Abstract
Increased extracellular pressure stimulates β1-integrin-dependent cancer cell adhesion. We asked whether pressure-induced adhesion is mediated by changes in β1-integrin binding affinity or avidity and whether these changes are phosphorylation dependent. We evaluated integrin affinity and clustering in human SW620 colon cancer cells by measuring differences in binding between soluble Arg-Gly-Asp (RGD)-Fc ligands and RGD-Fc-F(ab′)2 multimeric complexes under ambient and 15-mmHg increased pressures. Phosphorylation of β1-integrin S785 and T788/9 residues in SW620 and primary malignant colonocytes was assessed in parallel. We further used GD25-β1-integrin-null murine fibroblasts stably transfected with either wild-type β1A-integrin, S785A, TT788/9AA, or T788D mutants to investigate the role of β1-integrin site-specific phosphorylation. SW620 binding of RGD-Fc-F(ab′)2 multimeric complexes, but not soluble RGD-Fc ligands, was sensitive to integrin clustering. RGD-Fc ligand binding was significantly increased under elevated pressure, suggesting that pressure modulates β1-integrin affinity. Pressure stimulated both β1-integrin S785 and T788/9 phosphorylation. GD25-β1A-integrin wild-type and S785A cells displayed an increase in adhesion to fibronectin under elevated pressure, an effect absent in β1-integrin-null and TT788/9AA cells. T788D substitution significantly elevated basal cell adhesion but displayed no further increase under pressure. These results suggest pressure-induced cell adhesion is mediated by β1-integrin T788/9 phosphorylation-dependent changes in integrin binding affinity.
Keywords: adhesion, mechanotransduction, metastasis
integrin-mediated tumor cell adhesion to extracellular matrix components or endothelial cells is an important step in the development of metastatic lesions. Integrin binding affinity is regulated by distinct receptor activation states (23). Initial cell attachment requires integrin activation by cytoplasmic signals that convey large conformational changes to the extracellular domain and enhance ligand-binding affinity (56). The avidity and strength of the interaction is further increased through the redistribution of integrins in the cell membrane, often referred to as receptor clustering (65). The tendency of some malignancies to exhibit reduced integrin expression while maintaining adhesive properties underscores the significance of integrin activation states in mediating tumor cell adhesion (33, 47). Likewise, mutations conferring constitutive integrin activation have been shown to directly contribute to the metastatic potential of cancer cells (20, 53). Mechanisms underlying integrin affinity and avidity modulation are therefore of significant interest in the control of tumor metastasis.
It is increasingly clear that integrin modulation may be sensitive to external mechanical stimuli. Physical forces including strain, pressure, and shear influence diverse physiological and pathological functions ranging from cardiac hypertrophy (48), atherosclerosis (29), and bone remodeling (15) to regulation of cell adhesion (2, 50), differentiation (11, 44) and proliferation (68). Although the mechanisms by which physical forces are translated into biological responses remain poorly understood, the positioning of integrin receptors as a direct bridge between the extracellular matrix and the internal cell cytoskeleton supports integrins as key transducers of such mechanical signals (51). The ability of integrins to transfer external loads across the plasma membrane has been demonstrated by focal adhesion formation and cytoskeletal stiffening following the application of force to beads coated with a β1-integrin ligand (69). Mechanical strain stimulates conformational activation of integrins (25) as well as β1-integrin clustering (28). Furthermore, shear force magnitude has been shown to positively correlate with focal adhesion assembly and stabilization (46). We have previously reported that a pathophysiologically relevant (15 mmHg) increase in extracellular pressure stimulates colon cancer cell adhesion to matrix proteins, endothelial cell monolayers, and surgical wounds in vivo by a β1-integrin-dependent mechanism (2, 58, 64). Nonlaminar shear has a similar effect (61). Pressure stimulates cell adhesion to collagen, fibronectin, laminin, and Matrigel (2, 26), suggesting that this effect is not restricted to a specific α/β1-integrin heterodimer pair. Furthermore, β1-integrin surface expression does not change under elevated pressure conditions (58). Thus, whether pressure-mediated changes in cell adhesion reflect β1-integrin conformational activation or integrin redistribution and clustering requires further investigation.
Phosphorylation of the β-subunit cytoplasmic domain of α/β-integrin heterodimers is thought to functionally regulate integrin activity (12, 18, 67). Two highly conserved NPXY/F-motifs and a serine-threonine cluster are present on the cytoplasmic domain of all β-subunits excluding β4 and β8 (45). This region of the β1-integrin cytoplasmic domain consists of five potential phosphorylation regulatory sites: Y783, Y795, S785, T788, and T789 (40, 53). Previous studies have found that murine fibroblasts expressing β1-integrin Y783F and Y795F tyrosine phosphorylation mutants are fully functional in promoting cell adhesion to fibronectin (70). β1-Integrin S785 phosphorylation has been shown to regulate localization to focal adhesions (1) and enhance cell attachment but to inhibit cell spreading and migration (40). Replacement of both T788 and T789 in β1-integrin with alanine residues disrupts fibroblast attachment to fibronectin and significantly reduces exposure of the β1-integrin conformational activation epitope 9EG7 (70), whereas introduction of a T788D substitution, mimicking phosphorylation, results in a constitutively active conformation, 9EG7 induction, and enhanced cell adhesion (43). Interestingly, loss of ligand-binding function associated with double TT788/9AA substitutions does not affect induction of focal adhesion kinase (FAK) phosphorylation by β1-antibody-mediated receptor cross-linking, suggesting that T788/9 phosphorylation is involved in “inside-out” signaling rather than “outside-in” (70). However, whether these residues are actually phosphorylated under physiological conditions has yet to be shown.
We therefore sought to assess whether pressure-induced cell adhesion is mediated by changes in β1-integrin-binding affinity or avidity and whether these changes are phosphorylation dependent. We used a flow cytometry-based assay to quantify changes in integrin affinity and clustering by measuring differences in binding between soluble Arg-Gly-Asp (RGD)-Fc ligands and RGD-Fc-F(ab′)2 multimeric complexes under ambient and 15-mmHg increased pressures. We similarly evaluated the effect of increased pressure on induction of the β1-integrin activation epitope 9EG7. Pressure-stimulated phosphorylation of β1-integrin S785 and T788/9 residues in human SW620 colon cancer cells and primary malignant colonocytes was assessed in parallel. We evaluated the dependence of pressure-stimulated cell adhesion on β1-integrin site-specific phosphorylation using GD25-β1-integrin-null murine fibroblasts stably transfected with either wild-type β1A-integrin, S785A, TT788/9AA, or T788D phosphorylation site mutants. Finally, to further elucidate the upstream inside-out signaling pathway modulating β1-integrin activity, we assessed the effects of inhibiting molecules known to be essential for pressure-stimulated cell adhesion on β1-integrin phosphorylation.
MATERIALS AND METHODS
Cell culture.
SW620 colon cancer cells were cultured as previously described (2). Primary human colonocytes were isolated from resected tumors by mincing and collagenase digestion (16). Human tumor use was approved by the Wayne State University Human Investigation Committee. Cos-7 fibroblasts (American Type Culture Collection, Manassas, VA) used for the production of RGD-Fc fusion protein were maintained under standard conditions in DMEM with 10% fetal bovine serum. The β1-integrin-null GD25 murine fibroblast line and its stably transfected β1-integrin-expressing derivatives GD25-β1A, GD25-β1A,S785A, GD25-β1A,T788/9A (kindly provided by Dr. M. Mulvey, University of Utah, Salt Lake City, UT), and GD25-β1A,T788D (kindly provided by Dr. S. Johansson, Uppsala University, Uppsala, Sweden) have been previously described (17, 19, 71).
Cell transfection and pharmacological treatments.
Cells were transfected with 50 nM double-stranded small interfering RNA (siRNA) directed toward the mRNA target 5′-CACAGAUCGAGAACAUCGAAG-3′ for α-actinin-1 or 5′-AAGCAUGUGGCCUGCUAUGGA-3′ for FAK (Dharmacon, Lafayette, CO) as previously described (9). A Dharmacon siCONTROL Non-Targeting siRNA no. 1 sequence was used as a control. Transfected cells were studied after 48 h. In parallel studies, cells were treated with either 1 μM cytochalasin D (Calbiochem, San Diego, CA) or 1 mM MnCl2 (Sigma-Aldrich, St. Louis, MO) at the initiation of each experiment or with 20 μM PP2, 20 μM LY-294002 (Calbiochem), or 20 μM U-0126 (BIOMOL, Plymouth Meeting, PA) for 30 min before use, then throughout the remainder of each experiment.
Pressure application.
