Abstract
Adiponectin, an adipokine secreted by the white adipose tissue, plays an important role in regulating glucose and lipid metabolism and controlling energy homeostasis in insulin-sensitive tissues. A decrease in the circulating level of adiponectin has been linked to insulin resistance, type 2 diabetes, atherosclerosis, and metabolic syndrome. Adiponectin exerts its effects through two membrane receptors, AdipoR1 and AdipoR2. APPL1 is the first identified protein that interacts directly with adiponectin receptors. APPL1 is an adaptor protein with multiple functional domains, the Bin1/amphiphysin/rvs167, pleckstrin homology, and phosphotyrosine binding domains. The PTB domain of APPL1 interacts directly with the intracellular region of adiponectin receptors. Through this interaction, APPL1 mediates adiponectin signaling and its effects on metabolism. APPL1 also functions in insulin-signaling pathway and is an important mediator of adiponectin-dependent insulin sensitization in skeletal muscle. Adiponectin signaling through APPL1 is necessary to exert its anti-inflammatory and cytoprotective effects on endothelial cells. APPL1 also acts as a mediator of other signaling pathways by interacting directly with membrane receptors or signaling proteins, thereby playing critical roles in cell proliferation, apoptosis, cell survival, endosomal trafficking, and chromatin remodeling. This review focuses mainly on our current understanding of adiponectin signaling in various tissues, the role of APPL1 in mediating adiponectin signaling, and also its role in the cross-talk between adiponectin/insulin-signaling pathways.
Keywords: adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain, and leucine zipper motif
a decrease in adiponectin level is observed in various clinical conditions like type 2 diabetes, obesity, insulin resistance (4, 67, 70, 148), cardiovascular disease (93, 134), and hypertension (1). Adiponectin is an adipokine synthesized and secreted mainly by adipose tissue (76, 142). However, recent reports indicate that adiponectin is also synthesized by cardiomyocytes, skeletal muscle, osteoblasts, placenta, and pituitary (11, 19, 40, 90, 133, 137, 146). Adiponectin exerts its beneficial effect on metabolism by improving insulin sensitivity, glucose tolerance, and lipid profile and by decreasing atherosclerosis and inflammation (77). Adiponectin is a 30-kDa protein having an NH2-terminal collagen domain and a COOH-terminal globular domain. In serum, adiponectin exists either as multimers of full-length adiponectin or as globular adiponectin generated by the cleavage of full-length adiponectin by neutrophil elastase and other proteases (52, 76, 163). Adiponectin exerts its effect mainly by activating AMP-activated protein kinase (AMPK), p38 mitogen-activated protein kinase (MAPK), and peroxisome proliferator-activated receptor-α (PPARα) in skeletal muscle and liver, thereby decreasing the level of glucose and lipid in vivo. This effect of adiponectin is mainly through enhancement of fatty acid oxidation and glucose uptake in muscle and inhibition of gluconeogenesis in liver (172, 173), which are mediated by two cell surface receptors, AdipoR1 and AdipoR2 (173), by a direct interaction with the extracellular COOH terminus of these receptors (110, 173). APPL1 is the “missing link” in the adiponectin-signaling cascade, transmitting signals from adiponectin receptors to downstream targets by directly interacting with the NH2-terminal intracellular region of AdipoR1 and AdipoR2 (110).
APPL was initially identified as an Akt2-binding protein in a yeast-two hybrid screening (119) and is named after its unique structure, an adaptor protein containing pleckstrin homology (PH) domain, phosphotyrosine binding (PTB) domain, and leucine zipper motif. Because of its ability to interact with the tumor suppressor protein, deleted in colorectal cancer (DCC), APPL is also known as DCC-interacting protein (DIP-13α) (101). DIP-13β is the isoform of DIP-13α (101), and currently, APPL/DIP-13α is known as APPL1, whereas DIP-13β is called APPL2 (110, 116). Encoded in the chromosomal region 3p14.3–21.1 (119), human APPL1 containing 709 amino acids is highly hydrophilic and has no transmembrane domain (101). APPL1 does not possess a classical nuclear localization signal or nuclear export signal; however, nuclear localization in response to epidermal growth factor (EGF) or oxidative stress has been reported (116). APPL1 interacts with nearly 14 proteins, including membrane receptors and signaling molecules in various signaling pathways to mediate apoptosis (101), cell proliferation, chromatin remodeling (116), endosomal localization of proteins (161), and cell survival (141). Recently, emerging data suggest that APPL1 also plays a key role in regulating metabolism and insulin sensitivity by mediating adiponectin signaling (28, 110). We begin this review by giving an overview of the current understanding of adiponectin signaling in various tissues. The main focus of this review is on the role of APPL1 in adiponectin and insulin-signaling pathways and the mechanism by which APPL1 exerts the insulin-sensitizing effects of adiponectin.
ADIPONECTIN SIGNALING IN VARIOUS TISSUES AND THE ROLE OF APPL1
Adiponectin exhibits antidiabetic, anti-inflammatory, and antiatherogenic effects and also functions as an insulin sensitizer; hence, it is a novel therapeutic target for diabetes and metabolic syndrome (76, 86, 128). Adiponectin also plays a central role in energy homeostasis through its action in hypothalamus, and a new role for adiponectin as a “starvation gene” has been proposed (78, 92). In the following section, we will review the signal transduction of adiponectin in different tissues and the role of APPL1 in mediating the effects of adiponectin.
Skeletal Muscle
In type 2 diabetes and obesity, impairment of glucose and lipid metabolism results in the accumulation of lipid in insulin target tissues leading to insulin resistance (79, 147). AdipoR1 is the most abundantly expressed adiponectin receptor in skeletal muscle, one of the insulin target tissues. Most of the adiponectin effects in muscle are mediated by globular adiponectin, since globular adiponectin has a higher affinity for AdipoR1 (173). In C2C12 myocytes, adiponectin activates AMPK, p38 MAPK, and PPARα ligand activities, which increase fatty acid oxidation and glucose uptake in these cells (173). AMPK is a key sensor of cellular energy status (61), and adiponectin-mediated activation of AMPK in skeletal muscle increases glucose uptake, fatty acid oxidation, and lactate production (172). p38 MAPK regulates various cellular processes, including inflammation, cell differentiation, cell growth, and cell death and is activated in response to a variety of extracellular stimuli, including metabolic stress (126). In C2C12 myocytes, globular adiponectin-mediated fatty acid oxidation and glucose uptake are not solely by activation of AMPK but also by p38 MAPK activation (173). A study by Yoon et al. (181) suggests that, in C2C12 myotubes, adiponectin-stimulated fatty acid oxidation is by the sequential activation of AMPK, p38 MAPK, and PPARα. PPARα is a key regulator of fatty acid oxidation and p38 MAPK phosphorylates and activates PPARα and its coactivator PPARγ coactivator-1α (8, 135). Unlike activated AMPK and p38 MAPK, PPARα is involved only in fatty acid metabolism, not glucose uptake, in response to adiponectin stimulation (173).
Skeletal muscle has a relatively high expression of APPL1 and AdipoR1, and in C2C12 myotubes interaction between APPL1 and AdipoR1 is stimulated by adiponectin (110). APPL1 acts as a positive regulator of adiponectin signaling in muscle cells, since overexpression of APPL1 in C2C12 myocytes significantly increases phosphorylation levels of AMPK and p38 MAPK. Overexpression of APPL1 (ΔPTB) (AdipoR1-binding deficient mutant of APPL1) or suppression of APPL1 by siRNA failed to mediate adiponectin-stimulated AMPK and p38 MAPK activation. Suppression of APPL1 in C2C12 myotubes significantly attenuated adiponectin-stimulated phosphorylation of AMPK, p38 MAPK, acetyl-CoA carboxylase (ACC), and fatty acid oxidation, suggesting the crucial role of APPL1 in mediating adiponectin-regulated lipid metabolism in skeletal muscle cells (110).
Adiponectin decreases the resting blood glucose level in rodents and also protects animals with diet-induced obesity from developing insulin resistance (52, 173, 174). This glucose-lowering effect of adiponectin is mainly through glucose uptake in skeletal muscle by AMPK activation (173) and also by glucose transporter 4 (GLUT4) membrane translocation (22). Both full-length and globular adiponectin stimulate GLUT4 membrane translocation in L6 cells (110). Whereas overexpression of APPL1 enhances GLUT4 membrane translocation, suppression of APPL1 significantly reduces GLUT4 membrane translocation in L6 cells and also adiponectin-stimulated glucose uptake in C2C12 myotubes (110). Adiponectin-mediated GLUT4 membrane translocation is partially through activated AMPK and p38 MAPK, whereas the rest is through the small GTPase Rab5. Adiponectin stimulated the interaction between APPL1 and Rab5, and the disruption of APPL1-Rab5 interaction blocked adiponectin-mediated translocation of GLUT4 to the membrane (110). Adiponectin-stimulated p38 MAPK activation is inhibited by the overexpression of dominant negative Rab5, indicating that activation of p38 MAPK by adiponectin is partly through APPL1-Rab5 association (110).
Vascular Endothelium
A decreased level of circulating adiponectin is correlated with inflammation and increased incidence of cardiovascular disease (9, 112, 134, 144). In mice, adiponectin deficiency causes severe neointimal thickening due to vascular smooth muscle cell proliferation in injured arteries. Adiponectin supplementation attenuates this effect by suppressing vascular muscle cell migration (111). This protective effect of adiponectin is mediated by increasing the production of nitric oxide (NO) through activation of AMPK and endothelial NO synthase (eNOS) (26, 94, 131, 155, 169). NO protects the endothelial cells by inhibiting platelet aggregation, monocyte adhesion, and smooth muscle cell proliferation and also by increasing vasodilation (59, 72, 131, 169). Cheng et al. (28) found that in human umbilical vein endothelial cells the effects of adiponectin are mediated through adiponectin receptors AdipoR1 and AdipoR2. Their study identified APPL1 as a signaling protein mediating the downstream signaling events from adiponectin receptors to eNOS for NO production. Similar to the finding by Mao et al. (110) in C2C12 myotubes, in human umbilical vein endothelial cells also the cytoplasmic tail of adiponectin receptors interacts with APPL1. The critical role of APPL1 in adiponectin-induced vasodilation is demonstrated by the inability of adiponectin to induce NO production in APPL1 knockdown cells. Suppression of APPL1 expression resulted in a significant decrease in the phosphorylation of AMPK and eNOS and a decrease in the complex formation between eNOS and heat shock protein 90, which in turn decreases NO production, in response to adiponectin (28). In db/db mice, adiponectin-induced vasodilation is significantly lower than in their lean littermates, and the expression level of APPL1 mRNA is selectively decreased in small mesenteric arteries in these animals (28). This suggests a direct relationship between decreased APPL1 expression and impaired vasodilation, which in turn leads to endothelial dysfunction in diabetes.
In addition to its protective effect against atherosclerosis (4, 66, 128), adiponectin also exhibits anti-inflammatory effects, especially in endothelial cells and macrophages (128, 129, 180). Whereas the high-molecular-weight form of adiponectin has anti-inflammatory and antiapoptotic roles (87), the globular domain of adiponectin has cytoprotective effects (100, 125, 130). An inverse relationship exists between inflammation and adiponectin level, since chronic inflammatory diseases such as coronary heart disease are characterized by hypoadiponectinemia (143). Not only adiponectin but also its receptors AdipoR1 and AdipoR2 are downregulated in chronic inflammation associated with diabetes, obesity, and insulin resistance (77). Interleukin-18 (IL-18) is a proinflammatory and proapoptotic cytokine, the increased expression of which is correlated with acute coronary syndromes (89, 108). Treatment of endothelial cells with IL-18 causes cell death by inhibiting Akt activity and also by inducing phosphatase and tensin homolog activation through IκB kinase-nuclear factor-κB (NF-κB)-dependent pathway. Adiponectin treatment blocks IL-18-mediated cell death through APPL1-mediated activation of AMPK. This in turn activates Akt and inhibits IκB kinase-NF-κB-phosphatase and tensin homolog signaling and caspase-3 activation (23). The reversal of IL-18-mediated cell death by adiponectin is APPL1 dependent, since APPL1 knockdown reverses adiponectin's prosurvival effects by inhibiting AMPK phosphorylation (23).
