Abstract
Four grams of glucose circulates in the blood of a person weighing 70 kg. This glucose is critical for normal function in many cell types. In accordance with the importance of these 4 g of glucose, a sophisticated control system is in place to maintain blood glucose constant. Our focus has been on the mechanisms by which the flux of glucose from liver to blood and from blood to skeletal muscle is regulated. The body has a remarkable capacity to satisfy the nutritional need for glucose, while still maintaining blood glucose homeostasis. The essential role of glucagon and insulin and the importance of distributed control of glucose fluxes are highlighted in this review. With regard to the latter, studies are presented that show how regulation of muscle glucose uptake is regulated by glucose delivery to muscle, glucose transport into muscle, and glucose phosphorylation within muscle.
Keywords: insulin, mice, rat, dog, glycogen, epinephrine, hexokinase, glucose transport, glucose delivery
four grams of glucose circulates in the blood of a person weighing 70 kg. This is the amount needed to fill a teaspoon. Although these 4 g constitute an infinitesimally small fraction of the mass of the total organism, a wide variety of cells rely on it and are sensitive to its presence. A serious fall in blood glucose can be characterized by metabolic dysfunction, neuroglycopenia, seizure, and death. A persistent elevation in blood glucose leads to “glucose toxicity.” Glucose toxicity contributes to β-cell dysfunction and the pathology grouped together as complications of diabetes. This review is based on the Solomon A. Berson Lecture presented at Experimental Biology 2008. It summarizes research conducted in our laboratory at Vanderbilt University that has examined the regulation of blood glucose homeostasis.
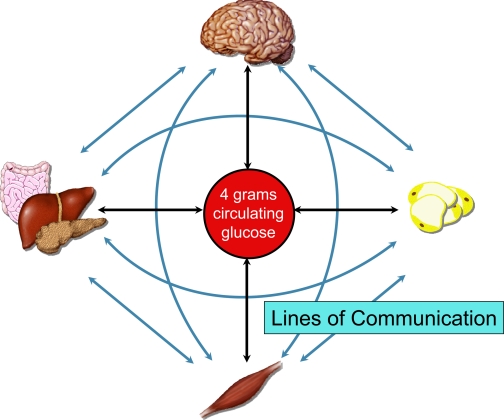
In accordance with the importance of blood glucose, a sophisticated control system is in place to protect it from marked deviations. Experiments in isolated organs, tissues, and cells have contributed extensively to our understanding of this control system. However, glucose homeostasis requires integrated control by the whole organism. The components essential to regulation of blood glucose communicate with each other. Communication can be by modification of glucose flux directly or through humoral and neural signaling mechanisms (Fig. 1). This level of control requires an in vivo model system to be fully delineated.
Fig. 1.
Lines of communication form a complex network. Much has been learned and remains to be learned about glucose metabolism by assessing organs, tissues, and cells independently. Understanding factors that protect blood glucose and maintain glucose homeostasis requires the communication between organs and integration of signals that is evident in in vivo model systems. Organs can exert effects by direct control of glucose flux (black lines) or indirectly by humoral and neural signals (blue lines).
Glucose is extracted from the blood to sustain metabolism in a variety of tissues. The brain consumes ∼60% of the blood glucose used in the sedentary, fasted person. The liver releases glucose formed by glycogenolysis and gluconeogenesis into the blood at rates equal to the uptake of blood glucose. Although the kidney can make glucose, it is a minor source compared with the liver. The real test of glucoregulation comes when it is challenged. Ingestion of a meal high in carbohydrates causes an incretin response and increased β-cell insulin secretion. Insulin suppresses the entry of glucose from liver to blood and stimulates glucose removal from blood in to muscle, liver, and fat. The result is that deviations in blood glucose are dampened. Insulin-independent mechanisms are activated that increase muscle glucose uptake during exercise. The liver is stimulated to produce glucose, again minimizing deviations in blood glucose. If glucose production does not increase, as sometimes happens in diabetics treated with too much insulin, hypoglycemia ensues.
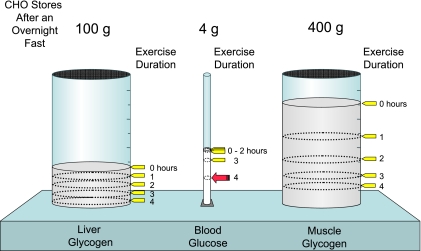
The amount of glucose in the blood is preserved at the expense of glycogen reservoirs (Fig. 2). In postabsorptive humans, there are ∼100 g of glycogen in the liver and ∼400 g of glycogen in muscle. Carbohydrate oxidation by the working muscle can go up by ∼10-fold with exercise, and yet after 1 h, blood glucose is maintained at ∼4 g. Blood glucose is preserved at the expense of liver and muscle glycogen. Liver glycogen breakdown protects blood glucose as the glucose moieties that comprise it are released into the blood. Muscle glycogen breakdown impedes the removal of glucose from the blood by increasing glucose 6-phosphate (G6P), which inhibits the hexokinase (HK) reaction, and by providing a source of fuel that diminishes the need for blood glucose. The amount of glucose in the blood can still be constant after 2 h of exercise in well-nourished subjects. Blood glucose is protected by liver gluconeogenesis after glycogen stores become critically low. Only after extremely prolonged exercise does blood glucose fall to concentrations that result in hypoglycemia severe enough to cause neuroglycopenia.
Fig. 2.
In the short-term, fasted healthy 70-kg human, liver, and muscle store ∼100 and 400 g glycogen, respectively. Four grams of glucose is present in the blood. During exercise, glucose is preserved at the expense of glycogen reservoirs. Carbohydrate (CHO) oxidation by the working muscle is increased in response to exercise. However, after 1 h, 4 g of glucose is maintained. Blood glucose is maintained at the expense of liver and muscle glycogen. The amount of glucose in the blood can still be constant after 2 h of exercise. Only after exercise of extremely long duration does blood glucose fall to concentrations that result in hypoglycemia severe enough to cause neuroglycopenia.
The Study of Glucose Metabolism In Vivo
A number of experimental models to study metabolic pathways in liver, gastrointestinal tissue, adipose tissue, and muscle have been used in our laboratory. Regardless of aim or hypothesis, we have relied on five principles of experimental design.
Glucose metabolism is all about flux control.
Glucose and metabolite concentrations are obviously keys to understanding metabolism. However, these alone do not tell the full story. The small changes in blood glucose during exercise and feeding belie the complicated interorgan movement of carbons. We have examined mechanisms of flux control and metabolite interconversion using isotopic and arterial-venous differences.
Glucose flux control is distributed amongst distinct systems and requires an in vivo model system to be fully understood.
Multiple pathways and organs participate in glucose homeostasis. It is difficult to gain insight from isolated organ, tissue, and cell preparations into mechanisms that protect blood glucose homeostasis. The deconvolution of glucoregulation using isolated preparations is, of course, vital. Ultimately what is learned from these systems must be conceptually reconstituted within the context of whole body metabolism to test how these systems interact to maintain glucose homeostasis.
Glucoregulatory mechanisms are isolated most effectively when blood glucose concentrations are clamped.
Changes in blood glucose can elicit compensatory hormone, neural, and autoregulatory responses. Redundant mechanisms that are invoked with a change in glucose can mask the role of a primary regulatory factor. For example, increasing insulin or decreasing glucagon causes a decrement in blood glucose. The effect of these hormonal changes can be masked or underestimated as other glucose-counterregulatory factors attempt to compensate for the fall in glucose. Only by clamping blood glucose can the true effectiveness of these hormones be assessed.
Glucose fluxes are most sensitively regulated and therefore best studied in the conscious state.
Cellular metabolism and hormone sensitivity are generally decreased by anesthesia. This results in the need for use of supraphysiological concentrations of hormones and other chemical activators. Conscious experimental animal models permit the most physiological examination of glucoregulation.
Exercise can precipitate phenotypes, amplify signals, and reveal functional limitations.
Exercise testing is used clinically to precipitate symptoms that facilitate disease diagnosis. Conditions that may be silent in the resting state may be provoked by the demands of physical exercise. Exercise is an effective research tool for the same reason that it is useful clinically. Exercise and other sensitizing tests can be used to reveal with more sensitivity the effect of a genetic, pharmacological, or experimental manipulation.
Studies on the Control of Glucose Mobilization from the Liver
Role of glucagon.
