Abstract
A decline in the bioavailability of nitric oxide (NO) that causes endothelial dysfunction is a hallmark of diabetes. The availability of NO to the vasculature is regulated by endothelial nitric oxide synthase (eNOS) activity and the involvement of heat shock protein-90 (Hsp-90) in the regulation of eNOS activity has been demonstrated. Hsp-90 has been shown to interact with upstream kinases [inhibitor κB kinases (IKK)α, β, and γ] in nonvascular cells. In this study, we have investigated the interaction of Hsp-90-IKKβ in endothelial cells under conditions of high glucose (HG) as a possible mechanism that diminishes Hsp-90-eNOS interaction, which could contribute to reduced bioavailability of NO. We report for the first time that IKKβ interacts with Hsp-90, and this interaction is augmented by HG in vascular endothelial cells. HG also augments transcriptional (3.5 ± 0.65-fold) and translational (1.97 ± 0.17-fold) expression as well as the catalytic activity of IKKβ (2.45 ± 0.4-fold). Both IKKβ and eNOS could be coimmunoprecipitated with Hsp-90. Inhibition of Hsp-90 with geldanamycin (2 μM) or Radicicol (20 μM) mitigated (0.45 ± 0.04-fold and 0.93 ± 0.16-fold, respectively) HG induced-IKKβ activity (2.5 ± 0.42-fold). Blocking of IKKβ expression by IKK inhibitor II (15 μM wedelolactone) or small interferring RNA (siRNA) improved Hsp-90-eNOS interaction and NO production under conditions of HG. These results illuminate a possible mechanism for the declining eNOS activity reported under conditions of HG.
Keywords: diabetes, endothelial nitric oxide synthase
accelerated atherosclerosis, one of the major vascular complications of diabetes, is attributed to hyperglycemia mediated-endothelial dysfunction (6). Endothelial dysfunction is characterized by the loss of nitric oxide (NO) bioactivity in the vessel wall with concomitant increase in superoxide release, which impairs vasodilatation, inhibits protection against leukocyte-endothelial interactions, platelet aggregation, and adhesion and smooth muscle cell proliferation (3, 17, 31). The optimal generation of NO from endothelial nitric oxide synthase (eNOS) activity is dependent on several factors, including availability of the substrate l-arginine (9) and the cofactor tetrahydrobiopterin (BH4) (1). The activation of eNOS is a complex process and may be regulated by 1) both transcriptional and posttranslational mechanisms, 2) site-specific phosphorylation of eNOS, 3) protein-protein interactions, 4) prosthetic groups, and 5) calcium and calmodulin (8).
eNOS has been shown to interact with several regulatory proteins such as heat shock protein-90 (Hsp-90), caveolin-1, G protein-coupled receptors, NO-interacting protein (NOSIP), Dynamin-2, and Porin (8). In endothelial cells, both BH4 and Hsp-90 have been shown to be important effectors in regulating eNOS activity. Hsp-90 is known to regulate calcium-dependent dissociation of eNOS from caveolin-1, enzyme activation, maturation, and trafficking, followed by the Akt-dependent activation and phosphorylation of serine 1177 (human eNOS) or serine 1179 (bovine eNOS) (8). Phosphorylation of eNOS is a key posttranslational modification that was believed to be a key determinant of eNOS activity associated with NO generation. The binding of Hsp-90 to eNOS ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of eNOS (18). Failure of this binding has been demonstrated to cause eNOS uncoupling and increased eNOS-dependent superoxide anion production (20, 28, 30).
A recent study in the diabetes literature has demonstrated that chronic exposure of endothelial cells to high glucose (HG) conditions downregulate both protein interaction between eNOS and Hsp-90 and the recruitment of activated Akt. The end result is the deactivation of eNOS and imbalance in NO versus reactive oxygen species (ROS) levels (18). Evidence of metformin-stimulated NOS in vivo (5) by promoting the association of Hsp-90 further supports the results of an earlier study (18) and confirms the concept that elevated glucose downregulates the interaction of eNOS and Hsp-90.
Parallel studies investigating NO production in endothelial cells under conditions of HG suggested that HG-mediated endothelial dysfunction involves activation of IKKβ, which inhibits insulin receptor substrate (IRS-1)/phosphatidylinositol 3-kinase (PI3 kinase) signaling and therefore attenuates NO production (14). This has been implicated in insulin resistance, a common complication of diabetes.
Requirement of Hsp-90 for the activation of IKK complex consisting of IKKα, -β, and -γ have been recently demonstrated in HeLa cells, COS cells, human colonic epithelial cells, and primary effusion lymphoma cell line (4, 2, 7, 24, 25). If such requirement is also experienced by endothelial cells, Hsp-90 will be required by both eNOS and IKKβ for their activity. Therefore, under conditions of HG, it is possible that there is increased IKKβ-Hsp-90 interaction in vascular cells due to HG-induced IKKβ, which may downregulate eNOS-Hsp-90 interaction. This could uncouple eNOS activity that results in reduced NO generation thus causing endothelial dysfunction.
In this study, we investigated the influence of HG on IKKβ-Hsp-90 interaction. Our results demonstrate that HG upregulates both the expression and activity of IKKβ and also favors the interaction of IKKβ-Hsp-90. Since HG favors the interaction of IKKβ with Hsp-90, this could compete with Hsp-90-eNOS interaction resulting in associated decrease in NO generation. Therefore, increased IKKβ-Hsp-90 interaction induced by HG could be an important mechanism by means of which HG causes endothelial dysfunction.
MATERIALS AND METHODS
Cell culture.
Bovine aortic endothelial cells (BAECs, Clonetics, San Diego, CA) were cultured in MCDB-131 medium (Sigma, St. Louis, MO) containing 10% bovine calf serum (BCS, Hyclone, Kansas City, KS) and enriched with 250 ng/ml fibroblast growth factor (PeproTech, Rocky Hill, NJ), 20 ng/ml of epidermal growth factor (PeproTech), 1 μg/ml of hydrocortisone (Sigma), 100 U/ml of penicillin, and 100 mg/ml streptomycin (Media-tech, Herndon, VA) (12, 21). Cells from passages 4 to 7 were used in all the experiments.
Treatment conditions.
BAECs maintained in MCDB-131 medium supplemented with 10% BCS were incubated in medium containing reduced serum (2%) for 20 h before treatments. MCDB-131 medium contains 5.5 mM glucose (that is normally present in the basal medium) and used as a normal glucose control. Cells were incubated with additional 5 or 25 mM of d(+)-glucose for different time intervals in time-course experiments. Additionally, as an osmotic control, cells were incubated with 25 mM mannitol. In the time-course experiments for evaluating IKKβ expression, cells incubated with 25 mM l-glucose were also included as controls. Cell viability determined by standard trypan blue dye exclusion method at these concentrations of glucose and mannitol after 24 or 48 h was more than 98% compared with untreated control.
RNA extraction and RT-PCR.