Hypothalamus
Hypothalamus plays a major role in the regulation of food intake and body weight. An increase in the activity of AMPK in arcuate hypothalamus has been shown to stimulate food intake (20, 60, 117). Kubota et al. (92) demonstrated that administration of adiponectin stimulates food intake and decreases energy expenditure by increasing the phosphorylation levels of AMPK and its downstream target ACC in hypothalamus and thereby plays an important role in maintaining energy homeostasis. Both AdipoR1 and AdipoR2 are expressed in the paraventricular nuclei of hypothalamus (35, 92) and also in the neurons of arcuate and lateral hypothalamic nuclei (35). Stimulation of food intake by adiponectin is mediated specifically through AdipoR1 in hypothalamus (92). APPL1, the mediator of adiponectin signaling, is also present in brain (110). Coope et al. (35) demonstrated that following an intracerebroventricular injection of adiponectin to rat hypothalamus, AdipoR1 and AdipoR2 interact with APPL1. The adiponectin injection to hypothalamus promoted anorexigenic condition in rats with a 40% reduction in food intake. In addition, adiponectin injection also increases signal transduction through the classical insulin- and leptin-signaling pathways, and these effects of adiponectin are mediated through AdipoR1, not AdipoR2 (35). Adiponectin injection to the hypothalamus increases phosphorylation levels of insulin receptor substrate (IRS)-1, IRS2, Akt, ERK, forkhead transcription factor 1 (FOXO1), JAK2, and signal transducer and activator of transcription 3, indicating the existence of cross-talk between adiponectin-insulin and adiponectin-leptin pathways in hypothalamus (35). How leptin pathway cross-talks with adiponectin pathway and what the role of APPL1 is in this cross-talk is not known. The discrepancy in terms of food intake as demonstrated by Kubota et al. (92) and Coope et al. (35) could account for the method used for adiponectin administration.
From these studies it is evident that APPL1-AdipoR1 interaction stimulated by adiponectin is a common mechanism for mediating adiponectin signal in various tissues like muscle (110), vascular endothelium (23, 28), and hypothalamus (35). At present, it is not clear whether such a relationship exists in other tissues that coexpress both APPL1 and AdipoR1.
Liver
In liver, adiponectin-stimulated activation of AMPK decreases gluconeogenesis by attenuating the expression level of phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (172), thereby reducing glucose level in vivo. db/db Mouse liver has decreased mRNA expression levels of AdipoR1 and AdipoR2, and overexpression of AdipoR1 or AdipoR2 in the liver of db/db mice markedly improves glucose intolerance and insulin resistance (175). Overexpression of AdipoR1 activates AMPK, reduces hepatic glucose production, and increases fatty acid oxidation. On the contrary, overexpression of AdipoR2 activates PPARα pathway, which increases fatty acid oxidation and inhibits inflammation and oxidative stress (175). Thus, in liver, AdipoR1 and AdipoR2 differ in their signaling pathways. In agreement with these findings, AdipoR1 knockout mice have increased expression of gluconeogenic enzymes in liver with impaired glucose tolerance and insulin resistance. AdipoR2 knockout mice have normal hepatic glucose production with increased insulin levels (175). AdipoR2 knockout mice are also resistant to high-fat-induced obesity and insulin resistance (14, 102). Whether APPL1 is involved in mediating these downstream effects of adiponectin through AdipoR1 and AdipoR2 in liver is unknown. However, overexpression of APPL1 in mouse hepatocyte cells can activate p38 MAPK, and adiponectin treatment could further enhance this effect, suggesting that APPL1 plays a role in adiponectin signaling in liver (110).
Adipocyte
Both AdipoR1 and AdipoR2 are expressed in adipose tissue (12). In rat adipocytes, globular adiponectin treatment increases glucose uptake by activating AMPK (168). In 3T3-L1 adipocytes, overexpression of adiponectin promotes adipocyte differentiation, increases lipid content, and enhances insulin sensitivity (54). These studies suggest that adiponectin exerts autocrine effects on adipocytes. ob/ob Mice overexpressing adiponectin are obese with higher contents of adipose tissue than ob/ob littermates. Hence, adiponectin promotes storage of fat preferentially in adipose tissue and thereby increases insulin sensitivity (86). AdipoR1 male knockout mice have increased total body fat mass, reproductive white adipose tissue, perirenal white adipose tissue, and brown adipose tissue, and this fat accumulation is due to decreased energy expenditure (14). Although presence of APPL1 in adipocytes is demonstrated (110, 140), it is not known whether adiponectin signaling in adipocytes is mediated through APPL1. A role for APPL1 in insulin signaling in adipocytes is reported. In primary rat adipocytes, APPL1 forms a complex with Akt2 that is dissociated with insulin treatment (140). In 3T3-L1 adipocytes, APPL1 is involved in insulin-mediated Akt phosphorylation, glucose uptake, and GLUT4 membrane translocation (140).
Bone
Adiponectin, AdipoR1, and AdipoR2 are expressed in osteoblasts (11, 105, 127, 146), suggesting that adiponectin has functions in bone. Osteoblast is a direct target of adiponectin, since adiponectin induces osteoblast proliferation through c-jun NH2-terminal kinase pathway and differentiation through p38 MAPK pathway, and these effects of adiponectin are mediated through AdipoR1 (105). Adiponectin plays a role in regulation of bone mass, since it increases bone mass by suppressing osteoclastogenesis and by activating osteoblastogenesis (125). Adiponectin-mediated activation of AMPK promotes differentiation and mineralization in MC3T3-E1 cells, and knockdown of AdipoR1 abrogates these effects (80). Adiponectin has a protective effect on bone metabolism in patients with type 2 diabetes, since adiponectin is positively associated with bone mineral density at the distal radius in these patients (154). In diseases associated with cytokine activation, adiponectin acts as a regulator of bone resorption by inhibiting tumor necrosis factor-α and receptor activator of NF-κB ligand-induced osteoclastogenesis through activation of AMPK (176). Expression of APPL1 mRNA in human bone marrow, which contains the precursors for osteoblasts and osteoclasts, has been reported (29). Since adiponectin can activate AMPK in bone cells through adiponectin receptors, the involvement of APPL1 in this activation can be expected.
Kidney
Expression of adiponectin receptors AdipoR1 and AdipoR2 in kidney is reported (145). A decrease in AMPK activity due to adiponectin deficiency causes albuminuria, which is an increased risk factor for cardiovascular diseases and is also associated with diabetes and obesity (145). Decreased AMPK activity causes podocyte dysfunction by increasing oxidative stress, and either administration of adiponectin or activation of AMPK reverses this effect. In podocytes, adiponectin exerts a direct effect through adiponectin receptors since both AdipoR1 and AdipoR2 are expressed in podocytes. Although the expression level of AdipoR1 in kidney and podocyte is comparable to that in liver, expression level of AdipoR2 is much lower (145). Although APPL1 is present in kidney (44), it is not known whether the effects of adiponectin are mediated through APPL1.
Other Tissues
Besides its major role in metabolism, adiponectin also regulates a wide range of biological functions, including tumor progression and pituitary hormone secretion (84, 88, 137). This finding is supported by a widespread expression of AdipoR1 and AdipoR2 not only in skeletal muscle, liver, brain, and heart but also in kidney, lung, spleen, testis (173), pituitary, pancreatic β-cells, and placenta (19, 85, 137). Interestingly, mRNA and protein expression of APPL1 is also reported in a wide range of tissues, including those expressing adiponectin receptors (29, 110). However, it is unknown whether APPL1 participates in the mediation of adiponectin signal through AdipoR1 and AdipoR2 in tissues that coexpress APPL1 and adiponectin receptors.
MECHANISM OF ADIPONECTIN-STIMULATED AMPK ACTIVATION MEDIATED THROUGH APPL1
Activation of AMPK by adiponectin is a key step in mediating most of the effects of this adipokine (Fig. 1). AMPK is a key sensor of cellular energy status, and activation of AMPK shuts down ATP-consuming processes like biosynthesis, cell growth, and proliferation while switching on catabolic pathways that generate ATP (61, 156). However, the molecular mechanism of adiponectin-mediated AMPK activation through APPL1 is unknown. The finding that selective phosphoinositide 3-kinase (PI3K) inhibitors can abolish adiponectin-stimulated AMPK phosphorylation in endothelial cells suggests a role for PI3K in AMPK activation (26, 169). The involvement of PI3K is further supported by the finding that activation of AMPK/eNOS pathway by metformin is dependent on PI3K (38). In addition, both p85 and p110 subunits of PI3K interact with APPL1 (119, 177). However, there is no evidence to show that PI3K is a direct upstream kinase of AMPK. In general, AMPK is activated either by an increase in cellular AMP/ATP ratio or by its upstream kinases LKB1 or calmodulin-dependent protein kinase kinase (13, 61). At the moment, it is unclear whether PI3K can activate AMPK via LKB1 or calmodulin-dependent protein kinase kinase and whether adiponectin has any effect on APPL1-PI3K interaction. Solving these questions will help the mechanism of adiponectin-mediated AMPK activation be fully understood.
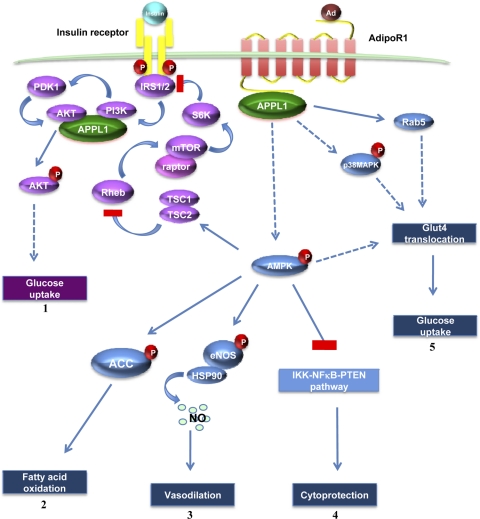
Fig. 1.
Model of adaptor protein containing pleckstrin homology (PH) domain, phosphotyrosine-binding (PTB) domain, and leucine zipper motif (APPL1)-mediated adiponectin and insulin-signaling pathways. Adiponectin (Ad) binding to the extracellular COOH terminus of adiponectin receptor 1 (AdipoR1) recruits APPL1 to the intracellular NH2 terminus of AdipoR1 and activation of AMPK, p38 MAPK, and Rab5. 1) Activation of the AMP-activated kinase (AMPK)/tuberous sclerosis complex (TSC)1/2 signaling pathway reduces mammalian target of rapamycin (mTOR)/p70 S6 (S6K)-mediated serine phosphorylation of insulin receptor substrate (IRS) proteins, which results in the enhancement of IRS tyrosine phosphorylation and insulin signaling to activate Akt, leading to glucose uptake. 2) Activated AMPK phosphorylates and thereby inactivates acetyl-CoA carboxylase (ACC), resulting in fatty acid oxidation. 3) Activated AMPK phosphorylates endothelial nitric oxide (NO) synthase (eNOS), leading to the formation of eNOS-heat shock protein 90 (Hsp90) complex and generation of NO to mediate the vasodilating effect of adiponectin. 4) Activated AMPK inhibits IKK-NF-κB-phosphatase and tensin homolog (PTEN) pathway, mediating the cytoprotective effect of adiponectin. 5) Binding of adiponectin to AdipoR1 stimulates glucose transporter 4 (GLUT4) translocation and glucose uptake through phosphorylated AMPK, phosphorylated p38 MAPK, and Rab5.
