Abstract
Arachidonic acid (AA) regulates intracellular calcium concentration ([Ca2+]i) in a variety of cell types including salivary cells. In the present study, the effects of serine/threonine phosphatases on AA-induced Ca2+ signaling in mouse parotid acini were determined. Mice were euthanized with CO2. Treatment of acini with the serine/threonine phosphatase inhibitor calyculin A blocked both thapsigargin- and carbachol-induced Ca2+ entry but resulted in an enhancement of AA-induced Ca2+ release and entry. Effects were mimicked by the protein phosphatase-1 (PP1) inhibitor tautomycin but were inhibited by the PP2A inhibitor okadaic acid. The protein kinase A (PKA) inhibitor PKI(14-22) significantly attenuated AA-induced enhancement of Ca2+ release and entry in the presence of calyculin A, whereas it had no effect on calyculin A-induced inhibition of thapsigargin-induced Ca2+ responses. The ryanodine receptor (RyR) inhibitor, tetracaine, and StHt-31, a peptide known to competitively inhibit type II PKA regulatory subunit binding to PKA-anchoring protein (AKAP), abolished calyculin A enhancement of AA-induced Ca2+ release and entry. StHt-31 also abolished forskolin potentiation of 4-chloro-3-ethylphenol (4-CEP) and AA on Ca2+ release but had no effect on 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cAMP potentiation of 4-CEP responses. Results suggest that inhibition of PP1 results in an enhancement of AA-induced [Ca2+]i via PKA, AKAP, and RyRs.
Keywords: calyculin A; protein kinase A-anchoring protein; ryanodine receptor; StHt-31; 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cAMP
regulation of the intracellular calcium concentration ([Ca2+]i) in nonexcitable cells is mediated by agonists that increase Ca2+ entry via depletion of intracellular Ca2+ stores, i.e., capacitative Ca2+ entry (CCE) (34). In several cell types, including parotid and pancreatic acinar cells (2, 19, 28, 30, 31, 40), Ca2+ entry is also regulated via a noncapacitative Ca2+ entry (NCCE) pathway. One activator of NCCE is arachidonic acid (AA) (28), an essential polyunsaturated fatty acid that plays an important role in intracellular signaling. Importantly, a distinction of the NCCE pathway is its dependence on low agonist concentrations (40). We recently reported that AA, at a concentration that inhibits the NCCE pathway [the arachidonate-regulated Ca2+ (ARC) channel (29)], regulates two Ca2+ entry pathways in mouse parotid acini; AA inhibited the CCE pathway and activated a second Ca2+ pathway that was not affected by gadolinium (Gd3+) but was dependent on depletion of Ca2+ from ryanodine-sensitive stores (46).
One way that [Ca2+]i can be regulated in cells is via serine/threonine phosphatases. Calcineurin, a protein phosphatase-2B (PP2B) inhibitor, for example, has been implicated in the modulation of ion channels including N-methyl-d-aspartate receptor channels (13); L-type Ca2+ channels (27); NCCE channels, i.e., ARC channels (29); and ryanodine receptor (RyR) channels (4). Less is known about serine/threonine phosphatases PP1 and PP2A, which have been reported to regulate carbachol- and thapsigargin-induced Ca2+ entry in parotid acinar cells (38, 43), lacrimal acini (51), and platelets (32). In parotid cells, the mechanism(s) involved was not defined, whereas in lacrimal cells and platelets, protein kinase C (PKC)-dependent phosphorylation of an unidentified target protein(s) was sensitive to PP1/PP2A.
Recent studies demonstrated that, in addition to store-operated Ca2+ entry, another pathway exists in thyroid FRTL-5 cells that is activated by calyculin A alone and that is not blocked by Gd3+ or 2-aminoethoxydiphenyl borate (14). Furthermore, this novel pathway is dependent on protein kinase A (PKA) (14). The aim of the present study was to determine the role of serine/threonine phosphatases in AA-induced regulation of [Ca2+]i in mouse parotid acini. We report that inhibition of serine/threonine phosphatase by calyculin A enhances AA-induced Ca2+ release and Ca2+ entry, whereas CCE is inhibited. Data further suggest that enhancement of AA-induced Ca2+ responses by calyculin A is dependent on PKA, acting via a PKA-anchoring protein (AKAP), and the release of Ca2+ from RyR-sensitive stores.
MATERIALS AND METHODS
Materials.
Materials were obtained as follows: arachidonic acid, thapsigargin, carbachol, forskolin, tetracaine, hyaluronidase, bovine serum albumin (BSA), HEPES, and 4-chloro-3-ethylphenol (4-CEP) from Sigma Chemical (St. Louis, MO); calyculin A, okadaic acid, and tautomycin from Calbiochem (La Jolla, CA); PKA-anchoring protein from Biomol Research Lab (Plymouth Meeting, PA); 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cAMP (8-pMeOPT-2′-O-Me-cAMP) from Axxora (San Diego, CA); collagenase type 2 (CLS2) from Worthington (Freehold, NJ); fura-2 AM from Molecular Probes (Eugene, OR); and StHt-31 from Promega (Madison, WI). Male Swiss Webster mice (27–30 g) were from Taconic (Germantown, NY). Incubation times for AA, thapsigargin, carbachol, and forskolin were based on our previous publications (47); StHt-31 and phosphatase inhibitor incubation times were based on the literature and preliminary studies.
Preparation of parotid acini.
Protocols and all experiments with animals were approved and conducted under the authority of the University of Washington Institutional Animal Care and Use Committee. Small groups of isolated mouse parotid cells (acini) from CO2-euthanized animals were prepared as described previously by Watson et al. (47).
Measurement of [Ca2+]i in intact cells.
Acini were suspended 1:50 (wt/vol) in Krebs-Henseleit bicarbonate (KHB) buffer containing 0.176 mg/ml ascorbic acid and 0.2% BSA, pH 7.4, and loaded with fura-2 AM at 3.3 μg/ml of cell suspension for 30 min at 37°C with continuous gassing (5% CO2-95% O2) and shaking. Fura-2 AM was prepared at 1 mg/ml in DMSO just before use. Loaded cells were washed three times in 0.2% BSA-KHB buffer, resuspended at 1:50 (wt/vol), and maintained at 24°C with gassing and shaking. Because AA binds to BSA (1), thus reducing the effective AA concentration, a concentration of AA (45 μM), in combination with a reduced level of BSA (0.025%), used to maintain cell integrity, was employed. The 0.025% BSA was sufficient to prevent cell leakage as determined by Trypan blue exclusion and illustrated by the thapsigargin studies conducted under identical incubation conditions (46). After a 25-min incubation period, an aliquot of cells was washed twice in the above buffers ± Ca2+, diluted 1:10, and placed in ultraviolet grade fluorometric cuvettes (Spectrocel). Calcium was measured with a Filterscan spectrofluorometer system equipped with a magnetic stirrer and constant temperature cuvette holder (Photon Technology International, South Brunswick, NJ). [Ca2+]i was calculated using the equation of Grynkiewicz et al. (15), where Kd = 224 nM. Data are expressed as the ratio of fura-2 fluorescence due to excitation at 340 nm to that due to excitation at 380 nm.
