Abstract
Ethanol (EtOH) has powerful effects on GABAA receptor-mediated neurotransmission, and we have previously shown that EtOH-induced enhancement of GABAA receptor-mediated synaptic transmission in the hippocampus is developmentally regulated. Because synaptic inhibition is determined in part by the firing properties of interneurons, we have investigated the mechanisms whereby EtOH influences the spontaneous firing characteristics and hyperpolarization-activated cation current (Ih) of hippocampal interneurons located in the near to the border of stratum lacunosum moleculare and s. radiatum of adolescent and adult rats. EtOH did not affect current injection-induced action potentials of interneurons that do not exhibit spontaneous firing. However, in neurons that fire spontaneously, EtOH enhanced the frequency of spontaneous action potentials (sAPs) in a concentration-dependent manner, an effect that was more pronounced in interneurons from adolescent rats, compared with adult rats. EtOH also modulated the afterhyperpolarization (AHP) that follows sAPs by shortening the τslow decay time constant, and this effect was more pronounced in slices from adolescent rats. EtOH increased Ih amplitudes, accelerated Ih activation kinetics, and increased the maximal Ih conductance in interneurons from animals in both age groups. These effects were also more pronounced in interneurons from adolescents and persisted in the presence of glutamatergic and GABAergic blockers. However, EtOH failed to affect sAP firing in the presence of ZD7288 or cesium chloride. These results suggest that Ih may be of mechanistic significance in the effect of EtOH on interneuron spontaneous firing.
INTRODUCTION
As one of the most important components of neural network function, hippocampal GABAergic interneurons control the excitability of thousands of pyramidal cells and synchronize neural activity in the hippocampus through divergent inhibitory connections (Freund and Gulyas 1997). The interneurons located at the border of stratum radiatum (SR) and s. lacunosum moleculare (S-LM) of the CA1 region of hippocampus exert inhibition on other CA1 neurons. Thus they have a powerful inhibitory influence on hippocampal circuit function and sculpt cortico-hippocampal interactions (Freund and Buzsaki 1996; Vida et al. 1998). The net effect of synaptic inhibition is determined, in part, by the firing properties of inhibitory cells as well as by the dynamics of GABA release and the location of inhibitory synapses.
EtOH increases the frequency of GABAA receptor-mediated spontaneous and miniature inhibitory postsynaptic currents in hippocampal CA1 (Ariwodola and Weiner 2004; Li et al. 2003, 2006, Sanna et al. 2004) or CA3 pyramidal cells (Galindo et al. 2005). In addition, EtOH inhibits hippocampal interneuron firing induced by kainate receptor activation (Carta et al. 2003). Although the effect of EtOH on the function of interneurons is clearly one mediator of its inhibitory effects, neither the parameters nor the mechanisms of these effects are known.
While initially observed in cardiac pacemaker cells (Brown and Difrancesco 1980), the slowly developing, hyperpolarization-activated cation current, Ih, is also observed in GABAergic hippocampal interneurons (Aponte et al. 2006; Lupica et al. 2001; Maccaferri and McBain 1996) and has become the subject of intensive investigation in neuroscience (see Pape 1996 for review). The physiological function of Ih is believed to contribute to the neuronal resting membrane potential, the generation of spontaneous firing discharges, and GABA release in hippocampal interneurons (Aponte et al. 2006; Lupica et al. 2001; Maccaferri and McBain 1996). Several reports (Brodie and Appel 1998; Okamoto et al. 2006) have suggested that enhancement of Ih current could be an important cellular mechanism whereby EtOH increases the firing rates of dopaminergic (DA) neurons in the ventral tegmental area (VTA) and that this effect can be blocked by the Ih antagonists, ZD7288 and cesium. However, another study (Appel at al. 2003) indicates that several types of delayed rectifier potassium currents may be responsible for EtOH-induced increases in VTA DA neuron excitability because it was blocked by quinidine but not cesium or ZD 7288. In the present study, we addressed this question by examining the effects of EtOH on spontaneous firing and Ih in hippocampal GABAergic interneurons.
It appears that the effects of EtOH on both behavioral sedation (Little et al. 1996; White et al. 2000; White and Swartzwelder 2004) and GABAA receptor-mediated activity in hippocampal CA1 pyramidal cells (Li et al. 2003, 2006) are developmentally dependent. Furthermore, EtOH promotes extrasynaptic GABAA receptor-mediated tonic currents more efficaciously in dentate granule cells from adolescent rats compared with those from adults (Fleming et al. 2007). Thus it seems that the developmental modulation of the effects of EtOH on GABA receptor-mediated activity is not simply a matter of developing tissue being less sensitive to the effects of EtOH. Therefore to begin a systematic assessment of the effects of EtOH on identified interneurons, we focused primarily on a subgroup of hippocampal interneurons with intrinsic spontaneous firing activity to assess the effect of EtOH on action potential firing and to determine if Ih contributes to the EtOH regulation of firing properties.
