Abstract
Electrophysiological studies in anesthetized animals have revealed that pathways carrying force information from Golgi tendon organs in antigravity muscles mediate widespread inhibition among other antigravity muscles in the feline hindlimb. More recent evidence in paralyzed or nonparalyzed decerebrate cats has shown that some inhibitory pathways are suppressed and separate excitatory pathways from Golgi tendon organ afferents are opened on the transition from steady force production to locomotor activity. To obtain additional insight into the functions of these pathways during locomotion, we investigated the distribution of force-dependent inhibition and excitation during spontaneous locomotion and during constant force exertion in the premammillary decerebrate cat. We used four servo-controlled stretching devices to apply controlled stretches in various combinations to the gastrocnemius muscles (G), plantaris muscle (PLAN), flexor hallucis longus muscle (FHL), and quadriceps muscles (QUADS) during treadmill stepping and the crossed-extension reflex (XER). We recorded the force responses from the same muscles and were therefore able to evaluate autogenic (intramuscular) and heterogenic (intermuscular) reflexes among this set of muscles. In previous studies using the intercollicular decerebrate cat, heterogenic inhibition among QUADS, G, FHL, and PLAN was bidirectional. During treadmill stepping, heterogenic feedback from QUADS onto G and G onto PLAN and FHL remained inhibitory and was force-dependent. However, heterogenic inhibition from PLAN and FHL onto G, and from G onto QUADS, was weaker than during the XER. We propose that pathways mediating heterogenic inhibition may remain inhibitory under some forms of locomotion on a level surface but that the strengths of these pathways change to result in a proximal to distal gradient of inhibition. The potential contributions of heterogenic inhibition to interjoint coordination and limb stability are discussed.
INTRODUCTION
Sensory feedback from muscles to spinal segments plays an integral role in regulating locomotion (Donelan and Pearson 2004; Duysens et al. 2000; Pearson 1995; Prochazka 1996; Sinkjaer et al. 2000; Stein et al. 2000). Afferents carrying length information from muscle spindles project mainly to parent motoneurons and close synergists and are thought to contribute to the regulation of muscular stiffness (Nichols and Houk 1976) and enhanced force output during stance (Mazzaro et al. 2005, 2006; Stein et al. 2000). Additionally, length feedback is subject to modulation during the step cycle (Ellaway et al. 2002; Sinkjaer et al. 1996; Taylor et al. 2000) at the stance-swing transition.
The functions of force feedback from Golgi tendon organs are less well understood. One proposed function of force feedback is to contribute to the regulation of muscle stiffness by projecting inhibition to the parent muscle (Houk 1979). Available evidence rejects this hypothesis because autogenic force feedback is weak (Duysens et al. 2000; Nichols 1999; Rymer and Hasan 1980). A second proposed function, and a major focus for the investigations reported here, is to promote interjoint coordination via inhibitory heterogenic connections (Harrison et al. 1983; Jankowska 1992; Nichols 1994). It is known that pathways from group Ib afferents project to the motoneurons of muscles that cross different joints and axes of rotation (Bonasera and Nichols 1994, 1996; Eccles et al. 1957; Wilmink and Nichols 2003). Furthermore, it has been found in anesthetized and spinalized preparations that interneurons mediating group Ib inhibition also receive inputs from group Ia afferents from muscle spindle receptors (Jankowska 1992). The significance of this sensory convergence is not understood, but it is clear at least that force feedback is widely distributed. More recent mechanographic studies of intermuscular reflexes have indicated that force responses of several muscles can be parsed into force-independent (Nichols and Koffler-Smulevitz 1991) and force-dependent components (Bonasera and Nichols 1994; Wilmink and Nichols 2003) and that the latter are likely to be due to feedback from Golgi tendon organs (Nichols 1999). A major question posed in the present work is whether this system of heterogenic, inhibitory pathways operates over a range of motor tasks or whether the expression of this network is limited to conditions of steady force exertion.
A third hypothesis concerning the functional role of pathways receiving input from Golgi tendon organs is that these pathways may serve as a “loading reflex” to support greater forces required during locomotion (Dietz and Duysens 2000; Donelan and Pearson 2004; Duysens and Pearson 1980; Pearson and Collins 1993). Recent studies have demonstrated electrophysiologically that inhibitory influences from group I afferents are suppressed during locomotion and are replaced by excitatory influences (Guertin et al. 1995; Hultborn 2001; Pearson and Collins 1993), thus yielding the third hypothesis. Studies employing either clonidine (a selective α2 adrenergic agonist) or stimulation of the mesencephalic locomotor region (MLR) to induce stepping (McCrea et al. 1995; Pearson and Collins 1993; Pearson et al. 1992) suggest that networks receiving group I feedback are modulated in a task-dependent manner during locomotion. Specifically, the effects of feedback from Ib afferents exhibit a reflex reversal during the stance phase of locomotion, thus acting as a loading reflex, while also regulating the transition from stance to swing phase in both the fictive and spontaneously locomoting decerebrate cat preparations (Conway et al. 1987; Duysens and Pearson 1980).
Further studies examining positive, or excitatory, force feedback have been completed in reduced preparations. During fictive locomotion, electrical stimulation of sensory afferents originating from extensor muscles elicits excitatory postsynaptic potentials (EPSPs) in extensor motoneurons following administration of L-DOPA or stimulation of the MLR (Gossard et al. 1994; McCrea et al. 1995). Furthermore, experiments in the spinalized cat and fictive preparations have provided evidence that feedback from group Ib afferents is involved in the entrainment of locomotor rhythm (Pearson et al. 1992). It has also been shown, however, that some inhibitory pathways arising from group I afferents can remain operational during stepping, such as the pathways from the quadriceps muscles (QUADS) to the triceps surae muscles (Guertin et al. 1995; Misiaszek and Pearson 1997). Based on this evidence, it has been proposed that on initiation of locomotion, an alternate excitatory pathway receiving input from both Ia and Ib afferents emerges, while the disynaptic inhibitory pathway for many intermuscular pathways is suppressed (Pearson and Collins 1993).
The preceding evidence supports the hypothesis of an excitatory loading reflex (Pearson 1995; Pearson and Collins 1993) and also suggests that the inhibition from pathways arising from Golgi tendon organ afferents is defeated during locomotion. Most studies have found excitatory feedback between ankle extensors, namely between the medial gastrocnemius muscle (MG) and plantaris muscle (PLAN) (Pearson and Collins 1993), although inhibitory feedback from the QUADS onto ankle extensor muscles has been observed in the stepping premammillary cat (Misiaszek and Pearson 1997) and the fictively locomoting cat (Guertin et al. 1995). In view of the potentially stabilizing influences of negative (inhibitory) force feedback, we sought to reinvestigate the distribution of force-related inhibition in an actively stepping preparation using the natural stimulus of muscle stretch. We examined the distribution of hetero- and autogenic feedback separately. We indeed found evidence for excitatory force-related feedback autogenically (Ross 2006; Ross et al. 2002, 2005) yet wanted to investigate further the distribution of heterogenic feedback during locomotion. Our experiments addressed the following central question: does heterogenic force feedback among gastrocnemius (G), PLAN, flexor hallucis longus (FHL), and QUADS, which is typically inhibitory during crossed-extension reflex (XER), exhibit a reflex reversal with the initiation of locomotion in this preparation? We hypothesized that under these conditions, there would not be a global change from inhibition to excitation with locomotion. Indeed we found that feedback from G onto ankle and knee extensors, namely FHL, PLAN, and QUADS, remained inhibitory during locomotion. Preliminary accounts of these results have been published in abstract form (Ross and Nichols 2004; Ross et al. 2002, 2003, 2005).
METHODS
Preparation
The method used to evaluate the distribution and contribution of feedback from muscle receptors is the mechanographic technique, which has been previously described (Nichols 1987). All protocols are in complete accordance with the guidelines of both the National Institutes of Health and the Emory Institutional Animal Care and Use Committee. Briefly, 19 cats ranging from 3 to 6 kg were deeply anesthetized using isoflurane gas. A tracheotomy was performed, loosened sutures were placed around the carotid arteries, and a cannula was inserted into the external jugular vein to administer intravenous fluids during the experimental procedure. Withdrawal responses were monitored, and the level of anesthetic was adjusted accordingly.
The right hindlimb was immobilized, and the limb was prepared for careful dissection. The animal was placed in the stereotaxic frame and supported above a variable-speed treadmill. A temperature probe was inserted rectally and a heating pad placed under the animal to maintain a core temperature of 37°C. Bone pins were inserted into the femur and tibia and then clamped to maintain the knee at an 110° angle, and the ankle and bone pins were clamped to the treadmill frame. Reference sutures were placed on the tendons of the muscles to represent physiological lengths of the muscle or the length of the muscle when the ankle is at 90°. We chose this angle for experimental convenience, but it also corresponds to the angle at the end of stance.
The appropriate muscles were dissected, carefully removing associated connective tissue to minimize mechanical coupling, yet preserving the blood supply and nerve innervation. The muscles, namely G, PLAN, and FHL, of the right hindlimb were each dissected. The tendons of both PLAN and FHL were cut near their insertion onto the flexor digitorum brevis muscle and anastomosis of the flexor digitorum longus muscle (FDL) tendon, respectively. A small bone fragment from the calcaneus was preserved during the G dissection. Each muscle was attached via the tendon to individual clamps. These tendon clamps were placed in series with myographs using strain gauges in a half bridge configuration, and four linear motors. In five experiments, the QUADS were dissected yet still remained attached to the patellar tendon. A small hole was drilled into the patellar tendon through which a cable was threaded and attached to a myograph and linear motor via a pulley system. Mineral oil was used to prevent drying of the exposed tissues.
A premammillary decerebration (Grillner and Shik 1973) was performed, whereby the brain stem was transected rostral to the superior colliculus while preserving the mammillary bodies and subthalamic nucleus. All brain matter rostral to the transection was removed. Gelfoam and cotton were placed on the base of the cranium to minimize bleeding. Anesthesia was then titrated down and withdrawn.