Data analysis.
Calcium release and entry data were collected as the ratio of the peak response, calculated as the average ± SE of n experiments and are expressed as the percent inhibition relative to maximal stimulation above control. Statistical analysis was performed using paired Student's t-test (P < 0.05).
RESULTS
Calyculin A augments AA-induced Ca2+ release and Ca2+ entry.
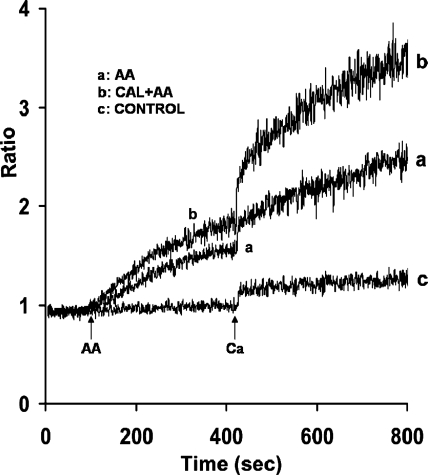
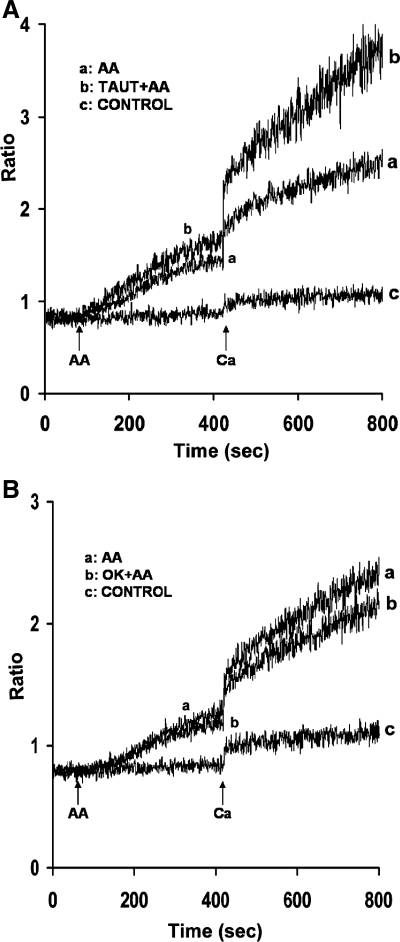
To determine whether serine/threonine phosphatases play a role in AA-induced Ca2+ release and entry, acini were incubated with specific serine/threonine phosphatase inhibitors, calyculin A, tautomycin, and okadaic acid. When parotid acini were preincubated for 10 min with calyculin A (100 nM), a PP1 and PP2A inhibitor, resting levels of [Ca2+]i were not affected. However, both AA-induced Ca2+ release and Ca2+ entry were significantly enhanced, i.e., by 30 ± 3.1% and 78.7 ± 7.1%, respectively (Fig. 1, trace b). Pretreatment of acini for 30 min with tautomycin, a PP1 inhibitor, mimicked the effects of calyculin A in enhancing AA-induced Ca2+ release by 29.3 ± 1.7% and Ca2+ entry by 88.8 ± 11% (Fig. 2A, trace b), whereas preincubation of acini for 45 min with okadaic acid, a PP2A inhibitor, had little effect on Ca2+ release, and inhibited Ca2+ entry, i.e., by 20 ± 3.9% (Fig. 2B, trace b).
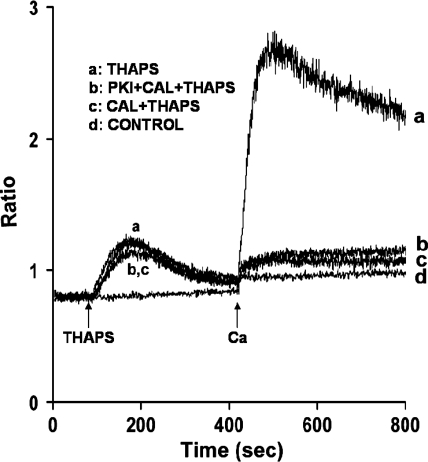
Fig. 1.
Effect of calyculin A (Cal) on arachidonic acid (AA)-induced Ca2+ release and Ca2+ entry in mouse parotid acini. Acini were incubated in nominally Ca2+-free Krebs-Henseleit bicarbonate (KHB) buffer in the absence (trace a) and presence (trace b) of calyculin A (100 nM) for 10 min (not recorded), before the addition, at 90 s, of AA (45 μM). Ca2+ (1.28 mM) was reintroduced at 420 s. Control (trace c) represents acini incubated in the presence of calyculin A alone, followed by the reintroduction of 1.28 mM Ca2+. Results are representative of 10 experiments (P < 0.05).
Fig. 2.
Effects of tautomycin (Taut) and okadaic acid (OK) on AA-induced Ca2+ release and Ca2+ entry in mouse parotid acini. Acini were incubated in nominally Ca2+-free KHB in the absence (trace a) and presence (trace b) of tautomycin (1 μM) (A) for 30 min (not recorded) and okadaic acid (1 μM) (B) for 30 min (not recorded), before the addition, at 90 s, of AA (45 μM). Ca2+ (1.28 mM) was reintroduced at 420 s. Control (trace c) represents acini incubated in the presence of tautomycin (A) and okadaic acid (B) alone, followed by the reintroduction of 1.28 mM Ca2+. Results are representative of four experiments (P < 0.05).