METHODS
Tissue preparation
Hippocampal slices were prepared from male, Sprague-Dawley, periadolescent [postnatal day (PD) 30–40] and adult (PD70-80) rats. Although the periadolescent period of development in the rat has been the subject of some controversy, in recent years, based on an accumulating literature, the period between PD 30 and 50 has become an accepted norm for this period in the male rat (see Spear 2000). The animals were handled and housed according to the guidelines of the National Institutes of Health Committee on Laboratory Animal Resources. All experimental procedures or protocols were approved by the Animal Care and Use Committee of the Duke University and Durham Veterans Affairs Medical Center. The rats were deeply anesthetized with halothane (Sigma, St. Louis, MO) and decapitated. The brains were quickly removed from the skulls and placed in ice-cold (<4°C), modified artificial cerebrospinal fluid (ACSF) containing (in mM) 120 NaCl, 3.3 KCl, 1.23 NaH2PO4, 1 MgSO4, 0.2 CaCl2, 25 NaHCO3, and 10 d-glucose with pH 7.3, previously saturated with 95%O2-5%CO2. The tissue was completely submerged into ice-cold modified ACSF and sectioned in 300- to 350-μm-thick slices using a Vibratome series 1000 sectioning system (Vibratome, St. Louis, MO). The brain slices were first transferred to ACSF containing 0.5 mM Ca2+ and incubated at room temperature for 20 min to allow gradual adaptation to the slight elevation of extracellular Ca2+, then the slices were allowed to equilibrate for ≥1 h, at 35°C, in normal ACSF containing (in mM) 120 NaCl, 3.3 KCl, 1.23 NaH2PO4, 1 MgSO4, 2.0 CaCl2, 25 NaHCO3, and 10 d-glucose, during which time they were continuously bubbled with a mixture of 95% O2-5% CO2 gas. After this period, the brain slices were maintained at room temperature (22–24°C) until recordings were initiated. This procedure was developed and utilized in our laboratory to facilitate the production of physiologically viable slices from adult rats (Li et al. 2003, 2006).
Whole cell electrophysiology
After incubation, one hippocampal slice was transferred to the recording chamber that was connected to a Masterflex C/l pump superfusion system (Cole-Parmer Instrument, Vernon Hills, IL). The slice was held to the bottom of the chamber with silver wires and superfused at a constant rate of 2 ml/min with ACSF, which was bubbled with a mixture of 95% O2-5% CO2 gas. The recording chamber temperature was kept at 29–30°C by Chamber System Temperature Controllers (TC-344B, Warner Instruments, Hamden, CT). The slice was visualized with infrared differential interference contrast (DIC, Zeiss Axioskope), using an upright microscope, with a ×40 water-immersion objective, and displayed on a monitor. Pyramidal cells and interneurons located in different hippocampal layers were easily distinguishable on visual inspection and were then selected for whole cell recording.
Recordings were made by using standard whole cell patch recording techniques. Patch pipettes were borosilicate glass capillaries (1.5 mm OD, 0.86 mm ID, Sutter Instrument, Novato, CA), pulled on a Flaming/Brown Micropipette Puller (Sutter Instrument, Model P-97) to produce electrodes with 3–5 MΩ resistance. The pipette solution for current-clamp experiments consisted of (in mM) 130 K-gluconate, 5 KCl, 1 MgCl2, 0.5 EGTA, 10 HEPES, 4 Mg-ATP, 0.5 Tris-GTP, and 10 phosphocreatine (pH = 7.3, 290 mosM). For voltage-clamp experiments (Ih), the patch pipettes were filled with (in mM) 125 KMeSO4, 5 KCl, 5 NaCl, 1 MgCl2, 11 HEPES, 0.02 EGTA, 4 Mg-ATP, 0.5 Na-GTP, and 10 phosphocreatine (pH = 7.3, 290 mosM). Tight seals (>1GΩ) were formed on cell bodies, and whole cell recordings were made by rupturing the cell membrane with negative pressure. Either an Axopatch 1D amplifier or an Axopatch 200B (Axon Instruments Inc., Union City, CA) was used for current- and voltage-clamp recordings which were low-pass filtered (2 kHz or 5 kHz, Bessel filter). Output signals were DC coupled to a digital oscilloscope (Model 410, Nicolet, Madison, WI), and data acquisition was performed using Strathclyde Electrophysiology Software, Whole Cell Program (WINWCP) (Courtesy of Dr. John Dempster) or pCLAMP 10 (Axon Instruments, Union City, CA), with an interface (BNC-2090, National Instruments, Austin, TX) or a DigiData 1440A (Axon Instruments), coupled with a PC computer. Resting membrane potential was directly measured in current-clamp mode after membrane rupture (range: −78 to −53 mV), and only cells with a resting membranes potential more negative than −58 mV were studied. The liquid junction potential was estimated to be 15.9 mV for the current-clamp solution and was not corrected. Input resistance was calculated from membrane voltage deflection, evoked by 600-ms hyperpolarizing current injections (0 to −300 pA in steps of 50 pA). To measure cell capacitance, interneurons were depolarized by applying 5 mV at a holding potential of −70 mV, and cell capacitance was measured from the change in membrane charge, determined from the integrated capacity transients. Series resistance was ∼15 MΩ and was monitored by small (depolarizing 5 mV, 150 ms) voltage steps during voltage-clamp recording or current steps (hyperpolarizing 25 pA, 50 ms) during current-clamp recording. Cells were rejected from analysis if the series resistance changed by >15%.
