Abstract
During free behaviors animals often experience lateral forces, such as collisions with obstacles or interactions with other animals. We studied postural reactions to lateral pulses of force (pushes) in the cat during standing and walking. During standing, a push applied to the hip region caused a lateral deviation of the caudal trunk, followed by a return to the initial position. The corrective hindlimb electromyographic (EMG) pattern included an initial wave of excitation in most extensors of the hindlimb contralateral to push and inhibition of those in the ipsilateral limb. In cats walking on a treadmill with only hindlimbs, application of force also caused lateral deviation of the caudal trunk, with subsequent return to the initial position. The type of corrective movement depended on the pulse timing relative to the step cycle. If the force was applied at the end of the stance phase of one of the limbs or during its swing phase, a lateral component appeared in the swing trajectory of this limb. The corrective step was directed either inward (when the corrective limb was ipsilateral to force application) or outward (when it was contralateral). The EMG pattern in the corrective limb was characterized by considerable modification of the hip abductor and adductor activity in the perturbed step. Thus the basic mechanisms for balance control in these two forms of behavior are different. They perform a redistribution of muscle activity between symmetrical limbs (in standing) and a reconfiguration of the base of support during a corrective lateral step (in walking).
INTRODUCTION
Maintenance of normal body posture and equilibrium is a necessary condition for standing and walking. In quadrupeds, the most critical aspect of postural control is lateral stability, involving maintenance of body orientation in the frontal plane. For example, when deprived of the ability to keep balance in their hindquarters after damage to the spinal cord, cats are not able to walk, although the basic mechanisms of stepping can function normally. However, locomotion in these animals becomes feasible if the lateral stability is maintained externally (Barbeau and Rossignol 1987).
Animals can use at least three different strategies to stabilize their body orientation in the frontal plane. These strategies depend on the type of postural perturbations and other factors.
It is well known that animals can adapt their posture to the lateral tilt of the supporting surface (inclination in the frontal plane). This is achieved by a differential change in the functional length of the left and right limbs. This type of adaptation is observed both when standing (Beloozerova et al. 2003; Deliagina et al. 2006b) and when walking (Karayannidou et al. 2008; Matsuyama and Drew 2000).
It is also known that animals and humans can maintain balance on unstable support, e.g., during lateral movements of the supporting platform imitating slip conditions. These postural corrections have been described for standing cats (Macpherson 1988a,b) and for walking cats (Misiaszek 2006), as well as for standing (e.g., Moor et al. 1988) and walking humans (Oddsson et al. 2004). In standing subjects, the postural system restores equilibrium by moving the body so that the projection of the center of mass appears closer to the center of the base of support. These postural corrections are enabled by specific forces produced by the limbs against the supporting surface. The situation is different in walking subjects, where postural corrections are due to specific modifications of the pattern of stepping limb movements.
Freely behaving animals are often subjected to lateral forces when colliding with obstacles or interacting with other animals. Thus an important postural task is compensation for body displacements in the frontal plane caused by lateral forces applied to the body. Standing cats well resist to lateral pushes and do not fall even with application of forces ≤0.5–1 kg in the hip region (Kato et al. 1985), suggesting a high efficacy of the posture-stabilizing mechanisms for such disturbances. However, analysis of the postural system that counteracts lateral pushes is lacking. Also, the corrective responses to perturbations of posture during locomotion have not been analyzed. The aim of the present study is to understand how cats maintain stability during standing and walking, when a lateral force perturbs their normal, dorsal side-up posture.
To simplify the analysis of postural corrections during locomotion, we examined the cat walking on the treadmill with only one pair of limbs (fore or hind), while keeping the other pair on a stationary platform. This simplification was based on the assumption that, in quadrupeds, the postural mechanisms of individual girdles for maintaining lateral stability are relatively autonomous, as was demonstrated for balancing on two independently tilted platforms (Beloozerova et al. 2003; Deliagina et al. 2006a,b). The step-generating mechanisms of the fore- and hindlimbs are also autonomous (Orlovsky et al. 1999). In the present study, we analyzed postural control in the hindquarters both when standing and when walking, using kinematic and electromyographic (EMG) data. Postural control in the forequarters was considered only for walking and in less detail (only kinematic data).
A brief account of a part of this study has been published in abstract form (Karayannidou et al. 2007).
METHODS
Recordings were obtained from four adult cats. Some of the methods were previously described (Beloozerova et al. 2005; Deliagina et al. 2006b) and will be reported only briefly here. All experiments were conducted with the approval of the Barrow Neurological Institute Animal Care and Use Committee.
Implantation of EMG electrodes
Surgery for implantation of EMG electrodes was performed under isoflurane anesthesia using aseptic procedures. The skin and fascia were removed from the dorsal surface of the skull. At 10 points around the circumference of the head, stainless steel screws were screwed into the skull and connected together with a wire; the screw heads and the wire were then inserted into a plastic cast to form a circular base.
EMG recording bipolar electrodes were constructed from Teflon-insulated multistrand stainless steel wire (0.2-mm outer diameter, AS633, Cooner Wire) and were implanted bilaterally into selected hindlimb muscles. Muscles were not the same for all cats; included were vastus lateralis (Vast, knee extensor), gastrocnemius lateralis (Gast, ankle extensor), gluteus medius (Glut Med, hip abductor and extensor), gracilis (Grac), and adductor femoris (Add fem); the two latter muscles function as hip adductors and extensors. Electrode placement was verified by stimulation through the implanted wires before closure of the incision. The wires were positioned subcutaneously and soldered to the connectors on the head base.
Postural tests
TESTS DURING WALKING.
Each animal was trained for a period of about 6 wk to walk on a treadmill with a constant belt speed of about 0.5 m/s. Positive reinforcement (food) was used to habituate cats to the experimental situation and to motivate locomotor behavior. When walking on the treadmill, cats continuously licked paste-like food, which was slowly ejected from a feeder positioned in front of the animal (Fig. 1 A). In doing this, the cat stabilized its spatial position and continuously kept its mouth against the feeder. Cats received all food from performance of the motor tasks for the duration of the experiment.
FIG. 1.
Experimental design for testing lateral stability in different motor tasks. A–C: postural tests during walking with hindlimbs. A and B: the side and back view of the walking cat, respectively, with mechanical sensors monitoring the anterior–posterior (AP) position of the left hindlimb, the medial–lateral (ML) position of the left hindlimb (LHL), and the ML position of the posterior part of the body (Bd). C: scheme of the step cycle (as derived from the AP position of limbs), with the phases of one-limb and two-limb support (nonshaded and shaded intervals, respectively). D: postural tests during walking with forelimbs. E and F: postural tests during standing. In different tests, lateral pushes were applied either in the hip area (A and E) or in the shoulder area (D). Correspondingly, video recording of the cat was done either from behind (A and E) or from the front (D).
