Abstract
The ability to adjust the amplitude of gaze shifts in response to persistent visual errors (“gaze adaptation”) has been investigated primarily by introducing visual errors at the end of saccades produced by head-restrained primates. Very little is known about the behavior and neural mechanisms underlying gaze adaptation when the head is free to move. We tested alternative hypotheses about the signals that are altered during gaze adaptation by increasing (25° → 50°; “forward adaptation”) or decreasing (50° → 25°; “backward adaptation”) the size of large, head-unrestrained gaze shifts. In our three rhesus monkey subjects, changes to primary gaze shift amplitude occurred regardless of the particular combinations of eye and head movements that made up the amplitude-altered gaze shifts. The relative changes to eye and head movements that occurred during adaptation could be predicted based on the magnitude of gaze adaptation and the positions of the eyes in the orbits at gaze onset. These results are consistent with the hypothesis that gaze adaptation occurs at the level of a gaze shift command and inconsistent with hypotheses based on the assumption that gaze adaptation results from alterations to eye- and/or head-specific signals.
INTRODUCTION
Adjustment of motor output in response to systematic movement errors is a critical function of the CNS. During visual orienting behaviors, this type of adaptation has been studied by introducing visual errors near the end of saccadic eye movements (Deubel 1987, 1991; Deubel et al. 1986; McLaughlin 1967; Miller et al. 1981; Noto et al. 1999; Robinson et al. 2003; Scudder et al. 1998; Semmlow et al. 1987; Straube et al. 1997). When the head is prevented from moving, a shift in target location triggered by the beginning of a saccade leads initially to a visual error at the end of the movement. As similar trials are repeated, the amplitude of the movements made to the first target location change (increasing or decreasing) so that the error at the end of the movement is reduced (Deubel 1987, 1991; Deubel et al. 1986; McLaughlin 1967; Miller et al. 1981; Noto et al. 1999; Robinson et al. 2003; Scudder et al. 1998; Semmlow et al. 1987; Straube et al. 1997). Changes in saccade amplitude follow a roughly exponential time course with “rate constants” around 30–60 saccades in humans (Albano 1996; Deubel 1987; Deubel et al. 1986; Frens and vanOpstal 1994) and between 100 and 800 saccades in monkeys (Straube et al. 1997).
When the head is free to move and gaze shifts are accomplished by combining movements of the eyes and head, a persistent visual error at the end of a gaze shift could be caused by a variety of deficits. For instance, a systematic decline in the amplitude of saccadic eye movements could result in consistently hypometric gaze shifts. Similarly, a reduced head contribution (produced either by a reduction in head-movement amplitudes or by altering the timing of eye and head movements) could lead to the same gaze dysmetria. The dysmetria caused by either hypometric saccades or reduced contribution of the head could be corrected by altering the command specifying the desired displacement of the line of sight. Consider, for example, a subject whose goal is to produce an accurate gaze shift to a visual target 30° to the right. However, the gaze shift is hypometric, resulting in a large visual error at the end of the movement. Rather than issuing a command for a 30° change in gaze position, a 40° command could be generated. The hypometria of eye or head movements would reduce the requested 40° change in gaze position so that the actual movement was closer to the required 30°; the error at the end of gaze shifts to a target displaced 30° would be reduced. This mechanism relies on detecting gaze error at the end of a movement and modifying the gaze shift command signal to compensate for deficits in either the eye or head machinery. It is also possible to conceive of a mechanism that determines that gaze hypometria resulted from a reduction in head movement or in saccade amplitudes. It would then be possible to modify head- or eye-specific command signals to compensate for the gaze hypometria. In this case, the gaze command signal would remain unaltered, but adaptive mechanisms would adjust the eye or head movements after these signals have been computed. From the subject's point of view, the cause of the hypometria matters very little and either approach to correcting it will accomplish the overall goal: gaze shifts that bring the images of visual targets close to the fovea. However, understanding the mechanism used by the nervous system to adapt to systematic movement errors is an important step in elucidating the neural control of visual orienting behaviors.
In an effort to distinguish between the above-outlined alternatives (modification of a gaze shift command vs. modification of separate eye and/or head specific signals) head-unrestrained gaze adaptation was investigated in three rhesus monkeys (Macaca mulatta) whose gaze shifts began from a variety of orbital eye positions. The relative contribution of the eyes and head to a particular amplitude gaze shift depends on the orbital position of the eyes at gaze onset and gaze vector (Freedman 2005; Freedman and Sparks 1997). Typically, head contribution is large when the eyes are deviated toward (head deviated away) and small when the eyes are deviated away (head deviated toward) from a potential visual target. During gaze adaptation, the eye- and head-command-only hypotheses predict that only one of these effectors is modified. In these instances, only the eye or head movement for gaze shifts from every eye position would be modified. However, an adaptive change to a gaze command would result in modifications to both eye and head movements and that the relative change in eye and head contribution during gaze adaptation would vary with starting eye position.
The data in this study illustrate that using the McLaughlin task in the head-unrestrained monkey, gaze shift amplitudes change systematically and with an exponential time course. As gaze amplitudes are modified, the amplitudes of eye and head contributions change. However, relative contributions remain appropriate (i.e., similar to control trials) for gaze shifts matched for amplitude and starting positions of the eyes in the orbits. Finally, gaze adaptation was carried out with the eyes always in one starting position relative to the head. After the amplitude of gaze shifts was altered, movements were made with the eyes in different (unadapted) initial positions. Although the contributions of the eyes and head were very different when movements were started with the eyes in these novel positions, the amplitudes of gaze shifts remained at the adapted values; gaze adaptation transferred to movements made under unadapted initial conditions. These data are consistent with the hypothesis that head-unrestrained gaze adaptation is mediated by changing gaze shift commands (Cecala and Freedman 2008; Phillips et al. 1997).
The data in this study extend those of our earlier study (Cecala and Freedman 2008) in several ways. First, we demonstrate the effects of adaptation on gaze, eye, and head movements in a nonhuman primate—a model system for future neurophysiological studies on the neural mechanisms of the adaptive process. In addition, herein we compare head-unrestrained adaptation with head-restrained saccadic adaptation and report on the rates of adaptive changes when the head is free to move using large intragaze target displacements during the McLaughlin paradigm.
METHODS
Three female rhesus monkeys weighing 4.5–6.0 kg served as subjects. A scleral coil was implanted for monitoring gaze position (Judge et al. 1980) and, during the same aseptic surgery, a small head-restraint device was secured to the skull. After full recovery, subjects were trained to make gaze shifts to visual targets. All surgical and experimental procedures were approved by the University of Rochester Animal Care and Use Committee and are in accordance with the National Institutes of Health Guide for the Care and Use of Animals.
During all training and experimental sessions, animals were seated in a primate chair designed to restrict movements of the hips and upper body while permitting unrestricted movements of the head. Data collection took place in one of two instruments that used either a pair of movable lasers or a light-emitting diode (LED) array as visual stimuli. When movable lasers were used as visual stimuli, the monkey was seated in the center of a 1.2-m cube that housed three pairs of magnetic field coils (CNC Engineering, Seattle, WA). The four vertical faces of the cube (front–back and left–right) contained two pairs of Helmholtz coils in spatial and phase quadrature (Collewijn 1977). The current produced in the scleral and matching head coil were linear within about 2% over 360°. The top and bottom faces of the cube contained a third pair of Helmholtz coils that were used to measure the vertical angle of the scleral and head-mounted coils. Visual targets were presented by pointing green (532 nm) or red (650 nm) laser diodes at the inside of a 1.5-m-diameter hemisphere (0.5-in. acrylic; Capital Plastics, Beltsville, MD). The center of the hemisphere's vertical face was aligned with the geometric center of the field coil frame. Positioning of the green lasers was accomplished using two independent, two-axis, motorized gimbals (custom designed using pairs of RGV 100 rotation stages; Newport, Irvine, CA). Each pair of rotation stages, arranged in a Fick gimbal, could direct a laser spot at any location within the hemisphere with >0.01° accuracy and precision rated at 0.0003°. The LED array was a flat panel array that subtended ±48° of visual angle horizontally and ±40° vertically (LEDs spaced every 2°). In other respects the two systems were similar (primate chair, coil system, etc.).
In both instruments, a lightweight cam-lock device, containing a coil similar to that implanted in the eye, was secured to the head. In addition, three laser diodes (red: 650 nm) were mounted on the head. The center laser was aligned with the midsagittal plane of the subject, whereas the others were directed about 18° to the left or right of center. Each trial began with the presentation of a central target (T0). Subjects were required to fixate T0 while aligning the head-mounted laser spot at the same location. When the central head-mounted laser was illuminated this served to align the eyes and head at the initial fixation target. When either the left-pointing or right-pointing head-mounted laser was lit, alignment of the head-mounted laser with the target (within a 3° computer-defined window) required a rotation of the head (to the right or the left) while the direction of gaze was maintained. As a result, the positions of the eyes in the orbits at the beginning of each gaze shift varied as a function of which head-mounted laser was turned on.
Trial types
All trials began as described earlier. Fixation of T0 and alignment of the head-mounted laser had to be maintained for an interval that varied from 250 to 1,250 ms (100-ms increments). At the end of this interval an additional target was illuminated. Head alignment and fixation of the T0 target had to be maintained during a delay period lasting between 250 and 1,250 ms (50-ms increments). The location of the new target was selected randomly by computer from a set of potential targets ranging from −50 to 50°. Typically, during any particular session 8–10 target locations were used and these always included the locations that were to be used for adaptation during the same session. At the end of the delay, the head-mounted laser and T0 were turned off, cuing the subject to make a gaze shift to the still-lit target. After the movement was completed, reward was delivered if the subject maintained fixation of the new location for 500 ms within a computer-defined window (5–7°) centered on the target location.