Pressure was applied using an airtight Lucite box with an inlet valve for gas application and an outlet valve connected to a manometer. The box was prewarmed to 37°C to prevent internal temperature and pressure fluctuations. Temperature was maintained within ±2°C and pressure within ± 1.5 mmHg of desired levels. Variation in Po2 and pH of culture medium was insignificant (2).
Preparation of soluble RGD-Fc fusion protein.
Secreted RGD peptide (ACDCRGDCFCG)-mouse IgG Fc fusion protein was isolated from Cos-7-conditioned medium 48–72 h following transfection with pAd-RGD/mFc recombinant DNA (36). The RGD-mFc fusion protein was concentrated using Amicon Ultra 50k and 10k centrifugal filters (Millipore, Billerica, MA). Successful isolation of the fusion protein was assessed by Western blot using an anti-mouse Fc fragment horseradish peroxidase-conjugated antibody (Cell Signaling, Beverly, MA). RGD-mFc fusion protein concentration was approximated by bicinchoninic acid assay (Pierce, Rockford, IL).
Soluble ligand and ligand-complex binding assay.
RGD-Fc-F(ab′)2 multimeric complexes were prepared by preincubation of soluble RGD-Fc ligands with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse Fc fragment-specific IgG F(ab′)2 fragments (Millipore) at a ratio of 6.25:1 in PBS for 30 min at 4°C as previously described (31). Ligand binding was assessed in bacteriologic plastic 96-well plates pretreated with 1% heat-inactivated BSA to prevent cell adhesion. Cells were suspended in a 200-μl volume of normal growth medium at 106 cells per well with either 4 μg RGD-Fc protein or an equivalent amount of complexed RGD-Fc ligand. Cells were treated with either 1 mM MnCl2, 1 μM cytochalasin D, or DMSO and exposed to ambient or 15-mmHg increased pressure. After 30 min, unbound ligand was washed away with PBS, and cells were fixed with 4% paraformaldehyde for 5 min. Binding of RGD-Fc-F(ab′)2 multimeric complexes was directly assessed by flow cytometric analysis. Cells incubated with soluble RGD-Fc ligand required secondary incubation with FITC-conjugated goat anti-mouse Fc fragment-specific IgG F(ab′)2 fragments for an additional 20 min at 4°C before flow cytometric analysis.
Cell adhesion assay.
Cells were incubated with 5 μM calcein AM (Invitrogen, Carlsbad, CA) in PBS for 15 min at 37°C. Cells were then washed, resuspended in growth medium, and allowed to adhere to bacteriologic plates (2.5 × 105 cells/well) precoated with 0.78–25 μg/ml fibronectin for 30 min at 37°C under ambient or increased pressure (+15 mmHg) conditions (59). After 30 min, nonadherent cells were gently washed away with warm PBS, and cell adhesion was determined by relative fluorescence per well using a FLx800 fluorescence microplate reader (BioTek, Winooski, VT).
Western blot analysis.
Cell lysates were prepared for immunoblotting as previously described (59). Equal amounts of protein were resolved by SDS-PAGE and transferred to Hybond ECL nitrocellulose membrane (GE Healthcare, Piscataway, NJ). Mouse anti-CD29 β1-integrin antibody (BD Biosciences, San Jose, CA), rabbit polyclonal phosphospecific β1-integrin S785, and phosphospecific β1-integrin T788/9 antibodies (Millipore) were coupled with appropriate horseradish peroxidase-conjugated secondary antibodies (Cell Signaling) for immunodetection of blotted proteins. Bands were detected with enhanced chemiluminescence (GE Healthcare) and analyzed with a Kodak Image Station 440CF (Perkin Elmer, Boston, MA).
Flow cytometry.
Cells were fixed with 4% paraformaldehyde for 10 min and washed with staining buffer containing 0.2% BSA and 0.02% sodium azide in PBS. Bound RGD-Fc ligand was assessed by incubation with FITC-conjugated goat anti-mouse Fc fragment-specific IgG F(ab′)2 fragments (Millipore), GD25-β1-integrin expression was determined by incubation with monoclonal hamster anti-mouse CD29-FITC antibody (HM β1–1; AbD Serotec, Kidlington, Oxford, United Kingdom), and 9EG7 epitope induction was measured by incubation with rat anti-mouse CD29 (9EG7) and FITC-conjugated goat anti rat Ig, all for 30 min at 4°C. Cells were then washed twice with staining buffer and analyzed with a FACSCalibur flow cytometer (BD Biosciences) and FlowJo software (Tree Star, Ashland, OR).
Fluorescence confocal microscopy.
After exposure to ambient or increased pressure conditions, SW620 cells were washed with PBS and fixed with 4% paraformaldehyde for 10 min at room temperature. Cell suspensions were stained with mouse anti-human CD29 β1-integrin antibody (BD Biosciences) and Alexa Fluor 350 goat anti-mouse IgG (Invitrogen), mounted on ProbeOn Plus glass slides (Fisher Scientific) and coverslipped using Geltol mounting medium (Immunon, Pittsburgh, PA). β1-Integrin spatial distribution was visualized on an LSM 510 confocal microscope (Zeiss, Jena, Germany) equipped with a ×40 objective and LSM software version 3.2 (Zeiss).
Statistical analysis.
All data are represented as means ± SE. Statistical analysis was by either a paired Student's t-test or a Wilcoxon matched-pairs signed-rank test as appropriate. Sidak's correction was used for multiple comparisons (49). A 95% confidence interval was set a priori as the desired level of statistical significance.
RESULTS
Increased extracellular pressure enhances integrin-binding affinity and cell adhesion.
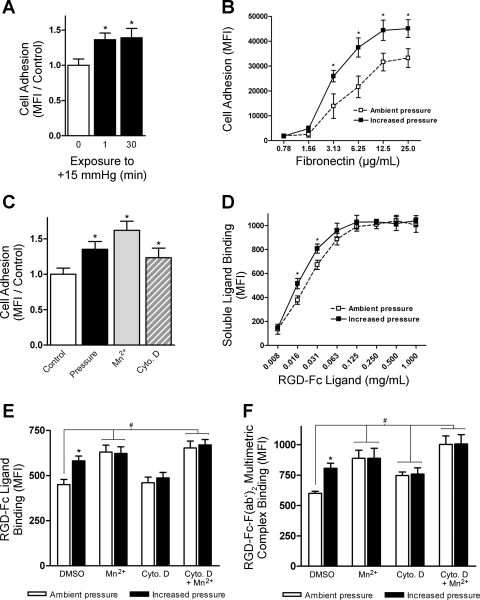
Consistent with our previous studies (26), 30-min exposure to 15 mmHg increased extracellular pressure-stimulated SW620 colon cancer cell adhesion to fibronectin by 39 ± 13% (n = 6; P < 0.04) compared with cells maintained under ambient conditions (Fig. 1A). A 1-min exposure to 15-mmHg increased pressure followed by a 29-min incubation under ambient conditions was sufficient to enhance cell adhesion by a similar magnitude (36 ± 9%; n = 6; P < 0.02), suggesting that cells are likely responding to the initial brief exposure to increased extracellular pressure, and longer-term exposure to such pressures may not be necessary to create this effect. Additional cell adhesion assays were conducted to assess the effect of fibronectin substrate concentration on pressure-stimulated SW620 cell adhesion (Fig. 1B). Basal cell adhesion to fibronectin appeared to saturate near 12.5 μg/ml, consistent with previous reports (37). A 30-min exposure to 15-mmHg increased pressure enhanced SW620 cell adhesion to fibronectin over a range of 1–25 μg/ml.
Fig. 1.
Effect of increased extracellular pressure on integrin affinity and avidity. SW620 cell adhesion to fibronectin or binding of soluble Arg-Gly-Asp (RGD)-Fc ligands and RGD-Fc-F(ab′)2 multimeric complexes was assessed under ambient and 15-mmHg increased pressures for 30 min in the absence or presence of 1 mM MnCl2 and 1 μM cytochalasin D (Ctyo D). A: effect of 1-min vs. 30-min exposure to increased pressure on cell adhesion to 12 μg/ml fibronectin (n = 6). Data from individual experiments were normalized to their respective ambient pressure controls. MFI, mean fluorescence intensity. B: effect of fibronectin substrate concentration (0.78–25 μg/ml) on pressure-stimulated cell adhesion (n = 3). C: effect of increased pressure, MnCl2, and cytochalasin D on cell adhesion to 12 μg/ml fibronectin (n = 6). Data from individual experiments were normalized to their respective DMSO-treated ambient pressure controls. D: dose response of RGD-Fc soluble ligand binding under ambient and increased pressure (n = 4). E: determination of affinity modulation by assessment of the effects of increased pressure, MnCl2, cytochalasin D, or MnCl2 and cytochalasin D in combination on soluble RGD-Fc ligand binding (n = 9). F: determination of affinity/avidity modulation by assessment of the effects of increased pressure, MnCl2, cytochalasin D, or MnCl2 and cytochalasin D in combination on RGD-Fc-F(ab′)2 multimeric complex binding (n = 9). Data from individual experiments are graphically expressed as means ± SE. *P < 0.05 compared with respective ambient pressure control. #P < 0.05 compared with DMSO-treated ambient pressure control.
