Abstract
Recent studies using a reaction time (RT) task have reported that a preprogrammed response could be triggered directly by a startling acoustic stimulus (115–124 dB) presented along with the usual “go” signal. It has been suggested that details of the upcoming response could be stored subcortically and are accessible by the startle volley, directly eliciting the correct movement. However, certain muscles (e.g., intrinsic hand) are heavily dependent on cortico-motoneuronal connections and thus would not be directly subject to the subcortical startle volley in a similar way to muscles whose innervations include extensive reticular connections. In this study, 14 participants performed 75 trials in each of two tasks within a RT paradigm: an arm extension task and an index finger abduction task. In 12 trials within each task, the regular go stimulus (82 dB) was replaced with a 115-dB startling stimulus. Results showed that, in the arm task, the presence of a startle reaction led to significantly shorter latency arm movements compared with the effect of the increased stimulus intensity alone. In contrast, for the finger task, no additional decrease in RT caused by startle was observed. Taken together, these results suggest that only movements that involve muscles more strongly innervated by subcortical pathways are susceptible to response advancement by startle.
INTRODUCTION
Recent studies using a reaction time (RT) paradigm have reported that premotor RT (time from stimulus presentation to EMG onset) in voluntary aiming tasks was substantially shortened when an unexpected loud acoustic stimulus (130 dB) was presented along with the usual “go” signal (Valls-Solé et al. 1995, 1999). Based on these findings, it was suggested that a loud startling stimulus could be used to directly elicit a preprogrammed response without the usual voluntary command. Specifically, Valls-Solé et al. (1999) stated that “the whole motor program [could] be triggered [by the startle] without the typical command from the cerebral cortex” (p. 937). This statement was mainly supported by the drastic nature of the RT decrease observed. That is, premotor RT (PMT) was very short compared with a control (no startle) condition. In the fastest reactions, PMT was 65 ms (Valls-Solé et al. 1999). In contrast, RTs of 180 ms are normally observed in response to visual stimuli, whereas RTs of 140 ms or more are commonly observed in response to acoustic stimuli (Brebner and Welford 1980). Valls-Solé et al. (1999) argued that because of the fixed amounts of time needed both to convert the acoustic stimulus to neural signals and for neural transmission, it was unlikely that cortical loops were involved in the initiation of movements in which PMT was <65 ms. Thus they suggested that sufficient details of a prepared movement may have been stored subcortically, possibly in the brain stem and spinal centers that were accessible to the startle volley, so that in some cases, it could be triggered early. Several later experiments replicated and extended these findings (Carlsen et al. 2003b, 2004b, 2007; Castellote et al. 2007; Cressman et al. 2006; MacKinnon et al. 2007; Siegmund et al. 2001). For example, it was found that response kinematics and EMG patterns are unchanged between control and startle elicited movements (Carlsen et al. 2004b) and that the paradigm can be extended to different effectors and movement types such as saccades (Castellote et al. 2007) and anticipatory postural adjustments (MacKinnon et al. 2007).
The defining requirement for a response to be elicited by a startle seems to be preprogramming. That is, for a startle to elicit a movement at short latencies, it must have been prepared in advance. When a startle was presented in a choice RT paradigm, it was found that PMT was unaffected by the startle. This indicated that the RT shortening effect of startle was not simply caused by increased neural excitability allowing for faster response transmission, because a similar speeding effect would be observed whether or not a response could be preprogrammed. It was only when the response was certain beforehand (i.e., a simple RT task) that the response was speeded by a startle (Carlsen et al. 2004a). Although this result supports the suggestion that a stored program is triggered early by a startle, it does not necessitate subcortical program storage (Carlsen et al. 2004b; Valls-Solé et al. 1999) because it cannot rule out an undescribed, fast transcortical route.
Although it has been traditionally thought that corticospinal connections are extremely important in the control of individual finger movements, recent preliminary data indicated that some reticulospinal connections with distal finger muscles exist and modulate their activity with movements of the finger (Baker and Riddle 2007; Soteropoulos et al. 2007). However, these reticulospinal connections are seen less frequently than corticospinal connections (Baker and Riddle 2007) and thus may be less functionally effective. This is evidenced by earlier studies showing that, following permanent lesions of the corticospinal tract, monkeys were unable to produce individual finger movements for tasks such as eating and grooming, although they recovered the ability to use more proximal muscles for climbing and walking (Lawrence and Kuypers 1968). Similar, although more variable, observations have been made in humans following stroke (Carroll 1965; Wade et al. 1983). More recently, similar deficits in fine finger control have been shown in monkeys through reversible chemical inactivation of primary motor cortex (Brochier et al. 1999). The aim of this experiment was to determine whether a startle acts to shorten RT in a finger movement (abduction of the index finger) that is thought to be strongly mediated by corticospinal connections. It was hypothesized that, if this finger movement was speeded by a startle, it would suggest that the startle effect likely includes a transcortical component. If, however, the movement was not speeded by startle, it would support the suggestion that, for other types of movements (e.g., arm extension, see Carlsen et al. 2004b), motor programs can be stored subcortically and that the startle can act to trigger preprogrammed movements without the involvement of cortex.
METHODS
Participants
Fourteen participants (9 males, 5 females; age, 24 ± 5 yr) with no obvious upper body abnormalities or sensory or motor dysfunctions volunteered to participate in the study. All participants gave written informed consent, and the study was conducted in accordance with the ethical guidelines set by the University of British Columbia.
Apparatus and task
Participants performed two tasks on separate days. These will be referred to as the finger task and the arm task.
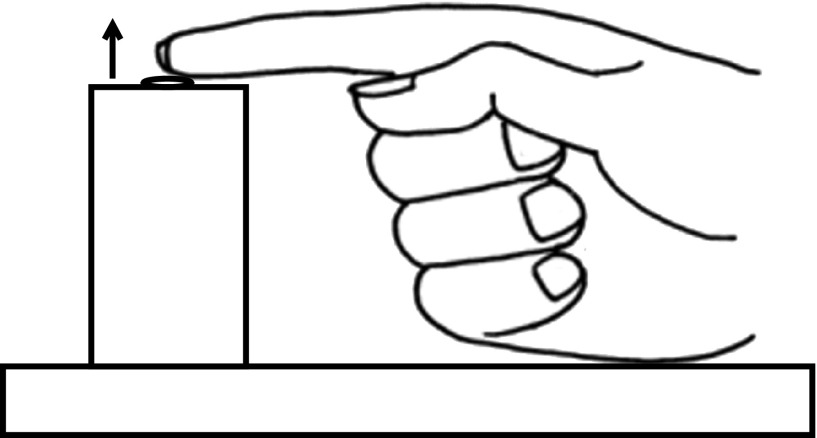
For the finger movement task, participants sat in a height-adjustable chair with their right arm secured to a table pointing forward and in a semipronated position. The arm was positioned so that the shoulder was both flexed and abducted ∼30° with the elbow flexed at 30°. The hand was also secured to the table using a Velcro strip attached to a clip and passing around fingers 3–5, which were bent 90° at the proximal interphalangeal joint leaving the index finger (digitus II) free to move. A simple contact switch requiring 0.04 N to close (i.e., simply resting the finger in the switch was sufficient to close it) was placed under the end of the outstreched index finger on the medial surface so that upwards movement (abduction) of the index finger opened the switch (Fig. 1). The finger movement task was a rapid finger abduction, which was just sufficient to open the switch using only the finger muscles, following an acoustic stimulus.