In the experiments of Banting and Best that led to the discovery of insulin in 1921 (5), a “contaminant” in pancreatic extracts was reported that caused a transient increase in blood glucose. Nevertheless, the hypoglycemic effect of the extract was sustained and predominated. Insulin was isolated from the extract and went into immediate use for treatment of diabetes. Working in the shadow cast by the discovery of insulin, Murlin and colleagues (48, 62) went on to define the hyperglycemic factor in pancreatic extracts. They wrote in 1923: “There are two substances in these aqueous extracts, one of which lowers the blood sugar and the D:N ratio and raises the R.Q. (insulin), The other raises the blood sugar of both normal and depancreatized animals. …” Based on their observations, these investigators proposed the existence of a second hormone secreted by the pancreas. They proposed the name glucagon, a contraction of the words “glucose” and “agonist,” for this putative hormone. The potent effects of glucagon on liver glycogenolysis and gluconeogenesis and the dependence of glucagon action on cAMP were defined in large part by the work of Vanderbilt scientists Earl W. Sutherland, Charles R. Park, and John H. Exton (22, 78). Sutherland was awarded the 1971 Nobel Prize “for his discoveries concerning the mechanisms of the action of hormones.”
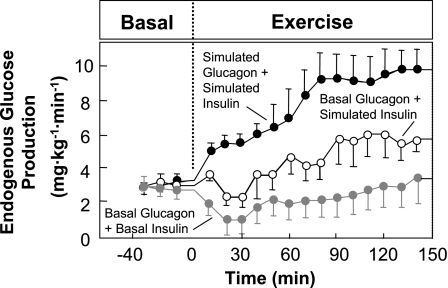
The full significance of glucagon in physiology and diabetes was not appreciated until the development of the radioimmunoassay, which made plasma glucagon measurement routine. Exercise was used to challenge the glucoregulatory system in a dog model and to define the roles of glucagon and other putative regulators. The key advantages of the dog are 1) access to the portal vein, which drains the pancreas and perfuses the liver; 2) a blood volume large enough to perform repeated transorgan sampling; and 3) the capacity to exercise for extended intervals. The first of these advantages of the dog, portal vein access, is not possible in conscious humans. Studies (45, 96, 97, 99) in the dog demonstrated the important role of glucagon in maintaining blood glucose. The pancreatic clamp technique in which somatostatin is infused to suppress pancreatic glucagon and insulin secretion was used. Glucagon and insulin were then replaced at basal rates or exercise-simulated rates directly in the portal vein. In the absence of the exercise-induced rise in glucagon, the increase in glucose production was attenuated by 60% (99; Fig. 3). The remaining 40% was due to potentiation of basal glucagon action in the liver resulting from the fall in insulin (96). Glucose production failed to increase when both the exercise-induced rise in glucagon and fall in insulin were prevented (Fig. 3). By combining isotopes and hepatic arterial-venous differences, glucagon was shown to be key to both the glycogenolytic and gluconeogenic responses to exercise (99). Studies (41, 49, 54, 103) conducted in human subjects were consistent with those findings.
Fig. 3.
Rates of endogenous glucose production in 3 protocols conducted in catheterized dogs. Glucagon and insulin were clamped in all protocols using somatostatin to suppress the endogenous release of these hormones. Glucagon and insulin were replaced at basal rates in the hepatic portal vein during rest. Glucagon and insulin responses to exercise were simulated (green), glucagon response to exercise was simulated and insulin was kept at basal (red), or glucagon and insulin concentrations were both maintained at basal (blue) during exercise. Data are means ± SE. Data are from Wasserman et al. (14, 99).
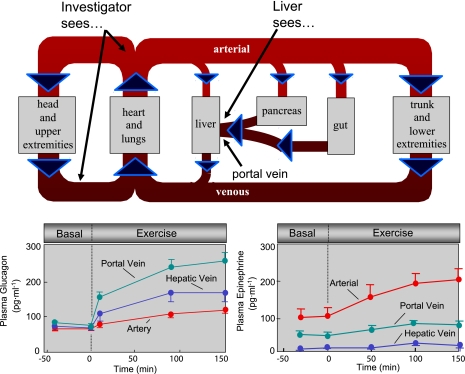
Despite experimental evidence showing the incredible sensitivity of the liver to glucagon (56), its role during exercise was slow to gain complete acceptance. This is because the exercise-induced increment in peripheral blood glucagon is delayed and dampened with respect to the increase in glucose released from the liver. In fact, depending on the specificity of the glucagon assay or the duration of exercise, peripheral blood glucagon may not increase at all. Glucagon released from the pancreas traverses the liver delaying its entry into the peripheral circulation (Fig. 4). Because the liver extracts glucagon, the increase in glucagon in peripheral blood is dampened (94). Figure 4 (bottom left) illustrates the marked increase in portal vein glucagon during exercise in the dog compared with the increases in arterial and hepatic vein blood. Having the pancreas and liver in series allows for increased glucagon in the blood perfusing the liver, without the need for the high glucagon secretion rates necessary to rapidly fill the general circulation. While this is an efficient physiological design, the placement of the liver between the pancreas and general circulation is experimentally inconvenient. Investigators working in human subjects have had difficulty in delineating the role of glucagon, as they do not have portal vein access and are blind to glucagon concentration at the liver.
Fig. 4.
An overview of the circulation is shown at top. In human subjects, blood is sampled from a peripheral vein, an arterialized hand vein, or an artery. Since the tissues of the splanchnic bed (including pancreas) consume and release hormones and metabolites, the content of the blood from these vessels does not reflect the content in portal vein blood. Glucagon and insulin are secreted by the pancreas into the portal vein (via pancreatic veins) and extracted from the portal vein by the liver. Data from a dog model show that arterial glucagon underestimates the glucagon concentration perfusing the liver (bottom left). Conversely, arterial epinephrine overestimates the epinephrine concentration perfusing the liver (bottom right). Data are means ± SE.
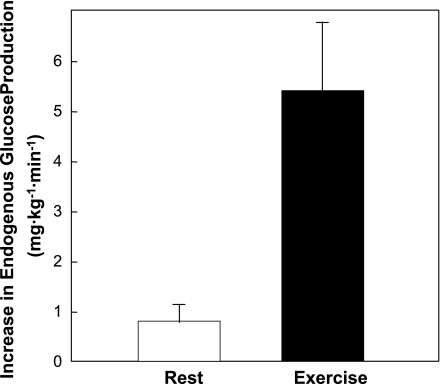
An increase in glucagon in the physiological context of exercise is considerably more potent than the effects of an experimental increase in glucagon of the same magnitude. A twofold increase in glucagon causes a peak increase in endogenous glucose production of ∼1 mg·kg−1·min−1 in the dog (84), while the same increase during exercise causes a peak increase of ∼5 mg·kg−1·min−1 (99; Fig. 5). Glucagon action is fully manifested during exercise for three reasons. First, the increased glucose utilization of working muscle prevents the hyperglycemia that accompanies an experimental increase in glucagon. Second, exercise puts the body into a gluconeogenic mode (93). Gluconeogenic substrates are mobilized from muscle, adipose, and intestine. Energy for gluconeogenesis in the form of free fatty acids is mobilized from adipose tissue. Finally, moderate exercise causes a fall in insulin that sensitizes the liver to glucagon (96).
Fig. 5.
Endogenous glucose production response to a 2-fold increase in glucagon during rest and exercise in catheterized dogs. Rates represent the peak response under each condition. It is important to note that the response to exercise was not only 5-fold higher, but it was also sustained compared with the transient response at rest. Data are means ± SE. Data were calculated from the data of Stevenson et al. (84) and Wasserman et al. (99).
Role of catecholamines.
Just as the splanchnic circulation masks true glucagon levels in the liver, it causes an overestimate of the epinephrine concentration at the liver (Fig. 4, bottom right). The postulate that the catecholamines are important for the stimulation of glucose release from the liver was based largely on high systemic catecholamine concentrations during exercise. The gut extracts a large fraction of the epinephrine from the blood (>50%). As a result, the increase with exercise in the portal vein of the dog is only ∼30% of the increase in arterial blood (15, 16). We have shown that hepatic denervation (100), selective hepatic β- and α-adrenergic receptor blockade (17), and adrenalectomy (60) have little or no direct effect on glucose production in the exercising dog. These findings are consistent with research in other species (90). Direct hepatic adrenergic stimulation was shown to be unimportant to the rate of glucose production during exercise even in the absence of changes in the primary regulators glucagon and insulin (14). Attempts to directly assess a role for catecholamines in stimulating endogenous glucose production during high-intensity exercise (>80% maximum O2 uptake) in humans have also been negative (43, 49, 59, 82, 83). This is not to say that other factors such as IL-6 (23) or an as yet undefined regulator do not play some role. It is possible, particularly during extremely intense or prolonged exercise, that some other mediator stimulates the liver to release glucose (13, 81). The most potent stimulant of hepatic glycogen breakdown that we have observed is 5′-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside (AICAR; Ref. 10). AICAR is converted by phosphorylation to the AMP analog ZMP inside cells. Liver AMP concentrations are increased during exercise to levels that match stimulatory concentrations of ZMP (9). A hormone, neurotransmitter, paracrine factor, or metabolite that increases hepatic AMP could potentially contribute to the control of glucose released by the liver.