Alterations in the expression of IKKβ due to HG incubation were examined by RT-PCR. Total RNA was isolated from BAECs using TRIzol reagent (GIBCO-BRL) and treated with DNase I (1 U/μg) to remove any contaminating genomic DNA. First-strand cDNA was reverse transcribed from 2 μg of total RNA using Murine Moloney Leukemia Virus Reverse transcriptase, RNAsin, olig-dT, and dNTP mix according to manufacturer's protocol (Invitrogen, Carlsbad, CA). Two microliters of RT reaction mixture were subjected to PCR using IKKβ-specific primers (10 μM each) and Taq polymerase in a final volume of 50 μl. Primers for GAPDH were used to ascertain that an equivalent amount of cDNA was synthesized. PCR amplification was performed over a range of cycle numbers to ensure that amplification was in the linear range of the curve. PCR products were analyzed in 12% polyacrylamide gels, stained with ethidium bromide, and photographed under UV illuminator. The density of each band was measured using ImageJ software (Rasband, W. S., ImageJ, National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997-2007). The densities of IKKβ mRNA bands were normalized relative to those of GAPDH mRNA bands. Data are expressed as relative mRNA expression where the ratio of the IKKβ band density in control cultures to the corresponding GAPDH signal was arbitrarily set at 1.
Immunoprecipitation and immunoblotting.
Lysates from BAECs treated either with 25 mM d-glucose, 25 mM mannitol, or regular medium were first precleared by incubating with protein A or protein G coupled Sepharose 4B beads for ∼6 h at 4°C. The cleared lysates were transferred into a fresh tube and incubated with 1–3 μg of immunoprecipitating primary antibody, [Rabbit (Rb)] anti-human Hsp-90, Rb anti-human eNOS, Rb anti-human IKKβ, or preimmune IgG (Millipore, Billerica, MA) followed by protein-A or protein-G coupled Sepharose 4B beads. The immune complex was separated by electrophoresis on a 10% SDS-polyacrylamide gel and then transferred to Hybond polyvinyldifluoride membrane (Amersham, Piscataway, NJ) for immunoblot analysis. Blots were blocked for 45 min using 3% low-fat milk solution (PBS containing 3% low-fat milk and 0.1% Tween-20) and further incubated overnight at 4°C in blocking solution containing primary antibody. Primary antibodies used were Rb anti-human eNOS (1:1,000 dilution), Rb anti-human IKKβ (1:1,000 dilution), and Rb anti-human Hsp-90 (1:1,000). Bound antibodies were detected with peroxidase-conjugated secondary antibodies (1:10,000) and visualized using enhanced chemiluminescence Western blotting detection reagent (Amersham, Arlington Heights, IL). Expression levels were quantified by densitometric scanning and using ImageJ software. α-Actin expression was used as an internal loading control in the immunoblots. In immunoprecipitation experiments, the efficiency of each immunoprecipitation was normalized by probing the membrane with the antibody to detect immunoprecipitating antigen. Loading differences were also normalized by heavy chain IgG expression.
Preparation of plasmid constructs.
The entire coding sequence of mammalian Hsp-90 was cleaved out from the pGEX vector (obtained from Dr. Richard Venema, Medical College of Georgia, Augusta, GA) with SmaI and NotI restriction endonucleases, and subsequently the NotI end was filled with the Klenow fragment of the DNA polymerase I. Hsp-90 coding sequence was then blunt-end ligated into SmaI site of pDS Red-C1 vector (Clontech, Mountain View, CA) to create a hybrid with NH2-terminal red fluorescent protein.
To subclone the mammalian IKKβ coding sequence from the pCR3-FLAG vector (obtained from Dr. Hiroyasu Nakano, Tokyo, Japan), the insert containing the complete ORF was excised using EcoRI and ApaI restriction enzymes. It was subsequently ligated into the pEYFP-C1 plasmid (Clontech) expressing yellow fluorescent protein. To keep the proper reading frame, it was reconstructed by changing the position of an EcoRI site using a synthetic linker/adapter. The pCR3-FL-Hsp-90 fusion plasmid was created by subcloning the Hsp-90 reading frame from pDSRed-Hsp-90 construct into the pCR3-FL2 vector bearing two repeats of the FLAG tag sequence upstream of the cloning site.
To generate a plasmid coding the Hsp-90 associated with the enhanced cyan fluorescent protein (ECFP), the fragment coding for ECFP was excised from pECFP-C1 plasmid (Clontech) with NheI and SacII restriction endonucleases, and ECFP fragment was used to replace the DsRed sequence upstream of the previously cloned Hsp-90 in pDsRed-C1 vector. For the removal of DsRed sequence, the same pair of restriction enzymes (NheI and SacII) was used. All the above constructs retained intact reading frames as confirmed individually by DNA sequencing.
FLAG-Hsp-90 pulldown and immunoblotting.
BAECs were transiently cotransfected with pCR3-FL-Hsp90 and pEY2-IKKβ plasmids. As controls, untransfected BAECs or cells transfected with pDS-Red-Hsp90 or pEY2-IKKβ construct alone were used. After 24 h of transfection, cells were incubated with high glucose and harvested for immunoprecipitation. To establish the specificity of Hsp-90 interaction with IKKβ, transfected cells were also incubated with 20 μM of radicicol. Equal amount of protein (∼100 μg) from cell lysates were immunoprecipited with EZview Red anti-Flag M2 affinity gel (Sigma), and the immune complex was analyzed for the presence of IKKβ using anti-GFP antibody (Molecular Probes, Eugene, OR).
In vitro kinase assays.
Kinase assays were performed as described in our previous study (21). In brief, cells induced by HG or mannitol were washed with ice-cold PBS and lysed on ice in 1 ml of lysis buffer containing 1% Nonidet P-40, 50 mM HEPES (pH 7.3), 150 mM NaCl, 2 mM EDTA, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 1 mM phenylmethylsulfonyl fluoride, 0.1 mM sodium orthovanadate, and 1 mM sodium fluoride. The cell lysates were precleared by incubating for 1 h at 4°C with protein A-agarose 4B (BD Pharmingen, San Diego, CA). After centrifugation, the cleared lysates were transferred into a fresh tube and incubated with anti-Flag mAb (1 μg/ml) tagged protein-A-agarose for 2 h at 4°C. The immunoprecipitates were then washed three times with the lysis buffer and twice in kinase buffer containing 20 mM HEPES (pH 7.3), 20 mM MgCl2, 20 mM MnCl2, 1 mM EDTA, 1 mM NaF, 0.1 mM sodium orthovanadate, and 1 mM DTT. The immunoprecipitates were then incubated with 1 μg of GST-1κBα (1–100 amino acids) and [γ-32P]ATP (10 μCi) in the kinase buffer for 20 min at 30°C. The reaction was stopped by the addition of the 1× Laemmli's sample buffer. The eluted proteins were subjected to SDS-PAGE, and the dried gels were exposed to X-ray film. The intensity of the bands was measured using ImageJ software. Equal loading of GST-IκBs in the reaction mixtures were also verified by Rapid Staining (G-Biosciences, St. Louis, MO) of the gel before drying to confirm equal loading.
Inhibition of IKKβ activity by siRNA transfection.
siGenome Smartpool siRNA reagents specific for IKKβ were purchased from Dharmacon (Lafayette, CO). Cells transfected with nonspecific control siRNA and untransfected cells were used as controls and to determine the efficiency of transfection. Transfections were performed as described earlier (21) using Hiperfect transfection reagent (Quiagen, Valencia, CA). To determine the efficiency of siRNA in blocking IKK activity, transfected cells were harvested after 48–72 h to measure protein levels of IKKβ expression by immunoblotting. Furthermore, cells transfected with siRNA for IKKβ were subjected to HG incubation for 24 h and harvested for immunoprecipitation analysis or NO determination.
Intracellular NO measurements using DAF-FM diacetate after blocking IKKβ expression.