APPL1 AS A MEDIATOR OF INSULIN SIGNALING
The location of adiponectin gene chromosome 3q27 is the susceptibility locus for type 2 diabetes and other metabolic syndromes (37, 139, 153). The mRNA expression and secretion of adiponectin is significantly decreased in type 2 diabetic patients and animal models of insulin resistance (4, 67, 166, 178). Interestingly, the insulin-sensitizing PPARγ agonist thiazolidinedione increases adiponectin level in animal models and human patients (107). Replenishment of adiponectin restores insulin sensitivity in high-fat-diet-fed and lipotropic mice (171). Thus, adiponectin level and insulin sensitivity are directly correlated, which suggests the insulin-sensitizing effect of adiponectin (76).
APPL1 is expected to play a role in insulin-signaling pathway since it interacts with Akt and PI3K (119). Activation of Akt2 by insulin stimulation plays a critical role in glucose transport (63, 74, 91). Akt is activated by phosphorylation at Thr308 by phosphoinositide-dependent kinase (PDK)1 (3, 151) and at Ser473 by PDK2 (43). PDK1 activation occurs by its binding to phosphatidylinositol 3,4,5-phosphate [PtdIns(3,4,5)P3] generated by PI3K, which is activated by binding to tyrosine-phosphorylated IRS (50, 53). Glucose clearance in response to insulin is mediated through the glucose transporter, GLUT4, that moves from intracellular membrane compartment to the cell surface during insulin stimulation. Mao et al. (110) demonstrated that APPL1 plays an important role in insulin signal transduction in C2C12 myocytes since overexpression of APPL1 stimulates insulin-mediated Akt phosphorylation, whereas APPL1 knockdown or overexpression of APPL1 (ΔPTB) mutant significantly reduces this effect in C2C12 myocytes. Similarly, in 3T3-L1 adipocytes, suppression of APPL1 attenuated Akt phosphorylation, GLUT4 translocation, and glucose uptake (140). Insulin stimulation has been shown to dissociate APPL1-Akt complex in primary rat adipocytes and skeletal muscle (140), which is in agreement with the previous finding that only the inactivated form of Akt can interact with APPL1 (119). These findings suggest a direct involvement of APPL1 in insulin-stimulated GLUT4 translocation and glucose uptake.
APPL1 AS A MEDIATOR OF INSULIN-SENSITIZING EFFECT OF ADIPONECTIN
Adiponectin is a well-documented insulin sensitizer, and adiponectin exerts this effect by 1) decreasing the triglyceride content in insulin-sensitizing tissues and thereby upregulating insulin signaling, 2) increasing fatty acid combustion and energy utilization by activating PPARα, and 3) increasing fatty acid oxidation and glucose uptake by activating AMPK (76). By itself, adiponectin is unable to phosphorylate Akt, although a cotreatment of C2C12 myotubes with adiponectin and insulin showed a synergistic increase in Akt phosphorylation, and this synergism disappeared in APPL1 knockdown cells (110). Hence, APPL1 plays a critical role in the cross-talk between adiponectin- and insulin-signaling pathways. APPL1-mediated cross-talk between insulin- and adiponectin-signaling pathways could be a novel mechanism for the insulin-sensitizing effect of adiponectin (110). The ability of adiponectin to decrease the phosphorylation of p70 S6 kinase (S6K) at Thr389 is a key step in this cross-talk (164), since S6K activation has a negative correlation with IRS-1 tyrosine phosphorylation and downstream signaling (62, 165). In addition to S6K, adiponectin treatment also decreased the phosphorylation of IRS-1 at Ser302 and Ser636/639 (164), which has inhibitory effects for insulin-stimulated Akt activation (15, 62, 157, 165). This decrease in S6K and IRS-1 Ser phosphorylation by adiponectin are mediated through AMPK. Binding of adiponectin to its receptor activates AMPK, which in turn activates tuberous sclerosis complex 2 (TSC2). Activated TSC2 together with TSC1 inhibits the activity of Ras homology enriched in brain, leading to the inhibition of mammalian target of rapamycin and S6K. This inhibition of S6K mediated by adiponectin enhances the ability of insulin to stimulate IRS-1 tyrosine phosphorylation and subsequent Akt phosphorylation and insulin-signaling activation (Fig. 1) (164).
APPL1: AN ADAPTOR PROTEIN WITH MULTIPLE FUNCTIONAL DOMAINS
Three major functional domains are present in APPL1, the NH2-terminal Bin1/amphiphysin/rvs167 (BAR) domain (initially identified as the leucine zipper motif, 18–226 amino acids) followed by a PH domain (278–377 amino acids) and a PTB domain (597–636 amino acids) near the COOH terminus (Fig. 2) (116). Recent analysis of the crystal structure of APPL1 gave more insight into the structure-function relationship of this interesting adaptor protein (97, 185).
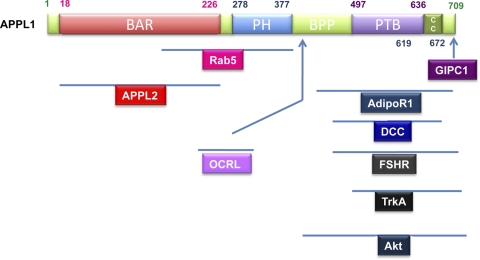
Fig. 2.
Domain structure of APPL1. Schematic representation of human APPL1 domain structure and the regions in APPL1 that interact with its associating proteins. BAR, Bin/amphiphysin/Rvs domain; BPP, region between PH and PTB domains; CC, coil-coiled region; GIPC1, GAIP-interacting protein, COOH terminus; OCRL, oculocerebrorenal syndrome of Lowe; DCC, deleted in colorectal cancer; FSHR, FSH receptor.
APPL1 belongs to a subfamily of BAR domain proteins in which the BAR and PH domain appear in tandem (16, 58, 106). The other members of this subfamily are APPL2, the GTPase-activating proteins centaurin-β1, -β2, and -β5, and oligophrenins (58). In general, the BAR domain is implicated in diverse biological processes like sensing and inducing membrane curvature, small GTPase binding (55, 58, 95, 186), transcriptional repression, apoptosis, and secretory vesicle fusion (39, 136). The BAR domain of APPL1 located near the NH2 terminus spans 251 amino acids and is unique in having four α-helixes (97, 185) compared with three α-helixes in the BAR domains of Bin1/amphiphysin II and endophilin (21, 55). APPL1-BAR domain is involved in the binding of the small GTPase Rab5 (116) and also in the formation of homo- or heterodimer with the BAR domain of APPL2 (29, 97, 124). APPL1-BAR dimer is crescent shaped, and only the first three α-helixes contribute to the structure of the concave inner face of dimer (97, 185). The fourth α-helix in the BAR domain is not necessary for dimerization or membrane targeting of APPL1 (29).
Unlike the BAR domain, PH domain of APPL1 is homologous to those in other proteins (97). In general, the PH domain targets proteins to specific membrane compartments by increasing the lipid specificity of the BAR domain (132) and also in GTPase binding (96). In APPL1, the BAR and PH domains function as a single unit rather than two individual domains (97, 116, 185). In cantaurin-β2 and oligophrenin, the other two members in the APPL family, the BAR-PH domain is involved in the tubulation of membranes, and hence, it is possible that APPL1 BAR-PH domain may induce membrane curvature (132). In APPL1 dimer, the PH domain of each monomer is positioned at the opposite ends of the BAR dimer like stretched arms, giving a crescent shape for the BAR-PH dimer with the concave face mediating membrane interaction (97, 185). The BAR-PH domain of APPL1 is involved in Rab5 binding (97, 116, 185), and Rab5 specifically binds the PH domain with marginal extension to the neighboring BAR domain (185). In agreement with this, a triple mutation in APPL1-PH domain (K280E/Y283C/G319R) disrupts its binding with Rab5 (116). A role for PH domain in membrane targeting is suggested, since isolated PH domain of APPL1 binds PtdIns(3)P, PtdIns(4)P, PtdIns(5)P, PtdIns(3,4)P2, and PtdIns(3,5)P2, which are the phospholipids in plasma membrane, early endosomes, late endosomes/multivesicular bodies, endoplasmic reticulum, Golgi, and the nucleus (29). The BAR and BAR-PH domains of APPL1 also interact with PtdIns(3,4,5)P3, consistent with the predicted membrane-binding function of BAR-PH domain of APPL1 (97). Consistent with this finding, isolated APPL1-PH is localized in plasma membrane, cytosolic vesicles, and nuclear and perinuclear structures (29), suggesting that BAR-PH domain is involved mainly in the membrane targeting of APPL1.
The general function of PTB domain is to act as an adaptor or scaffold for the binding of proteins, particularly those in signaling pathways. The PTB domain of APPL1 is located near the COOH terminus, away from BAR-PH domain, making it an easily accessible structure for its binding partners. This domain interacts with a diverse set of receptors, including netrin-1 receptor DCC (101), nerve growth factor (NGF) receptor TrkA (98, 161), follicle-stimulating hormone (FSH) receptor (FSHR) (123, 124), and adiponectin receptors AdipoR1 and AdipoR2 (28, 110). APPL1-PTB domain also interacts with various signaling proteins, such as Akt (119, 124, 140), PI3K subunits (119, 177), oculocerebrorenal syndrome of Lowe (OCRL), and inositol polyphosphate-5-phosphatase (INPP5B) (44), indicating the ability of APPL1 to act as an adaptor or scaffold protein for distinct signaling pathways. The secondary structure of APPL1-PTB domain is similar to the PTB domain of adaptor protein Shc (119), which recognizes phosphorylated tyrosine residues within the consensus sequence ΦNPXphosphoY (where Φ is a hydrophobic amino acid, X refers to any amino acid, and N, P, and Y represent Asn, Pro, and Tyr, respectively) (82, 184). However, APPL1-PTB domain-interacting proteins do not contain this consensus sequence, indicating that APPL1-PTB domain-mediated interaction is through a novel, unidentified mechanism (110). The PTB domain of APPL1 also interacts with PtdIns(3,4,5)P3 (97), PtdIns(3)P, PtdIns(4)P, PtdIns(5)P, PtdIns(3,4)P2, and PtdIns(3,5)P2 (29). Contrary to APPL1-PH domain, which also binds these phospholipids, isolated APPL1-PTB domain is localized in the cytosolic membrane structures and nucleus (29), suggesting that APPL1-PTB domain functions as the binding site for signaling molecules in various pathways.
APPL1 INTERACTS WITH VARIOUS PROTEINS IN ADIPONECTIN- AND INSULIN-SIGNALING PATHWAYS
With multiple functional domains described above, APPL1 has a high potential for binding various proteins in cells. In fact, APPL1 interacts with adiponectin receptors in adiponectin-signaling pathway and with signaling molecules Akt and PI3K in the insulin-signaling pathway.
AdipoR1 and AdipoR2
AdipoR1 and AdipoR2 are the two transmembrane receptors mediating the effects of adiponectin (173). AdipoR1 and AdipoR2 are highly related structurally and share 67.5% identity of protein sequence and are conserved from yeast to human (173). They are structurally and functionally different from the G protein-coupled receptors and contain seven transmembrane domains with an intracellular NH2 terminus and an extracellular COOH terminus end (173). AdipoR1 is expressed ubiquitously with high expression in skeletal muscle, and AdipoR2 is expressed predominantly in liver (173). Using a yeast-two hybrid study, we found that adiponectin interacts with the COOH-terminal extracellular part of AdipoR1 (110). We also identified APPL1 as an AdipoR1- and AdipoR2-interacting protein using NH2-terminal intracellular portion of AdipoR1 or AdipoR2 as bait. In C2C12 myocytes, APPL1 interacts directly with AdipoR1 through its COOH-terminal PTB domain, and adiponectin stimulates this interaction (110). A similar interaction of APPL1 with AdipoR1 and AdipoR2 stimulated by adiponectin is observed in endothelial cells (28). Although adiponectin-stimulated phosphorylation of AdipoR1 was expected to mediate AdipoR1-APPL1 interaction, the phosphorylation of AdipoR1 remains unchanged after adiponectin stimulation, and mutations of three tyrosine residues in the NH2 terminus of AdipoR1 did not alter its binding to APPL1, indicating the existence of an unidentified mechanism for APPL1-AdipoR1 interaction (110). Similarly, APPL1 interacts with DCC (101) and FSHR (123) by a phosphorylation-independent mechanism. Homo- or heterodimer formation by AdipoR1 and AdipoR2 (173) could be a possible mechanism by which APPL1 recognizes its receptors, as reported for FSHR (123). It is likely that oligomerization of receptors by adiponectin leads to curvature in the membrane, which is sensed by the APPL1 dimer via its BAR-PH domain to mediate downstream signals. However, whether adiponectin stimulates the oligomerization of its receptors is currently unknown.
