Abstract
During late gestation, amino acids and insulin promote skeletal muscle protein synthesis. However, the independent effects of amino acids and insulin on the regulation of mRNA translation initiation in the fetus are relatively unknown. The purpose of this study was to determine whether acute amino acid infusion in the late-gestation ovine fetus, with and without a simultaneous increase in fetal insulin concentration, activates translation initiation pathway(s) in skeletal muscle. Fetuses received saline (C), mixed amino acid infusion plus somatostatin infusion to suppress amino acid-stimulated fetal insulin secretion (AA+S), mixed amino acid infusion with concomitant physiological increase in fetal insulin (AA), or high-dose insulin infusion with euglycemia and euaminoacidemia (HI). After a 2-h infusion period, fetal skeletal muscle was harvested under in vivo steady-state conditions and frozen for quantification of proteins both upstream and downstream of mammalian target of rapamycin (mTOR). In the AA group, we found a threefold increase in ribosomal protein S6 kinase (p70S6k) and Erk1/2 phosphorylation; however, blocking the physiological rise in insulin with somatostatin in the AA+S group prevented this increase. In the HI group, Akt, Erk1/2, p70S6k, and ribosomal protein S6 were highly phosphorylated and 4E-binding protein 1 (4E-BP1) associated with eukaryotic initiation factor (eIF)4E decreased by 30%. These data show that insulin is a significant regulator of intermediates involved in translation initiation in ovine fetal skeletal muscle. Furthermore, the effect of amino acids is dependent on a concomitant increase in fetal insulin concentrations, because amino acid infusion upregulates p70S6k and Erk only when amino acid-stimulated increase in insulin occurs.
Keywords: translation initiation, mitogen-activated protein kinase pathway, ribosomal protein S6 kinase, protein synthesis
skeletal muscle protein synthesis and somatic growth increase rapidly during late fetal and early neonatal development (13, 18, 23). During late gestation, insulin stimulates amino acid utilization, whole body protein accretion, and skeletal muscle fractional protein synthesis under conditions of amino acid sufficiency (10, 30, 41, 44). The fetus also responds to an amino acid infusion by increasing protein synthesis and accretion (24). Moreover, experiments in the neonatal piglet have demonstrated independent, additive growth-promoting effects of insulin and amino acids (14, 32).
The cellular mechanisms that govern protein synthesis in response to nutrients and hormones are an area of intense investigation, given the wide variety of pathological conditions in the fetus and neonate that result from either nutrient and hormonal excess or restriction. Insulin, amino acids, and other anabolic growth factors initiate protein synthesis through signaling pathways that involve the mammalian target of rapamycin (mTOR). Many in vitro studies have defined mTOR as a nutrient sensor, coordinating both nutrient and energy availability with hormonal and growth factor signals to regulate protein synthesis and cell growth. Insulin enhances protein synthesis in part by activation of Akt-1 (protein kinase B) through the well-studied insulin receptor-insulin receptor substrate (IRS)-phosphatidylinositol 3-kinase (PI3-kinase) pathway that renders mTOR active (11). mTOR signaling to its downstream effectors, ribosomal protein S6 kinase (p70S6k) and 4E-binding protein 1 (4E-BP1), plays a key role in the regulation of translation initiation. On phosphorylation, p70S6k increases the translational capacity by stimulating ribosome biogenesis and by activating other translation factors such as ribosomal protein (rp) S6 (46). The rate-limiting step in translation initiation is phosphorylation of 4E-BP1. In its dephosphorylated state, 4E-BP1 functions as a repressor protein by binding eukaryotic initiation factor (eIF)4E and rendering it incapable of binding to the scaffold protein eIF4G (39). mTOR-mediated phosphorylation of 4E-BP1 allows eIF4E to bind eIF4G and promote the formation of translation initiation complexes (36). Other growth-promoting pathways contribute to translational control, such as the Ras/Mek/Erk pathway. Insulin activates MAPK (Erk1/2) to promote protein synthesis and cell growth by activating mTOR signaling, either through mTOR directly or through other downstream components such as p70S6k, rp S6, and 4E-BP1 (25, 29, 34, 38).
In vivo studies in the adult rat and the adult human have demonstrated that a mixed amino acid infusion independent of changes in insulin concentration stimulates phosphorylation of both 4E-BP1 and p70S6k in skeletal muscle (26, 28). Amino acid effects on intermediates within the mTOR signaling pathway in skeletal muscle in the fetus, however, are relatively unknown. Studies in skeletal muscle of the neonatal piglet were the first to evaluate the independent and interactive regulatory effects of insulin and amino acids on upregulating translation initiation early in development (33, 42). Those investigators found potent insulin-stimulated activation of the proximal insulin signal transduction pathway and activation of the downstream mediators of translation initiation. Amino acids also signaled the phosphorylation of 4E-BP1 and p70S6k and the assembly of the eIF4F translation initiation complex only in the presence of postprandial increase in insulin concentrations. Fetal studies in sheep demonstrated that insulin regulates the PI3-kinase pathway (2) and translation initiation complex assembly (40) in skeletal muscle. Mechanisms by which amino acids independently upregulate this pathway during fetal growth have not been investigated. Therefore, the objective of our study was to determine whether amino acids independently activate the key signal transduction proteins that regulate translation initiation in fetal skeletal muscle or whether insulin also is required. To meet this objective, we infused amino acids into normal late-gestation fetal sheep, allowing or inhibiting the secondary, amino acid-stimulated increase in fetal plasma insulin concentration, and compared activation of selected key translation initiation signal transduction proteins in skeletal muscle with hyperinsulinemia alone and with saline-infused control animals.