BAECs were seeded onto two-well chamber slides (Nalge Nunc International, Naperville, IL). Once the cells were 80% confluent, cells were transfected with siRNA for IKKβ. Transfected cells were incubated with 25 mM glucose for additional 24–48 h. Cells incubated with HG were also treated with 15 μM wedeloactone for 6 h before staining. To measure NO production, cells were washed with HBSS twice followed by Krebs-Ringer Phosphate (KRP) buffer containing 100 μM l-arginine. 4-Amino-5-methylamino-2,7′-difluorofluorescein diacetate (DAF-FM, Molecular Probes, Eugene, OR) at a final concentration of 5 μM (1:1,000 dilutions from a 5 mM stock prepared in DMSO) was added, and the cells were incubated at 37°C for 20 min. DAF-FM fluorescence is very weak. Acetate hydrolysis and subsequent reaction with nitrite or NO generates a highly fluorescent benzotriazole derivative in living cells with absorbance = 495 nm and emission = 515 nm in pH 7.4 buffer. DAF-FM is a more sensitive reagent for NO measurement than is DAF-2, which was originally used (16, 22, 26) and the NO and NO2− detection limit for DAF-FM is ∼3 nM vs. ∼5 nM for DAF-2. After DAF-FM loading was completed, all procedures were performed under yellow lights. At the completion of incubation, cells were washed and imaged using the Zeiss LSM 510 inverted confocal microscope (Carl Zeiss, Thornwood, NY). The temperature of the stage was maintained at 37°C with a microwarm plate. Cells were viewed using excitation and emission wavelengths of 490 and 515 nm, respectively.
Generation of constructs for mammalian two-hybrid analysis.
To investigate the interactions of the IKKβ with Hsp-90, two sets of constructs were prepared using pCMV-AD and pCMV-BD vector plasmids provided by the Mammalian Two-Hybrid Assay Kit (Stratagene, La Jolla, CA). We preferred mammalian two-hybrid system over yeast-two-hybrid system for this study because mammalian two-hybrid assay system enables us to study interactions between mammalian proteins that may fold incorrectly or require posttranslational modification when used in yeast system. In addition, selective inducers that are pertinent to mammalian cells cannot be used in yeast system. Mammalian protein interactions frequently depend on additional microenvironmental factors that are present within the mammalian but not in yeast cell.
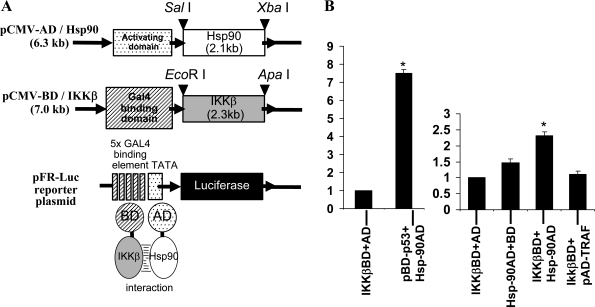
The full-length Hsp-90 coding sequence was excised from the pGEX-Hsp90 construct with SalI and XbaI restriction endonucleases and inserted downstream of the pCMV-AD cloning vector using the same set of restriction enzymes to prepare the target plasmid before ligation. To subclone the IKKβ coding sequence from the pCR2-flag vector, the insert containing the complete ORF was excised using EcoRI and ApaI restriction enzymes and subsequently ligated into pCMV-BD plasmid downstream of the Gal4 binding domain (see Fig. 7A).
Fig. 7.
A: illustration showing the construction of Hsp-90-IKKβ mammalian two hybrid system. B: interaction of Hsp-90 and IKKβ examined by mammalian two-hybrid system. The cDNA fragments encoding Hsp-90 or IKKβ were cloned into pCMV-AD cloning vector and pCMV-BD plasmid downstream of the Gal4 binding domain. Control plasmids pBD-p53 and pAD-TRAF were also transfected in pairs as shown in the histogram. These plasmids along with pFR-Luc reporter plasmid were cotransfected into BAECs. At 24 h posttransfection, cells were lysed and luciferase activity was measured. Histogram shows means ± SD of three independent experiments. *Statistical significance (P < 0.05).
All constructs were verified by DNA sequencing for intact reading frame. BAECs grown in six-well plates were transfected with plasmids containing pCMV-AD-Hsp-90, pCMV-BD-IKKβ, and pFR-Luc using Effectene reagent (Qiagen) as described in our previous study (21). Control plasmids pBD-p53 and pAD SV40T (large T-antigen) provided in the kit were cotransfected as positive control pairs. Also, pAD-Hsp-90/pBD-p53 and pBD-IKKβ/pADTRAF were cotransfected with pFR-Luc plasmid as controls. Luciferase assays were performed in 24 h posttransfected cells as per manufacturer's instructions (Promega, Madison, WI).
Fluorescence resonance energy transfer analysis by acceptor photobleaching method.
BAECs grown on coverslips were transfected with pEYFP-IKKβ and pECFP-Hsp-90 constructs using Effectene reagent (Qiagen). Transfected cells were either incubated with normal growth medium or HG medium containing 25 mM for 24 h. Cells were fixed and imaged using the Zeiss LSM 510 confocal microscope (Carl Zeiss). Images were acquired using the 63× 1.40 NA Plan-ApoCHROMAT lens (Carl Zeiss). The CFP (donor) and the YFP (acceptor) signals were imaged sequentially using detector channels optimized for each fluorophore. CFP was excited using the 458-nm line of an Argon laser, and the emission was collected through an HQ 487/37 nm band-pass filter. YFP was excited by the 514-nm line of an argon laser, and the emission was collected through an HQ 550/50 nm bandpass filter (Chroma Technology). Images of each channel were acquired before and after photobleaching the YFP. Photobleaching was performed by repeatedly scanning a defined region of the cell to achieve at least 85% reduction in the original fluorescence intensity. The reduction in intensity was assessed by comparing the YFP signal before and after photobleaching. The percent fluorescence resonance energy transfer (FRET) efficiency was calculated by %ET = 1 − (IDA/ID) × 100, where IDA is the intensity of the CFP (donor) signal in the presence of the acceptor (i.e., before photobleaching) and ID is the intensity of the CFP (donor) signal in the absence of the acceptor (i.e., after photobleaching). A total of 20 cells were imaged for the control and 20 cells for the HG exposed groups. Measurements of FRET efficiency were determined from at least 20 different regions in each cell.
Statistical analysis.
Each experiment was repeated at least three times independently. All results were expressed as means ± SD and P < 0.05 was used for significance. Student's t-test was used to determine statistical significance.
RESULTS
HG induces both transcriptional and translational expression and catalytic activity of IKKβ.
