Abstract
Parasitic infection with Plasmodium falciparum is responsible for the most severe form of human malaria in which patients suffer from periodic fever. It is well established that during intra-erythrocytic maturation of the parasite in the red blood cell (RBC), the RBC becomes significantly more cytoadhesive and less deformable; these and other biochemical factors together with human host factors such as compromised immune status are important contributors to the disease pathology. There is currently substantial interest in understanding the loss of RBC deformability due to P. falciparum infection, but few results are available concerning effects of febrile conditions or parasitization on RBC membrane rheology. Here, for the first time, we report rheology of the single, isolated RBC with and without P. falciparum merozoite invasion, spanning a range from room temperature to febrile conditions (41°C), over all the stages of parasite maturation. As expected, stiffness increased with parasite maturation. Surprisingly, however, stiffness increased acutely with temperature on a scale of minutes, particularly in late trophozoite and schizont stages. This acute stiffening in late falciparum stages may contribute to fever-dependent pathological consequences in the microcirculation.
Keywords: deformability, magnetic twisting cytometry, malaria, fever, environmental stress
malaria affects ∼400 million people and causes 1–3 million deaths annually. The asexual stage of Plasmodium falciparum is responsible for the most severe form of human malaria. A cardinal symptom of human malaria is the occurrence of febrile episodes resulting in body temperatures as high as 41°C. The malarial fever is associated with rupture of intra-erythrocytic schizonts and the release of the merozoites in the circulation; the released merozoites then invade other red blood cells (RBCs) and start a new intra-erythrocytic cycle. This causes fevers that repeatedly occur with the same frequency as the parasite maturation cycle inside the RBC (14).
During intra-erythrocytic development of the parasite, the cell undergoes striking morphological and rheological alterations (19). The parasite synthesizes, exports, and inserts different proteins to the RBC phospholipid bilayer membrane, where they interact with the spectrin network of the RBC (3, 18). As a result, the membrane of the infected cell is extensively remodeled, creating new permeation channels and pathways (34) and forming nano-scale “knobs” on the RBC surface that are electron-dense protrusions. These changes reduce RBC deformability (4, 24) and increase RBC cytoadhesion (29), both of which impair the ability of the RBC to circulate. RBC cytoadhesion in mature stages has received much attention in recent years, and these studies have led to identification of several parasite-derived molecules involved in adhesion, knob formation, and their interaction with the RBC cytoskeleton (3, 31). RBC deformability has received much attention as well (4, 11, 20, 21, 24, 26, 27, 32, 33), but effects of parasite maturational stage on membrane deformability at febrile temperature have not been examined.
Here we used magnetic twisting cytometry with optical detection (OMTC) (28) to provide the most comprehensive characterization to date of the membrane deformability of the isolated human RBC. We examined the influence of two variables: parasite maturation (P. falciparum, strain 3D7) and temperature.
MATERIALS AND METHODS
Materials.
Concanavalin A (ConA), poly-l-lysine, RPMI 1640, hypoxanthine, gentamycin, NaHCO3, glutaraldehyde, sodium cacodylate buffer, and hexamethyldisilazane (HMDS) were purchased from Sigma (St. Louis, MO). Phosphate-buffered saline (PBS), HEPES buffer, and albumax II were purchased from GIBCO (Gaithersburg, MD). Human serum and human erythrocytes of blood group O+ were purchased from Interstate Blood Bank (Memphis, TN).
Parasitized red blood cells.
Experiments involving cultures of P. falciparum (strain 3D7) in vitro used methods described previously (35). Cultures were grown at 5% hematocrit in 25-ml tissue culture flasks in a complete medium that consisted of RPMI 1640 supplemented with 25 mM HEPES buffer, 23.8 mM NaHCO3, 200 mM hypoxanthine, 0.25% wt/vol albumax II, and 5% vol/vol human serum. Cultures were gassed with 3% O2-5% CO2-92% N2 and incubated at 37°C in complete medium. Rings, trophozoites, and schizonts were identified accordingly to conventional criteria.