After the cats were habituated to treadmill locomotion, they were trained to walk with only one pair of limbs, keeping the other pair stationary on a platform; these two forms of bipedal locomotion are shown schematically in Fig. 1A (hindlimb walking) and Fig. 1D (forelimb walking).
The postural perturbations used in this study were brief pulses of force (pushes) applied in the medial–lateral (ML) direction in the hip joint area or in the shoulder joint area (gray circles in Fig. 1, A and D, respectively). This was done manually using a “pusher” (cylindrical tool 1.5 cm in diameter and 10 cm in length) with a force sensor on the side facing the cat. Each push lasted 150–250 ms, providing 100–300 g of force. During the push, the corresponding part of the trunk was displaced by a few centimeters in the ML direction; displacements in vertical or longitudinal directions were practically absent. The direction of perturbation (leftward or rightward with respect to the cat) was randomized. Pushes were applied randomly in different phases of the step cycle, approximately once every 5–10 cycles. Typically, each experimental session included 200 undisturbed step cycles and 20–30 cycles with perturbations.
In tests with walking hindlimbs, the ML positions of the caudal part of the trunk and hindlimbs were monitored by a video camera (30 frames/s) placed 2 m behind the animal (Fig. 1A). Video recordings also allowed for estimation of the phase of the step cycle. Five mechanical sensors monitored: 1) applied force (Push); 2) medial–lateral position of the caudal trunk (ML-Bd); 3) medial–lateral position of the left hindlimb (ML-LHL); 4) anterior–posterior position of the left hindlimb (AP-LHL); and 5) anterior–posterior position of the right hindlimb (AP-RHL). Figure 1C shows schematically the five values recorded by mechanical sensors in these experiments. The recordings by video and electronic systems were synchronized.
In tests with walking forelimbs, the ML position of the rostral part of the trunk and that of the forelimbs were monitored by video camera placed 2 m in front of the animal (Fig. 1D). Mechanical sensors were not used in forelimb tests.
TESTS DURING STANDING.
Standing tests had many features in common with those of walking. Each animal was trained to stand on the treadmill while it was stationary. Positive reinforcement (food) was used to habituate cats to the experimental situation and to keep standing during postural perturbations. When standing, cats continuously licked food from a feeder positioned in front of the animal (Fig. 1E). Postural perturbations were caused by the previously described pushes applied to the hip region (pulse duration 150–250 ms, force amplitude 100–300 g, ML force direction). In these experiments, the cat's view was recorded from behind by video camera and the ML position of the caudal part of the trunk was recorded by the mechanical sensor (Fig. 1, E and F).
Data presentation and analysis
Data related to the hindlimbs and obtained by the two recording systems were synchronized and combined in the following way. The records of AP position of the two symmetrical limbs and of the push were used to determine the phase of pushes within the step cycle. The step cycle was divided into four intervals, as shown schematically in Fig. 1C, involving one-leg support of the girdle (intervals 1 and 3) and two-leg support (intervals 2 and 4). The phase of the push onset was used to classify the pulses into these four categories.
Both video and mechanical sensor data were used to calculate the ML position of the right and left paws during stance (ML-R and ML-L), as well as the ML position of the trunk (Bd) in sequential steps. The data on the ML positions of limbs and trunk were thus combined with the data on the AP leg position (Fig. 1C).
RESULTS
Postural corrections during standing
Pushes applied to the hip region during standing (Fig. 1, E and F) evoked a distinct pattern of response that was very similar in all cats. Four characteristic positions of a cat during the response to rightward push are shown in Fig. 2, A–D. The body outline immediately before force application is shown in Fig. 2A. The push caused a postural perturbation (i.e., a rightward displacement of the caudal part of the trunk), with its maximal deviation depicted in Fig. 2B. Then, due to the activity of the mechanism of postural corrections, the body started to move leftward, toward the initial position, but overshot this position. The position corresponding to the peak overshoot is shown in Fig. 2C. The body then shifted to the right until it reached a position similar to the initial one and stopped moving (Fig. 2D). The postural correction was finished in <0.6 s after perturbation.
FIG. 2.
Kinematic responses to push in the standing cat. A–D: 4 sequential positions of the standing cat in response to the rightward push. The lateral deviation of the caudal trunk (H) is indicated. A: the position just before push. B: the maximal deviation of the caudal part of the trunk. C: under the effect of postural corrective mechanism, the trunk moved toward the initial position but passed it over. D: the trunk returned to the initial position. E: 3 examples of temporal patterns of postural responses (trunk deviation, H) with overshot. F: the maximal body deviation caused by push (Peak), the overshoot (Overshoot), and the final body position (Final) (mean ± SE), averaged over 21 responses recorded in 3 cats. Positive and negative values correspond to displacement in the direction of force and in the opposite direction, respectively.
An overshoot was observed in cats 1–3 (Fig. 2E), but not in cat 4, in 42% of all trials, whereas in 58% of trials no overshoot occurred (as in Fig. 3 A). Figure 2F shows different characteristics of the postural correction with overshoot: the peak body deviation (Peak), the overshoot value (Overshoot), and the final body position (Final), averaged over 21 responses recorded in three cats. These responses were elicited by pushes with force amplitude of 171 ± 16 g. Table 1 shows that the response characteristics in individual cats are rather similar and thus can be pooled together.
FIG. 3.
Electromyographic (EMG) responses to push in the standing cat. A: a representative example of EMG responses in 6 selected hindlimb muscles during standing. B: average EMG responses to a leftward push in 8 hindlimb muscles (averaging over 12 trials in cat 3; mean ± SE).
TABLE 1.
Characteristics of postural responses with overshoot
| Cat Number | Push, g | Peak, cm | Overshoot, cm | Final, cm |
|---|---|---|---|---|
| 1 | 210 ± 57 | 2.6 ± 0.2 | −1.8 ± 0.5 | −0.8 ± 0.2 |
| 2 | 171 ± 32 | 2.8 ± 0.2 | −2.8 ± 0.7 | −1.7 ± 0.7 |
| 3 | 137 ± 16 | 2.8 ± 0.2 | −1.6 ± 0.3 | −0.7 ± 0.2 |
| All | 171 ± 16 | 2.7 ± 0.1 | −2.0 ± 0.3 | −1.0 ± 0.2 |
Values are means ± SE, for individual cats (1–3) and for all three cats together. Number of trials for cats 1, 2, and 3: n = 7, n = 6; and n = 8, respectively. Designations: Push, the force amplitude; Peak, the peak body deviation caused by push; Overshoot, the overshoot value; Final, the final body position (see Fig. 2F).