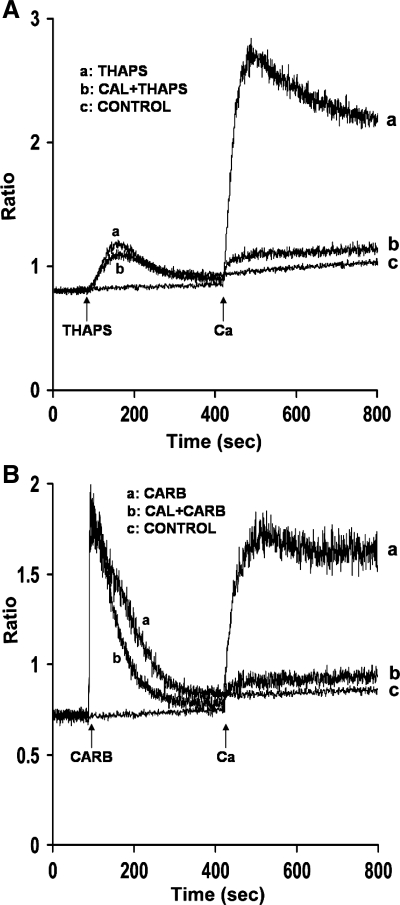
Because AA-induced Ca2+ entry in mouse parotid acini is distinct from CCE (46), we compared the effects of the serine/threonine phosphatase inhibitor, calyculin A, on AA-induced [Ca2+]i with effects on thapsigargin, a known activator of CCE. As shown in Fig. 3A (trace b), calyculin A (100 nM) attenuated thapsigargin (2 μM)-induced Ca2+ release by 29.3 ± 1.7% and Ca2+ entry by 84.0 ± 4.5%. A similar inhibiting effect by serine/threonine phosphatase inhibitors on thapsigargin-induced Ca2+ responses was previously observed in rat parotid acinar cells (38, 43) and thyroid FRTL-5 cells (14). Calyculin A also inhibited the effects of the G protein-coupled receptor agonist carbachol (10 μM) on Ca2+ entry, and it decreased the time required for Ca2+ to return to basal values (Fig. 3B, trace b). Whether calyculin A increases Ca2+ uptake into intracellular stores or increases removal from the cell has yet to be determined. Importantly, data indicate that inhibition of serine/threonine phosphatase, specifically PP1, results in augmentation of AA-induced [Ca2+]i, suggesting that a phosphorylation event is involved in regulating both Ca2+ release and Ca2+ entry.
Fig. 3.
Effects of calyculin A on thapsigargin (Thaps) and carbachol (Carb)-induced Ca2+ release and Ca2+ entry in mouse parotid acini. Acini were incubated in nominally Ca2+-free KHB in the absence (trace a) and presence (trace b) of calyculin A (100 nM) for 10 min (not recorded), before the addition, at 90 s, of thapsigargin (2 μM) (A) and carbachol (10 μM) (B). Ca2+ (1.28 mM) was reintroduced at 420 s. Control (trace c) represents acini incubated in the presence of calyculin A alone, followed by the reintroduction of 1.28 mM Ca2+. Results are representative of four experiments (P < 0.05).
Calyculin A enhancement of AA-induced Ca2+ release and Ca2+ entry involves PKA.
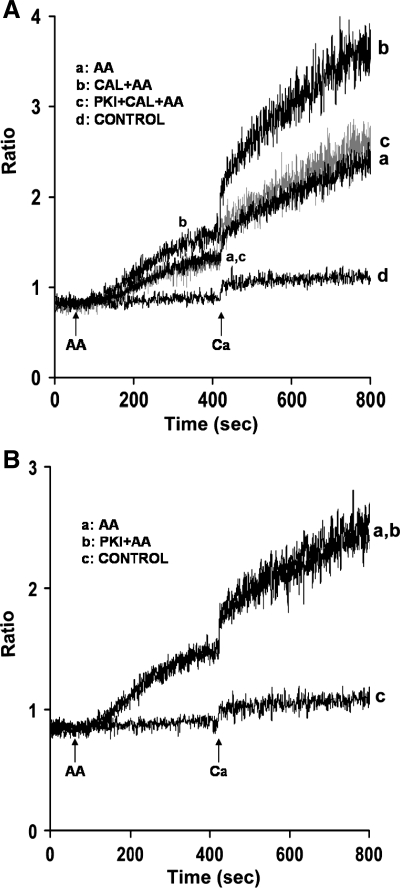
In the next series of experiments, the mechanism(s) by which calyculin A enhances AA-induced Ca2+ release and entry was determined. In thyroid FRTL-5 cells, phosphatase inhibition by calyculin A revealed a Ca2+ entry pathway dependent on PKA (14). To determine whether PKA is also involved in calyculin A augmentation of AA-induced Ca2+ release and entry in mouse parotid cells, acini were incubated with the PKA inhibitor PKI(14-22) (1 μM) for 30 min before the addition of AA. In the presence of calyculin A, PKI(14-22) reversed AA-induced augmentation of Ca2+ release and significantly inhibited Ca2+ entry by 87.6 ± 2.4% (Fig. 4A, trace c). In the absence of calyculin A, PKI(14-22) had no effect on either AA-induced Ca2+ release or Ca2+ entry (Fig. 4B). In contrast to PKA playing a role in calyculin A augmentation of AA-induced Ca2+ responses, thapsigargin-induced Ca2+ release and entry (Fig. 5, trace b), as well as carbachol-induced Ca2+ responses (data not shown), in the presence of calyculin A, were unaffected by PKA.
Fig. 4.
Effects of the PKA inhibitor, PKI(14-22) on AA-induced Ca2+ release and Ca2+ entry in the absence and presence of calyculin A in mouse parotid acini. A: acini were incubated in nominally Ca2+-free buffer, in the absence (traces a and b) and presence (trace c) of PKI(14-22) (1 μM) for 30 min (not recorded), and in the absence (trace a) and presence (traces b and c) of calyculin A (100 nM) for an additional 10 min, before the addition, at 60 s, of AA (45 μM). Ca2+ (1.28 mM) was reintroduced at 420 s. Control (trace d) represents acini incubated in the presence of calyculin A (100 nM) and PKI(14-22) (1 μM), followed by the reintroduction of 1.28 mM Ca2+. Results are representative of five experiments (P < 0.05). B: acini were incubated in nominally Ca2+-free buffer in the absence (trace a) and presence (trace b) of PKI(14-22) (1 μM) for 30 min (not recorded), before the addition, at 60 s, of AA (45 μM). Ca2+ (1.28 mM) was reintroduced at 420 s. Control (trace c) represents acini incubated with PKI(14-22) (1 μM) followed by the reintroduction of 1.28 mM Ca2+. Results are representative of five experiments (P < 0.05).
Fig. 5.
Effects of calyculin A on thapsigargin-induced Ca2+ release and Ca2+ entry in the absence and presence of the PKA inhibitor PKI(14-22) in mouse parotid acini. Acini were incubated in nominally Ca2+-free buffer, in the absence (traces a and c) and presence (trace b) of PKI(14-22) (1 μM), for 30 min (not recorded), and in the absence (trace a) and presence (traces b and c) of calyculin A (100 nM) for an additional 10 min (not recorded), before the addition, at 90 s, of thapsigargin (2 μM). Ca2+ (1.28 mM) was reintroduced at 420 s. Control (trace d) represents acini incubated in the presence of calyculin A (100 nM) plus PKI(14-22) (1 μM) followed by the reintroduction of 1.28 mM Ca2+. Results are representative of three experiments (P < 0.05).