Ferrimagnetic microbeads and binding.
Ferrimagnetic beads (solid Fe3O4, 2.27 μm ± 11.3% diameter) were prepared as described previously (8). Beads (1 mg) were coated with ConA by incubating overnight at 4°C in ConA solution (1 mg/ml). ConA is not blood group specific but has an affinity for terminal α-d-mannosyl and α-d-glucosyl residues. A wide variety of serum and membrane glycoproteins have a “core oligosaccharide” structure, which includes α-linked mannose residues.
Electron microscopy.
As described in our previous work (28), each sample of RBCs with beads attached was fixed by treating with a solution containing 2.5% glutaraldehyde in 0.085 M of sodium cacodylate buffer for 1 h at room temperature. The cells were washed twice in sodium cacodylate buffer for 5 min each. Fixed cells were then dehydrated through a graded series of ethanol (EtOH) baths for 10 min each: 25%, 50%, 70%, 95%, and 100% (three times). Cells were treated with 50:50 HMDS in 100% EtOH for 2 h, followed by treatment with 100% HMDS twice for 30 min. The sample was allowed to air dry overnight and then sputter-coated with a layer of Au in a Hummer V (Anatech, Alexandria, VA). The cells were examined with a 1450VP scanning electron microscope (Carl Zeiss SMT, Thornwood, NY).
Magnetic twisting cytometry with optical detection (OMTC).
The experimental setup is described elsewhere (8, 9). Briefly, cells adherent on the bottom of a glass well with beads attached were placed on an inverted microscope (Leica DM IRBE, Leica Microsystems, Weitzler, Germany) and viewed under brightfield with an oil immersion ×63 objective and optically magnified 1.5×. Beads were magnetized horizontally and then subjected to a vertical magnetic oscillatory field. This oscillatory field causes a specific torque on the bead. The lateral displacements of the bead in response to the oscillatory torque was tracked through images captured by a CCD camera (JAI CV-M10, Gloatrup, Denmark) with an exposure time of 0.1 ms and acquisition frequency of 24 Hz. From recorded images, bead position was computed using an intensity-weighted center-of-mass algorithm (9).
Mechanical tests at 20°C, 37°C and 41°C.
Clean, 35-mm glass bottom wells (MatTek, Ashland, MA) were treated with 0.1 mg/ml poly-l-lysine for 5 min at room temperature (temperature = 20°C). At this concentration, the RBCs adhere firmly to the wells, but retain their biconcave shape (12). RBCs were allowed to adhere for 10 min to the wells before the beads were added. Measurements were performed at oscillatory frequencies between 10−1 and 102 Hz and amplitudes of 2 or 4 Gauss depending on the parasite maturation stage. As described previously (9), heterodyning was used to sample the displacement response in tests where the sinusoidal forcing frequency was greater than 4 Hz. The temperature in the microscope stage (±0.2°C) and inside the sample was well controlled (±0.5°C). Each individual cell was sequentially measured at room temperature, at 37°C and then at 41°C, with 20 min allowed between each subsequent temperature level to ensure thermal equilibrium between sample and microscope stage. We measured five groups: unparasitized unexposed cells, unparasitized cells exposed to parasitized cells, ring stage, trophozoite stage, and schizont stage. The unparasitized unexposed cells are cultured similar to the parasitized cells and are referred to here as healthy RBCs.
Measurement of the complex viscoelastic modulus.
Storage, g′, and loss, g″, moduli were extracted from the torque and corresponding lateral bead displacement following Fourier analysis (8–10, 28).
Technical issues.
Similar to the noninvaded but exposed cells, the P. falciparum RBCs showed increasing irreversible damage at 41°C, and this was especially true in the schizont stage. This prevented a cell-by-cell paired analysis.