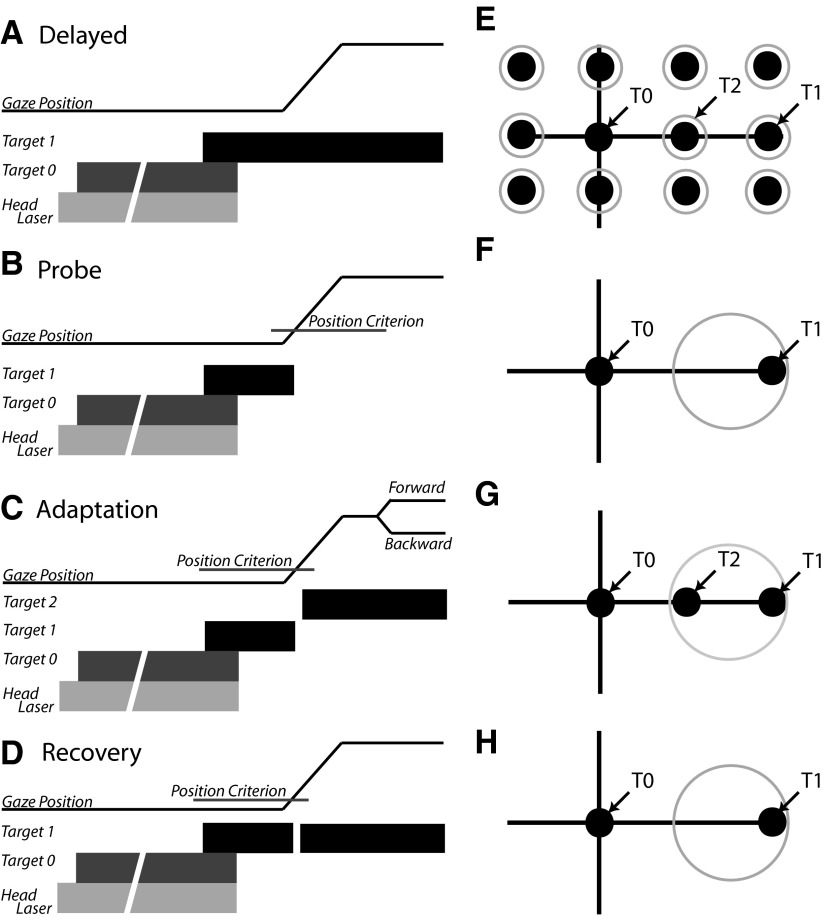
Adaptation trials differed from delayed gaze shift trials in two ways. First, the location of the gaze shift target remained the same for all adaptation trials on a given day (this target is referred to as the T1 target throughout the text and in Fig. 1). The second important difference in adaptation trials was that after the gaze shift was initiated (when gaze position exceeded the computer-defined fixation window centered on T0) the T1 target was turned off and, 20 ms later, another target (T2) was illuminated. During backward adaptation, the T2 target was located between T0 and T1; during forward adaptation the T2 target was further away from T0 than was T1. Example target locations for a backward adaptation session are illustrated in Fig. 1G. Because the amplitude of gaze shifts to the T1 location are expected to become smaller over the course of backward adaptation, the reward window size was increased and centered on a location halfway between T1 and T2 so that subjects continued to be rewarded despite making hypometric movements. Note that if they made normometric movements subjects would still be rewarded; there was no penalty for failing to change gaze shift amplitudes. To assess gaze adaptation without the potentially confounding effects of a visible target at T2 during part of the gaze shift, on randomly interleaved trials the T2 target was not illuminated (“probe” trials: Fig. 1, B and F). When time permitted, recovery from adaptation was facilitated by reilluminating the target at the T1 location (Fig. 1, D and H).
FIG. 1.
A–C: schematic diagrams of trial types used. In each panel, gaze position is represented by a thin black line and plotted as a function of time. Below this trace, targets are represented by thick bars indicating when within the trial each target was illuminated and extinguished. In each case, trials began with the illumination of a head-mounted laser (light gray bar) followed by presentation of T0. If behavioral contingencies were satisfied, the head-mounted laser and T0 target were extinguished and a second target (T1) was illuminated at one of the spatial locations shown in E (black circles). A: the T1 target remained illuminated for the duration of the “delayed” trials. B: during “probe” trials, the T1 target was extinguished when the line of sight moved beyond a computer-defined window (“position criterion”) surrounding T0. C: “adaptation” trials were similar to probe trials until 20 ms after the position criterion was satisfied, at which time T1 was turned off and a target at location T2 was illuminated. D: recovery trials were identical to adaptation trials except that the T1 target was reilluminated and remained illuminated for the duration of the trial. E: target locations used during adaptation and probe trials. F–H: example target and reward window locations during probe (F), adaptation (G), and recovery (H) trials during a backward adaptation experiment. See text for further details.
Experimental sessions were carried out according to the following pattern. Delayed gaze shift and probe trials to a variety of targets were randomly interleaved. Movements to the T1 and T2 target locations were included. After approximately 200 trials, adaptation trials using T1 and T2 locations began; probe trials to the T1 target and also to 8–10 other target locations continued to be presented during the adaptation period and were randomly interleaved with the adaptation trials. Probe trials made up about 30% of trials presented. Adaptation (and probe) trials continued to be presented for between 600 and 800 trials. At the end of this period of adaptation, only probe trials were presented (recall that there was no visual feedback about movement accuracy during probe trials). Following this period, recovery trials to T1 were presented until the end of the session. With the exception of transfer experiments, each trial began by the random illumination of one of the three head-mounted lasers throughout the duration of the experimental session.
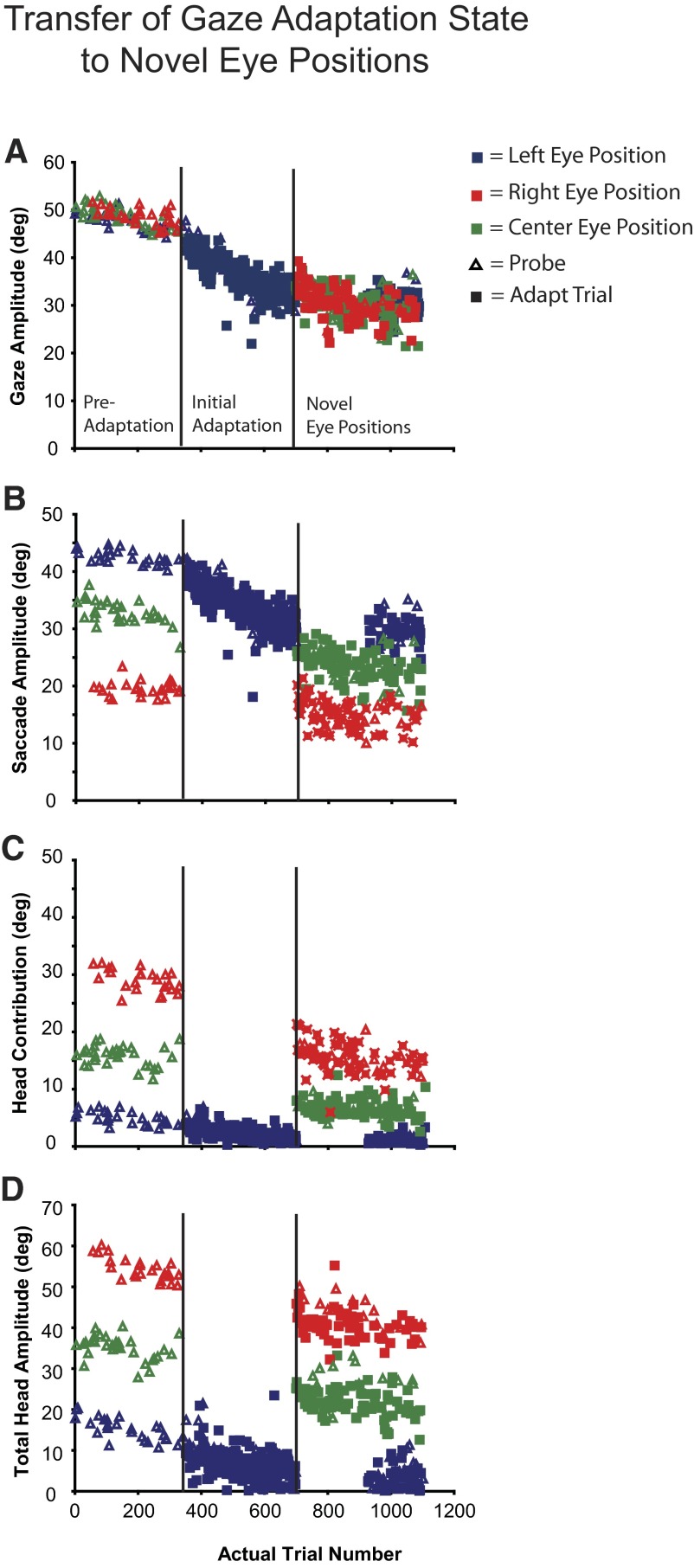
During transfer experiments, all three head lasers were randomly intermixed during the pre- and postprobe epochs. However, at the beginning of the adaptation epoch (“initial adaptation epoch”) gaze shifts were initiated from a single eye position (e.g., “centered”). At the beginning of the “novel eye position epoch,” approximately halfway through the adaptation session (300–400 trials), gaze shifts were initiated from only the remaining two eye positions (e.g., “leftward” and “rightward”). Note that toward the end of the adaptation epoch the original eye position (in this example case the centered eye position) was reintroduced to assess the magnitude of adaptation from this eye position at the end of the adaptation epoch. Adaptation sessions were separated by at least one behavioral session in which the subject performed only delayed and/or probe trials.
Data acquisition and analysis
Custom behavioral control software running on a PC with an extended PCI bus (National Instruments, Austin, TX) controlled the switching on and off of the head-mounted lasers, movable lasers, and LEDs in real time with 1-ms resolution. Search coil signals were filtered to remove the coil carrier frequencies and stored for off-line analysis using MATLAB (The MathWorks, Natick, MA). Using velocity and acceleration criteria, gaze, eye, and head movements were identified and their amplitudes calculated.1 In addition, the contribution of the head to the accomplishment of each gaze shift (the amplitude of head movements that occurred during the gaze shift) was determined. The average amplitudes of movements made during probe trials to the T1 target before adaptation were compared with average amplitudes observed during the last 15 adaptation trials and/or with the first 15 probe trials collected after adaptation was complete. Note that during three experiments (P10, P11, P12) we were unable to collect enough trials during the postprobe epoch for comparison. Unless otherwise noted, comparisons of means were made using a one-way ANOVA (Tukey–Kramer post hoc) in the MATLAB Statistical Toolbox. Significance was determined using the criterion P < 0.05.
Rates of adaptation were assessed by plotting movement amplitudes as functions of the number of completed adaptation trials (note that probe trials were randomly interleaved during adaptation; these trials were removed and excluded from adaptation rate calculations). Data were fit (least squares) with exponentials (Sigma Plot) and “trial constants” (analogous to rate constants) calculated.
RESULTS
Eyes and head aligned
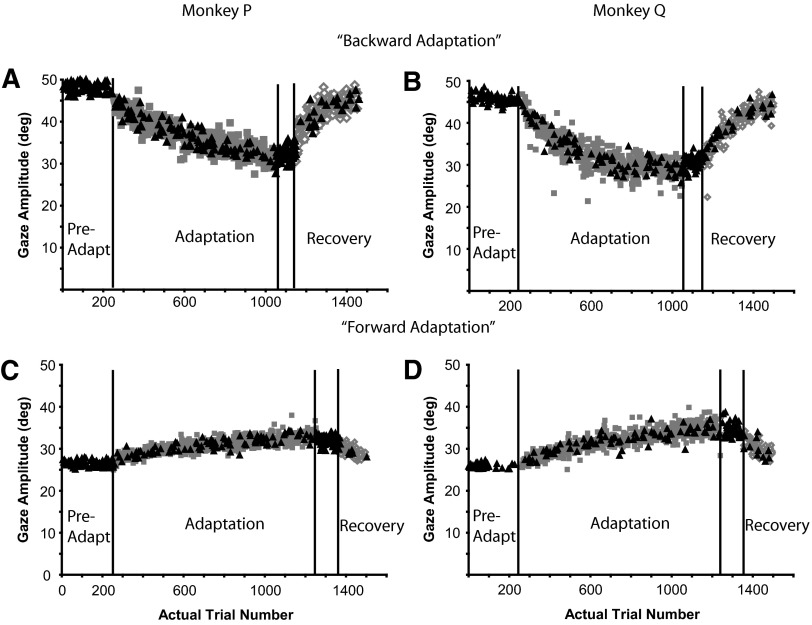
Primary gaze amplitude was altered in all 28 gaze adaptation experiments (17 backward, 11 forward) collected from three monkeys (P, Q, S). When the central head-mounted laser was illuminated, the eyes and head were aligned at the beginning of gaze shifts. During “backward” adaptation, the amplitude of gaze shifts made in response to presentation of T1 decreased for each subject. In Fig. 2, A–D, gaze (green), eye (red), and head (blue) positions are plotted as functions of time for typical gaze shifts made by subject Q during backward adaptation (session Q1). The initial target displacement (T1 − T0) was 50° in each case and movements began after subjects aligned the eyes and head. Average initial eye position (IEP) for these four representative trials was −0.3 ± 1.1°.