Cell treatment with Mn2+ divalent cations or cytochalasin D has also been shown to influence adhesion. Binding of Mn2+ cations to the integrin metal ion-dependent adhesion site induces a structural change in the extracellular domain to a high-affinity conformation and enhances cell adhesion (13, 57). Conversely, disruption of cell cytoskeletal constraints using cytochalasin D has been shown to induce ligand-dependent integrin clustering and enhance cell adhesion without affecting receptor affinity (27). In agreement with these reports, exposure of SW620 cells to increased pressure or incubation with either 1 mM MnCl2 or 1 μM cytochalasin D increased cell adhesion to fibronectin by 35 ± 6% (n = 6; P < 0.01), 62 ± 8% (n = 6; P < 0.01), and 23 ± 9% (n = 6; P < 0.05), respectively, compared with untreated cells (Fig. 1C). Whether pressure-activated cell adhesion is mediated by changes in integrin binding affinity or avidity is unknown.
The RGD peptide (ACDCRGDCFCG) has been demonstrated to bind α5β1-, αvβ1-, αIIbβ3-, αvβ3-, and αvβ5-integrins (30). SW620 cells bound the soluble RGD-Fc fusion protein in a dose-dependent manner (Fig. 1D). Pressure significantly influenced RGD-Fc ligand binding between 20% and 30% (n = 4; P < 0.05) at concentrations in the range of 16–31 μg/ml. We used flow cytometric analysis of integrin binding to 20 μg/ml single RGD-Fc ligands and RGD-Fc-F(ab′)2 multimeric complexes to assess changes in integrin affinity and clustering. Multimeric ligand complexes are capable of binding integrins in a low-affinity state in the presence of receptor clustering and may therefore be used for the detection of changes in integrin avidity apart from affinity (31). RGD-Fc-F(ab′)2 multimeric complex binding is therefore sensitive to changes in both integrin affinity and avidity, whereas single RGD-Fc ligand binding is sensitive only to changes in integrin affinity. Consistent with this idea, treatment of SW620 cells with 1 μM cytochalasin D stimulated cell binding to RGD-Fc-F(ab′)2 multimeric complexes by 23 ± 5% (Fig. 1F, n = 9; P < 0.01), but it failed to stimulate soluble RGD-Fc ligand binding (Fig. 1E). Incubation of SW620 cells with 1 mM MnCl2 conferred a 40 ± 9% (Fig. 1E, n = 9; P < 0.01) increase in RGD-Fc soluble ligand binding and a 48 ± 11% (Fig. 1F, n = 9; P < 0.01) increase in RGD-Fc-F(ab′)2 multimeric complex binding compared with control cell populations.
Using the RGD-Fc-F(ab′)2 multimeric complex-based assay, increased pressure enhanced ligand-complex binding by 34 ± 7% (Fig. 1E; n = 9; P < 0.01). Although basal levels of ligand-complex binding were significantly increased in cells pretreated with cytochalasin D, pressure failed to elicit any further response. This is consistent with our previous findings that an intact cytoskeleton is required for pressure-mediated promotion of cell adhesion (59). Cells pretreated with MnCl2 or both MnCl2 and cytochalasin D bound ligand at high enough levels to mask any effect of pressure. Because of the more subtle changes in ligand binding influenced by pressure compared with MnCl2 and cytochalasin D cell treatments, coupled with the nature by which MnCl2 and cytochalasin D modulate integrin affinity and avidity, any synergistic effect between MnCl2, cytochalasin D, and pressure would be unexpected. Furthermore, it is unlikely that the lack of pressure effect displayed by MnCl2 and cytochalasin D-treated cells is due to receptor saturation since mean fluorescence intensity values under these conditions were still below those observed at saturating doses of ligand in Fig. 1D. Using the single RGD-Fc ligand-based assay, pressure stimulated a 29 ± 6% (Fig. 1E; n = 9; P < 0.01) increase in ligand binding compared with cells kept under ambient conditions. Soluble ligand binding was not significantly affected by increased pressure in cells pretreated with cytochalasin D. As in the multimeric complex-based assay, basal levels of bound ligand in cells pretreated with either MnCl2 or MnCl2 and cytochalasin D were too high to discern a pressure-mediated effect. The significant increase in single RGD-Fc ligand binding by control populations observed under elevated pressure beyond that detected by the multimeric complex-based assay in cytochalasin D-treated cells suggests that pressure is predominantly modulating integrin affinity rather than avidity.
Pressure-mediated changes in integrin distribution or clustering were further investigated by fluorescence confocal analysis of β1-integrin cell surface distribution on suspended SW620 cells. Comparison of cells exposed to either ambient or increased pressure conditions failed to display any differences in β1-integrin localization between the two groups (data not shown).
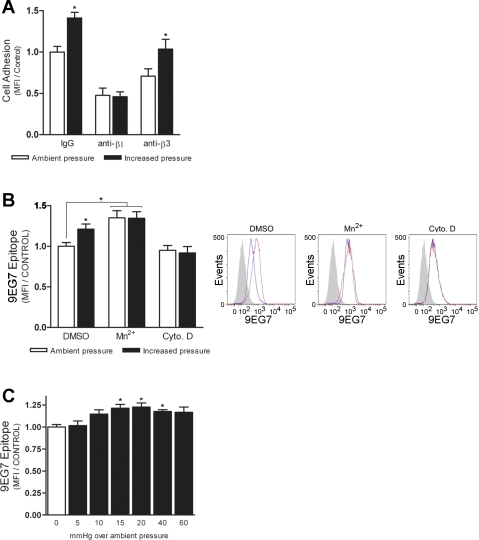
Pressure influences β1-integrin conformational activation.
Consistent with previous results (2), pressure-stimulated cell adhesion to fibronectin was completely abolished by preincubation with 1 mg/ml anti-β1-integrin blocking antibody (Fig. 2A). In contrast, pressure still induced a 32 ± 12% (n = 6; P < 0.05) increase in cell adhesion in the presence of anti-β3-integrin blocking antibody. Exposure of the extracellular 9EG7 epitope on β1-integrin has been shown to correlate with ligand occupancy or activated receptor conformations (4, 34). We sought to further validate our results by flow cytometric analysis of 9EG7 epitope induction on SW620 cells under ambient and increased pressure conditions (Fig. 2B). In agreement with previous reports (3, 70), cell treatment with MnCl2 increased 9EG7 exposure by 35 ± 9% (n = 4; P < 0.03) compared with control cells. Likewise, elevated pressure increased 9EG7 exposure by 21 ± 6% (n = 4; P < 0.05) over that of cells incubated under ambient conditions. Pressure failed to further increase exposure of the 9EG7 epitope on MnCl2-treated cells and had no effect on cells pretreated with cytochalasin D. Exposure to pressures <15 mmHg also did not have a statistically significant effect on receptor activation (Fig. 2C). Conversely, we observed a significant increase in 9EG7 epitope induction at pressures up to 40 mmHg over ambient (n = 3; P < 0.05). We have previously shown that pressures of similar magnitude impose a corresponding stimulatory effect on cell adhesion (2). Exposure to 60-mmHg increased pressure also tended to induce the 9EG7 epitope, but the effect did not achieve statistical significance in this series of studies.
Fig. 2.
Effect of increased pressure on exposure of the β1-integrin 9EG7 epitope. A: effect of functional blockade of β1- and β3-integrin on pressure-stimulated SW620 cell adhesion to 12 μg/ml fibronectin (n = 6). B: induction of the 9EG7 epitope on SW620 cells was assessed following exposure to ambient or 15-mmHg increased pressure for 30 min in the presence of DMSO, 1 mM MnCl2, or 1 μM cytochalasin D (n = 4). Representative histograms are shown (right) of typical 9EG7 staining following exposure to increased (red) or ambient (blue) pressure conditions. Solid gray peaks represent the unstained cell populations. C: effect of increasing pressures on 9EG7 induction (n = 3). MFI values of 9EG7-positive cells were determined by flow cytometric analysis. Data from individual experiments were normalized to respective IgG- or DMSO-treated ambient pressure controls and are graphically expressed as means ± SE. *P < 0.05 compared with ambient pressure control.