FIG. 1.
Illustration of the finger movement task. The right hand was placed with the index finger extended and relaxed, resting on a switch. Participants were instructed to make a rapid finger abduction movement in the upward direction (indicated by the arrow) following the acoustic stimulus.
For the arm task, participants sat in a height-adjustable chair outfitted with an automobile racing harness (Racer Components) to constrain any movement to the right elbow joint. The right arm was secured, in a pronated position with the palm down, to a custom-made aluminum manipulandum that moved in the transverse plane with an axis of rotation at the elbow. The starting position (90° of flexion at the elbow with the shoulder flexed 30°) was indicated by a mechanical stop. Participants were instructed to perform a 20° arm extension movement to a fixed target as quickly and as accurately as possible following an acoustic stimulus. For both tasks, participants were offered a monetary bonus for fast reactions.
Instrumentation and stimuli
Trials started with a warning consisting of a short acoustic tone (100 ms, 300 Hz, 80 dB) generated by the computer using a 16-bit sound card (Creative SoundBlaster 16) and standard computer speakers (Juster sp-691n). A variable foreperiod of 2–3 s spanned the time between the end of the warning tone and the imperative stimulus. A computer program generated the imperative stimulus consisting of a narrow band noise pulse (1 kHz, 40-ms duration). The signal was amplified and presented via a loudspeaker (<1-ms rise time) placed directly behind the head of the participant. The intensity of the acoustic go signal (imperative stimulus) was either 82 (control trials) or 115 dB (startle trials) and was measured using a sound level meter (model CR: 252B, Cirrus Research) at a distance of 30 cm from the loudspeaker (approximately the distance to the ears of the participant).
It was previously shown that 124 dB was the most effective intensity for eliciting a startle response (i.e., EMG activity in the sternocleidomastiod muscle) and startle RT facilitation in the greatest proportion of trials. In contrast, lower intensities resulted in a higher proportion of trials in which a startle response was not observed. Although RT was decreased in nonstartle trials, it was shown that when a startle response was observed, there was an associated further dramatic decrease in premotor RT to 80 ms irrespective of stimulus intensity (Carlsen et al. 2007). Here we used 115 dB so that several startled [sternocleidomastoid (SCM) activity present] and nonstartled (no SCM activity) loud (115-dB stimulus) trials would be achieved from each participant. This was done to control for stimulus intensity facilitation (Woodworth 1938; p. 318) where increases in intensity are associated with decreases in RT.
Each participant performed 75 trials in each of the two tasks, comprised of 57 control, 12 startle, and 6 catch (no acoustic imperative stimulus) trials. Catch trials were included to discourage false starts. Startle trials and catch trials occurred randomly among the control trials with the stipulation that no two consecutive trials were startle trials, and no startles occurred within the first five trials. The approximate trial to trial interval was 10 s, although this varied because of the random foreperiod.
For the arm movement task, surface EMG data were collected from the right elbow prime movers: the long head of the biceps brachii (BIC) and the lateral head of triceps brachii (TRI), as well as from the startle response indicator sternocleidomastoid (SCM). For the finger task, EMG data were collected from the first dorsal interosseous (FDI, primary index finger abductor), as well as from SCM. The recording sites were prepared and cleansed to decrease electrical impedance, and bipolar preamplified Ag/AgCl surface EMG electrodes were attached in the middle of the muscle bellies parallel to the line of force of the muscles. These electrodes were connected via shielded cabling to an external amplifier system (model 544, Therapeutics Unlimited). A grounding electrode was placed on the participant's left radial styloid process. Arm angular displacement data in the arm task was collected using a potentiometer attached to the pivot point of the manipulandum. Time of initiation of finger displacement in the finger task was monitored using the contact switch described above. All raw data were digitally sampled for 2 s at 1 kHz (PCI-6023E, National Instruments) using a personal computer running a customized program written with LabVIEW software (National Instruments). Data collection was automatically initiated 500 ms before the imperative stimulus.
Target and feedback
The target for the arm task was a fixed point in space located at 20° of angular displacement into extension with respect to the right arm's starting position. A computer screen placed directly in front of the participant provided real-time position feedback. The position of the manipulandum was represented with a yellow marker line (1 cm tall) whose movement in the horizontal plane corresponded directly to movement of the manipulandum. The starting position of the marker was approximately in the center of the computer screen. The target was represented by a stationary blue target line (1 cm tall), located 10 cm from the right edge of the screen. After each trial, feedback information including target error (°) and RT (ms) was displayed on the same computer monitor display. For the finger task, RT (ms) was displayed following each trial.
Training
Participants were allowed to practice the task before testing to familiarize themselves with the task and equipment. The participants were instructed that they would first hear a warning tone, followed by a variable foreperiod, and finally a go tone (imperative stimulus). Instructions emphasized fast RTs and fast movement times. Participants were also instructed that the loudness of the stimulus would be variable. For each task, participants received a single block of 10 practice trials in which no startle tone occurred.
Data reduction and analysis
For the arm task, movement onset (displacement RT) was defined as the first point of a change of >0.2° of angular displacement from the starting position following the stimulus. For the finger task, movement onset was the moment that the finger switch registered a positive voltage >1 V. Additional variables were calculated for the arm task. The final position of the movement was defined as the first point at which angular velocity remained <8°/s for ≥150 ms. Movement time was defined as the time (in ms) between movement onset and final position.
For both tasks, surface EMG burst onsets were defined as the point at which the EMG first began a sustained rise above baseline levels. The location of this point was determined by first displaying the EMG pattern on a computer monitor with a superimposed line indicating the point at which rectified, filtered EMG activity increased to >2 SD above baseline (mean of 100 ms of EMG activity preceding onset). Onset was verified by visually locating and manually adjusting the onset mark to the point at which the activity first increased on the raw EMG trace. This method allows for correction of errors caused by the strictness of the algorithm. PMT was defined as EMG onset in the TRI muscle for the arm task and EMG onset in the FDI for the finger task. PMT was the main measure of RT for this study because it represents an estimate of the total central processing time. EMG offsets were marked in a similar fashion, using a mean of EMG activity following the end of movement as a baseline level to account for any residual activity time between bursts. These were also verified and manually adjusted, with the activity between EMG onset and EMG offset being defined as a distinct burst. PMTs >1,000 and <40 ms were discarded if observed in the control (82 dB) condition.
Startle trials in which SCM activity was not present before 120 ms following the stimulus were treated separately from startle trials in which SCM activity was observed, because this distinction can be used to control for the effect of stimulus intensity versus the effect of startle (Carlsen et al. 2007). This led to the designation of a third stimulus category for analysis (see results).