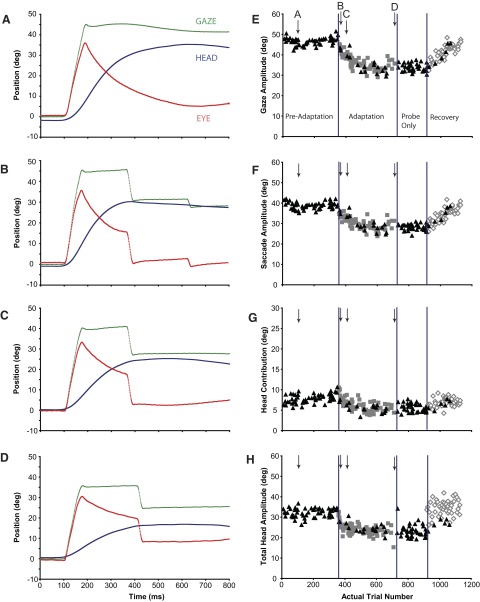
FIG. 2.
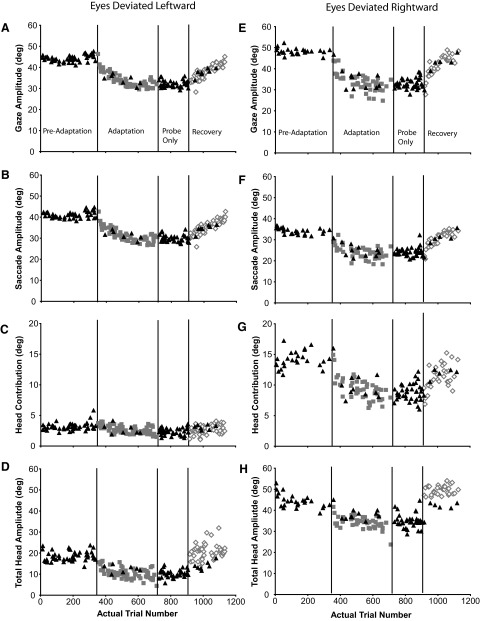
A–D: gaze (green), eye (red), and head (blue) positions as functions of time during a backward adaptation session (Q1) in which the eyes were initially centered in the orbits (±5°). Each panel illustrates a gaze shift made at a different stage of the adaptation process. Position traces are aligned at gaze onset (“100 ms”) in each plot. E–H: gaze, saccade, head contribution, and total head amplitudes of the primary gaze shifts made during preadaptation, adaptation, postprobe, and recovery segments of experiment Q1. Individual examples in A–D are indicated with labeled arrows. Black triangles: probe trials; gray squares: adaptation trials; open diamonds: recovery trials.
Figure 2A illustrates a gaze shift made toward T1 during a probe trial before the introduction of adaptation trials. In Fig. 2A, and subsequent similar plots (Figs. 2, B–D and 3, A–D), position traces are aligned to gaze onset; for display purposes, 100 ms of data before movement onset are also shown. The 44.7° gaze shift (green) in this example was accomplished by combining a 35.1° saccadic eye movement (red) with a head contribution of 9.6°. The head moved a total of 36.7° on this trial (blue); most of this large head movement occurred after the line of sight was already directed toward T1. During the continuing head movement, gaze position remained relatively constant—most likely a result of the vestibuloocular reflex. This subject's primary gaze shift during the first adaptation trial (Fig. 2B) was similar in amplitude to that shown in Fig. 2A. The 45.1° gaze shift consisted of a large saccade (34.7°) associated with a small head contribution (10.4°). The primary gaze shift was followed by two “corrective” gaze shifts that redirected the line of sight so that the subject's final gaze position was closer to T2 (T2 − T0 = 26°).
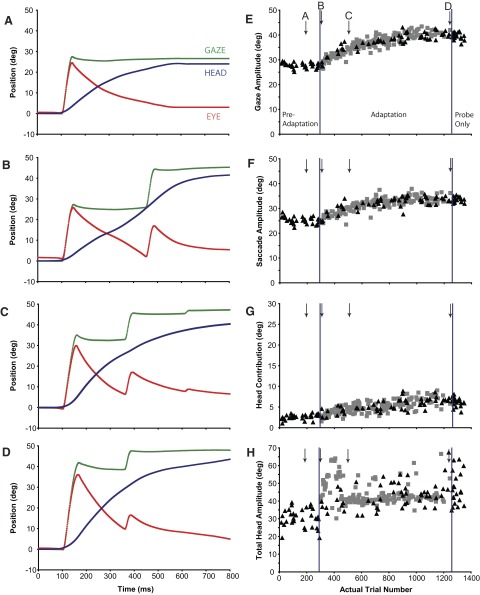
FIG. 3.
Gaze (green), eye (red), and head (blue) positions as functions of time during a forward adaptation session (Q3) in which the eyes were initially centered in the orbits (±5°). Each panel illustrates a gaze shift made at a different stage of the adaptation process. Position traces are aligned at gaze onset (“100 ms”) in each plot. E–H: gaze, saccade, head contribution, and total head amplitudes, respectively, of the primary gaze shifts made during preadaptation, adaptation, and postprobe segments of experiment Q3. Individual examples in A–D are indicated with labeled arrows. Black triangles: probe trials; gray squares: adaptation trials.
In a later adaptation trial (Fig. 2C), the primary gaze shift (40.1°) was smaller than that seen during preadaptation probe trials (Fig. 2A). Note that both the eye movement (33.6°) and head contribution (6.5°) amplitudes were smaller compared with movements made before or at the beginning of adaptation (Fig. 2, A and B); the gaze shift in Fig. 2C was also followed by a small corrective saccade. During the last adaptation trial presented in this experimental session, gaze shift amplitude was 35.7° (Fig. 2D), nearly 10° shorter than gaze shifts to T1 before adaptation. In this example, saccade amplitude was 31.3° and the contribution of the head was 4.3°. Eye movement amplitudes changed from 35.1 to 31.3° and changes in head contribution from 9.6 to 4.3° during the course of adaptation.
All movements to T1 made during this session (Q1) are shown in Fig. 2, E–H. Gaze shift amplitude is plotted as a function of trial number in Fig. 2E. Triangles indicate trials during which T1 was turned off when the gaze shift was initiated and no other targets were illuminated (“probe” trials). For all movements illustrated, the eyes and head initially were aligned (mean eye position at gaze shift onset = 0.1 ± 1.1°). Before adaptation trials were presented (“preadaptation”) mean gaze shift amplitude was 46.6 ± 1.8°. The trial indicated with the down-directed arrow (A) corresponds to the trial shown in Fig. 2A. During these gaze shifts, saccadic eye movements (Fig. 2F) made up about 83% of the change in gaze direction (saccade amplitudes were 38.7 ± 1.7°); the head contributed (Fig. 2G) the remaining 17% of gaze shift amplitude (7.8 ± 1.0°). As shown in Fig. 2, A–D, the head continued to move for several hundred milliseconds after the gaze shift ended and, as a result, the total amplitude of head movement (Fig. 2H) was much larger than the head contribution to the gaze shift (32.1 ± 2.1°).
Referring to the adaptation session illustrated in Fig. 2, after 350 control trials, adaptation trials were introduced (gray squares). These trials were identical to the ongoing “probe” trials except that after the movement is begun and T1 turned off, a second target (T2; in this example located between T1 and T0) is illuminated (Fig. 1C). Recall that during the entire session, probe trials to targets other than the T1 target were randomly interleaved with adaptation trials as were probe trials to the T1 location. As adaptation trials were presented, the amplitude of gaze shifts to the T1 target gradually became smaller, as did gaze shifts during probe trials to the same location (black triangles). Arrows labeled B–D indicate individual trials shown in Fig. 2, B–D. During the final 15 adaptation trials gaze shift amplitude was reduced from about 47 to 33.6° (±2.8°). Saccade amplitude (Fig. 2F; 28.1 ± 2.8°), head contribution (Fig. 2G; 5.5 ± 0.7°), and total head movement amplitude (Fig. 2H; 20.2 ± 4.2°) were also smaller after adaptation.
At this point adaptation trials were stopped. However, probe trials to the T1 location continued (“probe only”). There is no visual feedback at the end of probe trials and movements to this target under these conditions continued to be much smaller compared with preadaptation probe trials. Subsequently, visual feedback was reintroduced (“recovery”) and gaze, eye, and head movement amplitudes progressively increased.
During a different session (Q3: “forward adaptation”), T1 was located 26° away from the fixation target (T0) and T2 was located 50° away. Figure 3 presents details of this session (layout the same as in Fig. 2). Figure 3, A–D illustrates gaze, eye, and head positions plotted as functions of time for four movements at different points (indicated with arrows in Fig. 3, E–H) during the adaptation. Figure 3A illustrates a preadaptation trial in which primary gaze amplitude was 27.6°, saccade amplitude was 24.5°, and head contribution was 3.1°. The first adaptation trial (Fig. 3B) was similar to those made during preadaptation probe trials (gaze = 27.3°, eye = 24.6°, head contribution = 2.7°), the one notable difference being the large “corrective” movement that followed the primary gaze shift by about 300 ms. Also shown are trials part way through (Fig. 3C) and at the end of the adaptation session (Fig. 3D). A clear increase in gaze shift amplitude occurred during adaptation (Fig. 3E). Average gaze shift amplitude increased 13.1° over the course of adaptation. Eye movement amplitude increased by 8.7° (Fig. 3F) and head contribution increased by 4.4° (Fig. 3G). Although total head movement amplitude (Fig. 3H) also increased during adaptation trials compared with head movement amplitude before adaptation, variability was quite high during this session and the increase appeared to be stepwise rather than progressive.
The backward (Fig. 2) and forward (Fig. 3) adaptation sessions illustrate the changes in gaze shift amplitudes observed during adaptation and indicate that these can be mediated by changes in both the amplitude of the saccadic portion of the gaze shift and concomitant changes in the contribution of the head. Figure 4 summarizes the differences in gaze, eye, head contribution, and total head movement amplitude during adaptation for all backward (Fig. 4, A–D) and forward (Fig. 4, E–H) sessions when gaze shifts began with the eyes and head aligned. Positive values indicate an increase and negative values indicate a decrease in movement amplitude. Black bars compare preadaptation amplitudes with amplitudes during the last 15 adaptation trials; gray bars compare movement amplitudes before adaptation with the first 15 probe trials during the “probe-only” period.