Lateral pushes elicited a robust pattern of EMG responses in the hindlimbs; a representative example of the responses is shown in Fig. 3A. A push directed to the left caused a leftward displacement of the body and a subsequent return to the initial position without overshoot. The three recorded muscles of the left limb produced large bursts of activity, but almost complete inhibition of activity occurred in two of three recorded muscles of the right limb.
Figure 3B shows responses of the four pairs of hindlimb muscles (Gast, Vast, Glut, and Grac), averaged over 13 trials with left-directed pushes (cat 3). These pushes evoked an initial, short-latency wave of excitation in all four muscles of the limb contralateral to force application. By contrast, in the ipsilateral limb, the response in Gast, Vast, and Glut included an initial, short-latency wave of inhibition followed by a smaller wave of excitation. The response in Grac differed from that in other ipsilateral muscles, consisting of a brief, short-latency wave of excitation. A similar EMG pattern was observed in all four cats.
Postural corrections during walking of hindlimbs
Kinematic and EMG patterns of hindlimb stepping movements in this test and the body configuration (Fig. 1, A and B) were similar to that of normal, four-legged walking (reviewed, e.g., in Orlovsky et al. 1999; Rossignol 1996).
Recording the ML trunk position showed that in the absence of external influences the pelvis exhibited lateral oscillations in the rhythm of stepping. Figure 4 A shows these oscillations in cats 2 and 4 (averaging over 51 and 49 step cycles of the right hindlimb, respectively). The peak-to-peak (P-P) value of oscillations was 4 cm in cat 3 and 1.4 cm in cat 4. In all four cats, this value ranged from 1 to 6 cm and depended on the “step width” (the distance between paws during stance in the frontal plane). We found that the animals spontaneously changed their step width, as illustrated in Fig. 4B for cat 2, in which this value ranged from 8.5 to 14.5 cm. The P-P value of oscillations positively correlated with the step width (also shown in Fig. 4B). Thus the value of lateral oscillations of the pelvis varied considerably in individual animals and across the population. Postural responses to lateral pushes were superimposed on these step-related lateral oscillations.
FIG. 4.
Lateral oscillations of the trunk during stepping. A: averaged lateral oscillations of the pelvis in cat 2 (n = 51) and cat 4 (n = 49), in relation to a step cycle of the right hindlimb. B: positive correlation between the peak-to-peak (P-P) excursions of the posterior part of the trunk and the width of the step (the distance between the stance paws in the frontal plane), illustrated for cat 2 (n = 31).
All four cats exhibited two distinct patterns of response to postural perturbations, differing in the direction of the lateral component of the corrective limb movement. We will refer to these patterns as “outward step” and “inward step,” in which the limb landed more laterally or more medially than in the previous step, respectively.
OUTWARD STEP.
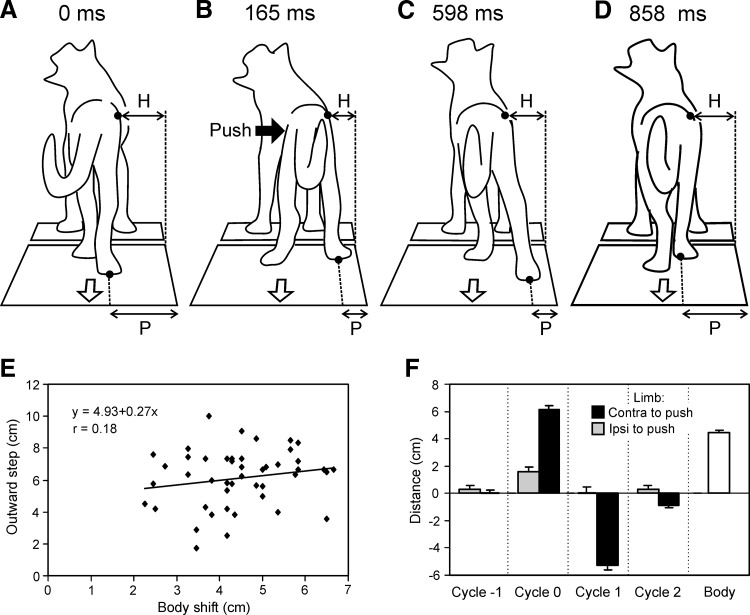
The four characteristic positions of cat 1 during this type of response are shown in Fig. 5, A–D. The push directed to the right was applied when the right hindlimb just finished its stance phase. The body outline before the push is shown in Fig. 5A. The push caused a rightward displacement of the caudal part of the trunk, as well as a large lateral component in the step of the right limb. These lateral displacements are well seen in the frame taken at the moment of landing the right foot (Fig. 5B). (In Fig. 5, A–D, the lateral foot position during stance is indicated by an interrupted line, P.) During the subsequent stance phase, extension of this limb evoked a corrective leftward displacement of the caudal part of the body (Fig. 5C) until the body returned to the initial ML position. After termination of stance, the right limb returned to the normal ML position during the subsequent swing phase of the step (Fig. 5D).
FIG. 5.
Outward step. A–D: 4 characteristic positions of the walking cat 1 resulting from the push applied in the hip region toward the right. A: configuration just before force application, at the moment when the right leg finished the stance phase. B: the push caused a rightward displacement of the trunk and landing of the foot at a more lateral position (P) than during unperturbed locomotion. C: extension of the leg in the subsequent stance phase caused a leftward displacement of the caudal trunk toward the initial position. D: during the subsequent swing phase, the right leg returned to the normal ML position. E and F: characteristics of outward steps of the hindlimbs. E: correlation between the postural perturbation (Body shift) and the response to this perturbation (Outward step). F: mean values (±SE) characterizing the lateral component of step in sequential cycles of the hindlimbs. Positive and negative values correspond to outward and inward displacement of the limb, respectively. Designation of cycles: (−1), the cycle before push; (0), the cycle including push; (1), the cycle next to the affected cycle; and (2), the cycle next to (1). The mean value of push-caused trunk displacement is also given (Body). For E and F, n = 3 and n = 46.
In all four cats, the outward step was consistently observed in the limb contralateral to force application, provided the push was performed close to the end of the stance phase of this limb or in its swing phase (periods 4 and 1, Fig. 1C). In some cases, however, the pushes appeared too forceful and dramatically affected equilibrium, causing a lateral displacement of the supporting limb. These cases were excluded from further analysis.
Using video recordings, we measured the body displacement caused by the push. We also measured the corrective response: the difference between the ML foot position during stance in the disturbed cycle and in the previous cycle. Figure 5E shows relationships between the postural perturbation (Body shift) and the postural response (Lateral step) for 46 individual outward steps (the outward displacements were considered as positive). There was no significant correlation between the two values (r = 0.18).