MATERIALS AND METHODS
Animal care and surgical procedure.
Studies were performed in 22 late-gestation [130 days gestational age (dga)] Columbia-Rambouillet mixed-breed ewes with singleton (n = 16, 16 fetuses) or twin (n = 6, 12 fetuses) pregnancies. Ewes were fasted 24 h before fetal surgery. Before surgical preparation, a maternal jugular catheter was placed for administration of diazepam (0.2 mg/kg) and ketamine (20 mg/kg) to induce anesthesia. Ewes were given Ketofen intramuscularly (0.6 mg/kg) for additional analgesia. The ewes were maintained on isoflurane inhalation anesthesia 2–4% for the remainder of the surgical procedures. Hysterotomy was performed, and fetal catheters were placed into the abdominal aorta via hindlimb arteries and into femoral veins via hindlimb veins for blood sampling and infusions, respectively. For maternal blood sampling, catheters were placed into the femoral artery and vein through a small incision in the ewe's groin. All catheters were filled with heparinized saline [50 U/ml heparin sodium in 0.9% (wt/vol) NaCl in water], tunneled subcutaneously through a maternal skin incision, and stored in a plastic pouch sutured to the ewe's flank. Ampicillin (500 mg) was administered into the amniotic fluid just before closure of the uterus, and procaine penicillin (6,000,000 U) was given intramuscularly to the ewes. The ewes were allowed to recover for 5–7 days before study. Buprinex (0.6 mg) was given every 12 h for 2 days postoperatively for pain management. During postoperative recovery ewes were housed in a temperature-controlled environment (18 ± 2°C) in standard carts. Ewes were fed alfalfa pellets ad libitum before surgery, during recovery, and during the study. All in vivo procedures and studies were performed at the University of Colorado Denver (UCD) Perinatal Research Center (PRC) under protocols approved by the UCD Institutional Animal Care and Use Committee. The PRC is accredited by the US Department of Agriculture, the National Institutes of Health, and the American Association for Accreditation of Laboratory Animal Care.
Experimental design.
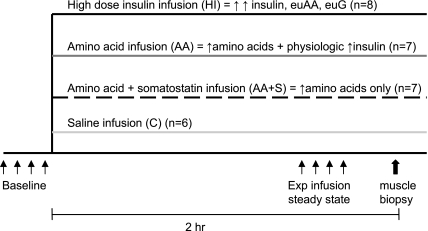
The experimental design is demonstrated schematically in Fig. 1. Each fetus was randomly assigned to one of four treatment groups: amino acid infusion (AA), amino acid and somatostatin infusion (AA+S), high-dose insulin infusion (HI), or saline-infused control (C). Both AA groups received a mixed amino acid infusion (Trophamine, Central Admixture Pharmacy Services, Denver, CO) at rates estimated to increase fetal amino acid concentrations by twofold greater than baseline. To determine amino acid effects independently of insulin, somatostatin infusion was used to suppress amino acid-stimulated fetal insulin secretion in the AA+S group. Animals in the HI group received pharmacological infusion rates of insulin (3 mU·min−1·kg−1 in 0.9% NaCl in water) and variable rates of 10% dextrose and Trophamine with glucose and branched-chain amino acid clamp techniques to keep glucose and amino acid concentrations at baseline (10). The C group received a saline infusion equivalent in volume to what experimental groups received. Fetal arterial plasma glucose, lactate, insulin, and amino acid concentrations and whole blood hematocrit, pH, arterial Po2 (PaO2), arterial Pco2 (PaCO2), oxygen saturation, and blood oxygen content values were measured at baseline and at experimental infusion steady state. Isovolemic transfusions of maternal blood were administered to the fetus at a constant rate during baseline and experimental steady-state blood sampling periods. At 120 min of experimental infusions, the ewes received diazepam and ketamine intravenously. The fetuses were delivered via maternal laparotomy and hysterotomy within 5 min of induction of maternal diazepam-ketamine anesthesia, and samples of the fetal biceps femoris skeletal muscle were obtained under acute experimental conditions. The skeletal muscle tissue was immediately frozen with liquid nitrogen and stored at −70°C. The ewes and fetuses were killed with intravenous pentobarbital sodium (Fatal Plus 85 mg/kg; Vortech Pharmaceuticals, Dearborn, MI). Fetal body weights were obtained at autopsy.
Fig. 1.
Schematic of experimental study design. euAA, euaminoacidemia; euG, euglycemia.
Biochemical analysis.
Fetal plasma glucose and lactate concentrations were measured rapidly with the YSI glucose and lactate model 2700 analyzer (Yellow Springs Instruments, Yellow Springs, OH). Plasma branched-chain amino acid (leucine, isoleucine, and valine) concentrations were measured with a spectrophotometric assay described by Beckett et al. (7) using an Ultrospec 4300 pro UV/Visible spectrophotometer (GE Healthcare BioSciences, Piscataway, NJ). Blood gas determinations, oxygen content, and hematocrit were measured in whole fetal blood (ABL 520 Hemoximeter, Copenhagen, Denmark). A portion of fetal plasma was collected by centrifugation for 3 min at 4°C and stored at −70°C for analysis of insulin and amino acid concentrations. Fetal plasma insulin concentrations were measured by ALPCO Ovine Insulin ELISA (Windham, NH). Individual plasma amino acid concentrations were determined with a Dionex HPLC Amino Acid Analyzer (Dionex, Sunnyvale, CA).