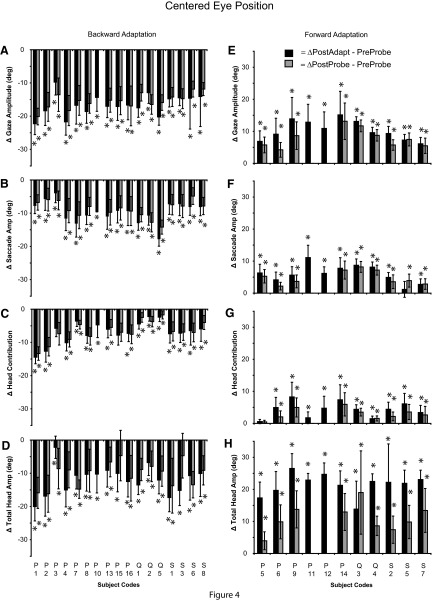
FIG. 4.
Summary of the change in gaze (A, E), saccade (B, F), head contribution (C, G), and total head amplitude (D, H) for gaze shifts initiated from the centered eye position from 28 sessions (17 backward and 11 forward). The difference (±SD) between the preadaptation probe mean and the postadaptation mean (black bars) or postprobe mean (gray bars) is illustrated. (*) denotes a change in movement amplitude between pre- and postmeans (t-test, P < 0.05).
The mean change in gaze amplitude for all 17 backward adaptation sessions (Fig. 4A) was −16.5 ± 3.1° (range: −9.7 to −22.3°). This decrease was mediated by significant decreases in head contribution (mean Δ = −6.8 ± 3.2°; range: −2.2 to −14.4°) and saccade amplitude (mean Δ = −9.7 ± 3.2°; range: −4.0 to −17.8°); decreases in gaze amplitude were accompanied by a decrease in total head movement amplitude (mean Δ = −11.9 ± 4.2°; range: −2.2 to −20.0°). Similar amplitude changes in gaze, saccade, head contribution, and total head movement were observed when comparing pre- and postadaptation probe trials to T1 (gray bars; Fig. 4, A–D).
During forward adaptation, gaze amplitude increased by 10.3 ± 3.0° (range: 6.9–15.1°). In the majority of cases, increases in gaze amplitude were mediated by significant increases in head contribution (mean Δ = 4.3 ± 2.4°; range: 0.6–8.3°), saccade amplitude (mean Δ = 6.1 ± 2.8°; range: 1.2–11.1°), and a large increase in total head amplitude (mean Δ = 21.5 ± 3.4°; range: 13.9–26.5°). Similar amplitude changes in gaze, saccade, and head contribution were observed when comparing pre- and postadaptation probe trials to T1 (gray bars; Fig. 4, E–H).
Eyes and head not aligned
The results discussed thus far describe the changes in gaze, eye, and head movements during backward and forward head-unrestrained gaze adaptation when the eyes and head were initially aligned. The results indicate that changes in gaze shift amplitude arise from changes in both eye and head components of head-unrestrained visual-orienting movements. This suggests that the gaze amplitude changes observed during adaptation may be due to changes in the gaze displacement command. However, parametric changes in both eye and head components of gaze remain a plausible alternative that could account for these results. To attempt to address this latter possibility, gaze shifts were initiated with the eyes in different positions relative to the head. Figure 5 plots gaze, saccade, head contribution, and total head amplitude data from gaze shifts initiated with the eyes directed to the left in the orbits (Fig. 5, A–D; mean IEP = −14.9 ± 2.1°). During the same adaptation session (Q1), on randomly interleaved trials, gaze shifts were also initiated with the eyes deviated to the right in the orbits (Fig. 5, E–H; mean IEP = 12.5 ± 1.0°). In each panel, data are plotted as a function of the actual trial number during the session. Recall that all data are plotted as if all gaze shifts were rightward. During this same adaptation session, additional trials initiated with the eyes and head aligned were also randomly presented (Fig. 2).
FIG. 5.
Gaze, saccade, head contribution, and total head amplitudes of the primary gaze shifts made during preadaptation, adaptation, postprobe, and recovery segments of backward adaptation experiment Q1. A–D: data from gaze shifts in which the eyes were deviated leftward (head deviated toward T1) in the orbits (mean initial eye position [IEP] = −14.9 ± 2.0°). E–H: data from gaze shifts in which the eyes were deviated rightward (head deviated away from T1) in the orbits (mean IEP = 12.5 ± 1.0°). Black triangles: probe trials; gray squares: adaptation trials; open diamonds: recovery trials.
When the eyes began deviated to the left, the majority of the preadaptation gaze shifts (Fig. 5A) to T1 were accomplished by large saccadic eye movements (Fig. 5B) accompanied by relatively small head movements (Fig. 5D); the head contribution to these gaze shifts was <5° (Fig. 5C). During adaptation, gaze amplitude decreased similarly during randomly interleaved adaptation (Fig. 5A, gray squares) and probe trials (Fig. 5A, black triangles) toward T1. The decrease in gaze amplitude was largely a result of reduced saccade amplitudes. Note in Fig. 5C that head contribution (already quite small) declined very little during adaptation. There was, however, a clear reduction in the overall head movement amplitudes throughout the course of adaptation (Fig. 5D). After adaptation, when only probe trials were presented, amplitudes of gaze, eye, and head remained at the reduced, adapted levels.
In contrast, when the eyes began deviated to the right, gaze shifts in the preadaptation phase (Fig. 5E) were accomplished by a smaller saccadic eye movement (Fig. 5F) and larger head contribution (Fig. 5G) than those initiated with the eyes to the left. Total head movement (Fig. 5H) was also significantly larger when the eyes began deviated to the right. During the adaptation phase, saccade, head contribution, and total head amplitude each decreased significantly. Similar to the centered (Fig. 2) and leftward eye position data described earlier, these changes were maintained during an epoch after adaptation where the subject performed only probe trials. Furthermore, the appropriate changes to eye and head movements from each of these eye positions were made during the recovery phase to produce primary gaze amplitudes that approached preadaptation amplitude values.
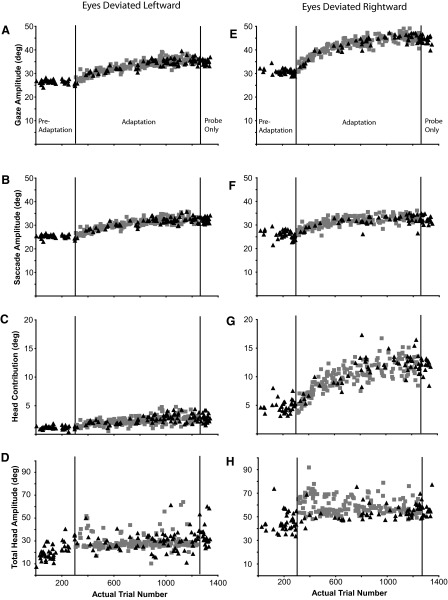
Figure 6 plots gaze, saccade, head contribution, and total head amplitude data from gaze shifts initiated from the leftward (Fig. 6, A–D; mean IEP = −13.0 ± 1.1°) and rightward (Fig. 6, E–H; mean IEP = 14.1 ± 1.4°) eye positions as a function of actual trial number for a single forward adaptation experiment (Q3). These data are from the same experiment used to illustrate changes to these components from the centered eye position (Fig. 3). Primary gaze shifts initiated from the leftward eye position were slightly (∼3°), but not significantly, smaller than gaze shifts initiated from the rightward eye position. Nonetheless, gaze amplitude increased consistently for both sets of movements during the adaptation phase. Similar to the preadaptation gaze shifts made in the backward adaptation experiment (Q1; Fig. 5), preadaptation gaze shifts to the T1 target initiated from the leftward eye position were primarily the result of a saccadic eye movement (Fig. 6B). The head did move during the preadaptation phase (Fig. 6D), but contributed very little to these gaze shifts (Fig. 6C). During the adaptation phase, changes in primary gaze amplitude (Fig. 6A) were accomplished by changes in saccade amplitude (Fig. 6B) and a very small, but significant, change in head contribution (Fig. 6C). In contrast, when gaze shifts began with the eyes deviated to the right, changes in gaze amplitude (Fig. 6E) were accomplished by increases in both saccade (Fig. 6F) and head contribution (Fig. 6G).
FIG. 6.
Gaze, saccade, head contribution, and total head amplitudes of the primary gaze shifts made during preadaptation, adaptation, and postprobe segments of backward adaptation experiment Q3. A–D: data from gaze shifts in which the eyes were deviated leftward (head deviated toward T1) in the orbits (mean IEP = −13.0 ± 1.1°). E–H: data from gaze shifts in which the eyes were deviated rightward (head deviated away from T1) in the orbits (mean IEP = 14.7 ± 1.0°). Black triangles: probe trials; gray squares: adaptation trials.
For all experiments, the changes to gaze, saccade, head contribution, and total head amplitude that occurred when the eyes and head are not aligned during backward and forward adaptation experiments are illustrated in Figs. 7 and 8, respectively. The black bars in each of these figures represent the mean difference (±SD) between preadaptation probes and adaptation trials, whereas gray bars represent the mean difference (±SD) between preadaptation probes and postprobe means.
FIG. 7.
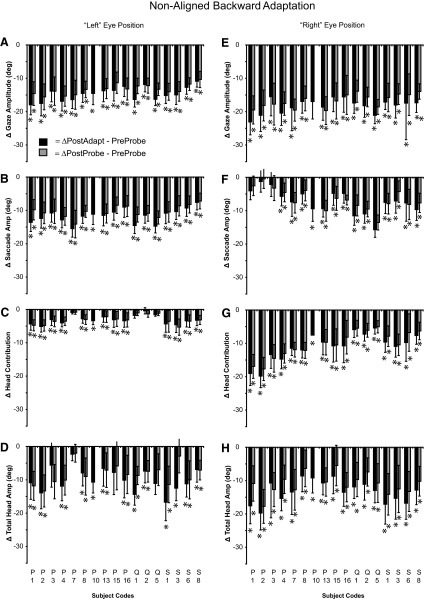
Summary of the change in gaze (A, E), saccade (B, F), head contribution (C, G), and total head amplitude (D, H) for gaze shifts initiated with the eyes and head not aligned during backward adaptation experiments. Data were extracted from the same 28 sessions shown in Fig. 4. The difference (±SD) between the preadaptation probe mean and the postadaptation mean (black bars) or postprobe mean (gray bars) is illustrated. (*) denotes a change in movement amplitude between pre- and postmeans (t-test, P < 0.05).
FIG. 8.