Coupling of glucose production to utilization.
The facet of the glucoregulatory response that is least understood is the initial signal informing the pancreas and ultimately the liver that the body is engaged in exercise. Studies from our laboratory have focused on feedback mechanisms related to the utilization of blood glucose. Glucoregulation during exercise is exquisitely sensitive to blood glucose or a closely related variable (6, 46, 95). The counterregulatory hormones do not begin to increase with insulin-induced hypoglycemia until blood glucose has fallen >25 mg/dl. During exercise, increased counterregulatory hormone responses are seen with a decrease in blood glucose of <8 mg/dl (95, 97). Moreover, an exogenous glucose infusion can affect pancreatic hormone secretion and shut down liver glucose output during exercise, before deviations in blood glucose can be measured (6, 46). Sites in the brain, portal vein, liver, intestine, carotid sinus, and pancreas are sensitive to blood glucose (55). We hypothesized that carotid bodies or receptors nearby detect a signal related to small deviations in arterial glucose. This was based on demonstrations that carotid bodies 1) sense glucose (2, 3, 51), 2) project afferent nerves to brain regions involved in glucoregulation (1), and 3) have unique characteristics (i.e., high blood flow and high metabolic rate) that make them highly sensitive to blood composition (66). Comparison of carotid body resected and sham-operated dogs (50) suggested that the carotid bodies or sensors close by are involved in arterial glucagon and sympathetic nerve responses to exercise. Carotid bodies, however, were not essential to glucose homeostasis during sustained exercise, indicating that compensatory mechanisms come into play in the carotid body resected dog. It is notable that these same structures are also necessary for the increment in glucagon secretion during insulin-induced hypoglycemia in the dog (51) and could mediate a common regulatory pathway activated by hypoglycemia and exercise.
Postexercise liver glucose metabolism.
For the liver to have the ability to mobilize glucose during a fast, it must have the ability to store it when fuels are abundant. Glycogen storage by the liver is controlled by many factors, most notably the rate of portal venous glucose delivery to the liver and insulin. Our laboratory showed using the conscious dog model that prior exercise accelerates the repletion of liver glycogen by increasing the rate of glucose absorption from the gut after ingestion (39, 70), by increasing the extraction of glucose by the liver (32), and by directing more of the glucose taken up after exercise to liver glycogen (69). Different mechanisms appear to govern the accelerated rates of hepatic glucose uptake and glycogen deposition. The postexercise increase in hepatic glucose uptake during a glucose load is closely linked to increased hepatic insulin sensitivity (69). On the other hand, the fate of glucose taken up by the liver (i.e., glycogen synthesis vs. glycolysis) is independent of insulin action. The fate of glucose taken up by the liver is more closely linked to glycogen mass (33). Preventing the decrease in glycogen mass during exercise by preventing the glucagon and insulin responses to exercise with somatostatin did not diminish the accelerated hepatic glucose uptake (68). It did, however, alter the metabolic fate of the glucose, as the percent glucose diverted to glycogen was greatly reduced (68).
Distributed Control of Blood Glucose Removal
Skeletal muscle is where glucose uptake is quantitatively the most important. It is the bulk of insulin-sensitive tissue and the primary site of glucose uptake during exercise. Blood glucose homeostasis cannot be understood without defining the control of muscle glucose uptake (MGU). The regulation of MGU has been a subject of intensive research at Vanderbilt University for over 50 yr. Dr. C. R. Park of the Vanderbilt Department of Physiology (now Department of Molecular Physiology and Biophysics) demonstrated in 1951 that insulin increases the permeability of muscle to glucose (67). A major breakthrough in understanding the mechanism by which insulin stimulates glucose uptake was made by Dr. Tetsuro Kono (86) also of the Vanderbilt Department of Physiology. Kono (86) and Cushman at the National Institutes of Health (18) showed that insulin causes translocation of a glucose transport protein (i.e., GLUT4) from an intracellular store to the plasma membrane. It is now well established that both insulin and exercise cause translocation of GLUT4 to the plasma membrane. Except for the fundamental process of GLUT4 translocation, MGU is controlled differently with exercise and insulin. Contraction-stimulated intracellular signaling (52, 80) and MGU (34, 75, 77, 88, 91, 98) are insulin independent. Moreover, the fate of glucose extracted from the blood is different in response to exercise and insulin (91, 105). For these reasons, barriers to glucose flux from blood to muscle must be defined independently for these two controllers of MGU.
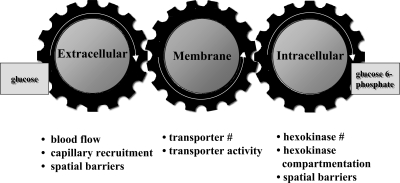
The physiological regulation of MGU in vivo is complex. It requires that glucose travel from blood to interstitium to intracellular space and then be phosphorylated to G6P. Figure 6 illustrates the coupling of these processes (92). Muscle blood flow and capillary recruitment determine glucose movement from blood to interstitium. The plasma membrane glucose transporter content determines glucose transport into the cell. Muscle HK activity, cellular HK compartmentalization, and the concentration of the HK inhibitor G6P determine the capacity to phosphorylate glucose. Phosphorylation of glucose is irreversible in muscle so, with this reaction, glucose is trapped and the uptake process is complete. Despite the characterization of the biochemical and histological composition of normal and insulin-resistant muscle, the factors that determine MGU in vivo have been difficult to define. This is because it is difficult to translate biochemical and histological characteristics into functional changes in glucose flux.
Fig. 6.
Distributed control of muscle glucose uptake; modified from Wasserman and Halseth (92) and Wasserman et al. (101).
Considerable emphasis has been placed on the role of the glucose transport step in glucose removal from the blood. The assertion that membrane transport is rate limiting for MGU is common in the scientific literature. There is no doubt that glucose transport by GLUT4 is essential to MGU. The issue is whether other steps also serve a regulatory function. Does the distributed control paradigm better define MGU than the rate-limiting step paradigm?
It is clear that membrane transport is the primary barrier to MGU in fasted, sedentary subjects, as membrane permeability to glucose is low. In response to both exercise and insulin, GLUT4 translocation to the muscle membrane is accelerated (21, 74), while the ability to phosphorylate glucose may be unaffected or inhibited by G6P. In addition to the effects on glucose transport, exercise and insulin increase muscle capillary blood flow (12, 20, 44, 87). Together these cellular events predict a shift in the barrier from transport to phosphorylation. The first clue we had that glucose phosphorylation may become a significant barrier to glucose influx was an observation in muscle from exercised rats. We showed that HK II mRNA, but not GLUT4 mRNA, was increased after exercise (64) due to an increase in gene transcription (63). Although increased GLUT4 expression has been reported after exercise (53, 76), the HK II gene has since been shown to be considerably more responsive (64, 73). The increase in HK II mRNA in response to a single bout of exercise only made sense from an adaptive standpoint if glucose phosphorylation was a barrier to MGU.
The three-step paradigm for MGU has been challenging to study because intracellular glucose, which is the product of membrane glucose transport and the substrate for glucose phosphorylation, cannot be measured. It can theoretically be calculated indirectly as the difference between total muscle and interstitial glucose. However, total muscle glucose and interstitial glucose are two comparatively large numbers, each with measurement error and assumptions. Intracellular glucose calculated using this indirect theoretical approach has a background signal that makes the method untenable. The close coupling of glucose delivery, transport, and phosphorylation and the existence of glucose compartmentalization and spatial gradients compelled us to develop new techniques to overcome these difficult facets associated with defining MGU.
Glucose countertransport approach for assessing distributed control.