Statistical analyses
Dependent measures were analyzed using two- (task) or three-factor (stimulus) repeated-measures ANOVA, where appropriate, to determine whether differences existed between tasks and/or conditions. EMG and kinematic measures were not analyzed between tasks because the mechanical features of the tasks differed greatly. Several dependent measures were available for the arm task only (e.g., final position) and were analyzed using a three-factor (stimulus) repeated-measures ANOVA. Proportion variables were subjected to an arcine square root transform before analysis. Greenhouse-Geisser corrected degrees of freedom were used to correct for violations of the assumption of sphericity. Differences with a probability of <0.05 were considered significant. Tukey's honestly significant difference (HSD) post hoc tests were administered to determine the locus of the differences.
RESULTS
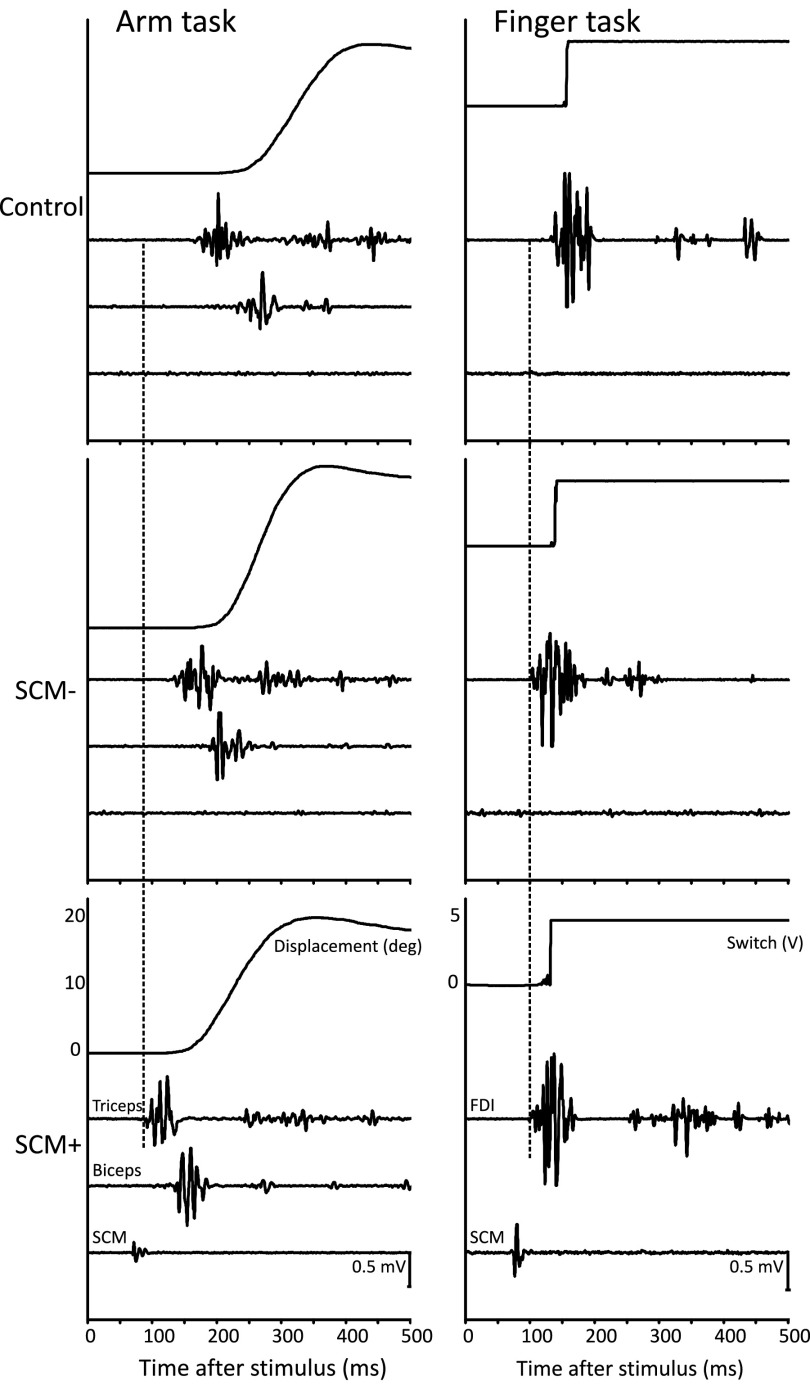
Raw data from a single participant is shown in Fig. 2, exemplifying the differences observed between the conditions in both the arm task (left) and the finger task (right). The presence of startle-related EMG activity in the sternocleidomastoid (SCM) was used to categorize startle trials into trials in which SCM activity was present (SCM+) versus absent (SCM−). The dashed line allows comparison of PMT observed in SCM+ trials with PMT observed in the control and SCM− conditions. Although the 115-dB stimulus led to shorter PMT in both movement tasks, only in the arm task did the presence of a startle response (SCM+) lead to a further reduction in PMT.
FIG. 2.
Example raw data from a single representative participant. Left panels contain data from the arm task; right panels from the finger task. Top 2 panels show control trials (82 dB), middle panels show startle trials (115 dB) where no sternocleidomastoid (SCM) activity was observed (SCM−), and bottom panels show startle trials with SCM activity (SCM+). Time 0 is stimulus onset. Arm task panels show displacement data, raw triceps, biceps, and SCM EMG activity. Finger task panels show displacement onset (moment of lift off switch), and raw FDI and SCM EMG activity. Dashed line shows prime mover EMG onset in the SCM+ condition allowing for comparison to the other conditions.
Startle response
In both the finger task and arm task, EMG activity in SCM was observed in startle trials for all participants. There was no significant difference in SCM onset latency between the arm task (72.6 ± 16.2 ms) and the finger task [76.2 ± 15.7 ms; F(1,13) = 1.213, P = 0.291]. Calculations showed that effect size was low, ηp2 = 0.085, indicating that only 8.5% of any difference observed was attributable to the difference between tasks. Although SCM activity was not observed in all 115-dB (startle) trials, the proportion of trials in which SCM activity was present (SCM+) versus absent (SCM−) did not differ between tasks [F(1,13) = 0.119, P = 0.736, ηp2 = 0.009], with a SCM response being observed in 59.5 ± 25.3% of startle trials in the arm task and in 59.5 ± 22.1% of startle trials in the finger task. Thus dependent measures were analyzed between three stimulus conditions: control trials, SCM+ trials, and SCM− trials. This made it possible to investigate the effect of stimulus intensity separately from the effect of a startle response because both SCM+ and SCM− trials involved the same stimulus (115 dB).