The right hindlimb remained immobilized while the three remaining limbs were free to step on the treadmill. Stimulation of the skin beneath the tail was used to initiate stepping when spontaneous locomotion did not occur. Once locomotion data were obtained, the XER was elicited with continuous electrical stimulation of 100-μs pulses at a frequency of 40 Hz of the left posterior tibial nerve at two times threshold (2T). Threshold corresponds to the minimum stimulation required to elicit a force response in the dissected muscles of the contralateral limb. The tibial nerve stimulation lasted for ∼30 s, during which time the force output of the muscle rapidly increases, plateaus, and gradually declines. At the end of each experiment, the animal was killed with an overdose of pentobarbital sodium (Nembutal) followed by a pneumothorax.
Data acquisition
The motors used in these experiments were Parker 406LXR linear motors with an encoder resolution of 0.1 μm, maximum acceleration of ∼50 m/s2, and maximum load capacity of 180 Kgf. Each of the four linear motors were mounted on a custom-built aluminum frame and could be adjusted in the horizontal, vertical, and diagonal directions to achieve proper alignment with the appropriate muscle. The four individual frames were mounted on a rigid, outer frame.
The motors were controlled using a 6000 series Gemini servo drive and dSPACE board, and Simulink program. Data were acquired digitally through the dSPACE board at a sampling rate of 1,000 Hz. The change in length, velocity, and hold time was specified using the data acquisition software built in Simulink with a graphical interface in ControlDesk. The typical paradigm was a 50-ms hold, 2-mm stretch at a velocity of 0.04 m/s, 100-ms hold period, and 2-mm release, for a total duration of 250 ms. All muscles were maintained at their referenced length (see above) when the knee was fixed at 110° and the ankle was at 90°. The eccentric length change of an ankle extensor that an intact animal experiences during walking and trotting is ∼4 mm (Goslow et al. 1973). The magnitude of stretch used in these experiments was 2 mm and within this physiological range. This conservative stretch was performed to preserve the tendon and to increase the longevity of the preparation while producing repeatable and robust results.
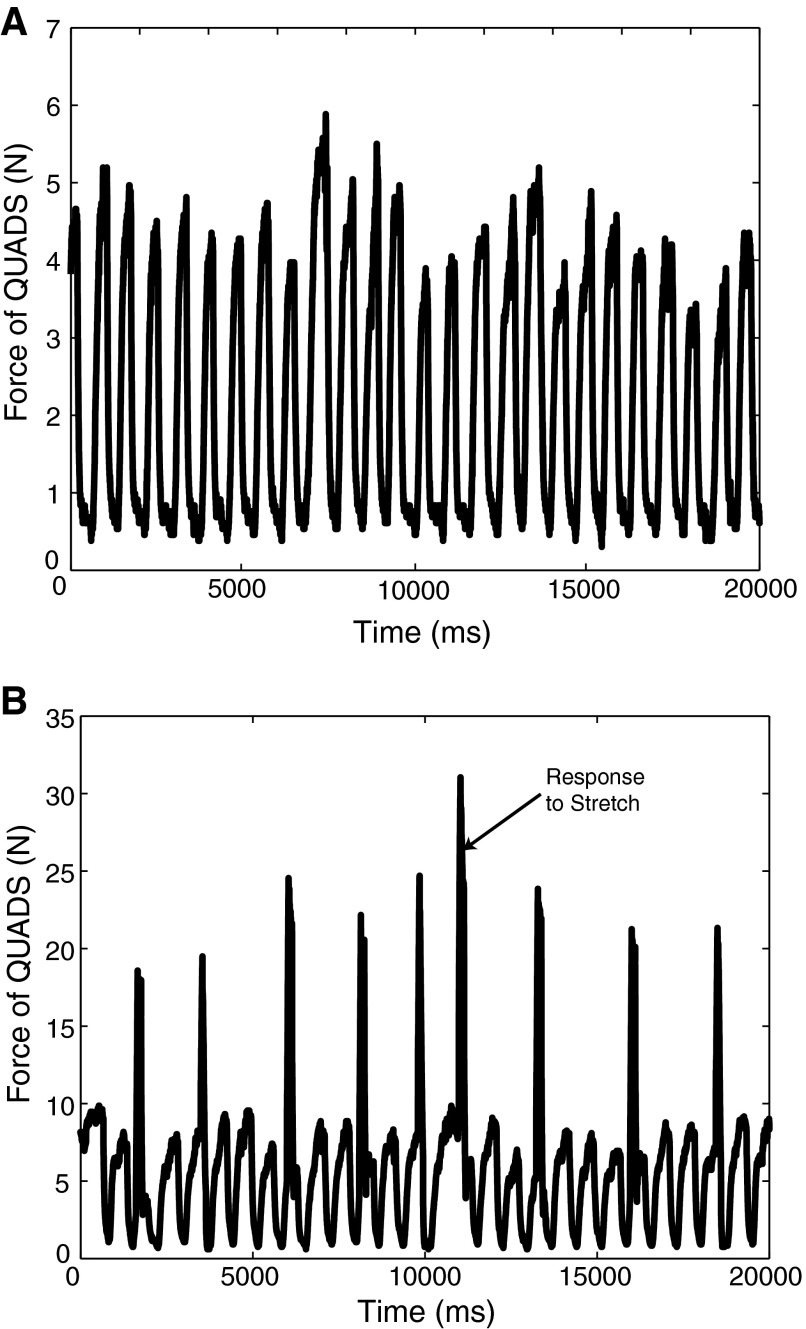
Once initiation of stepping from the three unfixed limbs commenced, the recorded force in the immobilized right hindlimb oscillated, as shown in Fig. 1A. Random stretches were manually triggered so as to capture trials in all phases of the step cycle. Figure 1B depicts the ongoing oscillations in the force with stretches administered during stepping.
FIG. 1.
A: oscillations in the background force in quadriceps (QUADS) of the immobilized right hindlimb during stepping. B: ramp-and-hold stretches delivered on top of the oscillating background force in QUADS. →, the force response to a single stretch during stepping.
The stretching paradigm in the right hindlimb was then repeated during the XER so that we could compare responses during the two behavioral states (locomotion vs. XER) to ultimately determine how feedback was reorganized with the initiation of stepping. In either locomotion or XER, the muscles were stretched in a two-state configuration. The recipient muscle was administered a ramp and hold stretch alone in state one (R) and the recipient and donor muscles were stretched simultaneously in state two (RD). Figure 2 depicts the force output and length input during XER of the recipient muscle (A and B) and donor muscle (C and D). Additionally, Fig. 2A contains symbols indicating the force responses obtained when the recipient muscle is stretched alone (•) and stretched along with the donor muscle (▵). Stretch-evoked force responses were used to discern heterogenic feedback pathways from the donor onto the recipient when the muscles were alternatively stretched. Measured forces of the recipient muscle consisted of intrinsic properties of muscles as well as contributions from reflex action (auto- and heterogenic); • represent the autogenic response, while the ▵ represent the autogenic response modified by the heterogenic input. This symbol convention remains consistent throughout this paper for evaluating the strength and sign of heterogenic feedback. Finally, calculating the voltage outputs with no load and with a 1-kg load completed a two-point calibration of the strain gauges. A linear interpolation between the two points was completed in Matlab version 7.01 to calculate fits for each of the four myographs.
FIG. 2.
A: recipient muscle stretch-evoked force response during crossed-extension reflex (XER). Symbols above recipient stretches indicate responses obtained when the muscle is stretched alone (•) and together with the donor muscle (▵). B: recipient muscle length input to 2-state stretch. C: donor muscle stretch-evoked force response during XER. D: donor muscle length input for 2-state stretch. A 2-state stretch is performed to ascertain strength and sign of heterogenic feedback between a recipient and donor muscle. The stimulation of the tibial nerve in the left hindlimb at 2 T evokes an increase in the background force of the recipient and donor muscles, flexor hallucis longus (FHL) and gastrocnemius (G) respectively (A and C). As the background force declines, ramp-and-hold stretches (2 mm, 0.04-m/s stretch, 100-ms hold period), are delivered to the recipient and donor muscles (B and D).
Data analysis
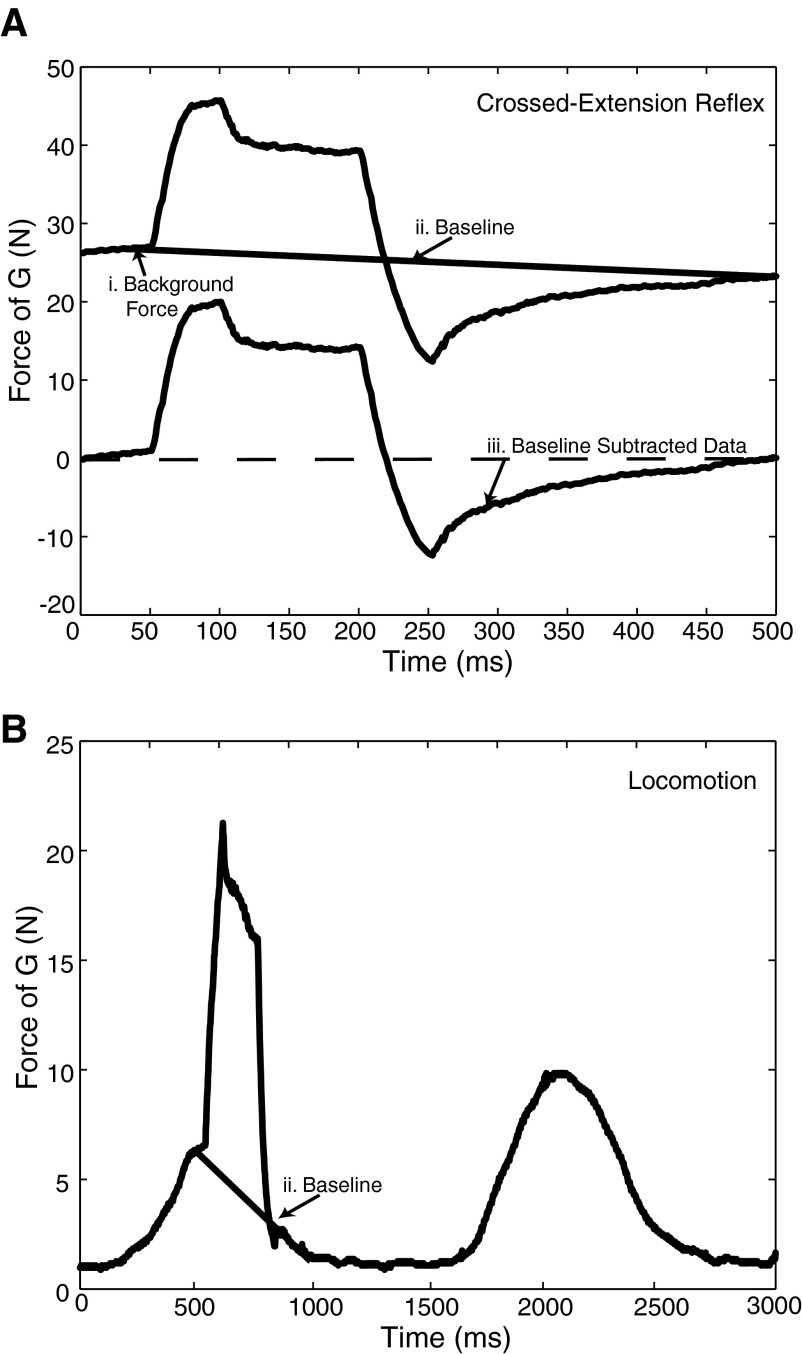
Software in Matlab version 7.01 was used to analyze the data. Briefly, the background force of the muscle was calculated as an average of the force during the interval 10 ms prior to the beginning of the stretch, during the isometric hold period. This brief period was used to account for any shift in the background force during locomotion. A baseline was then fit to the 10 ms prior to stretch and the 10 ms following the return to the initial position to account for a shifting baseline. Force responses were eliminated if the deviation in the force during either of these 10-ms periods prevented the calculation of an accurate baseline. The baseline was constructed by performing a linear interpolation from the mean force response just prior to the stretch to the mean force after the end of the release. The entire baseline was then subtracted from the overall force response. Figure 3A depicts a sample force trace, the baseline calculation described above, and the resulting baseline subtracted force data. Figure 3B depicts a single stretch administered during stepping; despite the oscillations in the background force, a baseline calculation can be performed.