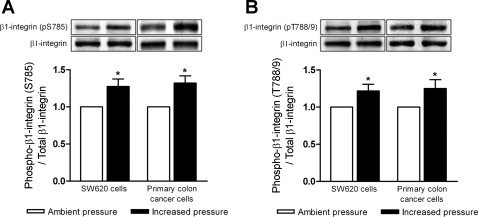
Pressure stimulates β1-integrin phosphorylation at S785 and T788/9.
Integrin activity may be influenced by site-specific phosphorylation of the cytoplasmic domain of the β-subunit (40, 53). We therefore next sought to determine the effect of increased pressure on β1-integrin phosphorylation. Human SW620 colon cancer cells and primary malignant colonocytes were exposed to either ambient or 15-mmHg increased pressure for 30 min, then lysed and assessed for β1-integrin phosphorylation at serine 785 and threonine 788/789 residues by Western blot. Pressure increased β1-integrin phosphorylation at S785 (Fig. 3A) in SW620 cells by 28 ± 9% (n = 6; P < 0.04) and in primary human colon cancer cells isolated directly from resected tumors of patients by 33 ± 10% (n = 7; P < 0.04). Likewise, pressure stimulated β1-integrin phosphorylation at T788/9 (Fig. 3B) in SW620 cells by 22 ± 8% (n = 6; P < 0.04) and in primary colon cancer cells by 25 ± 11% (n = 7; P < 0.04).
Fig. 3.
Effect of increased pressure on β1-integrin phosphorylation at serine 785 and threonines 788/789. Protein lysates from suspended SW620 cells (n = 6) and surgically resected human primary colon cancer cells (n = 7) exposed to either ambient or increased pressure conditions were assessed for β1-integrin phosphorylation at S785 and T788/9 by Western blot. A: effect of increased pressure on β1-integrin S785 phosphorylation (pS785) in SW620 cells and primary colon cancer cells. B: effect of elevated pressure on β1-integrin phosphorylation at T788/9 in SW620 cells and primary colon cancer cells. Data from individual experiments were normalized to respective ambient pressure controls and are graphically expressed as means ± SE. *P < 0.05 compared with respective ambient pressure controls.
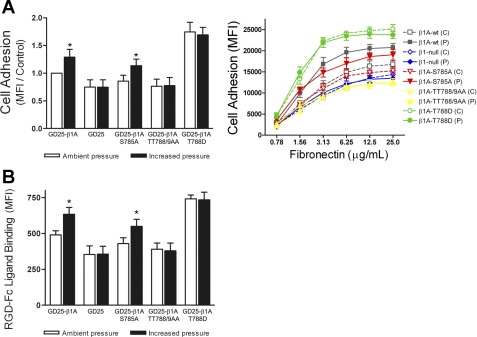
Pressure-stimulated cell adhesion requires phosphorylation of β1-integrin at T788/9.
To address the specific roles of these β1-integrin phosphorylation sites in pressure-stimulated cell adhesion, we used the GD25-β1-knockout murine fibroblast line and four stably transfected derivatives: GD25-β1A, GD25-β1A,S785A, GD25-β1A,TT788/9AA, and GD25-β1A,T788D. β1-Integrin expression was reconstituted in the GD25 cells with either the wild-type β1A-splice variant or one of three β1A-mutants containing alanine or aspartic acid substitutions at S785, T788/9, and T788 phosphorylation sites as previously described (19, 70). The β1-integrin phosphospecific antibodies used to detect S785 and T788/9 phosphorylation in human colon cancer cells failed to cross-react with murine β1A expressed by the GD25 lines. Thus, we next assessed the effect of the various phosphorylation site mutations on pressure-mediated GD25 cell adhesion to fibronectin (Fig. 4A). A 30-min exposure to 15-mmHg increased pressure enhanced GD25-β1A cell adhesion by 31 ± 14% (n = 6; P < 0.04) compared with cells maintained under ambient conditions. GD25 (β1-null) cells failed to display any pressure-mediated adhesion effect. While GD25-β1A,S785A transfectants displayed a modest reduction in basal cell adhesion, elevated pressure stimulated a 27 ± 12% (n = 6; P < 0.04) increase in adhesion to fibronectin. However, pressure-activated cell adhesion was completely blocked in the GD25-β1A,TT788/9AA transfectants. β1-Integrin T788D substitution, mimicking constitutive T788 phosphorylation, caused a 76 ± 17% (n = 6; P < 0.04) increase in basal GD25-β1A,T788D cell adhesion, but it failed to display a further increase under elevated pressure.
Fig. 4.
Effect of β1-integrin phosphorylation site-specific mutations on pressure-stimulated cell adhesion and soluble ligand binding. GD25-β1-null murine fibroblast cells and stably transfected derivatives GD25-β1A [wild-type (WT)], GD25-β1A,S785A, GD25-β1A,TT788/9AA, and GD25-β1A,T788D were exposed to either ambient [control (C)] or 15-mmHg increased pressure [pressure (P)] conditions for 30 min and assessed for adhesion to fibronectin and RGD-Fc soluble ligand binding. A: effect of β1-integrin site-specific mutations on pressure-stimulated adhesion to 12.5 μg/ml fibronectin (left; n = 6) and 0.78–25 μg/ml fibronectin (right; n = 3). B: effect of β1-integrin site-specific mutations on RGD-Fc soluble ligand binding under ambient and increased pressure conditions (n = 9). Data from individual experiments were normalized to respective GD25-β1A ambient pressure controls and are graphically expressed as means ± SE. *P < 0.05 compared with respective GD25-β1A ambient pressure controls.
To strengthen the relationship between the observed effects of pressure on integrin binding affinity and on cell adhesion, we assessed the ability of the GD25 lines to bind RGD-Fc ligand under ambient and increased pressure conditions (Fig. 4B). Consistent with adhesion results, exposure of GD25-β1A cells to elevated pressure stimulated a 29 ± 8% (n = 9; P < 0.05) increase in ligand binding compared with cells kept under ambient conditions. Again, GD25 cells were unresponsive to increased extracellular pressure. GD25-β1A,S785A transfectants displayed a 25 ± 6% (n = 9; P < 0.05) increase in binding affinity under elevated pressure conditions. Similar to β1-null cells, GD25-β1A,TT788/9AA cell transfectants exhibited a modest reduction in basal RGD-Fc ligand binding and displayed no effect under pressure. Again, GD25-β1A,T788D cells bound 51 ± 5% (n = 5; P < 0.02) greater RGD-Fc ligand than GD25-β1A cells under ambient pressure and bound equivalent levels of ligand under increased pressure conditions.
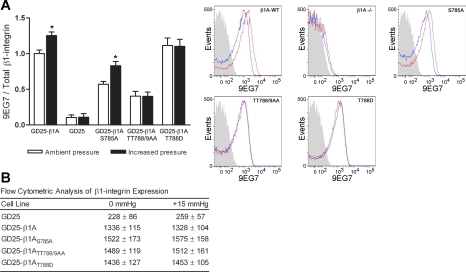
We next evaluated the influence of pressure on GD25-β1-integrin 9EG7 epitope induction and assessed total β1-integrin expression under ambient and increased pressure conditions (Fig. 5). Basal 9EG7 staining was reduced by 43 ± 4% (n = 4; P < 0.03) in GD25-β1A,S785A transfectants and by 59 ± 8% (n = 4; P < 0.01) in GD25-β1A,TT788/9AA transfectants compared with GD25-β1A cells. Conversely, basal 9EG7 exposure on GD25-β1A,T788D cells was elevated by 11 ± 8% (n = 4). All GD25 (β1-null) 9EG7 staining was considered background. Consistent with adhesion and ligand binding results, pressure enhanced GD25-β1A 9EG7 exposure by 25 ± 5% (n = 4; P < 0.05) and GD25-β1A,S785A 9EG7 exposure by 26 ± 7% (n = 4; P < 0.05). Increased pressure failed to stimulate further 9EG7 induction in both the GD25-β1A,TT788/9AA and GD25-β1A,T788D transfectants. No significant changes were observed in GD25-β1A surface expression under ambient versus increased pressure conditions (Fig. 5B), consistent with previous observations that β1-integrin surface expression on SW620 cells remains unchanged between ambient and increased pressure conditions (58).
Fig. 5.