PMT
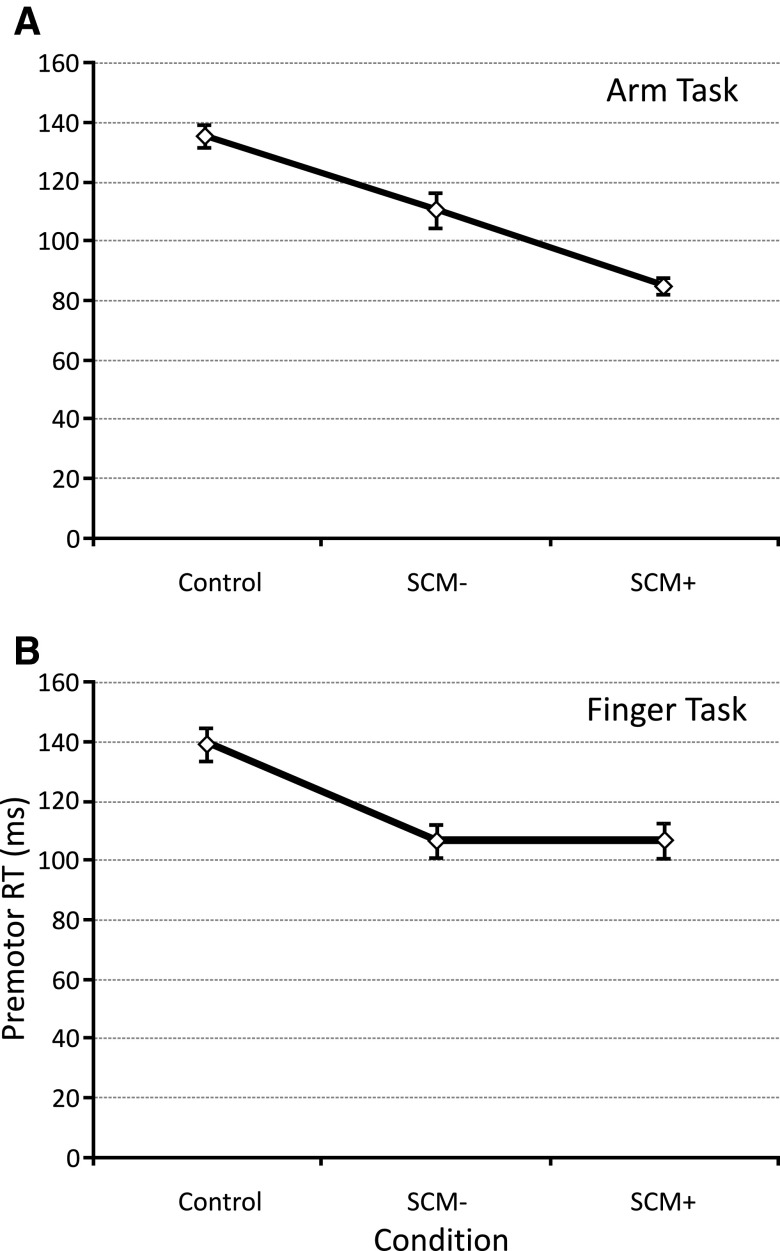
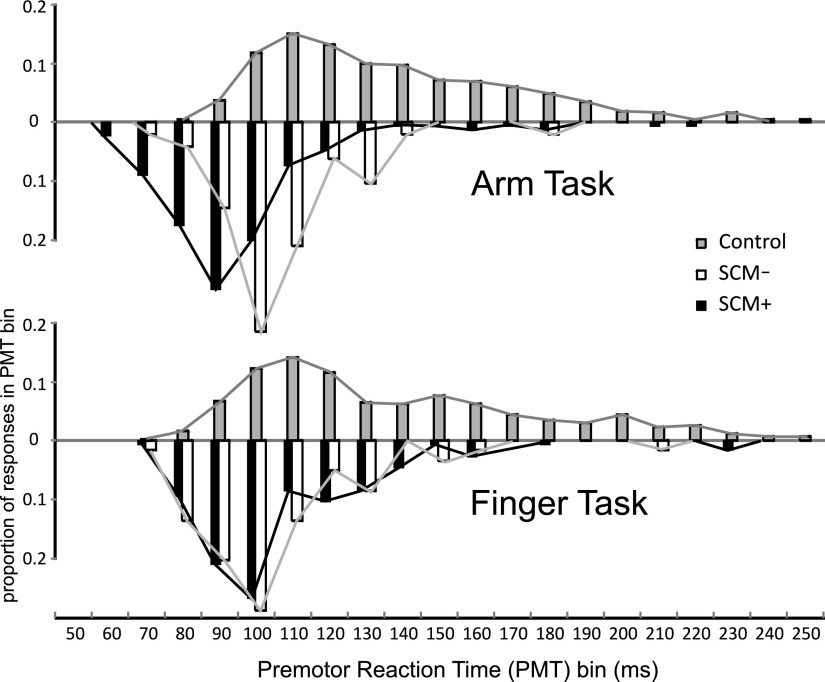
Because participants were not always startled by the 115-dB stimulus, as defined by EMG activity in SCM (Carlsen et al. 2007), PMT (i.e., time from stimulus to prime mover EMG onset) was analyzed between control trials, SCM+ trials, and SCM− trials for each task. Results are presented in Figs. 3 and 4. For the arm task, a significant main effect for stimulus was found [F(2,26) = 48.894, P < 0.001, ηp2 = 0.790]. Post hoc tests showed that PMT was shortest for SCM+ trials (P < 0.05), with PMT for SCM− trials being both significantly longer than for SCM+ trials and shorter than control trials (P < 0.05; Fig. 3A). This difference is further shown in Fig. 4, where it can be seen that the SCM+ and SCM− PMT distributions are separated. For the finger task, a significant main effect for stimulus was also found [F(2,26) = 18.580, P < 0.001, ηp2 = 0.588]. However, whereas post hoc tests showed that control trial PMT was significantly longer than both 115-dB conditions (P < 0.05), post hoc tests showed no difference (P > 0.05) in PMT between SCM+ and SCM− trials (Fig. 3B). In fact, Tukey's post hoc calculations showed that a PMT difference of 15.5 ms between conditions was required to reach significance at α = 0.05, whereas only a negligible mean PMT difference of 0.05 ms was observed between these conditions. Secondary analysis also showed that there was no significant difference [F(1,13) < 0.001, P = 0.995, ηp2 < 0.001] between the two 115-dB stimulus conditions for the finger task. This can also be seen in Fig. 4, where there is considerable overlap between the SCM+ and SCM− PMT distributions for the finger task. Using the arm task mean PMT difference between the SCM+ and SCM− conditions as an estimate, it was calculated that the effect size for the task was >1.36. Given the sample size (14), it was calculated that the power to detect any difference that existed between these two conditions was 0.996. No catch trial false starts were observed, and all were discarded from analysis.
FIG. 3.
Mean premotor reaction time (RT; ±SE) in the arm extension task (A) and the finger abduction task (B) for each stimulus condition: control (82 dB), SCM− (115 dB, no sternocleidomastiod activity observed), and SCM+ (115 dB, sternocleidomastiod activity observed).
FIG. 4.
Premotor RT (PMT) distributions in the arm extension task and the finger abduction task. Data are proportions of the total number of trials observed across all participants in 10-ms PMT bins for each stimulus condition: control (82 dB), SCM− (115 dB, no sternocleidomastiod activity observed), and SCM+ (115 dB, sternocleidomastiod activity observed). PMT bin values are upper limits for that bin.
Kinematic and EMG measures
For the arm task, displacement RT, movement final position, and movement time (time from displacement onset to final position) were analyzed between stimulus conditions. Displacement RT was also calculated and analyzed for the finger task. Results are presented in Table 1. None of the kinematic measures were significantly different between stimulus conditions except displacement RT for both the arm task [F(2,26) = 63.366, P < 0.001, ηp2 = 0.830] and for the finger task [F(2,26) = 15.932, P < 0.001, ηp2 = 0.551]. Post hoc tests showed the same pattern as observed for premotor RT: displacement RT was significantly different between SCM+ and SCM− in the arm task (P < 0.05) but not different in the finger task.