PI3K
PI3K is a critical component in the insulin-signaling pathway mediating a wide range of functions of insulin. PI3K catalyzes the phosphorylation of D-3 position of inositol ring in phosphoinositides (167). PI3K contains a 110-kDa catalytic subunit and an 85-kDa regulatory subunit that is constitutively associated with the p110 subunit. The SH2 domain in p85 subunit recognizes phosphorylated IRS-1, activated by insulin receptor or insulin-like growth factor I (IGF-I) receptor. This follows the recruitment of IRS proteins along with p110 subunit to the plasma membrane (7, 122), where it catalyzes the phosphorylation of PtdIns(4,5)P2 to PtdIns(3,4,5)P3. PtdIns(3,4,5)P3 can interact with PH domains of various proteins and thereby orchestrate a range of signaling pathways (160). APPL1 interacts with p110α (119) and p85 (177) subunits of PI3K; however, the interacting domain in APPL1 is not known. APPL1 tethers inactive Akt2 to p110α (119) and is an important adaptor protein in IGF-I-mediated Akt activation through PI3K (177). IGF-I-mediated Akt phosphorylation is enhanced by APPL1 in a PI3K-dependent manner, and knockdown of APPL1 abrogates this effect (177). It is not clear how APPL1 activates Akt; it is likely that the PH domain of APPL1 binds PI3K and serves as an adaptor to recruit PI3K to the cell membrane, where PI3K is activated by IGF-I receptor or insulin receptor, leading to Akt activation (177).
Akt
Akt/protein kinase B is a serine/threonine protein kinase, and signaling through Akt plays an important role in cell growth, survival, proliferation, and metabolism (17, 109). Akt is one of the critical components in the insulin-signaling pathway, and its activation regulates glucose metabolism in insulin-sensitive tissues (30, 74). APPL1 was initially identified as an Akt2-interacting protein in a yeast-two hybrid screen. APPL1 interacts only with the inactive form of Akt2, and the COOH-terminal PTB domain of APPL1 is implicated in this binding (119). In A2780 cells, APPL1 acts as an adaptor protein that anchors the inactive Akt2 to p110α subunit of PI3K in the cytoplasm (119). Although the physiological significance of this interaction in the A2780 cell is not clear, APPL1 may play a central role in insulin signaling, since Akt2 can phosphorylate and inactivate glycogen synthase kinase-3β (GSK-3β) (118). APPL1 plays an important role in insulin-stimulated Akt phosphorylation in C2C12 myocytes, since overexpression of APPL1 stimulates and suppression of APPL1 inhibits insulin-mediated Akt phosphorylation (110). Insulin treatment dissociates APPL1-Akt2 complex in primary rat adipocyte and skeletal muscle (140), which is in agreement with the findings of Mitsuuchi et al. (119) that only the inactive form of Akt associates with APPL1. Because knockdown of APPL1 attenuates insulin-mediated Akt phosphorylation, glucose uptake, and GLUT4 translocation in adipocytes, APPL1 is essential for mediating these effects of insulin (140). Insulin receptor trafficking necessary for insulin signaling to Akt2 should have been disrupted in APPL1 knockdown cells (140). In support of this idea, insulin cannot activate Akt in Rab5 knockdown cells (152), and APPL1 is an effector of Rab5 (116). Even though COOH-terminal PTB domain of APPL1 interacts with Akt2, overexpression of full-length and NH2-terminal APPL1 (BAR-PH) suppressed insulin-stimulated GLUT4 translocation, indicating that NH2-terminal portion may be critical for APPL1 function (140). Perhaps APPL1-PH domain competes with those of PDK1 and Akt2 and causes Akt2 mislocalization, resulting in the inhibition of GLUT4 translocation in response to insulin treatment (140).
APPL1 also plays a critical role in cell survival during development of zebrafish, and knockdown of APPL1 induces apoptosis, an effect mediated through Akt (141). APPL1 is specifically required for Akt activity and cell survival, since APPL1 knockdown significantly reduces the level of Akt phosphorylation (141). In addition, APPL1 knockdown significantly attenuates GSK-3β activation, a downstream target of Akt implicated in cell survival (75, 109). Activation of TSC2, another downstream target of Akt implicated in growth control (109), is unaffected by suppression of APPL1. Hence, APPL1 regulates the substrate specificity and activity of Akt. Endosomal localization of APPL1 is required for its activity (116), and APPL1-mediated Akt phosphorylation occurs only through endosomal APPL1, not through the soluble or nuclear APPL1 (141). Consistent with this, only GSK-3β, not TSC2, is localized in endosomes. Besides the Akt-mediated survival pathway mediated through GSK-3β, APPL1 also mediates cell survival through FOXO1a, another direct substrate of Akt (124).
EXPRESSION, CELLULAR LOCALIZATION, AND REGULATION OF APPL1
Protein and mRNA expression of APPL1 has been reported in various human and mouse tissues (29, 110, 119). In HeLa cells, APPL1 is present in both cytoplasm and nucleus as punctuate structures, and in the cytosol it is located on membrane structures like endosomes with high concentrations in tubular-vesicular structures under the plasma membrane (116). Interestingly, localization of APPL1 undergoes dramatic changes with respect to environmental stimuli. In HeLa cells, serum starvation shifts APPL1 localization entirely to the cytosol, and treatment with H2O2, simulating oxidative stress, translocates APPL1 from cytosol to the nuclei (116). In HeLa cells, in response to EGF treatment, APPL1 translocates from punctuate cytosolic structures to a subset of EGF-containing endosomes and then to the nuclei and, finally, back to membrane structures in cytoplasm (116). This whole cycle of translocation occurs in 30 min, indicating the presence of efficient machinery for the import and export of APPL1 to and from the nuclei, although APPL1 does not contain a nuclear localization signal or nuclear export signal. Although endosomes act as the shuttling cargo for APPL1 import to nuclei (116), the mechanism of APPL1 export from the nuclei is currently unknown. Truncation studies have shown that BAR-PH domain determines the membrane anchoring of APPL1 (116). In primary rat adipocytes, the majority of APPL1 is localized in the cytosol, with small proportions in nuclear/mitochondrial fraction and light microsomal fraction. In these cells, insulin treatment increased the proportion of APPL1 in light microsomes, plasma membrane, and nuclear/mitochondrial fraction (140). On the contrary, in cultured adipocyte, this increase was primarily observed only in light microsomes and plasma membrane (140). However, chronic insulin treatment for 6–24 h caused a translocation of APPL1 to nucleus (140). During early embryogenesis in zebrafish, a ubiquitous expression of APPL1 is observed, with elevated expression in telencephalon, pronephros, olfactory organ, and neural tube, and is also present in the characteristic endosomes beneath the plasma membrane (141).
Single nucleotide polymorphisms (SNPs) within AdipoR1 and AdipoR2 gene are risk factors for type 2 diabetes in white populations (36, 150, 162), and SNPs in AMPK gene are also associated with alterations in plasma lipids and increased risk for type 2 diabetes (65, 170), which suggests that adiponectin-signaling pathway contributes to genetic regulation of metabolism. APPL1 locus, a 78-kb genomic region, on chromosome 3p21.1-p14.3 is not a susceptibility region for type 2 diabetes (149). Four common SNPs (rs6774584, rs3087684, rs17791685, and rs528035) were identified in healthy white populations, and this genetic variation within the APPL1 locus does not contribute to insulin resistance, changes in lipid metabolism, or inflammatory parameters, indicating that APPL1 gene may not play a major role in the development of prediabetic conditions (149). Interestingly, studies by Fang et al. (45) in a Chinese population indicate that two SNPs (rs3806622 and rs4640525) in APPL1 gene are correlated with body fat distribution in type 2 diabetes.
APPL1 AS AN ADAPTOR IN MULTIPLE SIGNALING PATHWAYS
To date, nearly 14 proteins have been reported to associate with APPL1 in various types of cells, and they could be categorized into three different groups: 1) membrane receptors, 2) signaling proteins, and 3) others.
Membrane Receptors
DCC.
DCC is a candidate tumor suppressor gene (47) that encodes a type 1 membrane protein (31). It acts as a receptor for netrin-1 and plays important roles in axon outgrowth and cell migration in the developing nervous system (46, 83). The tumor suppression effect of DCC is through induction of apoptosis (27, 51, 115), and Liu et al. (101) identified APPL1 as a binding partner of DCC. The interaction between APPL1 and DCC enhances DCC-mediated apoptosis in the colon adenocarcinoma cell line DLD1. Cytoplasmic domain of DCC interacts with the COOH-terminal region of APPL1 (454–646 amino acids), which contains the PTB domain. DCC-induced apoptosis is dependent on APPL1, since inhibition of endogenous APPL1 blocks apoptosis (101). The molecular mechanism by which APPL1 induces apoptosis through DCC is not clear. It could be through caspase-9 (101), since the same region in DCC (1,243–1,264 amino acids) interacts with both APPL1 and caspase-9 (51).
Androgen receptor.
Androgen receptor (AR) is an androgen-dependent transcription factor belonging to the nuclear receptor superfamily (25), which plays an important role in male sexual differentiation and prostate cell proliferation. Binding of the ligand testosterone to AR attaches it to the androgen response element on the 5′-promoter of the target gene, resulting in the modulation of cell growth (24, 69). AR can be regulated by growth factor-signaling pathways, which contributes to the development and progression of prostate cancer (48). PI3K/Akt pathway suppresses AR transactivation (99), and APPL1 can enhance Akt-mediated suppression of AR transactivation in prostate cancer cell lines (177). AR does not directly interact with APPL1; rather, Akt acts as a bridge to mediate APPL1-AR interaction (177). Overexpression of APPL1 suppresses the expression of p27Kip1, a target gene of PI3K/Akt signaling (177). Hence, APPL1 controls AR transactivation by regulating PI3K/Akt pathway and downregulating the gene expression of p27Kip1.
FSHR.
During follicular development of the ovary, selection of a dominant follicle capable of ovulation is through a competing phenomenon of apoptosis and cell survival (121) mainly by the action of FSH and its receptor FSHR. FSH is capable of activating MAPK and Akt pathways through FSHR (32, 57). Using yeast two hybrid screen, Nechamen et al. (123) identified APPL1 as a FHSR-interacting protein. The COOH-terminal part of APPL1 (461–709 amino acids) containing the PTB domain interacts with FSHR, and this interaction is stimulated by FSH. Surprisingly, FSH also stimulates the interaction between FSHR and the APPL1 NH2-terminal end containing BAR-PH domain (1–460 amino acids). In 293 FSHR cells, FSH treatment phosphorylates and inactivates FOXO1a, a downstream effector of the antiapoptotic PI3K/Akt signaling pathway (123). FOXO1a is involved in the transcription of proteins involved in apoptosis, and hence, its activation by PI3K/Akt pathway leads to suppression of apoptotic gene expression (18) and ultimately cell survival. FSH regulated APPL1-FSHR association could be the survival mechanism for dominant follicle selection. However, it is not known how FSH stimulates APPL1-FSHR interaction. FOXO1a has been shown to interact directly with FSHR but not with APPL1 (124). FSH stimulation has been reported to enhance constitutive formation of FSHR oligomers, and this oligomerization may provide a mechanism for signal transduction (123).