Visualization of recorded cells
We initially visualized and selected interneurons for recording based on somatic shape and electrophysiological properties as described elsewhere (Christie et al. 2000; Gulyas et al. 1998). We employed several electrophysiological criteria, including the response to depolarizing current injections, and the observation of short-duration and fast spike action potentials that were followed by large afterhyperpolarizing potentials (AHPs) (Lacaille and Schwartzkroin 1988; Schwartzkroin and Mathers 1978). In contrast, CA1 pyramidal neurons generated action potentials that accommodated during maintained depolarization (Madison and Nicoll 1984). The recorded cells were also filled with Alexa Fluor 568 hydrazide or Alexa Fluor 488 hydrazide (50–80 μM, Invitrogen, Carlsbad, CA) to reveal their morphological characteristics for post hoc analysis. At the end of recordings, the florescence filled slices were fixed with 4% paraformaldehyde for 20–30 min and rinsed three times using a 0.2 M phosphate buffer. The slices were then mounted on gelatin-coated slides with Prolong Gold Antifade (Invitrogen, Molecular Probes, Carlsbad, CA), and visualized with a Leica TCS SP5 confocal laser scanning microscope (Leica Microsystems, Exton, PA).
Pharmacology
To isolate Ih, tetraethyl-ammonium chloride (TEA-Cl, 2 mM), tetrodotoxin (TTX, 1 μM), and BaCl2 (1 mM) were substituted for equimolar NaCl to block unwanted potassium, sodium, and inward rectifier potassium currents, respectively. TTX, ZD7288, and d-2-amino-5-phosphonovaleric acid (APV) were purchased from Tocris Cookson (Ellisville, MO). (−)-bicuculline methiodide (BMI), 6,7-dinitroquinoxaline-2,3-dione (DNQX), and the other chemicals were obtained from Sigma-Aldrich. The drugs were dissolved in distilled water or dimethyl sulfoxide (DMSO) to make stock solutions. The stock solutions were stored, frozen, in 1 ml aliquots, and before each experiment were diluted in ACSF to their final concentrations. The drugs were infused into the recording chamber using a standard perfusion system. After the establishment of stable baseline recordings, EtOH was added to the bath solution in incremental concentrations of 3, 10, 30, and 50 mM, and each concentration was maintained for 5–10 min followed by a washout period of 10–20 min.
Data analysis
The stored data signals were processed using either the Clampfit 10 (Axon Instruments) or Mini Analysis Program (Synaptosoft, Decatur, GA). Numerical data are presented as means ± SE, and n represents the number of cells tested per condition. Action potential amplitude, frequency, rise time (10–90%), half-width, and afterhyperpolarization (AHP) decay time were analyzed with the Mini Analysis Program (Synaptosoft). Paired or unpaired t-test, and one- or two-way ANOVAs followed by Tukey post hoc tests, when appropriate, were used to test statistical inferences related to grouped data. Calculated P values of ≤0.05 were accepted as evidence of statistically significant differences.
Ih was evoked by 1.2-s hyperpolarizing steps to −130 or −140 mV from −50 mV. Ih amplitude was measured as the difference between the current level at the end of a 1.2-s hyperpolarizing step command and at the beginning, after the capacitive transient had subsided. The EtOH concentration response curves were fitted by the Hill equation: Rexp = Rmax/{1 + [EC50/(A)]n}, where Rexp is the expected response, Rmax is the maximal response, EC50 is the concentration of EtOH that induced the half-maximal response, (A) is the concentration of the EtOH, and n is the Hill coefficient. AHP decay time constants were obtained by fitting a two-exponential function, I(t) = If exp(−t/τfast) + Is exp (−-t/τslow). If and Is are the amplitudes of fast and slow components in the AHP, respectively. τfast and τslow are decay time constants. Ih activation time constants were fitting with a single-exponential function of the equation: I(t) = Ih exp(−t/τ) + ISS, where I(t) is the amplitude of the current at time t, ISS is the steady-state current during a single voltage step, Ih is instantaneous current subtracted from ISS, and τ is the time constant of activation. The Ih activation curve was obtained from the Ih current amplitude at each test potential and was converted into conductance (G) using the relation G = I/(V − Er), where Er is the reversal potential for Ih. In the present experiment, the value of Er is −30.5 ± 3.02 mV (n = 7) calculated by the method of Mayer and Westbrook (1983). The conductance values (G) for each cell were fitted with Boltzmann relations of the form G = Gmax/[1 + exp(V − V1/2)/k], where Gmax is the extrapolated maximum conductance, V is the test voltage, V1/2 is the half-activation voltage, and k is the slope factor. In each cell, sAPs and AHPs were assessed using data from 2-min periods before and during each treatment.