FIG. 3.
A: individual force response during XER: background force (i) of an individual stretch is calculated as the average of the force over 10 ms just prior to the ramp and hold stretch; baseline (ii) is calculated for each stretch by performing a linear interpolation between the 1st 10 ms and last 10 ms of data; baseline is subtracted from each individual trace to yield the baseline-subtracted data (iii). B: baseline calculated for an individual stretch despite a shifting background force during stepping.
To evaluate the strength and sign of heterogenic feedback during either locomotion or XER, individual force responses at specific time points were obtained from the baseline subtracted force data, and background force was obtained from the original force trace. Force responses for a specific time point were plotted as a function of background force. Polynomial fits and 95% confidence intervals were fit to each population of data for a given time point to establish that populations of points under different conditions were significantly different. Data of this type usually require that at least a quadratic polynomial be used to fit the relationship between force response and background force (Nichols 1999). In this study, variability did increase for those force responses acquired during locomotion (see Fig. 4), although in cases where variability was decreased, force responses adhered to the polynomial curve.
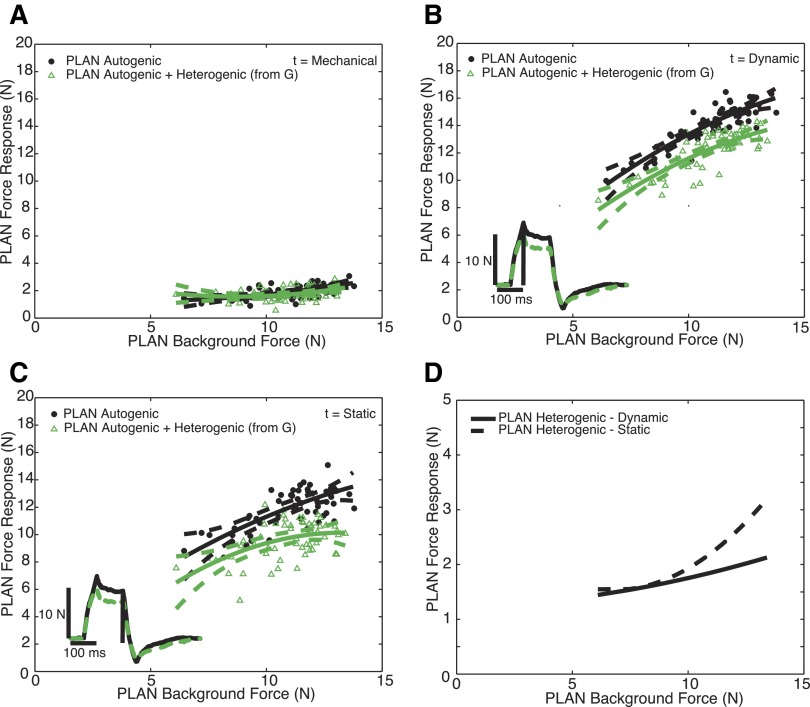
FIG. 4.
Heterogenic inhibition from G onto FHL, where G is the donor muscle and FHL is the recipient muscle in 1 animal during locomotion with force oscillations for the mechanical phase (A), dynamic phase (B), and static phase (C). • and ▵, FHL force responses from stretches occurring in ramp-and-hold stretch alone (R) and stretching the recipient and donor muscles simultaneously (RD), respectively. Polynomials and 95% confidence intervals are fit to each population of data, and statistical tests reveal that the populations for the dynamic and static phases are distinctly separated (P < 0.01). Two traces matched at 10 N background force in FHL from R (—) and RD (- - -) have been superimposed in the inset of B and C to illustrate the magnitude of inhibition from G onto FHL during locomotion, and the vertical line indicates the sample time. Heterogenic inhibition from G onto FHL is more force-dependent during the dynamic phase than during the static phase. The magnitude of heterogenic inhibition onto FHL for the dynamic and static time points (D) are represented as the difference between polynomials depicted in B and C.
Force responses were obtained at three separate time points to assess the strength and sign of heterogenic feedback throughout the ramp-and-hold stretch. Responses obtained 10 ms following the beginning of the stretch represent the mechanical phase, in that this time point occurs prior to any influence of reflex activity. Mechanical coupling occurs when insufficient muscle separation during dissection results in the transmission of force from a stretched muscle to a nonstretched muscle. Therefore substantial overlap of the data points and polynomial fits from the two populations of data for the 10-ms time point suggests a lack of mechanical coupling between the two muscles. Force responses that occur 50 ms following the beginning of the stretch contain both mechanical and reflexive responses and represent the dynamic phase of the response. A similar analysis was done for the end of the hold period, the static phase, which corresponds to 100 ms following the beginning of the stretch. To address the influence of the donor's afferent input to the recipient muscle, force responses were measured in the donor muscle when both muscles were stretched.
Statistics were performed using Statistica 6.0 and Excel to test the separation of the data populations. Multiple regression analysis was performed to first test the validity of comparing the two populations of data and then to test the overall separation of the populations. Specifically, a step-wise series of regression models and F-statistic calculations were performed to analyze the relationship between force responses and predictor variables. For each series, a full and reduced model was applied to the data, and an F-statistic was calculated. The sum of squared errors (SSE) and degrees of freedom (DF) were obtained from both the full and reduced models, as well as the mean squared errors (MSE) from the full model. An F statistic was calculated using the following equation
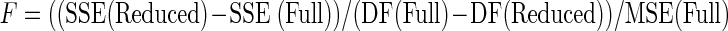 |
Once the first regression series confirmed that the slopes for the two populations were not significantly different and that the populations could validly be compared, the second regression could be applied to test for population separation. For this series, the full model, force response at the specified time point was the dependent variable (Y). The predictors in the full model included the grouping variable (X2), representing R or RD, background force (x1), background force squared (x12), the grouping and background force crossed term (x1X2), and the grouping variable multiplied by the squared background force term (x12X2). The reduced model lacked all terms containing the grouping variable, thus pooling the data into one population. The following equation (Kutner et al. 1996) represents the full model
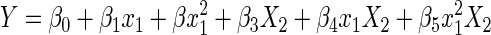 |
The reduced model eliminates all grouping variables, and thus reduces to the following equation
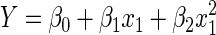 |
This F test was performed to test the following null hypothesis
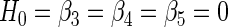 |
While the 95% confidence intervals represent the validity of the polynomial fit to the data points, a P value <0.01 from the statistical test stated in the preceding text rejects the null hypothesis and demonstrates that the two populations are statistically different.
The magnitude of the inhibition was also explored for the entire time course of the ramp and hold profile. To achieve this, polynomial fits of the two populations of data were calculated for every 5 ms of the entire response. For each 5-ms time point, the polynomial representing the data obtained when the muscle was stretched alone was subtracted from the polynomial representing the data obtained when two muscles were stretched together. A surface plot was then created from each of the difference calculations. A common range in background force of the muscle for both populations was also computed and represented on the z axis.
RESULTS
The purpose of these studies was to determine the distribution of the feedback among ankle extensor muscles in the hindlimb of the cat. Heterogenic feedback pathways among G, PLAN, FHL, and QUADS are predominately force-dependent and inhibitory under conditions of tonic force generation (Bonasera and Nichols 1994; Nichols 1999; Wilmink and Nichols 2003). This paper details results from 19 experiments and examines the feedback pathways among G, PLAN, FHL, and QUADS. The main observation from these studies was that heterogenic inhibition was maintained between G, PLAN, FHL, and QUADS during locomotion despite the variability in the preparations.