Effect of increased pressure on 9EG7 induction and β1-integrin surface expression on GD25-β1A phosphorylation mutants. A: induction of the 9EG7 epitope on GD25, GD25-β1A, GD25-β1A,S785A, GD25-β1A,TT788/9AA, and GD25-β1A,T788D cells was assessed following exposure to ambient or 15-mmHg increased pressure for 30 min (n = 4). Individual MFI values for 9EG7 staining were divided by respective MFI values for total β1-integrin expression and were normalized to the GD25-β1A ambient pressure controls. Data from individual experiments are graphically expressed as means ± SE. Representative histograms are shown (right) of typical 9EG7 staining following exposure to increased (red) or ambient (blue) pressure conditions. Solid gray peaks represent the unstained cell populations. B: flow cytometric analysis of β1-integrin surface expression on GD25 cell transfectants following 30-min exposure to ambient or increased pressure. Data are representative of MFI values from 3 individual experiments and are expressed as means ± SE. *P < 0.05 compared with respective GD25-β1A ambient pressure controls.
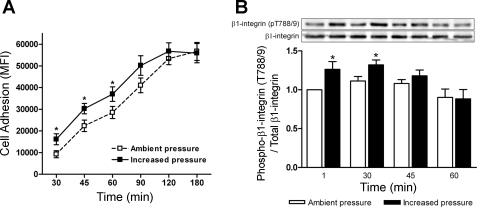
Transient exposure to elevated pressure is sufficient to stimulate prolonged β1-integrin T788/9 phosphorylation and increased tumor cell adhesion.
We sought to expand on our initial observation that a 1-min exposure to increased pressure was sufficient to induce tumor cell adhesion of a similar magnitude to 30 min under elevated pressure conditions. SW620 tumor cells were exposed to 15-mmHg increased pressure for 1 min and were then maintained under ambient pressure conditions and evaluated for relative adhesion trends to fibronectin at 30-, 45-, 60-, 90-, and 180-min time points (Fig. 6A). Compared with cell populations maintained solely under ambient pressure, we observed a significant increase in cell adhesion for up to 60 min following pressure treatment (n = 6; P < 0.05). β1-Integrin T788/9 phosphorylation was assessed in parallel (Fig. 6B). Pressure stimulated a 26 ± 9% (n = 5; P < 0.05) increase in T788/9 phosphorylation at 1 min. β1-Integrin T788/9 phosphorylation remained significantly elevated at 30 min (n = 5; P < 0.02). Differences between cells transiently exposed to elevated pressure and control cell populations were no longer significant at 45 min and were undetectable by 60 min (n = 5). The discrepancy between pressure-stimulated SW620 cell adhesion and β1-integrin T788/9 phosphorylation can likely be explained by the assertion that the effects of pressure on cell adhesion are only functionally relevant during the initial 30–45 min following exposure. Since we are using a fixed number of cells, the elevated cell adhesion observed at later time points is evident only until the numbers of adherent control cells equilibrates under basal adhesion rates and masks the effects of pressure.
Fig. 6.
Temporal effects of 1-min exposure to increased extracellular pressure on tumor cell adhesion and β1-integrin T788/9 phosphorylation. A: effect of 1-min exposure to 15-mmHg increased extracellular pressure on SW620 cell adhesion to 12 μg/ml fibronectin at 30, 45, 60, 90, and 180 min (n = 6). B: protein lysates from parallel nonadherent cell populations were collected and assessed for pressure-induced β1-integrin T788/9 phosphorylation at 1, 30, 45, and 60 min (n = 5). Data from individual experiments were normalized to respective (1 min) ambient pressure controls and are graphically expressed as means ± SE. *P < 0.05 compared with respective ambient pressure controls at individual time points.
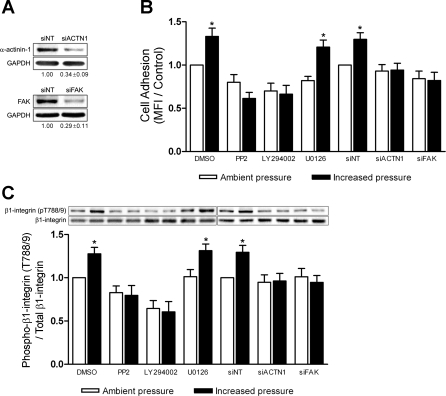
Pressure stimulates β1-integrin T788/9 phosphorylation via an α-actinin-1, FAK, Src, and phosphatidylinositol 3-kinase (PI3K)-dependent pathway.
Finally, we sought to further elucidate the upstream signaling molecules mediating inside-out β1-integrin affinity modulation and to determine whether β1-integrin T788/9 phosphorylation serves as a reliable end point for the pressure-stimulated mechanical signaling pathway governing cell adhesion. Consistent with previous results on collagen (9, 58, 60), pressure-stimulated cell adhesion to fibronectin requires α-actinin-1, FAK, Src, and PI3K (Fig. 7B). Although ERK 1/2 signaling has been reported to mediate integrin activation (5, 74), we have previously found ERK 1/2 phosphorylation in nonadherent cells to be insensitive to elevated pressure (58). In agreement with both of these reports, ERK 1/2 inhibition by the MEK-1 inhibitor, U-0126, significantly reduced basal cell adhesion by 18 ± 5% (n = 5; P < 0.05), while pressure still induced a 38 ± 8% (n = 5; P < 0.03) increase in adhesion compared with U-0126-treated cells maintained under ambient conditions.
Fig. 7.
Effect of α-actinin-1, focal adhesion kinase (FAK), Src, phosphatidylinositol 3-kinase, and ERK 1/2 inhibition on pressure-stimulated cell adhesion and β1-integrin T788/9 phosphorylation. A: typical reduction of total α-actinin-1 and FAK protein in SW620 cells transfected with small interfering RNA (siRNA) targeted to either α-actinin-1 (siACTN1) or FAK (siFAK) as measured by Western blot. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control, and α-actinin-1 and FAK protein expression was normalized against that of nontarget siRNA (siNT) transfectant controls (n = 7). B: SW620 cells were treated with either DMSO (n = 9), 20 μM PP2 (n = 9), 20 μM LY-294002 (n = 9), 20 μM U–0126 (n = 5), siNT (n = 7), siACTN1 (n = 7), or siFAK (n = 7) and assessed for ability to adhere to 12.5 μg/ml fibronectin under ambient or increased pressure conditions. C: protein lysates from parallel nonadherent cell populations were collected and assessed for pressure-induced β1-integrin T788/9 phosphorylation by Western blot. Data from individual experiments were normalized to respective ambient pressure DMSO or siNT controls and are graphically expressed as means ± SE. *P < 0.05 compared with respective ambient pressure DMSO or siNT controls.
We next assessed the effect of inhibiting these molecules on pressure activation of β1-integrin T788/9 phosphorylation (Fig. 7C). Reduction of either α-actinin-1 or FAK expression by siRNA completely blocked pressure-stimulated β1-integrin T788/9 phosphorylation in SW620 cells (n = 7). Cell pretreatment with 20 μM PP2, a Src-family kinase inhibitor, moderately affected basal T788/9 phosphorylation and blocked any additional T788/9 phosphorylation under elevated pressure. Likewise, cell pretreatment with 20 μM LY-294002, a PI3K inhibitor, significantly reduced T788/9 phosphorylation under both ambient and increased pressure conditions by more than 30% (n = 9; P < 0.05) compared with ambient control cell populations. Despite its inhibition of basal cell adhesion at ambient pressure, ERK 1/2 inhibition with 20 μM U-0126 did not alter basal β1-integrin T788/9 phosphorylation levels. Furthermore, U-0126-treated cells displayed a further 30 ± 8% (n = 5; P < 0.05) increase in T788/9 phosphorylation under elevated pressure (Fig. 7C, bars 7 and 8), paralleling the increase in adhesion observed in U-0126-treated cells in response to pressure (Fig. 7B, bars 7 and 8).
DISCUSSION
Viable cancer cells can be recovered from surgical sites, the lymphatics, and venous circulation during both tumor resection and metastasis (21, 22, 55, 62). The formation of secondary tumors requires the adhesion of these cells to surrounding tissues. Shed tumor cells may be subjected to increased pressure and shear forces during vascular and lymphatic transit, in the tumor microenvironment, or iatrogenically through surgical manipulation, laparoscopic insufflation, and postoperative bowel edema (14, 35, 38, 41, 72). Exposure to these forces may influence the ability of shed cells to adhere and form new tumors. Although metastasis may also require cell invasion, we recently reported that a 30-min exposure to 15-mmHg increased pressure does not affect tumor cell invasion in a Boyden-chamber assay (10). Although these results do not exclude an effect of physical forces on tumor cell motility, brief increases in extracellular pressure seem to affect tumor cell adhesion more importantly than tumor cell motility.