TABLE 1.
EMG and kinematic data values for each task and stimulus type
| Task Stimulus | Arm |
Finger | ||||
|---|---|---|---|---|---|---|
| Control | SCM+ | SCM− | Control | SCM+ | SCM− | |
| EMG measures | ||||||
| Premotor RT, ms | 135.1 (12.5) | 85.3 (9.5) | 110.8 (18.3)*† | 139.2 (17.3) | 106.3 (18.7) | 106.3 (17.8)* |
| Initial agonist burst duration, ms | 96.8 (15.3) | 92.2 (14.9) | 89.8 (18.1) | 90.0 (20.8) | 93.6 (28.1) | 90.5 (26.0) |
| TR1 to BIC inter-onset time, ms | 77.2 (34.5) | 73.5 (25.6) | 68.5 (25.2) | — | — | — |
| TR1 to TR2 inter-onset time, ms | 152.0 (40.3) | 156.8 (39.5) | 146.2 (31.6) | — | — | — |
| Kinematic measures | ||||||
| Displacement RT, ms | 199.8 (21.5) | 149.6 (11.5) | 165.6 (13.6)*† | 178.3 (16.8) | 143.6 (26.0) | 139.5 (20.1)* |
| Final position, deg | 20.1 (1.4) | 21.6 (3.3) | 21.7 (3.6) | — | — | — |
| Movement time, ms | 303.3 (29.1) | 325.1 (50.5) | 319.2 (42.1) | — | — | — |
Values are mean ± SD.
Significant main effect for auditory tone condition.
Significant difference between trials in which sternocleidomastiod setivity was present (SCM+) and absent (SCM−). TR1, triceps burst; BIC, biceps burst; TR2, second triceps burst; Premotor reaction time (RT), time from stimulus to initial agonist EMG onset; Displacement RT, time from stimulus to onset of displacement.
In addition to premotor RT, EMG measures were analyzed and are presented in Table 1. Initial agonist durations (TRI for the arm task; FDI for the finger task) were analyzed for differences between stimulus conditions; however, no significant differences were observed for either task (ηp2 = 0.017 and 0.166 for the finger and arm tasks, respectively). Second, burst onset timing was analyzed for differences between stimulus conditions for the arm task. Although a characteristic triphasic pattern of activity (agonist-antagonist-agonist) was observed for the arm task, no significant differences in burst timing were observed between the stimulus conditions.
DISCUSSION
Previous studies have shown that, during a RT task, preprogrammed movements can be elicited early if the go signal is accompanied by a startling acoustic stimulus (Carlsen et al. 2004a,b; Castellote et al. 2007; Cressman et al. 2006; Siegmund et al. 2001; Valls-Solé et al. 1999). Although it has been suggested that the startle effect acts by releasing a motor program that was stored subcortically (Carlsen et al. 2004b; Valls-Solé et al. 1999), there have been no direct tests of this hypothesis. In this study, a 115-dB startling stimulus replaced the usual RT go stimulus on several trials of two movement tasks. Here we show that, for a finger abduction movement, which seems to be more strongly mediated by corticospinal connections, the loud stimulus led to a reduction in PMT, yet no difference was observed whether or not a startle response was observed. For an arm extension movement, however, when a startle reaction was detected in response to the loud stimulus, a larger decrease in premotor RT was observed than when no startle response was elicited. These data indicate that, for early response triggering by startle to occur, the movement must involve more extensive subcortical brain stem connections.
To infer any effect of a startle on RT, it is important to measure the presence of a startle response in the participants. Without a startle response, the acoustic stimulus is simply “loud.” It may be suggested that the startle effect is merely an extreme case of stimulus intensity facilitation (Woodworth 1938, p. 318) where increases in intensity are associated with decreases in RT; however, it has been recently shown that when a startle reaction was detected, the RT facilitation was different and larger than that brought on by increases in stimulus intensity alone (Carlsen et al. 2007): A startle response can be detected using EMG activity in SCM, because it has been shown to be the electrophysiological indicator of startle in muscle EMG that is the among most reliable and one of the last to habituate to repeated stimuli (Brown et al. 1991). This SCM activity was associated with substantially shorter RTs at all stimulus intensities (93–123 dB). Thus irrespective of the intensity of the stimulus, if SCM activity was observed, mean PMT was shortened to ∼80 ms (Carlsen et al. 2007). However, lower intensities were also associated with a lower probability of observing a startle (i.e., SCM) response (see Carlsen et al. 2007). In this study, an acoustic stimulus intensity (115 dB) was chosen that would elicit a startle response in about one half of the loud trials. Although varying numbers of trials in which a startle response was (SCM+) or was not (SCM−) detected between participants (yet all exhibited some SCM+ and some SCM− trials), the mean proportion of SCM +115-dB trials was not different between the two tasks (59.5% for both the arm and finger tasks). Thus by comparing dependent measures between control trials, 115-dB SCM+ trials, and 115-dB SCM− trials, it was possible to examine the effect of the stimulus intensity on RT separately from the effect of an overt startle response.
PMT results for the arm task were similar to those reported in previous studies (Carlsen et al. 2003a, 2004a,b, 2007; Cressman et al. 2006; Siegmund et al. 2001; Valls-Solé et al. 1995, 1999). Specifically, when a startle reaction (i.e., EMG activity in SCM) was detected in trials where a startling stimulus (115 dB, SCM+) replaced the usual go stimulus (82 dB, control), mean PMT was dramatically shortened from 135 to 85 ms (Fig. 3A). However, many individual trials with PMT much shorter than this were observed (Fig. 4). Previously, it was hypothesized that, under certain circumstances, the details of the motor program are stored subcortically and can be triggered directly by the startle (Valls-Solé et al. 1999).
This subcortical storage and triggering hypothesis (Valls-Solé et al. 1999) is based on interconnections between the neural pathways involved in both voluntary reactions and startle. The startle reflex pathway involves connections between the cochlear nucleus and the caudal reticular formation, with the giant neurons of the nucleus reticularis pontis caudalis (NRPc) acting as control neurons for the startle reflex (Koch 1999; Yeomans and Frankland 1996). In addition, voluntary movement preparation-related activity has also been recorded from the NRPc in animal models (Buford and Davidson 2004; Schepens and Drew 2004). Thus it was suggested that the startle reflex may interact with the voluntary response at the level of the reticular formation (Carlsen et al. 2004b; Rothwell 2006; Rothwell et al. 2002), leading to early release of the intended movement. This was suggested because of the drastic nature of the RT decrease observed and because estimates of stimulus transduction and nerve conduction delays precluded a transcortical pathway for responses with PMTs of <65 ms (Valls-Solé et al. 1999). This value was calculated by summing the time between an acoustic stimulus and the first volley of activity arriving at the auditory cortex (35 ms, Erwin and Buchwald 1986), with the time required for neural conduction between the primary motor cortex and the muscles (20–30 ms, Jones et al. 1996; Rothwell 1997). This left almost no time for cortico-cortical transmission, let alone any cortical processing for the shortest RTs observed. However, these previous results could not rule out an unknown fast transcortical route. In this study, the fastest PMT observed in SCM+ trials (arm task) was 52 ms, with many more <80 ms, replicating the observed response speeding effect caused by startle. Importantly, these fast reactions all belong to the same RT distribution, which is significantly different from the control trial distribution (Fig. 4).