There was a range in the characteristics of locomotion among the 19 preparations. One of 19 animals did not exhibit any stepping despite skin stimulation, 6 of 19 and 9 of 19 animals produced stepping with the aid of skin stimulation with and without oscillations in the background force of the muscles in the immobilized limb respectively, and 3 of 19 animals exhibited stepping and oscillations without stimulation. The single animal that did not demonstrate stepping behavior was eliminated from the summary of results as the strength and sign of feedback could not be compared between the two behavioral states (locomotion and XER). Of the remaining 18 preparations, 9 exhibited both stepping and force oscillations in the investigated muscles. All preparations, including those with and without force oscillations, those requiring skin stimulation to evoke stepping, and those exhibiting spontaneous locomotion, exhibited heterogenic inhibition during stepping. Although stance and swing phases could not be accurately separated in the immobilized limb, responses obtained during swing corresponded to those at the lowest background forces. Phase effects were not observed in those preparations with background force oscillations; specifically, heterogenic inhibition was present in all phases. Furthermore, no clear trend between the quality of locomotion and the magnitude of inhibition was evident nor were there observable differences in the magnitude of heterogenic inhibition with or without skin stimulation. Plots of force responses versus background force in Figs. 4 and 6 are from animals exhibiting oscillatory forces. Data obtained during stepping frequently showed more scatter than those obtained during XER. However, because data with a low scatter require at least a quadratic polynomial fit, we utilized polynomial regression throughout this study. The trend in these data are heterogenic inhibition throughout the range of background forces with no hysteresis, thus indicating no effects of cycle phase. Furthermore, preparations exhibiting oscillations during stepping resulted in the internal shortening and lengthening of the muscle fibers that was sufficient to decrease the autogenic force responses. Because of the lack of significant phase effects, the data obtained in all stepping preparations were pooled.
FIG. 6.
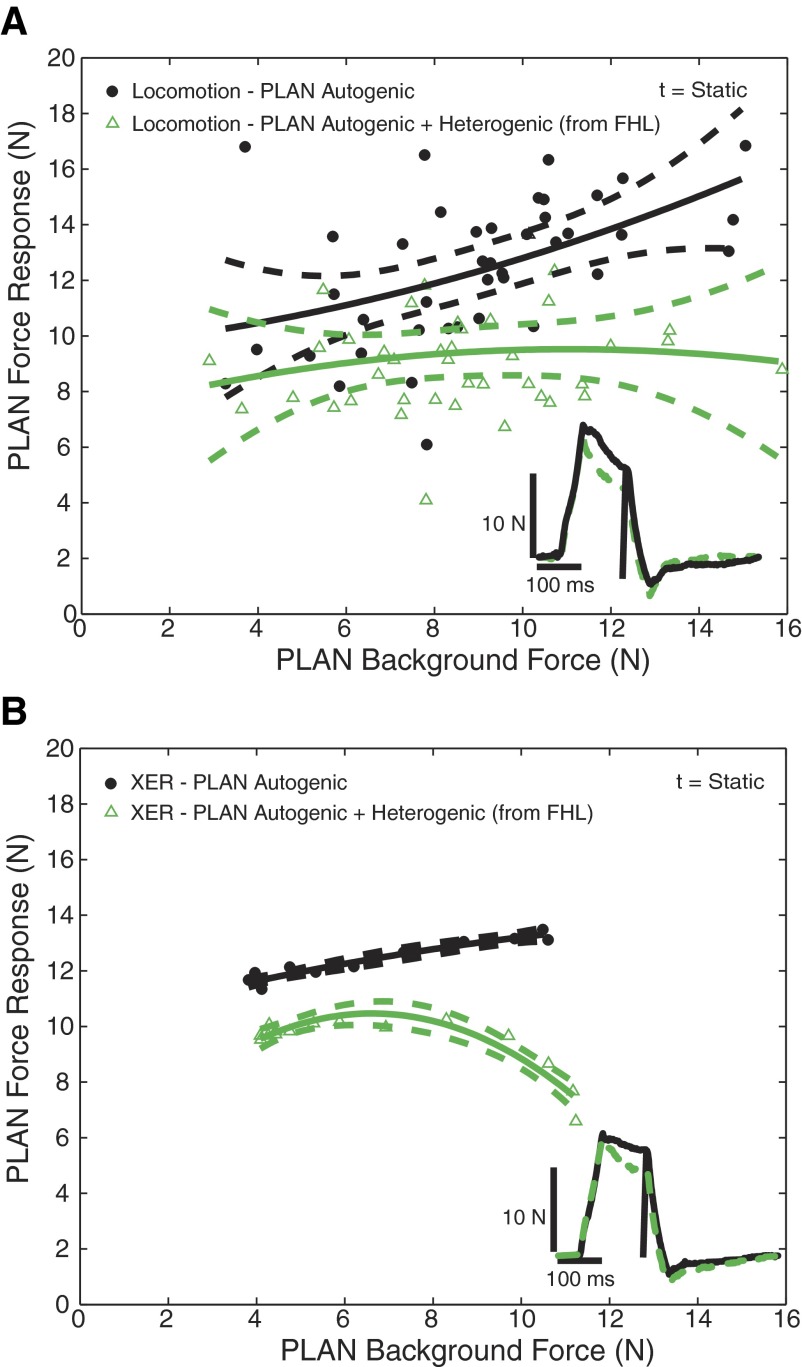
Heterogenic inhibition from G onto plantaris muscle (PLAN), where G is the donor muscle and PLAN is the recipient muscle in 1 animal during locomotion with force oscillations for the mechanical phase (A). B: heterogenic inhibition from G onto PLAN during locomotion for the dynamic phase. C: heterogenic inhibition from G onto PLAN during locomotion for the static phase. The same conventions as Fig. 5 apply. Two traces matched at 10 N background force in PLAN from R (—) and RD (- - -) have been superimposed to illustrate the magnitude of inhibition from G onto PLAN during locomotion, and the vertical line indicates the sample time. Heterogenic inhibition from G onto PLAN during locomotion remains independent of force during the dynamic phase yet increases with increasing force during the static phase. Variability also increases with increasing time. The magnitude of heterogenic inhibition onto PLAN for the dynamic and static time points (D) are represented as the difference between polynomials depicted in B and C.
Stretch of G leads to inhibition of FHL during locomotion
The heterogenic feedback from G onto FHL was examined in 17 preparations, 16 of which exhibited stepping behavior. Of these experiments in which we evaluated the interaction between G and FHL during locomotion, 12 demonstrated inhibition from G onto FHL as previously shown in the intercollicular decerebrate cat (Bonasera 1994). Of these 12 experiments, 7 exhibited oscillations in the background force during stepping and 5 exhibited no background force oscillations. The remaining four preparations did not exhibit heterogenic inhibition in either the locomotion or XER states.
Figure 4 depicts a representative example of the heterogenic inhibition from G onto FHL during stepping with background force oscillations. Heterogenic inhibition is present when force responses measured during RD are less than those force responses measured during R. The expression of heterogenic inhibition is not confounded by mechanical coupling between two muscles, as data points and polynomials from both populations (R and RD) are not significantly different (Fig. 4A). Furthermore, heterogenic inhibition from G onto FHL increases with increasing background force for the dynamic response as indicated by the divergence in the polynomial fits in Fig. 4B. While there is some variability and scatter among the data points, the relatively tight 95% confidence intervals in both conditions indicate that the polynomial fits effectively represent the data. Our criterion for significant inhibition is that the P value <0.01. In most, but not all cases, this also corresponds to nonoverlapping confidence intervals. In this example, the confidence intervals are clearly separate for the two populations of data, confirming that there is heterogenic inhibition from G onto FHL. Additionally, multiple regression yielded P < 0.01, thus these populations are statistically different. The magnitude of inhibition was estimated by subtracting the polynomials corresponding to R and RD for the dynamic (B) and static (C) responses. The resulting difference polynomials are shown in Fig. 4D and indicate that inhibition is force-dependent, particularly for the dynamic response.
Force traces inset in Fig. 4, B and C, are force responses for R (—) and RD (- - -). The background force for both conditions was matched at the mean background force of ∼10 N. Baselines were subtracted from both traces to better illustrate the magnitude and time course of the inhibition from G onto FHL. The difference between R (—) and RD (- - -) is indicative of the magnitude of heterogenic inhibition from G onto FHL at the given background force. As shown by these traces, the magnitude of inhibition remains relatively constant during the hold period. While individual traces demonstrate inhibition for one background force, the scatter plot is composed of multiple stretches and therefore illustrates inhibition across forces and phases. Data points corresponding to RD form one population, are well represented by the quadratic polynomial, and contain no indication of hysteresis or phase-dependent modulation. Although stance and swing could not be accurately separated in the immobilized limb, the points for low background forces do not form a separate population from those at high forces.
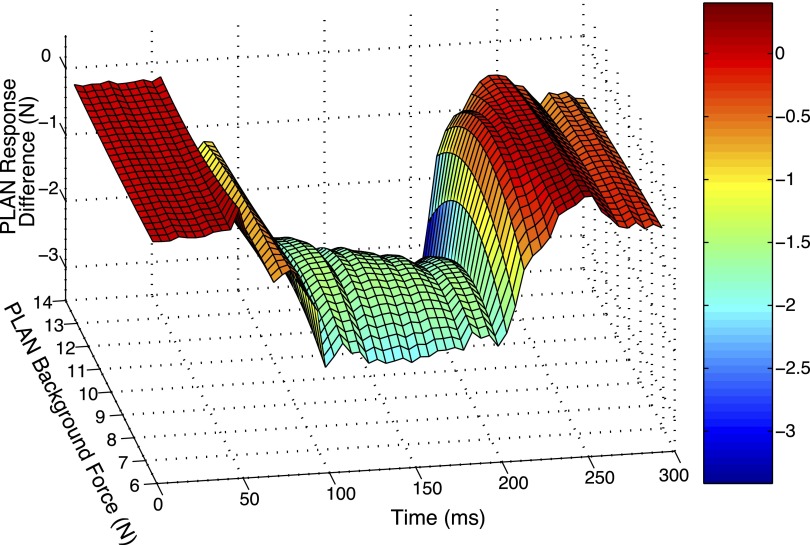
Figure 4, B and C, show heterogenic inhibition from G to FHL data during locomotion for one time point, dynamic and static, respectively. To test whether or not the inhibition remains throughout the time course of the length perturbations for all forces, a three-dimensional plot was created as previously described in methods. Figure 5 represents the time course of heterogenic feedback from G onto FHL during locomotion. The surface represents the difference between the two populations of data as a function of time and FHL background force. The maximum response difference is ∼5 N and is sustained throughout the 100-ms hold period. There is some slight force dependency at the earlier time points that is reduced at the end of the hold period.
FIG. 5.