Western blot analysis.
Frozen muscle biopsies (100 mg) were sonicated in 700 μl of lysis buffer (in mM: 20 Tris·HCl, pH 7.4, 150 NaCl, 20 NaF, 2 EDTA, pH 8.0, 2.5 NaPP, and 20 β-glycerophosphate, with 1% NP-40 and 10% glycerol). Pefabloc SC (Roche Pharmaceuticals, Basel, Switzerland), Complete mini tablet EDTA-free (Roche), and 1% phosphatase inhibitor II (Sigma, St. Louis, MO) were added to the lysis buffer just before use. Homogenates were rotated on an orbital rocker for 1 h at 4°C, followed by centrifugation at 15,000 rpm for 15 min to collect supernatant. Total protein in the supernatant was quantified with a bicinchoninic acid (BCA) protein quantification assay (Pierce, Rockford, IL). The supernatant was diluted with SDS-sample buffer and heated for 10 min at 90°C, and 80 μg of protein per sample was subjected to 10% SDS-PAGE. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane with a Mini Trans-Blot Transfer cell (Bio-Rad, Hercules, CA). The membrane was blocked for 1 h at room temperature (RT) in 5% nonfat dry milk with Tris-buffered saline-0.05% Tween (TBST). The membrane was serially incubated overnight at 4°C in antibodies to phospho-Akt (Ser473), total Akt, phospho-p44/42 MAPK (Thr202/Tyr204), total p44/42 MAPK, phospho-p70S6k (Thr421/Ser424), total p70S6k, phospho-rp S6 (Ser235/236), total rp S6, phospho-4E-BP1 (Thr37/46), or total 4E-BP1 [all 1:500 in TBST with 5% BSA except phospho-rp S6 (1:5,000) and total rp S6 (1:10,000); Cell Signaling Technology]. All proteins were normalized to monoclonal mouse anti-actin (MP Biomedical, Solon, OH) to correct for loading error.
The association of eIF4E with 4E-BP1 in fetal ovine skeletal muscle was determined by eIF4E extraction with m7GTP-Sepharose 4B (GE Healthcare Bio-Sciences) as described by Shen et al. (40). Briefly, 100 mg of tissue was homogenized in 7 volumes of lysis buffer containing (in mM) 20 HEPES (pH 7.4), 100 KCl, 0.2 EDTA, 2 EGTA, 1 DTT, 50 NaF, 50 mM β-glycerolphosphate, 0.1 PMSF, 1 benzamidine, and 0.5 mM sodium vanadate, with 1 μmol/l microcystin LR (Sigma). The homogenate was centrifuged at 10,400 rpm for 10 min at 4°C. Total protein in the supernatant was quantified with a DC protein quantification assay (Bio-Rad). Five hundred micrograms of protein was added to 60 μl of prewashed m7GTP-Sepharose 4B and mixed for 2 h at 4°C. After incubation, samples were centrifuged at 3,000 rpm for 4 min at 4°C and washed three times with 0.5 ml of ice-cold lysis buffer. Samples were resuspended in 30 μl of 1× Laemmli sample buffer with 5% β-mercaptoethanol, vortexed, and heated for 10 min at 95°C. Samples were centrifuged again at 3,000 rpm for 4 min at 4°C to separate beads from supernatant. The supernatant was subjected to electrophoresis, and proteins were transferred to a nitrocellulose membrane. The membrane was blocked for 1 h at RT in 5% nonfat dry milk and serially incubated overnight in goat polyclonal antibody to 4E-BP1 and mouse monoclonal antibody to eIF4E (1:500 in TBST with 5% nonfat dry milk; Cell Signaling Technology).
Horseradish peroxidase-conjugated secondary antibody was added for 1 h at RT to all blots. Blots were developed with enhanced chemiluminescence reagents (ECL; GE Healthcare Bio-Sciences) exposed to X-ray film. Chemiluminescence was measured and quantified with Alpha Innotech Fluorochem Digital Imaging System software (San Leandro, CA).
Statistical analysis.
Results are expressed as means ± SE. Physiological measurements obtained under baseline and experimental conditions for all treatment groups were compared with a two-way ANOVA followed by a post hoc Bonferroni test. A loge transformation was used to normalize insulin concentrations. Insulin signal transduction protein measurements were analyzed with a one-way ANOVA followed by a post hoc Tukey's test. For phosphorylation of rp S6, a post hoc Student's t-test also was used. P values <0.05 were considered significant.
RESULTS
Fetal gestational age for the entire study population was 131 ± 1 dga, and average fetal weight was 3.44 ± 0.14 kg (C 136 ± 1.7 dga, 4.13 ± 0.37 kg; AA 130 ± 0.5 dga, 3.33 ± 0.19 kg; AA+S 132 ± 1.0 dga, 3.73 ± 0.20 kg; HI 129 ± 0.9 dga, 2.80 ± 0.12 kg). Measurements of fetal hematocrit, arterial pH, PaCO2, PaO2, oxygen saturation, oxygen content, and plasma lactate concentration at baseline and experimental steady state are listed in Table 1. An increase in fetal lactate concentration was observed in the AA infusion groups only. Consistent with previous studies in our laboratory (19), fetal hyperinsulinemia decreased PaO2, O2 saturation, and O2 content and increased fetal CO2 production.
