Abstract
Diets high in fat are associated with increased susceptibility to obesity and metabolic syndrome. Increased adipose tissue that is caused by high-fat diets (HFD) results in altered storage of lipophilic toxicants like 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), which may further increase susceptibility to metabolic syndrome. Because both TCDD and HFD are associated with increased breast cancer risk, we examined their effects on metabolic syndrome-associated phenotypes in three mouse models of breast cancer: 7,12-dimethylbenz[a]anthracene (DMBA), Tg(MMTV-Neu)202Mul/J (HER2), and TgN(MMTV-PyMT)634Mul/J (PyMT), all on an FVB/N genetic background. Pregnant mice dosed with 1 μg/kg of TCDD or vehicle on gestational day 12.5 were placed on a HFD or low-fat diet (LFD) at parturition. Body weights, percent body fat, and fasting blood glucose were measured longitudinally, and triglycerides were measured at study termination. On HFD, all cancer models reached the pubertal growth spurt ahead of FVB controls. Among mice fed HFD, the HER2 model had a greater increase in body weight and adipose tissue from puberty through adulthood compared with the PyMT and DMBA models. However, the DMBA model consistently had higher fasting blood glucose levels than the PyMT and HER2 models. TCDD only impacted serum triglycerides in the PyMT model maintained on HFD. Because the estrogenic activity of the HFD was three times lower than that of the LFD, differential dietary estrogenic activities did not drive the observed phenotypic differences. Rather, the HFD-dependent changes were cancer model dependent. These results show that cancer models can have differential effects on metabolic syndrome-associated phenotypes even before cancers arise.
Keywords: obesity; metabolic syndrome; 2,3,7,8-tetrachlorodibenzo-p-dioxin; Tg(MMTV-Neu)202Mul/J; TgN(MMTV-PyMT)634Mul/J; dimethylbenz[a]anthracene
metabolic syndrome represents a constellation of disease-associated physiological changes affecting over 47 million Unite States residents (11). The key etiological component of metabolic syndrome is obesity, which can promote insulin insensitivity and type II diabetes. Additionally, excess adipose tissue results in increased storage of fatty acids and triglycerides in peripheral tissues (38). Abundant adipose tissue also causes excess endocrine signaling and estrogen aromatization, which in turn increase adipose proliferation, body weight, and fasting blood glucose (2, 31, 46, 47). Peripheral estrogen aromatization by excess adipose tissue is also thought to contribute to the increased postmenopausal breast cancer risk among obese women (14, 41).
Individuals that have metabolic syndrome or that are obese usually have higher consumption of fatty foods from animal origin, which is associated with a greater body burden of lipophilic endocrine disruptors such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (16, 18, 21, 34). Elimination of TCDD is slowed substantially as body fat increases (10, 32). Consequently, obese individuals may have unique susceptibilities to the effects of TCDD and other lipophilic endocrine disruptors.
Although the effects of high doses of TCDD are a depletion of both lean and adipose tissue that results in a wasting syndrome (36), the effects of low doses of TCDD seem to shift away from body compositional changes to endocrine disruption and promotion of diseases associated with metabolic syndrome like type II diabetes. Human exposure to TCDD results in an increase in serum insulin levels, an earlier diabetes onset, and diabetes-associated mortality in females (7, 8, 19).
While there is evidence that TCDD exposure and high-fat diet (HFD) interact and affect metabolic syndrome, little is known about how these exposures may differentially impact metabolic syndrome based on the type of breast cancer. Breast cancers can be partitioned into distinct subtypes that represent unique histopathologies, genetic alterations, treatment responses, and perhaps risk factors (20). Because of this human breast cancer diversity, multiple mouse models have been used to recapitulate clinically significant aspects of initiation, progression, and invasion.
Two human breast cancer subtypes are estrogen receptor (ER)-negative and have poor prognosis (42, 43); one of these subtypes has basal/myoepthilial cell characteristics (basal-like). Basal-like subtypes represent 5–10% of breast cancers and include those with BRCA1 and HRAS mutations (20), which are involved in proliferation and invasion (33). The other ER-negative breast cancer subtype has high ERBB2 (HER2) expression (49). Similar to these human breast cancers, the 7,12-dimethylbenz[a]anthracene (DMBA) carcinogen-induced mouse model of breast cancer results in hyperactivation of Hras and heightened proliferation (3, 20). Luminal-type breast cancers represent a third subtype, and prognosis for these can be good (luminal A) or bad (luminal B) (42, 43). The TgN(MMTV-PyMT)634Mul/J (PyMT) transgenic mouse model has characteristics of the luminal subtype of breast cancer (20).
To elucidate differential consequences of the interaction between dietary fat and TCDD exposure on metabolic syndrome-associated phenotypes, three mouse models of breast cancer were exposed maternally to low-dose TCDD in combination with either a HFD or a lower-fat, matched control diet (LFD). To directly compare metabolic syndrome-associated phenotypes, all three models were maintained on a uniform FVB/N genetic background, which is susceptible to TCDD toxicity and diet-induced obesity (5, 14, 25, 29, 30).
MATERIALS AND METHODS
Chemicals.
DMBA (98% pure), 17β-estradiol (98% pure E2), and 5α-androstan-17β-OL-3-one (99% pure DHT) were purchased from Sigma-Aldrich (St. Louis, MO). TCDD was purchased from Ultra Scientific (99.9% pure, North Kingstown, RI). DMBA and TCDD were dissolved in 95:5% olive oil-toluene by volume (Certified ACS; Sigma-Aldrich) and used at 25 mg/ml and 0.5 ng/μl stock concentrations, respectively. Dosing was at 2.4 μl DMBA solution/g mouse and 1.8–1.9 μl TCDD solution/g mouse. In the diet activity assays, TCDD, E2, and DHT were dissolved in dimethyl sulfoxide (DMSO; Certified ACS, Fisher Scientific, Pittsburg, PA), and activity levels were determined as described below.
Diets.