TrkA.
The TrkA receptor tyrosine kinase is a receptor for neurotrophin NGF transducing signaling cascades required for survival, differentiation, and growth of neurons during development (10, 159). Following activation by ligand binding, TrkA is internalized and the signal is transduced as endosomes (68). Within the endosomes, activated TrkA together with the components of the TrkA signaling pathway form a signaling unit that is propagated along the axon to cell body (41, 179). GAIP-interacting protein, COOH terminus (GIPC1), is a scaffold protein that is constitutively associated with TrkA and is involved in the recruitment of other proteins involved in endocytosis and early trafficking events (5, 103, 104). Lin et al. (98) found that, in NGF-treated cells, APPL1 is present in endosomal fractions along with phosphorylated TrkA and GIPC1. APPL1 associates with TrkA either directly through the COOH-terminal PTB domain (472–709 amino acids) or indirectly through GIPC1. The COOH-terminal end of APPL1 associates with the postsynaptic density protein, Drosophila disc large tumor suppressor, and zonula occludens-1 protein (PDZ) domain of GIPC1 (98). In the early stages of endosomal trafficking, APPL1 interacts with TrkA through GIPC1 (98). During trafficking, GIPC1 dissociates from the endosomes (5), and APPL1 may interact directly with TrkA at this stage. Through these interactions, APPL1 is involved in the NGF-mediated Akt and ERK phosphorylation (98), necessary for neuronal survival and neuritogenesis (81). Suppression of APPL1 attenuated these effects (98), suggesting that APPL1-mediated signaling is essential for neuronal survival and neuritogenesis.
Signaling Proteins
Rab5.
Recently, endosomes have emerged as an important machinery for signal transduction from cell membrane to the nuclei (42, 113). The small GTPase Rab5 has been identified as a key regulator of transport from the plasma membrane to early endosomes, and continuous cycles of GDP/GTP exchange and hydrolysis regulate endocytosis (138). Rab5 is involved in the organization of early endosomes that are enriched in PtdIns(3)P and a set of PtdIns(3)P-binding effectors (34, 183). Miaczynska et al. (116) identified APPL1 as a Rab5 effector localized in endocytic compartments receiving internalized EGF. EGF internalization releases APPL1 from membranes, which depends on the GTPase cycle of Rab5, and translocates APPL1 to the nuclei. In the nuclei, APPL1 interacts with components of the nucleosome remodeling and histone deacetylase complex (NuRD/MeCP1) necessary for efficient cell proliferation (116). The interaction with Rab5 is part of the control mechanism to coordinate the release of APPL1 from endosomes and its trafficking following growth factor stimulation. APPL1 interacts with Rab5 through the NH2-terminal BAR-PH domain (97, 116, 185). Three mutations in the PH domain of APPL1 (K280E, Y283C, and G319R) abolished the binding of APPL1 to Rab5, and these mutants accumulated in the cytosol and nucleus, which resulted in decreased rate of cell proliferation. Hence, Rab5-dependent localization of APPL1 is necessary for downstream cytoplasmic interactions and also for transmitting proliferative signals. Rab5 also plays a role in glucose metabolism in response to insulin and adiponectin stimulations. Rab5 has been shown to regulate GLUT4 internalization by an insulin-dependent mechanism in 3T3-L1 adipocytes (71). It is not known whether APPL1 has any role in this process. In L6 cells, adiponectin-stimulated interaction between APPL1 and Rab5 plays a role in adiponectin-regulated GLUT4 translocation (106).
GIPC1.
Originally identified as a binding partner of regulator of the G protein-signaling protein GAIP, GIPC1 plays an important role in TrkA-mediated NGF signaling (98). The PDZ domain of GIPC1 interacts with the COOH-terminal PDZ binding domain of APPL1 (a 3-amino acid, Ser-Glu-Ala domain at the COOH terminus of APPL1), and a mutant lacking the four COOH-terminal amino acids did not bind GIPC1 (161). During activation of TrkA with NGF, GIPC1 and APPL1 relocate from cytoplasm to the endocytic vesicles carrying TrkA. Knocking down APPL1 inhibits NGF-induced GIPC recruitment to endosomes, which in turn inhibits MAPK activation and neurite outgrowth (161). Hence, recruitment of GIPC1 to TrkA-containing endosomes by APPL1 plays an important role in TrkA trafficking and signaling.
Rab21.
Rab21 is a member of the Rab5 subfamily, and APPL1 is a Rab21 effector since it binds Rab21 in a GTP-dependent manner (185). Rab21 and Rab5 exhibit differential binding affinity toward various APPL1 mutants (185), but the physiological significance of APPL1-Rab21 interaction is not known.
OCRL and INPP5B.
OCRL is an inositol 5-phophatase, mutation of which causes OCRL, characterized by congenital cataracts, mental retardation, and renal Fanconi syndrome (6). OCRL mutation is also identified in Dent disease, a condition associated with the loss of low-molecular-weight proteins and electrolytes in urine (64). OCRL is implicated in membrane trafficking from endosomes to Golgi and is a Rab effector that binds clathrin and clathrin adaptors (33, 73, 158). INPP5B is another inositol 5-phosphatase similar to OCRL, and COOH-terminal region of both INPP5B and OCRL binds APPL1 (44). A short peptide comprising 11 amino acids in human APPL1 (403–413 amino acids), located between the PH and PTB domains, is the minimal binding site for OCRL and INPPB5. Interestingly, phosphorylation at Ser410 catalyzed by protein kinase A inhibited the binding of OCRL to the 11-mer peptide, suggesting that modification in APPL1 regulates its interaction with OCRL (44). Three single amino acid mutations in the COOH-terminal of OCRL are associated with Lowe syndrome (120), and these mutants do not bind APPL1, indicating that impairment in APPL1-OCRL interaction plays a major role in this disease. OCRL in the endocytic clathrin coated pits has shown to bind APPL1 on early endosomes, and APPL1-OCRL association is important for trafficking and signaling in kidney and brain (44). APPL1 participates in the formation of a protein network along with OCRL and GIPC, an oligomeric endocytic adaptor protein implicated in receptor endocytosis and recycling in brain and kidney, and disruption of this network is implicated in the renal defects and mental retardation in Lowe syndrome.
Others
NuRD/MeCP1.
NuRD/MeCP1 is an important regulator of chromatin structure and gene expression (49). In response to EGF or oxidative stress, APPL1 is transported from early endosomes to nuclei, where it interacts with NuRD/MeCP1 (116). It is not known whether APPL1 directly associates with NuRD/MeCP1 and which domain of APPL1 mediates this interaction. Mass spectra analysis of the interacted NuRD/MeCP1 revealed six out of 10 components of the complex, viz., PID/MTA2, p66, HDAC1 and/or HDAC2, RbAp46, RbAp48, and MBD3. Histone deacetylase (HDAC) activity is an essential factor for the progression of cell cycle and development (2, 182). The nuclear localization of APPL1 together with its binding to NuRD/MeCP1 complex suggests a role for APPL1 in cell proliferation. In agreement with this, APPL1 knocking down cells are less proliferative with less number of cells entering the S-phase (116). Hence, APPL1 is a critical element of signaling pathways leading to cell proliferation.
APPL2.
Initially identified because of its high homology with APPL1, APPL2/DIP-13β (101) shares 54% identity with APPL1 and is encoded in human chromosome 12, with the translated protein containing 664 amino acids (116). Both APPL1 and APPL2 share the same domain structure except that, in APPL2, 45 amino acids in the COOH terminus are absent compared with APPL1. Unlike APPL1, APPL2 posses a putative nuclear localization signal and is more concentrated in the nucleus with marginal distribution in the cytoplasm as punctuate structures (116). APPL1 forms a complex with APPL2, and the NH2-terminal region of APPL1 (1–460 amino acids) containing the BAR domain is implicated in this dimerization (124). The BAR domain, devoid of the fourth α-helix, is the minimal requirement for the dimerization of APPL isoforms to form homo- and heterodimers (29). The coexpression of APPL1 and APPL2 mRNAs in various human and mouse tissues suggests that APPL homo- and hetero-oligomer may be present in many tissues, whereas some tissues (kidney and lung in humans and lung, uterus, prostate, and certain breast tissues in mouse) may be rich in APPL1 isoforms or APPL2 isoforms (bone marrow and fetal brain in humans) (29). However, the potential function of APPL homo- or hetero-oligomer is not clear. Like APPL1, APPL2 PH and PTB domains also mediate phosphoinositide binding (29) and interact with Rab5 (116), NuRD/MeCP1 (116), GIPC1 (161), and FSHR (124) but not with Akt2 (124). APPL2 may also play a role in cell proliferation, since knockdown of APPL2 reduced the number of cells entering the S-phase (116).
CONCLUDING REMARKS
Adiponectin is a promising therapeutic target for the treatment of insulin resistance, type 2 diabetes, and related metabolic diseases. Identification of APPL1 as a mediator of adiponectin signaling enhances our current understanding on adiponectin and insulin-signaling pathways. APPL1 is indispensable for the signaling and insulin-sensitizing effect of adiponectin. However, our current knowledge of the mechanism by which APPL1 regulates adiponectin signaling is limited, and many questions need to be answered for a better understanding of this adaptor protein. How does APPL1 activate its downstream targets in response to adiponectin stimulation? What posttranslational modifications exist in APPL1, and how do these modifications affect the interaction with its binding partners? What is the functional significance of homo-oligomer of APPL1 or hetero-oligomers of APPL isoforms, and how they are regulated? How does APPL1 recognize adiponectin receptors? Do adiponectin receptors undergo endocytosis following adiponectin stimulation, and is endosomal localization of APPL1 necessary for adiponectin signaling? Do APPL1-dependent and/or -independent pathways exist for adiponectin signaling in tissues like liver, kidney, and bone? What is the physiological significance of this adaptor protein in metabolism? APPL1 knockout or conditional knockout mice will be helpful models to study the physiological significance of APPL1 in vivo. Since most drugs used to treat type 2 diabetes act as insulin sensitizers, similarly to adiponectin, it will be interesting to test whether they also modulate the expression or function of APPL1. Overexpression of APPL1 in C2C12 myoblasts could mimic the effect of adiponectin, and decreased expression of APPL1 in the small mesenteric arteries of db/db mice is related to defects in vasodilation, suggesting that regulation of APPL1 expression or function is a promising approach to enhance insulin sensitivity. This opens an avenue for the search of factors (like diet and exercise) and development of small molecules that can modulate expression and function of APPL1 in insulin target tissues. More insight into the regulation and expression of APPL1 will help to develop new strategies for treating type 2 diabetes and metabolic syndrome.
GRANTS
This work was supported in part by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-69930 (to L. Q. Dong) and by a Career Development Award from the American Diabetes Association (to L. Q. Dong).
Acknowledgments
We acknowledge the critical review and comments from Dr. Feng Liu, Xiaoban Xin, and Dr. Jiyoon Ryu.