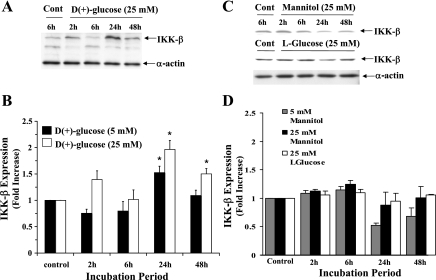
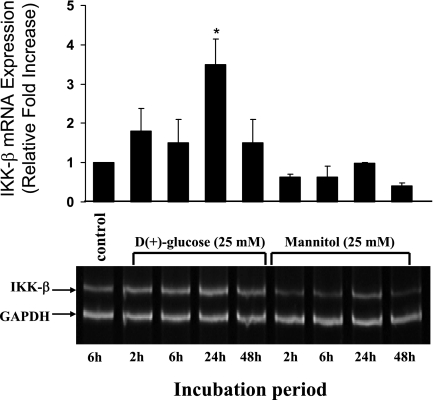
We have previously reported HG induced dose-dependent increase of NF-κB DNA binding activity showing a maximum level of induction at 30 mM glucose concentration (12). Furthermore, in this study we have examined whether HG could induce IKKβ, an upstream mediator involved in the canonical NF-κB signaling pathway. BAECs incubated with 5 mM glucose for 2, 6, 24, and 48 h revealed an almost basal level of IKKβ protein expression at 2 and 6 h; whereas the expression of IKKβ increased at 24 h (1.53 ± 0.12-fold) (Fig. 1B) and reduced again to constitutive level at 48 h. Unlike 5 mM, the 25 mM concentration of glucose rapidly induced IKKβ activity. The activity was seen as soon as 2 h (1.4 ± 0.16-fold) compared with control cells incubated with regular growth medium and reduced to basal level at 6 h. The expression of IKKβ increased again and reached a maximum level at 24 h (2.0 ± 0.17-fold) and then reduced at 48 h (1.5 ± 0.1-fold) close to the levels seen at 2-h posttreatment (Fig. 1B). Therefore, in both 5 and 25 mM glucose, the maximum induction was observed at 24 h (P < 0.05). This increased IKKβ protein expression correlated with the HG-induced increased transcriptional activity of IKKβ. A 3.5 ± 0.65-fold increase in mRNA expression was observed when cells were incubated with 25 mM glucose for 24 h (P < 0.05, Fig. 2). In contrast, cells incubated with mannitol (25 mM) for the same time points did not induce IKKβ protein (Fig. 1, C and D) or the mRNA expression (Fig. 2). In addition to mannitol control, l-glucose (25 mM) was also used as a control in IKKβ protein expression studies because mannitol also acts as a free radical scavenger. Similar to mannitol, l-glucose also did not induce IKKβ protein expression (Fig. 1, C and D). These results indicate that the induction of IKKβ expression after HG treatment at both transcriptional and translational level is not due to osmotic shock.
Fig. 1.
A: representative immunoblot showing time-dependent IKKβ protein expression in bovine aortic endothelial endotheials cells (BAECs) incubated with high glucose. Equal amount of protein from cells harvested at indicated time points were immunoblotted with inhibitor κB kinase (IKKβ) antibody or with α-actin to validate equal protein loading. B: histogram showing the levels of expression of IKKβ in cells treated with 5 or 25 mM glucose added in excess of basal amount. The expression level was determined from the ratio of IKKβ to α-actin. The fold increase was calculated considering control cells treated with complete growth medium as onefold. C and D: representative immunoblot and the histogram showing the quantitative results of IKKβ expression in BAEC after incubation with 5 or 25 mM mannitol or 25 mM l-glucose. Data are means ± SD of three independent experiments. *Statistical significance (P < 0.05).
Fig. 2.
High glucose increases IKKβ mRNA expression. RT-PCR analysis showing a time-dependent increase of IKKβ mRNA expression in BAEC after 25 mM glucose treatment. Parallel cultures of BAEC were incubated with mannitol (25 mM), which was used as an osmotic control. Total RNA was extracted using TRIzol reagent from BAECs incubated with 25 mM glucose or mannitol at indicated time points. Equal RNA loading and RNA integrity was verified by evaluating GAPDH expression. Quantitative measurements shown as histogram were determined from the ratio of IKKβ versus GAPDH expression and represented as relative fold increase compared with control. *Statistical significance (P < 0.05).
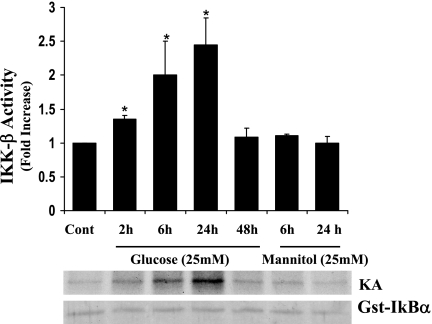
In addition, as shown in Fig. 3, corresponding to the protein and mRNA expression, a time-dependent increase of IKK kinase activity was observed after HG treatment. An increased kinase activity was seen as early as 2 h (1.4 ± 0.06-fold). The activity was high at 6 h (2.0 ± 0.5-fold) and increased further at 24 h (2.45 ± 0.4-fold) and declined at 48 h (1.08 ± 0.14-fold) post-HG treatment. Cells incubated with 25 mM mannitol for 6 and 24 h exhibited basal activity equivalent to control. Increased transcription with parallel increase in protein expression and kinase activity clearly indicated that HG could selectively augment IKKβ activity. Next, we examined a novel concept that IKKβ establishes an interaction with Hsp-90 in vascular endothelial cells, and HG imposes a positive influence over this interaction.
Fig. 3.
High glucose induces IKKβ kinase activity in a time-dependent manner. BAECs were incubated with 25 mM glucose for 2, 6, 24, and 48 h. Mannitol (25 mM) incubated cells for 6 h and 24 h were used as osmotic controls. Immunoprecipitated IKKβ protein with anti-IKKβ antibody was assayed for kinase activity using GST-IκBα (1–100 amino acids) and [γ-32P]ATP as substrates. Histogram represents fold increase ± SD from three independent experiments. *Statistical significance (P < 0.05).
HG augments Hsp-90-IKKβ interaction but downregulates Hsp-90-eNOS interaction.
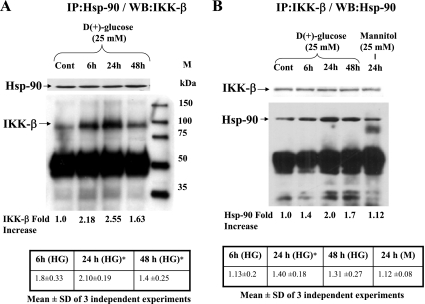
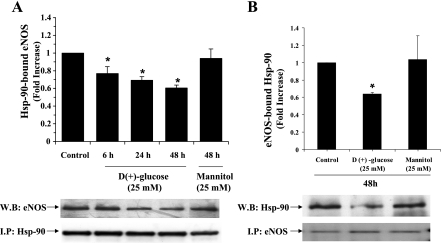
Immunoprecipitation of endogenous Hsp-90 from BAEC lysates resulted in coprecipitation of IKKβ. As shown in Fig. 4A, there was a significant increase in the IKKβ interaction with Hsp-90 in cells exposed to 25 mM glucose compared with control cells incubated with regular growth medium. Cells exposed to HG for 24 and 48 h showed a significant increase of IKKβ 1.55 ± 0.19-fold and 1.29 ± 0.25-fold; P < 0.05) bound to Hsp-90. Next, IKKβ was immunoprecipitated and the immune complex was further analyzed for the presence of bound Hsp-90 by immunoblotting. The results (Fig. 4B) showed a significantly increased coprecipitation of Hsp-90 when the cells were incubated with 25 mM glucose for 24 h (1.40 ± 0.18-fold; P < 0.05) compared with control cells incubated with regular growth medium or 25 mM mannitol thus corroborating our results demonstrated in Fig. 4A. These results strongly support our hypothesis that IKKβ not only interacts with Hsp-90 in endothelial cells, but this interaction is also positively influenced by HG. On the contrary, as reported earlier (8), there was a concurrent decrease in eNOS interaction with Hsp-90 when the cells were treated with 25 mM glucose for 6, 24, and 48 h. Immunoprecipitated Hsp-90-bound eNOS after 25 mM glucose incubation was found to be 0.77 ± 0.07, 0.69 ± 0.04, and 0.60 ± 0.03-fold at 6, 24, and 48 h, respectively compared with control (Fig. 5A). Similarly, eNOS-bound Hsp-90 significantly decreased to 0.64 ± 0.02-fold (P < 0.05; Fig. 5B) from basal level seen in control cells at 48 h. These results provide evidence for the HG-induced interaction of IKKβ and Hsp-90 and the simultaneous downregulation of eNOS-Hsp-90 interaction. To confirm this selective protein-protein interaction, we examined the binding of Hsp-90 with IKKβ after forcefully expressing them as fusion proteins.