RESULTS
Identification of pyramidal neurons and inhibitory interneurons located at the border of SL-M and SR
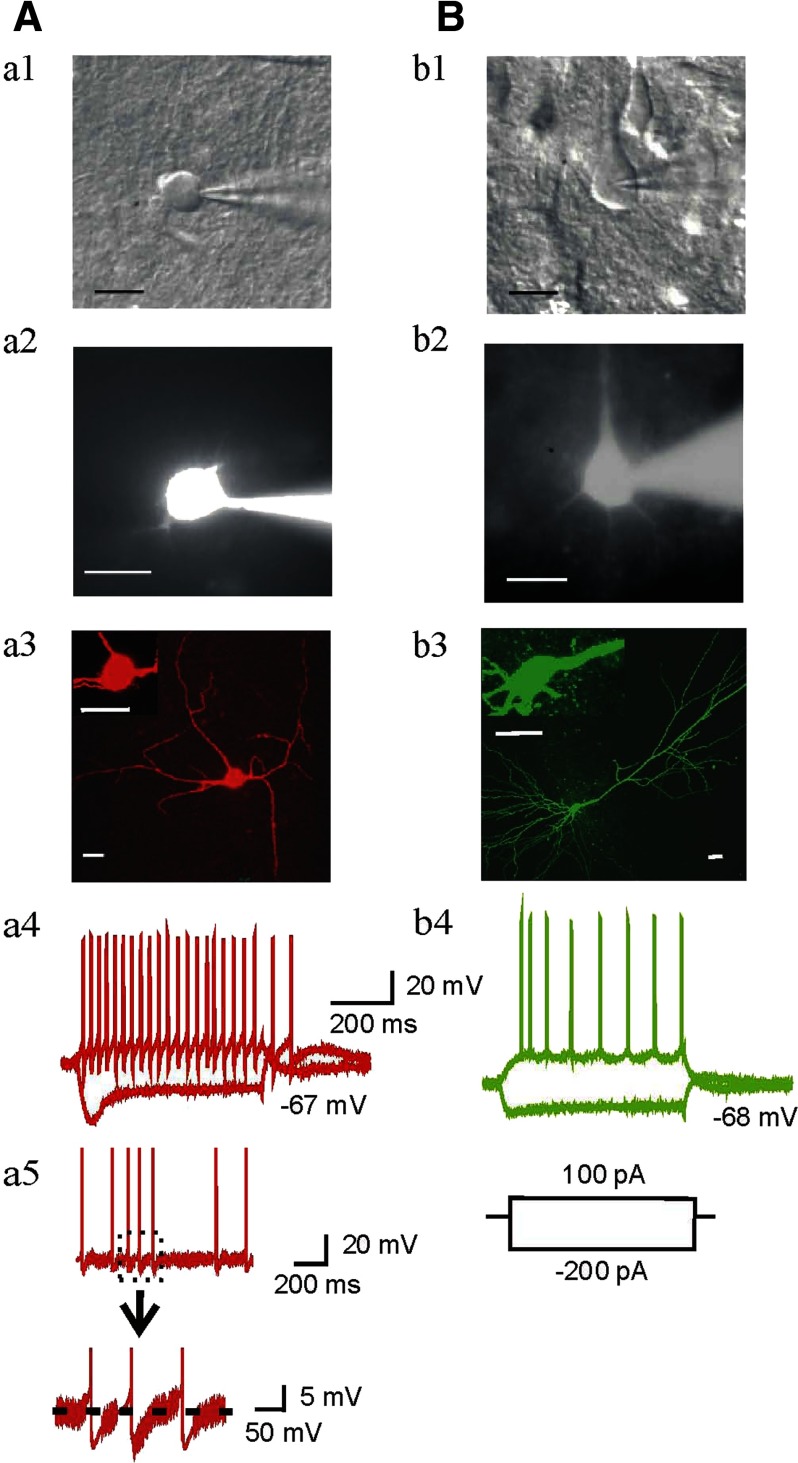
Interneurons located in or near the border of SL-M and SR (Fig. 1 A) were first identified electrophysiologically by their unique characteristic features distinguished from pyramidal neurons (Fig. 1B) and other cells as described previously (Christie et al. 2000; Gulyas et al. 1998). Typical examples of an interneuron (Fig. 1A) and a pyramidal cell (B) are shown along with their morphological (a3 and b3) and electrophysiological (a4 and b4) properties. The soma of the interneuron in Fig. 1A was situated in SL-M, near the SR border, was <20 μm in diameter, and the dendrites were smooth (Fig. 1A, a3). This cell demonstrated a typical response to depolarizing and hyperpolarizing current injections, and fired spontaneous action potentials (7 Hz) which were followed by large AHPs (a5). It also exhibited trains of minimally accommodating action potentials in response to depolarizing current injections (a4). These characteristics are consistent with interneurons as described previously (Bertrand and Lacaille 2001; Cope et al. 2002; Lacaille and Schwartzkroin 1988; Schwartzkroin and Mathers 1978; Vita et al. 1998). In contrast, CA1 pyramidal neurons typically fired action potentials of longer duration, which accommodated markedly during maintained depolarization (Fig. 1B, b4). These data are consistent with previous descriptions of the physiological characteristics of pyramidal cells (Madison and Nicoll 1984).
FIG. 1.
Identification of interneurons and pyramidal neurons by morphological and electrophysiological properties in rat hippocampal CA1 near to the border of stratum radiatum (SR) and s. lacunosum moleculare (S-LM) area. A: recorded interneuron near the border of SL-M and SR. B: recorded pyramidal cell from s. pyramidale area. a1 and b1: example images of whole cell pipettes attached to bodies of an interneuron and a pyramidal neuron under DIC. a2 and b2: images of same cells revealed by intracellular dialysis with Alexa Fluor 568 and 488 hydrazide 3–5 min after cell rupture. a3 and b3: fluorescence images of same cells (red, interneuron; green, pyramidal neuron) were taken with confocal microscopy. Scale bars = 20 μm. a4 and b4: current-clamp recordings in the same cells show voltage responses to depolarizing (600 ms, +100 pA) and hyperpolarizing (600 ms, −200 pA) current injection. Typical responses of an interneuron and a pyramidal cell (a4 and b4) respectively, were observed after current injection. Spontaneous action potentials (sAPs) and afterhyperpolarizations (AHPs) recorded from an interneuron are shown in a5.