REFERENCES
- 1.Adamczak M, Wiecek A, Funahashi T, Chudek J, Kokot F, Matsuzawa Y. Decreased plasma adiponectin concentration in patients with essential hypertension. Am J Hypertens 16: 72–75, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Ahringer J NuRD and SIN3 histone deacetylase complexes in development. Trends Genet 16: 351–356, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase B alpha. Curr Biol 7: 261–269, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun 257: 79–83, 1999. [DOI] [PubMed] [Google Scholar]
- 5.Aschenbrenner L, Lee TT, Hasson T. Myo6 facilitates the translocation of endocytic vesicles from cell peripheries. Mol Biol Cell 14: 2728–2743, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL. The Lowe's oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 358: 239–242, 1992. [DOI] [PubMed] [Google Scholar]
- 7.Backer JM, Myers MG Jr, Shoelson SE, Chin DJ, Sun XJ, Miralpeix M, Hu P, Margolis B, Skolnik EY, Schlessinger J, Whit MF. Phosphatidylinositol 3′-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J 11: 3469–3479, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barger PM, Browning AC, Garner AN, Kelly DP. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. J Biol Chem 276: 44495–44501, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Becker B, Kronenberg F, Kielstein JT, Haller H, Morath C, Ritz E, Fliser D. Renal insulin resistance syndrome, adiponectin and cardiovascular events in patients with kidney disease: the mild and moderate kidney disease study. J Am Soc Nephrol 16: 1091–1098, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Belliveau DJ, Krivko I, Kohn J, Lachance C, Pozniak C, Rusakov D, Kaplan D, Miller FD. NGF and neurotrophin-3 both activate TrkA on sympathetic neurons but differentially regulate survival and neuritogenesis. J Cell Biol 136: 375–388, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, Drevon CA, Syversen U, Reseland JE. Adiponectin and its receptors are expressed in bone-forming cells. Bone 35: 842–849, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Beylot M, Pinteur C, Peroni O. Expression of the adiponectin receptors AdipoR1 and AdipoR2 in lean rats and in obese Zucker rats. Metabolism 55: 396–401, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Birnbaum MJ Activating AMP-activated protein kinase without AMP. Mol Cell 19: 289–290, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Bjursell M, Ahnmark A, Bohlooly-Y M, William-Olsson L, Rhedin M, Peng XR, Ploj K, Gerdin AK, Arnerup G, Elmgren A, Berg AL, Oscarsson J, Lindén D. Opposing effects of adiponectin receptors 1 and 2 on energy metabolism. Diabetes 56: 583–593, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes 52: 1319–1325, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw JM, Waksman G. Molecular recognition by SH2 domains. Adv Protein Chem 61: 161–210, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci 29: 233–242, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Caminos JE, Nogueiras R, Gallego R, Bravo S, Tovar S, Garcia-Caballero T, Casanueva FF, Dieguez C. Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab 90: 4276–4286, 2005. [DOI] [PubMed] [Google Scholar]
- 20.Carling D AMP-activated protein kinase: balancing the scales. Biochimie 87: 87–91, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Casal E, Federici L, Zhang W, Fernandez-Recio J, Priego EM, Miguel RN, DuHadaway JB, Prendergast GC, Luisi BF, Laue ED. The crystal structure of the BAR domain from human Bin1/amphiphysin II and its implications for molecular recognition. Biochemistry 45: 12917–12928, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceddia RB, Somwar R, Maida A, Fang X, Bikopoulos G, Sweeney G. Globular adiponectin increases GLUT4 translocation and glucose uptake but reduces glycogen synthesis in rat skeletal muscle cells. Diabetologia 48: 132–139, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekar B, Boylston WH, Venkatachalam K, Webster NJ, Prabhu SD, Valente AJ. Adiponectin blocks interleukin-18-mediated endothelial cell death via APPL1-dependent AMP-activated protein kinase (AMPK) activation and IKK/NF-kappaB/PTEN suppression. J Biol Chem 283: 24889–24898, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang C, Saltzman A, Yeh S, Young W, Keller E, Lee HJ, Wang C, Mizokami A. Androgen receptor: an overview. Crit Rev Eukaryot Gene Expr 5: 97–125, 1995. [DOI] [PubMed] [Google Scholar]
- 25.Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science 240: 324–326, 1988. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem 278: 45021–45026, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Chen YQ, Hsieh JT, Yao F, Fang B, Pong RC, Cipriano SC, Krepulat F. Induction of apoptosis and G2/M cell cycle arrest by DCC. Oncogene 18: 2747–2754, 1999. [DOI] [PubMed] [Google Scholar]
- 28.Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes 56: 1387–1394, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Chial HJ, Wu R, Ustach CV, McPhail LC, Mobley WC, Chen YQ. Membrane targeting by APPL1 and APPL2: dynamic scaffolds that oligomerize and bind phosphoinositides. Traffic 9: 215–229, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, Kaestner KH, Bartolomei MS, Shulman GI, Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731, 2001. [DOI] [PubMed] [Google Scholar]
- 31.Cho KR, Oliner JD, Simons JW, Hedrick L, Fearon ER, Preisinger AC, Hedge P, Silverman GA, Vogelstein B. The DCC gene: structural analysis and mutations in colorectal carcinomas. Genomics 19: 525–531, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Choi KC, Kang SK, Tai CJ, Auersperg N, Leung PC. Follicle-stimulating hormone activates mitogen-activated protein kinase in preneoplastic and neoplastic ovarian surface epithelial cells. J Clin Endocrinol Metab 87: 2245–2253, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell 16: 3467–3479, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christoforidis S, Miaczynska M, Ashman K, Wilm M, Zhao L, Yip SC, Waterfield MD, Backer JM, Zerial M. Phosphoinositide-3-kinases are Rab5 effectors. Nat Cell Biol 1: 249–252, 1999. [DOI] [PubMed] [Google Scholar]
- 35.Coope A, Milanski M, Araújo EP, Tambascia M, Saad MJ, Geloneze B, Velloso LA. AdipoR1 mediates the anorexigenic and insulin/leptin-like actions of adiponectin in the hypothalamus. FEBS Lett 582: 1471–1476, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Damcott CM, Ott SH, Pollin TI, Reinhart LJ, Wang J, O'connell JR, Mitchell BD, Shuldiner AR. Genetic variation in adiponectin receptor 1 and adiponectin receptor 2 is associated with type 2 diabetes in the Old Order Amish. Diabetes 54: 2245–2250, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Das K, Lin Y, Widen E, Zhang YH, Scherer PE. Chromosomal localization, expression pattern, and promoter analysis of the mouse gene encoding adipocyte-specific secretory protein Acrp30. Biochem Biophys Res Commun 280: 1120–1129, 2001. [DOI] [PubMed] [Google Scholar]
- 38.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55: 496–505, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Dawson JC, Legg JA, Machesky LM. Bar domain proteins: a role in tubulation, scission and actin assembly in clathrin mediated endocytosis. Trends Cell Biol 16: 493–498, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Delaigle AM, Senou M, Guiot Y, Many MC, Brichard SM. Induction of adiponectin in skeletal muscle of type 2 diabetic mice: in vivo and in vitro studies. Diabetologia 49: 1311–1323, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGFretrograde signals. Neuron 39: 69–84, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Di Fiore PP, De Camilli P. Endocytosis and signaling: an inseparable partnership. Cell 106: 1–4, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab 289: E187–E196, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P. A role of the Lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell 13: 377–390, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang QC, Jia WP, Gao F, Zhang R, Hu C, Wang CR, Wang C, Ma XJ, Lu JX, Xu J, Chen HZ, Xiang KS. Association of variants in APPL1 gene with body fat and its distribution in Chinese patients with type 2 diabetic mellitus. Zhonghua Yi Xue Za Zhi 88: 369–373, 2008. [PubMed] [Google Scholar]
- 46.Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, Simons J, Bronson RT, Gordon JI, Tessier-Lavigne M, Weinberg RA. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature 386: 796–804, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Fearon ER, Cho KR, Nigro JM, Kern SE, Simons JW, Ruppert JM, Hamilton SR, Preisinger AC, Thomas G, Kinzler KW, Vogelstein B. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science 247: 49–56, 1990. [DOI] [PubMed] [Google Scholar]
- 48.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer 1: 34–45, 2001. [DOI] [PubMed] [Google Scholar]
- 49.Feng Q, Zhang Y. The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomes. Genes Dev 15: 827–832, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filippa N, Sable CL, Hemmings BA, Van Obberghen E. Effect of phosphoinositide-dependent kinase 1 on protein kinase B translocation and its subsequent activation. Mol Cell Biol 20: 5712–5721, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Forcet C, Ye X, Granger L, Corset V, Shin H, Bredesen DE, Mehlen P. The dependence receptor DCC (deleted in colorectal cancer) defines an alternative mechanism for caspase activation. Proc Natl Acad Sci USA 98: 3416–3421, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci USA 98: 2005–2010, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem 67: 481–507, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Fu Y, Luo N, Klein RL, Garvey WT. Adiponectin promotes adipocyte differentiation, insulin sensitivity, and lipid accumulation. J Lipid Res 46: 1369–1379, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Gallop JL, McMahon HT. BAR domains and membrane curvature: bringing your curves to the BAR. Biochem Soc Symp 72: 223–231, 2005. [DOI] [PubMed] [Google Scholar]
- 56.Gallop JL, Jao CC, Kent HM, Butler PJ, Evans PR, Langen R, McMahon HT. Mechanism of endophilin N-BAR domain-mediated membrane curvature. EMBO J 25: 2898–2910, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Robayna IJ, Falender AE, Ochsner S, Firestone GL, Richards JS. Follicle-Stimulating hormone (FSH) stimulates phosphorylation and activation of protein kinase B (PKB/Akt) and serum and glucocorticoid-lnduced kinase (Sgk): evidence for A kinase-independent signaling by FSH in granulosa cells. Mol Endocrinol 14: 1283–1300, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Habermann B The BAR-domain family of proteins: a case of bending and binding? EMBO Rep 5: 250–255, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hattori Y, Suzuki M, Hattori S, Kasai K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia 46: 1543–1549, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Hardie DG The AMP-activated protein kinase pathway—new players upstream and downstream. J Cell Sci 117: 5479–5487, 2004. [DOI] [PubMed] [Google Scholar]
- 61.Hardie DG AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 8: 774–785, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, Gout I, Downes CP, Lamb RF. The TSC1–2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol 166: 213–223, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hill MM, Clark SF, Tucker DF, Birnbaum MJ, James DE, Macaulay SL. A role for protein kinase Bbeta/Akt2 in insulin-stimulated GLUT4 translocation in adipocytes. Mol Cell Biol 19: 7771–7781, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoopes RR, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ. Dent disease with mutations in OCRL1. Am J Hum Genet 76: 260–267, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Horikoshi M, Hara K, Ohashi J, Miyake K, Tokunaga K, Ito C, Kasuga M, Nagai R, Kadowaki T. A polymorphism in the AMPKalpha2 subunit gene is associated with insulin resistance and type 2 diabetes in the Japanese population. Diabetes 55: 919–923, 2006. [DOI] [PubMed] [Google Scholar]
