-13C]oleate (18:1 n-9), and [U-13C] palmitate (16:0). We specifically isolated VLDL using immunoaffinity chromatography techniques.
MATERIALS AND METHODS
Subjects.
A total of 12 healthy men and women was studied. Subjects were recruited from the wider Oxford community via advertisement. Subjects were free from ongoing or chronic disease and were not taking any lipid lowering medication or medication that would alter lipid metabolism. The study was approved by the Oxfordshire Clinical Research Ethics Committee, and all subjects gave written informed consent before starting the study.
Study protocol.
Before the study day, subjects were asked to avoid foodstuffs naturally enriched in 13C for 48 h and refrain from strenuous exercise and alcohol for 24 h before the study.
Subjects arrived at the clinical research unit after an overnight fast. A cannula was inserted into an antecubital vein, and a baseline blood sample was taken for background isotopic enrichment. At time 0, subjects were fed a test meal that represented approximately one-third of their total energy requirement, which had been determined using the equation of Schofield (44). The test meal consisted of Rice Krispies (Kelloggs, Manchester, UK), skimmed milk, cottage cheese, and a warm chocolate shake containing 100 mg each of [U-13C]linoleate, [U-13C]oleate, and [U-13C]palmitate such that fat, carbohydrate, and protein contributed 40, 45, and 15% of total energy. The macronutrient composition was chosen as it was within the range of current UK dietary intake (20). As such, a relatively high proportion of fat in the meal ensured a good lipemic response, which facilitated the study of fatty acid tracers. A mixture of safflower and palm oil was used as the carrier fat to balance the meal fatty acids to the added stable isotope fatty acids. At 6 h, subjects were given a glucose drink (75 g glucose) to assess the second meal effect (40). After 7 h, subjects left the clinical research unit, went back to consuming their habitual diet, and then returned the after morning (24 h after the baseline sample) for a final fasting blood sample.
Analyses.
Whole blood was collected into heparinized syringes (Sarstedt, Leicester, UK), plasma was rapidly separated by centrifugation at 4°C, and plasma NEFA and lipoprotein-TG concentrations were determined as previously described (3). After the removal of plasma and upper buffy coat, containing platelets and white blood cells, the packed erythrocytes were isolated and washed as previously described (23). Aliquots used for analysis of erythrocyte total PL fatty acids were stored at −80°C.
Separations of chylomicrons of Sf >400 and VLDL-rich fraction (Sf 20-400) were made by sequential flotation using density gradient ultracentrifugation (28). Ultracentrifugation was performed in a SW40Ti swinging bucket rotor (Beckman Instruments, Palo Alto, CA) at 40,000 rpm at 15°C. The gradients were run for 32 min to float Sf >400 lipoproteins and for a further 16 h to float Sf 20-400 lipoproteins. The Sf >400 fraction will hereafter be called chylomicrons. The Sf 20-400 fraction was then further separated by immunoaffinity chromatography as previously described by Heath et al. (19). The bound fraction [containing lipoproteins bound by anti-apolipoprotein (apo)B-100] was collected, and this was completely devoid of apoB-48 and will hereafter be called VLDL.
Blood samples were taken at −30 min and 0, 1, 2, 4, 5, 6, 6.5, 7, and 24 h after the mixed meal for the analysis of plasma NEFA and 0, 4, 6, 6.5, 7, and 24 h for the analysis of chylomicrons and VLDL. Blood samples were taken at 0, 7, and 24 h for the analysis of plasma PL and CE, which were available from 11 subjects and erythrocyte PL, which was available from 10 subjects. The particular time points were chosen as follows. Peak enrichment of meal fatty acid tracers in lipoprotein fractions occurs within the time frame of 4–6 h (21). At 6 h, a glucose drink was given; therefore, we expected further partitioning of fatty acids at 6.5 and 7 h due to the “second meal effect.” At 24 h, we expected to find enrichment remaining in VLDL-TG and beginning to appear in other lipid pools such as erythrocyte PL (47).
Fatty acid analysis and isotopic enrichment.
Fatty acid methyl esters (FAMEs) were prepared from NEFA, chylomicron-TG, VLDL-TG fractions, and the test meal (19) and from erythrocyte PL and plasma PL and CE (23, 24). Known weights of internal standards were added before lipid extraction so that fatty acid concentrations could be determined.
Fatty acid compositions (μmol/100 μmol total fatty acids) in these fractions were determined by gas chromatography (GC) (12), and specific fatty acid concentrations were determined by multiplying the proportion of the specific fatty acid by the corresponding relevant plasma concentration as determined enzymatically for plasma NEFA, chylomicron-TG, and VLDL-TG, or by GC analysis for plasma PL and CE. The approximate PL concentration of erythrocytes was determined using literature values for blood erythrocyte composition and proportion of PLs in erythrocytes (13, 48).
Analysis of [U-13C]fatty acid enrichments.
The 13C-to-12C ratio in [U-13C]linoleate, [U-13C]oleate, and [U-13C]palmitate was measured in the plasma NEFA, chylomicron-TG, VLDL-TG, erythrocyte PL, plasma PL and CE, and the test meal FAME derivatives using a Delta Plus XP GC-combustion-isotope ratio mass spectrometry (GC-C-IRMS; Thermo Electron, Bremen, Germany). The 13C enrichment results (from FAME derivatives) expressed as δ13C0/00 were converted to tracer-tracee (TTR) ratio using the following formula: TTR (13C/12C) = [(δ13C0/00/1,000) + 1)]·0.0112372.
The TTR of a baseline measurement (before administration of the stable isotope tracer) was subtracted from each sample TTR to account for natural abundance. The TTRs for [U-13C]linoleate, [U-13C]oleate, and [U-13C]palmitate were multiplied by the corresponding linoleate-, oleate-, and palmitate-plasma NEFA, PL, CE, lipoprotein-TG, or erythrocyte PL concentrations to give plasma, lipoprotein, and erythrocyte tracer concentrations.