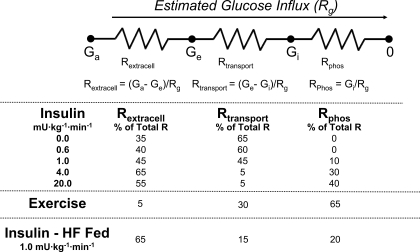
The first approach used isotopic glucose analogs in the conscious rat to obtain a surrogate for intracellular glucose by applying the concept of glucose countertransport (36–38, 65, 72). Countertransport creates a situation where the steady-state distribution of one sugar between intracellular and extracellular water is induced by a transmembrane gradient of a second sugar (61). With this technique, the distribution of trace 3–0-[3H]methyl-glucose between intracellular and extracellular water can be used to calculate the glucose concentration at the outer ([G]om) and inner ([G]im) membrane surfaces. This information allows the extracellular glucose gradient (arterial glucose − [G]om), the transmembrane gradient ([G]om − [G]im), and the intracellular glucose available for phosphorylation ([G]im) to be calculated. An index of MGU (Rg) was derived from the accumulation of phosphorylated 2-deoxy-[3H]glucose (2[3H]DG). Extracellular, membrane, and intracellular resistances to muscle glucose influx were calculated using a variation of Ohm's law for electrical circuits where glucose gradients and Rg are analogous to voltage gradient and current, respectively (Fig. 7).
Fig. 7.
Estimated muscle extracellular (Rextracell), transport (Rtransport), and phoshorylation (Rphos) resistances to muscle glucose uptake (MGU) during insulin clamps, exercise, and during insulin clamps in rats fed a high-fat (HF) diet. Glucose gradients were calculated using the countertransport method, and the index of MGU (Rg) was calculating using 2-deoxy-[3H]glucose (2[3H]DG; Ref. 65). Values are expressed as %total resistance to MGU. Ga, arterial glucose; Ge, extracellular glucose; Gi, intracellular glucose.
The countertransport method showed that insulin (36, 37, 65) and exercise (37) both decrease the muscle membrane glucose gradient, reflecting a shift in control of MGU away from glucose transport to delivery and/or phosphorylation (Fig. 7). Results were consistent with those obtained by muscle interstitial microdialysis (42, 79), showing that glucose delivery is a barrier to MGU over a range of insulin doses (65). As one would predict from the marked exercise hyperemia, muscle glucose delivery was not a barrier to MGU during exercise (37). This again was consistent with results obtained with microdialysis (58).
Insulin resistance can be associated with deficits in muscle blood flow (11, 12, 19, 89), membrane glucose transport (40, 57, 104), and intracellular capacity to phosphorylate glucose (7, 8, 71, 102). A corollary to distributed control of MGU is that one or all of these deficits may contribute to insulin resistance. We delineated the steps that cause the functional impairment in MGU by applying the countertransport method to high-fat-fed insulin-resistant rats. Results showed that extracellular and intracellular resistances were the two chief causes of the resistance of MGU to insulin. This is not to say that transport is normal. Glucose transport is, in fact, defective with diet-induced insulin resistance. The countertransport experiments, however, show that the primary functional limitations are in glucose delivery and phosphorylation in this model. The pathogenesis of insulin resistance is further complicated when one considers that extracellular barriers to glucose delivery are also apt to be barriers to insulin delivery. Impaired insulin delivery will effect MGU and metabolic regulation in general, if the barrier to insulin delivery is sufficiently large.
Transgenic mouse models to study distributed control.
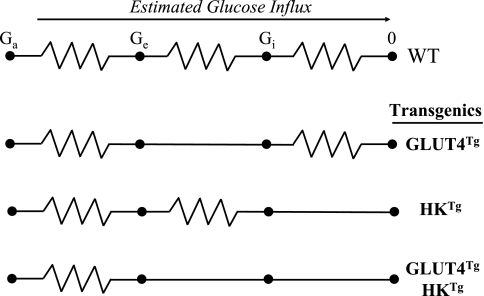
The second approach used to dissect control of MGU was the application of isotopic techniques to genetic increases in muscle GLUT4 and HK II in mice. As is the case with the countertransport model, this approach was also predicated on the principles set forth by Ohm's Law (Fig. 8). The hypotheses that HK II overexpression would increase the capacity of muscle to consume glucose, while GLUT4 overexpression would have no effect was tested (24, 35). Mice overexpressing GLUT4 (GLUT4Tg) and/or HK II (HKTg) were catheterized and underwent experiments >5 days later. An index of MGU was measured using 2[3H]DG (Fig. 9). Consistent with predictions of the countertransport approach, HKTg mice had increased exercise- and insulin-stimulated Rg, while GLUT4Tg mice did not. A variation of the “control coefficient” concept was applied to the three steps of MGU (Table 1) and calculated by the equation derived from control theory (47): CTg = δln(Rg)/δln(PTg), where CTg is the control coefficient for the regulatory site of interest and PTg is calculated from the GLUT4 and HK II expression in GLUT4Tg and HKTg mice relative to their wild-type littermates. In a closed system, the control coefficients sum to 1.0. A “control coefficient” for glucose delivery was calculated as 1.0 minus the sum of the transport and phosphorylation control coefficients. A key assumption is that the fold overexpression equals the functional increase in transgene product. Control of MGU using the three-step paradigm shows the control coefficient in the fasted sedentary state is highest at the transport step. During conditions that result in increased GLUT4 translocation, the control coefficient for transport may fall to zero suggesting that the muscle membrane is highly permeable to glucose. The difference between insulin stimulation and exercise is that with insulin control of MGU is shared between glucose delivery and phosphorylation, while during exercise the onus of control rests with the phosphorylation step. These results are the same as those obtained using the countertransport approach.
Fig. 8.
Mouse models for assessing distributed control of MGU are depicted by segments of an electrical circuit. WT, GLUT4Tg, HKTg, and GLUT4TgHKTg: wild-type, GLUT4 overexpressing, HK II overexpressing, and combined GLUT4 and HK II overexpressors, respectively. Resistances to transport and phosphorylation are reduced by overexpression of GLUT4 and HK II proteins. Glucose influx was estimated using Rg measured with 2[3H]DG in these mouse models.
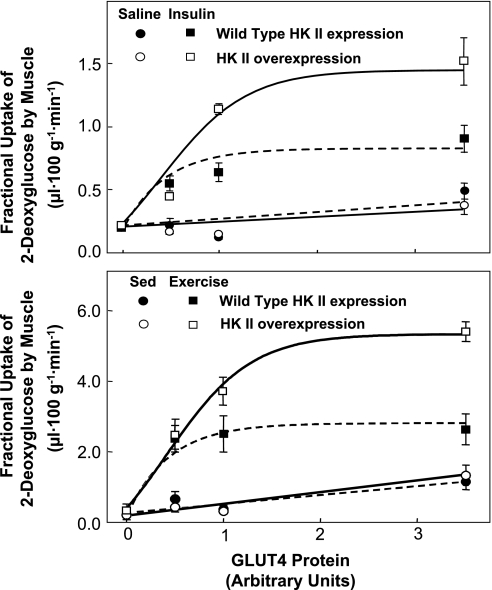
Fig. 9.
Fractional 2[3H]DG uptake in gastrocnemius of mice expressing 0, 0.5, 1.0 and ∼3.5 fold WT GLUT4 levels during the steady state period of a 4.0 mU·kg−1·min−1 insulin clamp or during an equal duration saline infusion (top). The same comparisons are made for sedentary (Sed) and exercised mice (bottom). HK II content was either normal (WT) or the protein was overexpressed. GLUT4 only affected fractional 2[3H]DG uptake during saline infusion when it was overexpressed. On the other hand, the absence of GLUT4 caused a marked attenuation of fractional 2[3H]DG uptake during insulin stimulation regardless of whether HK II was overexpressed. HK II overexpression had no effect on the response during saline infusion but increased the maximal response to insulin. Each point is means ± SE for 8–11 in vivo mouse experiments. Data are from Fueger and colleagues (25, 31).
Table 1.
“Control coefficients” for steps involved in muscle glucose index in gastrocnemius muscle from fasted, sedentary, insulin-clamped, and exercised mice
| Control Steps | Delivery | Transport | Phosphorylation |
|---|---|---|---|
| Rest | 0.1 | 0.9 | 0.0 |
| Insulin clamp, 4.0 mU·kg−1·min−1 | 0.5 | 0.1 | 0.4 |
| Exercise, 0.6 mph at 0% grade | 0.2 | 0.0 | 0.8 |
See text for description of the calculation.
Mice with a heterozygous or homozygous deletion of GLUT4 and mice with a heterozygous HK II deletion (complete knockout is lethal) were tested to extend the results obtained in GLUT4 and HK II overexpressing mice. The Rg response to exercise was diminished in mice with a partial HK II knockout (26). A reduction in HK II also impaired whole body insulin sensitivity and heart Rg but did not impair Rg of the gastrocnemius muscle (29). A heterozygous deletion of GLUT4 did not impair Rg in exercised and insulin-stimulated states (27, 28). However, a complete deletion of GLUT4 prevented the increase in Rg with exercise and led to marked hyperglycemia (30). We tested the hypothesis that if the glucose phosphorylation barrier were lowered by HK II overexpression, muscle would then be sensitive to a 50% reduction in GLUT4. This was in fact the case. Lowering the phosphorylation barrier resulted in a shift in control so that MGU was sensitive to a quantitative reduction in GLUT4 during insulin stimulation (27) and exercise (28).