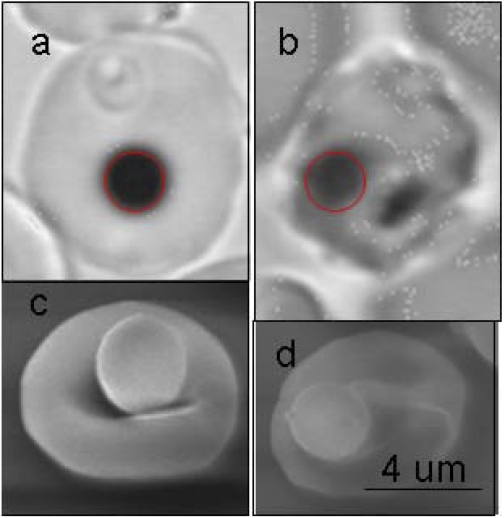
RBC membrane morphology and composition vary extensively as the parasite matures within the cell (2, 30). De novo malarial proteins have been identified in the surface of the cell at late stages. Hence, the bead-to-cell attachment might be different at late stages. Using scanning electron microscope, we saw little variation in the bead-cell contact area (Fig. 1). Although a change in membrane composition could also contribute to the stiffening seen at late stages, this is unlikely the main factor responsible for the stiffening seen at febrile conditions.
Fig. 1.
Red blood cells (RBCs) with beads bound to their surface viewed under brightfield with an oil immersion ×63 objective and optically magnified by 1.5× ring stage (a) and late stage (b). Scanning electron micrograph (EM) of a RBC with a bead bound to the surface, uninfected RBC, and ring stage are indistinguishable in EM (c), and late stages are recognized based on shape and roughness on the surface in EM (d).
RESULTS
Stiffness spectra at baseline conditions.
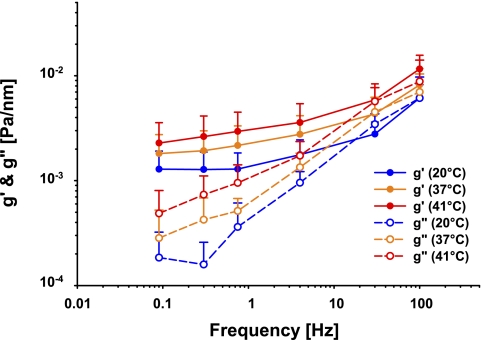
At room temperature (20°C), RBC stiffness g′ increased slowly with increasing frequency of deformation (Fig. 2, blue symbols). The loss modulus g" was substantially smaller than g′ indicating that within the RBC elastic stresses are larger than frictional stresses by about an order of magnitude. However, g″ exhibited a much stronger dependence upon frequency than did g′ and was well approximated by the power law (g″ ∼ fa). These trends are comparable to those we have reported previously (28).
Fig. 2.
Apparent storage (g′; closed symbols) and loss (g″; open symbols) moduli (in units of Pa/nm) measured at frequencies from 0.1 to 100 Hz, room temperature (blue, N = 28), 37°C (orange, N = 18), and 41°C (red, N = 18). Data plotted are means and error bars represent SD.
Mechanical changes with temperature in control RBCs.
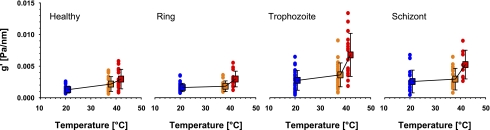
As temperature was increased from room temperature (20°C) to normal physiological temperature (37°C) and then to a high febrile temperature (41°C), both g′ and g″ systematically increased (Fig. 3). Over this range, increases in g′ were typically 50 to 100%, depending upon the frequency, whereas increases in g″ were somewhat greater, ranging from 100 to 200%.
Fig. 3.
Stiffness g′ (in units of Pa/nm) as a function of temperature. All panels have a common ordinate scale shown on the left. Blue, 20°C; orange, 37°C; red, 41°C. Data are shown separately for healthy RBCs (N = 28), ring stage (N = 32), trophozoite (N = 30), and schizont (N = 18) stages. Square symbols are means and error bars represent SD.