A 3-dimensional surface that quantifies the magnitude of heterogenic inhibition from G onto FHL during locomotion as a function of force and time. To quantify the magnitude of heterogenic inhibition from G onto FHL during locomotion, response differences are calculated for every 5 ms over the ramp-and-hold stretch by subtracting the polynomial fits for RD from the polynomial fits from R. A 3-dimensional surface is created from the series of response difference calculations. The heterogenic inhibition from G onto FHL during locomotion remains relatively constant over time and FHL background force.
Of the 12 experiments exhibiting heterogenic inhibition from G onto FHL, 6 exhibited this force-dependent trend in the inhibition for the dynamic response, 3 exhibited inhibition that remained constant with increasing background force, 2 displayed greater inhibition at lower and higher background forces (parabolic), and 1 decreased inhibition with increasing background force. Force dependency decreased and variability increased for the static response (Fig. 4C). For the static response, 3 of 12 demonstrated an increasing inhibition from G onto FHL, 6 of 12 remained constant with increasing background force, and 3 of 12 exhibited greater inhibition at lower and higher background forces (parabolic). While heterogenic inhibition from G onto FHL during locomotion was robust, the opposing interaction was much weaker, meaning the inhibition was significantly less. Weak, yet statistically significant, inhibition from FHL onto G occurred in 6 of the 15 experiments devoted to exploring the interaction (not illustrated). Two of these six experiments exhibited oscillations in the background force of G during stepping.
Stretch of G leads to inhibition of PLAN during locomotion
Feedback from G onto PLAN was examined in 12 experiments, 11 of which included stepping. Of these 12 experiments, 9 exhibited heterogenic inhibition from G onto PLAN. Of these 9 experiments, 3 exhibited oscillations in the background force during stepping and 6 exhibited no background force oscillations. The remaining preparations did not exhibit heterogenic inhibition in either locomotion or XER. Figure 6 depicts a representative example of the heterogenic inhibition from G onto PLAN during stepping with background force oscillations. The data in Fig. 6A demonstrate that there is no statistical difference between the two populations of data during the mechanical phase, thus ruling out the possibility of mechanical coupling between these two muscles. Figure 6B depicts responses acquired 50 ms following the beginning of the ramp, representing the dynamic phase of the feedback from G onto PLAN during locomotion. While there is slight overlap in the confidence intervals at the lowest background force, ∼6 N, the two populations are clearly separated at higher background forces. Multiple regression yielded P < 0.01, therefore these populations are statistically different. Of the 9 experiments exhibiting heterogenic inhibition from G onto PLAN, 5 exhibited this force-dependent trend in the inhibition for the dynamic response, 2 displayed greater inhibition at lower and higher background forces (parabolic), 1 exhibited inhibition that remained constant with increasing background force, and 1 decreased inhibition with increasing background force.
Force responses in the recipient muscle, PLAN, for R (—) and RD (- - -) are inset in Fig. 6, B and C, to illustrate the magnitude and timing of heterogenic inhibition from G onto PLAN. Each trace was chosen at the mean PLAN background force of 10 N, and the baselines were subtracted to better visualize the inhibition. The difference between R (—) and RD (- - -) is indicative of the magnitude of heterogenic inhibition from G onto PLAN at the given background force. While individual traces demonstrate inhibition for one background force, the scatter plot is comprised of multiple stretches and therefore illustrates inhibition across forces and phases. The inhibition from G onto PLAN remained constant over the 100-ms hold period. Additionally, during locomotion, the magnitude of inhibition from G onto PLAN (Fig. 6B) was less than the inhibition from G onto FHL (Fig. 4B).
Figure 6B depicts force responses in the static phase of feedback and illustrates the increase in variability when compared with the dynamic phase (Fig. 6C). For the static response, 3 of 9 preparations exhibited the force-dependent trend in inhibition from G onto PLAN, 3 of 9 remained constant with increasing background force, 2 of 9 exhibited a parabolic distribution, and 1 of 9 decreased with increasing force. The magnitude of inhibition was estimated by subtracting the polynomials corresponding to R and RD for the dynamic (B) and static (C) responses. The resulting difference polynomials are shown in Fig. 6D and indicate that the heterogenic inhibition increases with force.
To assess the magnitude and time-course of the heterogenic inhibition from G onto PLAN during locomotion, a three-dimensional difference plot was created as previously described in methods, as shown in Fig. 7. The inhibition reaches a peak of 2 N and increases in force dependency at the end of the hold period. The feedback from PLAN onto G was also examined during locomotion yet yielded less robust results than the opposing interaction. Weak yet statistically significant inhibition from PLAN onto G occurred in 3 of the 13 experiments devoted to exploring the interaction; none of these 3 experiments exhibited oscillations in the background force during stepping.
FIG. 7.
A 3-dimensional surface that quantifies the magnitude of heterogenic inhibition from G onto PLAN during locomotion as a function of force and time. The magnitude of heterogenic inhibition from G onto PLAN during locomotion for the entire time course of the ramp-and-hold stretch was calculated in the same manner as Fig. 6. The heterogenic inhibition from G onto PLAN during locomotion increases slightly over time and remains slightly dependent on PLAN background force at longer latencies.
Heterogenic inhibition between QUADS and G during stepping
In view of the distribution of force feedback pathways that cross joints and influence interjoint coordination, we explored the interactions between QUADS and G during locomotion in 5 experiments, all of which exhibited stepping. In these 5 experiments, 3 exhibited oscillations in the background force and 2 did not exhibit any background force oscillations. Figure 8A illustrates the heterogenic inhibition that exists during locomotion from QUADS onto G without oscillations. Force traces from R (—) and RD (- - -) are matched at a G background force of 6 N. While there is some overlap in the confidence intervals of the two polynomial fits, these two populations are statistically distinct (P < 0.01). Force traces from R (—) and RD (- - -) are matched at a G background force of 3.5 N. Statistical tests reveal that these two populations are statistically separate (P < 0.01). The opposing interaction of the feedback from G onto QUADS was also inhibitory during locomotion. However, the magnitude of inhibition from G onto QUADS was significantly smaller, yet still statistically significant (P < 0.01), than that from QUADS onto G, and it diminished at longer latencies (not shown). Statistically significant heterogenic inhibition from G onto QUADS and QUADS onto G during locomotion was found in 3 of 5 of the experiments.
FIG. 8.
A: heterogenic inhibition from QUADS onto G where QUADS is the donor muscle and G is the recipient muscle in 1 animal during locomotion without force oscillations for the dynamic phase. B: heterogenic inhibition from QUADS onto G during XER for the dynamic phase. The same conventions as Fig. 5 apply. Two traces matched at 6 N (A) and 3.5 N (B) background force from R (—) and RD (- - -) have been superimposed to illustrate the magnitude of inhibition from QUADS onto G during locomotion and XER, respectively, and the vertical line indicates the sample time.
Stretch of FHL leads to inhibition of PLAN during locomotion
Feedback from FHL onto PLAN was examined in 12 experiments, 11 of which produced stepping. Of these experiments, 4 exhibited heterogenic inhibition from FHL onto PLAN, and half of these experiments exhibited oscillations in the background force during stepping. The remaining preparations did not exhibit heterogenic inhibition in either the locomotion or XER state. Figure 9A depicts responses acquired at the 200-ms time point, representing the static phase of the feedback from FHL onto PLAN during locomotion. While there is slight overlap in the confidence intervals at the lowest background force, a P value <0.01 indicates that these populations are distinctly different. While this preparation presented force-dependent inhibition, this was not a consistent result across locomoting preparations. Figure 9B depicts the inhibition from FHL onto PLAN during the XER in the same preparation. It is evident that the magnitude of inhibition at the static time point is similar in both behavioral conditions. Force responses in the recipient muscle, PLAN, for R (—) and RD (- - -) are inset in Fig. 9, A and B, to illustrate the magnitude and timing of heterogenic inhibition from FHL onto PLAN. Each trace was chosen at the mean PLAN background force of 7 N, and the baselines were subtracted to better visualize the inhibition. The separation of these data are statistically significant (P < 0.01). Feedback from PLAN onto FHL was examined in 13 experiments, 12 of which exhibited stepping. Of these experiments, only 1 experiment exhibited weak inhibition from PLAN onto FHL during locomotion, and this experiment did not exhibit oscillations in the background force during stepping.
FIG. 9.
A: heterogenic inhibition from FHL onto PLAN, where FHL is the donor muscle and PLAN is the recipient muscle during locomotion without force oscillations for the static phase. B: heterogenic inhibition from FHL onto PLAN during XER for the static phase. Heterogenic inhibition from FHL onto PLAN is similar in strength and sign during locomotion (A) and XER (B). The same conventions as Fig. 5 apply. Matched traces at a background force of 7 N for R (—) and RD (- - -) have been superimposed and inset to demonstrate the trend of heterogenic inhibition in both behavioral states, and the vertical line indicates the sample time.
Comparison of heterogenic inhibition during locomotion and XER
The magnitude of heterogenic inhibition from QUADS to G, G to FHL, and FHL onto PLAN were similar during stepping and XER. Previous studies have mapped the organization of heterogenic feedback in the intercollicular decerebrate cat under conditions of XER (Bonasera and Nichols 1994; Nichols 1999). Figures 8–10 compare force responses during locomotion and XER. Figure 8 compares interactions from QUADS onto G during locomotion (Fig. 8A) and XER (Fig. 8B), and Fig. 9 compares feedback from FHL onto PLAN during locomotion (Fig. 9A) and XER (Fig. 9B) as outlined previously. Figure 10 compares the heterogenic inhibition exhibited from G onto FHL during locomotion (Fig. 10A) with that during XER (Fig. 10B) in the same premammillary decerebrate animal. Although FHL achieved higher force responses during XER than during locomotion for comparable background forces in FHL, the relative separation between the polynomial fits remains similar in both conditions. Inset traces are responses for R (—) and RD (- - -) matched at an FHL background force of 5 N. Under both locomotion and XER, the separation of the populations of data are statistically significant (P < 0.01). These traces further support the similar magnitude of heterogenic inhibition from QUADS onto G and G onto FHL during locomotion and XER. It was also observed that, particularly at the lower forces, the autogenic force responses (obtained in state R) were lower during locomotion than during XER (Figs. 8–10). This reduction was observed whether or not there were oscillations in the muscle force.
FIG. 10.