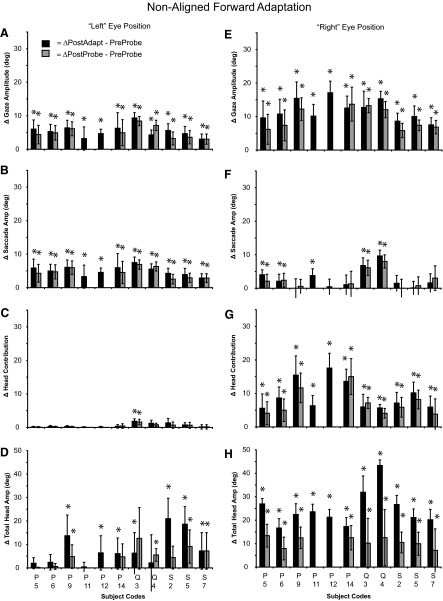
Summary of the change in gaze (A, E), saccade (B, F), head contribution (C, G), and total head amplitude (D, H) for gaze shifts initiated with the eyes and head not aligned during forward adaptation experiments. Data were extracted from the same 11 sessions shown in Fig. 4, E–H. The difference (±SD) between the preadaptation probe mean and the postadaptation mean (black bars) or postprobe mean (gray bars) is illustrated. (*) denotes a change in movement amplitude between pre- and postmeans (t-test, P < 0.05).
During backward adaptation when gaze shifts were initiated with the eyes deviated to the left in the orbits (Fig. 7, A–D), mean gaze and saccade amplitudes decreased significantly in every experiment regardless of the mean comparison (preprobe vs. either adaptation or postprobe mean). Head contribution decreased significantly in 13 of 17 experiments during adaptation trials (black bars) and 12 of 16 experiments during adapt-probe trials (gray bars). Total head movement decreased significantly in 13 of 17 experiments during adaptation trials and 11 of 16 probe trials.
During backward adaptation when gaze shifts were initiated with the eyes deviated to the right in the orbits (Fig. 7, E–H), mean gaze and head contribution amplitude decreased significantly in every case regardless of the mean comparison. Postadaptation mean saccade amplitude decreased significantly in 13 of 17 experiments and postprobe mean saccade amplitude decreased significantly in 12 of 16 experiments. Both postadaptation mean head contribution and total head amplitude decreased significantly in every experiment.
Figure 8 (A–D) illustrates the changes in gaze, saccade, head contribution, and total head movement amplitude during forward adaptation when gaze shifts were initiated with the eyes deviated to the left. As shown, postadaptation and postprobe mean gaze and saccade amplitudes increased significantly in every experiment regardless of mean comparison. However, postadaptation mean head contribution (Fig. 8C) increased significantly in only one experiment (Q3). Mean total head amplitude increased in 7 of 11 experiments during adaptation trials.
When the eyes began deviated to the right during forward adaptation experiments (Fig. 8, E–H), gaze amplitude (Fig. 8E), head contribution (Fig. 8G), and total head movement increased significantly during both adaptation (11/11) and probe trials (9/9). In half of the experiments, saccade amplitude increased during both adaptation and probe trials.
Head-unrestrained transfer experiments
In our subjects, changes to eye and head movements depended on the magnitude of gaze adaptation and the orbital positions of the eyes at gaze onset. However, it could be argued that initial eye position provided a sufficient context (Alahyane and Pelisson 2004) so that different combinations of eye–head changes were simultaneously specified during adaptation from each starting position. The alternative assumes that a gaze displacement command is altered and, in this case, initial eye position acts not as a context cue but instead determines the relative contributions of the eyes and head to a gaze shift of specific amplitude and direction. In the latter case, gaze amplitude adapted from a small range of eye positions should transfer to gaze shifts initiated from significantly different eye positions, whereas in the former, they should not.
Figure 9 illustrates data from an example backward adaptation transfer experiment (P5). In the preadaptation phase of all transfer experiments, subjects initiated gaze shifts from all three eye positions. As illustrated, monkey P produced accurate gaze shifts toward the T1 target located 50° to the right of T0 (Fig. 9A, “preadaptation”). These gaze shifts were composed of different eye and head movements depending on IEP (Fig. 9, B–D). For instance, when the eyes began centered in the orbits (green) 50° gaze shifts were composed of 35° saccadic eye movements and head contributions of 15°. However, head contribution was 30° and saccade amplitudes 20° when the eyes began deviated to the right (red). When deviated to the left (blue), eye movement amplitudes were 45° and head contributions were 5°.
FIG. 9.
Gaze transfer experiment (P4). Gaze shifts were initiated from all 3 eye positions in the preadaptation phase. During the initial adaptation phase gaze shifts were initiated only from the leftward eye position (blue). After about 300 trials novel eye positions (centered: green; rightward: red) were introduced. Note that the gaze amplitude at the end of the initial adaptation and the novel eye position phases is similar; however, the saccade, head contribution, and total head movements are not.
In the example shown in Fig. 9, adaptation trials were initiated with the eyes in the same starting positions (deviated to the left in the orbits in this example). A significant decrease in gaze amplitude during this epoch occurred as a result of a large decrease in saccade amplitude and a very small decrease in head contribution and total head amplitude. At the beginning of the “novel eye position” epoch, gaze shifts were initiated from the remaining two IEPs (centered and rightward eye positions in this case). Two key observations can be made. First, gaze amplitude at the beginning of the “novel eye position” phase is the same as that at the end of the “initial adaptation position” phase [before transition = 32.2 ± 1.9°; after transition = 31.9 ± 2.7° (eyes right) and 32.6 ± 2.5° (eyes centered)]. In contrast, saccade amplitude was significantly smaller and head contribution (and total head movement) was significantly larger when initiated from the centered and rightward eye positions compared with the initially adapted leftward position. The change in gaze amplitude, produced during adaptation with the eyes starting in one position, transferred immediately and completely to gaze shifts made with the eyes in different positions. Eye and head movement amplitudes, however, were very different under the different conditions.
Similar observations were made in each of our 13 transfer experiments (8 backward; 5 forward). The metrics of gaze shifts before and after transfer were assessed using the following ratios:
-
Gaze ratio = GIEP2/GIEP1
Eye ratio = EIEP2/EIEP1
Head contribution ratio = HCIEP2/HCIEP1
Total head movement ratio = THIEP2/THIEP1
where IEP1 is the initial eye position from which all gaze shifts began during the initial adaptation epoch and IEP2 indicates a novel eye position introduced in the novel eye position epoch. Mean pre- and posttransfer values were calculated using data from the 15 trials before (data just before vertical black line in Fig. 9) or after (data just after vertical black line) transfer, respectively. Supplementary Table S1 contains the gaze, eye, head contribution, and total head ratios and mean amplitudes (±SD) from these epochs for all 13 of our transfer experiments.2 Although there was some variation between experiments, the average gaze ratio across all conditions was 1.02 ± 0.13° (n = 26). However, eye and head movement amplitudes (both total head movement and head contribution) in the postadaptation phase were different and varied in an initial eye-dependent fashion.
Rate of gaze adaptation
For each adaptation experiment, we assessed the rate of gaze adaptation by fitting a plot of primary gaze amplitude versus adaptation trial number with either an exponential growth or decay function (see methods for details). The trial constant (analogous to a rate constant) and R2 values from each experiment are listed in Supplementary Table S2. Trial constants varied between and within animals during both forward and backward experiments. For example, during backward adaptation, trial constants ranged from 25 (P7) to 500 (S1) adaptation trials and trial constants for monkey S ranged from 100 to 500 adaptation trials. Similar ranges and variability can been noted for forward adaptation experiments. Average trial constants for backward and forward adaptation were 115.7 ± 117.0 and 236.9 ± 139.1, respectively.
“Adapted” versus “normal” gaze shifts
We compared the relative contributions of the eyes and head to gaze shifts produced before adaptation (pooled across data sessions) with those made during the last 15 adaptation trials of each data session. Figure 10 plots mean (±SD) saccade (Fig. 10, A–C) and head contribution (Fig. 10, D–F) amplitude of control gaze shifts against those made during adaptation trials for each adaptation experiment. Data were separated into leftward (Fig. 10, A and D), centered (Fig. 10, B and E), and rightward (Fig. 10, C and F) eye positions. Note that mean gaze shift amplitude and initial eye position of control and “adapted” gaze shifts were not significantly different in any of these comparisons (P > 0.05; Mann–Whitney U test). During backward adaptation the relative amplitudes of the eyes and head were consistently different during adaptation trials versus control trials in subjects P and S (P < 0.05; Mann–Whitney U test), but not subject Q. When the eyes were centered or deviated rightward, gaze shifts during adaptation trials had larger head contribution and smaller saccade amplitudes than control movements (gray symbols, Fig. 10, B, C, E, and F). This was not observed for gaze shifts initiated from the leftward eye position in these animals. In contrast, there were no consistent changes in eye–head coordination during forward adaptation experiments in any of our subjects (Fig. 10, black symbols). Total head amplitude was consistently larger during forward adaptation and smaller during backward adaptation for gaze shifts initiated from all three eye positions by monkeys P and S; this trend was not observed for monkey Q (data not shown).
FIG. 10.
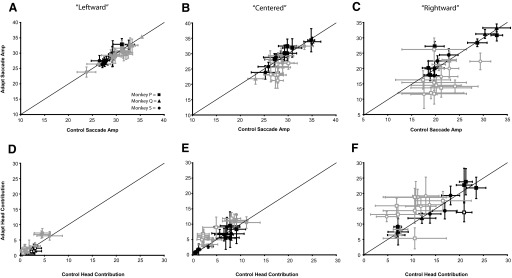
Eye–head coordination during adapted vs. control movements. Gaze shifts were matched for gaze amplitude and initial orbital eye position (“leftward,” “centered,” “rightward”). A–C: mean (±SD) control saccade plotted as a function of saccades during adapted movements. D–F: mean (±SD) control head contribution plotted as a function of head contribution during adapted movements. Data from monkey Q (▴), monkey S (), and monkey P (▪) are shown. Black symbols represent comparisons for forward adaptation experiments. Gray symbols represent comparisons for backward adaptation experiments. Open symbols represent statistically significant differences between adapted and control values (Mann–Whitney U test, P < 0.05).
Comparison with head-restrained adaptation
The general characteristics of head-unrestrained gaze adaptation described earlier are remarkably similar to head-restrained saccadic adaptation using smaller intrasaccade target displacements. However, it was not clear, a priori, that large target displacements during large-amplitude head-restrained saccades would produce the same large changes in amplitude. To address this issue, we collected a small number of head-restrained adaptation data from two monkeys (P and S) for comparison with head-unrestrained data. Figure 11 illustrates examples of head-restrained backward (Fig. 11, A and B) and forward (Fig. 11, C and D) gaze adaptation from each subject. During backward adaptation, T1 was located 50° to the right of the initial fixation point (T0) and T2 was 26° to the right of fixation. Both subjects made slightly hypometric primary gaze shifts toward T1 during probe trials (black triangles) in the preadaptation period (P: 48.1 ± 1.3°, n = 75; Q: 45.9 ± 1.3°, n = 62). Movement amplitudes systematically declined during adaptation such that the amplitudes of the last 15 adaptation (P: 32.2 ± 2.0°; Q: 28.4 ± 1.8°) and probe (P: 31.5 ± 1.3°; Q: 29.5 ± 1.6°) trials were significantly different from preadaptation probe trials. The last 15 adaptation and probe trials of the adaptation epoch were not significantly different from each other (P = 0.48). After adaptation, when only probe trials were presented, both subjects continued to produce movements that were significantly shorter than preadaptation probes (P: 32.4 ± 1.6°, n = 60; Q: 30.4 ± 1.4°, n = 51; P < 0.05), but indistinguishable from either the adaptation or probe trials at the end of the adaptation phase (P > 0.05). As illustrated (Fig. 11, A and B), movement amplitudes returned toward preadaptation amplitudes during recovery. Note that the amplitudes of movements made during probe trials in the recovery period also returned toward preadaptation levels.