- 66.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol 20: 1595–1599, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Hotta K, Funahashi T, Bodkin NL, Ortmeyer HK, Arita Y, Hansen BC, Matsuzawa Y. Circulating concentrations of the adipocyte protein adiponectin are decreased in parallel with reduced insulin sensitivity during the progression to type 2 diabetes in rhesus monkeys. Diabetes 50: 1126–1133, 2001. [DOI] [PubMed] [Google Scholar]
- 68.Howe CL, Mobley WC. Signaling endosome hypothesis: a cellular mechanism for long distance communication. J Neurobiol 58: 207–216, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Hsiao PW, Thin TH, Lin DL, Chang C. Differential regulation of testosterone vs. 5alpha-dihydrotestosterone by selective androgen response elements. Mol Cell Biochem 206: 169–175, 2000. [DOI] [PubMed] [Google Scholar]
- 70.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem 271: 10697–10703, 1996. [DOI] [PubMed] [Google Scholar]
- 71.Huang J, Imamura T, Olefsky JM. Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc Natl Acad Sci USA 98: 13084–13089, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang PL Endothelial nitric oxide synthase and endothelial dysfunction. Curr Hypertens Rep 5: 473–480, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J 25: 3750–3761, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang ZY, Zhou QL, Coleman KA, Chouinard M, Boese Q, Czech MP. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA 100: 7569–7574, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase-3. Trends Biochem Sci 29: 95–102, 2004. [DOI] [PubMed] [Google Scholar]
- 76.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev 26: 439–451, 2005. [DOI] [PubMed] [Google Scholar]
- 77.Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K. Adiponectin and adiponectin receptors in obesity-linked insulin resistance. Novartis Found Symp 286: 164–176; discussion 176–182, 2007. [DOI] [PubMed] [Google Scholar]
- 78.Kadowaki T, Yamauchi T, Kubota N. The physiological and pathophysiological role of adiponectin and adiponectin receptors in the peripheral tissues and CNS. FEBS Lett 582: 74–80, 2008. [DOI] [PubMed] [Google Scholar]
- 79.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 106: 473–481, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. Adiponectin and AMP kinase activator stimulate proliferation, differentiation, and mineralization of osteoblastic MC3T3-E1 cells. BMC Cell Biol 8: 51, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol 10: 381–391, 2000. [DOI] [PubMed] [Google Scholar]
- 82.Kavanaugh WM, Turck CW, Williams LT. PTB domain binding to signaling proteins through a sequence motif containing phosphotyrosine. Science 268: 1177–1179, 1995. [DOI] [PubMed] [Google Scholar]
- 83.Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell 87: 175–185, 1996. [DOI] [PubMed] [Google Scholar]
- 84.Kelesidis I, Kelesidis T, Mantzoros CS. Adiponectin and cancer: a systematic review. Br J Cancer 94: 1221–1225, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kharroubi I, Rasschaert J, Eizirik DL, Cnop M. Expression of adiponectin receptors in pancreatic β cells. Biochem Biophys Res Commun 312: 1118–1122, 2003. [DOI] [PubMed] [Google Scholar]
- 86.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest 117: 2621–2637, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res 94: e27–e31, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koerner A, Kratzsch J, Kiess W. Adipocytokines: leptin—the classical, resistin—the controversial, adiponectin—the promising, and more to come. Best Pract Res Clin Endocrinol Metab 19: 525–546, 2005. [DOI] [PubMed] [Google Scholar]
- 89.Korshunov VA, Nikonenko TA, Tkachuk VA, Brooks A, Berk BC. Interleukin-18 and macrophage migration inhibitory factor are associated with increased carotid intima-media thickening. Arterioscler Thromb Vasc Biol 26: 295–300, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Krause MP, Liu Y, Vu V, Chan L, Xu A, Riddell MC, Sweeney G, Hawke TJ. Adiponectin is expressed by skeletal muscle fibers and influences muscle phenotype and function. Am J Physiol Cell Physiol 295: C203–C212, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Krook A, Kawano Y, Song XM, Efendic S, Roth RA, Wallberg-Henriksson H, Zierath JR. Improved glucose tolerance restores insulin-stimulated Akt kinase activity and glucose transport in skeletal muscle from diabetic Goto-Kakizaki rats. Diabetes 46: 2110–2114, 1997. [DOI] [PubMed] [Google Scholar]
- 92.Kubota N, Yano W, Kubota T, Yamauchi T, Itoh S, Kumagai H, Kozono H, Takamoto I, Okamoto S, Shiuchi T, Suzuki R, Satoh H, Tsuchida A, Moroi M, Sugi K, Noda T, Ebinuma H, Ueta Y, Kondo T, Araki E, Ezaki O, Nagai R, Tobe K, Terauchi Y, Ueki K, Minokoshi Y, Kadowaki T. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6: 55–68, 2007. [DOI] [PubMed] [Google Scholar]
- 93.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol 23: 85–89, 2003. [DOI] [PubMed] [Google Scholar]
- 94.Lam KS, Xu A. Adiponectin: protection of the endothelium. Curr Diab Rep 5: 254–259, 2005. [DOI] [PubMed] [Google Scholar]
- 95.Lee MC, Schekman R. BAR domains go on a bender. Science 303: 479–480, 2004. [DOI] [PubMed] [Google Scholar]
- 96.Lemmon MA Pleckstrin homology domains: not just for phosphoinositides. Biochem Soc Trans 32: 707–711, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Li J, Mao X, Dong LQ, Liu F, Tong L. Crystal structures of the BAR-PH and PTB domains of human APPL1. Structure 15: 525–533, 2007. [DOI] [PubMed] [Google Scholar]
- 98.Lin DC, Quevedo C, Brewer NE, Bell A, Testa JR, Grimes ML, Miller FD, Kaplan DR. APPL1 associates with TrkA and GIPC1 and is required for nerve growth factor-mediated signal transduction. Mol Cell Biol 26: 8928–8941, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci USA 98: 7200–7205, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin LY, Lin CY, Su TC, Liau CS. Angiotensin II-induced apoptosis in human endothelial cells is inhibited by adiponectin through restoration of the association between endothelial nitric oxide synthase and heat shock protein 90. FEBS Lett 574: 106–110, 2004. [DOI] [PubMed] [Google Scholar]
- 101.Liu J, Yao F, Wu R, Morgan M, Thorburn A, Finley RL Jr, Chen YQ. Mediation of the DCC apoptotic signal by DIP13α. J Biol Chem 277: 26281–26285, 2002. [DOI] [PubMed] [Google Scholar]
- 102.Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, Otto KA, Syed SK, Bhanot S, Sloop KW, Sullivan JM, Reifel-Miller A. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology 148: 683–692, 2007. [DOI] [PubMed] [Google Scholar]
- 103.Lou X, Yano H, Lee F, Chao MV, Farquhar MG. GIPC and GAIP form a complex with TrkA: a putative link between G protein and receptor tyrosine kinase pathways. Mol Biol Cell 12: 615–627, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lou X, McQuistan T, Orlando RA, Farquhar MG. GAIP, GIPC and Gi3 are concentrated in endocytic compartments of proximal tubule cells: putative role in regulating megalin's function. J Am Soc Nephrol 13: 918–927, 2002. [DOI] [PubMed] [Google Scholar]
- 105.Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, Wu XP, Liao EY. Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res 309: 99–109, 2005. [DOI] [PubMed] [Google Scholar]
- 106.Machida K, Mayer BJ. The SH2 domain: versatile signaling module and pharmaceutical target. Biochim Biophys Acta 1747: 1–25, 2005. [DOI] [PubMed] [Google Scholar]
- 107.Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, Nagaretani H, Matsuda M, Komuro R, Ouchi N, Kuriyama H, Hotta K, Nakamura T, Shimomura I, Matsuzawa Y. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. Diabetes 50: 2094–2099, 2001. [DOI] [PubMed] [Google Scholar]
- 108.Mallat Z, Henry P, Fressonnet R, Alouani S, Scoazec A, Beaufils P, Chvatchko Y, Tedgui A. Increased plasma concentrations of interleukin-18 in acute coronary syndromes. Heart 88: 467–469, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Manning BD, Cantley LC. AKT/PKB signaling navigating downstream. Cell 129: 1261–1274, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mao X, Kikani CK, Riojas RA, Langlais P, Wang L, Ramos FJ, Fang Q, Christ-Roberts CY, Hong JY, Kim RY, Liu F, Dong LQ. APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8: 516–523, 2006. [DOI] [PubMed] [Google Scholar]
- 111.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, Kumada M, Okamoto Y, Nagaretani H, Nishizawa H, Kishida K, Komuro R, Ouchi N, Kihara S, Nagai R, Funahashi T, Matsuzawa Y. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem 277: 37487–37491, 2002. [DOI] [PubMed] [Google Scholar]
- 112.Matsushita K, Yatsuya H, Tamakoshi K, Wada K, Otsuka R, Takefuji S, Sugiura K, Kondo T, Murohara T, Toyoshima H. Comparison of circulating adiponectin and proinflammatory markers regarding their association with metabolic syndrome in Japanese men. Arterioscler Thromb Vasc Biol 26: 871–876, 2006. [DOI] [PubMed] [Google Scholar]
- 113.McPherson PS, Kay BK, Hussain NK. Signaling on the endocytic pathway. Traffic 2: 375–384, 2001. [DOI] [PubMed] [Google Scholar]
- 114.Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature 404: 782–787, 2000. [DOI] [PubMed] [Google Scholar]
- 115.Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 395: 801–804, 1998. [DOI] [PubMed] [Google Scholar]
- 116.Miaczynska M, Christoforidis S, Giner A, Shevchenko A, Uttenweiler-Joseph S, Habermann B, Wilm M, Parton RG, Zerial M. APPL proteins link Rab5 to nuclear signal transduction via an endosomal compartment. Cell 116: 445–456, 2004. [DOI] [PubMed] [Google Scholar]
- 117.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004. [DOI] [PubMed] [Google Scholar]
- 118.Mitsuuchi Y, Johnson SW, Moonblatt S, Testa JR. Translocation and activation of AKT2 in response to stimulation by insulin. J Cell Biochem 70: 433–441, 1998. [PubMed] [Google Scholar]
- 119.Mitsuuchi Y, Johnson SW, Sonoda G, Tanno S, Golemis EA, Testa JR. Identification of a chromosome 3p14.3–21.1 gene, APPL, encoding an adaptor molecule that interacts with the oncoprotein-serine/threonine kinase AKT2. Oncogene 18: 4891–4898, 1999. [DOI] [PubMed] [Google Scholar]
- 120.Monnier N, Satre V, Lerouge E, Berthoin F, Lunardi J. OCRL1 mutation analysis in French Lowe syndrome patients: implications for molecular diagnosis strategy and genetic counseling. Hum Mutat 16: 157–165, 2000. [DOI] [PubMed] [Google Scholar]
- 121.Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol 213: 1–17, 1999. [DOI] [PubMed] [Google Scholar]
- 122.Myers MG, Backer JM, Sun XJ, Shoelson S, Hu P, Schlessinger J, Yoakim M, Schaffhausen B, White MF. IRS-1 activates phosphatidylinositol 3′-kinase by associating with src homology 2 domains of p85. Proc Natl Acad Sci USA 89: 10350–10354, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nechamen CA, Thomas RM, Cohen BD, Acevedo G, Poulikakos PI, Testa JR, Dias JA. Human follicle-stimulating hormone (FSH) receptor interacts with the adaptor protein APPL1 in HEK 293 cells: potential involvement of the PI3K pathway in FSH signaling. Biol Reprod 71: 629–636, 2004. [DOI] [PubMed] [Google Scholar]
- 124.Nechamen CA, Thomas RM, Dias JA. APPL1, APPL2, Akt2 and FOXO1a interact with FSHR in a potential signaling complex. Mol Cell Endocrinol 260–262: 93–99, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension 47: 1108–1116, 2006. [DOI] [PubMed] [Google Scholar]
- 126.Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal 12: 1–13, 2000. [DOI] [PubMed] [Google Scholar]
- 127.Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, Hashimoto J, Yoshikawa H, Shimomura I. Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 331: 520–526, 2005. [DOI] [PubMed] [Google Scholar]
- 128.Ouchi N, Kihara S, Arita Y. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation 100: 2473–2476, 1999. [DOI] [PubMed] [Google Scholar]