The HFD (4.73 kcal/g, D12451; Research Diets, New Brunswick, NJ) was composed of 20% protein, 35% carbohydrate, and 45% fat by total kilocalories, whereas the LFD (3.85 kcal/g, D12450B; Research Diets) was composed of 20% protein, 70% carbohydrate, and 10% fat by total kilocalories. The primary differences between the matched diets are increased maltodextrin and lard and decreased cornstarch and sucrose in the HFD compared with the LFD (400, 1,598, 291, and 691 kcal vs. 140, 180, 1,260, and 1,400 kcal, respectively). The LFD has fat levels that fall within the range commonly found in standard rodent chows, but much lower than the typical human diet in the United States.
Mice and husbandry.
All three mouse models of breast cancer were on the FVB/N (FVB) background. Nulliparous female FVB mice were mated with male hemizygous FVB-Tg(MMTVNeu)202Mul/J (HER2) mice (Jackson Laboratories, Bar Harbor, ME) to generate female pups for the HER2 model, representing ER-negative, HER2-positive breast cancers (20, 48). Nulliparous female FVB and male hemizygous FVB-TgN(MMTV-PyMT)634Mul/J (PyMT) mice (NCI MMHCC Repository, Frederick, MD) were crossed to generate female offspring for the PyMT model, representing ER-negative luminal breast cancers (13, 20, 37). Nulliparous female and male FVB mice were mated to produce female pups used in the DMBA model, representing basal-like breast cancers (20), as well as untreated FVB controls. Six litters per treatment were used for the DMBA model because of the added variability associated with carcinogen models, while three litters were used per treatment for the HER2 and PyMT models.
Mice carrying the PyMT transgene were identified using primers 5′-AACGGCGGAGCGAGGAACTG and 5′-ATCGGGCTCAGCAACACAAG (Operon, Huntsville, AL) and a PCR protocol previously described (Jackson Laboratories) (23). Mice carrying the HER2 transgene were identified using PCR primers 5′-TTTCCTGCAGCAGCCTACGC and 5′-CGGAACCCACATCAGGCC (37). Mice were housed in HEPA-filtered ventilated cages with food and water provided ad libitum. Care and treatment of the mice complied with the guidelines of the Animal Welfare Act under an Institutional Animal Care and Use Committee-approved protocol. Mice were killed by carbon dioxide asphyxiation once tumors reached 1 cm in diameter or at 11 mo of age, whichever came first. FVB control mice were killed by carbon dioxide asphyxiation at postnatal day (PND) 121.
Treatment and experimental groups.
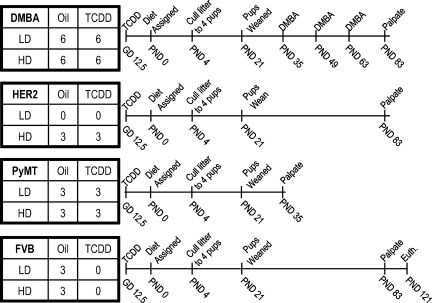
The effects of diet and TCDD were studied in a randomized 2 × 2 factorial design (Fig. 1). Noon on the day that a vaginal plug was observed was designated at 0.5 days postcoitus (dpc). On 12.5 dpc, 1 μg/kg of TCDD was administered by oral gavage to pregnant FVB (n = 12), PyMT (n = 6), and HER2 (n = 3) dams or the equivalent volume of vehicle to pregnant FVB (n = 18), PyMT (n = 6), and HER2 (n = 3) dams. From parturition at PND 0, dams were fed one of the two standardized diets, and their respective pups were weaned at PND 21 on the same diet until death. FVB and PyMT litters were assigned to HFD or LFD symmetrically with respect to TCDD exposure status, whereas HER2 litters from TCDD-exposed dams were assigned to HFD only; the HER2 model has been analyzed extensively previously using diets equivalent to the LFD and shown to not differ from FVB controls (5). In total, 30 FVB, 12 PyMT, and 6 HER2 litters were fed HFD or LFD. Litters were culled to four mice at PND 4, maximizing the number of females with the desired genotype. At PND 35, 49, and 63, 60 mg/kg of DMBA were administered by oral gavage to 24 FVB litters. The remaining six FVB litters served as controls. DMBA and HER2 mice were palpated weekly for tumors beginning at PND 83; PyMT mice were palpated for tumors three times per week beginning at PND 35.
Fig. 1.
Schematic of 7,12-dimethylbenz[a]anthracene (DMBA), Tg(MMTV-Neu)202Mul/J (HER2), and TgN(MMTV-PyMT)634Mul/J (PyMT) breast cancer models and FVB controls. Numbers in 2 × 2 tables represent the number of litters per treatment group. Euth, death of FVB. Cancer models were killed after palpation when lesions reached >1 cm in diameter or at postnatal day (PND) 330.
Body composition.
At PND 0, body mass was determined by dividing the total litter mass by the number of pups within the litter. Individual body masses were evaluated at PND 4, 7, 10, 14, 21, 35, 49, 63, 90, 120, 150, 180, 210, 240, 270, 300, and 330. At PND 35, 90, and 180, all mice were placed under isoflourane anesthesia for ∼5 min while undergoing dual-energy X-ray absorptiometry (DEXA; GE Lunar PIXImus, Madison, WI) to evaluate percent body fat.
Blood chemistry.
Following a 24-h fast at PND 35, 120, 180, 240, and 300, blood glucose levels were measured longitudinally using a ThermaSense FreeStyle blood glucose kit (Alameda, CA). Immediately following euthanasia, blood was drawn from the inferior vena cava, and serum was stored at −80°C for triglyceride analysis using the StanBio Laboratories Enzymatic Triglycerides Procedure 2150 (Boerne, TX).
Nuclear receptor activity assays.
Approximately 10 g of each diet were crushed with a mortar and pestle and extracted three times with 20 ml of toluene (nonpolar extract) or ethanol (polar extract). The extract was allowed to settle before passing the supernatant through a Celite filter column. The column was rinsed with 20 ml of toluene or ethanol. The toluene or ethanol extracts were dried and resuspended in 4 ml of the same solvent. Aliquots of polar (200 μl) and nonpolar (300 μl) suspensions were mixed with 3.5 μl of DMSO, and the solvents were removed by vacuum centrifugation before the remaining DMSO was mixed with 350 μl of culture media. Each diet extract was analyzed for its ability to activate expression of an ER-, androgen receptor (AR)-, or aryl hydrocarbon receptor (AhR)-dependent firefly luciferase reporter gene in stably transfected human ovarian (BG1), human breast cancer (T47D), and mouse hepatoma (Hepa1c1c7) cell lines, respectively (15, 39, 50).