A: heterogenic inhibition from G onto FHL where G is the donor muscle and FHL is the recipient muscle in 1 animal during locomotion with force oscillations for the dynamic phase. B: heterogenic inhibition from G onto FHL during XER for the dynamic phase. Heterogenic inhibition from G onto FHL is similar in strength and sign during locomotion (A) and XER (B). The same conventions as Fig. 5 apply. Matched traces at a background force of 5 N for R (—) and RD (- - -) have been superimposed and inset to demonstrate the trend of heterogenic inhibition in both behavioral states, and the vertical line indicates the sample time.
DISCUSSION
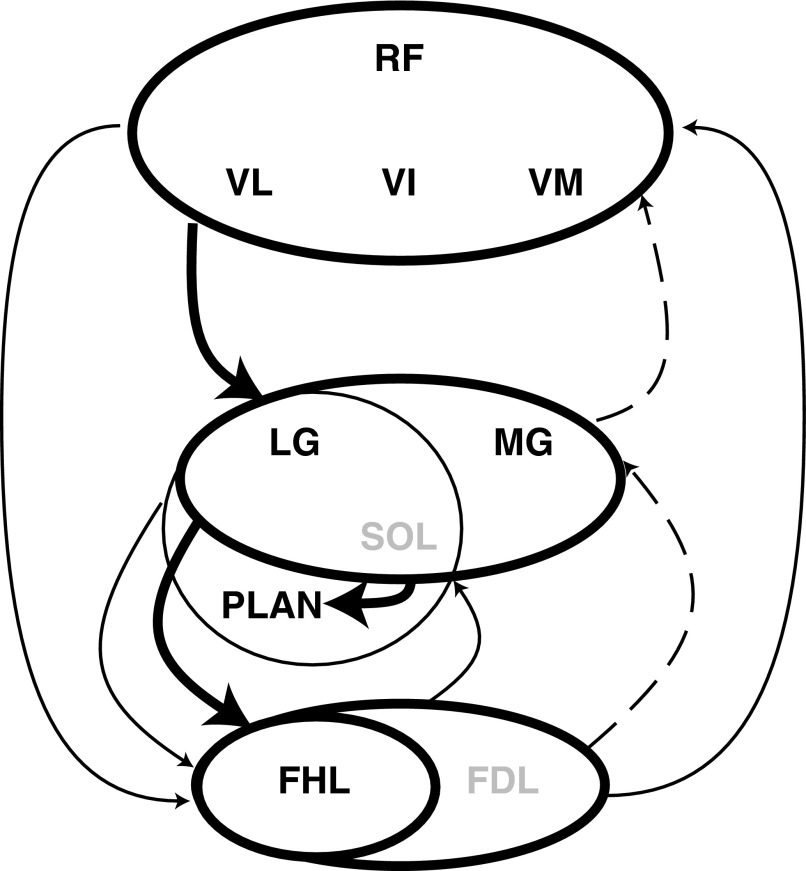
In this study, we have investigated the distribution of heterogenic, force-dependent feedback among ankle extensors, namely G, PLAN, FHL, and QUADS, during spontaneous locomotion and during XER in premammillary decerebrate cats. Heterogenic inhibition remained operational during both treadmill stepping and the XER for all of the preceding muscles. These results stand in contrast to other studies using different methods that showed that heterogenic inhibition between some muscle groups is suppressed and replaced by group Ia excitation during locomotion. Although the underlying inhibitory pathways were not suppressed in our study, the weightings of heterogenic inhibition did change from XER to stepping. Overall heterogenic inhibition was weighted in a proximal to distal direction in contrast to the more symmetrical distribution observed during the XER. Heterogenic inhibition from QUADS onto G and G onto FHL and PLAN was strong during both stepping and the XER, whereas in the reverse direction, it was weaker during stepping. Between the two more distal muscles, FHL and PLAN, heterogenic inhibition was asymmetrical during stepping, with the greater inhibition extending from FHL onto PLAN. The summary of these results, including the relative weightings of these inhibitory heterogenic pathways among ankle and knee extensors, has been depicted graphically in Fig. 11.
FIG. 11.
Summary diagram of the heterogenic inhibition among ankle and knee extensors present during locomotion in the spontaneously locomoting premammillary cat and during XER in the intercollicular decerebrate cat. Heterogenic inhibition from G onto PLAN and FHL in the premammillary cat during locomotion remained of similar strength and sign to heterogenic inhibition found in the intercollicular cat during XER, whereas heterogenic inhibition from PLAN or FHL onto G was significantly weaker. - - -, those reflexes that appeared to be modulated between XER and treadmill stepping. The net effect of the modulation was to produce a proximal to distal gradient of inhibition.
Pathways mediating heterogenic inhibition during stepping
The pattern of inhibition observed in the present studies and in previous investigations indicates the presence of an extensive neural network that can impact the mechanical properties of the limb as well as interjoint coordination. This network includes interneurons that receive inputs from group Ia and Ib afferents as well as other sensory afferents and descending signals (Jankowska 1992). These interneurons are thought to mediate the force-dependent reflexes observed in previous studies (Bonasera and Nichols 1994, 1996; Nichols 1999; Wilmink and Nichols 2003) and in the work presented here. The demonstration that these interneurons also receive inputs from group Ia afferents (Jankowska 1992: see introduction) might suggest that the signals reaching motoneurons contain length as well as force information. However, it has also been noted that the relative strengths of these inputs are different, suggesting that the input from spindle afferents plays a supportive (Fetz et al. 1979) or modulatory (Jankowska and McCrea 1983) role for the transmission of force information from Golgi tendon organs. How transmission in this network is organized in the intact or decerebrate animal during stepping is not known. In this and previous studies (Wilmink and Nichols 2003), a few heterogenic reflexes were only weakly force-dependent, suggesting that the underlying pathways in these cases included interneurons driven by both Ia and Ib afferents, and possibly group II afferents (Jankowska 1992).
The reduction in strength of the inhibition from FHL to G and from G to QUADS during locomotion could arise from at least two mechanisms. First, the strength of the inhibitory pathway could decrease in the locomotion state as suggested by many electrophysiological studies (for example, Angel et al. 2005; Conway et al 1987; Gossard et al. 1994; McCrea et al. 1995). Alternatively, the disynaptic excitation from group Ia afferents that becomes operational during stepping in extensors (Angel et al. 1996, 2005; McCrea et al. 1995; Schomburg and Behrends 1978) and flexors (Degtyarenko et al. 1998; Quevedo et al. 2000) could reduce the magnitude of inhibition if it was distributed among muscles in the appropriate way.
Changes in the distribution of heterogenic inhibition from XER to treadmill locomotion
An issue central to this study was the comparison of data obtained during two behavioral states: locomotion and XER. Of the 19 experiments exhibiting stepping, with or without oscillations, the heterogenic reflexes remained inhibitory during both behavioral states. In those experiments where no heterogenic inhibition was observed, it was likely that the activation levels were insufficient to yield inhibition or excitation in either behavioral state. That is, animals exhibiting heterogenic inhibition during locomotion also exhibited inhibition during XER. While the magnitude of heterogenic inhibition emanating from G was similar during both locomotion and XER in these premammillary animals, the autogenic force responses were actually higher during XER than during locomotion for a given background force.
Further conclusions can be drawn from previous studies in the intercollicular decerebrate cat. It should be noted that it is possible that different response magnitudes may result from the level of brain transection (i.e., premammillary vs. intercollicular) (Nichols and Steeves 1986). It has been shown that bidirectional inhibition is present between QUADS and the triceps surae during XER (Wilmink and Nichols 2003), whereas results in this study suggest that inhibition is stronger from QUADS onto G than from G onto QUADS. Similarly, inhibition is greater from G onto the toe flexors, PLAN or FHL, than from PLAN or FHL onto G during locomotion; this same trend is not observed during XER in the intercollicular cat (Bonasera and Nichols 1994, 1996; Wilmink and Nichols 2003). In summary, the proximal to distal magnitudes of inhibition were similar in locomotion and XER in intercollicular animals, whereas in premammillary animals, the distal to proximal magnitudes were weaker during treadmill stepping. To highlight these differences, Fig. 11 depicts the relative weightings of heterogenic inhibition observed during locomotion and XER. The pathways indicated by - - - become reduced in strength during stepping.
Lack of heterogenic excitation during treadmill locomotion
Previous studies in reduced preparations demonstrated that excitatory feedback from group I receptors is widespread during locomotion (Conway et al. 1987; Guertin et al. 1995; McCrea et al. 1995; Pearson and Collins 1993). Group I excitation was observed for autogenic pathways as well as a number of heterogenic pathways, although a small but variable number of heterogenic pathways remained inhibitory (Guertin et al. 1995). In the present experiments, however, heterogenic inhibition remained operational and widespread during locomotion for all of the muscle combinations tested. Factors that might explain the differences in results from these studies include the experimental preparation, the manner of inducing locomotion or activation of Ib or force-related afferents. Studies have utilized electrical nerve stimulation at group I strength to evaluate force feedback for pathways lacking Ia connections (Guertin et al. 1995; Pearson and Collins 1993) or electrical stimulation of the MLR to evoke stepping (Donelan and Pearson 2004; Guertin et al. 1995; McCrea et al. 1995). Previous work has revealed important differences between synchronous electrical stimulation and more repetitive natural stimulation (i.e., muscle stretch) such that stretch-evoked Ia afferents are less sensitive to presynaptic inhibition than electrically evoked Ia afferents (Enriquez-Denton et al. 2002). Our use of natural stimulation is one factor that might explain the different patterns of force feedback from previous studies. However, some previous studies also employed natural stimulation (Duysens and Pearson 1980; Guertin et al. 1995), so it appears that natural stimulation does not explain the difference in results in our study. It is not clear from the literature whether the difference in results might be due to quantitative differences in the distribution of positive force feedback between experiments using spontaneous locomotion, MLR stimulation, or different behavioral conditions. The use of external manipulations to induce locomotion, such as MLR, could underlie the different experimental results. Finally, the one consistent difference in the experimental preparation described here from many of the other studies of afferent regulation of locomotion quoted in the preceding text is that the preparation for the other studies included extensive denervation of the limb except for any muscles used as sources for natural inputs. In the studies reported here, the innervation to most muscles in the hind limb was left intact. The preservation of innervation throughout the limb was necessary to investigate a number of heterogenic pathways, and to obtain strong rhythmic contracts of the muscles in association with the locomotor pattern. The preservation of afferent input from many muscles in the limb could have helped to maintain activation of those interneuronal populations mediating the relevant inhibitory pathways.