FIG. 11.
Large-amplitude, head-restrained saccade adaptation. Summary of primary saccades made during preadaptation, adaptation, postprobe, and recovery segments of backward (A, B) and forward (C, D) adaptation experiments from 2 monkeys (P, Q). Black triangles: probe trials; gray squares: adaptation trials; open diamonds: recovery trials.
Both monkeys increased the size of their primary gaze shifts during forward adaptation (Fig. 11, C and D). In these sessions, T1 was displaced 26° and T2 was displaced 50° to the right of the initial fixation point (T0). Both subjects made fairly accurate movements toward T1 during probe trials (black triangles) in the preadaptation phase (monkey P: 27.0 ± 0.7°, n = 92; monkey Q: 23.2 ± 0.9°, n = 81). Gaze amplitude increased significantly during both adaptation and probe trials by the end of the adaptation epoch (P < 0.05). Gaze shifts during the postprobe epoch were also significantly larger than those in the preadaptation epoch (P < 0.05). Similar observations were made in each of our eight (four backward, four forward) head-restrained, saccade amplitude adaptation experiments (Supplementary Table S3).
In summary, when the head is restrained, large changes in movement amplitudes can occur when the intrasaccadic target step (T2 − T1) is large (∼25°). Furthermore, in accordance with previous accounts of head-restrained saccadic adaptation (Bahcall and Kowler 2000; Deubel 1991; Miller et al. 1981; Noto et al. 1999; Robinson et al. 2003; Scudder et al. 1998; Straube et al. 1997), backward adaptation was larger in magnitude than forward adaptation in response to the same postsaccadic visual error.
Head-restrained to head-unrestrained transfer
Phillips and colleagues (1997) showed that head-restrained saccadic adaptation transfers to head-unrestrained gaze shifts. We sought to replicate and expand their observations while testing the hypothesis that saccade amplitude adaptation results from a modification of a gaze shift command. The preadaptation epoch during each head-restrained to head-unrestrained transfer experiment consisted of two phases. At the beginning of the preadaptation epoch, monkey P produced head-unrestrained gaze shifts to a variety of T1 targets (including the T1 used during adaptation trials; ΔT1 − T0 = 50°). After about 200–300 trials, the subject's head was restrained using a vertical post and clamp such that gaze shifts could be achieved only using movements of the eyes. Between 100 and 150 trials were presented using the same targets as used when the head was unrestrained. The adaptation epoch began with the introduction of intrasaccade back-step trials intermixed with probe trials while the head remained restrained (ΔT1 − T2 = 25°). At the end of the adaptation epoch (∼400–500 trials), the monkey's head was manually released by the experimenter while the subject sat in the dark. The postadaptation phase consisted of only probe trials in which the subject produced movements toward each of the T1 targets presented in the preadaptation epoch. Note that head-unrestrained gaze shifts in the pre- and postadaptation phases always began with the random illumination of one of the three head lasers.
The transfer of head-restrained saccade adaptation to head-unrestrained gaze shifts was quantified by comparing the change in head-restrained saccade amplitude to the change in head-unrestrained gaze amplitude. Comparisons were made for gaze shifts initiated from each of the different eye positions (left, center, right). The ratio of these values (“transfer ratio”) is presented for each experiment and condition (Table 1). Again, note that data are presented as if all gaze shifts were rightward. As shown, the transfer from head-restrained saccades to head-unrestrained gaze shifts was incomplete and varied slightly for gaze shifts initiated from the different eye positions. However, note the following: 1) head-unrestrained gaze shifts after head-restrained adaptation were significantly smaller than preadaptation gaze shifts regardless of the initial starting eye position (gaze ratio); 2) changes in head-unrestrained gaze amplitude between pre- and postadaptation epochs were the result of changes to both eye (eye ratio) and head contribution (head contribution ratio); and 3) total head movement was smaller during the postadaptation epoch than that during the preadaptation epoch regardless of starting eye position (total head ratio). The changes in head contribution and total head movement are not consistent with the hypothesis that only an eye movement command is modified during head-restrained saccadic adaptation. However, they are consistent with a change in a gaze command upstream of the separate eye and head signals.
TABLE 1.
Changes to gaze, saccade, head contribution, and total head amplitude during head-restrained transfer experiments
| Experiment | Eye Position | Transfer Ratio | Gaze Ratio, Δamp | Eye Ratio, Δamp | Head Contribution Ratio, Δamp | Total Head Ratio, Δamp |
|---|---|---|---|---|---|---|
| PA19AUG08 | Left | 0.77 | 0.76 (−11.0)* | 0.83 (−6.5)* | 0.44 (−4.5)* | 0.55 (−11.0)* |
| Center | 0.76 | 0.78 (−10.9)* | 0.87 (−4.1)* | 0.64 (−6.8)* | 0.80 (−9.0)* | |
| Right | 0.68 | 0.82 (−9.7)* | 0.92 (−1.6)* | 0.75 (−8.1)* | 0.84 (−10.0)* | |
| PA26AUG08 | Left | 0.57 | 0.85 (−7.3)* | 0.89 (−4.4)* | 0.58 (−2.9)* | 0.62 (−8.7)* |
| Center | 0.55 | 0.86 (−7.1)* | 0.93 (−2.3)* | 0.72 (−4.8)* | 0.82 (−8.0)* | |
| Right | 0.38 | 0.91 (−4.9)* | 0.95 (−1.2)* | 0.87 (−3.7)* | 0.86 (−8.6)* | |
| PA28AUG08 | Left | 0.66 | 0.81 (−8.8)* | 0.84 (−6.5)* | 0.57 (−2.3)* | 0.87 (−6.5)* |
| Center | 0.57 | 0.85 (−7.5)* | 0.90 (−3.4)* | 0.72 (−4.1)* | 0.77 (−9.0)* | |
| Right | 0.57 | 0.86 (−7.5)* | 0.88 (−3.2)* | 0.84 (−4.3)* | 0.61 (−7.3)* | |
| PA02SEP08 | Left | 0.57 | 0.85 (−7.0)* | 0.87 (−5.4)* | 0.65 (−1.6)* | 0.67 (−5.8)* |
| Center | 0.37 | 0.91 (−4.4)* | 0.90 (−3.4)* | 0.93 (−1.0) | 0.87 (−5.0)* | |
| Right | 0.46 | 0.91 (−5.7)* | 0.89 (−2.9)* | 0.90 (−2.8)* | 0.87 (−7.4)* |
Transfer ratio = ΔHead-unrestrained gaze amplitude/ΔHead-restrained gaze amplitude.
Head-unrestrained comparisons (pre- vs. postprobes; average of all gaze shifts during these epochs):
Gaze ratio = postadaptation gaze amplitude/preadaptation gaze amplitude
Eye ratio = postadaptation eye amplitude/preadaptation eye amplitude
Head contribution ratio = postadaptation head contribution amplitude/preadaptation head contribution amplitude
Total head ratio = postadaptation total head amplitude/preadaptation total head amplitude
Significantly different pre- versus postadaptation amplitudes (two-tailed t-test).
DISCUSSION
In rhesus monkeys, some changes in the line of sight (gaze) are accomplished by coordinated movements of the eyes and head. The ability to adjust the accuracy of gaze shifts in response to persistent visual errors is important for maintaining high acuity vision. One possible adaptive control mechanism adjusts gaze accuracy by modifying a gaze displacement command. Alternatively, changes in gaze accuracy could result from modifications to either of the separate signals used to move the eyes or head. Our results describe the changes in eye and head movements during a short-term gaze adaptation task under conditions in which these alternative hypotheses are dissociable (Cecala and Freedman 2008).
Major findings
The results of our study of rhesus monkey gaze adaptation indicate that large changes in gaze amplitude can be elicited during backward and forward versions of the McLaughlin task regardless of whether the head is restrained or allowed to move. When our subjects' heads were allowed to move, changes in gaze amplitude resulted from changes to both saccade amplitude and head contribution (Figs. 4, 7, and 8). Changes in gaze shift amplitude occurred systematically as a function of the number of adaptation trials presented to each subject. These changes in gaze shift amplitude were mirrored by changes in the amplitude of gaze shifts made during randomly interleaved “probe” trials. Note that during probe trials the T1 target was turned off when the gaze shift was initiated and the T2 target was never illuminated. This reveals some underlying change in the sensorimotor apparatus linking a visual target in a particular spatial location to a movement of a particular amplitude; the movements associated with presentation of T1 were altered. After adaptation when only probe trials were presented, the changes in gaze shift amplitudes that arose during adaptation persisted. Changes in gaze shift amplitudes were necessarily caused by changes in the eye and head movements that lead to changes in the direction of the line of sight. However, as gaze amplitudes were altered during adaptation, the changes in eye and head movement amplitudes varied and were determined by the starting positions of the eyes in the orbits and by the amplitude of the ongoing gaze shift. The clearest demonstration of this point occurs during gaze transfer experiments in which changes in gaze amplitude during the initial adaptation phase resulted from specific changes in eye and head movements. However, when movements were initiated from eye positions not used during adaptation, gaze amplitudes were unaltered but the eye and head movements used to produce these gaze shifts were very different (Fig. 9; Table 1). These data suggest changes to a gaze command signal and are inconsistent with specific changes to separate eye and head movement commands.
Previous findings
Two previous studies involving head-unrestrained primates have concluded that gaze adaptation results from modifying a gaze displacement command signal (Cecala and Freedman 2008; Phillips et al. 1997). The study by Phillips et al. (1997) emphasized the transfer of head-restrained saccade amplitude adaptation to head-unrestrained gaze shifts produced by rhesus monkeys. Phillips and colleagues were successful in reducing 50° head-restrained saccades by back-stepping a target by 20°. When the head was subsequently allowed to move, the induced changes in gaze amplitude persisted and were shown to be a result of changes in both eye and head movement amplitudes. The authors concluded that these changes were likely due to changes in a gaze-related command. However, they could not rule out the possibility that the observed changes in amplitude were a result of specific changes to separate eye and head movement commands. To rule out this alternative, movements must be initiated with the eyes and head in different positions.
Phillips and colleagues (1997) described an average of 81% transfer from head-restrained to head-unrestrained gaze shifts (n = 8). It is not clear why our head-restrained to head-unrestrained gaze transfer values are less (average across all conditions = 58%). One possible explanation for our observation is the manual interaction between experimenter and subject when switching from head-restrained to head-unrestrained after the adaptation epoch; Phillips and colleagues released their subject's head remotely thus not interrupting the subject's performance (J. O. Phillips, personal communication). However, the rest of our observations are consistent with those of Phillips and colleagues.