Calculations and statistics.
The stable isotope fatty acids were given as equal weights in the test meal, but we report tracer concentrations as micromoles. Therefore, we adjusted the results accordingly to account for the small differences in the moles of fatty acid consumed by subjects.
The contribution of meal fatty acids to the chylomicron-TG, plasma NEFA, VLDL-TG, plasma PL and CE, and erythrocyte PL was calculated at 4, 7, and 24 h, based on previously described algorithms using the meal TTR determined by GC-C-IRMS (21). For example, the contribution of linoleate from the breakfast test meal to linoleate in VLDL-TG at the specific time points (expressed as a proportion and concentration) was calculated.
Data were analyzed using SPSS for Windows v15 (SPSS, Chertsey, UK). Statistical significance was set at P < 0.05. For ease of presentation, data are presented as means (SE) unless otherwise stated. All data sets were tested for normality according to the Shapiro-Wilk test. Comparisons were made between the three tracers, i.e., [U-13C]linoleate, [U-13C]oleate, and [U-13C]palmitate using repeated measures ANOVA with time and tracer as within-subject factors. Where statistical significance was found, we did a post hoc analysis with Bonferonni corrections. The contributions of breakfast test meal fatty acids to fatty acids in each lipid fraction were assessed using a Wilcoxon-signed rank test.
RESULTS
Subject characteristics are given in Table 1.
Table 1.
Participant characteristics
| Characteristics | |
|---|---|
| Sex | 6 female/6 male |
| Age, yr | 27 (5) |
| BMI, kg/m2 | 23 (2) |
| Fasting plasma, μmol/l | |
| NEFA | 411 (101) |
| TG | 846 (428) |
Data are presented as means (SD). BMI, body mass index; NEFA, nonesterified fatty acids; TG, triacylglycerol.
Chylomicron-TG, VLDL-TG, and meal fatty acid composition.
We measured the fatty acid composition of chylomicron-TG at time 0, and the composition appeared to resemble that of VLDL-TG, suggesting that in fact the chylomicron-TG fraction at this time contained large VLDL particles; however, the concentration was very low [25 (7) μmol/l; means (SE)]. This is probably true for chylomicron-TG at 24 h. By 4 h, the fatty acid composition of chylomicron-TG reflected (but was not identical to) that of the meal (Table 2) and the fatty acid composition of the chylomicron-TG remained unchanged until 7 h.
Table 2.
Fatty acid composition (mol%) of the test meal and chylomicron-TG over 24 h
| Fatty Acid |
Time, h |
Meal | |||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 6 | 6.5 | 7 | 24 | ||
| 14:0 | 1.8 (0.8) | 0.7 (0.2) | 1.6 (0.6) | 0.9 (0.3) | 1.0 (0.4) | 1.8 (0.8) | 0.7 |
| 16:0 | 30.7 (2.3) | 26.9 (0.9) | 25.8 (1.4) | 26.4 (1.1) | 26.8 (0.8) | 25.5 (2.0) | 30.4 |
| 16:1 n-7 | 1.4 (0.7) | 0.3 (0.1) | 1.2 (0.7) | 0.8 (0.4) | 0.8 (0.3) | 1.4 (0.5) | |
| 18:0 | 7.5 (1.4) | 5.8 (0.4) | 5.9 (0.5) | 5.4 (0.3) | 6.1 (0.6) | 7.7 (0.8) | 4.6 |
| 18:1 n-9 | 36.9 (1.5) | 29.6 (0.4) | 29.6 (0.9) | 29.9 (0.7) | 31.0 (0.8) | 40.7 (1.7) | 29.6 |
| 18:2 n-6 | 12.8 (2.2) | 32.8 (0.9) | 31.1 (1.8) | 32.9 (1.4) | 32.2 (1.0) | 16.6 (1.3) | 33.9 |
Data are presented as means (SE).
The linoleate content of VLDL-TG increased by 5.4 mol% over the 7-h postprandial period; however, by 24 h, the proportion of linoleate had decreased to be similar to the initial baseline value (Table 3). As the proportion of linoleate increased over time, there was a subsequent decrease in the amount of oleate in VLDL-TG, with palmitate remaining reasonably constant, ∼27 mol% until 24 h (Table 3).
Table 3.
Fatty acid composition (mol%) of the test meal and VLDL-TG over 24 h
| Fatty Acid |
Time, h |
Meal | |||||
|---|---|---|---|---|---|---|---|
| 0 | 4 | 6 | 6.5 | 7 | 24 | ||
| 14:0 | 3.0 (0.5) | 2.5 (0.5) | 2.1 (0.6) | 1.6 (0.4) | 1.5 (0.5) | 2.9 (0.5) | 0.7 |
| 16:0 | 26.6 (1.4) | 27.6 (1.0) | 26.5 (1.1) | 27.7 (0.8) | 27.9 (1.1) | 29.6 (1.6) | 30.4 |
| 16:1 n-7 | 3.6 (0.4) | 2.9 (0.5) | 2.6 (0.5) | 2.6 (0.5) | 2.5 (0.7) | 4.0 (0.6) | |
| 18:0 | 3.4 (0.2) | 3.9 (0.3) | 4.0 (0.4) | 4.2 (0.5) | 4.4 (0.6) | 3.8 (0.6) | 4.6 |
| 18:1 n-9 | 41.4 (2.2) | 40.4 (2.0) | 39.4 (1.5) | 40.7 (1.6) | 37.2 (1.9) | 43.0 (1.8) | 29.6 |
| 18:2 n-6 | 14.3 (1.4) | 18.0 (1.7) | 20.1 (1.3) | 19.6 (2.0) | 19.9 (2.1) | 13.2 (1.8) | 33.9 |
Data are presented as means (SE). VLDL, very low-density lipoprotein.