Task dependence of the distribution of force feedback
Although the contrasting results from studies of reduced preparations may be due in part to methodological differences in the studies, an additional factor may be that the distribution of force feedback and its relative weightings depends on motor task. This has important implications for previous studies evaluating the reflex reversal of force-dependent inhibition to excitation in fictively locomoting cats (Conway et al. 1987; Gossard et al. 1994; McCrea et al. 1995), whereby the lack of sensory feedback modulation renders the specific motor task unknown. We propose that this array of experimental paradigms, from intercollicular, to spontaneously stepping preparations, to paralyzed fictive preparations, represents a behavioral spectrum that ranges from predominately inhibitory force feedback to more widespread excitatory force feedback. In support of a task dependency in the distribution and sign of force feedback, other investigators have found evidence of variability in the distribution of excitatory and inhibitory group I feedback across preparations (Guertin et al. 1995). Also the observations of inhibitory actions from QUADS to the triceps surae muscles in paralyzed (Guertin et al. 1995) and nonparalyzed (Misiaszek and Pearson 1997) preparations suggest that the reflex reversal on initiation of locomotion is not an all-or-none phenomenon but may be subject to regulation for different forms of locomotion or other movements.
Recent experiments in intact animals as well as reduced preparations have provided strong evidence that impulses from Golgi tendon organs reinforce the contractions of the medial gastrocnemius muscle during locomotion (reviewed in Donelan and Pearson 2004). These and previous studies leave little doubt that positive force feedback contributes importantly to propulsion and stance during level walking in the cat. However, the sources of this feedback are difficult to determine in the intact animal, and so little information exists concerning the distribution and sign of heterogenic feedback in intact animals. In view of the study reported here and of previous results from reduced preparations quoted in the preceding text, it is reasonable to suggest that both excitatory and inhibitory pathways from group I afferents can coexist during natural movements.
Muscle specificity
In this study, rather than heterogenic, force-dependent feedback becoming excitatory during locomotion (i.e., positive force feedback), force-dependent feedback remains inhibitory (i.e., negative force feedback) with strong inhibition emanating from G. There are several physiological explanations for G serving as the major source of inhibition during locomotion. First, the distribution of Golgi tendon organs in G, FHL, and PLAN could influence the amount of inhibition present during locomotion. It is known that the combined medial and lateral heads of G possess ∼60% more muscle spindles than FHL (Chin et al. 1962), and the ratio of muscle spindles to Golgi tendon organs may be preserved across muscles (Eldred et al. 1974). Second, there could be a presumed dependence of inhibition on the background force and response of the donor muscle as seen in previous studies (Bonasera and Nichols 1994). It is known that G, including both the medial and lateral heads, has the greatest physiological cross-sectional area and largest pinnation angle (Sacks and Roy 1982) and is therefore poised to generate the greatest force. However, the magnitudes of inhibition did not always correlate with the donor responses or forces in our study. Third, G generates a large, nonsagittal abduction torque about the ankle joint as demonstrated in intercollicular decerebrate cats using nerve stimulation (Lawrence and Nichols 1999). This could play an important role in stability during locomotion and possibly implicates a central organization of neural inhibitory connections among these muscles to create a stabilizing effect.
The question then arises as to whether or not mechanical effects could explain the decreased autogenic force responses in hindlimb extensors during locomotion when compared with XER. Despite isometric muscle-tendon units, internal movement of muscle fibers during locomotion could explain the decreased forces. Stepping is likely to increase muscle fiber cross-bridge detachment and turnover rate, which decreases force and intrinsic muscle stiffness (Joyce et al. 1969; Kirsch et al. 1994; Rack and Westbury 1969). Therefore the response to stretch during locomotion is lower than that during XER, where the activation and internal muscle fiber length and stiffness remain constant. In some cases without detectable oscillations in muscular force, force responses were still lower during treadmill stepping than during the XER; this suggests alternatively that the reduction could have been associated with the difference in state of spinal circuitry, or a combination of the two mechanisms.
Mechanical actions of inhibitory feedback
A mechanical consequence of heterogenic inhibition is the reinforcement of interjoint coordination. Mechanically, G has action at both the ankle and knee joints. PLAN also exerts a high ankle torque and has mechanical action on the metatarsalphalangeal (MTP) joint. While FHL has a smaller ankle torque, it also produces an adduction torque and acts on the MTP and distal interphalangeal (IP) joints (Goslow et al. 1972; Lawrence and Nichols 1999). Like the biomechanical actions of these muscles, the distribution of force feedback crosses multiple joints and axes of rotation (Wilmink and Nichols 2003). Therefore heterogenic inhibition provides cross-joint and -axis coupling that, in some cases, parallels the mechanical coupling of biarticular muscles (Nichols 1994).
Furthermore, heterogenic inhibition, in combination with excitatory length and force feedback, can contribute to the regulation of whole-limb stiffness (Nichols and Houk 1976; Nichols et al. 1999). It is important to also note that while the greatest amount of heterogenic inhibition emanates from G in our study, we have also provided evidence for positive force feedback that is autogenic and mainly associated with G (Ross et al. 2002, 2005). The asymmetrical distribution of inhibitory force feedback observed in the present studies suggests that these pathways preferentially reduce the stiffness of the distal joints. This prediction, combined with the observation that the yield during stance is absorbed mainly at the knee and ankle joints (Goslow et al. 1973) is consistent with a recent suggestion (Biewener and Daley 2007) that the distal joints provide the main mechanical interface with the ground, while the proximal joints serve mainly to drive the movement. These results reported here are not inconsistent with the evidence that group I excitation contributes importantly to extensor activity during stance (Donelan and Pearson 2004). This contribution may come primarily from autogenic excitatory feedback in muscles that receive weak inhibition.
GRANTS
National Institute of Neruological Disorders and Stroke Grants NS-20855 to T. R. Nichols and NS-23640 to K. T. Ross provided financial support for this work.
Acknowledgments
The authors thank Drs. Young-Hui Chang, Jinger Gotschall, and Clotilde Huyghues-Despointes and A. Auyang, A. Burgess, C. Honeycutt, K. Murinas, and V. Stahl for assistance with data collection, Dr. J. Alex Bragg, Emory University, Dept. of Neurology, for assistance with software development for the data acquisition system, and M. Kutner and G. Cotsonis for statistics consultation.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Angel 1996.Angel MJ, Guertin P, Jimenez I, McCrea DA. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurons during fictive locomotion. J Physiol 494: 851–861, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angel 2005.Angel MJ, Jankowska E, McCrea DA. Candidate interneurons mediating group I disynaptic EPSPs in extensor motoneurons during fictive locomotion in the cat. J Physiol 563: 597–610, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biewener 2007.Biewener AA, Daley MA. Unsteady locomotion: integrating muscle function with whole body dynamics and neuromuscular control. J Exp Biol 210: 2949–2960, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonasera 1994.Bonasera SJ Toward a Neural Representation of the Feline Ankle Joint. Atlanta, GA: Emory University, 1994.
- Bonasera 1994.Bonasera SJ, Nichols TR. Mechanical actions of heterogenic reflexes linking long toe flexors and extensors of the knee and ankle in the cat. J Neurophysiol 71: 1096–1110, 1994. [DOI] [PubMed] [Google Scholar]
- Bonasera 1996.Bonasera SJ, Nichols TR. Mechanical actions of heterogenic reflexes among ankle stabilizers and their interactions with plantar flexors of the cat hindlimb. J Neurophysiol 75: 2050–2070, 1996. [DOI] [PubMed] [Google Scholar]
- Brink 1983.Brink E, Jankowska E, McCrea DA, Skoog B. Inhibitory interactions between interneurons in reflex pathways from group Ia and group Ib afferents in the cat. J Physiol 343: 361–373, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin 1962.Chin NK, Cope M, Pang M. Number and distribution of spindle capsules in seven hindlimb muscles of the cat. In: Symposium on Muscle Receptors, edited by Barker D. Hong Kong: Hong Kong University Press, 1962, p. 241–248.