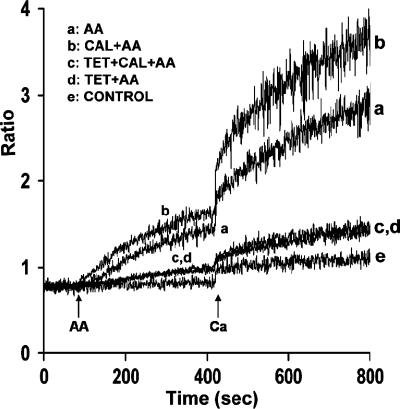
Enhancement of AA-induced- Ca2+ release is dependent on ryanodine-sensitive stores.
Previous studies from our laboratory demonstrated that RyRs are present in mouse parotid acini (9) and that AA induces release of Ca2+ from ryanodine-sensitive stores (46). Thus we determined whether AA-induced augmentation of Ca2+ entry by calyculin A was dependent on the release of Ca2+ from ryanodine-sensitive stores. For these studies, we used the RyR-sensitive store antagonist tetracaine, previously shown to inhibit AA-induced Ca2+ increase from parotid acini (46). As shown in Fig. 6 (trace b), calyculin A augmented AA-induced Ca2+ release and Ca2+ entry as described in Fig. 1. In the presence of tetracaine (500 μM), augmentation of AA-induced Ca2+ release and Ca2+ entry by calyculin A was reduced by 82.1 ± 3% and 76.4 ± 4%, respectively (Fig. 6, trace c). Tetracaine also blocked AA-induced Ca2+ release and Ca2+ entry by 82.6 ± 3.8% and 74.7% ± 2.3%, respectively, in the absence of calyculin A (Fig. 6, trace d), as previously reported (46).
Fig. 6.
Effects of tetracaine (Tet) on AA-induced Ca2+ release and Ca2+ entry in the absence and presence of calyculin A. Acini were incubated in nominally Ca2+-free buffer in the absence (traces a and b) and presence (traces c and d) of tetracaine (500 μM) for 5 min (not recorded), and in the absence (traces a and d) and presence (traces b and c) of calyculin A for 10 min (not recorded), before the addition, at 90 s, of AA (45 μM). Ca2+ (1.28 mM) was reintroduced at 420 s. Control (trace e) represents acini incubated in the presence of calyculin A (100 nM) plus tetracaine (500 μM) followed by the reintroduction of 1.28 mM Ca2+. Results are representative of three experiments (P < 0.05).
Forskolin potentiation of 4-CEP and AA on [Ca2+]i.
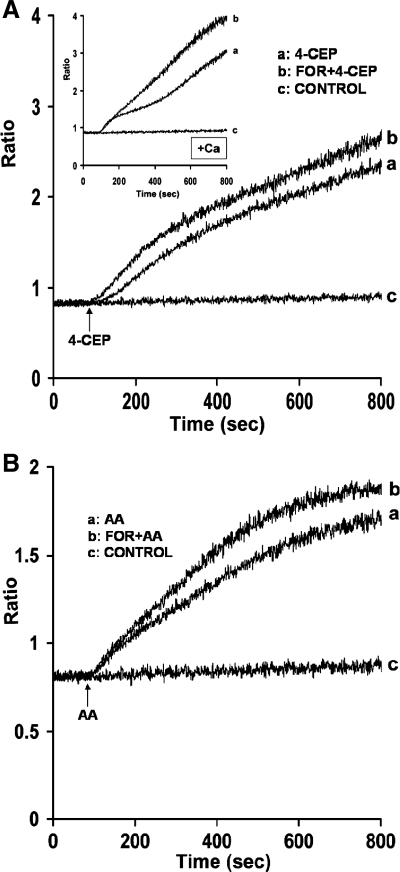
Since data suggest that AA releases Ca2+ from ryanodine-sensitive stores in parotid acini, we determined whether PKA directly activates RyRs. In initial experiments, we determined whether forskolin, a known activator of PKA, releases Ca2+ from RyR-sensitive stores activated by 4-CEP. Acini were treated with forskolin (10 μM) for 10 min, and the effects of 4-CEP on Ca2+ release and entry were determined. As shown in Fig. 7A, trace a, 4-CEP (200 μM) produced a slow increase in Ca2+ release as previously reported in mouse parotid acini (3, 46). In the presence of forskolin (10 μM), effects of 4-CEP on Ca2+ release were potentiated (trace b). Forskolin (10 μM) also potentiated the effects of 4-CEP on Ca2+ entry (Fig. 7A, inset trace b). Forskolin alone, however, had no effect on resting [Ca2+]. In the second experiment, we determined whether forskolin also potentiates the effects of AA (45 μM) on Ca2+ release, because this compound was reported to enhance the binding of [3H]ryanodine to mouse parotid microsomal membranes (9) and to activate RyRs in isolated parotid acini (46). As shown in Fig. 7B (trace b), addition of forskolin (25 μM) to parotid acini also potentiated the effects of AA (45 μM) on Ca2+ release. Forskolin (10 μM) also potentiated AA-induced Ca2+ release, but to a lesser extent. Results thus suggested that under these conditions, PKA directly releases Ca2+ from ryanodine stores in mouse parotid acini.
Fig. 7.
Effects of forskolin (For) on 4-chloro-3-ethylphenol (4-CEP) and AA-induced Ca2+ release from mouse parotid acini. A: acini were incubated in a nominally Ca2+-free buffer in the absence (trace a) and presence (trace b) of forskolin (10 μM) for 10 min (not recorded), before the addition, at 90 s, of 4-CEP (200 μM). Inset: acini were incubated in a Ca2+-containing buffer in the absence (trace a) and presence (trace b) of forskolin (10 μM) for 10 min (not recorded), before the addition of 4-CEP (200 μM). B: acini were incubated in a nominally Ca2+-free buffer in the absence (trace a) and presence (trace b) of forskolin (25 μM) for 10 min (not recorded), before the addition, at 90 s, of AA (45 μM). Controls (trace c) represent acini incubated without agonists. Results are representative of three experiments (P < 0.05).
AA-induces Ca2+ release via an A-kinase PKA-anchoring protein (AKAP).