Fig. 4.
Representative autoradiogram showing the increased association of IKKβ with heat shock protein (Hsp)-90 under high glucose condition. Lysates from BAECs incubated with 25 mM glucose or 25 mM mannitol for indicated time points were immunoprecipitated using monoclonal antibody against Hsp-90 and immunoblotted for IKKβ (A) or immunoprecipitated with IKKβ and immunoblotted for Hsp-90 (B). Blots were also reprobed for immunoprecipitating antigens. Blots were quantified and the level of Hsp-90-bound IKKβ or IKKβ-bound Hsp-90 was expressed after determining the ratio of IKKβ or Hsp-90 versus IP antigen or IgGH. Fold increase of the representative blot and mean values ± SD of 3 independent experiments are shown below the figure. *Statistical significance (P < 0.05).
Fig. 5.
Histogram and representative blots showing the reduced association of endothelial nitric oxide synthase (eNOS) with Hsp-90 under high glucose condition. Lysates from BAECs incubated with 25 mM glucose or mannitol for indicated time points were immunoprecipitated using Hsp-90 antibody and immunoblotted for eNOS (A) or immunoprecipitated with eNOS and immunoblotted for Hsp-90 (B). Blots were quantified and the level of Hsp-90-bound eNOS or eNOS-bound Hsp-90 is expressed after determining the ratio of eNOS or Hsp-90 versus IP antigen or IgGH. *Statistical significance (P < 0.05).
Interaction of forcefully expressed fusion proteins (FL-Hsp-90 and EYFP-IKKβ).
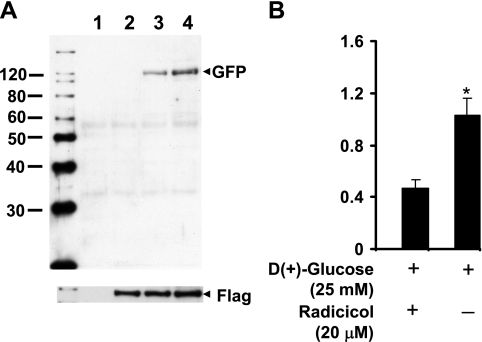
We performed similar immunoprecipitation experiments in cells where Hsp-90 and IKKβ were forcefully expressed by transient transfection using pCR3-FL-Hsp-90 and pEYFP-IKKβ expression plasmids. Pull-down assays using EZview Red anti-Flag M2 affinity gel followed by immunoblotting analyses of the immune complex with anti-GFP antibody clearly demonstrated the interaction of exogenously introduced FLAG-Hsp-90 with EYFP-IKKβ (Fig. 6). The presence of radicicol significantly decreased (0.5 ± 0.06 vs. 1.03 ± 0.13, P < 0.01) the interaction of overexpressed pCR3-FL-Hsp-90 and pEYFP-IKKβ. However, experiments performed to determine the influence of HG on this interaction, as assessed by forcefully expressed and coimmunoprecipitated fusion proteins did not demonstrate significant increase over control. This could be due to modified protein structure of the fusion proteins that could have influenced the binding and/or due to inherent variability in the in vitro overexpression system.
Fig. 6.
A representative autoradiogram showing the association of forcefully expressed Hsp-90 and IKKβ in BAECs (A). Cells were cotransfected with cDNA encoding flag-tagged Hsp-90 or pDS Red-C1-Hsp-90 along with yellow fluorescent protein tagged IKKβ. The flag-epitope was immunoprecipitated and immunoblotted for IKKβ using anti-GFP antibody (lane 4). Plasmid DS/Red Hsp-90 transfected cells (lane 1) and mock transfected (vectors only, lane 2) cells were used as controls. Radicicol-mediated inhibition of Flag-Hsp-90 and GFP-IKKβ interaction is demonstrated in lane 3. Histogram shows the quantitation of GFP-IKKβ/Flag-Hsp-90 interaction in the presence or absence of radiciol (B).
Interaction of Hsp-90-IKKβ demonstrated by mammalian two-hybrid system and FRET analysis.
Furthermore, to confirm the selective interaction of IKKβ with Hsp-90, mammalian two-hybrid assay and FRET analysis were performed. As shown in Fig. 7B, luciferase reporter assays showed increased luciferase activity in cells transfected with both IKKβ-BD and Hsp-90-AD constructs (2.32 ± 0.12-fold) compared with cells transfected with IKKβ-BD with AD vector alone (without target gene) or Hsp-90-AD construct with BD vector alone (without bait gene). The basal level luciferase activity in these cells transfected with pCMV-BD vector or pCMV-AD vector independently was between 1- and 1.4-fold. Interaction between positive control plasmid pairs pBD-p53 and pAD-SV-40T resulted in enhanced (200-fold) luciferase activity. Interaction between pBD-p53 (bait) and pAD-Hsp-90 (target) resulted in 7.5 ± 0.2-fold higher luciferase activity than basal activity. The interaction between pBD-IKKβ (bait) and pAD-TRAF (target) was negative.
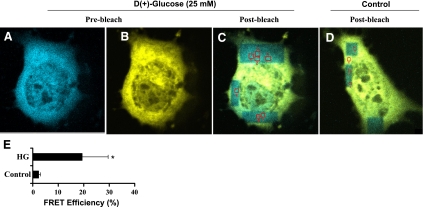
In addition, FRET analysis by acceptor photobleaching method as described in materials and methods clearly demonstrated (Fig. 8, A–C) a significant increase in percent FRET efficiency of 19.36 ± 10.22 (Fig. 8E) in cells overexpressed with pEYFP-IKKβ and pECFP-Hsp-90 and further exposed to 25 mM glucose for 24 h. Under the influence of normal glucose condition (Fig. 8D), the FRET efficiency was only 2.28 ± 0.73%.
Fig. 8.
Fluorescence resonance energy transfer (FRET) imaging of Hsp-90-IKKβ interaction. BAECs were cotransfected with Hsp-90-ECFP and IKKβ-EYFP constructs. Transfected cells were incubated with regular growth medium or HG medium (25 mM glucose) for 24 h. Cells were fixed with 3.7% paraformaldehyde and analyzed for FRET as described in materials and methods. A–C: images captured using the CFP and YFP imaging configurations before and after selectively photobleaching of YFP. D: postbleached control cell incubated in regular medium. E: histogram shows the level of FRET efficiencies of cotransfected cells incubated with HG (25 mM) versus control.
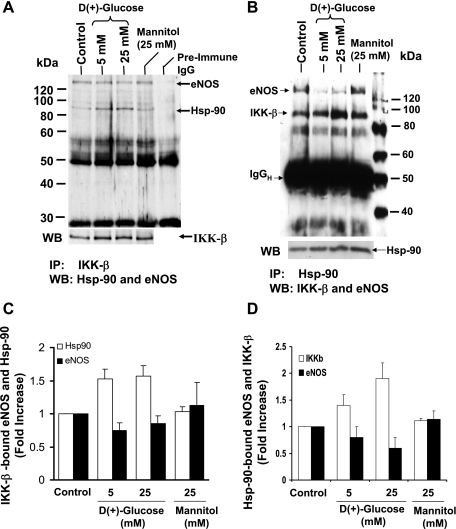
IKKβ and eNOS could be coimmunoprecipitated with Hsp-90.