Incorporation of dietary fatty acids into chylomicron-TG, plasma NEFA, and VLDL-TG.
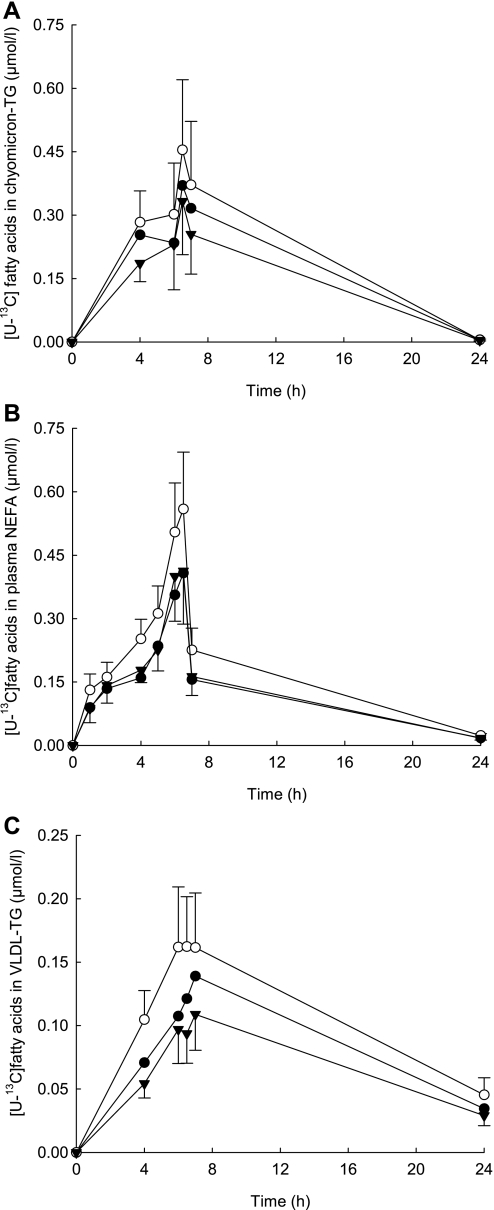
A similar pattern of incorporation of the [U-13C]fatty acids was observed in chylomicron-TG, with an increase in the amount of [U-13C]fatty acids occurring after the consumption of the second meal (at 6 h). There was a tendency for [U-13C]oleate to be higher than [U-13C]palmitate (P = 0.076) and [U-13C]linoleate (P = 0.066), and enrichment was not significantly different between [U-13C]palmitate and [U-13C]linoleate (Fig. 1A). There was a significant difference in the amount and pattern of [U-13C]fatty acids in plasma NEFA (P = 0.003); the concentration of [U-13C]oleate was significantly higher than [U-13C]palmitate and [U-13C]linoleate (P = 0.010 and P = 0.006, respectively; Fig. 1B). This pattern of difference between the fatty acids was maintained in VLDL-TG, with a significant difference in the amount of [U-13C]oleate compared with [U-13C]palmitate and [U-13C]linoleate (P = 0.02 and P = 0.018, respectively; Fig. 1C).
Fig. 1.
Dietary [U-13C]linoleate (▾), [U-13C]oleate (○), and [U-13C]palmitate (•) incorporation into chylomicron-triacylglycerol (TG; A), plasma nonesterified fatty acid (NEFA; B), and very low-density lipoprotein (VLDL)-TG (C) after a mixed meal (0 h), a 75-g glucose drink (6 h), and a habitual diet. Values are means ± SE; n = 12.
Incorporation of dietary fatty acids into plasma CE, PL, and erythrocyte PL.
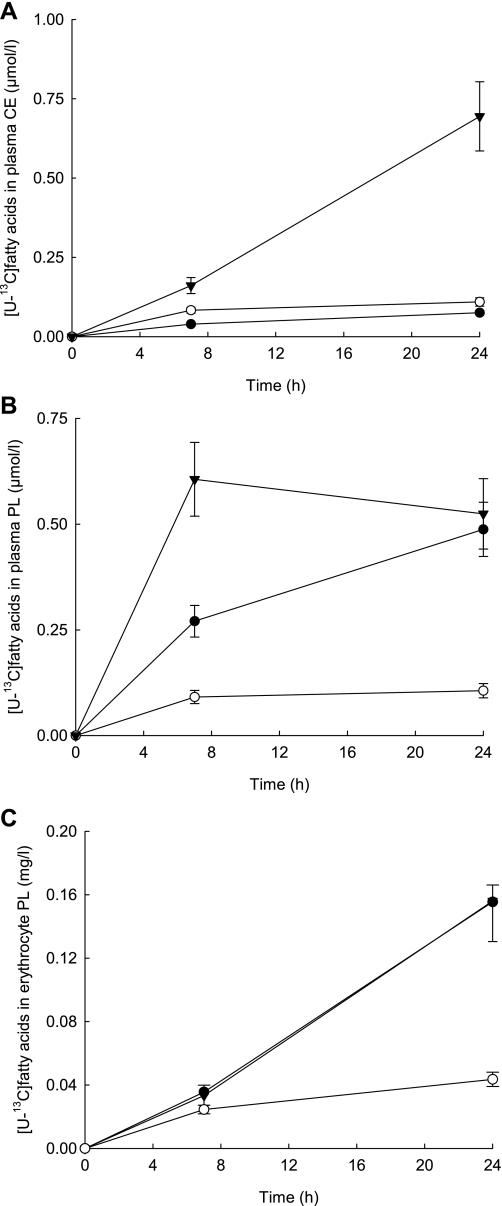
Incorporation of the stable isotope tracers into plasma CE was lower for palmitate and oleate. However, there was marked incorporation of [U-13C]linoleate; the incorporation was over six times greater than the other two fatty acid tracers at 24 h (P = 0.000 for both; Fig. 2A). The increase in [U-13C]linoleate in plasma PL over time was greater and more dynamic compared with [U-13C]palmitate (P = 0.028) and [U-13C]oleate (P = 0.000; Fig. 2B). The concentration of [U-13C]oleate changed little over time, with the concentration only increasing to 0.11 μmol/l by 24 h compared with 0.49 μmol/l for [U-13C]palmitate (P = 0.000) and 0.52 μmol/l for [U-13C]linoleate (P = 0.000). At time 0, the proportion of linoleate, oleate, and palmitate in erythrocyte PL was 7.11 (0.92), 15.1 (0.23), and 38.0 (1.05) mol%, respectively. By 7 h after ingestion of the test meal, fatty acid tracers were detectable in erythrocyte PL. The incorporation of [U-13C]palmitate and [U-13C]linoleate into erythrocyte PL over time was identical such that by 24 h the concentration was 0.16 mg/l for both (Fig. 2C). The concentration of [U-13C]oleate was 75% lower than [U-13C]palmitate (P = 0.000) and [U-13C]linoleate (P = 0.008) by 24 h.