Concentration-dependent reporter gene induction was determined by analysis of the inducing potency of a serial dilution of each sample extract (100 μl/well). Cells (75,000) were plated in sterile COSTAR white clear-bottomed 96-well tissue culture microplates (Corning, Corning, NY) and allowed to attach for 24 h before chemical treatment. For the ER bioassay, cells were maintained in estrogen-stripped media for 5 days before plating to reduce background estrogen activity. Cells were incubated with diet extracts, carrier DMSO solvent (1% final solvent concentration), or increasing concentrations of E2, DHT, or TCDD standards for 24 h at 37°C. After 24 h of incubation, all microplate wells were washed two times with PBS before addition of 50 μl of cell lysis buffer (Promega, Madison, WI) and shaking for 20 min at room temperature to facilitate cell lysis. The plates were inserted in a Berthold microplate luminometer (Spectranalyzed Berthold Detection Systems, Bleichstrasse 56–68, Pforzheim, Germany), and luciferase activity in each well was measured over 10 s after a 2-s delay following automatic injection of 50 μl Promega-stabilized luciferase reagent (15, 39, 50). Luciferase activity in each well was expressed relative to the maximal inducing concentration of the respective positive control (E2, DHT, or TCDD).
Statistical analysis.
All statistical analyses were performed with SAS software, version 9.0 (SAS Institute, Cary, NC). Monophasic- and diphasic-sigmoidal models of change in body mass over time (26, 28) were evaluated for all cancer models and controls according to yt = ∑i (ai {1 + tanh[bi (t − ci)]}) using the nonlinear regression procedure (PROC NLIN), where yt is the predicted body weight in grams (g) at age t, i is the number of phases, ai is half the asymptotic value of y in phase i, bi is a growth parameter in phase i, and ci is the age at the inflection point of phase i. It follows that, when i = 1, parameters a1, b1, and c1 describe growth in prepubertal mice and, when i = 2, parameters a2, b2, and c2 describe growth in postpubertal mice. a1 is the body weight (g) at the maximum prepubertal growth rate, 2a1 + a2 is the body weight (g) at the maximum postpubertal growth rate, a1b1 is the maximum prepubertal growth rate (g/PND), a2b2 is the maximum postpubertal growth rate (g/PND), c1 is the age at the maximum prepubertal growth rate (PND), and c2 is the age at the maximum postpubertal growth rate (PND).
Changes in fasting blood glucose and percent body fat over time were modeled longitudinally with PROC MIXED, a model that accounts for both fixed (e.g., exposures) and random effects. Because pregnant dams were exposed to TCDD, the litter was used as a random effect. Median triglycerides per litter were evaluated using ANOVA and Student's two-way t-test for all pairwise comparisons at an α level of 0.05. Tumor presence and tumor size had no effect on fasting blood glucose, body weight, or percent body fat and were not included as random effects. This model is robust against sample size variance due to attrition, weekends, and holidays.
For nuclear receptor activity assays, mean relative light units were determined at each concentration in both toluene and ethanol extracts for standard and diet samples. From this, the effective extract concentration giving 50% of the maximal induction response was calculated for each standard in both toluene and ethanol extracts. The percent effective concentration (EC[x]) of each diet was then determined in both toluene and ethanol extracts. ANOVA was used to assess significance of induction equivalents, as a ratio of the EC[x] value of the sample and the equivalent value from the standard curves, between diet samples for each standard and solvent combination.
RESULTS
HFD increases longitudinal body weight.
The effect of dietary fat on body weight was determined by analyzing the interaction between body weight and age using individual mice. HFD increased the body weight and growth of mice from the DMBA and PyMT models, but not of FVB control mice. Because mice from the HER2 model were only raised on HFD, no diet comparisons were performed with this model. Neither TCDD nor tumor presence had an effect on body weight for any model.
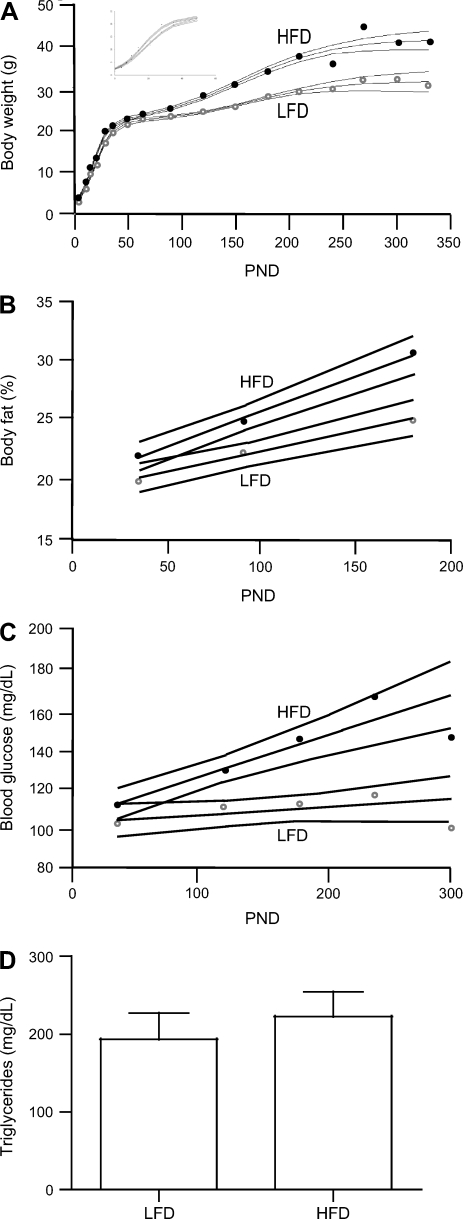
For mice from the DMBA model, the diphasic sigmoidal model of growth fit best (r2 = 0.945) (28). At prepubertal time points before DMBA treatment, HFD increased the maximum growth rate and decreased the age at the maximum growth rate of mice compared with LFD (P < 0.01; Fig. 2A and Table 1). Similarly, HFD also increased the maximum growth rate of mice from the DMBA model at postpubertal time points as well as the weight at the maximum growth rate (P < 0.001). DMBA mice maintained on HFD had higher body weights compared with mice maintained on LFD from PND 4 until PND 300 (P < 0.01), when there were insufficient mice on HFD still surviving for comparison.