Elucidation of the role of the β1-integrin subunit in pressure-stimulated cell adhesion is important to understanding tumor metastasis as well as of scientific interest as a paradigm for mechanotransduction and the inside-out signaling events that influence cell-matrix interactions at the cell membrane. In the present study, we have characterized the effect of pressure on integrin affinity and avidity modulation, the role of β1-integrin phosphorylation, and the mechanotransduced signal pathway by which β1-integrin T788/9 phosphorylation is regulated in response to increased extracellular pressure. Our data indicate that pressure-induced cell adhesion results primarily, if not entirely, from enhanced integrin affinity. Furthermore, β1-integrin phosphorylation at T788/9 is a critical regulatory event in pressure-induced integrin activation and cell adhesion. Finally, our data suggest that β1-integrin T788/9 phosphorylation is regulated by an α-actinin-1, FAK, Src, and PI3K-dependent mechanical signaling pathway.
Konstandin et al. (31) recently reported that T lymphocyte binding of soluble ICAM-1-Fc ligands is strictly dependent on integrin activation, whereas binding to ICAM-1-Fc-F(ab′)2 multimeric complexes can be accomplished by integrins in a low-affinity conformation only in the presence of PMA- and cytochalasin D-induced integrin clustering. In the current study, increased pressure stimulated SW620 soluble RGD-Fc ligand binding beyond that observed with RGD-Fc-F(ab′)2 multimeric complexes following cytochalasin D-induced clustering. These results taken together with observed trends in 9EG7 staining and the unresponsiveness of high-affinity GD25-β1A,T788D cell transfectants to increased pressure are consistent with the idea that increased pressure stimulates cell adhesion by inducing integrin activation and enhancing receptor affinity. The multimeric ligand complexes contain multiple FITC molecules per complex, providing proportionally greater fluorescence intensity than soluble RGD-Fc ligands bound by single FITC-conjugated antibodies. Although this allows for greater sensitivity in detecting small changes in clustering in the absence of integrin activation, it fails to distinguish clustering among integrins in a high-affinity conformation. Therefore, these results cannot be used to completely rule out the possibility that pressure-mediated integrin clustering or avidity modulation may occur in addition to conformational activation.
While it is well established that cell treatment with phorbol esters and cytoskeleton-disrupting agents can promote cell adhesion in the absence of integrin activation (32, 42, 75), Kim and colleagues (27) recently used fluorescence resonance energy transfer to demonstrate that cell treatment with PMA, cytochalasin D, and latrunculin A did not affect β2-integrin distribution patterns or clustering in the absence of multivalent ligand. It was proposed that cytoskeleton-disrupting agents may promote integrin diffusion within the cell membrane and thereby enhance cell adhesion through facilitation of ligand-driven integrin accumulation at contact sites rather than through formation of proactive clusters. In our current study, cytochalasin D-mediated clustering was assessed in the presence of multimeric ligand complexes and therefore still allows reconciliation of our results with this previous report. Moreover, Kim's observations are consistent with our inability to detect pressure-mediated integrin clustering in suspended cells by fluorescence microscopy. Thus, whether proactive clustering may actually precede integrin activation in the absence of multivalent ligand is debatable. Furthermore, this issue underscores the complexity of attributing primacy to either affinity or avidity modulation in the promotion of cell adhesion. Alternate studies have shown that the expression of constitutively activated, high-affinity β2-integrin receptors in leukocytes has no effect on cell adhesion, suggesting the predominance of avidity modulation in regulating leukocyte adhesion (66), whereas kinetic analysis of coinciding α4β1-integrin affinity and avidity modulation rates in human monoblastoid cells displayed a marked dependence on receptor activation and affinity regulation (8). Nonetheless, our current data seem more consistent with the predominance of integrin affinity modulation in regulating increased cell adhesion under pressure.
GD25-β1A cells are known to express α3β1-, α5β1-, α6β1-, αvβ3-, αvβ5-, and α6β4-integrin heterodimers based on previous characterization (71). The relative maintenance of basal adhesion to fibronectin and ability to bind RGD ligand among mutant GD25-β1A derivatives is most likely due to the overlap in matrix specificity between α5β1-, αvβ3-, and αvβ5-integrins. Furthermore, the RGD-Fc ligand used for these studies has been demonstrated to bind αvβ3- and αvβ5-integrins with higher affinity than α5β1 (30, 36). Although partial functionality of specific β1A mutants may be slightly obscured at the basal level by not blocking the αvβ3- and αvβ5-integrins, the absence of any pressure-mediated adhesion and ligand binding effects in GD25-β1-null cells under these conditions allows us to conclude that β3- and β5-integrins are not the primary mediators of this response. This is consistent with our finding that pressure-stimulated cell adhesion to fibronectin can be blocked by coincubation with anti-β1-blocking antibody, but not anti-β3.
Although it could be speculated that the role of β3-integrin in pressure-stimulated GD25-β1A cell adhesion was masked by the overexpression of β1-integrin, and that the GD25-null cells failed to display the pressure effect due to a critical reduction in total integrin surface expression, our finding that SW620 cells exhibit a similar magnitude of pressure-stimulated adhesion following treatment with either IgG or anti-β3 suggests that pressure-stimulated adhesion is not overtly sensitive to partial reduction of total surface integrin functionality, and more specifically to that of β3-integrin heterodimers. Conversely, the complete blockade of this effect by anti-β1 antibody suggests that it is unlikely that other non-β1-integrins play a critical role in this process. However, these results do not exclude the potential that β3- and β5-integrins may also respond to changes in extracellular pressure. β1-, β3-, and β5-integrins all share two highly conserved cytoplasmic domain NPXY/F-motifs and a serine-threonine cluster containing similar potential phosphorylation sites (45). β3-Integrin tyrosine phosphorylation at Y747 and Y759 has recently been shown to regulate integrin activation (54, 73). Whether changes in extracellular pressure influence such phosphorylation sites on β3- or β5-integrins awaits further study. However, our current data would suggest that any such pressure-mediated effects on β3- or β5-integrins are not likely to contribute substantially to the stimulation of adhesion by increased extracellular pressure.
To date, phosphorylation-dependent regulation of β-chain affinity has been the most thoroughly investigated with regard to β2-integrin (7, 63). Although increased β1-integrin tyrosine phosphorylation has been observed in v-Src-transformed cells (24) and increased serine phosphorylation in parietal endoderm has been demonstrated following treatment with okadaic acid (39), the transient nature of β1-integrin phosphorylation has limited most site-specific functional studies to using mutational analysis of conserved cytoplasmic serine-threonine residues without direct evidence of conditional phosphorylation. Increased pressure stimulates robust phosphorylation of β1-integrin S785 and T788/9 residues and provides a unique model by which to study the role of site-specific phosphorylation events on β1-integrin conformational activation and cell adhesion. GD25-β1A,S785A transfectants displayed reduced adhesion to fibronectin compared with wild-type β1A-expressing cells. Consistent with these results, site-directed substitution of chicken β1-integrin S785 to methionine, mimicking a dephosphorylated residue, in F9 and GD25 cells is reported to interfere with cell attachment to laminin (40). However, GD25-β1A,S785A mutants still retained sensitivity to pressure-stimulated adhesion signals. In contrast, RGD-Fc ligand binding and adhesion of GD25 cells expressing β1A,TT788/9AA was unresponsive to increased pressure. While these results are similar to those of Wennerberg et al. (70) demonstrating that the TT788/9AA double-substitution gives rise to an altered extracellular conformation that is defective in mediating cell attachment, our study is distinct in that it provides direct evidence that T788/9 phosphorylation actually occurs before cell adhesion and correlates with β1-integrin affinity states. Moreover, β1A-T788D substitution, mimicking a phosphorylated residue, significantly enhanced GD25-β1A,T788D cell adhesion. Taken together with recent work by Nilsson et al. (43), showing that β1A-T789A substitution has little effect on cell adhesion, these results suggest that T788 phosphorylation holds the greater regulatory importance of the two residues.