Comparison between healthy RBC and unparasitized but exposed RBC.
Viscoelastic moduli from the healthy RBC were no different from those of the unparasitized RBC in contact with parasitized cells (data not shown). However, RBCs in healthy preparations retained their characteristic biconcave shape regardless of temperature, whereas the unparasitized but exposed cells in asynchronous cultures showed an overall degradation at 41°C, with increasing numbers of irreversibly damaged cells as seen by morphology assessed by their appearance under the microscope.
Temperature dependence of viscoelasticity in parasitized RBCs.
Regardless of the parasitization stage, frequency dependencies of g′ and g′ were similar to those seen in healthy cells (data not shown). Therefore, to quantify changes with parasitization and temperature, we focused on responses of g′ measured at 0.75 Hz (Fig. 3). At room temperature there was little change in g′ from healthy to ring stage, but a marked increase, approximating twofold, with maturation from the ring to trophozoite stages (P < 0.001), and no change between the trophozoite and schizont stages. Healthy cells showed gradual stiffening with increasing temperature, with the change in stiffness being roughly linear with temperature. By contrast, the parasitized cells, regardless of parasite stage, showed little change in stiffness from room temperature to body temperature but an abrupt increase in stiffness at febrile temperature. This abrupt increase was completed as quickly as the experimental system could be thermally equilibrated, which was on the order of minutes.
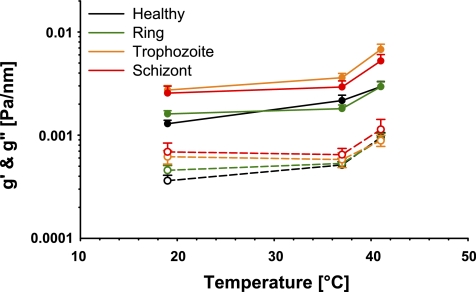
Figure 4 summarizes the results of storage and loss moduli as a function of temperature for the different stages of infection of RBC; we include the data on healthy cells for comparison. For the purpose of specific comparison of cell behavior between parasite stages (including control) at each given temperature, means are plotted with standard error. Over the range of temperature and infection stage studied here, the change in RBC stiffness spanned a fivefold range (Fig. 4).
Fig. 4.
Apparent storage (g′, 0.75 Hz; closed symbols) and loss moduli (g″; 0.75 Hz, open symbols) for healthy RBC (black), ring stage (green), trophozoite (orange), and schizont (red). Data plotted are means and error bars represent SE.
DISCUSSION
As the P. falciparum merozoite undergoes intra-erythrocytic maturation, the parasitized RBC exhibits striking morphological and rheological alterations. Here we have quantified the effects of temperature and P. falciparum maturation on RBC rheology. Changes in deformability as characterized by storage modulus g′ over a wide frequency range approached fivefold and as such are large enough to alter dynamic behavior of the RBC in the microcirculation.
Changes in rheological properties with temperature—control RBCs.
The healthy RBC showed gradual stiffening with temperature (Figs. 2 and 3). A significant entropic component would not be surprising given that the spectrin network that forms the main component of the RBC membrane can be conceptualized as polymeric chains with a high degree of flexibility and mobility (5). Failure to find strict proportionality with temperature, as in rubber, does not imply an insignificant role for entropy; indeed, the sign of the change of stiffness with temperature argues that entropic contribution might dominate the contribution of internal energy to the Helmholtz free energy of the system (17, 36).
The stiffening with temperature that we found here in the healthy RBC has been seen as a trend in previous studies using ektacytometry (39) and using optical tweezers (20) but failed to reach statistical significance. By contrast, Waugh and Evans (38) found with micropipette aspiration that the elastic shear modulus of the RBC membrane decreases with temperature, although the decrease was small. A likely explanation for this discrepancy is that RBC aspiration engenders very large deformations that may magnify the contribution of internal energy to stiffness, leading to a net softening with temperature. This discrepancy requires further investigation, and, more important, reveals the need for a more accurate physical picture of the RBC.