Using a paradigm similar to that used in the current report, we have shown that large changes in gaze amplitude can be elicited in head-unrestrained humans (Cecala and Freedman 2008). In this study of human gaze adaptation, preadaptation gaze shifts of about 30° (forward adaptation) and about 60° (backward adaptation) were adjusted using a 30° intragaze target displacement. On average, the 30° visual error was reduced by about 50% during backward adaptation and by about 40% during forward adaptation. In the current study, monkey subjects adjusted gaze amplitude by amounts similar to human subjects when normalized to the 25° back- or forward-stepped target (backward mean: −16.5 ± 2.8° or 66%; forward mean: 9.2 ± 3.8° or 37%). Also, similar to the head-unrestrained transfer experiments in the current study, when gaze amplitude is altered from a small range of orbital eye positions in human subjects, the magnitude of gaze change transfers to other novel eye positions and the contribution of the eyes and head to gaze shifts in the novel eye position epoch are markedly different from those in the initial adaptation phase.
The rate of head-unrestrained gaze adaptation has never been quantified. Gaze adaptation (both head-restrained and head-unrestrained) in our subjects followed an exponential time course (Figs. 2, 3, 5, 6, 9, and 11) like that noted in previous head-restrained saccadic adaptation studies (e.g., Straube et al. 1997). Thus the ability to produce changes in gaze movement amplitude using the McLaughlin task appears to be similar regardless of the displacement of the targets and independent of whether the head is free to move (Cecala and Freedman 2008).
Differences in eye–head coordination
Phillips and colleagues (1997) observed no differences in eye–head coordination when comparing equal-amplitude gaze shifts before and after adaptation. In the current report, we compared the relative contribution of the eyes and head to gaze shifts made at the end of each adaptation epoch with those produced during control trials. When gaze shifts were matched for amplitude, initial eye position, and direction we consistently observed increases (forward adaptation) or decreases (backward adaptation) in total head amplitude in two of our three subjects (P and S). We also observed alterations in eye–head coordination in these monkeys during backward adaptation from particular eye positions (“centered” and “rightward”) where the head was most likely to contribute substantially to gaze shifts. Typically, saccade amplitude was smaller and head contribution was larger compared with matched control movements. However, as illustrated in the data from monkey Q, alterations to the normal eye–head coordination and total head movement need not accompany decreases in gaze amplitude during backward adaptation. Furthermore, consistent changes in eye–head coordination were not observed during forward adaptation in any of our subjects.
It is not entirely clear that the differences in eye–head coordination observed in monkeys P and S are unique to the adaptive process. The spatial and temporal predictability of the target and gaze shift amplitude has been shown to influence the relative contribution of the eyes and head to gaze shifts (Moschner and Zangemeister 1993; Zangemeister and Stark 1982). We attempted to reduce our subjects' ability to predict target location by intermixing probe trials in a variety of locations with adaptation trials during the adaptation epoch. However, during 75% of the trials our subjects produced movements toward the same T1 location. We cannot rule out the possibility that the differences in eye–head coordination are a spurious result caused by a highly predictable target location.
Physiological implications
Our data are consistent with the hypothesis that a gaze signal is modified prior to its separation into commands to move the eyes and head. Evidence gathered from microstimulation (Freedman and Sparks 1996), inactivation (Walton et al. 2008), and single-unit recording (Freedman and Sparks 1997b) experiments in rhesus monkeys supports a hypothesis that activity in the primate superior colliculus (SC) encodes a gaze-displacement command that is parsed downstream into commands to move the eyes and/or head. For example, suprathreshold stimulation of the deeper layers of the SC produces contraversive gaze shifts whose metrical relationships are comparable to visually guided gaze shifts matched for amplitude, direction, and initial eye position (Freedman and Sparks 1996). Two alternative hypotheses describing collicular activity could account for the change in gaze output we observed during the McLaughlin paradigm. First, as gaze amplitudes change, the locus of SC motor activity could change systematically. In this case, the motor output of the SC specifies the motor output that is actually observed. Alternatively, the locus of SC motor activity could remain unchanged, thereby specifying a change in the line of sight toward the first visual target presented to the subject (T1). In the latter case, the collicular command must be altered “downstream” of the SC to account for the systematic change in gaze amplitude observed during adaptation.
To our knowledge, the SC has been studied only during head-restrained adaptation. Frens and van Opstal (1997) recorded single units from the deep layers of the SC during backward adaptation. These authors reported that about 60% of units recorded did not change activity throughout the adaptation epoch even though saccade amplitude decreased. These results, along with those of microstimulation experiments (Edelman and Goldberg 2000; Meils and van Gisbergen 1996), suggest a modification to collicular output occurs downstream from the SC. In contrast, Takeichi and colleagues (2007) recently described modifications to the movement fields of SC units that correlated with changes in saccade amplitude, which they interpreted as the gaze command being altered at the level of the SC (or its afferents) during adaptation. Interestingly, when Takeichi and colleagues compared the burst metrics of units recorded during backward adaptation in a fashion similar to that used by Frens and van Opstal (1997), the majority (79%) of cells “showed no change in the number of spikes at the desired saccade size after adaptation.” Note that the somewhat mixed results from these studies may be due to the relatively small target displacements used to produce saccadic adaptation. The small changes in saccade amplitude in response to these target steps may have resulted in difficulty dissociating changes in SC neuronal burst metrics from noise. The majority of evidence suggests that SC activity during adaptation represents a desired displacement signal toward T1 during saccadic adaptation and that the SC command must be modified downstream of the SC to account for changes in gaze amplitude.
The medioposterior cerebellum has been implicated in the adaptive control of saccadic eye movements (for review see Robinson and Fuchs 2001). The deeper layers of the SC are connected to the medioposterior cerebellum (caudal fastigial nucleus and oculomotor vermis; Ohtsuka and Noda 1990) via nucleus reticularis tegmenti pontis (NRTP; May et al. 1990; Ohtsuka and Noda 1990) and dorsal lateral pontine nucleus (Thier and Mock 2005). Lesions to the medioposterior cerebellum impair rapid saccade amplitude adaptation during the McLaughlin task (Barash et al. 1999; Robinson et al. 2002; Takagi et al. 2000) and the burst metrics of neurons in NRTP (Takeichi et al. 2005), caudal fastigial nucleus (Inaba et al. 2003; Scudder and McGee 2003), and oculomotor vermis (Catz et al. 2005, 2008; Soetedjo and Fuchs 2006) are altered along with saccade amplitude during head-restrained saccadic adaptation. Future studies are required to classify the types of motor command signals (gaze, eye, or head) represented at each level of this circuit prior to describing modifications to these signals during gaze adaptation using the McLaughlin task.
In summary, reliable changes in gaze amplitude can be elicited from the rhesus monkey using the McLaughlin (1967) task. These changes are the result of modifications to both eye and head movements that are dependent on the magnitude of change in gaze amplitude and the positions of the eyes at gaze shift onset. This result along with results of our transfer experiments suggest that a gaze displacement command signal upstream of those used to drive separately the eyes and the head is altered systematically during gaze adaptation.
GRANTS
This work was supported in part by National Eye Institute Grant EY-13239.
Supplementary Material
Acknowledgments
We thank G. Parker for technical support, G. Rivlis for continuous efforts in maintaining and upgrading data acquisition software, and Drs. S. Quessy, J. Quinet, and M. Walton for valuable comments on an earlier version of this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Gaze/eye onset was defined as the time at which velocity exceeded 30 and 5,000°·s−1·s−1 acceleration. Gaze/eye offset was defined as the time at which velocity fell below 30 and 5,000°·s−1·s−1. Head movement onset and offset were defined using 25 and 10°/s and 5,000 and 200°·s−1·s−1 acceleration/deceleration criteria, respectively.
The online version of this article contains supplemental data.
REFERENCES
- Alahyane 2004.Alahyane N, Pelisson D. Eye position specificity of saccadic adaptation. Invest Ophthalmol Vis Sci 45: 123–130, 2004. [DOI] [PubMed] [Google Scholar]
- Albano 1996.Albano J Adaptive changes in saccade amplitude: oculocentric or orbitocentric mapping. Vision Res 36: 2087–2098, 1996. [DOI] [PubMed] [Google Scholar]
- Bahill 1975.Bahill AT, Clark MR, Stark L. The main sequence, a tool for studying human eye movements. Math Biosci 24: 191–204, 1975. [Google Scholar]
- Baloh 1975.Baloh RW, Sills AW, Kumley WE, Honrubia V. Quantitative measurement of saccade amplitude, duration, and velocity. Neurology 25: 1065–1070, 1975. [DOI] [PubMed] [Google Scholar]
- Barash 1999.Barash S, Melikyan A, Sivakov A, Zhang M, Glickstein M, Their P. Saccadic dysmetria and adaptation after lesions of the cerebellar cortex. J Neurosci 19: 10931–10939, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker 1972.Becker W The control of eye movements in the saccadic system. Bibl Ophthalmol 82: 233–243, 1972. [PubMed] [Google Scholar]
- Becker 1989.Becker W Metrics. In: The Neurobiology of Saccadic Eye Movements, edited by Wurtz R, Goldberg ME. Amsterdam: Elsevier, 1989, p. 13–67. [PubMed]
- Becker 1969.Becker W, Fuchs AF. Further properties of the human saccadic syste: eye movements and correction saccadic with and without visual fixation points. Vision Res 9: 1247–1258, 1969. [DOI] [PubMed] [Google Scholar]
- Catz 2005.Catz N, Dicke PW, Their P. Cerebellar complex spike firing is suitable to induce as well as to stabilize motor learning. Curr Biol 15: 2179–2189, 2005. [DOI] [PubMed] [Google Scholar]
- Catz 2008.Catz N, Dicke PW, Their P. Cerebellar-dependent motor learning is based on pruning a Purkinje cell population response. Proc Natl Acad Sci USA 105: 7309–7314, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecala 2008.Cecala AL, Freedman EG. Amplitude changes in response to target displacements during human eye–head movements. Vision Res 48: 149–166, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collewijn 1977.Collewijn H Eye- and head movements in freely moving rabbits. J Physiol 266: 471–498, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delreux 1991.Delreux V, Abeele SV, Lefevre P, Roucoux A. Eye–head coordination: influence of eye position on the control of head movement amplitude. In: Brain and Space, edited by Paillard J. London: Oxford Univ. Press, 1991, p. 38–48.
- Deubel 1987.Deubel H Adaptivity of gain and direction in oblique saccades. In: Eye Movements: From Physiology to Cognition, edited by O'Regan J, Levy-Schoen A. Amsterdam: Elsevier, 1987, p. 181–191.
- Deubel 1991.Deubel H Adaptive control of saccade metrics. In: Presbyopia Research, edited by Obrecht G, Stark L. New York: Plenum Press, 1991, p. 93–100.
- Deubel 1995.Deubel H Is saccadic adaptation context-specific? In: Eye Movement Research: Mechanisms, Processes and Applications, edited by Findlay J, Kentridge R, Walker R. Amsterdam: Elsevier, 1995, p. 177–187.