Fig. 2.
Dietary [U-13C]linoleate (▾), [U-13C]oleate (○), and [U-13C]palmitate (•) incorporation into plasma cholesteryl esters (CE; A), plasma phospholipids (PL; B), and erythrocyte PL (C) after a mixed meal (0 h), a 75-g glucose drink (6 h), and a habitual diet. Values are means ± SE; n = 11 for PL and CE; n = 10 for erythrocyte PL.
Contribution of meal fatty acids to specific lipid fractions.
Within the lipid pools measured, we determined the contribution of the respective dietary fatty acids at 4, 7, and 24 h (Table 4). The absolute contribution of dietary linoleate to chylomicron-TG was significantly lower than that of oleate (P < 0.05; Table 4). Surprisingly, the proportion of meal fatty acids contributing to chylomicron-TG at 4 and 7 h was substantially lower than 100%. This implies “dilution” of chylomicron-TG fatty acids by an endogenous pool, which was greatest for linoleate. There were no significant differences among the three fatty acids that each contributed approximately one-third of these specific fatty acids in plasma NEFA. At 4 h, the absolute contribution of oleate to VLDL-TG was significantly (P < 0.05) higher than linoleate and palmitate; however, these differences were not noted at 7 and 24 h, although as discussed above, over the whole time course significant differences were found (Fig. 1C).
Table 4.
Postprandial contribution of meal-derived fatty acids to specific lipid pools within the body at 4, 7, and 24 h
| Lipid Fraction |
Absolute, μmol/l |
Proportion, %
|
||||
|---|---|---|---|---|---|---|
| Linoleate | Oleate | Palmitate | Linoleate | Oleate | Palmitate | |
| Chylomicron-TG | ||||||
| 4 h | 20 (5) | 28 (7)* | 26 (7) | 51 (5) | 72 (5)* | 72 (6)* |
| 7 h | 28 (10) | 36 (15)* | 33 (13) | 49 (5) | 70 (7)* | 72 (7)* |
| Plasma NEFA | ||||||
| 4 h | 19 (3) | 25 (5) | 17 (3) | 31 (3) | 29 (4) | 27 (4) |
| 7 h | 18 (5) | 22 (5) | 16 (4) | 29 (5) | 35 (6) | 32 (5) |
| VLDL-TG | ||||||
| 4 h | 6 (1) | 10 (2)* | 7 (2)† | 15 (2) | 11 (2)* | 11 (2)* |
| 7 h | 12 (3) | 16 (4) | 14 (4) | 22 (2) | 16 (2)* | 17 (2) |
| 24 h | 3 (1) | 4 (1) | 4 (1) | 8 (1) | 3 (1)* | 3 (0)* |
| Plasma CE | ||||||
| 7 h | 17 (3) | 8 (1)* | 4 (0)*† | 1 (0.3) | 2 (0.1) | 1 (0.1)† |
| 24 h | 75 (12) | 11 (1)* | 7 (1)*† | 5 (0.7) | 2 (0.2)* | 2 (0.1)* |
| Plasma PL | ||||||
| 7 h | 60 (12) | 9 (2)* | 24 (4)*† | 10 (2) | 3 (0.3)* | 3 (0.3)* |
| 24 h | 55 (10) | 10 (2)* | 43 (7)† | 10 (2) | 4 (0.4)* | 5 (0.5)*† |
| Erythrocyte PL‡ | ||||||
| 7 h | 4 (1) | 2 (0) | 3 (0) | 1.3 (0.1) | 0.3 (0)* | 0.2 (0)*† |
| 24 h | 17 (3) | 4 (0)* | 14 (1)† | 2.6 (0.5) | 0.5 (0.1)* | 0.7 (0.1)*† |
Data are presented as means (SE). Postprandial contribution was not detectable at 24 h for chylomicron-TG and plasma NEFA and not detectable at 4 h for plasma cholesteryl ester (CE), plasma phospholipid (PL), and erythrocyte PL.
Significantly different from linoleate (P < 0.05);
significantly different from oleate (P < 0.05);
erythrocyte PL was measured as mg/l.