In our attempts to investigate the possibility of whether both IKKβ and eNOS could interact simultaneously with the available pool of Hsp-90, we performed immunoprecipitations of IKKβ (Fig. 9A) or Hsp-90 (Fig. 9B) from cell lysates derived from BAECs incubated with 5 or 25 mM glucose for 48 h. The IKKβ immune complex was analyzed by immunoblotting using both anti-Hsp-90 and anti-eNOS antibodies. The Hsp-90 immune complex was immunoblotted using anti-IKKβ and anti-eNOS antibody. When BAECs maintained under conditions of 5 and 25 mM glucose for 48 h were immunoprecipitated with anti-IKKβ antibody (shown in Fig. 9, A and C), the immune complex contained significantly increased levels of Hsp-90 (1.53 ± 0.15 and 1.56 ± 0.16-fold in 5 and 25 mM glucose, respectively) compared with control. However, these complexes contained significantly less eNOS (0.75 ± 0.11 and 0.85 ± 0.11-fold in 5 and 25 mM glucose, respectively) compared with control.
Fig. 9.
IKKβ and eNOS could be co-immunoprecipitated with Hsp-90. A: lysates from BAECs incubated with 5 or 25 mM glucose for 48 h were immunoprecipitated using IKKβ antibody. Immunoprecipitates were immunoblotted simultaneously for both Hsp-90 and eNOS. B: lysates from BAECs incubated with 5 or 25 mM glucose for 48 h were immunoprecipitated using Hsp-90 antibody. Immunoprecipitates were immunoblotted for both IKKβ and eNOS. Blots were also reprobed for immunoprecipitating antigens and quantitated by scanning the autoradiogram. ImageJ software package was used for analysis. IKKβ bound Hsp-90/eNOS (C) and Hsp-90-bound eNOS/IKKβ (D) are expressed as fold increase compared with control. Mannitol (25 mM)-treated cells were also included as osmotic controls.
Similarly, the Hsp-90 immune complex showed reduced eNOS (0.8 ± 0.2-fold and 0.6 ± 0.2-fold) and increased IKKβ content (1.41 ± 0.18-fold and 1.90 ± 0.3-fold) in 5 and 25 mM glucose. Similar to untreated control, the osmotic control mannitol did not show differences in the level of IKKβ or eNOS in Hsp-90 immune complex and the content of Hsp-90 or eNOS in IKKβ immune complex. These data indicate that the amounts of IKKβ and eNOS that associate with the pool of Hsp-90 could be influenced by the chemical milieu and therefore could be altered by high concentrations of glucose.
Functional significance of Hsp-90-IKK interaction.
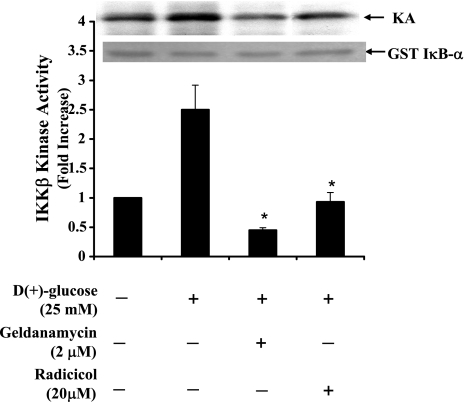
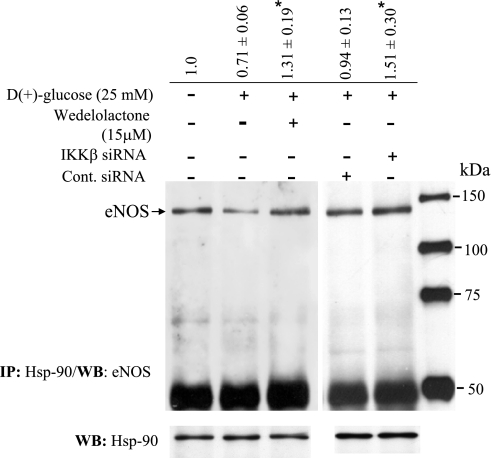
We then determined whether the HG-induced IKKβ kinase activity, as demonstrated in Fig. 3, could be downregulated by inhibiting Hsp-90. Both geldanamycin and radicicol block the ATP binding site of Hsp-90. BAECs incubated with geldanamycin (2 μM) or radicicol (20 μM) for 30 min before HG induction for 6 h exhibited significantly reduced kinase activity. Kinase activity in the presence of geldanamycin or radicicol under conditions of HG were 0.45 ± 0.04 and 0.93 ± 0.16-fold, respectively, compared with control (Fig. 10). In addition, we attempted to inhibit IKKβ expression to determine whether depletion of IKKβ would restore or augment Hsp-90-eNOS interaction under conditions of HG. BAECs were incubated with 15 μM of wedelolactone (IKK inhibitor II, Calbiochem, La Jolla, CA) for 2 h followed by incubation with 25 mM glucose for 48 h. Alternatively, IKKβ expression was blocked by transfecting target-specific siRNA, and these IKKβ-depleted cells were further incubated with 25 mM glucose for 24 h. As shown in Fig. 11, while HG-treated cells contained significantly less (0.71 ± 0.16-fold) eNOS bound to the Hsp-90 immune complex, wedeloactone-treated and IKKβ-specific siRNA-transfected cells contained increased amount of eNOS (1.3 ± 0.19-fold and 1.51 ± 0.20-fold, respectively) in the Hsp-90 immune complex indicating the possibility that eNOS interaction with Hsp-90 could be augmented by downregulating the expression of IKKβ.
Fig. 10.
Blocking of high glucose induced-IKKβ kinase activity by Hsp-90 inhibitors. Cells were incubated with geldanamycin (2 μM) or radicicol (20 μM) for 30 min before incubation with HG (25 mM). Cells harvested at 24 h after incubation was immunoprecipitated with anti-IKKβ antibody. The kinase activity was performed using GST-IκBα (1–100 amino acids) and [γ-3P]ATP as substrates. Bar graph represents kinase activity in terms of fold increase ± SD from three independent experiments. *Statistical significance (P < 0.05) compared with HG-treated cells.
Fig. 11.
Inhibition of IKKβ augments eNOS-Hsp-90 interaction. BAECs were incubated with wedelolactone (15 μM) for 2 h, transiently transfected with small interferring RNA (siRNA) specific for IKKβ or transfected with control siRNA. Cells were incubated with HG (25 mM) and harvested after 24 h for immunoprecipitation analysis using anti Hsp-90 antibody. The immune complex was further analyzed for the presence of eNOS by immuno blotting using anti eNOS antibody. Blots were reprobed for Hsp-90 to show efficiency of immunoprecipitation. *Statistical significance (P < 0.05) compared with control.
Blocking IKKβ expression and activity upregulates NO generation.