- Deubel 1986.Deubel H, Wolf W, Hauske G. Adaptive gain control of saccadic eye movements. Hum Neurobiol 5: 245–253, 1986. [PubMed] [Google Scholar]
- Edelman 2002.Edelman J, Goldberg M. Effect of short-term saccadic adaptation on saccades evoked by electrical stimulation of the primate superior colliculus. J Neurophysiol 87: 1915–1923, 2002. [DOI] [PubMed] [Google Scholar]
- Freedman 2005.Freedman EG Head–eye interactions during vertical gaze shifts made by rhesus monkeys. Exp Brain Res 167: 557–570, 2005. [DOI] [PubMed] [Google Scholar]
- Freedman 1997a.Freedman EG, Sparks DL. Eye–head coordination during head-unrestrained gaze shifts in rhesus monkey. J Neurophysiol 77: 2328–2348, 1997a. [DOI] [PubMed] [Google Scholar]
- Freedman 1997b.Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol 78: 1669–1690, 1997b. [DOI] [PubMed] [Google Scholar]
- Freedman 1996.Freedman EG, Stanford TR, Sparks DL. Combined eye–head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol 76: 927–952, 1996. [DOI] [PubMed] [Google Scholar]
- Frens 1994.Frens M, van Opstal A. Transfer of short-term adaptation in human saccadic eye movements. Exp Brain Res 100: 293–306, 1994. [DOI] [PubMed] [Google Scholar]
- Frens 1997.Frens M, van Opstal A. Monkey superior colliculus activity during short-term saccadic adaptation. Brain Res Bull 43: 473–483, 1997. [DOI] [PubMed] [Google Scholar]
- Fuchs 1996.Fuchs A, Reiner D, Pong M. Transfer of gain changes from targeting to other types of saccade in the monkey: constraints on possible sites of saccadic gain adaptation. J Neurophysiol 74: 2522–2535, 1996. [DOI] [PubMed] [Google Scholar]
- Fuchs 1985.Fuchs AF, Kaneko CR, Scudder CA. Brainstem control of saccadic eye movements. Annu Rev Neurosci 8: 307–337, 1985. [DOI] [PubMed] [Google Scholar]
- Gonshor 1973.Gonshor A, Melvill Jones G. Changes of human vestibuloocular response induced by vision-reversal during head rotation. J Physiol 234: 102P–103P, 1973. [PubMed] [Google Scholar]
- Guitton 1987.Guitton D, Volle M. Gaze control in humans: coordination during orienting movements to targets within and beyond the oculomotor range. J Neurophysiol 427–459, 1987. [DOI] [PubMed]
- Henson 1978.Henson D Corrective saccades: effects of altering visual feedback. Vision Res 18: 63–67, 1978. [DOI] [PubMed] [Google Scholar]
- Henson 1979.Henson D Investigation into corrective saccadic eye movements for refixation amplitudes of 10 degrees and below. Vision Res 19: 57–61, 1979. [DOI] [PubMed] [Google Scholar]
- Hopp 2004.Hopp JJ, Fuchs AF. The characteristics and neuronal substrate of saccadic eye movement plasticity. Prog Neurobiol 72: 27–53, 2004. [DOI] [PubMed] [Google Scholar]
- Hopp 2006.Hopp JJ, Fuchs AF. Amplitude adaptation occurs where a saccade is represented as a vector and not as its components. Vision Res 46: 3121–3128, 2006. [DOI] [PubMed] [Google Scholar]
- Hyde 1959.Hyde JE Some characteristics of voluntary human ocular movements in the horizontal plane. Am J Ophthalmol 48: 85–94, 1959. [DOI] [PubMed] [Google Scholar]
- Inaba 2003.Inaba N, Iwamoto Y, Yoshida K. Changes in cerebellar fastigial burst activity related to saccadic gain adaptation in the monkey. Neurosci Res 46: 359–368, 2003. [DOI] [PubMed] [Google Scholar]
- Judge 1980.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980. [DOI] [PubMed] [Google Scholar]
- Kahlon 1996.Kahlon M, Lisberger SG. Coordinate system for learning in the smooth pursuit eye movements of monkeys. J Neurosci 16: 7270–7283, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima 2008.Kojima Y, Iwamoto Y, Robinson FR, Noto CT, Yoshida K. Premotor inhibitory neurons carry signals related to saccade adaptation in the monkey. J Neurophysiol 99: 220–230, 2008. [DOI] [PubMed] [Google Scholar]
- Kojima 2005.Kojima Y, Iwamoto Y, Yoshida K. Effect of saccadic amplitude adaptation on subsequent adaptation of saccades in different directions. Neurosci Res 53: 404–412, 2005. [DOI] [PubMed] [Google Scholar]
- Kowler 1995.Kowler E, Blaser E. The accuracy and precision of saccades to small and large targets. Vision Res 35: 1741–1754, 1995. [DOI] [PubMed] [Google Scholar]
- McLaughlin 1967.McLaughlin S Parametric adjustment in saccadic eye movements. Percept Psychophys 2: 359–262, 1967. [Google Scholar]
- Melis 1996.Melis B, van Gisbergen JAM. Short-term adaptation of electrically induced saccades in monkey superior colliculus. J Neurophysiol 76: 1744–1758, 1996. [DOI] [PubMed] [Google Scholar]
- Miller 1981.Miller J, Anstis T, Templeton W. Saccadic plasticity: parametric adaptive control by retinal feedback. J Exp Psychol 7: 356–366, 1981. [DOI] [PubMed] [Google Scholar]
- Moschner 1993.Moschner C, Zangemeister WH. Preview control of gaze saccades: efficacy of prediction modulates eye–head interaction during human gaze saccades. Neurol Res 15: 417–432, 1993. [DOI] [PubMed] [Google Scholar]
- Noto 1999.Noto C, Watanabe S, Fuchs A. Characteristics of adaptation fields produced by behavioral changes in saccadic gain and direction. J Neurophysiol 81: 2798–2813, 1999. [DOI] [PubMed] [Google Scholar]
- Ohtsuka 1990.Ohtsuka K, Noda H. Direction selective saccadic burst neurons in the fastigial oculomotor region of the macaque. Exp Brain Res 81: 659–662, 1990. [DOI] [PubMed] [Google Scholar]
- Phillips 1997.Phillips JO, Fuchs AF, Ling L, Iwamotor Y, Votaw S. Gain adaptation of eye and head movement components of simian gaze shifts. J Neurophysiol 78: 2817–2821, 1997. [DOI] [PubMed] [Google Scholar]
- Prablanc 1978.Prablanc C, Masse D, Echallier JF. Error-correcting mechanisms in large saccades. Vision Res 18: 557–560, 1978. [DOI] [PubMed] [Google Scholar]
- Robinson 2001.Robinson FR, Fuchs AF. The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci 24: 981–1004, 2001. [DOI] [PubMed] [Google Scholar]
- Robinson 2003.Robinson FR, Noto CT, Bevans SE. Effect of visual error size on saccade adaptation in monkey. J Neurophysiol 90: 1235–1244, 2003. [DOI] [PubMed] [Google Scholar]
- Scudder 1998.Scudder CA, Batournia E, Tunder G. Comparison of two methods of producing adaptation of saccade size and implications for the site of plasticity. J Neurophysiol 79: 704–715, 1998. [DOI] [PubMed] [Google Scholar]
- Scudder 2002.Scudder CA, Kaneko CS, Fuchs AF. The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res 142: 439–462, 2002. [DOI] [PubMed] [Google Scholar]
- Scudder 2003.Scudder CA, McGee DM. Adaptive modification of saccade size produces correlated changes in the discharges of fastigial nucleus neurons. J Neurophysiol 90: 1011–1026, 2003. [DOI] [PubMed] [Google Scholar]
- Semmelow 1987.Semmelow J, Gauthier G, Vercher JL. Short-term adaptive modification of saccade amplitude. In: Eye Movements: From Physiology to Cognition, edited by O'Regan JK, Levy-Schoen A. Amsterdam: Elsevier, 1987, p. 191–200.
- Soetedjo 2006.Soetedjo R, Fuchs AF. Complex spike activity of Purkinje cells in the oculomotor vermis during behavioral adaptation of monkey saccades. J Neurosci 26: 7741–7755, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks 2002.Sparks DL The brainstem control of saccadic eye movements. Nat Rev Neurosci 3: 952–964, 2002. [DOI] [PubMed] [Google Scholar]
- Stahl 1999.Stahl J Amplitude of human head movements associated with horizontal saccades. Exp Brain Res 126: 41–54, 1999. [DOI] [PubMed] [Google Scholar]
- Stahl 2001.Stahl J Eye–head coordination and the variation of eye movement accuracy with orbital eccentricity. Exp Brain Res 136: 200–210, 2001. [DOI] [PubMed] [Google Scholar]
- Straube 1997.Straube A, Robinson F, Fuchs AF. Characteristics of saccadic gain adaptation in rhesus macaques. J Neurophysiol 77: 874–895, 1997. [DOI] [PubMed] [Google Scholar]
- Takagi 1998.Takagi J, Zee D, Tamargo R. Effect of lesions of the oculomotor vermis on eye movements in primate: saccades. J Neurophysiol 80: 1911–1931, 1998. [DOI] [PubMed] [Google Scholar]
- Takeichi 2005.Takeichi N, Kaneko CR, Fuchs AF. Discharge of monkey nucleus reticularis tegmenti pontis neurons changes during adaptation. J Neurophysiol 94: 1938–1951, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi 2007.Takeichi N, Kaneko CR, Fuchs AF. Activity changes in monkey superior colliculus during saccade adaptation. J Neurophysiol 97: 4096–4107, 2007. [DOI] [PubMed] [Google Scholar]
- Thier 2000.Thier P, Dicke PW, Haas R, Barash S. Encoding of movement time by populations of cerebellar Purkinje cells. Nature 405: 72–76, 2000. [DOI] [PubMed] [Google Scholar]
- Van Gisbergen 1984.Van Gisbergen JAM, Van Opstal AJ, Ottes FP. Parameterization of saccadic velocity profiles in man. In: Theoretical and Applied Aspects of Eye Movement Research, edited by Gale AG, Johnson F. Amsterdam: Elsevier, 1984, p. 87–94.
- Volle 1993.Volle M, Guitton D. Human gaze shifts in which the head and eyes are not initially aligned. Exp Brain Res 94: 463–470, 1993. [DOI] [PubMed] [Google Scholar]
- Wallman 1998.Wallman J, Fuchs A. Saccadic gain modification: visual error drives motor adaptation. J Neurophysiol 80: 2405–2416, 1998. [DOI] [PubMed] [Google Scholar]
- Walton 2008.Walton MM, Bechara B, Gandhi NJ. Effect of reversible inactivation of superior colliculus on head movements. J Neurophysiol 99: 2479–2495, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangemeister 1982.Zangemeister WH, Stark L. Types of gaze movement: variable interactions of eye and head movements. Exp Neurol 77: 563–577, 1982. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.