To assess some of the active and passive membrane properties of interneurons recorded from both age groups, we analyzed 84 interneurons in slices from adolescent rats and 75 interneurons in slices from adult rats. Twenty three of the interneurons from adolescent rats (27.4%) exhibited sAPs with firing rates of 4.75 ± 1.30 Hz, amplitudes 74.93 ± 2.36 mV, and prominent AHPs. The other 61 interneurons from adolescents did not display sAP firing. In slices from adult rats, 20 (26.7%) interneurons generated sAP firing with AHPs, had firing rates of 4.63 ± 0.88 Hz, and amplitudes of 79.30 ± 2.87 mV. The remaining 55 neurons from adult animals did not spontaneously fire APs. The membrane properties of each group of cells, including resting membrane potential, input resistance, cell capacitance, sAP amplitude and frequency are summarized in Table 1. The resting membrane potential and input resistance of interneurons and pyramidal neurons did not differ in cells from adolescent animals compared with those from adults. However, in general, interneurons exhibited higher membrane input resistance than pyramidal neurons in both age groups (Table 1, unpaired-t-test, for adolescent group, t(32) = −3.66, P = 0.00089, interneurons with sAPs and t(70) = −3.23, P = 0.00185 without sAPs; for adult group, t(29) = −2.85, P = 0.0085, interneurons with sAPs and t(64) = −3.07, P = 0.00312 without sAPs).
TABLE 1.
Membrane properties of CA1 interneurons and pyramidal neurons from adolescent and adult rats
| Parameters | Adolescent | n | Adult | n |
|---|---|---|---|---|
| Interneurons with sAP | ||||
| Resting membrane potential, mV | −67.4 ± 0.5 | 23 | −67.7 ± 0.6 | 20 |
| Input resistance, MΩ | 169.5 ± 8.7** | 23 | 179.1 ± 13.9** | 20 |
| sAP frequency, Hz | 4.7 ± 1.3 | 23 | 4.6 ± 0.9 | 20 |
| sAP amplitude, mV | 74.9 ± 2.4 | 23 | 79.3 ± 2.9 | 20 |
| Capacitance, pF | 23.9 ± 1.1 | 23 | 22.1 ± 1.0 | 20 |
| Interneurons without sAP | ||||
| Resting membrane potential, mV | −68.3 ± 0.5 | 61 | −68.6 ± 0.5 | 55 |
| Input resistance, MΩ | 173.9 ± 11.5** | 61 | 171.5 ± 15.0* | 55 |
| Capacitance, pF | 23.6 ± 0.8 | 61 | 22.7 ± 0.9 | 55 |
| Pyramidal neurons | ||||
| Resting membrane potential, mV | −67.8 ± 1.0 | 11 | −67.2 ± 0.5 | 11 |
| Input resistance, MΩ | 105.8 ± 16.3 | 11 | 104.4 ± 15.7 | 11 |
| Capacitance, pF | 25.5 ± 1.5 | 11 | 25.0 ± 1.5 | 11 |
Values are mean ± SE. sAP amplitudes were measured from the action potential threshold. sAP, spontaneous action potential.
P < 0.05,
P < 0.01 (Compared with pyramidal neurons in each age group).
EtOH effects on evoked APs of interneurons in adolescent and adult rats
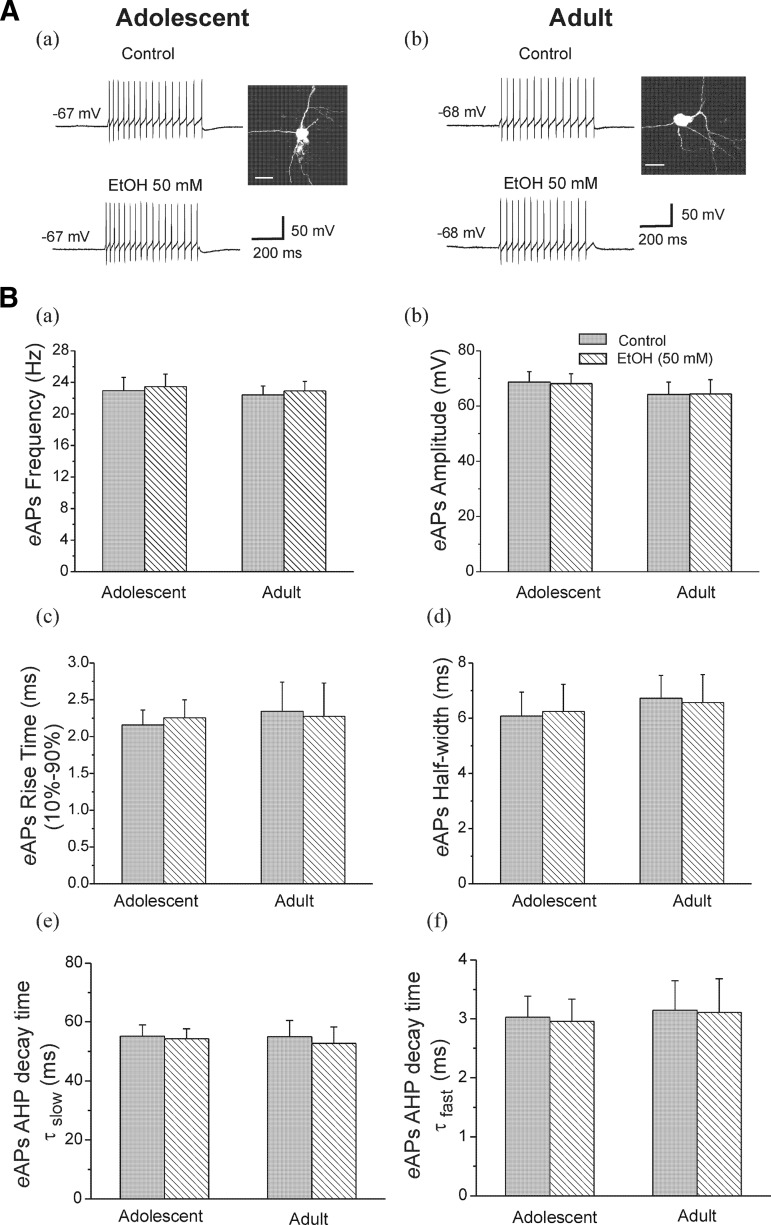