The data for individual disturbances were then averaged over different trials. Figure 5F shows the mean values (±SE) characterizing the lateral component of step in sequential cycles for both hindlimbs. In the cycle before push (cycle −1), no lateral component was present. In the cycle with a push (cycle 0), the contralateral (effective) limb performed a large outward step (6.2 cm). A small (1.5-cm) lateral component was also observed in the ipsilateral limb. In the cycle following the affected cycle (cycle 1), the effective limb performed a large (∼5.4 cm) inward step to return to the normal ML position. In the next cycle (cycle 2), the lateral component was largely absent. The mean value of lateral (outward) step in cycle 0 was larger than the mean value of body displacement in this cycle (shown as an unfilled bar, Body, in Fig. 5F): 6.2 ± 0.3 versus 4.3 ± 0.2 cm (P < 0.05). Table 2 shows that the ML component of the hindlimb corrective outward steps was rather similar in individual cats and thus these data can be pooled together. Table 2 also shows that the ML component was significantly larger in corrective steps compared with ordinary steps.
TABLE 2.
Medial–lateral displacement of foot in ordinary steps and in corrective steps
| Ordinary Steps, cm |
Corrective Steps, cm | |||||||
|---|---|---|---|---|---|---|---|---|
| Forelimbs |
Hindlimbs | Forelimbs | Hindlimbs | |||||
| Cat Number | Out | In | Out | In | Out | In | Out | In |
| 1 | 0.9 ± 0.5 (n = 8) | −1.1 ± 0.8 (n = 3) | 1.2 ± 0.2 (n = 22) | −0.5 ± 0.4 (n = 11) | 3.5 ± 1.3 (n = 7) | −4.7 ± 0.7 (n = 4) | 6.5 ± 0.4 (n = 17) | −4.0 ± 0.6 (n = 16) |
| 2 | 0.9 ± 0.4 (n = 3) | −1.0 ± 0.4 (n = 7) | 0.3 ± 0.1 (n = 8) | −1.5 ± 0.3 (n = 8) | 6.6 ± 0.4 (n = 7) | −9.9 ± 1.2 (n = 3) | 6.0 ± 0.6 (n = 9) | −4.6 ± 1.1 (n = 7) |
| 3 | 0.5 ± 0.2 (n = 7) | −1.0 ± 0.4 (n = 6) | 0.6 ± 0.2 (n = 14) | −1.4 ± 0.6 (n = 6) | 4.7 ± 1.2 (n = 7) | −5.2 ± 0.8 (n = 6) | 5.5 ± 0.7 (n = 12) | −4.4 ± 1.2 (n = 8) |
| 4 | 0.8 ± 0.2 (n = 8) | −1.0 ± 0.3 (n = 5) | 1.4 ± 0.4 (n = 11) | −1.4 ± 0.5 (n = 10) | 6.7 ± 0.5 (n = 7) | −5.2 ± 0.9 (n = 6) | 5.1 ± 0.9 (n = 11) | −4.0 ± 1.5 (n = 10) |
| All | 0.7 ± 0.2 (n = 26) | −1.1 ± 0.2 (n = 21) | 1.0 ± 0.1 (n = 55) | −1.2 ± 0.2 (n = 35) | 5.4 ± 0.5 (n = 28) | −6.0 ± 0.6 (n = 19) | 5.9 ± 0.3 (n = 49) | −4.2 ± 0.5 (n = 41 |
Values are means ± SE of medial–lateral displacements of the foot-on-support position in sequential steps, given for individual cats (1–4) and for all four cats together; number of trials, n, in parentheses. Positive and negative values correspond to outward and inward displacement of the limb, respectively. Out, steps with outward component; In, steps with inward component. In each cat, there was a significant difference (P < 0.05) between the corresponding values in ordinary steps and in corrective steps.
EMGs were recorded along with kinematic data. Figure 6 A shows a direct recording, in seven sequential steps, of the AP positions of the left and right limbs, ML positions of the left limb and trunk, and four selected EMGs (cat 3). A leftward push was applied in cycle 3, shortly before the onset of the left limb's swing phase. This caused a leftward displacement of the trunk and an outward step of the left limb. There were considerable changes to EMG patterns in the perturbed cycle.
FIG. 6.
EMG pattern of the outward step. A: a representative example of EMG responses to a leftward push (Force) in 4 selected hindlimb muscles during walking (cat 3). Shown also are AP position of the left and right hindlimbs (L AP and R AP), ML position of the left hindlimb (L ML), and ML position of the trunk (Body). B and C: average EMG responses to a push in 8 hindlimb muscles. EMGs shown are for the limb performing the outward step (B) and for the opposite limb (C). Blue traces indicate EMGs recorded in the cycle preceding the outward step and red traces are those in the cycle with the outward step—averaging over 12 trials in cat 3. Stance and swing phases are shown by the filled (black) and the empty bar, respectively. The red bar indicates the push position in the cycle.
To evaluate these changes, we performed cycle-linked averaging of individual EMGs. This is illustrated in Fig. 6, B and C, for the leftward push applied at the beginning of the swing phase of the left limb, eliciting its outward step (cat 3). The start of the swing phase of this limb was taken as the cycle onset. Shown are the EMGs of the effective (left) limb (Fig. 6B) and of the opposite limb (Fig. 6C). One can compare the EMGs in the cycle preceding the outward step (blue traces) with those in the affected cycle (red traces). The knee and ankle extensors (Vast and Gast) only slightly changed their pattern in the affected cycle, both in the effective limb and in the opposite limb. By contrast, hip adductors (Grac and Add fem) and abductor (Glut) in the effective limb dramatically increased their activity. In the opposite limb, the activity increased dramatically in Grac, slightly increased and changed the phase of one of the peaks in Glut, and did not change in Add fem. Similar EMG responses were observed in two other cats.
INWARD STEP.
Four characteristic positions of cat 1 during this response type are shown in Fig. 7, A–D. A push directed to the right was applied when the left hindlimb finished the stance phase. The body outline immediately before pushing is shown in Fig. 7A. The push caused a rightward displacement of the caudal part of the body as well as a step of the left limb with a large lateral component. These lateral displacements are well seen in the frame taken at the moment of landing the left foot (Fig. 7B). During the subsequent stance phase, extension of this limb caused a corrective leftward displacement of the caudal part of the body (Fig. 7C) until the body returned to the initial ML position. After termination of stance, the right limb returned to the normal ML position during the subsequent swing phase (Fig. 7D). (In Fig. 7, A–D, the lateral foot position during stance is indicated by an interrupted line, P.)