- 129.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-κB signaling through a cAMP-dependent pathway. Circulation 102: 1296–1301, 2000. [DOI] [PubMed] [Google Scholar]
- 130.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, Kumada M, Ohashi K, Okamoto Y, Nishizawa H, Kishida K, Maeda N, Nagasawa A, Kobayashi H, Hiraoka H, Komai N, Kaibe M, Rakugi H, Ogihara T, Matsuzawa Y. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension 42: 231–234, 2003. [DOI] [PubMed] [Google Scholar]
- 131.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem 279: 1304–1309, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJ, Evans PR, McMahon HT. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303: 495–499, 2004. [DOI] [PubMed] [Google Scholar]
- 133.Piñeiro R, Iglesias MJ, Gallego R, Raghay K, Eiras S, Rubio J, Diéguez C, Gualillo O, González-Juanatey JR, Lago F. Adiponectin is synthesized and secreted by human and murine cardiomyocytes. FEBS Lett 579: 5163–5169, 2005. [DOI] [PubMed] [Google Scholar]
- 134.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 291: 1730–1737, 2004. [DOI] [PubMed] [Google Scholar]
- 135.Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell 8: 971–982, 2001. [DOI] [PubMed] [Google Scholar]
- 136.Ren G, Vajjhala P, Lee JS, Winsor B, Munn AL. The BAR domain proteins: molding membranes in fission, fusion, and phagy. Microbiol Mol Biol Rev 70: 37–120, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rodriguez-Pacheco F, Martinez-Fuentes AJ, Tovar S, Pinilla L, Tena-Sempere M, Dieguez C, Castano JP, Malagon MM. Regulation of pituitary cell function by adiponectin. Endocrinology 148: 401–410, 2007. [DOI] [PubMed] [Google Scholar]
- 138.Rybin V, Ullrich O, Rubino M, Alexandrov K, Simon I, Seabra MC, Goody R, Zerial M. GTPase activity of rab5 acts as a timer for endocytic membrane fusion. Nature 383: 266–269, 1996. [DOI] [PubMed] [Google Scholar]
- 139.Saito K, Tobe T, Minoshima S, Asakawa S, Sumiya J, Yoda M, Nakano Y, Shimizu N, Tomita M. Organization of the gene for gelatin-binding protein (GBP28). Gene 229: 67–73, 1999. [DOI] [PubMed] [Google Scholar]
- 140.Saito T, Jones CC, Huang S, Czech MP, Pilch PF. The interaction of Akt with APPL1 is required for insulin-stimulated Glut4 translocation. J Biol Chem 282: 32280–32287, 2007. [DOI] [PubMed] [Google Scholar]
- 141.Schenck A, Goto-Silva L, Collinet C, Rhinn M, Giner A, Habermann B, Brand M, Zerial M. The endosomal protein Appl1 mediates Akt substrate specificity and cell survival in vertebrate development. Cell 133: 486–497, 2008. [DOI] [PubMed] [Google Scholar]
- 142.Scherer P, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q produced exclusively in adipocytes. J Biol Chem 270: 26746–26749, 1995. [DOI] [PubMed] [Google Scholar]
- 143.Schnabel R, Messow CM, Lubos E, Espinola-Klein C, Rupprecht HJ, Bickel C, Sinning C, Tzikas S, Keller T, Genth-Zotz S, Lackner KJ, Munzel TF, Blankenberg S. Association of adiponectin with adverse outcome in coronary artery disease patients: results from the AtheroGene study. Eur Heart J 29: 649–657, 2008. [DOI] [PubMed] [Google Scholar]
- 144.Schulze MB, Shai I, Rimm EB, Li T, Rifai N, Hu FB. Adiponectin and future coronary heart disease events among men with type 2 diabetes. Diabetes 54: 534–539, 2005. [DOI] [PubMed] [Google Scholar]
- 145.Sharma K, Ramachandrarao S, Qiu G, Usui HK, Zhu Y, Dunn SR, Ouedraogo R, Hough K, McCue P, Chan L, Falkner B, Goldstein BJ. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest 118: 1645–1656, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, Yamauchi T, Terauchi Y, Kadowaki T, Takeuchi Y, Fukumoto S, Ikeda T, Hoshi K, Chung U, Nakamura K, Kawaguchi H. Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem 99: 196–208, 2006. [DOI] [PubMed] [Google Scholar]
- 147.Shulman GI Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Spranger J, Kroke A, Möhlig M, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Adiponectin and protection against type 2 diabetes mellitus. Lancet 361: 226–228, 2003. [DOI] [PubMed] [Google Scholar]
- 149.Staiger H, Machicao F, Machann J, Schick F, Kuulasmaa T, Laakso M, Fritsche A, Stefan N, Häring HU. Genetic variation within the APPL locus is not associated with metabolic or inflammatory traits in a healthy White population. Diabet Med 24: 817–822, 2007. [DOI] [PubMed] [Google Scholar]
- 150.Stefan N, Machicao F, Staiger H, Machann J, Schick F, Tschritter O, Spieth C, Weigert C, Fritsche A, Stumvoll M, Häring HU. Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat. Diabetologia 48: 2282–2291, 2005. [DOI] [PubMed] [Google Scholar]
- 151.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, Gaffney PR, Reese CB, McCormick F, Tempst P, Coadwell J, Hawkins PT. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science 279: 710–714, 1998. [DOI] [PubMed] [Google Scholar]
- 152.Su X, Lodhi IJ, Saltiel AR, Stahl PD. Insulin-stimulated interaction between insulin receptor substrate 1 and p85alpha and activation of protein kinase B/Akt require Rab5. J Biol Chem 281: 27982–27990, 2006. [DOI] [PubMed] [Google Scholar]
- 153.Takahashi M, Arita Y, Yamagata K, Matsukawa Y, Okutomi K, Horie M, Shimomura I, Hotta K, Kuriyama H, Kihara S, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Genomic structure and mutations in adipose-specific gene, adiponectin. Int J Obes 24: 861–868, 2000. [DOI] [PubMed] [Google Scholar]
- 154.Tamura T, Yoneda M, Yamane K, Nakanishi S, Nakashima R, Okubo M, Kohno N. Serum leptin and adiponectin are positively associated with bone mineral density at the distal radius in patients with type 2 diabetes mellitus. Metabolism 56: 623–628, 2007. [DOI] [PubMed] [Google Scholar]
- 155.Tan KC, Xu A, Chow WS, Lam MC, Ai VH, Tam SC, Lam KS. Hypoadiponectinemia is associated with impaired endothelium-dependent vasodilation. J Clin Endocrinol Metab 89: 765–769, 2004. [DOI] [PubMed] [Google Scholar]
- 156.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–341, 2007. [DOI] [PubMed] [Google Scholar]
- 157.Tzatsos A, Kandror KV. Nutrients suppress phosphatidylinositol 3-kinase/Akt signaling via raptor-dependent mTOR-mediated insulin receptor substrate 1 phosphorylation. Mol Cell Biol 26: 63–76, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ungewickell A, Ward ME, Ungewickell E, Majerus PW. The inositol polyphosphate 5-phosphatase Ocrl associates with endosomes that are partially coated with clathrin. Proc Natl Acad Sci USA 101: 13501–13506, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Valdez G, Akmentin W, Philippidou P, Kuruvilla R, Ginty DD, Halegoua S. Pincher-mediated macroendocytosis underlies retrograde signaling by neurotrophin receptors. J Neurosci 25: 5236–5247, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: more than just a road to PKB. Biochem J 346: 561–576, 2000. [PMC free article] [PubMed] [Google Scholar]
- 161.Varsano T, Dong MQ, Niesman I, Gacula H, Lou X, Ma T, Testa JR, Yates JR 3rd, Farquhar MG. GIPC is recruited by APPL to peripheral TrkA endosomes and regulates TrkA trafficking and signaling. Mol Cell Biol 26: 8942–8952, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Vaxillaire M, Dechaume A, Vasseur-Delannoy V, Lahmidi S, Vatin V, Leprêtre F, Boutin P, Hercberg S, Charpentier G, Dina C, Froguel P. Genetic analysis of ADIPOR1 and ADIPOR2 candidate polymorphisms for type 2 diabetes in the Caucasian population. Diabetes 55: 856–861, 2006. [DOI] [PubMed] [Google Scholar]
- 163.Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y, Uchida S, Tsuchida A, Takekawa S, Kadowaki T. Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology 146: 790–796, 2005. [DOI] [PubMed] [Google Scholar]
- 164.Wang C, Mao X, Wang L, Liu M, Wetzel MD, Guan KL, Dong LQ, Liu F. Adiponectin sensitizes insulin signaling by reducing p70 S6 kinase-mediated serine phosphorylation of IRS-1. J Biol Chem 282: 7991–7996, 2007. [DOI] [PubMed] [Google Scholar]
- 165.Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem 279: 35298–35305, 2004. [DOI] [PubMed] [Google Scholar]
- 166.Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, Tataranni PA. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab 86: 1930–1935, 2001. [DOI] [PubMed] [Google Scholar]
- 167.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 332: 644–646, 1988. [DOI] [PubMed] [Google Scholar]
- 168.Wu X, Motoshima H, Mahadev K, Stalker TJ, Scalia R, Goldstein BJ. Involvement of AMP-activated protein kinase in glucose uptake stimulated by the globular domain of adiponectin in primary rat adipocytes. Diabetes 52: 1355–1363, 2003. [DOI] [PubMed] [Google Scholar]
- 169.Xi W, Satoh H, Kase H, Suzuki K, Hattori Y. Stimulated HSP90 binding to eNOS and activation of the PI3-Akt pathway contribute to globular adiponectin-induced NO production: vasorelaxation in response to globular adiponectin. Biochem Biophys Res Commun 332: 200–205, 2005. [DOI] [PubMed] [Google Scholar]
- 170.Xu M, Li X, Wang JG, Du P, Hong J, Gu W, Zhang Y, Ning G. Glucose and lipid metabolism in relation to novel polymorphisms in the 5′-AMP-activated protein kinase gamma2 gene in Chinese. Mol Genet Metab 86: 372–378, 2005. [DOI] [PubMed] [Google Scholar]
- 171.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med 7: 941–946, 2001. [DOI] [PubMed] [Google Scholar]
- 172.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med 8: 1288–1295, 2002. [DOI] [PubMed] [Google Scholar]
- 173.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423: 762–769, 2003. [DOI] [PubMed] [Google Scholar]
- 174.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and apoE-deficient mice from atherosclerosis. J Biol Chem 278: 2461–2468, 2003. [DOI] [PubMed] [Google Scholar]
- 175.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13: 332–339, 2007. [DOI] [PubMed] [Google Scholar]
- 176.Yamaguchi N, Kukita T, Li YJ, Kamio N, Fukumoto S, Nonaka K, Ninomiya Y, Hanazawa S, Yamashita Y. Adiponectin inhibits induction of TNF-alpha/RANKL-stimulated NFATc1 via the AMPK signaling. FEBS Lett 582: 451–456, 2008. [DOI] [PubMed] [Google Scholar]
- 177.Yang L, Lin HK, Altuwaijri S, Xie S, Wang L, Chang C. APPL suppresses androgen receptor transactivation via potentiating Akt activity. J Biol Chem 278: 16820–16827, 2003. [DOI] [PubMed] [Google Scholar]
- 178.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, Chen CL, Tai TY, Chuang LM. Weight reduction increases plasma levels of an adipose-derived anti-inflammatory protein, adiponectin. J Clin Endocrinol Metab 86: 3815–3819, 2001. [DOI] [PubMed] [Google Scholar]
- 179.Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron 39: 57–68, 2003. [DOI] [PubMed] [Google Scholar]
- 180.Yokota T, Oritani K, Takahashi I, Ishikawa J, Matsuyama A, Ouchi N, Kihara S, Funahashi T, Tenner AJ, Tomiyama Y, Matsuzawa Y. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96: 1723–1732, 2000. [PubMed] [Google Scholar]
- 181.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor. Diabetes 55: 2562–2570, 2006. [DOI] [PubMed] [Google Scholar]
- 182.Yoshida M, Horinouchi S, Beppu T. Trichostatin A and trapoxin: novel chemical probes for the role of histone acetylation in chromatin structure and function. Bioessays 17: 423–430, 1995. [DOI] [PubMed] [Google Scholar]
- 183.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2: 107–117, 2001. [DOI] [PubMed] [Google Scholar]
- 184.Zhou MM, Ravichandran KS, Olejniczak EF, Petros AM, Meadows RP, Sattler M, Harlan JE, Wade WS, Burakoff SJ, Fesik SW. Structure and ligand recognition of the phosphotyrosine binding domain of Shc. Nature 378: 584–592, 1995. [DOI] [PubMed] [Google Scholar]
- 185.Zhu G, Chen J, Liu J, Brunzelle JS, Huang B, Wakeham N, Terzyan S, Li X, Rao Z, Li G, Zhang XC. Structure of the APPL1 BAR-PH domain and characterization of its interaction with Rab5. EMBO J 26: 3484–3493, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zimmerberg J, McLaughlin S. Membrane curvature: how BAR domains bend bilayers. Curr Biol 14: R250–R252, 2004. [DOI] [PubMed] [Google Scholar]