We first assessed the effects of EtOH on evoked action potentials (eAP) of interneurons that exhibited no spontaneous firing. eAPs were elicited by injection of a depolarizing pulse (100 pA, 600-ms duration) in interneurons from both age groups. The basal amplitude and firing frequency of eAPs were not significantly different between interneurons from adolescent (68.68 ± 3.79 mV and 22.94 ± 1.68 Hz, n = 12) and adult rats (64.16 ± 4.50 mV and 22.41 ± 1.11 Hz, n = 7). There were no significant developmental differences in resting membrane potentials (adolescent, −65.67 ± 1.09 mV, n = 12; adult, −65.28 ± 1.15 mV, n = 7). Typical voltage traces are illustrated in Fig. 2. Data from an interneuron from an adolescent rat is shown in Fig. 2Aa, this neuron fired 16 APs per evoked response under control conditions and 17 APs after bath application of EtOH (50 mM). Figure 2Ab shows an interneuron from an adult rat, EtOH (50 mM) did not change eAP firing (14 APs to 14 APs per evoked response). The averaged data for repetitive eAP firing frequency (a), amplitude (b), 10–90% rise time (c), and half-width (d) are shown in Fig. 2B. The results indicate that EtOH did not affect the repetitive firing frequency in response to depolarizing current injection in interneurons from adolescent (from 22.94 ± 1.68 to 23.47 ± 1.58 Hz, n = 12) and adult (from 22.41 ± 1.11 to 22.91 ± 1.20 Hz, n = 7) rats. EtOH had no significant effects on eAP amplitude (adolescent, from 68.68 ± 3.79 mV in control to 68.11 ± 3.56 mV, n = 12; adult, from 64.16 ± 4.50 mV in control to 64.38 ± 5.17 mV, n = 7), 10–90% rise time (adolescent, from 2.16 ± 0.2 ms in control to 2.25 ± 0.24 ms, n = 12; adult, from 2.34 ± 0.39 ms in control to 2.28 ± 0.45 ms, n = 7), or half-width (adolescent, from 6.08 ± 0.86 ms in control to 6.24 ± 0.98 ms, n = 12; adult, from 6.72 ± 0.82 ms in control to 6.56 ± 1.01 ms, n = 7). The effects of EtOH on AHP decay time after eAPs are shown in Fig. 2B, d and e. EtOH (50 mM) did not change either AHP τslow or τfast decay time in neurons from adolescent (τslow, from 55.15 ± 3.86 to 54.29 ± 3.34 ms; τfast, from 3.03 ± 0.36 to 2.96 ± 0.38 ms) and adult rats (τslow, from 54.98 ± 5.47 to 52.70 ± 5.61 ms; τfast, from 3.15 ± 0.50 to 3.11 ± 0.57 ms). These results are consistent with those of Carta et al. (2003), in which hippocampal CA1 interneurons were recorded in the same region as we recorded, though without a developmental comparison.
FIG. 2.
Ethanol (EtOH) did not affect evoked action potential (eAP) firing in CA1 interneurons from adolescent and adult rats. A: interneurons identified by basic morphological and physiological properties from an adolescent (a) and an adult (b) rat. Photomicrography is fluorescence image of interneurons (scale bar = 20 μm), the traces were evoked by injecting a depolarizing current (100 pA for 600-ms duration). eAPs were obtained before (control) and after application of 50 mM EtOH. B: summary graph of the effects of EtOH on depolarization-induced eAP firing frequency (a), amplitude (b), 10–90% rise time (c), half-width (d), eAP AHP decay time τslow (e), and τfast (f) in the neurons from adolescent (n = 12) and adult (n = 7) rats.