- Cleland 1990.Cleland CL, Hayward L, Rymer WZ. Neural mechanisms underlying the clasp-knife reflex in the cat. II. Stretch-sensitive muscular-free nerve endings. J Neurophysiol 64: 1319–1330, 1990. [DOI] [PubMed] [Google Scholar]
- Conway 1987.Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cat. Exp Brain Res 68: 643–656, 1987. [DOI] [PubMed] [Google Scholar]
- Degtyarenko 1998.Degtyarenko AM, Simon ES, Burke RE. Locomotor modulation of disynaptic EPSPs from the mesencephalic locomotor region in cat motoneurons. J Neurophysiol 80: 3284–3296, 1998. [DOI] [PubMed] [Google Scholar]
- Dietz 2000.Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait Posture 11: 102–110, 2000. [DOI] [PubMed] [Google Scholar]
- Donelan 2004.Donelan JM, Pearson KG. Contribution of force feedback to ankle extensor activity in decerebrate walking cats. J Neurophysiol 92: 2093–2104, 2004. [DOI] [PubMed] [Google Scholar]
- Duysens 2000.Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiol Rev 80: 83–133, 2000. [DOI] [PubMed] [Google Scholar]
- Duysens 1980.Duysens J, Pearson KG. Inhibition of flexor burst generation by loading ankle extensor muscles in walking cats. Brain Res 187: 321–332, 1980. [DOI] [PubMed] [Google Scholar]
- Eccles 1957.Eccles JC, Eccles RM, Lundberg A. Synaptic actions on motoneurons caused by impulses in the Golgi tendon organ afferents. J Physiol 138: 227–252, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldred 1974.Eldred E, Maier A, Bridgman CF. Differences in intrafusal fibres content of spindles in several muscle of the cat. Exp Neurol 45: 8–18, 1974. [DOI] [PubMed] [Google Scholar]
- Ellaway 2002.Ellaway PH, Taylor A, Durbaba R, Rawlinson S. Role of the fusimotor system in locomotion. Adv Exp Med Biol 508: 335–343, 2002. [DOI] [PubMed] [Google Scholar]
- Enriquez-Denton 2002.Enriquez-Denton M, Morita H, Christensen LO, Petersen N, Sinkjaer T, Nielsen JB. Interaction between peripheral afferent activity and presynaptic inhibition of Ia afferents in the cat. J Neurophysiol 88: 1664–1674, 2002. [DOI] [PubMed] [Google Scholar]
- Fetz 1979.Fetz EE, Jankowska E, Johannisson T, Lipski J. Autogenetic inhibition of motoneurons by impulses in group Ia muscle spindle afferents. J Physiol 293: 173–195, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goslow 1973.Goslow GE, Reinking RM, Stuart DG. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol 141: 1–42, 1973. [DOI] [PubMed] [Google Scholar]
- Goslow 1972.Goslow GE, Stauffer EK, Nemeth WC, Stuart DG. Digit flexor muscles in the cat: their action and motor units. J Morphol 137: 335–352, 1972. [DOI] [PubMed] [Google Scholar]
- Gossard 1994.Gossard J-P, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Exp Brain Res 98: 213–228, 1994. [DOI] [PubMed] [Google Scholar]
- Grillner 1973.Grillner S, Shik ML. On the descending control of the lumbosacral spinal cord from the “mesencephalic locomotor region.” Acta Physiol Scand 87: 320–333, 1973. [DOI] [PubMed] [Google Scholar]
- Guertin 1995.Guertin P, Angel M, Perreault M-C, McCrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during MLR-evoked fictive locomotion in the cat. J Physiol 487: 197–209, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haftel 2004.Haftel VK, Bichler EK, Nichols TR, Pinter MJ, Cope TC. Movement reduces the dynamic response of muscle spindle afferents and motoneuron synaptic potentials in rat. J Neurophysiol 91: 2164–2171, 2004. [DOI] [PubMed] [Google Scholar]
- Harrison 1983.Harrison PJ, Jankowska E, Johannisson T. Shared reflex pathways of group I afferents of different cat hind-limb muscles. J Physiol 338: 113–128, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk 1979.Houk JC Regulation of stiffness by skeletomotor reflexes. Annu Rev Physiol 41: 99–114, 1979. [DOI] [PubMed] [Google Scholar]
- Hultborn 2001.Hultborn H State-dependent modulation of sensory feedback. J Physiol 533: 5–13, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska 1992.Jankowska E, McCrea DA. Shared reflex pathways from Ib tendon organ afferents and Ia muscle spindle afferents in the cat. J Physiol 338: 99–111, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska 1992.Jankowska E Interneuronal relay in spinal pathways from proprioceptors. Prog Neurobiol 38: 335–378, 1992. [DOI] [PubMed] [Google Scholar]
- Joyce 1969.Joyce GC, Rack PMH, Westbury DR. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. J Physiol 204: 461–474, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch 1994.Kirsch RF, Boskov D, Rymer WZ. Muscle stiffness during transient and continuous movements of cat muscle: perturbation characteristics and physiological relevance. IEEE Trans Biomed Eng 41: 758–770, 1994. [DOI] [PubMed] [Google Scholar]
- Kutner 1996.Kutner MH, Nachtsheim CJ, Neter J, Wasserman W. Applied Linear Statistical Models. Chicago: Irwin, 1996.
- Lawrence 1999.Lawrence JH, Nichols TR. A three-dimensional biomechanical analysis of the cat ankle joint complex. I. Active and passive postural mechanisms. J Appl Biomechan 15: 95–105, 1999. [Google Scholar]
- Mazzaro 2006.Mazzaro N, Grey MJ, do Nascimento OF, Sinkjaer T. Afferent-mediated modulation of the soleus muscle activity during the stance phase of human walking. Exp Brain Res 2006. [DOI] [PubMed]
- Mazzaro et al. 2005.Mazzaro N, Grey MJ, Sinkjaer T. Contribution of afferent feedback to the soleus muscle activity during human locomotion. J Neurophysiol 93: 167–177, 2005. [DOI] [PubMed] [Google Scholar]
- McCrea 1995.McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. J Physiol 487: 527–539, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiaszek 1997.Misiaszek JE, Pearson KG. Stretch of quadriceps inhibits soleus H reflex during locomotion in decerebrate cats. J Neurophysiol 78: 2975–2984, 1997. [DOI] [PubMed] [Google Scholar]
- Nichols 1987.Nichols TR A technique for measuring the mechanical actions of heterogenic (intermuscular) reflexes in the decerebrate cat. J Neurosci Methods 21: 265–273, 1987. [DOI] [PubMed] [Google Scholar]
- Nichols 1989.Nichols TR The organization of heterogenic reflexes among muscles crossing the ankle joint in the decerebrate cat. J Physiol 410: 463–477, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols 1994.Nichols TR A biomechanical perspective on spinal mechanisms of coordinated muscular action: an architecture principle. Acta Anat 151: 1–13, 1994. [DOI] [PubMed] [Google Scholar]
- Nichols 1999.Nichols TR Receptor mechanisms underlying heterogenic reflexes among the triceps surae muscles of the cat. J Neurophysiol 81: 467–478, 1999. [DOI] [PubMed] [Google Scholar]
- Nichols 2001.Nichols TR, Cope TC. The organization of distributed proprioceptive feedback in the chronic spinal cat. In: Motor Neurobiology of the Spinal Cord, edited by Cope TC. Boca Raton, FL: CRC, 2001, p. 305–326.
- Nichols 1999.Nichols TR, Cope TC, Abelew TA. Rapid spinal mechanisms of motor coordination. Exercise Sport Sci Rev 27: 255–284, 1999. [PubMed] [Google Scholar]
- Nichols 1976.Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of the stretch reflex. J Neurophysiol 39: 119–142, 1976. [DOI] [PubMed] [Google Scholar]
- Nichols 1991.Nichols TR, Koffler-Smulevitz D. Mechanical analysis of heterogenic inhibition between soleus muscle and the pretibial flexors in the cat. J Neurophysiol 66: 1139–1155, 1991. [DOI] [PubMed] [Google Scholar]
- Nichols 1986.Nichols TR, Steeves JD. Resetting of resultant stiffness in ankle flexor and extensor muscles in the decerebrate cat. Exp Brain Res 62: 401–410, 1986. [DOI] [PubMed] [Google Scholar]
- Pearson 1995.Pearson KG Proprioceptive regulation of locomotion. Curr Opin Neurobiol 5: 786–791, 1995. [DOI] [PubMed] [Google Scholar]
- Pearson 1993.Pearson KG, Collins DF. Reversal of the influence of group Ib afferents from plantaris on activity in medial gastrocnemius muscle during locomotor activity. J Neurophysiol 70: 1009–1017, 1993. [DOI] [PubMed] [Google Scholar]
- Pearson 1992.Pearson KG, Ramirez JM, Jiang W. Entrainment of the locomotor rhythm by group Ib afferents from ankle extensor muscles in spinal cats. Exp Brain Res 90: 557–566, 1992. [DOI] [PubMed] [Google Scholar]
- Prochazka 1996.Prochazka A Proprioceptive feedback and movement regulation. In: Handbook of Physiology. Excercise: Regulation and Integration of Multiple Systems. New York: Oxford, 1996, sect. 12, p. 89–127.
- Quevedo 2000.Quevedo J, Fedirchuk B, Gosgnach S, McCrea DA. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurons during fictive locomotion. J Physiol 525: 549–64, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rack 1969.Rack PMH, Westbury DR. The effects of length and stimulus rate on tension in the isometric cat soleus muscle. J Physiol 204: 443–460, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross 2006.Ross KT Quantitative analysis of feedback during locomotion. In: Biomedical Engineering. Atlanta: Emory University and Georgia Institute of Technology, 2006, 1–194.
- Ross 2005.Ross KT, Duysens JE, Smith VA, Nichols TR. Modulation of cutaneous and proprioceptive feedback in the premammillary locomoting cat. Soc Neurosci Abstr 630.7, 2005.
- Ross 2002.Ross KT, Fan RH, Nichols TR. Distribution of force feedback during spontaneous locomotion in premammillary cats. Soc Neurosci Abstr 65.16, 2002.
- Ross 2003.Ross KT, Huyghues-Despointes CMJ, Nichols TR. Heterogenic feedback among quadriceps and ankle extensors during spontaneous locomotion in premammillary cats. Soc Neurosci Abstr 276.11, 2003.
- Ross 2004.Ross KT, Nichols TR. Inhibitory force feedback to and from the plantaris muscle in the locomoting premammillary cat. Soc Neurosci Abstr 882.16, 2004.
- Rymer 1980.Rymer WZ, Hasan Z. Absence of force-feedback regulation in soleus of the decerebrate cat. Brain Res 184: 203–209, 1980. [DOI] [PubMed] [Google Scholar]
- Sacks 1982.Sacks RD, Roy RR. Architecture of the hind limb muscles of cats: functional significance. J Morphol 173: 185–195, 1982. [DOI] [PubMed] [Google Scholar]
- Schomburg 1978.Schomburg ED, Behrends HB. The possibility of phase-dependent monosynaptic and polysynaptic is excitation to homonymous motoneurons during fictive locomotion. Brain Res 143: 533–537, 1978. [DOI] [PubMed] [Google Scholar]
- Sinkjaer 2000.Sinkjaer T, Andersen JB, Ladouceur M, Christensen LO, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. J Physiol 523: 817–827, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer 1996.Sinkjaer T, Andersen JB, Larsen B. Soleus stretch reflex modulation during gait in humans. J Neurophysiol 76: 1112–1120, 1996. [DOI] [PubMed] [Google Scholar]
- Stein 2000.Stein RB, Misiaszek JE, Pearson KG. Functional role of muscle reflexes for force generation in the decerebrate walking cat. J Physiol 525: 781–791, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor 2000.Taylor A, Durbaba R, Ellaway PH, Rawlinson S. Patterns of fusimotor activity during locomotion in the decerebrate cat deduced from recordings from hindlimb muscle spindles. J Physiol 522: 515–532, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmink 2003.Wilmink RJH, Nichols TR. Distribution of heterogenic reflexes among the quadriceps and triceps surae muscles of the cat hindlimb. J Neurophysiol 90: 2310–2324, 2003. [DOI] [PubMed] [Google Scholar]