After 7 h, the absolute contribution of dietary fatty acids to plasma CE was highest for linoleate (P < 0.05). By 24 h, linoleate from the test meal contributed 5% of linoleate pool in CE compared with oleate and palmitate, which remained virtually unchanged from 7 h. The contribution of linoleate to plasma PL was greater than for oleate and palmitate (P < 0.05; 60 vs. 9 and 24 μmol/l) at 7 h and made up 10% of the PL-linoleate. This high contribution was maintained at 24 h.
At 24 h, the absolute contribution of linoleate and palmitate to erythrocyte PL was approximately four times higher than oleate (P < 0.05).
DISCUSSION
We found differences in the concentrations of fatty acid tracers from the test meal in almost all blood lipid fractions analyzed. The pattern of differences ([U-13C]oleate > [U-13C]palmitate > [U-13C]linoleate) was maintained in chylomicron-TG, plasma NEFA, and VLDL-TG but altered in plasma CE, PL, and erythrocyte PL.
Chylomicron-TG was extensively composed of meal fatty acids as shown by the fatty acid composition. Using stable isotope tracers and a different test meal, we confirmed the findings of Summers et al. (50) that oleate and palmitate were overrepresented in chylomicron-TG compared with linoleate. This suggests partitioning of linoleate to other lipid pools within the enterocyte, such as PLs (4, 55), and assumes that all three fatty acids are absorbed equally in the intestine. This assumption is likely to hold as studies (27) with tracer enrichment in fecal samples have shown that >98% of meal palmitate and oleate are absorbed. It is also assumed that there is no selectivity of chylomicron hydrolysis (50). Furthermore, we have shown that between 30–50% of chylomicron-TG fatty acids at 4 and 7 h were composed of endogenous fatty acids. This may represent the contribution of fatty acids from a storage pool of TG within the enterocyte, derived from previous meals and then released in response to further ingestion of nutrients (14, 40).
The appearance of [U-13C]fatty acids in the plasma NEFA pool was consistent with “spillover” from chylomicron-TG hydrolysis (15). The higher plasma concentration of [U-13C]oleate could be partly explained by lower systemic uptake. However, this is not supported by studies (16, 17) investigating liver and skeletal muscle fatty acid uptake. There was a sharp rise in the concentration of [U-13C]fatty acids after the glucose drink at 6 h. This reflects the hydrolysis of a second wave of chylomicron release from the enterocytes due to the “second meal effect” (14). At 4 and 7 h, approximately one-third of the specific fatty acids in plasma NEFA was replaced by meal fatty acids. This high incorporation is partly due to the low contribution of endogenous fatty acids (decrease in plasma NEFA concentration) in response to the glucose drink.
In the postprandial period, the fatty acid composition of the meal was not directly reflected in VLDL-TG, as others (6, 41) have previously reported for a nonspecific, i.e., Sf 20-400 VLDL fraction. For example, meal linoleate was 34 mol% but only reached 20 mol% in VLDL-TG. The abundance of oleate in VLDL-TG remained at ∼40 mol% through the postprandial period despite being 30 mol% in the meal. The incorporation of stable isotope tracers revealed that as much as 20% of specific fatty acids were replaced by dietary fatty acids. The magnitude of incorporation, as a percent, was related to the abundance of specific fatty acids, e.g., linoleate had the lowest proportion compared with oleate and palmitate (<20 mol%) but had the highest percent incorporation into VLDL-TG. Over the course of the study, the concentration of [U-13C]oleate was significantly higher than [U-13C]linoleate and [U-13C]palmitate, representing incorporation of dietary fatty acids from plasma NEFA or chylomicron remnants into hepatic pathways (21) but suggesting greater hepatic partitioning than in the intestine or plasma. This has not previously been shown in a postprandial study but concurs with Aarsland and Wolfe (1) who reported that oleate, compared with other fatty acids, is predominantly used for VLDL-TG synthesis. McCloy et al. (31) similarly found greater incorporation of meal [U-13C]oleate compared with [U-13C]linoleate into plasma TG.
The incorporation of [U-13C]linoleate into plasma CE was markedly higher than for the other two tracers. CEs can be formed in the intestine and liver by the action of acyl-CoA-cholesterol acyl transferase (ACAT), which has specificity for oleate (49). Additionally, CEs can be formed in the blood under the influence of lecithin-cholesterol acyl transferase (LCAT), which transfers a fatty acid from phosphatidylcholine (PC) in lipoproteins containing apoA1 (such as HDL) (45). LCAT has specificity for the sn-2 position of PC, whereas the sn-1 position is often taken by a saturated fatty acid (in this case palmitate) (30), which shows high incorporation into PL but not in CE. Our data confirm that the majority of plasma CEs are derived from the action of LCAT rather than ACAT (52). Markedly greater incorporation of meal [U-13C]linoleate compared with [U-13C]oleate into plasma CE has previously been reported (31).
There were striking differences in the incorporation of [U-13C]fatty acids into plasma PLs at 7 h, with the order of magnitude being greatest for [U-13C]linoleate and lowest for [U-13C]oleate as found previously for [U-13C]linoleate compared with [U-13C]oleate (31). By 24 h, the incorporation of [U-13C]linoleate and [U-13C]palmitate was similar. The synthesis of PLs (Kennedy pathway) and remodeling pathway (Lands’ cycle) is a complex pathway, and therefore, our results probably reflect a combination of known specificities of multiple acyl-CoA:lysophospholipid acyltransferases (46) and CoA-dependent transacylases (30, 56).
By 7 h, meal linoleate had replaced 10% of the plasma PL-linoleate pool, and this contribution was three times greater than the other specific meal fatty acids. These fatty acids are most likely to be found in HDL and VLDL. HDL particles have the greatest proportion of PL and are secreted from the intestine and liver and acquire PLs during chylomicron remnant formation (37). Therefore, it is likely in the postprandial period meal fatty acids become incorporated into chylomicron-PL as previously described (4, 55). Hepatic partitioning and storage of dietary fatty acids have previously been demonstrated using an animal model. Moir and Zammit (34–36) demonstrated that meal fatty acids are incorporated into liver PL and that only a small proportion of this is secreted into the blood.