In the arm task, PMT for the 115-dB trials where no startle response was detected (SCM−) was 111 ms, which is significantly longer than for SCM+ trials (85 ms) also agrees well with previous studies that have shown that PMT for startle facilitated responses are different from stimulus intensity facilitated responses (Carlsen et al. 2003a, 2007). It was argued that only when SCM activity was observed, was there sufficient activation to directly trigger a response that was stored subcortically (Carlsen et al. 2003a, 2007). Otherwise, PMT was sufficiently long (based on the above calculation) to allow the involvement of a normal cortical route for response initiation. Thus in this study, it seems that SCM− trials were only affected by the increased intensity of stimulus (Kohfeld 1969; Luce 1986; Woodworth 1938, p. 318) and not by a triggering effect caused by startle.
Previously, it was suggested that the speeding effect caused by startle may simply be because of a later voluntary response adding on to an earlier startle reflex, resulting in an apparent decrease in voluntary RT where none truly existed (Siegmund et al. 2001). However, unless somehow seamlessly blended together, this would result in differences in EMG timing characteristics. For example, the duration of the initial agonist burst and the time from EMG onset until antagonist onset would be lengthened. This was exemplified in an experiment in which participants produced a required arm extension movement to a target located at 20, 40, or 60° from the starting position. Although the burst durations were different between the movement distances, when startled, no differences were observed in either the kinematic or EMG characteristics (Carlsen et al. 2004b). This provided evidence that the startle triggered the intended movement and was not simply a movement superimposed on an early startle. In this experiment, as in previous studies, the movement produced when participants were startled (or simply had a loud stimulus) was indistinguishable from that produced in response to the control stimulus (except for RT differences). That is, no differences in either EMG timing patterns or response kinematics were observed between the conditions for either the finger task or the arm task. Additionally, the small nonsignificant difference shown in the initial agonist burst duration data (Table 1) was opposite to the hypothesized outcome if a startle added onto a later voluntary response. If that were the case, the burst durations observed in response to startle should be longer than control and not shorter as observed here.
In the finger task, however, a somewhat different RT result was observed compared with the arm task. That is, like for the arm task, PMT was significantly shorter for the 115-dB stimulus compared with control when the primary movement task was index finger abduction. However, although some responses were observed at what would be considered “startle like” latencies in the finger task following the 115-dB stimulus, the PMT distributions were not different whether or not a startle (SCM) response was observed (Figs. 3B and 4). Given that the power to detect a difference that existed between these two conditions was 0.996, this implies there was a very low probability of a type II error (< 0.005) and a high probability that no PMT difference existed between these conditions.
In addition, mean PMT in both SCM+ and SCM− trials was sufficiently long (>106 ms; Table 1) to allow for traditional transcortical pathways to be used to initiate the response and was also similar to mean PMT for SCM− trials in the arm task (Fig. 3; Table 1). Thus the results of this experiment indicate that, unlike the arm task, the finger task was not directly triggered by startle. Indeed, it seems that the response pathway may be different for the finger task compared with the arm task because of the differential effect of a startle reaction on RT between the two tasks. Therefore it is suggested that the RT advancement observed in the finger task may have been caused by stimulus intensity facilitation, which is thought to have a perceptual basis and is the result of faster cortical perceptual processing (Levick 1973). This is in contrast to the hypothesized subcortical origin for the RT speeding effect of startle.
This result is important in determining the mechanism of the startle effect. As previously stated, there is considerable evidence that the intrinsic muscles of the hand [such as first dorsal interosseous (FDI)] are strongly mediated by cortico-motoneuronal connections (Brochier et al. 1999; Carroll 1965; Krakauer and Ghez 2000; Lawrence and Kuypers 1968; Wade et al. 1983). However, although preliminary reports indicated that some subcortical connections exist with the distal finger muscles in the monkey (Baker and Riddle 2007; Soteropoulos et al. 2007), these are weak compared with corticospinal effects. Additionally, stimulation of the reticulospinal tracts in cats and monkeys has not been reported to evoke paw or hand movements (Drew and Rossignol 1990; Davidson and Buford 2006). If the mechanism of startle advancement is to release a motor program that is stored in subcortical structures, as has been proposed (Carlsen et al. 2004b; Valls-Solé et al. 1999), a movement involving intrinsic hand muscles, such as the one used in this experiment, should not be triggered by startle. This is precisely what was observed in this study. On the other hand, reticulospinal pathways and parallel subcortical (e.g., reticulospinal / rubrospinal) connections in the voluntary activation pathway can have strong effects on more proximal limb muscles such as the elbow prime movers (Buford and Davidson 2004; Davidson and Buford 2006; Drew and Rossignol 1990; Schepens and Drew 2004). Furthermore, there seems to be strong projections to the reticular formation from cortical preparatory areas (Keizer and Kuypers 1989), as well as from cerebellar nuclei with preparatory activity (Allen et al. 1997). Thus because PMT in the finger task was not facilitated by startle (over and above the effect of stimulus intensity), whereasd PMT in the arm task was facilitated by startle, it seems that the involvement of subcortical (particularly reticulospinal) pathways for voluntary activation is a requirement to elicit a prepared movement via startle.
As noted earlier, the cortical preparatory areas and cerebellar nuclei could create a preparatory state in the reticulospinal neurons such that, when the startle occurred, those prepared brain stem neurons were the ones most likely to respond produce an output. There is also evidence that spinal cord interneurons can display preparatory motor activity for wrist movements in monkeys (Prut and Fetz 1999; Prut et al. 2001). Buford and Davidson (2004) noted this and argued that, in addition to preparatory activity in the reticulospinal system, corticospinal projections from the premotor areas to the spinal cord could set a state in the spinal cord that would be favorable for transmission of brain stem outputs to the appropriate muscles. Drew and colleagues have suggested this type of cortical gating of brain stem output as an explanation for the observation that the effects of reticulospinal outputs to upper limb muscles can vary with the phase of locomotion or if the limb is standing or reaching (Drew et al. 2004; Schepens and Drew 2006). Hence, there is ample evidence for preparation in the brain stem and the spinal cord that could serve to direct the output of startle-evoked responses from the brain stem to appropriate proximal upper limb muscles.
The most likely alternative explanation for our findings is that startle-evoked responses can be observed in intrinsic hand muscles through a direct subcortical route, but our intensity here was simply not high enough to evoke these effects because thresholds are higher in this pathway (Brown et al. 1991; Rothwell 2006). We chose an intensity high enough to elicit a startle in ∼60% of the trials. It is possible that if we had used a higher intensity (e.g., 124 dB, see Carlsen et al. 2007) such that a much higher percentage of trials included a startle, we could have overcome this threshold and observed a startle-associated advancement of response latencies in finger muscles. However, if a startle was elicited in all trials, there would be no way to reliably differentiate between stimulus intensity facilitated and startle-elicited responses. Nevertheless, this remains a possibility requiring further study. It should be noted that acoustic stimulus intensities of 130 dB or greater should not be used because of the possibility of damaging the auditory apparatus (NIOSH 1998).