Figure 9 is a compilation of studies (24, 31) in which mice with varying degrees of muscle GLUT4 and HK II expression were studied during exercise and insulin stimulation. Several significant points emerge: 1) GLUT4 overexpression increases fractional muscle 2[3H]DG uptake in sedentary, fasted mice, while HK II overexpression does not; 2) GLUT4 overexpression does not further increase the stimulatory effect of exercise and insulin on fractional 2[3H]DG uptake; 3) muscle can tolerate a 50% reduction in GLUT4 expression without compromising sedentary, exercise-stimulated, or insulin-stimulated MGU, provided that the phosphorylation barrier is not reduced by HK II overexpression; and 4) HK II overexpression increases the Vmax of fractional 2[3H]DG uptake during exercise and insulin stimulation. The shift in control from transport to phosphorylation should not be interpreted as meaning that GLUT4 is unimportant in the regulation of MGU. GLUT4 translocation is the fulcrum that distributes the balance of control among the three steps that control MGU. It is the sensitive regulation of GLUT4 translocation that shifts the burden of control from glucose transport to phosphorylation and/or delivery.
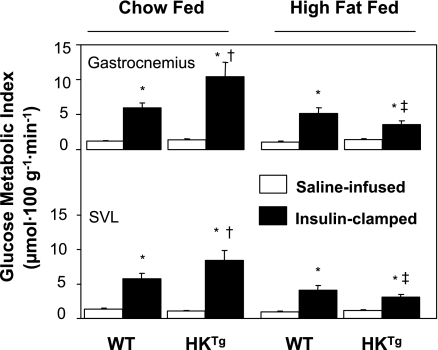
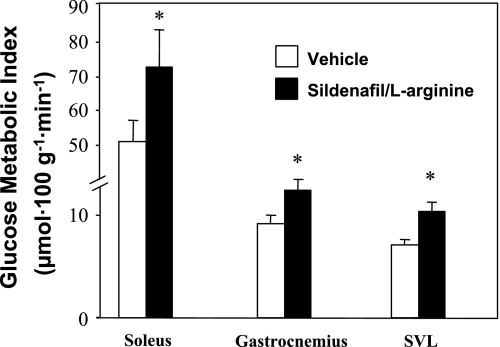
C57Bl/6J mice develop insulin resistance on a high-fat diet (85) and were used to determine the functional deficits that make muscle insulin resistant. We tested whether HK II overexpression could correct insulin-stimulated MGU in high-fat-fed mice (Fig. 10). In contrast to the marked effect of HK II overexpression on insulin-stimulated MGU in chow-fed mice, there was no effect in high-fat-fed mice (24). This suggested that the glucose phosphorylation barrier was not the functional limitation causing insulin resistance to MGU. The countertransport data showed that extracellular resistance was the chief site of resistance to MGU in the high-fat-fed rat. To further test this finding, we treated high-fat-fed mice for 3 mo with the PDE5 inhibitor sildenafil (4). PDE5 is expressed in vascular smooth muscle and causes breakdown of cGMP. cGMP signals relaxation of vascular smooth muscle. Sildenafil inhibition of PDE5 results in increased cGMP levels and decreased vascular resistance. We showed that this compound increases insulin-stimulated Rg in high-fat-fed mice (Fig. 11) and that it did so without improving muscle insulin signaling. This supports the theory that inhibition of PDE5 acts by lowering the barrier to glucose delivery and by so doing decreases insulin resistance. One would predict that sildenafil increases insulin delivery to the muscle as well. The fact that insulin signaling was not enhanced would suggest that the improved Rg response was not due to greater insulin availability. Chronic sildenafil treatment had other actions, however, including increased energy expenditure and decreased body weight. Therefore, although sildenafil is effective at improving MGU during an insulin clamp in the high-fat-fed mouse, more work still needs to be done before the mechanism of action can be fully defined (4).
Fig. 10.
Glucose metabolic index measured using 2[3H]DG was measured for the gastrocnemius and superficial vastus lateralis (SVL) during the last 30 min of a 120-min saline infusion or hyperinsulinemic euglycemic clamp (4.0 mU·kg−1·min−1) experiment on 5-h-fasted conscious, unrestrained mice. WT or HKTg mice were fed either a standard diet or a high-fat diet up to 4 mo of age and fasted for 5 h. Data are means ± SE for 7–14 mice/group. *P < 0.05 vs. saline condition; †P < 0.05 vs. WT standard diet fed; ‡P < 0.05 vs. HKTg standard diet fed. Data are from Fueger et al. (24).
Fig. 11.
Hyperinsulinemic-euglycemic clamps on 5-h-fasted, conscious, unrestrained C57BL/6J mice chronically treated with either vehicle or sildenafil plus arginine subcutaneously for 3 mo. Glucose metabolic index measured using 2[3H]DG is shown for soleus, gastrocnemius, and SVL. Data are means ± SE for 7–8 mice/group. *P ≤ 0.05 vs. vehicle. Data are from Ayala et al. (4).
Four Grams and Holding: Boundaries of Distributed Control
Blood glucose control has two attributes that make it so effective. First, there is redundancy of control due to multiple layers of the glucoregulatory response. Changes in pancreatic islet glucagon and insulin secretion normally prevent hypoglycemia. Should the response of the pancreas be inadequate, other controllers (e.g., catecholamines, cortisol, growth hormone, and glucose autoregulation) are in place to prevent a deeper fall in blood glucose. Second, there is distribution of control. Control at the level of the muscle was defined as distributed between glucose delivery to muscle, membrane transport into muscle, and intracellular glucose phosphorylation. Built into this model is a wider distribution of control that includes nutrition, liver function, and energy metabolism. Glucose delivery is a function of glucose concentration and vascular regulation. The ability of the gut to supply glucose in the postprandial state and the liver to do so in the postabsorptive state maintains blood glucose concentration. By doing so, these organs sustain glucose delivery and exert control over MGU. In fact, in the postabsorptive state the rate that glucose is released from the liver is the most sensitive regulator of MGU. With prolonged exercise or mild hyperinsulinemia, the muscle is highly permeable to glucose but MGU still declines. During prolonged exercise, the glycogen-depleted liver cannot sustain blood glucose. With iatrogenic hyperinsulinemia (i.e., treatment of diabetes), the release of glucose from the liver is suppressed, causing a decrease in blood glucose. In short, factors that control the release of glucose from the liver such as insulin, glucagon, and nutritional state are part of distributed control.
Distributed control of MGU also extends downstream of the intracellular glucose phosphorylation step. Feedback inhibition of HK II by G6P is the means by which the distribution of control is transferred to pathways downstream of glucose phosphorylation (i.e., glycogen synthesis and glycolysis). This provides a means for linking energy metabolism/storage to glucose flux.
Future Directions
The ability to manipulate the mouse genome and other approaches to alter gene expression creates many exciting avenues for future research in the study of metabolic control and glucose homeostasis. The implications for treatment of insulin resistance, obesity, diabetes, and metabolic syndrome are far reaching. The proliferation of mouse models has, in many ways, exceeded technical advances to characterize metabolism in mice where tissue mass and blood volume are limited. Discovery in metabolism and metabolic diseases will be accelerated by development and improvements in isotopic and nonisotopic methods to quantify the metabolome. Novel metabolomic signatures defined for specific mouse models can be compared with the metabolome of human diseases, giving valuable insight into their pathogenesis.
A number of genetic and pharmacological manipulations have been shown to reduce insulin resistance. Some of these genetic manipulations have led to the development of promising drug targeting strategies for treating metabolic syndrome, insulin resistance, or associated symptoms. Treatment strategies for insulin resistance are attempting to mimic a beneficial effect that happens normally in subjects that exercise regularly. Perhaps the major difference between targeted therapies and physical activity is that the health benefits of physical activity involve the interaction of physiological systems. This may, in fact, be what makes physical activity so effective in disease prevention. The beneficial effects of exercise generally occur without complications. Physical activity is an impractical therapy for many because compliance for a variety of reasons can be low. Like feeding behavior, spontaneous physical activity is controlled in large part by neural pathways. An important future research direction is to define the neural pathways that determine activity patterns. The hope is that the knowledge gained by identification of such pathways could be used to make a more physically active population.