EtOH effects on sAP firing of interneurons in adolescent and adult rats
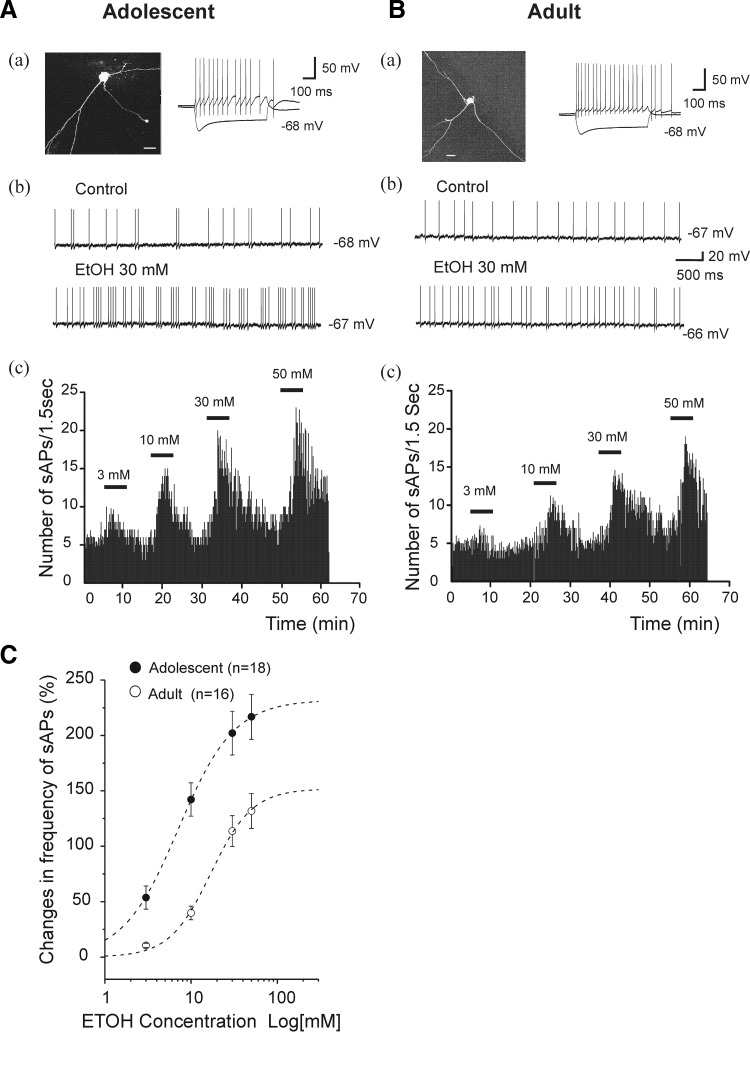
We found that EtOH enhanced the frequency of sAPs but did not significantly affect the resting membrane potential of interneurons from the hippocampal region described in the preceding text. However, in a few cases (2 cells from adolescent rats and 1 cell from an adult rat), even 3 mM EtOH produced a reversible depolarization (>5 mV) that was accompanied by high-frequency firing (>50 Hz). Data from these cells were not included in the analyses for this study. In addition, EtOH 50 mM did not significantly affect action potential firing that was evoked by a depolarizing current (100 pA for 600 ms duration) injection into spontaneously firing interneurons from adolescent rats (from 31.67 ± 1.41 and 32.22 ± 1.70 Hz, respectively, n = 6, paired t-test t(5) = −0.79, P = 0.47). EtOH (3, 10, 30, and 50 mM) did not significantly alter the sAP amplitude of interneurons from adolescent [1-way ANOVA, F(3, 68) = 1.15, P = 0.34] or adult [1-way ANOVA, F(3, 60) = 1.54, P = 0.21] animals. However, bath application of EtOH caused a dose-dependent increase in the frequency of sAPs in cells from animals of either age group. The EtOH-induced increase in frequency of sAPs was reversed after the EtOH was washed out of the recording chamber for 15–30 min. Figure 3 Ab shows action potentials recorded from an interneuron from an adolescent rat, and an increase in sAP frequency to 12 Hz from 4.22 Hz, 10 min after 30 mM EtOH was applied. EtOH did not alter the resting membrane potential. In slices from adolescent rats, we completed the EtOH concentration protocol in 18 of 23 interneurons that had sAP firing. In those cells, EtOH increased sAP frequency in a concentration-dependent manner. A representative example of interneuron responsiveness to EtOH from 3 to 50 mM is shown in Fig. 3Ac. On average, EtOH, at concentrations of 3, 10, 30, and 50 mM, increased sAP frequency by 53.79 ± 10.44, 142.19 ± 14.98, 202.04 ± 19.74, and 216.83 ± 20.23%, respectively (Fig. 3C, •). Among cells from adolescent rats, all EtOH concentrations resulted in increases of sAP frequency relative to control conditions [1-way ANOVA, F(3, 68) = 18.278, P = 8.38E-9]. Figure 3Bb shows that EtOH (30 mM) also significantly enhanced the frequency of sAPs of interneurons recorded from adult rats. In slices from adult rats, we completed the EtOH concentration protocol in 16 of 20 interneurons that had sAP firing. A representative example is shown in Fig. 3Bc. EtOH, at concentrations of 3, 10, 30, and 50 mM, increased firing frequency by 10.54 ± 1.96, 39.84 ± 6.14, 113.72 ± 13.91, and 131.85 ± 15.69% (n = 16), respectively (Fig. 3C, ○). Among cells from adult rats, EtOH concentrations of ≥10 mM resulted in increases of sAP frequency relative to control conditions [1-way ANOVA, F(3, 60) = 26.29, P = 5.57E-11]. EtOH increased sAP frequency more powerfully in interneurons from adolescent rats, compared with those from adults [2-way ANOVA, Tukey test, F(1,128) = 56.25, P = 9.41E-12, Fig. 3C]. The average concentration-response curves for the effect of EtOH on sAP firing frequency are shown Fig. 3C. The curves were fitted to averaged percentage increase in sAP frequency at each EtOH concentration by Hill equation (see methods). Best-fit curves, which fitted to data obtained from individual neurons revealed that EC50s for EtOH were 10.78 ± 1.38 mM (n = 18) for cells from adolescent rats and 20.54 ± 2.24 mM (n = 16) for cells from adults. The Rmax values were 238.30 ± 21.07 and 154.99 ± 16.81% for cells from adolescent rats and adult rats, respectively. These were significant differences for EC50 (unpaired t-test, t(32) = −3.68, P = 0.0085) and Rmax (t(32) = 2.95, P = 0.0059) between the two age groups. The concentration-response curve for interneurons from adolescents was shifted significantly to the left compared with the curve for interneurons from the adult group (Fig. 3C). There was no significant difference in the Hill coefficient between two age groups (adolescent, 1.56 ± 0.20; adult, 1.89 ± 0.23; unpaired t-test, t(32) = 1.05, P = 0.300).