FIG. 7.
Inward step. A–D: 4 characteristic positions of the walking cat resulting from the push applied in the hip region toward the right. A: configuration just before force application, at the end of the left leg's stance phase. B: the push caused a rightward displacement of the trunk and landing of the foot at a more medial position (P) than during unperturbed locomotion. C: leftward displacement of the caudal trunk toward the initial position in the subsequent stance phase. D: return to the normal ML position of the right leg in the subsequent swing phase. E and F: characteristics of inward steps of the hindlimbs. E: correlation between the postural perturbation (Body shift) and the response to this perturbation (Inward step). F: mean values characterizing the lateral component of step in sequential cycles of the hindlimbs. Designations as in Fig. 5. The mean value of push-caused trunk displacement is also given (Body). For E and F, n = 3 and n = 46.
In all four cats, the inward step was consistently observed in the limb ipsilateral to force application, provided the push was performed close to the end of the stance phase of this limb or in its swing phase (periods 2 and 3, Fig. 1C). Thus with the same stimulus applied, the type of postural response (outward or inward step) depends on the phase of push application in the step cycle.
Using video recordings, we measured the body displacement caused by the push. We also measured the difference between the ML foot position during stance in the disturbed cycle and in the previous cycle. Figure 7E shows relationships between the postural perturbation (Body shift) and the postural response (Lateral step) for 46 individual inward steps (the inward displacements were considered as negative). There was no significant correlation between the two values (r = 0.23).
The data for individual inward steps were averaged over all steps. Figure 7F shows the mean values characterizing the lateral component of step in sequential cycles for both hindlimbs. In the cycle before push (cycle −1), no lateral component was present. In the cycle with push (cycle 0), the contralateral (effective) limb performed a large inward step (5.2 cm). A small (∼1-cm) lateral component was also observed in the ipsilateral limb. In the cycle following the affected cycle (cycle 1), the effective limb performed a large (about 4.4 cm) outward step to return to the normal ML position. In the next cycle (cycle 2) the lateral component was practically absent. The mean value of lateral (inward) step in cycle 0 was larger than the mean value of body displacement in this cycle (shown as unfilled bar, Body, in Fig. 7F): 5.2 ± 0.3 against 3.5 ± 0.2 cm (P < 0.05). Table 2 shows that the ML component of the hindlimb corrective inward steps was rather similar in individual cats and thus these data can be pooled together. Table 2 also shows that the ML component was significantly larger in corrective steps compared with ordinary steps.
Figure 8 A shows a representative recording of the AP positions of the left and right limbs, ML positions of the left limb and trunk, and four selected EMGs in seven sequential steps (cat 3). The rightward push was applied in cycle 3, at the onset of the swing phase of the left limb. This caused a rightward displacement of the trunk and an inward step of the left limb. There were considerable changes to the EMG patterns of the perturbed cycle.
FIG. 8.
EMG pattern of the inward step. A: a representative example of EMG responses to a rightward push in 4 selected hindlimb muscles during walking (cat 3) (designations as in Fig. 6A). B and C: average EMG responses to a push in 8 hindlimb muscles. EMGs shown are for the limb performing the inward step (A) and for the opposite limb (B). Blue traces indicate EMGs recorded in the cycle preceding the inward step and red traces are those in the cycle with the inward step—averaging over 12 trials in cat 3. Stance and swing phases are shown by the filled (black) and empty bar, respectively. The red bar indicates the push position in the cycle.
To evaluate these changes, we performed cycle-linked averaging of individual EMGs (cat 3). These are illustrated in Fig. 8, B and C, for the rightward push applied at the beginning of the swing phase of the right limb. The start of the swing phase of this limb was taken as the cycle onset. EMGs of the effective (right) limb (Fig. 8B) and of the opposite limb (Fig. 8C) are shown. One can compare the EMGs in the cycle preceding the outward step (blue traces) with those in the affected cycle (red traces). The ankle extensor (Gast) practically did not change its pattern in the affected cycle, in either the effective or opposite limb. Some increase of activity was observed in the knee extensor (Vast) and hip abductor (Glut) in both limbs. A large increase was observed in hip adductors (Grac, Add fem) in the effective limb, whereas in the opposite limb the increase was smaller. Similar EMG responses were observed in other cats.
Both the outward step and the inward step only slightly affected the temporal pattern of stepping. Figure 9 shows the average duration of the step cycle and its components in normal and perturbed steps, for the outward steps (Fig. 9A) and inward steps (Fig. 9B). The values in affected cycles were slightly reduced compared with control (by 10–15%), although the differences were statistically significant (paired t-test, P < 0.05).
FIG. 9.
Duration of the cycle and its components in normal and perturbed steps. A: outward steps (n = 4, n = 36). B: inward steps (n = 4, n = 47). For the leg performing the lateral step, the mean value of cycle duration and the mean value of duration of swing and stance phases are shown. This was done for control (cycle before push) and for the affected cycle (which started with the lateral step). The values in affected cycles only slightly differed from control, but the differences were statistically significant (paired t-test, P < 0.05).
Postural corrections during walking of forelimbs
In these experiments, we used the experimental design shown in Fig. 1D; pushes were applied in the shoulder region. In all four cats, the main result was similar to that obtained for the hindlimbs: the cats exhibited two distinct patterns of response to pushes, the outward and inward steps. The outward step was observed in the forelimb contralateral to force application, if the push was performed close to the end of the stance phase of this limb or in its swing phase. The inward step was observed in the forelimb ipsilateral to force application, if the push was performed close to the end of the stance phase of this limb or in its swing phase.
Characteristics of individual corrective steps are shown in Fig. 10. In both outward steps (n = 4, n = 28; Fig. 10A) and inward steps (n = 4, n = 22; Fig. 10C), the value of the ML component (Lateral step) correlated well with the value of postural perturbation (Body shift).
FIG. 10.
Characteristics of outward and inward steps of the forelimbs. A and C: correlation between the postural perturbation (Body shift) and the response to this perturbation (Lateral step) for outward steps (A) and inward steps (C). B and D: mean values characterizing the lateral component of step in sequential cycles of the forelimbs, for outward steps (B) and inward steps (D). Designation of cycles as in Fig. 5. The mean value of push-caused trunk displacement is also given (Body). In A–D, n = 3. In A, n = 28; in B, n = 29; in C, n = 22; in D, n = 25.