It has been suggested that the unparasitized RBC cultured in the presence of the malaria parasite is less deformable due to release of exoantigens that bind to normal RBCs (22). With the use of ektacytometry, some studies confirmed that deformability is reduced in exposed but unparasitized RBCs, mainly in cases of severe falciparum malaria (6, 7, 32), whereas others using micropipette aspiration did not (11, 24). We found no significant difference in viscoelastic moduli between healthy RBCs and those that were exposed but unparasitized.
Changes in rheological properties with temperature parasitized RBCs.
The behavior of P. falciparum parasitized RBCs in the circulation depends strongly on the stage of maturation. For example, during the ring stage, a parasitized RBC retains its biconcave shape and circulates freely through the body. However, during the later trophozoite and subsequent schizont stages, the rigid and very viscous parasite enlarges (21). This causes the parasitized RBC to lose its biconcavity and becomes more irregular in shape and more adhesive to the vascular endothelium and to other cells. Consequently, there is a marked increase in RBC sequestration; RBCs at these late stages of maturation are rarely seen in free circulation (16). Consistent with this overall picture, we found little change in stiffness in the ring stage compared with control RBCs but a twofold increase in stiffness from ring to trophozoite stage, remaining elevated through the schizont stage (Fig. 3, blue points). These general features of increased stiffness with P. falciparum maturation are consistent with previous studies employing a variety of techniques, including fluid shear and cone-plate rheometry (4, 33), micropipette aspiration (11, 21, 24), and recent work using optical tweezers (20, 32), all at room temperature. On the basis of observed morphological alterations of parasitized cells, as well as the presence of stage-specific parasite-encoded proteins that associate with the RBC membrane (30), including parasite exported proteins such as P. falciparum erythrocyte membrane protein 3 (Pf-EMP3) (27) or mature parasite-infected erythrocyte surface antigen (MESA), a membrane contribution to stiffening at later maturation stages is not surprising.
The relative lack of membrane stiffening that we found in ring stage compared with healthy RBCs (Fig. 3, blue data points in healthy and ring panels) is in apparent contradiction to some previous studies using micropipette aspiration (21) and shear flow rheometry (4), which show that ring stages have impaired deformability. Those reports utilized techniques that deform the entire cell globally and therefore probe changes that may occur both in membrane viscoelasticity and in the internal cytoplasm due to the presence of the parasite. Hence, techniques that involve large deformations of the entire cell reflect the lumped contribution from all sources throughout the cell into a global response and independent contributions from the cell membrane cannot be distinguished. By contrast, the OMTC technique used here involves deformations that are relatively small and, in the case of the healthy RBC, are localized mainly to the vicinity of the bead, and therefore measure primarily the contribution of membrane viscoelasticity (Fig. 5) (for more details see Ref. 28). In the case of the parasitized RBC it remains unclear how OMTC might be influenced by the presence of the parasite, but in the ring stage these influences are expected to be small. Taken together, these factors suggest caution in interpretation of globally deformed cell rheology purely in terms of membrane characteristics, with no contribution from the cell's internal structure. In particular, it is not known to what extent the sources of the stiffening response are additive, and therefore it is premature to assign quantitatively differential roles to the membrane and the intracellular milieu. Nevertheless, we stress that our technique uniquely probes the primary contribution of the RBC membrane in its response both to infection and to stiffness changes associated with temperature.
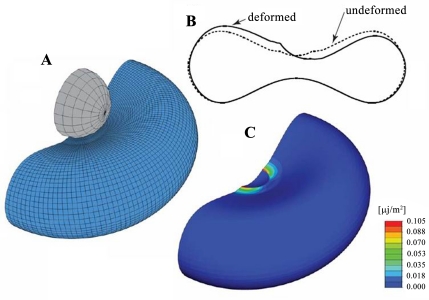
Fig. 5.