Fig. 2.
Metabolic syndrome-related phenotypes in DMBA mice. High-fat diet (HFD) increases body weight (A), percent body fat (B), and blood glucose (C) over the low-fat diet (LFD). Longitudinal models are depicted per diet with 95% confidence intervals, and diet-based mean is plotted for LFD (○) and HFD (•). For body weight measurement at PND 0, 4, 7, 10, 14, 18, 21, 28, 35, 49, 63, 90, 120, 150, 180, 210, 240, 270, 300, and 330, there were 62, 54, 62, 62, 61, 45, 62, 20, 61, 54, 58, 58, 56, 35, 39, 32, 19, 15, 12, and 11 mice on LFD and 66, 65, 66, 62, 66, 43, 66, 28, 66, 65, 65, 58, 53, 40, 20, 5, 5, 4, 2, and 0 mice on HFD, respectively. For body fat measurement at PND 35, 90, and 180, there were 62, 59, and 33 mice on LFD and 64, 62, and 27 mice on HFD, respectively. For blood glucose measurement at PND 35, 120, 180, 240, and 300, there were 50, 51, 26, 19, and 11 and mice on LFD and 65, 56, 15, 5, and 2 mice on HFD, respectively. D: triglycerides are not impacted by diet (mean ± SE; n = 21 and 23 mice for LFD and HFD, respectively).
Table 1.
Effects of dietary fat on body growth characteristics in the DMBA model
| Parameter | Interpretation | LFD | HFD | |||
|---|---|---|---|---|---|---|
| Prepubertal phase | ||||||
| a1 | Weight at maximum growth rate, g | 11.0 (0.27) | 10.7 (0.45) | |||
| a1 × b1 | Maximum growth rate, g/PND | 0.640 (0.02)* | 0.730 (0.02)* | |||
| d1 | Age at maximum growth rate, PND | 18.8 (0.35)* | 16.7 (0.29)* | |||
| Postpubertal phase | ||||||
| 2a1 + a2 | Weight at maximum growth rate, g | 26.8 (0.28)* | 31.5 (0.52)* | |||
| a2 × b2 | Maximum growth rate, g/PND | 0.062 (0.01)* | 0.116 (0.01)* | |||
| d2 | Age at maximum growth rate, PND | 164.3 (6.7) | 154.2 (5.8) | |||
Values are means (+SE). DMBA, 7,12-dimethylbenz[a]anthracene; LFD, low-fat diet; HFD, high-fat diet; PND, postnatal day. Parameters were estimated from a diphasic sigmoidal curve (28). See text for parameter definitions.
P < 0.05, significant differences between diets.
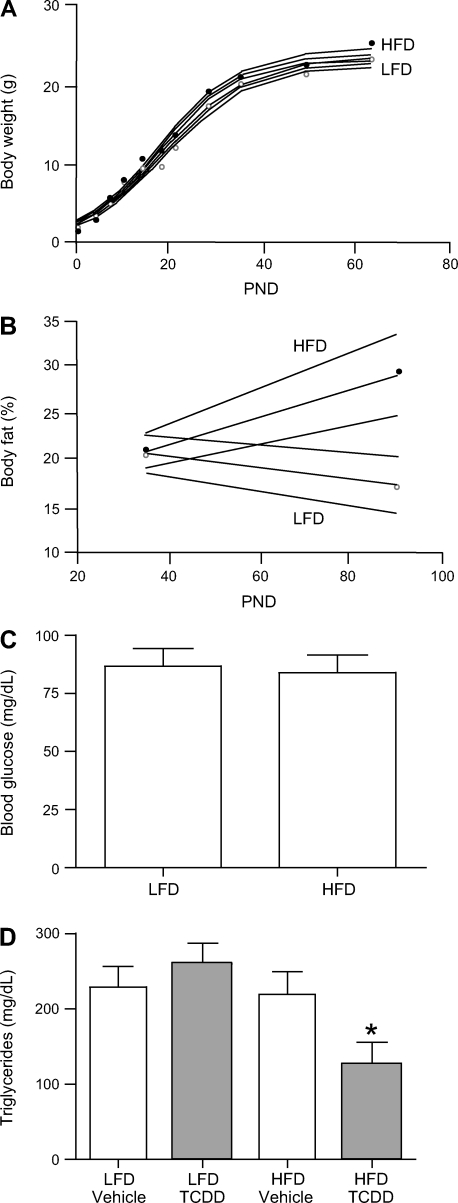
The monophasic sigmoidal model of growth fit best for mice from the PyMT model (r2 = 0.989) (28). Similar to mice from the DMBA model, prepubertal PyMT mice maintained on HFD had a faster maximum growth rate and decreased age at the maximum growth rate than those maintained on LFD (P < 0.01 and 0.05, respectively; Fig. 3A and Table 2). Mice from the PyMT model maintained on HFD were also heavier at the maximum growth rate than those maintained on LFD (P < 0.05); there was a trend of increased body weight with the HFD in the PyMT model across all ages, although it was only significant between PND 14 and 35 (P < 0.05).
Fig. 3.
Metabolic syndrome-related phenotypes in PyMT mice. HFD increases body weight (A), percent body fat (B), but not blood glucose (C; n = 17 and 18 mice for LFD and HFD, respectively). D: triglycerides are altered by HFD and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) (means ± SE; n = 6, 6, 5, and 5 mice for LFD + vehicle, LFD + TCDD, HFD + vehicle, and HFD + TCDD, respectively). In A–C, longitudinal models are depicted per diet with 95% confidence intervals, and diet-based mean is plotted for LFD (○) and HFD (•). For body weight measurements at PND 0, 4, 7, 10, 14, 18, 21, 28, 35, 49, and 63, there were 17, 17, 17, 17, 17, 11, 17, 12, 17, 15, and 12 mice on LFD and 18, 18, 18, 18, 18, 18, 15, 13, 18, 10, and 8 mice on HFD, respectively. For body fat at PND 35 and 90, there were 16 and 5 mice on LFD and 18 and 2 mice on HFD, respectively.