The data for individual steps were averaged over all trials, for outward steps (Fig. 10B) and for inward steps (Fig. 10D). The mean values characterizing the lateral component of step in sequential cycles for both forelimbs are shown. In the cycle before push (cycle −1), no lateral component was present. In the cycle with push (cycle 0), the effective limb (contralateral one in Fig. 10B and ipsilateral in Fig. 10D) performed a large lateral step. In the cycle following the affected cycle (cycle 1), the effective limb performed a large lateral step in the opposite direction and returned to the normal ML position. In the next cycle (cycle 2) the lateral component was practically absent. The mean value of lateral step (cycle 0) was larger than the mean value of body displacement (Body): 5.5 ± 0.4 versus 4.0 ± 0.1 cm in Fig. 10B, and 6.0 ± 0.4 versus 4.1 ± 0.1 cm in Fig. 10D (P < 0.05). Table 2 shows that the ML component of the forelimb corrective steps was rather similar in individual cats and thus these data can be pooled together. Table 2 also shows that the ML component was significantly larger in corrective steps compared with ordinary steps.
DISCUSSION
We investigated the effects of postural perturbations—medial–lateral (ML) body displacements—in standing and walking cats. These perturbations are very frequent in natural habitat; they can happen during the animal's self-motion (e.g., due to colliding with obstacles) or when interacting with other moving animals. This type of perturbations differs from the perturbations caused by unstable support surface (e.g., by rapid ML translation of the supporting platform), which primarily affect feet positions rather than trunk position (Macpherson 1988a,b; Misiaszek 2006).
Despite the obvious importance of effective stabilization of ML body position in quadrupeds, this issue has not been investigated in any detail (however, see Kato et al. 1985). One of the reasons for this is related to technical difficulties in inducing ML perturbations of a certain magnitude and timing. In the present study, the cat was walking on the treadmill and, due to continuous licking of food from the feeder, was keeping a stationary position in space, which allowed us to easily perform external perturbations of posture. We managed to cover a wide range of displacements of the trunk (2–8 cm), with a relatively standard duration of push (150–250 ms).
In the standing animal, a postural perturbation (lateral displacement of the caudal part of the trunk) elicited a robust postural response and a rapid return to the normal position (Figs. 2 and 3). The response included the initial wave of excitation of the extensor muscles (Gast, Vast) of the hindlimb contralateral to force application and inhibition of the corresponding muscles in the ipsilateral limb (Fig. 3). Activation of extensors of the contralateral limb allows the limb to take on itself an additional load caused by a displacement of the center of mass (COM) toward this limb and provides a support for the trunk until it returns to the initial position with equal loading of the two limbs. Both the hip abductor (Glut) and adductor (Grac) in the contralateral limb were activated by push, suggesting an increase of stiffness of the hip joint during postural corrections. Similar responses to push were observed in the rabbit, both intact and decerebrated (Musienko et al. 2008). A similar pattern of extensor activity was also observed in response to ML translation of the supporting platform (Macpherson 1988b).
This type of postural corrections occurs without any modification of the base of support, in contrast to corrections during walking, which are based on a specific reconfiguration of the base of support due to a lateral step (see following text). It should be noted, however, that standing animals can also use a lateral step when there is a risk of losing balance (Beloozerova et al. 2003). Also, in the present study, there were a few cases when, in response to a stronger push, the animal performed a lateral step and thus changed the configuration of the base of support. This strategy was similar to that used during walking.
During undisturbed stationary walking, landing of the foot at the end of the swing (transfer) phase in sequential steps occurs at approximately the same ML position (see also Misiaszek 2006). A postural perturbation (lateral displacement of the trunk) caused a lateral component in the transfer movement of the next step, so that landing of the foot occurred at the ML position considerably differing from the normal one. The rightward displacements of the trunk caused the rightward lateral step and, vice versa, the leftward displacement caused the leftward step. This pattern of response was observed both when walking with hindlimbs and when walking with forelimbs (Figs. 5, 7, and 10). This pattern differs from that caused by a lateral translation of the supporting platform, in which the lateral steps were performed by both left and right limbs (Misiaszek 2006).
The lateral step is an efficient way to compensate for the type of postural perturbations used in the present study. After the lateral step, the limb is in a better position not only to counteract the push-induced movement of the trunk, but also to return the trunk to its normal (undisturbed) posture. As shown in humans, lateral placement of the feet with respect to the COM position provides a mechanical moment, which is the main factor controlling ML stability during walking (Oddsson et al. 2004; Powell 1994; Winter 1995).
We found that the choice of limb (left or right) for performing the lateral step (the corrective limb) depends on the phase of the locomotor cycle—step modification occurred in the limb that was approaching the end of the stance phase or was moving forward in the swing phase. This choice did not depend on the direction of the forthcoming lateral step; consequently, each limb could perform either outward step (Fig. 5, A–D) or inward step (Fig. 7, A–D). The magnitudes of outward and inward steps on average were similar (see Figs. 5F and 7F for the hindlimbs and Fig. 10, B and D, for the forelimbs). Thus this type of postural correction is based on specific modifications to the base of support, in contrast to postural corrections during standing (see preceding text).
Push-evoked lateral body displacement could be seen as a characteristic of postural perturbation and the lateral step would function as a corrective motor response to this perturbation. In the forelimbs, there was a significant correlation between these variables (Fig. 10, A and C), suggesting that the displacement value of lateral step depends on the magnitude of postural perturbation in a graded manner. Surprisingly, we found rather weak correlation between these variables for the hindlimbs (Figs. 5E and 7E). A possible explanation for this finding could be that, in the hindlimbs control system (in contrast to the forelimbs), the value of corrective response (lateral step) is scaled only roughly to the value of body displacement. A simplest case would be the generation of a standard lateral step in response to a suprathreshold disturbance. One should also take into account that the pelvis of a walking cat is subjected to large-scale lateral oscillations in the rhythm of stepping; the amplitude of these oscillations varies considerably (Fig. 4). This factor might be a reason for variability in the push-evoked postural perturbations and in the responses to these perturbations, which will make the correlation between these characteristics less evident. One can suggest that lateral oscillations in the anterior part of the body are considerably reduced compared with the pelvis region. As shown previously, when the cat is licking food from the feeder, the anterior part of the body is stabilized better than its posterior part (Beloozerova et al. 2005).
Regular stepping movements of limbs take place mainly in a parasagittal plane (although a small ML component can be observed; see Misiaszek 2006). They are due to a highly specific pattern of flexion and extension of limb joints in this plane. In a simplified form, this pattern can be considered as alternating activity of flexor and extensor muscles (see, e.g., Engberg and Lundberg 1969; Rasmussen et al. 1978). However, postural corrections during stepping, in the form of outward and inward steps, require a considerable lateral component and could be expected to require significant participation of corresponding muscular groups.