The observation that β1-integrin T788/9 phosphorylation may be stimulated in suspended cells in the absence of ligand strongly supports its involvement in inside-out signaling, in which intracellular signaling events modulate cellular interactions with the external environment. The altered T788/9 phosphorylation patterns resulting from blockade of α-actinin-1, FAK, Src, and PI3K further suggest that phosphorylation of these residues is dynamically regulated by cytoplasmic signals. Moreover, the ability of ERK 1/2 inhibition to impact cell adhesion without affecting basal β1-integrin T788/9 phosphorylation levels suggests that the mechanical signaling pathway by which increases in extracellular pressure stimulate cell adhesion and T788/9 phosphorylation is differentially regulated or at least partially independent of the signaling mechanisms by which cell adhesion is governed at constant ambient pressures. In previous investigations, we have demonstrated that α-actinin-1 functions as a β1-integrin-associated adapter protein and is crucial for Src localization to focal adhesions and formation of the FAK-Src complex (9). In turn, FAK is activated under increased pressure and is required for pressure-mediated activation of Src (58). Inhibition of Src has then been shown to disrupt pressure-induced PI3K activation and cell adhesion (60). PI3K inhibition had the largest impact on β1-integrin T788/9 phosphorylation. While PI3K appears to be the furthest downstream mediator of the pressure-stimulated signaling pathway, it is also known to be upstream of integrin-linked kinase (ILK), the most commonly proposed serine-threonine kinase to directly phosphorylate β1-integrin (6, 52). Whether pressure stimuli can activate ILK is unknown. Alternatively, our results displayed a tendency for increased pressure to inhibit cell adhesion to fibronectin compared with cells maintained under ambient conditions following treatment with the Src inhibitor PP2. This observation may reflect an additional counterregulatory adhesion mechanism also sensitive to fluctuations in extracellular pressure. However, this effect as well as the role of Src in this process await clarification and are beyond the scope of the current article.
In summary, these results suggest that mechanical forces such as pressure and shear increase cell adhesion predominantly via β1-integrin T788/9 phosphorylation-dependent changes in integrin binding affinity. The current study not only serves to strengthen the specific mechanism by which the described force-activated signaling pathway regulates cell adhesion, but further supports the possibility that the β1-integrin T788/9 residues and associated regulatory kinases may prove useful therapeutic targets in the inhibition of tumor cell implantation and metastasis.
GRANTS
This work was supported in part by National Institutes of Health Grant RO1-DK-06771 (to M. D. Basson) and by a VA Merit Review (to M. D. Basson).
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Barreuther MF, Grabel LB. The role of phosphorylation in modulating beta 1 integrin localization. Exp Cell Res 222: 10–15, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Basson MD, Yu CF, Herden-Kirchoff O, Ellermeier M, Sanders MA, Merrell RC, Sumpio BE. Effects of increased ambient pressure on colon cancer cell adhesion. J Cell Biochem 78: 47–61, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Bazzoni G, Ma L, Blue ML, Hemler ME. Divalent cations and ligands induce conformational changes that are highly divergent among beta1 integrins. J Biol Chem 273: 6670–6678, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem 270: 25570–25577, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Blaschke F, Stawowy P, Goetze S, Hintz O, Grafe M, Kintscher U, Fleck E, Graf K. Hypoxia activates beta(1)-integrin via ERK 1/2 and p38 MAP kinase in human vascular smooth muscle cells. Biochem Biophys Res Commun 296: 890–896, 2002. [DOI] [PubMed] [Google Scholar]
- 6.Boulter E, Van Obberghen-Schilling E. Integrin-linked kinase and its partners: a modular platform regulating cell-matrix adhesion dynamics and cytoskeletal organization. Eur J Cell Biol 85: 255–263, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Chatila TA, Geha RS, Arnaout MA. Constitutive and stimulus-induced phosphorylation of CD11/CD18 leukocyte adhesion molecules. J Cell Biol 109: 3435–3444, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chigaev A, Zwartz G, Graves SW, Dwyer DC, Tsuji H, Foutz TD, Edwards BS, Prossnitz ER, Larson RS, Sklar LA. Alpha4beta1 integrin affinity changes govern cell adhesion. J Biol Chem 278: 38174–38182, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Craig DH, Haimovich B, Basson MD. α-Actinin-1 phosphorylation modulates pressure-induced colon cancer cell adhesion through regulation of focal adhesion kinase-Src interaction. Am J Physiol Cell Physiol 293: C1862–C1874, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Craig DH, Owen CR, Conway WC, Walsh MF, Downey C, Basson MD. Colchicine inhibits pressure-induced tumor cell implantation within surgical wounds and enhances tumor-free survival in mice. J Clin Invest 118: 3170–3180, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig DH, Schaubert KL, Shiratsuchi H, Kan-Mitchell J, Basson MD. Increased pressure stimulates aberrant dendritic cell maturation. Cell Mol Biol Lett 13: 260–270, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dahl SC, Grabel LB. Integrin phosphorylation is modulated during the differentiation of F-9 teratocarcinoma stem cells. J Cell Biol 108: 183–190, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond MS, Springer TA. The dynamic regulation of integrin adhesiveness. Curr Biol 4: 506–517, 1994. [DOI] [PubMed] [Google Scholar]
- 14.Dregelid E, Svendsen E. Endothelial cell injury in human saphenous veins after manipulation and tweezer grasping. J Cardiovasc Surg (Torino) 29: 464–469, 1988. [PubMed] [Google Scholar]
- 15.Duncan RL, Turner CH. Mechanotransduction and the functional response of bone to mechanical strain. Calcif Tissue Int 57: 344–358, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Emenaker NJ, Basson MD. Short chain fatty acids inhibit human (SW1116) colon cancer cell invasion by reducing urokinase plasminogen activator activity and stimulating TIMP-1 and TIMP-2 activities, rather than via MMP modulation. J Surg Res 76: 41–46, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Eto DS, Jones TA, Sundsbak JL, Mulvey MA. Integrin-mediated host cell invasion by type 1-piliated uropathogenic Escherichia coli. PLoS Pathog 3: e100, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagerholm SC, Hilden TJ, Gahmberg CG. P marks the spot: site-specific integrin phosphorylation regulates molecular interactions. Trends Biochem Sci 29: 504–512, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Fassler R, Pfaff M, Murphy J, Noegel AA, Johansson S, Timpl R, Albrecht R. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J Cell Biol 128: 979–988, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felding-Habermann B, O'Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci USA 98: 1853–1858, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita S, Kudo N, Akasu T, Moriya Y. Detection of cytokeratin 19 and 20 mRNA in peripheral and mesenteric blood from colorectal cancer patients and their prognosis. Int J Colorectal Dis 16: 141–146, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Guller U, Zajac P, Schnider A, Bosch B, Vorburger S, Zuber M, Spagnoli GC, Oertli D, Maurer R, Metzger U, Harder F, Heberer M, Marti WR. Disseminated single tumor cells as detected by real-time quantitative polymerase chain reaction represent a prognostic factor in patients undergoing surgery for colorectal cancer. Ann Surg 236: 768–775, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hynes RO Integrins: bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Johansson MW, Larsson E, Luning B, Pasquale EB, Ruoslahti E. Altered localization and cytoplasmic domain-binding properties of tyrosine-phosphorylated beta 1 integrin. J Cell Biol 126: 1299–1309, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem 280: 16546–16549, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Kavic SM, Basson MD. Environmental factors of temperature, humidity, serum accumulation, and cell seeding increase colon cancer cell adhesion in vitro, with partial characterization of the serum component responsible for pressure-stimulated adhesion. J Surg Res 98: 89–96, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Kim M, Carman CV, Yang W, Salas A, Springer TA. The primacy of affinity over clustering in regulation of adhesiveness of the integrin (alpha)L(beta)2. J Cell Biol 167: 1241–1253, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knies Y, Bernd A, Kaufmann R, Bereiter-Hahn J, Kippenberger S. Mechanical stretch induces clustering of beta1-integrins and facilitates adhesion. Exp Dermatol 15: 347–355, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Knoll R, Hoshijima M, Chien K. Cardiac mechanotransduction and implications for heart disease. J Mol Med 81: 750–756, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology 13: 265–270, 1995. [DOI] [PubMed] [Google Scholar]