Table 2.
Effects of dietary fat on body growth characteristics in the PyMT model
| Parameter | Interpretation | LFD | HFD |
|---|---|---|---|
| a1 | Weight at maximum growth rate, g | 11.5 (0.16)* | 12.0 (0.18)* |
| a1×b1 | Maximum growth rate, g/PND | 0.639 (0.02)* | 0.721 (0.02)* |
| d1 | Age at maximum growth rate, PND | 18.9 (0.45)* | 17.6 (0.41)* |
Values are means (+SE). PyMT, TgN(MMTV-PyMT)634Mul/J. Parameters were estimated from a monophasic sigmoidal curve (28). See text for parameter definitions.
P < 0.05, significant differences between diets.
HFD increases longitudinal percent body fat.
The effect of dietary fat on percent body fat was analyzed as an interaction between diet and PND for individual mice over time, with litter as a mixed effect. Similar to body weight, neither perinatal TCDD nor tumor presence caused a change in percent body fat.
Percent body fat was greater in mice from the DMBA model maintained on HFD compared with those maintained on LFD at all ages measured (P < 0.01; Fig. 2B), with the effect of HFD becoming more pronounced with aging (P < 0.0001). This trend was similar to that seen for the PyMT model, where percent body fat increased with time in mice maintained on HFD but not on LFD, causing a significant dietary-induced difference in percent body fat by PND 90 (P < 0.05; Fig. 3B). Relative to LFD, maintenance on HFD resulted in increased percent body fat in FVB control mice at PND 35 (P = 0.05) but not at PND 90 (data not shown). Unlike mice from the DMBA and PyMT breast cancer models, control FVB mice were resistant to diet-induced adiposity.
HFD increases longitudinal fasting blood glucose.
Fasting blood glucose was modeled as an interaction between dietary fat and PND on individual mice using litter as a mixed effect. Consistent with elevated percent body fat, fasting blood glucose was significantly elevated in mice from the DMBA model maintained on HFD compared with those on LFD at all ages after PND 35 (P < 0.05; Fig. 2C). The differential effects of diet increased with aging (P < 0.0001). Fasting blood glucose was not affected by diet in FVB control mice at PND 35 or 120 (data not shown), nor in the PyMT cancer model mice at PND 35 (Fig. 3C).
HFD and TCDD depress serum triglycerides.
Unlike body fat and fasting blood glucose, serum triglycerides were affected by TCDD; thus, ANOVAs were performed on the medians of litter triglyceride values. Dietary fat and TCDD had no effect on triglycerides in mice from the DMBA model or FVB control mice (Fig. 2D). However, triglycerides were significantly depressed in mice from the PyMT model by a combined exposure of HFD and TCDD (P < 0.01; Fig. 3D).
Differences among models in the longitudinal effects of HFD.
Mice from the DMBA and PyMT breast cancer models had faster maximum prepubertal growth rates than FVB control mice (P < 0.05; Table 3). Mice from the DMBA and HER2 models weighed the least and most, respectively, at their maximum prepubertal growth rates, possibly reflecting differences in their age of maximum prepubertal growth rate. FVB control mice reached their maximum prepubertal growth rate later than mice from the breast cancer models. By PND 35 and continuing through adulthood at PND 180, mice from the HER2 model were heavier than all others (P < 0.01; Table 4). After PND 180, there was no difference between weights or growth trajectories of mice from the HER2 and DMBA models.
Table 3.
Comparison of body growth characteristics among models of breast cancer maintained on HFD
| Parameter | Interpretation | FVB | PyMT | HER2 | DMBA | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prepubertal phase | ||||||||||
| a1 | Weight at maximum growth rate, g | 12.1 (0.12)a | 12.0 (0.18)b | 13.5 (1.6)a,b | 10.7 (0.45)c | |||||
| a1 × b1 | Maximum growth rate, g/PND | 0.681 (0.03)b | 0.721 (0.02)a | 0.761 (0.07)a | 0.729 (0.02)a | |||||
| d1 | Age at maximum growth rate, PND | 21.3 (0.43)a | 17.6 (0.41)b | 20.5 (1.3)a | 16.7 (0.29)b | |||||
| Postpubertal phase | ||||||||||
| 2a1 + a2 | Weight at maximum growth rate, g | 44.8 (23.9)a | 31.5 (0.5)a | |||||||
| a2 × b2 | Maximum growth rate, g/PND | 0.0947 (0.07)a | 0.116 (0.01)a | |||||||
| d2 | Age at maximum growth rate, PND | 332.7 (233.7)a | 154.2 (5.8)a | |||||||
Values are means (±SE), with significant differences between breast cancer models that do not share a letter a, b, or c (P < 0.05). Parameters were estimated from monophasic and diphasic sigmoidal curves (27).
Table 4.
Comparison of metabolic syndrome phenotypes among models of breast cancer maintained on HFD
| Phenotype | FVB | DMBA | PyMT | HER2 |
|---|---|---|---|---|
| BW at PND 35 | 20.4 (0.57)b | 20.7 (0.21)b | 21.0 (0.40)b | 23.7 (0.31)a |
| Body fat at PND 35 | 21.0 (2.3)b | 22.0 (3.3)b | 20.8 (2.6)b | 26.2 (2.2)a |
| Glucose at PND 35 | 104.9 (27.0)a,b | 111.4 (29.1)a | 83.9 (23.5)b | 92.1 (15.8)b |
| BW at PND 90 | 25.2 (0.74)b | 25.1 (0.32)b | 20.1 (0.48)c | 29.4 (0.85)a |
| Body fat at PND 90 | 27.3 (4.2)b | 25.0 (3.5)b | 29.4 (4.0)a,b | 31.9 (4.5)a |
| BW at PND 120 | 27.8 (1.3)a | 28.1 (0.44)a | 29.5 (0.92)a | |
| Glucose at PND 120 | 118.2 (26.9)a | 129.3 (24.6)a | 93.8 (21.7)b | |
| TAG at sacrifice | 128.6 (15.4)a | 193.5 (31.3)a | 172.6 (20.8)a | 179.8 (49.6)a |
Values are means (±SE), with significant differences between breast cancer models that do not share a letter a or b (P < 0.05). Means were estimated from monophasic and diphasic sigmoidal curves for BW (body weight, g) (27), from linear mixed models for fasting blood glucose (glucose, mg/dl) and body fat (%), and from ANOVA for serum triglycerides (TAG, mg/dl).