It has been suggested that at least part of the RT advancement observed in startle trials may be caused by increased neural activation. In some cases, startle activity may lower the thresholds of spinal circuits, resulting in subthreshold activation of alpha motor neurons. Thus when the voluntary response is triggered, less time is required for the central command to reach the muscles. Although one experiment involving a choice RT task found no evidence of decreased RTs caused by startle (Carlsen et al. 2004a), others have described some RT advancement in a choice RT task (Kumru et al. 2006; Oude Nijhuis et al. 2007; Reynolds and Day 2007). One explanation for this result was that the increased neural excitability may have been responsible for at least some of the response shortening observed. Indeed, an alternative explanation for the differential effect of startle between the two tasks in this experiment involves the startle response threshold of the hand muscles. Specifically, hand muscles such as FDI may have much higher thresholds required to elicit a startle response, as outlined above, indicating that a different pathway is used for activation caused by startle. This pathway possibly involves caudo-rostral pattern of activation beginning at the NRPc resulting in late corticospinal startle activation of the hand muscles. Therefore even though a startle response was detected in SCM on some trials, there was not necessarily sufficient activation in the FDI pathway to lead to a further decrease in PMT. These PMT results could be explained if the only effect of startle was to increase neural excitability; however, it seems unlikely that simply a decrease in neural transmission time could account for the entirety of the 45-ms PMT decrease (larger for the fastest reactions) from 133 to 88 ms observed in the arm task. A similar argument has been made previously (Valls-Solé et al. 1999). Although decreased conduction time may explain some of the observed PMT decrease, it is our contention that a modified response pathway stemming from the elicitation of a startle response is also at least partially responsible for the observed PMT decrease.
Conclusion
This experiment studied the effect of a startling acoustic stimulus on the performance of a finger abduction movement and an arm extension movement within the context of a RT task. Consistent with previous literature, the presence of a startle response (activity in SCM) during the arm movement task resulted in the early release of the intended response. In contrast, no increased difference was observed in PMT for the finger abduction task when a startle response was observed. Because voluntary activation of the intrinsic muscles of the hand depends largely on corticospinal connections, we feel that the most likely explananation for these results is that only movements involving muscles with strong subcortical circuit connections as part of the voluntary response pathway are susceptible to full response speeding by startle.
GRANTS
This research was supported by a grant from the Natural Sciences and Engineering Research Council of Canada awarded to I. M. Franks.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Allen et al. 1997.Allen G, Buxton RB, Wong EC, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science 275: 1940–1943, 1997. [DOI] [PubMed] [Google Scholar]
- Baker and Riddle 2007.Baker SN, Riddle CN. The macaque reticulospinal tract forms monosynaptic connections with motoneurons in the cervical spinal cord controlling distal arm and hand muscle. Soc Neurosci Abstr 191.3, 2007.
- Brochier et al. 1999.Brochier T, Boudreau M-J, Pare M, Smith AM. The effects of muscimol inactivation of small regions of motor and somatosensory cortex on independent finger movements and force control in the precision grip. Exp Brain Res 128: 31–40, 1999. [DOI] [PubMed] [Google Scholar]
- Brebner and Welford 1980.Brebner JMT, Welford AT. Introduction: an historical background sketch. In: Reaction Times, edited by AT Welford. London: Academic Press, 1980.
- Brown et al. 1991.Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD. New observations on the normal auditory startle reflex in man. Brain 114: 1891–1902, 1991. [DOI] [PubMed] [Google Scholar]
- Buford and Davidson 2004.Buford JA, Davidson AG. Movement-related and preparatory activity in the reticulospinal system of the monkey. Exp Brain Res 159: 284–300, 2004. [DOI] [PubMed] [Google Scholar]
- Carlsen et al. 2003a.Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Startle response is dishabituated during a reaction time task. Exp Brain Res 152: 510–518, 2003a. [DOI] [PubMed] [Google Scholar]
- Carlsen et al. 2004a.Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Can prepared responses be stored subcortically? Exp Brain Res 159: 301–309, 2004a. [DOI] [PubMed] [Google Scholar]
- Carlsen et al. 2004b.Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Motor Behav 36: 253–264, 2004b. [DOI] [PubMed] [Google Scholar]
- Carlsen et al. 2007.Carlsen AN, Dakin CJ, Chua R, Franks IM. Startle produces early response latencies that are distinct from stimulus intensity effects. Exp Brain Res 176: 199–205, 2007. [DOI] [PubMed] [Google Scholar]
- Carlsen et al. 2003b.Carlsen AN, Hunt MA, Inglis JT, Sanderson DJ, Chua R. Altered triggering of a prepared movement by a startling stimulus. J Neurophysiol 89: 1857–1863, 2003b. [DOI] [PubMed] [Google Scholar]
- Carroll 1965.Carroll D Hand function in hemiplegia. J Chronic Dis 18: 493–500, 1965. [DOI] [PubMed] [Google Scholar]
- Castellote et al. 2007.Castellote JM, Kumru H, Queralt A, Valls-Solé J. A startle speeds up the execution of externally guided saccades. Exp Brain Res 177: 129–136, 2007. [DOI] [PubMed] [Google Scholar]
- Cressman et al. 2006.Cressman EK, Carlsen AN, Chua R, Franks IM. Temporal uncertainty does not affect response latencies of movements produced during startle reactions. Exp Brain Res 171: 278–282, 2006. [DOI] [PubMed] [Google Scholar]
- Davidson and Buford 2006.Davidson AG, Buford JA. Bilateral actions of the reticulospinal tract on arm and shoulder muscles in the monkey: stimulus triggered averaging. Exp Brain Res 173: 25–39, 2006. [DOI] [PubMed] [Google Scholar]
- Drew et al. 2004.Drew T, Prentice S, Schepens B. Cortical and brainstem control of locomotion. Prog Brain Res 143: 251–261, 2004. [DOI] [PubMed] [Google Scholar]
- Drew and Rossignol 1990.Drew T, Rossignol S. Functional organization within the medullary reticular formation of intact unanesthetized cat. I. Movements evoked by microstimulation. J Neurophysiol 64: 767–781, 1990. [DOI] [PubMed] [Google Scholar]
- Erwin and Bushwald 1986.Erwin RJ, Bushwald JS. Midlatency auditory evoked responses: differential recovery cycle characteristics. Electroencephalogr Clin Neurophysiol 64: 417–423, 1986. [DOI] [PubMed] [Google Scholar]
- Ghez and Krakauer 2000.Ghez C, Krakauer J. The organization of movement. In: Principles of Neural Science (4th ed.), edited by Kandel ER, Schwartz JH, Jessel TM. New York: McGraw-Hill, 2000.
- Jones et al. 1996.Jones KE, Calancie B, Hall A, Bawa P. Comparison of peripheral Ia and corticomotoneuronal composite EPSPs in human motoneurons. Electroencephalogr Clin Neurophysiol 101: 431–437, 1996. [PubMed] [Google Scholar]
- Keizer and Kuypers 1989.Keizer K, Kuypers HJGM. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp Brain Res 74: 311–318, 1989. [DOI] [PubMed] [Google Scholar]
- Koch 1999.Koch M The neurobiology of startle. Prog Neurobiol 59: 107–128, 1999. [DOI] [PubMed] [Google Scholar]
- Kohfeld 1969.Kohfeld DL Effects of the intensity of auditory and visual ready signals on simple reaction time. J Exp Psychol 82: 88–95, 1969. [DOI] [PubMed] [Google Scholar]
- Krakauer and Ghez 2000.Krakauer J, Ghez C. Voluntary movement. In: Principles of Neural Science (4th ed.), edited by Kandel ER, Schwartz JH, Jessel TM. New York: McGraw-Hill, 2000.