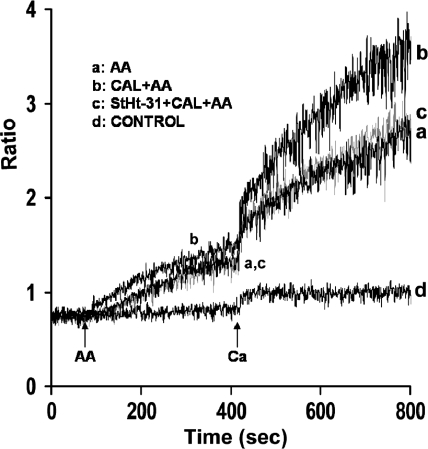
RyRs have been reported to exist in a macromolecular signaling complex with PKA, AKAP, and phosphatases (6, 26). Furthermore, PKA is known to be targeted to specific subcellular locations through the association with AKAPs (7, 11, 24, 33, 36). Because results indicated that calyculin A produces augmentation of AA-induced Ca2+ entry by releasing Ca2+ from ryanodine stores sensitive to PKA, further studies were conducted to evaluate the role of AKAP in PKA regulation of Ca2+ release and entry in parotid acini. For these studies, we used the cell- permeable peptide inhibitor StHt-31 (10, 45), which inhibits type II PKA regulatory subunit (RII) binding to AKAP (12). Acini were pretreated with StHt-31 (10 μM) for 30 min before the addition of calyculin A (100 nM) for an additional 10 min before the addition of AA (45 μM). As shown in Fig. 8 (trace c), augmentation of AA-induced Ca2+ release and entry by calyculin A (100 nM) was almost completely blocked in the presence of StHt-31. StHt-31, alone, elicited no effect on AA-induced Ca2+ responses (data not shown), consistent with the lack of effect of PKA noted under these conditions.
Fig. 8.
Effects of StHt-31 on AA-induced Ca2+ release and Ca2+ entry in mouse parotid acini. Acini were incubated in a nominally Ca2+-free buffer, in the absence (traces a and b) and presence (trace c) of StHt-31 (10 μM) for 30 min (not recorded), and in the absence (trace a) and presence (traces b and c) of calyculin A (100 nM) for an additional 10 min (not recorded), before the addition, at 90 s, of AA (45 μM). Ca2+ (1.28 mM) was reintroduced at 420 s. Control (trace d) represents acini incubated in the presence of StHt-31 (10 μM) and calyculin A (100 nM) followed by the reintroduction of 1.28 mM Ca2+. Results are representative of three experiments (P < 0.05).
Forskolin potentiates 4-CEP and AA-induced Ca2+ release via an A-kinase PKA-anchoring protein (AKAP).
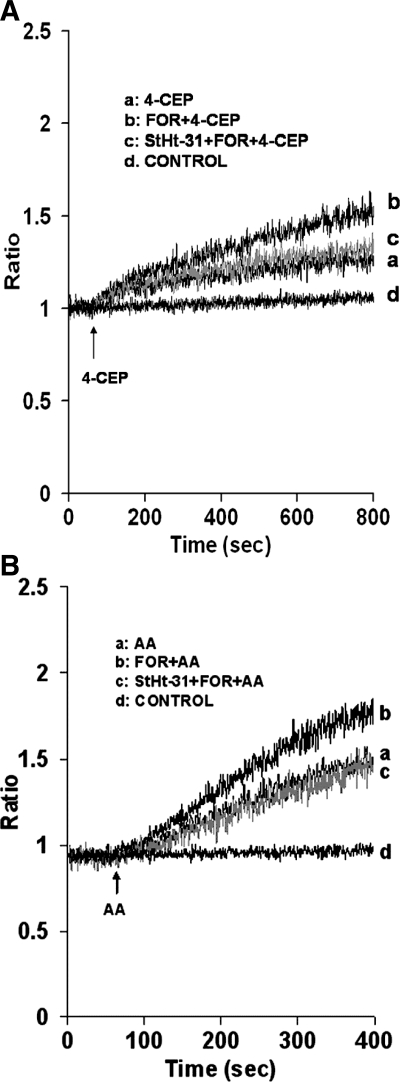
As proof of specificity and association between AKAP and RyRs in parotid cells, we further examined the effects of StHt-31 on forskolin enhancement of 4-CEP, as well as on forskolin enhancement of AA-induced Ca2+ release. Acini were treated with StHt-31 (10 μM) for 30 min and forskolin (25 μM) for an additional 10 min before the addition of 4-CEP (200 μM; Fig. 9A) or AA (45 μM; Fig. 9B). StHt-31 reversed forskolin augmentation of both 4-CEP and AA-induced Ca2+ release by 88 ± 7% and 94% ± 2.3, respectively (Fig. 9, A and B, trace c). StHt-31, alone, elicited no effect on Ca2+ release (Fig. 9, A and B, trace d).
Fig. 9.
Effects of StHt-31 on forskolin augmentation of 4-CEP and AA-induced Ca2+ release in mouse parotid acini. Acini were incubated in a nominally Ca2+-free buffer, in the absence (traces a and b) and presence (trace c) of StHt-31 (10 μM) for 30 min (not recorded), and in the absence (trace a) and presence (traces b and c) of forskolin (25 μM) for an additional 10 min (not recorded), before the addition, at 60 s, of 4-CEP (200 μM) (A) and AA (45 μM) (B). Controls (trace d) represent acini incubated with StHt-31 alone. Results are representative of four experiments (P < 0.05).
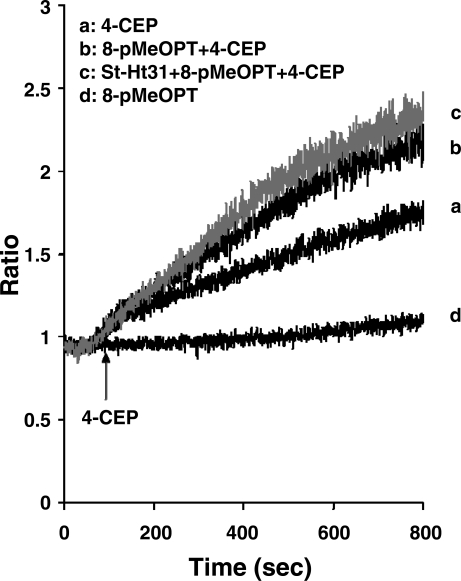
As further proof that forskolin augmentation of 4-CEP-induced Ca2+ release is due to PKA, rather than to stimulation of Epac, a cAMP-regulated guanine nucleotide exchange factor reported to affect the release of Ca2+ from intracellular stores (17, 20), we examined the effect of StHT-31 on Epac augmentation of 4-CEP-induced Ca2+ release. Acini were treated with the Epac activator 8-pMeOPT-2′-O-Me-cAMP (100 μM) for 10 min before the addition of 4-CEP (200 μM). Results presented in Fig. 10 show that 8-pMeOPT-2′-O-Me-cAMP also augmented 4-CEP-stimulated Ca2+ release. However, treatment of acini with StHt-31 (10 μM) for 30 min before the addition of 8-pMeOPT-2′-O-Me-cAMP (100 μM) did not abolish this response.
Fig. 10.
Effects of StHt-31 on 8-(4-methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cAMP (8-pMeOPT) augmentation of 4-CEP in mouse parotid acini. Acini were incubated in a nominally Ca2+-free buffer, in the absence (traces a and b), and presence (trace c) of StHt-31 (10 μM) for 30 min (not recorded), and in the absence (trace a) and presence (traces b and c) of 8-pMeOPT (100 μM) for an additional 10 min (not recorded), before the addition, at 60 s, of 4-CEP (200 μM). Controls (trace d) represent acini incubated with StHt-31 alone. Results are representative of four experiments (P < 0.05).