Trends for percent body fat in the three models of breast cancer were similar to trends in body mass. Mice from the HER2 model had a higher percent body fat early in life than mice from the DMBA and PyMT models, or FVB control mice (P < 0.01), but by PND 180 percent body fat was similar between mice from the DMBA and HER2 models (Table 4). Percent body fat did not differ at any age among mice from the DMBA and PyMT models, or the FVB control mice.
Mice from the DMBA model had higher fasting blood glucose than those from the PyMT and HER2 models at PND 35 (P < 0.05; Table 4). However, by PND 120, mice from both the DMBA model and FVB control had higher fasting blood glucose than those from the HER2 model (P < 0.05). This divergence increased throughout the duration of the study. Mice from the HER2 model maintained constant levels of fasting blood glucose over time, whereas mice from the DMBA model continued to increase until PND 300. There were no differences among the models of breast cancer or controls in their serum triglyceride levels, despite differences in age at death (Table 4).
LFD has higher nuclear receptor activity than HFD.
Differences between HFD and LFD with regard to the presence of endocrine-disrupting chemicals could underlie their differential effects on metabolic syndrome-associated phenotypes (45). Accordingly, both diets were analyzed for their ability to activate ER, AR, or AhR using luciferase reporter gene assays. Dose-response studies revealed that little or no AR- or AhR-dependent reporter gene expression was observed using polar and nonpolar extracts from either diet (data not shown). In contrast, significant induction of ER-dependent reporter gene expression was observed using nonpolar extracts from both diets (Table 5); little or no ER-dependent activity was observed using polar extracts. Comparison of ER-dependent reporter gene expression levels using dose-response analysis revealed that the overall estrogenic activity of the LFD was three times greater than that of the HFD (P < 0.01), opposite to that expected if differences in diet-derived estrogenic activities were responsible for the metabolic syndrome-associated phenotype differences.
Table 5.
Estrogenic activity of organic fraction from diets
| Diet | Extract Concentration, g/ml | EC50 Standards, pg E2 | EC[x] for Sample, g diet equivalents | Induction Equivalent, pg E2/g diet equivalents |
|---|---|---|---|---|
| LFD | 2.500 | 0.07352 | 0.00705 [50.0%] | 10.43* |
| HFD | 2.502 | 0.19172 | 0.05150 [18.6%] | 3.72 |
Values are meanss, based on triplicates. E2, 17β-estradiol.
Interdiet significant differences using ANOVA (P < 0.05).
DISCUSSION
Metabolic syndrome and breast cancer are traits with complex etiologies driven in part by disrupted endocrine signaling. With the increasing prevalence of metabolic syndrome in humans, it is critical to characterize the susceptibility of those with metabolic syndrome to environmental endocrine disruptors. Through a cross-model comparison using three mouse models of breast cancer on a uniform genetic background, we have provided insights into the contribution of different types of breast cancers to diet-induced metabolic syndrome-associated phenotypes.
Body composition.
We observed that age-dependent differences in percent body fat due to diet, consistent with reports that the DMBA, PyMT, and HER2 models are sensitive to dietary fat and obesity (12, 14, 29). We did not observe any dietary effects on FVB control body weight or percent body fat, as expected, based on the literature (30). This difference may be due to several differences between studies, such as mouse age and specific HFD used. Whereas DMBA and FVB did not differ in body weight or percent body fat at PND 35 (before DMBA exposure), growth modeling suggested DMBA and FVB were growing differently before DMBA exposure. Sampling error may have caused an apparent effect in the DMBA model, or, alternatively, the sum growth trajectory due to DMBA treatment may have impacted the apparent growth model enough to result in differential growth between DMBA and FVB before DMBA treatment. Although HER2 mice were not fed LFD in the present study, in another study using a comparable LFD, the body weight of HER2 was similar to what was seen in FVB control mice here [23.3 ± 0.4 vs. 24.4 ± 0.4 (SE) g] (5).
The body weight and fat accumulation seen in the HER2 model, relative to the other models or FVB control, may be due to the biology of the model (27). HER2 is part of the receptor family involved in the mitogen-activated protein kinase proliferation pathway, and overexpression of HER2 may lead to greater mammary adiposity. Consistent with a link between HER2 and obesity, women with HER2-positive breast cancer are more likely to be overweight/obese than women without cancer (24). The increased adiposity in the HER2 model is also supported by results showing that higher ERBB2 (HER2) activity levels decrease preadipocyte differentiation, which is permissive of clonal adipocyte expansion (17). Because low transcript levels of the HER2 transgene have been detected in the thymus, it is also possible that the adiposity of this model was driven by thymic endocrine changes (22, 40).
Fasting blood glucose.
We observed a general resistance to dietary effects on fasting blood glucose levels among the models of breast cancer. The PyMT and HER2 models as well as the FVB controls were resistant to HFD induction of elevated blood glucose levels. In contrast, the DMBA model appeared to develop impaired glucose tolerance, a risk factor of both metabolic syndrome and type II diabetes (10a), in response to HFD.
The variation in fasting blood glucose levels among cancer models demonstrates the independent etiologies of their metabolic syndrome-associated phenotypes. The HER2 model had greater adiposity than the DMBA model, yet the inverse was true with respect to fasting blood glucose levels. These two metabolic syndrome-associated phenotypes are typically highly correlated (9).
TCDD.
Exposure to TCDD in humans is associated with an increased risk for diseases linked to metabolic syndrome like type II diabetes. However, there are yet no animal models for low-dose TCDD-associated changes in diabetes risk. Our results are consistent with previous observations that the TCDD wasting syndrome is only a high-dose phenomena since we did not detect any influence of maternal TCDD exposure on longitudinal changes in body mass, percent body fat, or fasting blood glucose. The only exception was serum triglycerides.
Triglycerides.
Our low-dose TCDD results clarify previous conflicting reports and suggest that serum triglycerides are decreased by acute TCDD exposure (1, 4, 35, 44). We also found that increased dietary fat increases susceptibility to TCDD-induced changes in triglycerides. Interestingly, the interaction between dietary fat and TCDD on triglycerides was only observed with the PyMT model, underscoring the importance of defining susceptibility in terms of specific gene-by-environment interactions.