Table 1.
Physiological measurements obtained at baseline and during experimental infusion in each treatment group
|
Control |
AA+S
|
AA
|
HI
|
|||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Exp | Baseline | Exp | Baseline | Exp | Baseline | Exp | |
| Hematocrit, % | 33.4±1.1 | 33.5±1.1 | 34.7±0.8 | 34.3±0.7 | 35.5±1.4 | 34.5±1.7 | 35.6±0.8 | 35.3±0.8 |
| pH | 7.37±0.01 | 7.36±0.01 | 7.36±0.01 | 7.36±0.01 | 7.37±0.004 | 7.36±0.006 | 7.37±0.003 | 7.34±0.01* |
| PaCO2, Torr | 50.5±1.5 | 51.0±1.3 | 50.7±1.4 | 50.8±1.3 | 50.0±0.9 | 50.1±0.8 | 48.7±1.0 | 50.5±1.0* |
| PaO2, Torr | 20.5±1.0 | 20.2±1.0 | 20.7±1.0 | 19.5±1.0 | 19.7±0.8 | 18.9±1.1 | 18.0±1.6 | 15.8±1.9* |
| O2 saturation, % | 48.8±3.9 | 48.2±3.2 | 51.3±3.0 | 47.1±3.1 | 47.4±2.9 | 44.2±2.9 | 53.8±2.3 | 42.0±3.5* |
| O2 content, mmol/l | 3.2±0.2 | 3.2±0.2 | 3.5±0.2 | 3.2±0.3 | 3.3±0.2 | 3.1±0.2 | 3.7±0.2 | 2.9±0.2* |
| Lactate, mmol/l | 1.81±0.21 | 1.77±0.21 | 1.66±0.10 | 2.10±0.19* | 1.82±0.08 | 2.18±0.13* | 2.01±0.2 | 2.07±0.16 |
| Insulin, μU/ml | 5.7±0.9 | 6.1±1.0 | 4.8±0.8 | 4.1±0.9 | 6.0±0.6 | 13.2±1.6* | 5.0±0.9 | 220.0±38.9* |
| Glucose, mg/dl | 22.3±3.2 | 21.4±2.5 | 18.8±1.7 | 20.3±2.0 | 20.6±1.4 | 22.3±1.6 | 22.1±2.4 | 19.8±1.9* |
| BCAA, μM/ml | 0.82±0.09 | 0.78±0.08 | 0.67±0.06 | 1.33±0.11* | 0.75±0.06 | 1.46±0.12* | 0.94±0.04 | 0.90±0.04 |
Values are means ± SE. Control, saline-infused control group; AA, mixed-amino acid infusion; AA+S, mixed-amino acid infusion + somatostatin; HI, high-dose insulin; Exp, experimental infusion; PaO2, arterial Po2; PaCO2, arterial Pco2; BCAA, branched-chain amino acids.
Significant difference (P ≤ 0.05) between study periods within a treatment group.
Steady-state fetal plasma insulin, glucose, and branched-chain amino acid concentrations during baseline and experimental clamp infusions are also shown in Table 1. In the AA+S group, somatostatin effectively suppressed fetal insulin secretion, because fetal plasma insulin concentrations did not change significantly with amino acid infusion. In the AA group, fetal plasma insulin concentrations increased 2.2-fold with mixed amino acid infusion alone (P < 0.01). In the HI group, fetal insulin infusion elevated fetal plasma insulin concentrations 44-fold (P < 0.001). There was a minor decrease in fetal plasma glucose concentrations with experimental infusion of insulin. In the AA and AA+S groups, fetal branched-chain amino acid concentrations were similarly raised twofold from baseline with mixed amino acid infusion (P < 0.001). Complete analyses of the fetal plasma concentrations of 20 amino acids in all four treatment groups are shown in Fig. 2. There were minor decreases in amino acid concentrations in the C and HI groups (Fig. 2, A and B). In the AA and AA+S groups (Fig. 2, C and D), all essential and most nonessential fetal plasma amino acids were increased with mixed amino acid infusion (1.3- to 3.2-fold, P < 0.001). There were some nonessential amino acids that did not change with mixed amino acid infusion, although none decreased significantly.
Fig. 2.
Fetal arterial plasma amino acid concentrations in four treatment groups. Open bars, values at baseline; gray bars, values at experimental infusion steady state. A: saline control (C). B: high-dose insulin infusion (HI). C: amino acid infusion (AA). D: amino acid + somatostatin infusion (AA+S). Minor changes were observed in some amino acids in the C and HI groups. Significant increases in all essential and most nonessential amino acids as a result of mixed amino acid infusion were demonstrated in the AA and AA+S groups. *P ≤ 0.05.