By 24 h, the pattern of incorporation of meal fatty acids in erythrocyte PL was similar to that observed in plasma PL. The experimental time may be insufficient for the release of a new generation of erythrocytes, as <1% of erythrocytes are replaced within 24 h (120-day life span) (9). Thus the contribution to the erythrocyte PL pool via endogenous production is likely to be minor. The rapid increase in dietary fatty acids (within 24 h) suggests incorporation into erythrocyte PL by the acylation of lysophospholipids (5, 8). It is known that fatty acids can be taken up directly by erythrocytes (30, 56). Alternatively, incorporation of dietary fatty acids could be by the direct exchange of PC from blood lipoproteins (5, 8, 38, 39). There was a doubling in the contribution (as a percent) of meal linoleate between 7 and 24 h such that the contribution of meal linoleate to erythrocyte PL-linoleate was over four times greater than meal oleate and palmitate. Nevertheless, equal amounts of linoleate and palmitate from the test meal were incorporated into erythrocyte PL, suggesting preferential incorporation of these fatty acids compared with oleate. This was an unexpected finding given that the proportion of palmitate was more than five times that of linoleate in erythrocyte PL at time 0. However, meal fatty acids in general contributed to only a small proportion (<3%) of the specific fatty acids in erythrocyte PL. To our knowledge, the rapid incorporation of meal linoleate, oleate, and palmitate into erythrocyte PL has not previously been reported.
We investigated the postprandial partitioning of the three most abundant fatty acids in blood. In our protocol, fatty acid stable isotope tracers were incorporated into a single test meal and studied simultaneously thereby mimicking a typical mixed meal. Moreover, we compared fatty acid metabolism over a 24-h period, which revealed the extent to which specific fatty acids are incorporated into blood lipid pools from a single test meal over time. Other studies (25, 26) have compared fatty acids in different test meals, e.g., olive oil vs. safflower oil. Results from such studies reported differences in lipoprotein size and composition, and therefore, such an approach would not be directly comparable with the present study in which the different fatty acids were incorporated into a single test meal. Our results demonstrate that the incorporation of linoleate, oleate, and palmitate follows a similar pattern in chylomicron and VLDL-TG and plasma NEFA. However, the incorporation of the three fatty acids into plasma and erythrocyte PL and plasma CE shows a significantly different pattern, consistent with transfer from plasma lipoproteins and in accordance with known enzyme specificities, and we are the first, to our knowledge, to report the magnitude to which meal fatty acids are incorporated. Our results suggest that substrate supply, in this case meal fatty acids, can also influence PL fatty acid composition. Of the three fatty acids studied, oleate was incorporated the least into PL and CE, implying that more could be available for oxidation, as has been previously found (2, 31, 43). The quantitatively significant incorporation of linoleate from a single meal into blood PL fractions within 24 h may help to maintain membrane integrity and may explain why linoleate is a useful biomarker of dietary intake. Obtaining a biomarker of dietary intake is important in relation to health and disease to provide an objective measure of dietary intake (22). For example, adipose tissue and platelet linoleate have been inversely associated with cardiovascular risk (54) and serum CE-linoleate has been inversely associated with the risk of developing type 2 diabetes (53). Further studies are required to specifically investigate fatty acid partitioning in different disease states.
GRANTS
L. Hodson held the Girdlers’ Health Research Council New Zealand Fellowship. S. E. McQuaid is a Wellcome Trust Clinical Training Fellow. F. Karpe is a Wellcome Trust Senior Clinical fellow.
Acknowledgments
We thank Ross Milne from the Ottawa Heart Institute for kindly providing the antibodies; Toralph Ruge, Louise Dennis, and Jane Cheeseman for expert assistance with the clinical studies; Marjorie Gilbert for sample analysis; and Sandy Humphreys for expert statistical advice.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aarsland A, Wolfe RR. Hepatic secretion of VLDL fatty acids during stimulated lipogenesis in men. J Lipid Res 39: 1280–1286, 1998. [PubMed] [Google Scholar]
- 2.Bergouignan A, Schoeller DA, Normand S, Gauquelin-Koch G, Laville M, Shriver T, Desage M, Le Maho Y, Ohshima H, Gharib C, Blanc S. Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary Fatty acids: results of a randomized trial. PLoS Clin Trials 1: e27, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bickerton AS, Roberts R, Fielding BA, Hodson L, Blaak EE, Wagenmakers AJ, Gilbert M, Karpe F, Frayn KN. Preferential uptake of dietary fatty acids in adipose tissue and muscle in the postprandial period. Diabetes 56: 168–176, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Bierman EL, Gordis E, Hamlin JT 3rd. Heterogeneity of fat particles in plasma during alimentary lipemia. J Clin Invest 41: 2254–2260, 1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brossard N, Croset M, Normand S, Pousin J, Lecerf J, Laville M, Tayot JL, Lagarde M. Human plasma albumin transports [13C]docosahexaenoic acid in two lipid forms to blood cells. J Lipid Res 38: 1571–1582, 1997. [PubMed] [Google Scholar]