DISCUSSION AND CONCLUSIONS
We previously reported that AA regulates Ca2+ entry by depletion of Ca2+ from ryanodine-sensitive intracellular stores (46). In the present study we used serine/threonine phosphatase inhibitors, i.e., calyculin A, okadaic acid, and tautomycin, to determine the identity and role of the phosphatases in regulating AA-induced Ca2+ signaling. The effects of calyculin A appear to be agonist dependent, because calyculin A enhanced AA-induced Ca2+ responses but inhibited both thapsigargin and carbachol-induced Ca2+ responses. The major finding of the present study is that inhibition of serine/threonine phosphatase PP1 augments AA-induced Ca2+ entry in mouse parotid acini by a mechanism involving PKA-mediated Ca2+ release from ryanodine-sensitive stores. Inhibition of serine/threonine phosphate by calyculin A, alone, was previously reported to increase Ca2+ entry in FRTL-5 cells (14), whereas, in parotid acini, pretreatment of acini with calyculin A for 30 min before the addition of AA did not change basal Ca2+ levels. Furthermore, in parotid acini, calyculin A increased AA-induced Ca2+ entry by releasing Ca2+ from ryanodine-sensitive stores, whereas calyculin A-induced Ca2+ entry in FRTL-5 cells was independent of intracellular stores (14).
Importantly, in the present study, PKA had no effect on AA-induced Ca2+ release or entry in the absence of calyculin A. Inhibition of endogenous PP1 was required to observe effects of PKA on [Ca2+]i . This suggests that inhibition of serine/threonine phosphatase increases the activity of PKA sufficient to release intracellular Ca2+ stores. Common to both FRTL-5 cells (14) and parotid acini, however, is the observation that increases in Ca2+ entry by calyculin A are not blocked by Gd3+ (data not shown), an inhibitor shown to block store-operated Ca2+ channels, but not AA-induced Ca2+ entry in mouse parotid acini (46). In both thyroid and FRTL-5 cells and parotid cells, the Ca2+ entry channel affected by calyculin A is not known and will require further investigation.
An important aspect of the present study is the dependence of AA-induced Ca2+ entry on PKA, observed only in the presence of calyculin A. This suggests that inhibition of serine/threonine phosphatase increases the activity of PKA sufficient to release intracellular Ca2+ stores. Protein phosphatases regulate a large number of cellular functions and signal transduction pathways in cooperation with protein kinase; they dephosphorylate a large number of substrates (6). PP1 is found in nearly all cellular compartments and is thought to be targeted to intracellular organelles including the sarcoplasmic and endoplasmic reticulum (8, 18). The free catalytic subunit of PP1 activity has been referred to as promiscuous because of its measured high activity and low specificity (22). High intrinsic activity is necessary to dephosphorylate and thereby downregulate ion channels (42).
A major mechanism for regulating native forms of PPI is phosphorylation of its regulatory subunits by PKA, PKC, myosin light-chain kinase, and casein kinase II (41). PP1 activity is also regulated by endogenous inhibitor proteins (39) as well as exogenous products that inhibit serine/threonine phosphatase 1, e.g., tautomycin (41). Among its many functions, PP1 is an enzyme that promotes the rational use of energy. Reversal of the cell to a basal and/or energy-conserving state plays a key role in recovery from stress, promotes apoptosis when cells are damaged beyond repair, downregulates ion pumps and transporters and ion channels in various tissues, and promotes the exit from mitosis and maintains cells in the G1 or G2 phases of the cell cycle (6). In the present study, ryanodine-associated PP1 is thought to counteract PKA, i.e., to dephosphorylate RyR subunits and the conductance of RyR channels (16, 49). Calcium release channels, i.e., RyR and inositol 1,4,5-trisphosphate (IP3) receptor (IP3R), are part of a macromolecular complex containing AKAP-6, PP1, and PP2A (37) and are targets for PP1 in parotid cells. PP1 activity is regulated in this complex to ensure appropriate responses to extracellular signals.
In our study, we found that inhibition of PP1 increased Ca2+ release/entry by increasing PKA phosphorylation of the RyR Ca2+ release channel. The localization of PKA near a particular substrate appears to be highly important for the regulation of specific physiological events (7). RyRs are known to be regulated by PKA phosphorylation (37). Previous studies provided evidence for PKA phosphorylation of IP3R following stimulation with physiological levels of CCK in pancreatic acinar cells (23) and carbachol in mouse parotid cells (3). In isolated parotid acinar cells, Bruce et al. (3) reported that forskolin potentiates carbachol-evoked increases in Ca2+ from IP3-sensitive stores, whereas no evidence was obtained to support direct PKA-mediated activation of RyRs. In contrast, calyculin A potentiated AA-induced Ca2+ release in the present study, by a mechanism involving PKA regulation of RyR-sensitive stores. That PKA regulates ryanodine-sensitive stores, and not IP3-sensitive stores, in the presence of AA and calyculin A, is supported by the following data: 1) AA alone increased Ca2+ release in mouse parotid acini from ryanodine and not IP3 stores (46); 2) tetracaine, an inhibitor of RyRs, prevented AA-induced Ca2+ release and entry in parotid acini treated without (46) and with calyculin A; 3) AA-enhancement of Ca2+ release by calyculin A was inhibited by PKI(14-22); and 4) forskolin enhanced 4-CEP and AA on Ca2+ release, both of which were reversed by StHt-31. A recent study by Luo et al. (25) also indicates that AA, at low and high concentrations, releases Ca2+ from RyR stores and increases Ca2+ entry in human embryonic kidney 293 cells.
In contrast to our findings, Bruce et al. (3) failed to observe forskolin augmentation of 4-chloro-m-cresol on Ca2+ release in mouse parotid cells, at a forskolin concentration used in the present study. Because RyRs are in low abundance, compared with IP3Rs in nonexcitable cells, including mouse parotid cells (9), Bruce et al. (3) were unable to observe phosphorylation of RyRs by PKA. To circumvent this problem, we determined whether there was an association between PKA and RyRs by demonstrating that forskolin enhancement of 4-CEP, as well as AA, on Ca2+ release is blocked by StHt-31. Although mouse parotid acini were used in both studies, there were differences in cell preparations as well as in mouse strains. Over the years, we have found that responses to a given agonist can differ significantly depending on the mouse strain. Differences in responses to forskolin may also be related to the use of cell suspensions in this study, rather than single cells. Cyclic AMP was also reported to release Ca2+ from ryanodine stores in rat parotid suspensions (48). Although Bruce et al. (3) reported PKA-mediated phosphorylation of IP3Rs, but not RyRs, forskolin had no affect on resting [Ca2+]i.