In summary, here we show that three mouse models of breast cancer, driven by specific oncogenes or induced by a carcinogen, can differentially impact the presentation of metabolic syndrome-associated phenotypes. Exposure to HFD caused variation in metabolic syndrome-associated phenotypes among these widely used mouse models. In the DMBA model, HFD caused a modest change in body fat but a large change in fasting glucose level. Conversely, HFD in the HER2 model resulted in a large change in body fat without changing fasting glucose levels. In the PyMT model, HFD caused a large change in body fat and triglycerides without changing fasting glucose levels. The variation observed among different mouse models of breast cancer in response to HFD and maternal TCDD exposure may have utility in elucidating the mechanisms of those metabolic syndrome-associated phenotypes that are etiologically linked to breast cancer risk.
GRANTS
This work was supported by National Cancer Institute grant (U01CA-105417) to D. W. Threadgill; Department of Defense Fellowship (BC050873) to M. La Merrill; National Institute of Environmental Health Sciences Training Grant (T32ES-007126); National Institute of Diabetes and Digestive and Kidney Diseases Grant (DK-56350); Superfund Basic Research Grant (P42E-S004699) to M. S. Denison; and National Institutes of Health Center Grants (P30ES-010126, P30DK-056350, and P30CA-016086).
Acknowledgments
We thank Kunjie Hua of the Animal Metabolism and Phenotyping Core of the University of North Carolina Clinical Nutrition Research Center for assistance with the Dual Energy X-ray Absorptiometry, Dr. Chris Wiesen of the Odum Institute for assistance with statistical analysis, and Drs. Michael De Vito, Suzanne Fenton, and Julian Preston for editorial feedback.
This document has been reviewed in accordance with U.S. EPA policy and approved for publication. Approval does not signify that the content necessarily reflect the view and policies of the agency, nor does mention of the trade names or commercial products constitutes endorsement or recommendation for use.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Birnbaum LS, McDonald MM, Blair PC, Clark AM, Harris MW. Differential toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57BL/6J mice congenic at the Ah Locus. Fundam Appl Toxicol 15: 186–200, 1990. [DOI] [PubMed] [Google Scholar]
- 2.Bunt JC Metabolic actions of estradiol: significance for acute and chronic exercise responses. Med Sci Sports Exerc 22: 286–290, 1990. [PubMed] [Google Scholar]
- 3.Cardiff RD, Gumerlock PH, Soong MM, Dandekar S, Barry PA, Young LJ, Meyers FJ. c-H-ras-1 expression in 7,12-dimethyl benzanthracene-induced Balb/c mouse mammary hyperplasias and their tumors. Oncogene 3: 205–213, 1988. [PubMed] [Google Scholar]
- 4.Chapman DE, Schiller CM. Dose-related effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57BL/6J and DBA/2J mice. Toxicol Appl Pharmacol 78: 147–157, 1985. [DOI] [PubMed] [Google Scholar]
- 5.Cleary MP, Grande JP, Juneja SC, Maihle NJ. Diet-induced obesity and mammary tumor development in MMTV-neu female mice. Nutr Cancer 50: 174–180, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Consonni D, Pesatori AC, Zocchetti C, Sindaco R, D'Oro LC, Rubagotti M, Bertazzi PA. Mortality in a population exposed to dioxin after the Seveso, Italy, accident in 1976: 25 years of follow-Up. Am J Epidemiol 167: 847–858, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Cranmer M, Louie S, Kennedy RH, Kern PA, Fonseca VA. Exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is associated with hyperinsulinemia and insulin resistance. Toxicol Sci 56: 431–436, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Eberhardt MS, Ogden CL, Engelgau M, Cadwell B, Hedley AA, Saydah SH. Prevalence of overweight and obesity among adults with diagnosed diabetes—United States, 1988–1994 and 1999–2002, edited by NCHS and CDC. MMWR Weekly 53: 1066–1068, 2004. [PubMed] [Google Scholar]
- 10.Emond C, Birnbaum LS, DeVito MJ. Use of a physiologically based pharmacokinetic model for rats to study the influence of body fat mass and induction of CYP1A2 on the pharmacokinetics of TCDD. Environ Health Perspect 114: 1394–1400, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). J Am Med Assoc 285: 2486–2497, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. J Am Med Assoc 287: 356–359, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Gordon RR, Hunter KW, La Merrill M, Sorensen P, Threadgill DW, Pomp D. Genotype X diet interactions in mice predisposed to mammary cancer. II. Tumors and metastasis. Mamm Genome 19: 179–189, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol 12: 954–961, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hakkak R, MacLeod S, Shaaf S, Holley AW, Simpson P, Fuchs G, Jo CH, Kieber-Emmons T, Korourian S. Obesity increases the incidence of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in an ovariectomized Zucker rat model. Int J Oncol 30: 557–563, 2007. [PubMed] [Google Scholar]
- 15.Han D, Nagy SR, Denison MS. Comparison of recombinant cell bioassays for the detection of Ah receptor agonists. Biofactors 20: 11–22, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Harrad S, Wang Y, Sandaradura S, Leeds A. Human dietary intake and excretion of dioxin-like compounds. J Environ Monit 5: 224–228, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Harrington M, Pond-Tor S, Boney CM. Role of epidermal growth factor and ErbB2 receptors in 3T3–L1 adipogenesis. Obesity (Silver Spring) 15: 563–571, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Hayward DG, Hooper K, Andrzejewski D. Tandem-in-time mass spectrometry method for the sub-parts-per-trillion determination of 2,3,7,8-chlorine-substituted dibenzo-p-dioxins and -furans in high-fat foods. Anal Chem 71: 212–220, 1999. [DOI] [PubMed] [Google Scholar]
- 19.Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology 8: 252–258, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Herschkowitz JI, Simin K, Weigman VJ, Mikaelian I, Usary J, Hu Z, Rasmussen KE, Jones LP, Assefnia S, Chandrasekharan S, Backlund MG, Yin Y, Khramtsov AI, Bastein R, Quackenbush J, Glazer RI, Brown PH, Green JE, Kopelovich L, Furth PA, Palazzo JP, Olopade OI, Bernard PS, Churchill GA, Van Dyke T, Perou CM. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors (Abstract). Genome Biol 8: R76, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hooper K, Petreas MX, Chuvakova T, Kazbekova G, Druz N, Seminova G, Sharmanov T, Hayward D, She J, Visita P, Winkler J, McKinney M, Wade TJ, Grassman J, Stephens RD. Analysis of breast milk to assess exposure to chlorinated contaminants in Kazakstan: high levels of 2,3,7, 8-tetrachlorodibenzo-p-dioxin (TCDD) in agricultural villages of southern Kazakstan. Environ Health Perspect 106: 797–806, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang Y, Kim SO, Jiang J, Frank SJ. Growth hormone-induced phosphorylation of epidermal growth factor (EGF) receptor in 3T3–F442A cells. Modulation of EGF-induced trafficking and signaling. J Biol Chem 278: 18902–18913, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Jackson Laboratories. JAX Mice Database. Bar Harbor, ME: Jackson Laboratories, 2008.