- 31.Konstandin MH, Sester U, Klemke M, Weschenfelder T, Wabnitz GH, Samstag Y. A novel flow-cytometry-based assay for quantification of affinity and avidity changes of integrins. J Immunol Methods 310: 67–77, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest 97: 2139–2144, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuphal S, Bauer R, Bosserhoff AK. Integrin signaling in malignant melanoma. Cancer Metastasis Rev 24: 195–222, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc Natl Acad Sci USA 90: 9051–9055, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Interstitial hypertension in human breast and colorectal tumors. Cancer Res 52: 6371–6374, 1992. [PubMed] [Google Scholar]
- 36.Li J, Ji J, Holmes LM, Burgin KE, Barton LB, Yu X, Wagner TE, Wei Y. Fusion protein from RGD peptide and Fc fragment of mouse immunoglobulin G inhibits angiogenesis in tumor. Cancer Gene Ther 11: 363–370, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Madri JA, Pratt BM, Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol 132: 18–27, 1988. [PMC free article] [PubMed] [Google Scholar]
- 38.Moore-Olufemi SD, Xue H, Allen SJ, Moore FA, Stewart RH, Laine GA, Cox CS Jr. Effects of primary and secondary intra-abdominal hypertension on mesenteric lymph flow: implications for the abdominal compartment syndrome. Shock 23: 571–575, 2005. [PubMed] [Google Scholar]
- 39.Mulrooney J, Foley K, Vineberg S, Barreuther M, Grabel L. Phosphorylation of the beta1 integrin cytoplasmic domain: toward an understanding of function and mechanism. Exp Cell Res 258: 332–341, 2000. [DOI] [PubMed] [Google Scholar]
- 40.Mulrooney JP, Hong T, Grabel LB. Serine 785 phosphorylation of the beta1 cytoplasmic domain modulates beta1A-integrin-dependent functions. J Cell Sci 114: 2525–2533, 2001. [DOI] [PubMed] [Google Scholar]
- 41.Nathan SS, DiResta GR, Casas-Ganem JE, Hoang BH, Sowers R, Yang R, Huvos AG, Gorlick R, Healey JH. Elevated physiologic tumor pressure promotes proliferation and chemosensitivity in human osteosarcoma. Clin Cancer Res 11: 2389–2397, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Ni N, Kevil CG, Bullard DC, Kucik DF. Avidity modulation activates adhesion under flow and requires cooperativity among adhesion receptors. Biophys J 85: 4122–4133, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nilsson S, Kaniowska D, Brakebusch C, Fassler R, Johansson S. Threonine 788 in integrin subunit beta1 regulates integrin activation. Exp Cell Res 312: 844–853, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Opitz F, Schenke-Layland K, Cohnert TU, Stock UA. Phenotypical plasticity of vascular smooth muscle cells-effect of in vitro and in vivo shear stress for tissue engineering of blood vessels. Tissue Eng 13: 2505–2514, 2007. [DOI] [PubMed] [Google Scholar]
- 45.O'Toole TE, Ylanne J, Culley BM. Regulation of integrin affinity states through an NPXY motif in the beta subunit cytoplasmic domain. J Biol Chem 270: 8553–8558, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM. Tensional homeostasis and the malignant phenotype. Cancer Cell 8: 241–254, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Plantefaber LC, Hynes RO. Changes in integrin receptors on oncogenically transformed cells. Cell 56: 281–290, 1989. [DOI] [PubMed] [Google Scholar]
- 48.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol 59: 551–571, 1997. [DOI] [PubMed] [Google Scholar]
- 49.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple endpoint adjustment methods in clinical trials. Stat Med 16: 2529–2542, 1997. [DOI] [PubMed] [Google Scholar]
- 50.Schaff UY, Yamayoshi I, Tse T, Griffin D, Kibathi L, Simon SI. Calcium flux in neutrophils synchronizes beta2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann Biomed Eng 36: 632–646, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwartz MA, Ingber DE. Integrating with integrins. Mol Biol Cell 5: 389–393, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens JM, Jordan PA, Sage T, Gibbins JM. The regulation of integrin-linked kinase in human platelets: evidence for involvement in the regulation of integrin alpha 2 beta 1. J Thromb Haemost 2: 1443–1452, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Stroeken PJ, van Rijthoven EA, Boer E, Geerts D, Roos E. Cytoplasmic domain mutants of beta1 integrin, expressed in beta 1-knockout lymphoma cells, have distinct effects on adhesion, invasion and metastasis. Oncogene 19: 1232–1238, 2000. [DOI] [PubMed] [Google Scholar]
- 54.Su X, Mi J, Yan J, Flevaris P, Lu Y, Liu H, Ruan Z, Wang X, Kieffer N, Chen S, Du X, Xi X. RGT, a synthetic peptide corresponding to the integrin beta 3 cytoplasmic C-terminal sequence, selectively inhibits outside-in signaling in human platelets by disrupting the interaction of integrin alpha IIb beta 3 with Src kinase. Blood 112: 592–602, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugarbaker PH Successful management of microscopic residual disease in large bowel cancer. Cancer Chemother Pharmacol 43, Suppl: S15–S25, 1999. [DOI] [PubMed] [Google Scholar]
- 56.Takagi J, Petre BM, Walz T, Springer TA. Global conformational rearrangements in integrin extracellular domains in outside-in and inside-out signaling. Cell 110: 599–511, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Takagi J, Springer TA. Integrin activation and structural rearrangement. Immunol Rev 186: 141–163, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Thamilselvan V, Basson MD. Pressure activates colon cancer cell adhesion by inside-out focal adhesion complex and actin cytoskeletal signaling. Gastroenterology 126: 8–18, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Thamilselvan V, Basson MD. The role of the cytoskeleton in differentially regulating pressure-mediated effects on malignant colonocyte focal adhesion signaling and cell adhesion. Carcinogenesis 26: 1687–1697, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Thamilselvan V, Craig DH, Basson MD. FAK association with multiple signal proteins mediates pressure-induced colon cancer cell adhesion via a Src-dependent PI3K/Akt pathway. FASEB J 21: 1730–1741, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Thamilselvan V, Patel A, van der Voort van Zyp J, Basson MD. Colon cancer cell adhesion in response to Src kinase activation and actin-cytoskeleton by non-laminar shear stress. J Cell Biochem 92: 361–371, 2004. [DOI] [PubMed] [Google Scholar]
- 62.Umpleby HC, Fermor B, Symes MO, Williamson RC. Viability of exfoliated colorectal carcinoma cells. Br J Surg 71: 659–663, 1984. [DOI] [PubMed] [Google Scholar]
- 63.Valmu L, Gahmberg CG. Treatment with okadaic acid reveals strong threonine phosphorylation of CD18 after activation of CD11/CD18 leukocyte integrins with phorbol esters or CD3 antibodies. J Immunol 155: 1175–1183, 1995. [PubMed] [Google Scholar]
- 64.Van der Voort van Zyp J, Conway WC, Thamilselvan V, Polin L, Basson MD. Divalent cations influence colon cancer cell adhesion in a murine transplantable tumor model. Am J Surg 190: 701–707, 2005. [DOI] [PubMed] [Google Scholar]
- 65.Van Kooyk Y, Figdor CG. Avidity regulation of integrins: the driving force in leukocyte adhesion. Curr Opin Cell Biol 12: 542–547, 2000. [DOI] [PubMed] [Google Scholar]
- 66.Van Kooyk Y, van Vliet SJ, Figdor CG. The actin cytoskeleton regulates LFA-1 ligand binding through avidity rather than affinity changes. J Biol Chem 274: 26869–26877, 1999. [DOI] [PubMed] [Google Scholar]
- 67.Van Willigen G, Hers I, Gorter G, Akkerman JW. Exposure of ligand-binding sites on platelet integrin alpha IIB/beta 3 by phosphorylation of the beta 3 subunit. Biochem J 314: 769–779, 1996. [PMC free article] [PubMed] [Google Scholar]
- 68.Walsh MF, Woo RK, Gomez R, Basson MD. Extracellular pressure stimulates colon cancer cell proliferation via a mechanism requiring PKC and tyrosine kinase signals. Cell Prolif 37: 427–441, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang N, Butler JP, Ingber DE. Mechanotransduction across the cell surface and through the cytoskeleton. Science 260: 1124–1127, 1993. [DOI] [PubMed] [Google Scholar]
- 70.Wennerberg K, Fassler R, Warmegard B, Johansson S. Mutational analysis of the potential phosphorylation sites in the cytoplasmic domain of integrin beta1A. Requirement for threonines 788–789 in receptor activation. J Cell Sci 111: 1117–1126, 1998. [DOI] [PubMed] [Google Scholar]
- 71.Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol 132: 227–238, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu JS, Brasfield EB, Guo LW, Ruiz M, Connett JM, Philpott GW, Jones DB, Fleshman JW. Implantation of colon cancer at trocar sites is increased by low pressure pneumoperitoneum. Surgery 122: 1–7, 1997. [DOI] [PubMed] [Google Scholar]
- 73.Xi X, Bodnar RJ, Li Z, Lam SC, Du X. Critical roles for the COOH-terminal NITY and RGT sequences of the integrin beta3 cytoplasmic domain in inside-out and outside-in signaling. J Cell Biol 162: 329–339, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Z, Vuori K, Wang H, Reed JC, Ruoslahti E. Integrin activation by R-ras. Cell 85: 61–69, 1996. [DOI] [PubMed] [Google Scholar]
- 75.Zhou X, Li J. Macrophage-enriched myristoylated alanine-rich C kinase substrate and its phosphorylation is required for the phorbol ester-stimulated diffusion of beta 2 integrin molecules. J Biol Chem 275: 20217–20222, 2000. [DOI] [PubMed] [Google Scholar]