In the present study, we also found that forskolin (10 μM) alone had no effect on [Ca2+]i. However, in the presence of either 4-CEP or AA, forskolin enhanced Ca2+ release via an effect of PKA on RyRs. Importantly, although augmentation of 4-CEP was mimicked by the Epac activator 8-pMeOPT-2′-O-Me-cAMP, Ca2+ release was not blocked by StHt-31. Therefore, an important question relates to how PKA activates the RyR. Failure to observe PKA phosphorylation of RyR may be related to the fact that RyRs may not be directly phosphorylated. A recent study by Zissimopoulos et al. (50) showed that RyRs, i.e., RyR1, RyR2, and RyR3, bind to snapin, a ubiquitously expressed SNARE-associated protein in nonneuronal cells (5). Further studies will be required to determine whether there is an association between RyRs and snapin in parotid cells, and whether PKA phosphorylation of snapin regulates Ca2+ release.
In establishing a role for PKA in calyculin A augmentation of AA-induced Ca2+ release and entry, we also found that StHt-31, a peptide known to inhibit RII binding to AKAP (12), significantly blocked enhancement of AA-induced Ca2+ release and entry. AKAPs are expressed in salivary cells (21) and are typically part of a complex that includes not only PKA (10, 12, 35) but also serine/threonine phosphatases and IP3R/RyR (37, 44). Importantly, by anchoring PKA in close proximity to RyR, AKAP regulates RyR function in muscle cells (37). To date, no studies have been reported that address the role of a PKA/AKAP/RyR complex in nonmuscle cells. Our data suggest that a similar complex may be operative in parotid cells and would represent the first report of such a complex existing in nonexcitable cells. Enhanced PKA activity causes a maintained potentiation of local Ca2+ release signals.
Data presented show that treatment of acini with calyculin A produced differential effects on AA and thapsigargin/carbachol-induced Ca2+ signaling. Whereas calyculin A attenuated thapsigargin- and carbachol-induced Ca2+ signaling, AA-induced Ca2+ release and entry were augmented, and by a novel mechanism involving the serine/threonine phosphatase, PP1, PKA, and an AKAP. Protein phosphorylation and dephosphorylation are considered to be key steps in the control of secretion. A major function of parotid acinar cells is to secrete water and electrolytes and proteins that are necessary for oral health. Acinar secretion is highly dependent on Ca2+. Thus, in our study, inhibition of PP1 by calyculin A and an increase in AA-induced [Ca2+]i would lead to an increase in amylase secretion from mouse parotid acini.
GRANTS
This work was supported by National Institute of Dental and Craniofacial Research Grant DE-05249.
Acknowledgments
We thank Kerry L. Jacobson for assisting in the Ca2+ experiments.
Permanent address of T. Saino, Department of Histology, School of Medicine, Iwate Medical University, Morioka, Iwate, Japan 020-8505 (e-mail: tsaino@iwate-med.ac.jp).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Bojesen IN, Bojesen E. Binding of arachidonate and oleate to bovine serum albumin. J Lipid Res 35: 770–778, 1994. [PubMed] [Google Scholar]
- 2.Broad LM, Cannon TR, Taylor CW. A non-capacitative pathway activated by arachidonic acid is the major Ca2+ entry mechanism in rat A7r5 smooth muscle cells stimulated with low concentrations of vasopressin. J Physiol 517: 121–134, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce JI, Shuttleworth TJ, Giovannucci DR, Yule DI. Phosphorylation of inositol 1,4,5-trisphosphate receptors in parotid acinar cells. A mechanism for the synergistic effects of cAMP on Ca2+ signaling. J Biol Chem 277: 1340–1348, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bultynck G, Vermassen E, Szlufcik K, De Smet P, Fissore RA, Callewaert G, Missiaen L, De Smedt H, Parys JB. Calcineurin and intracellular Ca2+-release channels: regulation or association? Biochem Biophys Res Commun 311: 1181–1193, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Buxton P, Zhang XM, Walsh B, Sriratana A, Schenberg I, Manickam E, Rowe T. Identification and characterization of Snapin as a ubiquitously expressed SNARE-binding protein that interacts with SNAP23 in non-neuronal cells. Biochem J 375: 433–440, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceulemans H, Bollen M. Functional diversity of protein phosphatase-1, a cellular economizer and reset button. Physiol Rev 84: 1–39, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Dell'Acqua ML, Scott JD. Protein kinase A anchoring. J Biol Chem 272: 12881–12884, 1997. [DOI] [PubMed] [Google Scholar]
- 8.DeSouza N, Reiken S, Ondrias K, Yang YM, Matkovich S, Marks AR. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J Biol Chem 277: 39397–39400, 2002. [DOI] [PubMed] [Google Scholar]
- 9.DiJulio DH, Watson EL, Pessah IN, Jacobson KL, Ott SM, Buck ED, Singh JC. Ryanodine receptor type III (Ry3R) identification in mouse parotid acini. Properties and modulation of [3H]ryanodine-binding sites. J Biol Chem 272: 15687–15696, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Dodge K, Scott JD. AKAP79 and the evolution of the AKAP model. FEBS Lett 476: 58–61, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Faux MC, Scott JD. More on target with protein phosphorylation: conferring specificity by location. Trends Biochem Sci 21: 312–315, 1996. [PubMed] [Google Scholar]
- 12.Feliciello A, Gottesman ME, Avvedimento EV. The biological functions of A-kinase anchor proteins. J Mol Biol 308: 99–114, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Ghetti A, Heinemann SF. NMDA-dependent modulation of hippocampal kainate receptors by calcineurin and Ca(2+)/calmodulin-dependent protein kinase. J Neurosci 20: 2766–2773, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratschev D, Blom T, Bjorklund S, Tornquist K. Phosphatase inhibition reveals a calcium entry pathway dependent on protein kinase A in thyroid FRTL-5 cells: comparison with store-operated calcium entry. J Biol Chem 279: 49816–49824, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 16.Hain J, Nath S, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from skeletal muscle. Biophys J 67: 1823–1833, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holz GG Epac: a new cAMP-binding protein in support of glucagon-like peptide-1 receptor-mediated signal transduction in the pancreatic beta-cell. Diabetes 53: 5–13, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubbard MJ, Dent P, Smythe C, Cohen P. Targetting of protein phosphatase 1 to the sarcoplasmic reticulum of rabbit skeletal muscle by a protein that is very similar or identical to the G subunit that directs the enzyme to glycogen. Eur J Biochem 189: 243–249, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Ito K, Rome C, Bouleau Y, Dulon D. Substance P mobilizes intracellular calcium and activates a nonselective cation conductance in rat spiral ganglion neurons. Eur J