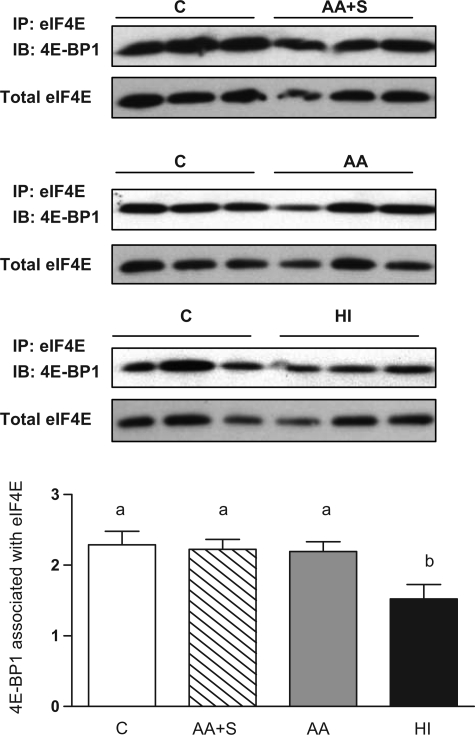
Figure 3 illustrates the changes in the phosphorylation of Akt, Erk1/2, p70S6k, rp S6, and 4E-BP1. All data are expressed as a ratio of the phosphorylated protein to the total amount of the respective protein, and total protein concentrations did not change as a result of treatment. Figure 3A shows that in the HI group, but not in the AA or AA+S groups, Akt phosphorylation was increased sevenfold compared with control in fetal skeletal muscle. In contrast, Erk1/2 phosphorylation was increased threefold in the AA and HI groups, but not in the AA+S group (Fig. 3B). p70S6k was not stimulated in the AA+S group, while in the AA and HI groups a stepwise increase in p70S6k phosphorylation was demonstrated (Fig. 3C). rp S6 phosphorylation was increased sevenfold in the HI group and by post hoc analysis was phosphorylated in response to amino acids in the presence and absence of increased insulin (Fig. 3D). Despite an increase in fetal plasma insulin concentrations, the phosphorylation of 4E-BP1 was not affected in the AA and HI groups (Fig. 3E). However, eIF4E was disassociated from the repressor protein 4E-BP1 in the HI group, evidence that 4E-BP1 was rendered inactive by insulin (Fig. 4). Thus, in summary, amino acids stimulated p70S6k and stimulated Erk1/2 only when a physiological increase in insulin was permitted. There was no effect of amino acids on Akt phosphorylation, and eIF4E remained bound to 4E-BP1 except in the presence of high fetal insulin concentrations.
Fig. 3.
Representative Western blots with data graphed to show phosphorylation differences in skeletal muscle from animals treated with saline (C), amino acids and somatostatin reflecting independent AA effects (AA+S), amino acids with permissive physiological rise in fetal plasma insulin (AA), and pharmacological hyperinsulinemia (HI). All data are expressed as a ratio of the phosphorylated protein to respective total protein content. A: Akt. B: Erk. C: ribosomal S6 kinase (p70S6k). D: ribosomal protein (rp) S6. E: 4E-binding protein 1 (4E-BP1). β-Actin was used as an internal control. Means with different superscripts differ at P ≤ 0.01. *Experimental group differs from C group by Student's t-test at P ≤ 0.01.
Fig. 4.
Association of eukaryotic initiation factor (eIF)4E with 4E-BP1 in C, AA+S, AA, and HI treatment groups. eIF4E and its bound proteins were immunoprecipitated (IP) with m7GTP-Sepharose 4B. Immunoblot (IB) analysis was performed on the resin-bound fraction with anti-4E-BP1 antibody and normalized to total eIF4E (representative blots shown at top). Means with different superscripts differ at P ≤ 0.05.
DISCUSSION
The purpose of these studies was to determine whether an acute infusion of a full complement of amino acids would upregulate signaling components involved in mRNA translation initiation in the ovine fetus during late gestation independently of the same effect of insulin. We proposed to perform this study in the unique nutrient and hormonal environment of the fetus, where insulin concentrations are relatively low (1) and amino acids are actively transported into the fetus by the placental trophoblast to concentrations higher than those in the mother. Our results show for the first time in the late-gestation fetus that amino acids activate signal transduction proteins p70S6k and Erk1/2 only when a simultaneous amino acid-stimulated increase in insulin occurs. We also confirm previous observations of insulin's significant, independent effect on regulating translation initiation in normal fetal skeletal muscle in vivo. Under physiological conditions in mammals, insulin and amino acids have interactive effects on amino acid metabolism. An acute insulin infusion will decrease plasma amino acid concentrations, which then limit the effectiveness of insulin to promote amino acid utilization (30, 41). Amino acids stimulate pancreatic insulin secretion, such that some studies of amino acid infusion have not been able to discriminate between insulin- and amino acid-specific effects. Our study design enabled us to study the independent effects of amino acids to activate proteins involved in the regulation of translation initiation by using somatostatin to suppress amino acid-stimulated insulin secretion. The study design also allowed us to assess the synergistic effects of amino acids and the physiological increase in insulin from amino acid stimulation of pancreatic insulin secretion. Finally, using glucose and amino acid clamp techniques, we were able to measure the independent effect of insulin on translation initiation signaling.