- 6.Bysted A, Holmer G, Lund P, Sandstrom B, Tholstrup T. Effect of dietary fatty acids on the postprandial fatty acid composition of triacylglycerol-rich lipoproteins in healthy male subjects. Eur J Clin Nutr 59: 24–34, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chong MFF, Fielding B, Frayn K. Mechanisms for the acute effect of fructose on postprandial lipemia. Am J Clin Nutr 85: 1511–1520, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Donabedian RK, Karmen A. Fatty acid transport and incorporation into human erythrocytes in vitro. J Clin Invest 46: 1017–1027, 1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebaugh FG, Emerson CP, Ross JF. The use of radioactive chromium 51 as an erythrocyte tagging agent for the determination or red cell survival in vivo. J Clin Invest 32: 1260–1276, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emken EA, Rohwedder WK, Adlof RO, Rakoff H, Gulley RM. Metabolism in humans of cis-12,trans-15-octadecadienoic acid relative to palmitic, stearic, oleic and linoleic acids. Lipids 22: 495–504, 1987. [DOI] [PubMed] [Google Scholar]
- 11.Emken EA, Rohwedder WK, Dutton HJ, Dejarlais WJ, Adlof RO. Incorporation of deuterium-labeled cis- and trans-9-octadecenoic acids in humans: plasma, erythrocyte, and platelet phospholipids. Lipids 14: 547–554, 1979. [DOI] [PubMed] [Google Scholar]
- 12.Evans K, Burdge GC, Wootton SA, Clark ML, Frayn KN. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes 51: 2684–2690, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Farquhar JW, Ahrens EH Jr. Effects of dietary fats on human erythrocyte fatty acid patterns. J Clin Invest 42: 675–685, 1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fielding BA, Callow J, Owen RM, Samra JS, Matthews DR, Frayn KN. Postprandial lipemia: the origin of an early peak studied by specific dietary fatty acid intake during sequential meals. Am J Clin Nutr 63: 36–41, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Fielding BA, Frayn KN. Lipoprotein lipase and the disposition of dietary fatty acids. Br J Nutr 80: 495–502, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Hagenfeldt L, Wahren J. Human forearm muscle metabolism during exercise. II. Uptake, release and oxidation of individual FFA and glycerol. Scand J Clin Lab Invest 21: 263–276, 1968. [DOI] [PubMed] [Google Scholar]
- 17.Hagenfeldt L, Wahren J, Pernow B, Raf L. Uptake of individual free fatty acids by skeletal muscle and liver in man. J Clin Invest 51: 2324–2330, 1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Dietary fatty acids make a rapid and substantial contribution to VLDL-triacylglycerol in the fed state. Am J Physiol Endocrinol Metab 292: E732–E739, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Heath RB, Karpe F, Milne RW, Burdge GC, Wootton SA, Frayn KN. Selective partitioning of dietary fatty acids into the VLDL TG pool in the early postprandial period. J Lipid Res 44: 2065–2072, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Henderson L, Gregory J, Irving K, Swan G. The National Diet and Nutrition Survey: Adults Aged 19 to 64 Years. London, UK: National Diet and Nutrition Survey Food Standards Agency and the Departments of Health by the Social Survey Division of the Office for National Statistics and Medical Research Council Human Nutrition Research, 2003, p. 1–106.
- 21.Hodson L, Bickerton AS, McQuaid SE, Roberts R, Karpe F, Frayn KN, Fielding BA. The contribution of splanchnic fat to VLDL triglyceride is greater in insulin-resistant than insulin-sensitive men and women: studies in the postprandial state. Diabetes 56: 2433–2441, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res 47: 348–380, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Hodson L, Skeaff CM, Wallace AJ, Arribas GL. Stability of plasma and erythrocyte fatty acid composition during cold storage. Clin Chim Acta 321: 63–67, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Holub BJ, Bakker DJ, Skeaff CM. Alterations in molecular species of cholesterol esters formed via plasma lecithin-cholesterol acyltransferase in human subjects consuming fish oil. Atherosclerosis 66: 11–18, 1987. [DOI] [PubMed] [Google Scholar]
- 25.Jackson KG, Robertson MD, Fielding BA, Frayn KN, Williams CM. Measurement of apolipoprotein B-48 in the Svedberg flotation rate [S(f)]>400, S(f) 60-400 and S(f) 20-60 lipoprotein fractions reveals novel findings with respect to the effects of dietary fatty acids on triacylglycerol-rich lipoproteins in postmenopausal women. Clin Sci (Lond) 103: 227–237, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Jackson KG, Robertson MD, Fielding BA, Frayn KN, Williams CM. Olive oil increases the number of triacylglycerol-rich chylomicron particles compared with other oils: an effect retained when a second standard meal is fed. Am J Clin Nutr 76: 942–949, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Jones AE, Stolinski M, Smith RD, Murphy JL, Wootton SA. Effect of fatty acid chain length and saturation on the gastrointestinal handling and metabolic disposal of dietary fatty acids in women. Br J Nutr 81: 37–43, 1999. [PubMed] [Google Scholar]
- 28.Karpe F, Steiner G, Olivecrona T, Carlson LA, Hamsten A. Metabolism of triglyceride-rich lipoproteins during alimentary lipemia. J Clin Invest 91: 748–758, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katan MB, Deslypere JP, van Birgelen AP, Penders M, Zegwaard M. Kinetics of the incorporation of dietary fatty acids into serum cholesteryl esters, erythrocyte membranes, and adipose tissue: an 18-month controlled study. J Lipid Res 38: 2012–2022, 1997. [PubMed] [Google Scholar]
- 30.MacDonald JI, Sprecher H. Phospholipid fatty acid remodeling in mammalian cells. Biochim Biophys Acta 1084: 105–121, 1991. [DOI] [PubMed] [Google Scholar]