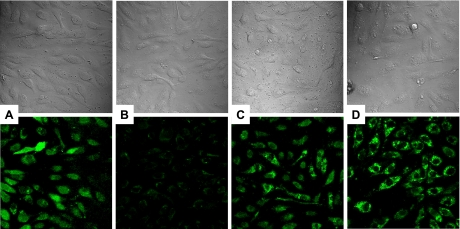
NO derived from the oxidation of l-arginine and generated by the eNOS activity plays a central role in vascular biology. Abnormal regulation of endothelial function is caused by reduced bioavailability of NO. Therefore, we determined whether blocking IKKβ-Hsp-90 interaction could improve Hsp-90-eNOS interaction (as shown in Fig. 11) and associated NO production using the fluorescent indicator DAF-FM diacetate. Direct visualization of the NO production with this fluorescent indicator was performed in combination with a laser scanning confocal microscope (Fig. 12). Total number of cells in a given area could also be visualized by differential interference contrast (DIC) microscopy (top, A, B, C, D). The microscopic images demonstrate the increased DAF staining intensity in BAECs treated with HG in the presence of wedelolactone or cells transfected with IKKβ-specific siRNA compared with cells incubated with HG alone for 24 h (bottom, C and D compared with B, respectively). Figure 12A shows basal level expression of NO in control BAECs grown in regular growth medium. Vehicle-treated controls also showed NO expression at basal level.
Fig. 12.
Nitric oxide production determined by using 4-amino-5-methyl amino-2,7′-difluorofluorescein diacetate (DAF-FM) diacetate fluorophore in BAEC treated with control medium (A), medium containing 25 mM d(+)-glucose (B), 25 mM d(+)-glucose plus 15 μM wedelolactone (C), and IKKβ specific siRNA-transfected BAECs treated with 25 mM d(+)-glucose (D). The top panels correspond to differential interference contrast (DIC) photomicrographs and the bottom panels show DAF-FM fluorescence images from the identical sites. Cells were viewed through a ×40 oil immersion plan-neofluar objective (numeric aperture = 1.3). Confocal images (512 × 512 × 16 bits) were acquired and saved to a disk. For each sample at least five areas were selected for image collection. Data showed are the representative image of three independent experiments. Saved images were adjusted for brightness uniformly on same scale using Adobe Photoshop7.0.1. software.
DISCUSSION
In this study, we have demonstrated a novel concept that endothelial dysfunction could result due to elevated glucose-induced interaction of Hsp-90 with IKKβ. Our results for the first time demonstrated the interaction of Hsp-90-IKKβ in primary vascular endothelial cells and its relevance to endothelial dysfunction, a hallmark of diabetes. Inhibition of Hsp-90 interaction with IKK using geldanamycin or radicicol downregulated HG-induced IKKβ activity. Additionally, blocking IKKβ expression resulted not only in increased eNOS-Hsp-90 interaction but also demonstrated its physiological relevance of improved NO production. These results indicate that IKKβ-Hsp-90 interaction is not a mere physical association but has potential function with pathophysiological relevance of causing vascular dysfunction in diabetic individuals thus linking hyperglycemia and reduced NO production to endothelial dysfunction.
So far, to our knowledge Hsp-90-IKK interaction has been demonstrated only in malignant or transformed cell lines. Requirement of Hsp-90 for the tumor necrosis factor-α-induced IKK activity was first demonstrated in HeLa cells (4) and was recognized as a bonafide subunit of the IKK complex. The requirement of Hsp-90 for the biosynthesis and activity of IKK has been demonstrated in lymphoma cells and alveolar epithelial cell line (25). A recent report from Park et al. (24) demonstrated PKC-dependent downregulation of nuclear factor (NF)-κB signaling by the dissociation of IKK-γ and Hsp-90 complex in colonic epithelial cells, hepatocellular, gastric and pancreatic carcinoma cells.
The functional significance of Hsp-90 interaction with eNOS, unlike IKK interaction, has been well established (10, 13, 23) in vascular endothelial cells. Hsp-90 is associated with eNOS in the resting state and upon stimulation with agonists like VEGF, histamine, shear stress, or statins; the association between these two proteins is increased with concomitant NO generation (1). In the absence of a stimulus, eNOS is associated with caveolin-1 and remains in an inactive membrane-bound state. Upon stimulation, the eNOS-caveolin-1 interaction is disrupted and the eNOS-Hsp-90 association is promoted resulting in the coupled activity of eNOS (11). With the use of in vitro cultures of human umbilical vein endothelial cells, the interaction between eNOS and Hsp-90 has been shown to be impaired by long-term HG (33 mM) incubation (for a period over 24 h) (18). However, during early hours of HG incubation (<24 h), eNOS expression was found to be high along with increased interaction with Hsp-90. The NO generated during this early phase has been implicated in the protective mechanism against apoptosis of vascular endothelial cells during HG exposure. These results uncover the functional significance of eNOS-Hsp-90 interaction in maintaining healthy endothelium by protecting it against apoptotic cell death. The significance of eNOS-Hsp-90 interaction has been further emphasized in studies conducted by Davis et al. (5) where eNOS-Hsp-90 interaction has been suggested as the mechanism by which metformin, one of the most commonly used drugs for the treatment of Type-2 diabetes, improves vascular endothelial function by activating AMP-activated protein kinase-dependent Hsp-90-eNOS activation. It has been suggested that metformin improved vascular function through enhanced NO release by increasing eNOS-Ser1179 phosphorylation and its association with Hsp-90 without enhancing eNOS expression. Our results presented in this study provide evidence for the possibility that long-term exposure of endothelial cells to HG could downregulate eNOS-Hsp-90 interaction by increasing the expression and activity of IKKβ.
Two similar reports from Kim et al. (14, 15) have showed corroborative evidence for the involvement of IKKβ that disrupts eNOS activity and downregulates NO generation via the IRS-1-pAkt-peNOS pathway in HG/insulin- and FFA-mediated eNOS phosphorylation and NO generation. The mechanism proposed in these reports could be one of many other mechanisms that explain endothelial dysfunction due to insulin resistance. Even though the involvement of IKKβ in mediating the decreased insulin-induced NO generation has been realized (14, 15), the precise role of IKKβ in these events that lead to endothelial dysfunction in diabetes has not been identified. IKKβ, the catalytic subunit required for the activation of NF-κB, has been demonstrated as a direct target of S-nitrosylation resulting in loss of IKKβ activity (27). Additionally, the p50 subunit of NF-κB has also been reported to be S-nitrosylated, causing an inhibition of its binding to DNA (19). A recent report from Wadham et al. (29) has shown a reduction in the S-nitrosylated proteins by HG in endothelial cells. Additionally, PKA-dependent phosphorylation of Hsp-90, in particular the α-subunit, has been implicated to impair eNOS activity in diabetic rats by translocating Hsp-90 out of the cell and thereby reducing the amount of available Hsp-90 to interact with eNOS (17). In this context, as discussed by Lei et al. (17), it is possible that Hsp-90 within the cell could exist as separate pools, and these may not be functionally interchangeable. Therefore, even though Hsp-90 is abundant overall within the cell, separate pools of Hsp-90 may get depleted under specific conditions. As shown in our study, the ability of IKKβ to interact with Hsp-90 could be a possible alternative mechanism by means of which HG downregulates the eNOS-Hsp-90 interaction. Since HG induces IKKβ expression, it is possible that the abundant availability of IKKβ could initiate Hsp-90-IKKβ interaction. Even though the precise mechanism of how HG induces IKKβ expression is not known at present, it is clear from our results that HG specifically induces IKKβ expression and activity. d(+)-glucose at a concentration of 25 mM was added to generate HG medium. Mannitol or l-glucose at a concentration of 25 mM was added to the MCDB-131 medium and used as osmotic controls. Mannitol at 25 mM concentration neither induced IKKβ protein expression nor its activity. Similarly, l-glucose (25 mM) also did not influence IKKβ expression indicating that high glucose selectively modulates IKKβ expression and activity. It is interesting to note that while we observed a maximum IKKβ protein expression at 24 h, eNOS expression under conditions of HG was shown to peak at 6 h and decline there after at 24 h (18). Investigations are underway in our laboratory to determine the putative binding sites of IKKβ that interact with Hsp-90. Results of these studies would help us to determine whether eNOS and IKKβ share a common binding site on Hsp-90, or they just overlap with each other.