GRANTS
The research was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants R01-DK-50277, R01-DK-54902, U24-DK-59637, and P60-DK-20593.
Acknowledgments
I thank the many colleagues that have contributed to the work presented in this paper.
REFERENCES
- 1.Alvarez-Buylla R, Alvarez-Buylla E, Mendoza H, Montero SA, Alvarez-Buylla A. Pituitary and adrenals are required for hyperglycemic reflex initiated by stimulation of CBR with cyanide. Am J Physiol Regul Integr Comp Physiol 272: R392–R399, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez-Buylla R, Alvarez-Buylla ER. Carotid sinus receptors participate in glucose homeostasis. Respir Physiol 72: 347–360, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Buylla R, Alvarez-Buylla ER. Changes in blood glucose concentration in the carotid-body sinus modify brain glucose retention. Brain Res 654: 167–170, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Ayala JE, Bracy DP, Julien BM, Rottman JN, Fueger PT, Wasserman DH. Chronic treatment with sildenafil improves energy balance and insulin action in high fat-fed conscious mice. Diabetes 56: 1025–1033, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Banting FG, Best CH. The internal secretions of the pancreas. J Lab Clin Med 5: 251–266, 1922. [Google Scholar]
- 6.Berger CM, Sharis PJ, Bracy DP, Lacy DB, Wasserman DH. Sensitivity of exercise-induced increase in hepatic glucose production to glucose supply and demand. Am J Physiol Endocrinol Metab 267: E411–E421, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Bonadonna R, Prato SD, Bonora E, Saccomani M, Gulli G, Natali A, Frascerra S, Pecori N, Ferrannini E, Bier D, Cobelli C, Defronzo R. Roles of glucose transport and glucose phosphorylation in muscle insulin resistance of NIDDM. Diabetes 45: 915–925, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Braithwaite SS, Palazuk B, Colca JR, Edwards CW, Hofmann C. Reduced expression of hexokinase II in insulin-resistant diabetes. Diabetes 44: 43–48, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Camacho RC, Donahue EP, James FD, Berglund ED, Wasserman DH. Energy state of the liver during short-term and exhaustive exercise in C57BL/6J mice. Am J Physiol Endocrinol Metab 290: E405–E408, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Camacho RC, Pencek RR, Lacy DB, James FD, Donahue EP, Wasserman DH. Portal venous 5-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside infusion overcomes hyperinsulinemic suppression of endogenous glucose output. Diabetes 54: 373–382, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab 284: E241–E258, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Clerk LH, Vincent MA, Barrett EJ, Lankford MF, Lindner JR. Skeletal muscle capillary responses to insulin are abnormal in late-stage diabetes and are restored by angiotensin-converting enzyme inhibition. Am J Physiol Endocrinol Metab 293: E1804–E1809, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Coggan AR, Raguso CA, Gastaldelli A, Williams BD, Wolfe RR. Regulation of glucose production during exercise at 80% of V̇o2 peak in untrained humans. Am J Physiol Endocrinol Metab 273: E348–E354, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Coker RH, Koyama Y, Denny JC, Camacho RC, Lacy DB, Wasserman DH. Prevention of overt hypoglycemia during exercise. Stimulation of endogenous glucose production independent of hepatic catecholamine action and changes in pancreatic hormone concentration. Diabetes 51: 1310–1318, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Coker RH, Krishna MG, Lacy DB, Allen EJ, Wasserman DH. Sympathetic drive to liver and nonhepatic splanchnic tissue during heavy exercise. J Appl Physiol 82: 1244–1249, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Coker RH, Krishna MG, Lacy DB, Zinker B, Wasserman DH. Sympathetic drive to liver and nonhepatic splanchnic tissue during prolonged exercise is increased in diabetes. Metabolism 46: 1327–1332, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Coker RH, Lacy DB, Williams PE, Wasserman DH. Hepatic α- and β-adrenergic receptors are not essential for the increase in Ra during exercise in diabetes. Am J Physiol Endocrinol Metab 278: E444–E451, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Cushman S, Wardzala L. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. J Biol Chem 255: 4578–4762, 1980. [PubMed] [Google Scholar]
- 19.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation 104: 342–345, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Eggleston EM, Jahn LA, Barrett EJ. Hyperinsulinemia rapidly increases human muscle microvascular perfusion but fails to increase muscle insulin clearance: evidence that a saturable process mediates muscle insulin uptake. Diabetes 56: 2958–2963, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Etgen GJ, Wilson CM, Jensen J, Cushman SW, Ivy JL. Glucose transport and cell surface GLUT-4 protein in skeletal muscle of the obese Zucker rat. Am J Physiol Endocrinol Metab 271: E294–E301, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Exton JH, Park CR. The role of cyclic AMP in the control of liver metabolism. Adv Enzyme Regul 6: 391–407, 1968. [DOI] [PubMed] [Google Scholar]
- 23.Febbraio MA, Hiscock N, Sacchetti M, Fischer CP, Pedersen BK. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes 53: 1643–1648, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Fueger PT, Bracy DP, Malabanan CM, Pencek RR, Granner DK, Wasserman DH. Hexokinase II overexpression improves exercise-stimulated but not insulin-stimulated muscle glucose uptake in high fat fed C57BL/6J. Diabetes 53: 306–314, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Fueger PT, Bracy DP, Malabanan CM, Pencek RR, Wasserman DH. Distributed control of glucose uptake by working muscles of conscious mice: roles of transport and phosphorylation. Am J Physiol Endocrinol Metab 286: E77–E84, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Fueger PT, Heikkinen S, Bracy DP, Malabanan CM, Laakso M, Wasserman DH. Hexokinase II partial knockout impairs exercise-stimulated muscle glucose uptake in oxidative muscles of mice. Am J Physiol Endocrinol Metab 285: E958–E963, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Fueger PT, Hess HS, Bracy DP, Pencek RR, Posey KA, Charron MJ, Wasserman DH. Regulation of insulin-stimulated muscle glucose uptake in the conscious mouse: role of glucose transport is dependent on glucose phosphorylation capacity. Endocrinology 145: 4912–4916, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Fueger PT, Hess HS, Posey KA, Bracy DP, Pencek RR, Charron MJ, Wasserman DH. Control of exercise-stimulated muscle glucose uptake by GLUT4 is dependent on glucose phosphorylation capacity in the conscious mouse. J Biol Chem 279: 50956–50961, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Fueger PT, Lee-Young RS, Shearer J, Bracy DP, Heikkinen S, Laakso M, Rottman JN, Wasserman DH. Phosphorylation barriers to skeletal and cardiac muscle glucose uptakes in high-fat fed mice: studies in mice with a 50% reduction of hexokinase II. Diabetes 56: 2476–2484, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Fueger PT, Li CY, Ayala JE, Shearer J, Bracy DP, Charron MJ, Rottman JN, Wasserman DH. Glucose kinetics and exercise tolerance in mice lacking the GLUT4 glucose transporter. J Physiol 582: 801–812, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fueger PT, Shearer J, Bracy DP, Posey KA, Pencek RR, McGuinness OP, Wasserman DH. Control of muscle glucose uptake: test of the rate-limiting step paradigm in conscious, unrestrained mice. J Physiol 562: 