We found that a mixed amino acid infusion relatively enriched in essential amino acids increased fetal plasma insulin concentrations by twofold and stimulated the phosphorylation of Erk1/2 and p70S6k. When somatostatin was administered to inhibit the amino acid-stimulated increase in fetal plasma insulin, these effects were abolished, demonstrating that the effect of the amino acid infusion was specific to insulin and not to the amino acids. Our results are consistent with a study in adult rat skeletal muscle, which demonstrated that when insulin was specifically blocked with diazoxide a high-protein diet did not upregulate p70S6k or 4E-BP1 (6). However, the majority of studies in the neonatal piglet, adult rat, and adult human in vivo have demonstrated that amino acids (and particularly leucine) promote translation initiation and muscle protein synthesis independently of changes in insulin concentrations (3, 4, 12, 15, 17, 27, 33). Postnatally, the anabolic effect of amino acids appears to be independent of any but a permissive effect of insulin.
Our results demonstrating that amino acid signaling depends on a simultaneous increase in insulin secretion differ from those obtained in postnatal studies. A possible explanation for this could be that a continuous and uninterrupted supply of amino acids from the placenta may be sufficiently high enough to maximally stimulate intermediates involved in the regulation of translation initiation in the presence of insulin. Thus acute addition of amino acids above and beyond this concentration would not be expected to further upregulate the pathway, unless accompanied by an increase in insulin. In studies in adult humans, an increase in amino acid concentrations within a physiological range upregulated protein synthesis, but very high concentrations could not further increase protein synthesis (8, 37). We also were limited to evaluating the in vivo activation of signal transduction after 2 h of acute stimulation; independent amino acid stimulatory effects may occur earlier or later than this time point (9). Finally, it also may be that amino acid signaling occurs through other pathways during fetal skeletal muscle growth. In a post hoc analysis, we found that rp S6, which is one of the furthest downstream intermediates in pathways that lead to translation initiation, was phosphorylated by amino acids independently of any change in insulin concentration. Further investigation is warranted into alternate signaling pathways in fetal muscle that might be activated by the presence of amino acids, such as other phosphorylation sites on p70S6k, GCN2 control of eIF2, and the regulation of elongation factor eEF2 (20, 35).
It is not surprising, however, that we found significant effects of increased fetal insulin concentrations, either as a result of an amino acid-induced pancreatic secretion or from exogenous infusion, on the signaling proteins generally thought to promote muscle protein synthesis. In the newborn animal, enhanced stimulation of skeletal muscle protein synthesis is primarily insulin mediated (13). Many studies have shown that amino acid delivery in utero and rates of protein synthesis and turnover in response to nutrient stimulation are highest early in development and decline with advancing age (21, 47, 48). Furthermore, upregulation of mTOR, p70S6k, and eIF4F complex assembly by both insulin and amino acids in skeletal muscle of neonatal pigs is higher at 6 days of life compared with 26 days of life (43). Our data are consistent with the concept that hormonal and nutrient regulation of growth pathways may differ depending on the stage of development of the organism. It should also be noted that in our study the group of animals that received insulin infusion were younger and therefore smaller than the control group of animals, which may have exaggerated their insulin response.
Previous studies in late-gestation fetal sheep have shown that acute hyperinsulinemia at pharmacological doses (∼110 μU/ml) activates the insulin signal transduction pathway through PI3-kinase/Akt, leading to substantial upregulation of p70S6k and 4E-BP1 and assembly of the translation initiation complex eIF4F (2, 40). Consistent with these results, we found a sevenfold increase in the phosphorylation of Akt, p70S6k, and rp S6 with high-dose fetal insulin infusion. Additionally, we demonstrated that a physiological twofold increase in fetal insulin concentration stimulates a threefold increase in phosphorylated p70S6k and that its stimulation by insulin follows a dose-response pattern. During late gestational muscle growth, p70S6k is sensitive to small physiological changes in insulin concentrations, indicating that the protein may function as a sensor of not only the presence but also the concentration of insulin available. We also have shown for the first time that both physiological and high plasma insulin concentrations will activate Erk1/2 in the Ras/Mek/Erk pathway in the ovine fetus. Erk is an activator of mTOR signaling, and in vitro studies have shown that Erk1/2 may activate p70S6k either by allowing additional activating phosphorylation events for maximal protein activation (5) or through inactivation of tuberin (TSC2), an inhibitor of mTOR (38). Insulin stimulates protein synthesis in myoblasts by upregulating the p70S6k and MAPK signal transduction pathways (22). Furthermore, recent studies in mice have demonstrated that S6 phosphorylation can occur through the MAPK pathway (34). Our data indicate that p70S6k and Erk may be activated by insulin in a coordinated and reciprocal fashion. More detailed in vitro studies are needed to identify potential synergistic and/or additive interactions between the Ras/Mek/Erk pathway and p70S6k under these experimental conditions.