To address this issue, we compared the EMG pattern of hindlimb muscles in the ordinary (undisturbed) step and in the lateral step (Figs. 6 and 8). In the effective limb, the adductors (Grac and Add fem), but not the abductor (Glut), significantly increased their activity during the inward step (Fig. 8B), which accounts for the limb adduction seen in this step. However, during the outward step (with limb abduction), the increase was observed not only in the abductor (Glut), but also in the adductors (Grac and Add fem) (Fig. 6B). It is possible that the net action of all these muscles is limb abduction and that activation of the antagonistic muscles (adductors) serves to increase the stiffness of the abducted limb. A considerable activation of the adductor (Grac) during the outward step was observed not only in the effective limb but also in the opposite limb supporting the body during correction of posture (Fig. 6C). This could generate a torque counteracting the effect of push. Thus abductors and adductors are strongly involved in the generation of postural corrections in this postural task. Previously it was shown that these muscles are involved in postural corrections caused by ML translation of the walkway (Misiaszek 2006). We also observed strong responses of these muscles to lateral tilts of the supporting surface during standing and during walking (Karayannidou et al. 2008). One can thus conclude that abductor and adductor muscles (in addition to their extensor function) are involved in the maintenance of lateral stability during different motor behaviors and with different postural disturbances in the frontal plane.
Postural corrections during walking were well incorporated into the basic locomotor pattern. First, the abnormal ML position of the corrective limb was maintained during only one stance period. Within the next swing period, the limb returned to its usual ML position and the normal locomotor pattern was restored. The correction of posture required only two steps (Figs. 5F, 7F, and 10, B and D). Second, changes to the duration of the locomotor cycle associated with postural corrections were relatively small (10–15%, Fig. 9). Corrections for other types of postural perturbations during walking in quadrupeds (lateral translation or dropping of the supporting platform) are also completed in one to three step cycles (Marple-Horvat et al. 1993; Misiaszek 2006). This is in contrast to humans, who require multiple steps to regain ML stability during walking (Maki et al. 2000; Oddsson et al. 2004).
After spinal cord injury, stroke, and some other diseases, a limiting factor to functional standing and locomotion is lateral stability. Unfortunately, the nervous mechanisms of this vital motor function have not been analyzed in any detail. In particular, the contribution of spinal and supraspinal motor centers to the generation of postural responses during standing and walking is poorly understood. During standing, postural reactions in the hindlimbs to a translation of the supporting platform dramatically decreased after a complete transection of the spinal cord (Macpherson and Fung 1999; Macpherson et al. 1997). Also, postural reactions to lateral tilt in the standing animal disappeared after a ventral hemisection of the spinal cord (Lyalka et al. 2005). These results suggest that spinal postural reflexes are either insufficient to maintain the standing posture or require a considerable excitatory supraspinal drive to be activated (Deliagina et al. 2006a; Horak and Macpherson 1996). The latter hypothesis appears more likely because partial restoration of postural limb reflexes in the acute spinal animal is possible by using combined electrical and pharmacological stimulation of the spinal cord below the lesion (Musienko et al. 2007).
Results of the present study suggest that the lateral stability during locomotion in the cat is largely based on the mechanisms of lateral step. Unfortunately, data on sensory elicitation of this reaction and on its motor pattern are rather scarce. In intact animals, lateral step of the forelimb can be triggered by an increased vestibular input caused by a large, noncompensated lateral tilt (Beloozerova et al. 2003). Continuous stepping of the forelimbs with a large lateral component (circling behavior) is observed in rats, guinea pig, rabbits, and cats after a unilateral labyrinthectomy (Deliagina et al. 1997; Magnus 1924). A lateral step can also be evoked by a lateral displacement of the limb (placing and hopping reactions; Rademaker 1931.). It was suggested that these reactions are triggered by proprioceptive input from the afferents of abductor and adductor muscles (Rademaker 1931; Rademaker and Hoogerwerf 1930). However, these reactions do not seem to contribute to postural responses in our experiments. During standing, lateral steps were observed very rarely, only in cases of a higher risk of losing balance. During walking, lateral steps were observed in the unloaded limb (during its swing phase), whereas the loaded limb (which was subjected to adduction/abduction by pushes) did not perform the lateral step.
The contribution of different supraspinal mechanisms to the generation of the lateral step is also not clear. It was found that rabbits decerebrated at the premammillary level (Musienko et al. 2008) and chronic decerebrated cats (Bard and Macht 1958) retain the ability of walking and keeping balance. These findings strongly suggest that the animals deprived of the forebrain can perform lateral steps to compensate for lateral perturbations, but the presence of such steps has not been documented. On the other hand, it was reported that damage to the sensorimotor cortical areas deprived the standing animal of the ability to make compensatory lateral steps during placing and hopping reactions (Bard 1933; Brooks 1933). From the latter studies it remained unclear whether cortical lesions could also affect the corrective lateral steps during walking. In future studies, it will be interesting to examine the ability of the animal to perform lateral steps after damage to different supraspinal motor centers and to different descending spinal tracts.
To conclude, in the present study we compared postural reactions in the cat to the same destabilizing stimulus (lateral push) during standing and during walking. We found that basic mechanisms for balance control in these two forms of behavior are largely different. They perform a redistribution of muscle activity between symmetrical limbs while standing and a reconfiguration of the base of support due to a lateral step in walking. Adductor and abductor limb muscles are strongly involved in the generation of postural corrections in these motor tasks.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants R01 NS-049884, R01 NS-39340, and R01 NS-058659; Swedish Research Council Grant 11554; Gösta Fraenckels Foundation grant; Erik and Edith Fernströms Foundation grant to T. G. Deliagina; and Barrow Neurological Foundation grant to I. N. Beloozerova.
Acknowledgments
We thank P. Wettenstein for exceptional engineering assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Barbeau and Rossignol 1987.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412: 84–95, 1987. [DOI] [PubMed] [Google Scholar]
- Bard 1933.Bard P Studies on the cerebral cortex. I. Localized control of placing and hopping reactions in the cat and their normal management by small cortical remnants. Arch Neurol Psychiatry 30: 40–74, 1933. [Google Scholar]
- Bard and Macht 1958.Bard P, Macht MB. The behavior of chronically decerebrate cats. In: Neurological Basis of Behavior, edited by Wolstenholme GEW, O'Connor CM. London: Churchill, 1958, p. 55–71.