Neurosci 16: 2095–2102, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta-cells. J Biol Chem 278: 8279–8285, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurihara K, Nakanishi N. Regulation of Na,K-ATPase by cAMP-dependent protein kinase anchored on membrane via A-kinase anchoring protein subtype, AKAP-150, in rat parotid gland. Ann NY Acad Sci 986: 636–638, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Lad C, Williams NH, Wolfenden R. The rate of hydrolysis of phosphomonoester dianions and the exceptional catalytic proficiencies of protein and inositol phosphatases. Proc Natl Acad Sci USA 100: 5607–5610, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeBeau AP, Yule DI, Groblewski GE, Sneyd J. Agonist-dependent phosphorylation of the inositol 1,4,5-trisphosphate receptor: a possible mechanism for agonist-specific calcium oscillations in pancreatic acinar cells. J Gen Physiol 113: 851–872, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lester LB, Scott JD. Anchoring and scaffold proteins for kinases and phosphatases. Recent Prog Horm Res 52: 409–429, 1997. [PubMed] [Google Scholar]
- 25.Luo D, Sun H, Lan X, Xiao R, Han Q. Direct coupling between arachidonic acid-induced Ca2+ release and Ca2+ entry in HEK293 cells. Prostaglandins Other Lipid Mediat 75: 141–151, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101: 365–376, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Matthes J, Jager A, Handrock R, Groner F, Mehlhorn U, Schwinger RH, Varadi G, Schwartz A, Herzig S. Ca2+-dependent modulation of single human cardiac L-type calcium channels by the calcineurin inhibitor cyclosporine. J Mol Cell Cardiol 36: 241–255, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Mignen O, Thompson JL, Shuttleworth TJ. Arachidonate-regulated Ca2+-selective (ARC) channel activity is modulated by phosphorylation and involves an A-kinase anchoring protein. J Physiol 567: 787–798, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mignen O, Thompson JL, Shuttleworth TJ. Ca2+ selectivity and fatty acid specificity of the noncapacitative, arachidonate-regulated Ca2+ (ARC) channels. J Biol Chem 278: 10174–10181, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Moneer Z, Dyer JL, Taylor CW. Nitric oxide co-ordinates the activities of the capacitative and non-capacitative Ca2+-entry pathways regulated by vasopressin. Biochem J 370: 439–448, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Munaron L, Antoniotti S, Distasi C, Lovisolo D. Arachidonic acid mediates calcium influx induced by basic fibroblast growth factor in Balb-c 3T3 fibroblasts. Cell Calcium 22: 179–188, 1997. [DOI] [PubMed] [Google Scholar]
- 32.Murphy CT, Bullock AJ, Westwick J. A role for protein phosphorylation in modulating Ca2+ elevation in rabbit platelets treated with thapsigargin. Biochem J 313: 83–89, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pawson T, Scott JD. Signaling through scaffold, anchoring, and adaptor proteins. Science 278: 2075–2080, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Putney JW Capacitative calcium entry revisited. Cell Calcium 11: 611–624, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Rosenmund C, Carr DW, Bergeson SE, Nilaver G, Scott JD, Westbrook GL. Anchoring of protein kinase A is required for modulation of AMPA/kainate receptors on hippocampal neurons. Nature 368: 853–856, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Rubin RP, Adolf MA. Cyclic AMP regulation of calcium mobilization and amylase release from isolated permeabilized rat parotid cells. J Pharmacol Exp Ther 268: 600–606, 1994. [PubMed] [Google Scholar]
- 37.Ruehr ML, Russell MA, Ferguson DG, Bhat M, Ma J, Damron DS, Scott JD, Bond M. Targeting of protein kinase A by muscle A kinase-anchoring protein (mAKAP) regulates phosphorylation and function of the skeletal muscle ryanodine receptor. J Biol Chem 278: 24831–24836, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Sakai T, Ambudkar IS. Role for protein phosphatase in the regulation of Ca2+ influx in parotid gland acinar cells. Am J Physiol Cell Physiol 271: C284–C294, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Shenolikar S Protein phosphatase regulation by endogenous inhibitors. Semin Cancer Biol 6: 219–227, 1995. [DOI] [PubMed] [Google Scholar]
- 40.Shuttleworth TJ Arachidonic acid activates the noncapacitative entry of Ca2+ during [Ca2+]i oscillations. J Biol Chem 271: 21720–21725, 1996. [DOI] [PubMed] [Google Scholar]
- 41.Sim AT, Baldwin ML, Rostas JA, Holst J, Ludowyke RI. The role of serine/threonine protein phosphatases in exocytosis. Biochem J 373: 641–659, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang TS, Tu H, Wang Z, Bezprozvanny I. Modulation of type 1 inositol (1,4,5)-trisphosphate receptor function by protein kinase a and protein phosphatase 1alpha. J Neurosci 23: 403–415, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tojyo Y, Tanimura A, Matsumoto Y. Suppression of capacitative Ca2+ entry by serine/threonine phosphatase inhibitors in rat parotid acinar cells. Jpn J Pharmacol 69: 381–389, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Tu H, Wang Z, Nosyreva E, De Smedt H, Bezprozvanny I. Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys J 88: 1046–1055, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vijayaraghavan S, Olson GE, NagDas S, Winfrey VP, Carr DW. Subcellular localization of the regulatory subunits of cyclic adenosine 3′,5′-monophosphate-dependent protein kinase in bovine spermatozoa. Biol Reprod 57: 1517–1523, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Watson EL, Jacobson KL, Singh JC, DiJulio DH. Arachidonic acid regulates two Ca2+ entry pathways via nitric oxide. Cell Signal 16: 157–165, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Watson EL, Wu Z, Jacobson KL, Storm DR, Singh JC, Ott SM. Capacitative Ca2+ entry is involved in cAMP synthesis in mouse parotid acini. Am J Physiol Cell Physiol 274: C557–C565, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Zhang X, Wen J, Bidasee KR, Besch HR Jr, Wojcikiewicz RJ, Lee B, Rubin RP. Ryanodine and inositol trisphosphate receptors are differentially distributed and expressed in rat parotid gland. Biochem J 340: 519–527, 1999. [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao S, Brandt NR, Caswell AH, Lee EY. Binding of the catalytic subunit of protein phosphatase-1 to the ryanodine-sensitive calcium release channel protein. Biochemistry 37: 18102–18109, 1998. [DOI] [PubMed] [Google Scholar]
- 50.Zissimopoulos S, West DJ, Williams AJ, Lai FA. Ryanodine receptor interaction with the SNARE-associated protein snapin. J Cell Sci 119: 2386–2397, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Zoukhri D, Hodges RR, Sergheraert C, Dartt DA. Cholinergic-induced Ca2+ elevation in rat lacrimal gland acini is negatively modulated by PKCdelta and PKCepsilon. Invest Ophthalmol Vis Sci 41: 386–392, 2000. [PubMed] [Google Scholar]