925–935, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galassetti P, Coker RH, Lacy DB, Cherrington AD, Wasserman DH. Prior exercise increases net hepatic glucose uptake during a glucose load. Am J Physiol Endocrinol Metab 276: E1022–E1029, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Galassetti P, Reed E, Messina A, Lacy DB, Wasserman DH. Effect of prior fast duration on the appearance and disposal of an intraduodenal glucose load. Am J Physiol Endocrinol Metab 276: E543–E552, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Goodyear LJ, King PA, Hirshman MF, Thompson CM, Horton ES. Contractile activity increases plasma membrane glucose transporters in absence of insulin. Am J Physiol Endocrinol Metab 258: E667–E672, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Halseth A, Bracy D, Wasserman D. Overexpression of hexokinase II increases insulin- and exercise-stimulated muscle glucose uptake in vivo. Am J Physiol Endocrinol Metab 276: E70–E77, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Halseth AE, Bracy DP, Wasserman DH. Functional limitations to glucose uptake in muscles comprised of different fiber types. Am J Physiol Endocrinol Metab 280: E994–E999, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Halseth AE, Bracy DP, Wasserman DH. Limitations to exercise- and maximal insulin-stimulated muscle glucose uptake in vivo. J Appl Physiol 85: 2305–2313, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Halseth AE, Bracy DP, Wasserman DH. Limitations to muscle glucose uptake due to high fat feeding. Am J Physiol Endocrinol Metab 279: E1064–E1071, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Hamilton KS, Gibbons FK, Bracy DP, Lacy DB, Cherrington AD, Wasserman DH. Effect of prior exercise on the partitioning of an intestinal glucose load between splanchnic bed and skeletal muscle. J Clin Invest 98: 125–135, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han X, Ploug T, Galbo H. Effect of diet on insulin- and contraction-mediated glucose transport and uptake in rat muscle. Am J Physiol Regul Integr Comp Physiol 269: R544–R551, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Hirsch IB, Marker JC, Smith LJ, Spina R, Parvin CA, Holloszy JO, Cryer PE. Insulin and glucagon in the prevention of hypoglycemia during exercise in humans. Am J Physiol Endocrinol Metab 260: E695–E704, 1991. [DOI] [PubMed] [Google Scholar]
- 42.Holmang A, Muller M, Andersson OK, Lonroth P. Minimal influence of blood flow on interstitial glucose and lactate-normal and insulin-resistant muscle. Am J Physiol Endocrinol Metab 274: E446–E452, 1998. [DOI] [PubMed] [Google Scholar]
- 43.Howlett KF, Watt MJ, Hargreaves M, Febbraio MA. Regulation of glucose kinetics during intense exercise in humans: effects of alpha- and beta-adrenergic blockade. Metabolism 52: 1615–1620, 2003. [DOI] [PubMed] [Google Scholar]
- 44.Inyard AC, Clerk LH, Vincent MA, Barrett EJ. Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 56: 2194–2200, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Issekutz B, Vranic M. Significance of glucagon in the control of glucose production during exercise. Am J Physiol Endocrinol Metab 238: E13–E20, 1980. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins AB, Chisholm DJ, Ho KY, Kraegen EW. Exercise induced hepatic glucose output is precisely sensitive to the rate of systemic glucose supply. Metabolism 34: 431–434, 1985. [DOI] [PubMed] [Google Scholar]
- 47.Kacser H, Burns JA. The control of flux. Symp Soc Exp Biol 27: 65–104, 1973. [PubMed] [Google Scholar]
- 48.Kimball CP, Murlin JR. Aqueous extracts of pancreas. III. Some precipitation reactions of insulin. J Biol Chem 58: 337–346, 1923. [Google Scholar]
- 49.Kjaer M, Engfred K, Fernandez A, Secher N, Galbo H. Regulation of hepatic glucose production during exercise in humans: role of sympathoadrenergic activity. Am J Physiol Endocrinol Metab 265: E275–E283, 1993. [DOI] [PubMed] [Google Scholar]
- 50.Koyama Y, Coker RH, Denny JC, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Role of carotid bodies in the neuroendocrine response to exercise. Am J Physiol Endocrinol Metab 281: E742–E748, 2001. [DOI] [PubMed] [Google Scholar]
- 51.Koyama Y, Coker RH, Stone EE, Lacy DB, Jabbour K, Williams PE, Wasserman DH. Evidence that carotid bodies play an important role in glucoregulation in vivo. Diabetes 49: 1434–1442, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Kramer HF, Taylor EB, Witczak CA, Fujii N, Hirshman MF, Goodyear LJ. Calmodulin-binding domain of AS160 regulates contraction- but not insulin-stimulated glucose uptake in skeletal muscle. Diabetes 56: 2854–2862, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Kraniou GN, Cameron-Smith D, Hargreaves M. Acute exercise and GLUT4 expression in human skeletal muscle: influence of exercise intensity. J Appl Physiol 101: 934–937, 2006. [DOI] [PubMed] [Google Scholar]
- 54.Lavoie C, Ducros F, Bourque J, Langelier H, Chiasson JL. Glucose metabolism during exercise in man: the role of insulin and glucagon in the regulation of hepatic glucose production and gluconeogenesis. Can J Physiol Pharmacol 75: 26–35, 1997. [DOI] [PubMed] [Google Scholar]
- 55.Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA. Neuronal glucosensing: what do we know after 50 years? Diabetes 53: 2521–2528, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Lins PE, Wajngot A, Adamson U, Vranic M, Efendic S. Minimal increases in glucagon levels enhance glucose production in man with partial hypoinsulinemia. Diabetes 32: 633–636, 1983. [DOI] [PubMed] [Google Scholar]
- 57.Liu S, Baracos VE, Quinney HA, Bricon TL, Clandinin MT. Parallel IGF-1 and insulin resistance in muscles of rats fed a high fat diet. Endocrinology 136: 3318–3324, 1995. [DOI] [PubMed] [Google Scholar]
- 58.MacLean DA, Bangsbo J, Saltin B. Muscle interstitial glucose and lactate levels during dynamic exercise in humans determined by microdialysis. J Appl Physiol 87: 1483–1490, 1999. [DOI] [PubMed] [Google Scholar]
- 59.Marliss EB, Purdon C, Halter JB, Sigal RJ, Vranic M. Glucoregulation During and After Intense Exercise in Control and Diabetic Subjects. London: Smith-Gordon, 1992.
- 60.Moates JM, Lacy DB, Cherrington AD, Goldstein RE, Wasserman DH. The metabolic role of the exercise-induced increment in epinephrine. Am J Physiol Endocrinol Metab 255: E428–E436, 1988. [DOI] [PubMed] [Google Scholar]
- 61.Morgan HE, Regen DM, Park CR. Identification of a mobile carrier-mediated sugar transport system in muscle. J Biol Chem 239: 369–374, 1964. [PubMed] [Google Scholar]
- 62.Murlin JR, Clough HD, Gibbs CBF, Stokes AM. Aqueous extracts of pancreas. I. Influence on the carbohydrate metabolism of depancreatized animals. J Biol Chem 56: 253–296, 1923. [Google Scholar]
- 63.O'Doherty RM, Bracy DP, Granner DK, Wasserman DH. Hexokinase II gene transcription is increased by acute exercise in rat skeletal muscle. J Appl Physiol 81: 789–793, 1996. [DOI] [PubMed] [Google Scholar]
- 64.O'Doherty RM, Bracy DP, Osawa H, Wasserman DH, Granner DK. Rat skeletal muscle hexokinase II mRNA and activity are increased by a single bout of acute exercise. Am J Physiol Endocrinol Metab 266: E171–E178, 1994. [DOI] [PubMed] [Google Scholar]
- 65.O'Doherty RM, Halseth AE, Granner DK, Bracy DP, Wasserman DH. Analysis of insulin-stimulated skeletal muscle glucose uptake in the conscious rat using isotopic glucose analogs. Am J Physiol Endocrinol Metab 274: E287–E296, 1998. [DOI] [PubMed] [Google Scholar]
- 66.Obeso A, Rocher A, Herreros B, Gonzalez C. The Carotid Body Chemoreceptors. Austin, TX: Landes Bioscience, 1997.