Schematic of RBC optical magnetic testing cytometry (OMTC) tests. A: RBC with a bead attached to its surface. B: undeformed and deformed shape. Displacements have been magnified by a factor of four. C: plot of the elastic strain energy density across the entire cell (the bead has been removed for clarity). Figure was reprinted from Puig-de-Morales-Marinkovic et al. (28).
Changes in stiffness of parasitized RBCs over the 48-h P. falciparum life cycle evolve gradually but are dramatic. Consistent with this, Mills et al. (20) found an increased shear elastic modulus at the late ring stage, using closely synchronized cultures, but not at the early ring stage. In addition to the fact that Mills et al. (20) used a different strain of P. falciparum and they applied large deformations, we note that the ring stage RBCs used in our study were early ring stage cells.
The repeated occurrence of fever at regular intervals is a key feature of malaria (25), and the behavior of parasitized RBCs in the febrile state is correspondingly important. The malarial fever is associated with the propensity of the parasites to grow synchronously with one another after several cycles. The outcome is recurrent paroxysmal fever that has the same periodicity as the maturation cycle of the parasite within the RBC (14). There are several reports in the literature addressing the influence of elevated temperature encountered during malarial fever on the infected individual (1) as well as on protein expression and survival of the parasite (13, 14) in vitro. The consequences of fever are well known to include increased cytoadherence of Pf-RBCs to CD36 and ICAM-1 host receptors over the time course of hours (37), inhibition of parasite growth (14, 15) and parasite death (23) over the time course of days. However, the effect of fever on the mechanical properties of parasitized RBC has been less well studied. We exposed the same cell to an increased temperature and found that there is little change in stiffness of the Pf-RBC from room temperature to body temperature but a large and prompt increase under febrile conditions especially during the second half of the growth cycle (Figs. 3 and 4). This temperature-related stiffening occurred within minutes and is therefore unlikely to be due to temperature-related changes in protein expression. This surprising observation highlights the importance of host temperature not just as an environmental stressor for the parasite but also in disease treatment.
Finally, we offer the following speculation regarding the specific effect of fever on RBC occlusion in the microvasculature. First, for those RBCs already occluding a small vessel, increased stiffness associated with temperature will have little effect. But for those cells cytoadhered elsewhere throughout the vasculature, the cell's stiffness may increase its probability of detachment through increased shear stress associated with the cell's inability to deform in response. The effect of stiffening on detachment kinetics is an open question, but if the above suggestion is true, this mechanism may in turn lead to increased visits to and occlusion of the microvasculature. Second, we note that strict synchrony in the cycle of maturation and rupture would not lead to any direct influence of temperature, insofar as it would lead to a uniform population of ring stage RBCs, which have little potential for cytoadherence or mechanical occlusion. But synchrony is unusual in acute falciparum malaria, and indeed the distribution of parasite ages may not even exhibit unimodal characteristics (37). To the extent that there is a substantial population of late stage RBCs present at and following the febrile episode, the mechanisms outlined above may lead to pathophysiological consequences.
In conclusion, here we have identified membrane viscoelasticity as a key factor in loss of RBC deformability during the 48 h intra-erythrocytic life cycle of P. falciparum, and shown for the first time that fever magnifies the stiffening over and above that associated with parasitic maturation. These temperature-related changes are prompt and may have important consequences in the microcirculation.
NOTE ADDED IN PROOF
In the Articles in Press Version of this manuscript, several sentences, which represented descriptive general information not integral to the reporting of the research results, appeared without appropriate attribution. In this final published version, these sentences have been corrected.
GRANTS
M. Diez-Silva acknowledges support from the Global Enterprise for Micro-Mechanics and Molecular Medicine (GEM4). J. J. Fredberg acknowledges support from the National Heart, Lung, and Blood Institute (NHLBI) Grants HL-33009, HL-65960, and HL-59682. M. Marinkovic acknowledges support from the NHLBI Grant HL-007118 and REG from Harvard University. S. Suresh acknowledges support from the NIH/National Institutes of General Medical Sciences Grant 1-R01-GM076689-01, the Computational System Biology Program, the Singapore-MIT Alliance, and the Interdisciplinary Research Group on Infectious Diseases at the Singapore-MIT Alliance for Research and Technology Center.