- 31.McCloy U, Ryan MA, Pencharz PB, Ross RJ, Cunnane SC. A comparison of the metabolism of eighteen-carbon 13C-unsaturated fatty acids in healthy women. J Lipid Res 45: 474–485, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Mekki N, Charbonnier M, Borel P, Leonardi J, Juhel C, Portugal H, Lairon D. Butter differs from olive oil and sunflower oil in its effects on postprandial lipemia and triacylglycerol-rich lipoproteins after single mixed meals in healthy young men. J Nutr 132: 3642–3649, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Mitchell DC, McMahon KE, Shively CA, Apgar JL, Kris-Etherton PM. Digestibility of cocoa butter and corn oil in human subjects: a preliminary study. Am J Clin Nutr 50: 983–986, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Moir AM, Zammit VA. Acute meal-induced changes in hepatic glycerolipid metabolism are unimpaired in severely diabetic rats: implications for the role of insulin. FEBS Lett 370: 255–258, 1995. [DOI] [PubMed] [Google Scholar]
- 35.Moir AM, Zammit VA. Monitoring of changes in hepatic fatty acid and glycerolipid metabolism during the starved-to-fed transition in vivo. Studies on awake, unrestrained rats. Biochem J 289: 49–55, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moir AM, Zammit VA. Selective labelling of hepatic fatty acids in vivo. Studies on the synthesis and secretion of glycerolipids in the rat. Biochem J 283: 145–149, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Redgrave TG, Small DM. Quantitation of the transfer of surface phospholipid of chylomicrons to the high density lipoprotein fraction during the catabolism of chylomicrons in the rat. J Clin Invest 64: 162–171, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed CF Phospholipid exchange between plasma and erythrocytes in man and the dog. J Clin Invest 47: 749–760, 1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renooij W, Van Golde LM. The transposition of molecular classes of phosphatidylcholine across the rat erythrocyte membrane and their exchange between the red cell membrane and plasma lipoproteins. Biochim Biophys Acta 470: 465–474, 1977. [DOI] [PubMed] [Google Scholar]
- 40.Robertson MD, Parkes M, Warren BF, Ferguson DJ, Jackson KG, Jewell DP, Frayn KN. Mobilisation of enterocyte fat stores by oral glucose in humans. Gut 52: 834–839, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruiz-Gutierrez V, Morgado N, Prada JL, Perez-Jimenez F, Muriana FJ. Composition of human VLDL triacylglycerols after ingestion of olive oil and high oleic sunflower oil. J Nutr 128: 570–576, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Sakr SW, Attia N, Haourigui M, Paul JL, Soni T, Vacher D, Girard-Globa A. Fatty acid composition of an oral load affects chylomicron size in human subjects. Br J Nutr 77: 19–31, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt DE, Allred JB, Kien CL. Fractional oxidation of chylomicron-derived oleate is greater than that of palmitate in healthy adults fed frequent small meals. J Lipid Res 40: 2322–2332, 1999. [PubMed] [Google Scholar]
- 44.Schofield WN Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr 39, Suppl 1: 5–41, 1985. [PubMed] [Google Scholar]
- 45.Sgoutas DS Fatty acid specificity of plasma phosphatidylcholine: cholesterol acyltransferase. Biochemistry 11: 293–296, 1972. [DOI] [PubMed] [Google Scholar]
- 46.Shindou H, Shimizu T. Acyl-CoA:lysophospholipid acyltransferases. J Biol Chem 2008. Aug 21 [Epub ahead of print]. [DOI] [PubMed]
- 47.Skeaff CM, Hodson L, McKenzie JE. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr 136: 565–569, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Snyder WS Report of the Task Force on Reference Man. Oxford, UK: Pergamon Press for the International Commission on Radiological Protection.
- 49.Spector AA, Mathur SN, Kaduce TL. Role of acylcoenzyme A:cholesterol o-acyltransferase in cholesterol metabolism. Prog Lipid Res 18: 31–53, 1979. [DOI] [PubMed] [Google Scholar]
- 50.Summers LK, Barnes SC, Fielding BA, Beysen C, Ilic V, Humphreys SM, Frayn KN. Uptake of individual fatty acids into adipose tissue in relation to their presence in the diet. Am J Clin Nutr 71: 1470–1477, 2000. [DOI] [PubMed] [Google Scholar]
- 51.Thomsen C, Rasmussen O, Lousen T, Holst JJ, Fenselau S, Schrezenmeir J, Hermansen K. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Am J Clin Nutr 69: 1135–1143, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Ueno K, Sakuma N, Kawaguchi M, Fujinami T, Okuyama H. Selectivity and contribution of lecithin: cholesterol acyltransferase to plasma cholesterol ester formation. J Biochem (Tokyo) 99: 541–547, 1986. [DOI] [PubMed] [Google Scholar]
- 53.Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes 43: 1353–1357, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Wood DA, Riemersma RA, Butler S, Thomson M, Macintyre C, Elton RA, Oliver MF. Linoleic and eicosapentaenoic acids in adipose tissue and platelets and risk of coronary heart disease. Lancet 1: 177–183, 1987. [DOI] [PubMed] [Google Scholar]
- 55.Wood P, Imaichi K, Knowles J, Michaels G, Kinsell L. The lipid composition of human plasma chylomicrons. J Lipid Res 5: 225–231, 1964. [PubMed] [Google Scholar]
- 56.Yamashita A, Sugiura T, Waku K. Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells. J Biochem (Tokyo) 122: 1–16, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Yli-Jokipii K, Kallio H, Schwab U, Mykkanen H, Kurvinen JP, Savolainen MJ, Tahvonen R. Effects of palm oil and transesterified palm oil on chylomicron and VLDL triacylglycerol structures and postprandial lipid response. J Lipid Res 42: 1618–1625, 2001. [PubMed] [Google Scholar]
- 58.Yli-Jokipii KM, Schwab US, Tahvonen RL, Kurvinen JP, Mykkanen HM, Kallio HP. Chylomicron and VLDL TAG structures and postprandial lipid response induced by lard and modified lard. Lipids 38: 693–703, 2003. [DOI] [PubMed] [Google Scholar]