- 67.Park CR, Johnson LH. Effect of insulin on transport of glucose and galactose into cells of rat muscle and brain. Am J Physiol 182: 17–23, 1955. [DOI] [PubMed] [Google Scholar]
- 68.Pencek RR, James FD, Lacy DB, Camacho RC, Fueger PT, Wasserman DH. Exercise-induced changes in insulin and glucagon are not required for enhanced glucose uptake by the liver following exercise but influence the fate of glucose. Diabetes 53: 3041–3047, 2004. [DOI] [PubMed] [Google Scholar]
- 69.Pencek RR, James FD, Lacy DB, Jabbour K, Williams PE, Fueger PT, Wasserman DH. Interaction of insulin and prior exercise in control of hepatic metabolism of a glucose load. Diabetes 52: 1897–1903, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Pencek RR, Koyama Y, Lacy DB, James FD, Fueger PT, Jabbour K, Williams PE, Wasserman DH. Prior exercise enhances passive absorption of intraduodenal glucose. J Appl Physiol 95: 1132–1138, 2003. [DOI] [PubMed] [Google Scholar]
- 71.Pendergrass M, Koval J, Vogt C, Yki-Jarvinen H, Iozzo P, Pipek R, Ardehali H, Printz R, Granner DK, DeFronzo RA, Mandarino LJ. Insulin-induced hexokinase II expression is reduced in obesity and NIDDM. Diabetes 47: 387–394, 1998. [DOI] [PubMed] [Google Scholar]
- 72.Petersen HA, Fueger PT, Bracy DP, Wasserman DH, Halseth AE. Fiber type-specific determinants of Vmax for insulin-stimulated muscle glucose uptake in vivo. Am J Physiol Endocrinol Metab 284: E541–E548, 2003. [DOI] [PubMed] [Google Scholar]
- 73.Pilegaard H, Osada T, Andersen LT, Helge JW, Saltin B, Neufer PD. Substrate availability and transcriptional regulation of metabolic genes in human skeletal muscle during recovery from exercise. Metabolism 54: 1048–1055, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Ploug T, Galbo H, Ohkuwa T, Tranum-Jensen J, Vinten J. Kinetics of glucose transport in rat skeletal muscle membrane vesicles: effects of insulin and contractions. Am J Physiol Endocrinol Metab 262: E700–E711, 1992. [DOI] [PubMed] [Google Scholar]
- 75.Ploug T, Galbo H, Richter EA. Increased muscle glucose uptake during contractions: no need for insulin. Am J Physiol Endocrinol Metab 247: E726–E731, 1984. [DOI] [PubMed] [Google Scholar]
- 76.Ren JM, Semenkovich CF, Gulve EA, Gao JG, Holloszy JO. Exercise induces rapid increases in GLUT4 expression, glucose transport capacity, and insulin-stimulated glycogen storage in muscle. J Biol Chem 269: 14396–14401, 1994. [PubMed] [Google Scholar]
- 77.Richter EA, Ploug T, Galbo H. Increased muscle glucose uptake after exercise. No need for insulin during exercise. Diabetes 34: 1041–1048, 1985. [DOI] [PubMed] [Google Scholar]
- 78.Robison GA, Butcher RW, Sutherland EW. Cyclic AMP. Annu Rev Biochem 37: 149–154, 1968. [DOI] [PubMed] [Google Scholar]
- 79.Rosdahl H, Lind L, Millgard J, Lithell H, Ungerstedt U, Henriksson J. Effect of physiological hyperinsulinemia on blood flow and interstitial glucose concentration in human skeletal muscle and adipose tissue studied by microdialysis. Diabetes 47: 1296–1301, 1998. [DOI] [PubMed] [Google Scholar]
- 80.Sakamoto K, Goodyear LJ. Intracellular signaling in contracting skeletal muscle. J Appl Physiol 93: 369–383, 2002. [DOI] [PubMed] [Google Scholar]
- 81.Sigal RJ, Fisher SF, Halter JB, Vranic M, Marliss EB. The roles of catecholamines in glucoregulation in intense exercise as defined by the islet cell clamp technique. Diabetes 45: 148–156, 1995. [DOI] [PubMed] [Google Scholar]
- 82.Sigal RJ, Fisher SJ, Manzon A, Morais JA, Halter JB, Vranic M, Marliss EB. Glucoregulation during and after intense exercise: effects of alpha-adrenergic blockade. Metabolism 49: 386–394, 2000. [DOI] [PubMed] [Google Scholar]
- 83.Sigal RJ, Purdon C, Bilinski D, Vranic M, Halter JB, Marliss EB. Glucoregulation during and after intense exercise: effects of beta-blockade. J Clin Endocrinol Metab 78: 359–366, 1994. [DOI] [PubMed] [Google Scholar]
- 84.Stevenson RW, Steiner KE, Davis MA, Williams PE, Lacy WW, Brown L, Donahue P, Lacy DB, Cherrington AD. Similar dose responsiveness of hepatic glycogenolysis and gluconeogenesis to glucagon in vivo. Diabetes 36: 382–389, 1987. [DOI] [PubMed] [Google Scholar]
- 85.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 37: 1163–1167, 1988. [DOI] [PubMed] [Google Scholar]
- 86.Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA 77: 2542–2545, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vincent MA, Dawson D, Clark AD, Lindner JR, Rattigan S, Clark MG, Barrett EJ. Skeletal muscle microvascular recruitment by physiological hyperinsulinemia precedes increases in total blood flow. Diabetes 51: 42–48, 2002. [DOI] [PubMed] [Google Scholar]
- 88.Wallberg-Henriksson H, Holloszy JO. Activation of glucose transport in diabetic muscle: responses to contraction and insulin. Am J Physiol Cell Physiol 249: C233–C237, 1985. [DOI] [PubMed] [Google Scholar]
- 89.Wallis MG, Wheatley CM, Rattigan S, Barrett EJ, Clark ADH, Clark MG. Insulin-mediated hemodynamic changes are impaired in muscle of Zucker obese rats. Diabetes 51: 3492–3498, 2002. [DOI] [PubMed] [Google Scholar]
- 90.Wasserman DH Control of glucose fluxes during exercise in the postabsorptive state. In: Annual Review Physiology. Palo Alto, CA: Annual Reviews, 1995, p. 191–218. [DOI] [PubMed]
- 91.Wasserman DH, Geer RJ, Rice DE, Bracy D, Flakoll PJ, Brown LL, Hill JO, Abumrad NN. Interaction of exercise and insulin action in man. Am J Physiol Endocrinol Metab 260: E37–E45, 1991. [DOI] [PubMed] [Google Scholar]
- 92.Wasserman DH, Halseth AE. An overview of muscle glucose uptake during exercise: sites of regulation. In: Advances in Experimental Medicine and Biology, edited by Richter EA, Kiens B, Galbo H, Saltin B. New York: Plenum, 1998, p. 1–16. [DOI] [PubMed]
- 93.Wasserman DH, Lacy DB, Bracy D, Williams PE. Metabolic regulation in peripheral tissues and transition to increased gluconeogenic mode during prolonged exercise. Am J Physiol Endocrinol Metab 263: E345–E354, 1992. [DOI] [PubMed] [Google Scholar]
- 94.Wasserman DH, Lacy DB, Bracy DP. Relationship between arterial and portal vein immunoreactive glucagon during exercise. J Appl Physiol 75: 724–729, 1993. [DOI] [PubMed] [Google Scholar]
- 95.Wasserman DH, Lacy DB, Colburn CA, Bracy DP, Cherrington AD. Efficiency of compensation for the absence of the fall in insulin during exercise. Am J Physiol Endocrinol Metab 261: E587–E597, 1991. [DOI] [PubMed] [Google Scholar]
- 96.Wasserman DH, Lacy DB, Goldstein RE, Williams PE, Cherrington AD. Exercise-induced fall in insulin and hepatic carbohydrate metabolism during exercise. Am J Physiol Endocrinol Metab 256: E500–E508, 1989. [DOI] [PubMed] [Google Scholar]
- 97.Wasserman DH, Lickley HLA, Vranic M. Interactions between glucagon and other counterregulatory hormones during normoglycemic and hypoglycemic exercise in dogs. J Clin Invest 74: 1404–1413, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wasserman DH, Mohr T, Kelly P, Lacy DB, Bracy D. The impact of insulin-deficiency on glucose fluxes and muscle glucose metabolism during exercise. Diabetes 41: 1229–1238, 1992. [DOI] [PubMed] [Google Scholar]
- 99.Wasserman DH, Spalding JS, Lacy DB, Colburn CA, Goldstein RE, Cherrington AD. Glucagon is a primary controller of the increments in hepatic glycogenolysis and gluconeogenesis during exercise. Am J Physiol Endocrinol Metab 257: E108–E117, 1989. [DOI] [PubMed] [Google Scholar]
- 100.Wasserman DH, Williams PE, Lacy DB, Bracy D, Cherrington AD. Hepatic nerves are not essential to the increase in hepatic glucose production during muscular work. Am J Physiol Endocrinol Metab 259: E195–E203, 1990. [DOI] [PubMed] [Google Scholar]
- 101.Wasserman K, VanKessel AL, Burton GG. Interaction of physiological mechanisms during exercise. J Appl Physiol 22: 71–85, 1967. [DOI] [PubMed] [Google Scholar]
- 102.Williams KV, Price JC, Kelley DE. Interactions of impaired glucose transport and phosphorylation in skeletal muscle insulin resistance: a dose-response assessment using positron emission tomography. Diabetes 50: 2069–2079, 2001. [DOI] [PubMed] [Google Scholar]
- 103.Wolfe RR, Nadel ER, Shaw JHF, Stephenson LA, Wolfe M. Role of changes in insulin and glucagon in glucose homeostasis in exercise. J Clin Invest 77: 900–907, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zierath JR, Houseknecht KL, Gnudi L, Kahn BB. High-fat feeding impairs insulin-stimulated GLUT4 recruitment via an early insulin-signaling defect. Diabetes 46: 215–223, 1997. [DOI] [PubMed] [Google Scholar]
- 105.Zinker BA, Bracy D, Lacy DB, Jacobs J, Wasserman DH. Regulation of glucose uptake and metabolism during exercise: an in vivo analysis. Diabetes 42: 956–965, 1993. [DOI] [PubMed] [Google Scholar]