DISCLOSURE
The authors declare no competing financial interest.
Supplementary Material
Acknowledgments
We thank Rebecca Stearns for helping with the electron microscopy, Sebastian D'Anna for assisting in the stage calibration, and Emil Millet for producing ferrimagnetic microbeads. We thank Dyann Wirth and Sarah Volkman for the use of their facilities.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Brandts CH, Ndjave M, Graninger W, Kremsner PG. Effect of paracetamol on parasite clearance time in Plasmodium falciparum malaria. Lancet 350: 704–709, 1997. [DOI] [PubMed] [Google Scholar]
- 2.Cooke BM, Mohandas N, Coppel RL. The malaria-infected red blood cell: structural and functional changes. Adv Parasitol 50: 1–86, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooke BM, Mohandas N, Coppel RL. Malaria and the red blood cell membrane. Semin Hematol 41: 173–188, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Cranston HA, Boylan CW, Carroll GL, Sutera SP, Williamson JR, Gluzman IY, Krogstad DJ. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science 223: 400–403, 1984. [DOI] [PubMed] [Google Scholar]
- 5.Discher DE, Carl P. New insights into red cell network structure, elasticity, and spectrin unfolding–a current review. Cell Mol Biol Lett 6: 593–606, 2001. [PubMed] [Google Scholar]
- 6.Dondorp AM, Angus BJ, Hardeman MR, Chotivanich KT, Silamut K, Ruangveerayuth R, Kager PA, White NJ, Vreeken J. Prognostic significance of reduced red blood cell deformability in severe falciparum malaria. Am J Trop Med Hyg 57: 507–511, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop 89: 309–317, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Fabry B, Maksym GN, Butler JP, Glogauer M, Navajas D, Taback NA, Millet EJ, Fredberg JJ. Time scale and other invariants of integrative mechanical behavior in living cells. Phys Rev E Stat Nonlin Soft Matter Phys 68: 041914, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Fabry B, Maksym GN, Shore SA, Moore PE, Panettieri RA Jr, Butler JP, Fredberg JJ. Selected contribution: time course and heterogeneity of contractile responses in cultured human airway smooth muscle cells. J Appl Physiol 91: 986–994, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol 67: 2408–2419, 1989. [DOI] [PubMed] [Google Scholar]
- 11.Glenister FK, Coppel RL, Cowman AF, Mohandas N, Cooke BM. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood 99: 1060–1063, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Hategan A, Sengupta K, Kahn S, Sackmann E, Discher DE. Topographical pattern dynamics in passive adhesion of cell membranes. Biophys J 87: 3547–3560, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser K, Camargo N, Kappe SH. Transformation of sporozoites into early exoerythrocytic malaria parasites does not require host cells. J Exp Med 197: 1045–1050, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwiatkowski D Febrile temperatures can synchronize the growth of Plasmodium falciparum in vitro. J Exp Med 169: 357–361, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Long HY, Lell B, Dietz K, Kremsner PG. Plasmodium falciparum: in vitro growth inhibition by febrile temperatures. Parasitol Res 87: 553–555, 2001. [DOI] [PubMed] [Google Scholar]
- 16.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol 119: 385–401, 1985. [PMC free article] [PubMed] [Google Scholar]
- 17.Mark JE, Erman B. Rubberlike Elasticity Cambridge. Cambridge University Press, 2007.