To examine the effect of amino acids and insulin on translation initiation complex formation, we used an mRNA cap structure-binding affinity matrix to isolate eIF4E and quantify its association with 4E-BP1. We found increased dissociation of 4E-BP1 from eIF4E with high doses of insulin, indicative of eIF4F translation initiation complex formation. However, we did not find changes in the phosphorylation of 4E-BP1 by either insulin or amino acids. Therefore, 4E-BP1 was triggered to release eIF4E under hyperinsulinemic conditions, despite our inability to detect differences in its phosphorylation. Our findings differ from a previous study in fetal sheep that demonstrated both phosphorylation of 4E-BP1 and eIF4F complex assembly with high doses of insulin (40). In that study, activation of 4E-BP1 was measured by a shift in electrophoretic migration of 4E-BP1, indicating an overall increase in the phosphorylation of the protein. In vitro studies have demonstrated that the regulation of 4E-BP1 occurs via five phosphorylation sites: Thr37, Thr46, Thr70, Ser64, and Ser82 (31). mTOR directly phosphorylates Thr37/46, with subsequent phosphorylation of sites Thr70 and Ser64 (16). We only evaluated the Thr37/46 phosphorylation site of the protein, which indicates that insulin may preferentially stimulate phosphorylation of certain sites of 4E-BP1, resulting in its release of eIF4E, independent of mTOR activation (45). We were unable to study the phosphorylation of sites Thr70 and Ser64 in sheep tissue with the commercially available phospho-specific antibodies. However, it would be interesting to pursue this evaluation in the future, given recent evidence that these sites are responsive to amino acid supply in vitro (16, 31), and Erk also has been shown to play a role in phosphorylating site Ser64 (25).
In conclusion, we demonstrate for the first time effects of a mixed amino acid infusion on key proteins involved in regulating translation initiation during fetal life. We show that increases in fetal insulin, both at physiological concentrations as a result of amino acid infusion and independently with fetal amino acids maintained at baseline values, significantly phosphorylate and activate p70S6k and Erk1/2, as well their downstream effector, rp S6. AA infusion independently of insulin did not result in activation of these pathways. These results indicate that when skeletal muscle is growing at a rapid rate during late gestation, insulin is a primary regulator of anabolic signaling pathways.
GRANTS
This work was supported by National Institutes of Health (NIH) Grants HD-07186 and DK-52138 (W. W. Hay Jr., principal investigator), NIH-Clinical Nutrition Research Unit Pilot and Feasibility Project 5-P30-DK-048520-13 (L. D. Brown, principal investigator), and The Children's Hospital of Denver Research Institute Scholar Award (L. D. Brown, principal investigator).
Acknowledgments
We thank Julie Torvik, Meredith Davidsen, Karen Trembler, and David Caprio for their excellent technical support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aldoretta PW, Carver TD, Hay WW Jr. Maturation of glucose-stimulated insulin secretion in fetal sheep. Biol Neonate 73: 375–386, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Anderson MS, Thamotharan M, Kao D, Devaskar SU, Qiao L, Friedman JE, Hay WW Jr. Effects of acute hyperinsulinemia on insulin signal transduction and glucose transporters in ovine fetal skeletal muscle. Am J Physiol Regul Integr Comp Physiol 288: R473–R481, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr 130: 139–145, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130: 2413–2419, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Avruch J Insulin signal transduction through protein kinase cascades. Mol Cell Biochem 182: 31–48, 1998. [PubMed] [Google Scholar]
- 6.Balage M, Sinaud S, Prod'Homme M, Dardevet D, Vary TC, Kimball SR, Jefferson LS, Grizard J. Amino acids and insulin are both required to regulate assembly of the eIF4E·eIF4G complex in rat skeletal muscle. Am J Physiol Endocrinol Metab 281: E565–E574, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Beckett PR, Hardin DS, Davis TA, Nguyen HV, Wray-Cahen D, Copeland KC. Spectrophotometric assay for measuring branched-chain amino acid concentrations: application for measuring the sensitivity of protein metabolism to insulin. Anal Biochem 240: 48–53, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Bohe J, Low A, Wolfe RR, Rennie MJ. Human muscle protein synthesis is modulated by extracellular, not intramuscular amino acid availability: a dose-response study. J Physiol 552: 315–324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohe J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol 532: 575–579, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown LD, Hay WW Jr. Effect of hyperinsulinemia on amino acid utilization and oxidation independent of glucose metabolism in the ovine fetus. Am J Physiol Endocrinol Metab 291: E1333–E1340, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Cai SL, Tee AR, Short JD, Bergeron JM, Kim J, Shen J, Guo R, Johnson CL, Kiguchi K, Walker CL. Activity of TSC2 is inhibited by AKT-mediated phosphorylation and membrane partitioning. J Cell Biol 173: 279–289, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Davis TA, Burrin DG, Fiorotto ML, Reeds PJ, Jahoor F. Roles of insulin and amino acids in the regulation of protein synthesis in the neonate. J Nutr 128: 347S–350S, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O'Connor PM. Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282: E880–E890, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA. Regulation of cardiac and skeletal muscle protein synthesis by individual branched-chain amino acids in neonatal pigs. Am J Physiol Endocrinol Metab 290: E612–E621, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev 15: 2852–2864, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greiwe JS, Kwon G, McDaniel ML, Semenkovich CF. Leucine and insulin activate p70 S6 kinase through different pathways in human skeletal muscle. Am J Physiol Endocrinol Metab 281: E466–E471, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Gulve EA, Dice JF. Regulation of protein synthesis and degradation in L8 myotubes. Effects of serum, insulin and insulin-like growth factors. Biochem J 260: 377–387, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hay WW, DiGiacomo JE, Meznarich HK, Hirst K, Zerbe G. Effects of glucose and insulin on fetal glucose oxidation and oxygen consumption. Am J Physiol Endocrinol Metab 256: E704–E713, 1989. [DOI] [PubMed] [Google Scholar]