- Beloozerova et al. 2005.Beloozerova IN, Sirota MG, Orlovsky GN, Deliagina TG. Activity of pyramidal tract neurons in the cat during postural corrections. J Neurophysiol 93: 1831–1844, 2005. [DOI] [PubMed] [Google Scholar]
- Beloozerova et al. 2003.Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol 90: 3783–3793, 2003. [DOI] [PubMed] [Google Scholar]
- Brooks 1933.Brooks CM Studies on the cerebral cortex. II. Localized control of hopping and placing reactions in the rat. Am J Physiol 105: 162–171, 1933. [Google Scholar]
- Deliagina et al. 2006a.Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology 21: 216–225, 2006a. [DOI] [PubMed] [Google Scholar]
- Deliagina et al. 1997.Deliagina TG, Popova LB, Grant G. The role of tonic vestibular input for postural control in rats. Arch Ital Biol 135: 239–261, 1997. [PubMed] [Google Scholar]
- Deliagina et al. 2006b.Deliagina TG, Sirota MG, Zelenin PV, Orlovsky GN, Beloozerova IN. Interlimb postural coordination in the standing cat. J Physiol 573: 211–224, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engberg and Lundberg 1969.Engberg I, Lundberg A. An electromyographic analysis of muscular activity in the hindlimb of the cat during unrestrained locomotion. Acta Physiol Scand 75: 614–630, 1969. [DOI] [PubMed] [Google Scholar]
- Horak and Macpherson 1996.Horak FB, Macpherson JM. Postural orientation and equilibrium. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Neural Control of Movement. Bethesda, MD: Am. Physiol. Soc., 1996, sect. 12, p. 255–292.
- Karayannidou et al. 2008.Karayannidou A, Beloozerova IN, Zelenin PV, Sirota MG, Stout EE, Orlovsky GN, Deliagina TG. Participation of pyramidal tract neurons in control of standing and walking on inclined surface. Soc Neurosci Abstr 34: 860.3, 2008.
- Karayannidou et al. 2007.Karayannidou A, Deliagina TG, Orlovsky GN, Sirota MG, Beloozerova IN. Compensation for lateral perturbations of body orientation in the walking cat. Soc Neurosci Abstr 33: 411.8, 2007.
- Kato et al. 1985.Kato M, Murakami S, Hirayama H, Hikino K. Recovery of postural control following chronic bilateral hemisections at different spinal cord levels in adult cats. Exp Neurol 90: 350–364, 1985. [DOI] [PubMed] [Google Scholar]
- Lyalka et al. 2005.Lyalka FV, Zelenin PV, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG. Impairment and recovery of postural control in rabbits with spinal cord lesions. J Neurophysiol 94: 3677–3690, 2005. [DOI] [PubMed] [Google Scholar]
- Macpherson 1988a.Macpherson JM Strategies that simplify the control of quadrupedal stance. I. Forces at the ground. J Neurophysiol 60: 204–217, 1988a. [DOI] [PubMed] [Google Scholar]
- Macpherson 1988b.Macpherson JM Strategies that simplify the control of quadrupedal stance. II. Electromyographic activity. J Neurophysiol 60: 218–231, 1988b. [DOI] [PubMed] [Google Scholar]
- Macpherson and Fung 1999.Macpherson JM, Fung J. Weight support and balance during perturbed stance in the chronic spinal cat. J Neurophysiol 82: 3066–3081, 1999. [DOI] [PubMed] [Google Scholar]
- Macpherson et al. 1997.Macpherson JM, Fung J, Jacobs R. Postural orientation, equilibrium, and the spinal cord. In: Advances in Neurology: Neuronal Regeneration, Reorganization, and Repair, edited by Seil FJ. Philadelphia PA: Lippincott-Raven, 1997, vol. 72, p. 227–232. [PubMed]
- Magnus 1924.Magnus R Korperstellung. Berlin: Springer-Verlag, 1924.
- Maki et al. 2000.Maki BE, Edmontstone MA, McIlroy WE. Age-related differences in laterally-directed compensatory stepping behavior. J Gerontol A Biol Sci Med Sci 55: M270–M277, 2000. [DOI] [PubMed] [Google Scholar]
- Marple-Horvat et al. 1993.Marple-Horvat DE, Amos AJ, Armstrong DM, Criado JM. Changes in the discharge patterns of cat motor cortex neurones during unexpected perturbations of on-going locomotion. J Physiol 462: 87–113, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama and Drew 2000.Matsuyama K, Drew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. II. Walking on an inclined surface. J Neurophysiol 84: 2257–2276, 2000. [DOI] [PubMed] [Google Scholar]
- Misiaszek 2006.Misiaszek JE Control of frontal plane motion of the hindlimbs in the unrestrained walking cat. J Neurophysiol 96: 1816–1828, 2006. [DOI] [PubMed] [Google Scholar]
- Moor et al. 1988.Moor SP, Rushmer DS, Windus SL, Nashner LM. Human automatic postural responses: responses to horizontal perturbations of stance in multiple directions. Exp Brain Res 73: 648–658, 1988. [DOI] [PubMed] [Google Scholar]
- Musienko et al. 2008.Musienko PE, Zelenin PV, Lylalka VF, Orlovsky GN, Deliagina TG. Postural performance in decerebrated rabbit. Behav Brain Res 190: 124–134, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko et al. 2007.Musienko PE, Zelenin PV, Orlovsky GN, Deliagina TG. Enhancement of limb reflexes in the spinal rabbit by stimulation of the spinal cord. Soc Neurosci Abstr 33: 75.17, 2007.
- Oddsson et al. 2004.Oddsson LI, Wall C, McPartland MD, Krebs DE, Tucker CA. Recovery from perturbations during paced walking. Gait Posture 19: 24–34, 2004. [DOI] [PubMed] [Google Scholar]
- Orlovsky et al. 1999.Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. From Mollusc to Man. Oxford, UK: Oxford Univ. Press, 1999.
- Powell 1994.Powell C Responses to Step Width Perturbation in Medial/Lateral Balance during Walking. Waterloo, Canada: Univ. of Waterloo, 1994.
- Rademaker 1931.Rademaker GGJ Das Stehen. Berlin: Julius Springer, 1931.
- Rademaker and Hoogerwerf 1930.Rademaker GGJ, Hoogerwerf S. Réactions provoquées par l'allongement passif du muscle semi-tendineux. Arch Néerl de Physiol 15: 338, 1930. [Google Scholar]
- Rasmussen et al. 1978.Rasmussen S, Chan AK, Goslow GE Jr. The cat step cycle: electromyographic patterns for hindlimb muscles during posture and unrestrained locomotion. J Morphol 155: 253–269, 1978. [DOI] [PubMed] [Google Scholar]
- Rossignol 1996.Rossignol S Neural control of stereotypic limb movements. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems. Neural Control of Movement. Betheda, MD: Am. Physiol. Soc., 1996, sect. 12, p. 173–216.
- Winter 1995.Winter DA Anatomy, Biomechanics and Control (ABC) of Balance Standing and Walking. Waterloo, Canada: Waterloo Biomechanics, 1995.