In conclusion, our results contribute to a novel mechanism that explains how hyperglycemic state impairs NO pathway. The impairment appeared to be through the enhanced IKKβ expression and activity. Further investigations on the role of IKKβ in regulating NO generation may illuminate modified therapeutic approaches of blocking IKKβ to improve eNOS coupling and increased NO generation in diabetes.
GRANTS
Major part of this work was supported by funds from Juvenile Diabetes Research Foundation (1-2007-558), National Institutes of Health (NIH) (Grant 63032 for S. Mohan), San Antonio Area Foundation Grants 118981 (for S. Mohan) and 120909 (for M. Natarajan). FRET analysis was performed in the Core Optical Imaging Facility, which is supported by UTHSCSA, NIH-NCI P30-CA54174 (San Antonio Cancer Institute), NIH-NIA P30-AG-013319 (Nathan Shock Center), and NIH-NIA P01-AG-19316.
Acknowledgments
We thank Jessica Rogge and Richard Tamfu for excellent technical support and assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Balligand JL Heat shock protein 90 in endothelial nitric oxide synthase signaling: following the lead(er)? Circ Res 90: 838–841, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Broemer M, Krappmann D, Scheidereit C. Requirement of Hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive and inducible IKK and NF-κB activation. Oncogene 23: 5378–5386, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000. [DOI] [PubMed] [Google Scholar]
- 4.Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell 9: 401–410, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Davis BJ, Xie Z, Viollet B, Zou MH. Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 55: 496–505, 2006. [DOI] [PubMed] [Google Scholar]
- 6.De Vriese S, Verbeuren TJ, Voorde JV, Lameire NH and Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol 130: 963–974, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Field N, Low W, Daniels M, Howell S, Daviet L, Boshoff C, Collins M. KSHV vFLIP binds to IKK-γ to activate IKK. J Cell Sci 116: 3721–3728, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R1–R12, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Forstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease. Circulation 113: 1708–1714, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Cardeña G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 821–824, 1998. [DOI] [PubMed] [Google Scholar]
- 11.Gratton JP, Fontana J, O'Connor DS, García-Cardeña G, McCabe TJ, Sessa WC. Reconstitution of an endothelial nitric-oxide synthase (eNOS), hsp90, and caveolin-1 complex in vitro. J Biol Chem 275: 22268–22272, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Hamuro M, Polan J, Natarajan M, Mohan S. High glucose induced nuclear factor kappa B mediated inhibition of endothelial cell migration. Atherosclerosis 162: 277–87, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Harris MB, Ju H, Venema VJ, Blackstone M, Venema RC. Role of heat shock protein 90 in bradykinin-stimulated endothelial nitric oxide release. Gen Pharmacol 35: 165–170, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Kim F, Tysseling KA, Rice J, Gallis B, Haji L, Giachelli CM, Raines EW, Corson MA, Schwartz MW. Activation of IKKβ by glucose is necessary and sufficient to impair insulin signaling and nitric oxide production in endothelial cells. J Mol Cell Cardiol 39: 327–334, 2005. [DOI] [PubMed] [Google Scholar]
- 15.Kim F, Tysseling KA, Rice J, Pham M, Haji L, Gallis BM, Baas AS, Paramsothy P, Giachelli CM, Corson MA, Raines EW. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKβ. Arterioscler Thromb Vasc Biol 25: 989–994, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kojima H, Nakatsubo N, Kikuchi K, Kawahara S, Kirino Y, Nagoshi H, Hirata Y, Nagao T. Detection and Imaging of nitric oxide with novel fluorescent indicators: diaminofluoresceins. Anal Chem 70: 2446–2453, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Lei H, Venkatakrishnan A, Yu. S, Kazlauskas A. Protein kinase A-dependent translocation of Hsp-90α impairs endothelial nitric oxide synthase activity in high glucose and diabetes. J Biol Chem 282: 9364–9371, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Lin LY, Lin CY, Ho FM, Liau CS. Up-regulation of the association between heat shock protein 90 and endothelial nitric oxide synthase prevents high glucose-induced apoptosis in human endothelial cells. J Cell Biochem 94: 194–201, 2005. [DOI] [PubMed] [Google Scholar]
- 19.Marshall HE, Stamler JS. Inhibition of NF-kappa B by S-nitrosylation. Biochemistry 40: 1688–1693, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Miao RQ, Fontana J, Fulton D, Lin MI, Harrison KD, Sessa WC. Dominant-negative Hsp90 reduces VEGF-stimulated nitric oxide release and migration in endothelial cells. Arterioscler Thromb Vasc Biol 28: 105–111, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Mohan S, Koyoma K, Thangasamy A, Nakano H, Glickman RD, Mohan N. Low shear stress preferentially enhances IKK activity through selective sources of reactive oxygen species for persistent activation of NF-κB in endothelial cells. Am J Physiol Cell Physiol 292: C362–C371, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Nakatsubo N, Kojima H, Kikuchi K, Nagoshi H, Hirata Y, Maeda D, Imai Y, Irimura T, Nagano T. Direct evidence of nitric oxide production from bovine aortic endothelial cells using new fluorescence indicators: diaminofluoresceins. FEBS Lett 427: 263–266, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Ou J, Ou Z, Ackerman, AW, Oldham, KT and Pritchard, KA, Jr. Inhibition of heat shock protein 90 (Hsp-90) in proliferating endothelial cells uncouples endothelial nitric oxide synthase activity. Free Radic Biol Med 34: 269–276, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Park KA, Byun HS, Won M, Yang KJ, Shin S, Piao L, Kim JM, Yoon WH, Junn E, Park J, Seok JH, Hur GM. Sustained activation of protein kinase C downregulates nuclear factor-κB signaling by dissociation of IKK-β and Hsp90 complex in human colonic epithelial cells. Carcinogenesis 28: 71–80, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Pittet JF, Lee H, Pespeni M, O'Mahoney A, Roux J, Welch WJ. Stress-induced inhibition of the NF-κB signaling pathway results from the insolubilization of the IκB kinase complex following its dissociation from heat shock protein 90. J Immune 174: 384–394, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Räthel TR, Leikert JF, Vollmar AM, Dirsch VM. Application of 4, 5-diaminofluorescein to reliably measure nitric oxide released from endothelial cells in vitro. Biol Proced Online 5: 136–142, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EFM, Vliet A, and Janssen-Heininger YMW. Nitric oxide represses inhibitory κB kinase through S-nitrosylation. Proc Natl Acad Sci USA 101: 8945–8950, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sud N, Sharma S, Wiseman DA, Harmon C, Kumar S, Venema RC, Fineman JR, Black SM. Nitric oxide and superoxide generation from endothelial NOS: modulation by HSP90. Am J Physiol Lung Cell Mol Physiol 293: L1444–L1453, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Wadham C, Parker A, Wang L, Xia P. High glucose attenuates protein S-nitrosylation in endothelial cells: role of oxidative stress. Diabetes 56: 2715–21, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Shi Y, Wang J, Jones D, Weilrauch D, Ying R, Wakim B, Pritchard KA Jr. A heat shock protein 90 binding domain in endothelial nitric-oxide synthase influences enzyme function. J Biol Chem 282: 37567–37574, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Yetik-Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vasc Pharmacol 45: 268–276, 2006. [DOI] [PubMed] [Google Scholar]