- 24.Jones LW, Haykowsky M, Peddle CJ, Joy AA, Pituskin EN, Tkachuk LM, Courneya KS, Slamon DJ, Mackey JR. Cardiovascular risk profile of patients with HER2/neu-positive breast cancer treated with anthracycline-taxane-containing adjuvant chemotherapy and/or trastuzumab. Cancer Epidemiol Biomarkers Prev 16: 1026–1031, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Jones SN, Jones PG, Ibarguen H, Caskey CT, Craigen WJ. Induction of the Cyp1a-1 dioxin-responsive enhancer in transgenic mice. Nucleic Acids Res 19: 6547–6551, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koops WJ Multiphasic growth curve analysis. Growth 50: 169–177, 1986. [PubMed] [Google Scholar]
- 27.Koops WJ, Grossman M. Multiphasic analysis of growth curves for progeny of a somatotropin transgenic male mouse. Growth Dev Aging 55: 193–202, 1991. [PubMed] [Google Scholar]
- 28.Koops WJ, Grossman M, Michalska E. Multiphasic growth curve analysis in mice. Growth 51: 372–382, 1987. [PubMed] [Google Scholar]
- 29.Luijten M, Thomsen AR, van den Berg JA, Wester PW, Verhoef A, Nagelkerke NJ, Adlercreutz H, van Kranen HJ, Piersma AH, Sorensen IK, Rao GN, van Kreijl CF. Effects of soy-derived isoflavones and a high-fat diet on spontaneous mammary tumor development in Tg.NK (MMTV/c-neu) mice. Nutr Cancer 50: 46–54, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Martin TL, Alquier T, Asakura K, Furukawa N, Preitner F, Kahn BB. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. J Biol Chem 281: 18933–18941, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Mayes JS, Watson GH. Direct effects of sex steroid hormones on adipose tissues and obesity. Obes Rev 5: 197–216, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Michalek JE, Tripathi RC. Pharmacokinetics of TCDD in veterans of Operation Ranch Hand: 15-year follow-up. J Toxicol Environ Health A 57: 369–378, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Moon A Differential functions of Ras for malignant phenotypic conversion. Arch Pharm Res 29: 113–122, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Muntean N, Jermini M, Small I, Falzon D, Furst P, Migliorati G, Scortichini G, Forti A, Elke A, von Holst C, Niyazmatov B, Bahkridinov S, Aertgeerts R, Bertollini R, Tirado C, Kolb A. Assessment of dietary exposure to some persistent organic pollutants in the Republic of Karakalpakstan of Uzbekistan. Environ Health Perspect 111: 1306–1311, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pelclova D, Fenclova Z, Dlaskova Z, Urban P, Lukas E, Prochazka B, Rappe C, Preiss J, Kocan A, Vejlupkova J. Biochemical, neuropsychological, and neurological abnormalities following 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure. Arch Environ Health 56: 493–500, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: effects, mechanisms, and animal models. Pharmacol Rev 46: 483–549, 1994. [PubMed] [Google Scholar]
- 37.Qiu TH, Chandramouli GV, Hunter KW, Alkharouf NW, Green JE, Liu ET. Global expression profiling identifies signatures of tumor virulence in MMTV-PyMT-transgenic mice: correlation to human disease. Cancer Res 64: 5973–5981, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Roche HM, Phillips C, Gibney MJ. The metabolic syndrome: the crossroads of diet and genetics. Proc Nutr Soc 64: 371–377, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Rogers JM, Denison MS. Analysis of the antiestrogenic activity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in human ovarian carcinoma BG-1 cells. Mol Pharmacol 61: 1393–1403, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Savino W Neuroendocrine control of T cell development in mammals: role of growth hormone in modulating thymocyte migration. Exp Physiol 92: 813–817, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Simpson ER Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86: 225–230, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen-Dale AL. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98: 10869–10874, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100: 8418–8423, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweeney MH, Mocarelli P. Human health effects after exposure to 2,3,7,8-TCDD. Food Addit Contam 17: 303–316, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Thigpen JE, Setchell KD, Saunders HE, Haseman JK, Grant MG, Forsythe DB. Selecting the appropriate rodent diet for endocrine disruptor research and testing studies. Ilar J 45: 401–416, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr 92: 347–355, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Vidal O, Lindberg M, Savendahl L, Lubahn DB, Ritzen EM, Gustafsson JA, Ohlsson C. Disproportional body growth in female estrogen receptor-alpha-inactivated mice. Biochem Biophys Res Commun 265: 569–571, 1999. [DOI] [PubMed] [Google Scholar]
- 48.Weinstein EJ, Kitsberg DI, Leder P. A mouse model for breast cancer induced by amplification and overexpression of the neu promoter and transgene. Mol Med 6: 4–16, 2000. [PMC free article] [PubMed] [Google Scholar]
- 49.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J Pathol 200: 290–297, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Ziccardi MH, Gardner IA, Denison MS. Development and modification of a recombinant cell bioassay to directly detect halogenated and polycyclic aromatic hydrocarbons in serum. Toxicol Sci 54: 183–193, 2000. [DOI] [PubMed] [Google Scholar]