- 20.Jefferson LS, Kimball SR. Amino acids as regulators of gene expression at the level of mRNA translation. J Nutr 133: 2046S–2051S, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kennaugh JM, Bell AW, Teng C, Meschia G, Battaglia FC. Ontogenetic changes in the rates of protein synthesis and leucine oxidation during fetal life. Pediatr Res 22: 688–692, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol Cell Physiol 274: C221–C228, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Lewis SE, Kelly FJ, Goldspink DF. Pre- and post-natal growth and protein turnover in smooth muscle, heart and slow- and fast-twitch skeletal muscles of the rat. Biochem J 217: 517–526, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liechty EA, Boyle DW, Moorehead H, Auble L, Denne SC. Aromatic amino acids are utilized and protein synthesis is stimulated during amino acid infusion in the ovine fetus. J Nutr 129: 1161–1166, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Lin TA, Kong X, Haystead TA, Pause A, Belsham G, Sonenberg N, Lawrence JC Jr. PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science 266: 653–656, 1994. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z, Jahn LA, Wei L, Long W, Barrett EJ. Amino acids stimulate translation initiation and protein synthesis through an Akt-independent pathway in human skeletal muscle. J Clin Endocrinol Metab 87: 5553–5558, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Wu Y, Nicklas EW, Jahn LA, Price WJ, Barrett EJ. Unlike insulin, amino acids stimulate p70S6k but not GSK-3 or glycogen synthase in human skeletal muscle. Am J Physiol Endocrinol Metab 286: E523–E528, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Long W, Saffer L, Wei L, Barrett EJ. Amino acids regulate skeletal muscle PHAS-I and p70 S6-kinase phosphorylation independently of insulin. Am J Physiol Endocrinol Metab 279: E301–E306, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk. Implications for tuberous sclerosis and cancer pathogenesis. Cell 121: 179–193, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Milley JR Effects of insulin on ovine fetal leucine kinetics and protein metabolism. J Clin Invest 93: 1616–1624, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mothe-Satney I, Yang D, Fadden P, Haystead TA, Lawrence JC Jr. Multiple mechanisms control phosphorylation of PHAS-I in five (S/T)P sites that govern translational repression. Mol Cell Biol 20: 3558–3567, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Connor PM, Bush JA, Suryawan A, Nguyen HV, Davis TA. Insulin and amino acids independently stimulate skeletal muscle protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 284: E110–E119, 2003. [DOI] [PubMed] [Google Scholar]
- 33.O'Connor PM, Kimball SR, Suryawan A, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Regulation of translation initiation by insulin and amino acids in skeletal muscle of neonatal pigs. Am J Physiol Endocrinol Metab 285: E40–E53, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1−/−/S6K2−/− mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol 24: 3112–3124, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proud CG mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun 313: 429–436, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Proud CG Amino acids and mTOR signalling in anabolic function. Biochem Soc Trans 35: 1187–1190, 2007. [DOI] [PubMed] [Google Scholar]
- 37.Rennie MJ, Bohe J, Wolfe RR. Latency, duration and dose response relationships of amino acid effects on human muscle protein synthesis. J Nutr 132: 3225S–3227S, 2002. [DOI] [PubMed] [Google Scholar]
- 38.Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc Natl Acad Sci USA 101: 13489–13494, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah OJ, Anthony JC, Kimball SR, Jefferson LS. 4E-BP1 and S6K1: translational integration sites for nutritional and hormonal information in muscle. Am J Physiol Endocrinol Metab 279: E715–E729, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Shen W, Mallon D, Boyle DW, Liechty EA. IGF-I and insulin regulate eIF4F formation by different mechanisms in muscle and liver in the ovine fetus. Am J Physiol Endocrinol Metab 283: E593–E603, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Shen W, Wisniowski P, Ahmed L, Boyle DW, Denne SC, Liechty EA. Protein anabolic effects of insulin and IGF-I in the ovine fetus. Am J Physiol Endocrinol Metab 284: E748–E756, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Suryawan A, O'Connor PM, Kimball SR, Bush JA, Nguyen HV, Jefferson LS, Davis TA. Amino acids do not alter the insulin-induced activation of the insulin signaling pathway in neonatal pigs. J Nutr 134: 24–30, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Suryawan A, Orellana RA, Nguyen HV, Jeyapalan AS, Fleming JR, Davis TA. Activation by insulin and amino acids of signaling components leading to translation initiation in skeletal muscle of neonatal pigs is developmentally regulated. Am J Physiol Endocrinol Metab 293: E1597–E1605, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thureen PJ, Scheer B, Anderson SM, Tooze JA, Young DA, Hay WW Jr. Effect of hyperinsulinemia on amino acid utilization in the ovine fetus. Am J Physiol Endocrinol Metab 279: E1294–E1304, 2000. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Beugnet A, Murakami M, Yamanaka S, Proud CG. Distinct signaling events downstream of mTOR cooperate to mediate the effects of amino acids and insulin on initiation factor 4E-binding proteins. Mol Cell Biol 25: 2558–2572, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21: 362–369, 2006. [DOI] [PubMed] [Google Scholar]
- 47.Wray-Cahen D, Beckett PR, Nguyen HV, Davis TA. Insulin-stimulated amino acid utilization during glucose and amino acid clamps decreases with development. Am J Physiol Endocrinol Metab 273: E305–E314, 1997. [DOI] [PubMed] [Google Scholar]
- 48.Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA. Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab 275: E602–E609, 1998. [DOI] [PubMed] [Google Scholar]