- Kumru et al. 2006.Kumru H, Urra X, Compta Y, Castellote JM, Turbau J, Valls-Solé J. Excitability of subcortical motor circuits in Go/noGo and forced choice reaction time tasks. Neurosci Lett 406: 66–70, 2006. [DOI] [PubMed] [Google Scholar]
- Lawrence and Kuypers 1968.Lawrence DG, Kuypers HGJM. The functional organization of the motor system in the monkey. I. The effects of bilateral pyramidal lesions. Brain 91: 1–14, 1968. [DOI] [PubMed] [Google Scholar]
- Levick 1973.Levick WR Variation in the response latency of cat retinal ganglion cells. Vision Res 13: 837–853, 1973. [DOI] [PubMed] [Google Scholar]
- Luce 1986.Luce RD Response Times: Their Role in Inferring Elementary Mental Organization. New York: Oxford, 1986.
- MacKinnon et al. 2007.MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, Zhang Y, Mille M-L, Rogers MW. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiol 97: 4368–4379, 2007. [DOI] [PubMed] [Google Scholar]
- McDowell et al. 2006.McDowell JE, Brown GG, Lazard N, Camchonga J, Sharp R, Krebs-Thomson K, Eylerc LT, Braff DL, Geyer MA. The neural correlates of habituation of response to startling tactile stimuli presented in a functional magnetic resonance imaging environment. Psychiatr Res Neuroimaging 148: 1–10, 2006. [DOI] [PubMed] [Google Scholar]
- Nambu et al. 1988.Nambu A, Yoshida S, Jinnai K. Projection on the motor cortex of thalamic neurons with pallidal input in the monkey. Exp Brain Res 71: 658–662, 1988. [DOI] [PubMed] [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH) 2007.National Institute for Occupational Safety and Health (NIOSH). Publication No. 98–126: Criteria for a Recommended Standard. Cincinnati: CDC, 1998.
- Oude Nijhuis et al. 2007.Oude Nijhuis LB, Janssen L, Bloem BR, vanDijk JG, Gielen SC, Borm GF, Overeem S. Choice reaction times for human head rotations are shortened by startling acoustic stimuli, irrespective of stimulus direction. J Physiol 514: 97–109, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma and Zanchetti 1956.Parma M, Zanchetti A. Ascending reticular influences upon thalamically evoked pyramidal discharges. Am J Physiol 185: 614–616, 1956. [DOI] [PubMed] [Google Scholar]
- Prut and Fetz 1999.Prut Y, Fetz EE. Primate spinal interneurons show pre-movement instructed delay activity. Nature 401: 590–594, 1999. [DOI] [PubMed] [Google Scholar]
- Prut et al. 2001.Prut Y, Perlmutter SI, Fetz EE. Distributed processing in the motor system: spinal cord perspective. Prog Brain Res 130: 267–278, 2001. [DOI] [PubMed] [Google Scholar]
- Reynolds and Day 2007.Reynolds RF, Day BL. Fast visuomotor processing made faster by sound. J Physiol 583: 1107–1115, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell 1997.Rothwell JC Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods 74: 113–122, 1997. [DOI] [PubMed] [Google Scholar]
- Rothwell 2006.Rothwell JC The startle reflex, voluntary movement, and the reticulospinal tract. In: Brainstem Function and Dysfunction, edited by Cruccu G, Hallett M. Amsterdam: Elsevier, 2006. [DOI] [PubMed]
- Rothwell et al. 2002.Rothwell JC, MacKinnon CD, Valls-Solé J. Role of brainstem-spinal projections in voluntary movement. Mov Disord 17: S27–S29, 2002. [DOI] [PubMed] [Google Scholar]
- Salami et al. 2003.Salami M, Itami C, Tsumoto T, Kimura F. Change of conduction velocity by regional myelination yields constant latency irrespective of distance between thalamus and cortex. Proc Natl Acad Sci USA 100: 6174–6179, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepens and Drew 2004.Schepens B, Drew T. Independent and convergent signals from the pontomedullary reticular formation contribute to the control of posture and movement during reaching in the cat. J Neurophysiol 92: 2217–2238, 2004. [DOI] [PubMed] [Google Scholar]
- Schepens and Drew 2006.Schepens B, Drew T. Descending signals from the pontomedullary reticular formation are bilateral, asymmetric, and gated during reaching movements in the cat. J Neurophysiol 96: 2229–2252, 2006. [DOI] [PubMed] [Google Scholar]
- Siegmund et al. 2001.Siegmund GP, Inglis JT, Sanderson DJ. Startle response of human neck muscles sculpted by readiness to perform ballistic head movements. J Physiol 535: 289–300, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner et al. 2004.Skinner RD, Homma Y, Garcia-Rill E. Arousal mechanisms related to posture and locomotion. II. Ascending modulation. Prog Brain Res 143: 291–298, 2004. [DOI] [PubMed] [Google Scholar]
- Soteropoulos et al. 2007.Soteropoulos DS, Williams ER, Baker SN. Primate reticular neurones modulate activity with a slow finger movement task. Soc Neurosci Abstr 191.4, 2007.
- Stelmack et al. 2003.Stelmack RM, Knott V, Beauchamp CM. Intelligence and neural transmission time: a brain stem auditory evoked potential analysis. Personality Indiv Diff 34: 97–107, 2003. [Google Scholar]
- Stockard et al. 1977.Stockard JJ, Stockard JE, Sharbrough FW. Detection and localization of occult lesions with brain stem auditory responses. Mayo Clin Proc 52: 761, 1977. [PubMed] [Google Scholar]
- Takakusaki et al. 2004.Takakusaki K, Saitoh K, Harada H, Kashiwayanagi M. Role of basal ganglia–brainstem pathways in the control of motor behaviors. Neurosci Res 50: 137–151, 2004. [DOI] [PubMed] [Google Scholar]
- Valls-Solé et al. 1999.Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516: 931–938, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Solé et al. 1995.Valls-Solé J, Solé A, Valldeoriola F, Muñoz E, Gonzalez LE, Tolosa ES. Reaction time and acoustic startle in normal human subjects. Neurosci Lett 195: 97–100, 1995. [DOI] [PubMed] [Google Scholar]
- Wade et al. 1983.Wade DT, Langton-Hewer R, Wood VA, Skilbeck CE, Ismail HM. The hemiplegic arm after stroke: measurement and recovery. J Neurol Neurosurg Psychiatry 46: 521–524, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth 1938.Woodworth RS Experimental Psychology. New York: Henry Holt, 1938.
- Yeomans and Frankland 1996.Yeomans JS, Frankland PW. The acoustic startle reflex: neurons and connections. Brain Res Rev 21: 301–314, 1996. [DOI] [PubMed] [Google Scholar]