- 18.Marti M, Good RT, Rug M, Knuepfer E, Cowman AF. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306: 1930–1933, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature 415: 673–679, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Mills JP, Diez-Silva M, Quinn DJ, Dao M, Lang MJ, Tan KS, Lim CT, Milon G, David PH, Mercereau-Puijalon O, Bonnefoy S, Suresh S. Effect of plasmodial RESA protein on deformability of human red blood cells harboring Plasmodium falciparum. Proc Natl Acad Sci USA 104: 9213–9217, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nash GB, O'Brien E, Gordon-Smith EC, Dormandy JA. Abnormalities in the mechanical properties of red blood cells caused by Plasmodium falciparum. Blood 74: 855–861, 1989. [PubMed] [Google Scholar]
- 22.Naumann KM, Jones GL, Saul A, Smith R. A Plasmodium falciparum exo-antigen alters erythrocyte membrane deformability. FEBS Lett 292: 95–97, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Oakley MS, Kumar S, Anantharaman V, Zheng H, Mahajan B, Haynes JD, Moch JK, Fairhurst R, McCutchan TF, Aravind L. Molecular factors and biochemical pathways induced by febrile temperature in intraerythrocytic Plasmodium falciparum parasites. Infect Immun 75: 2012–2025, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulitschke M, Nash GB. Membrane rigidity of red blood cells parasitized by different strains of Plasmodium falciparum. J Lab Clin Med 122: 581–589, 1993. [PubMed] [Google Scholar]
- 25.Pavithra SR, Banumathy G, Joy O, Singh V, Tatu U. Recurrent fever promotes Plasmodium falciparum development in human erythrocytes. J Biol Chem 279: 46692–46699, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Pei X, Guo X, Coppel R, Bhattacharjee S, Haldar K, Gratzer W, Mohandas N, An X. The ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum stabilizes spectrin tetramers and suppresses further invasion. Blood 110: 1036–1042, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pei X, Guo X, Coppel R, Mohandas N, An X. Plasmodium falciparum erythrocyte membrane protein 3 (PfEMP3) destabilizes erythrocyte membrane skeleton. J Biol Chem 282: 26754–26758, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Puig-de-Morales-Marinkovic M, Turner KT, Butler JP, Fredberg JJ, Suresh S. Viscoelasticity of the human red blood cell. Am J Physiol Cell Physiol 293: C597–C605, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Raventos-Suarez C, Kaul DK, Macaluso F, Nagel RL. Membrane knobs are required for the microcirculatory obstruction induced by Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci USA 82: 3829–3833, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sherman IW Biochemistry of Plasmodium (malarial parasites). Microbiol Rev 43: 453–495, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sherman IW, Eda S, Winograd E. Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect 5: 897–909, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Suresh S, Spatz J, Mills JP, Micoulet A, Dao M, Lim CT, Beil M, Sefferlein T. Connections between single-cell biomechanics and human disease states: gastrointestinal cancer and malaria. Acta Biomaterialia 1: 16–30, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Suwanarusk R, Cooke BM, Dondorp AM, Silamut K, Sattabongkot J, White NJ, Udomsangpetch R. The deformability of red blood cells parasitized by Plasmodium falciparum and P. vivax J Infect Dis 189: 190–194, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Thomas SL, Lew VL. Plasmodium falciparum and the permeation pathway of the host red blood cell. Trends Parasitol 20: 122–125, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 193: 673–675, 1976. [DOI] [PubMed] [Google Scholar]
- 36.Treloar LRG The Physics of Rubber Elasticity: Oxford, 1958.
- 37.Udomsangpetch R, Pipitaporn B, Silamut K, Pinches R, Kyes S, Looareesuwan S, Newbold C, White NJ. Febrile temperatures induce cytoadherence of ring-stage Plasmodium falciparum-infected erythrocytes. Proc Natl Acad Sci USA 99: 11825–11829, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waugh R, Evans EA. Thermoelasticity of red blood cell membrane. Biophys J 26: 115–131, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yawata Y Red cell membrane protein band 4.2: phenotypic, genetic and electron microscopic aspects. Biochim Biophys Acta 1204: 131–148, 1994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.