FIG. 3.
EtOH increased sAP firing rate in CA1 interneurons from adolescent and adult rats. A, a: an interneuron identified by basic morphological and physiological properties from an adolescent rat. Photomicrography is fluorescence image of an interneuron (scale bar = 20 μm), the current trace was evoked by injection of depolarizing (100 pA) and hyperpolarizing (−200 pA) current for 600 ms. b: sAP firings were recorded from the same interneuron after addition of 30 mM EtOH. c: time course of sAP firing was recorded continuously after addition of 3, 10, 30, and 50 mM EtOH. EtOH caused a concentration-dependent increase in the number of sAP firings. B: following same experimental protocol, the results were obtained in an interneuron from an adult animal. C: EtOH concentration-response curves for changes in frequency of sAPs (%) from adolescent and adult rats. The individual data points show mean percentage change in sAP frequency plotted against log of the EtOH concentration, suggesting that EtOH enhanced sAP firing more powerfully in neurons from adolescent rats than adult rats at each concentration level (2-way ANOVA, P < 0.01). Averaged EtOH concentration-response curves were fitted by the Hill equation (adolescent, n = 18; adult, n = 16, - - -). The concentration-response curve for adult rats was shifted to the right compared with the curves from adolescent rats.
Effects of EtOH on sAP AHP decay time of interneurons in adolescent and adult rats
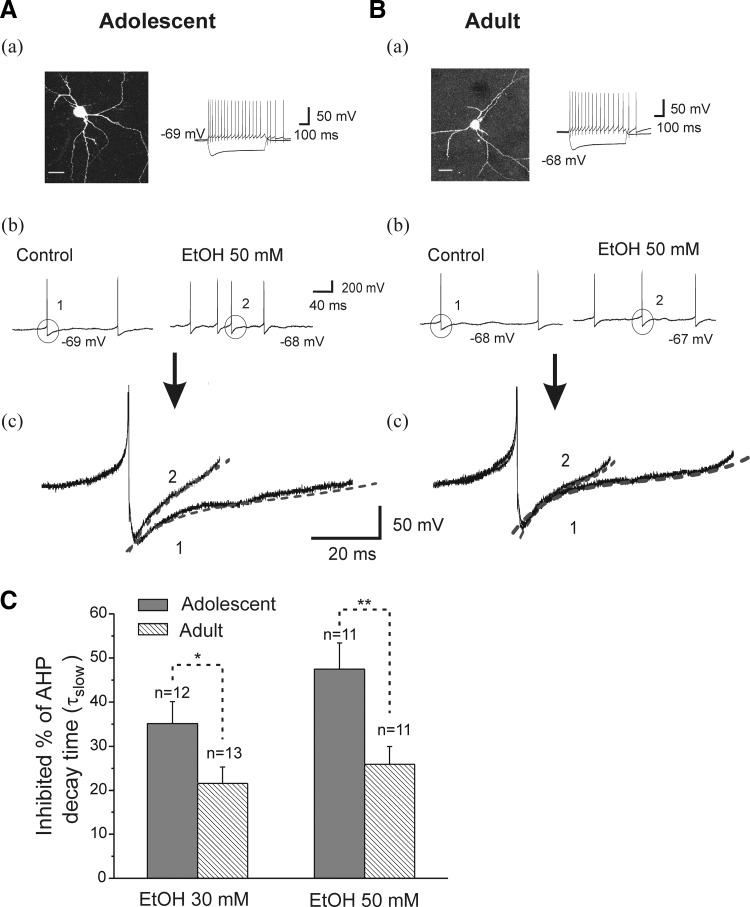
In CA1 hippocampal interneurons, AHPs regulate firing pattern and discharge frequency. Thus an effect of EtOH on AHPs could have substantial impact on circuit level inhibition in the hippocampus and could represent an important mechanism driving the effects of EtOH on hippocampal function. We next evaluated EtOH-induced changes in the time course of AHP decay in interneurons from animals in both age groups. AHP decay time constants were obtained by fitting a two-exponential function (see methods). EtOH (30 or 50 mM) did not change the τfast decay time components in interneurons from adolescent or adult rats. In cells from adolescent rats, the AHP τfast decay time (10–90%) was 9.73 ± 1.71 ms (n = 12), which did not change significantly after application of EtOH 30 mM (9.39 ± 1.82 ms, n = 12; t(11) = 1.02, P = 0.32). Bath application of EtOH (50 mM) also did not affect the AHP τfast decay time (from 9.48 ± 1.35 to 9.12 ± 1.35 ms, n = 11; paired t-test, t(10) = 1.63, P = 0.13). In addition, neither 30 nor 50 mM EtOH significantly altered AHP τfast decay time (30 mM, from 8.79 ± 0.95 to 8.39 ± 0.89 ms, n = 13, paired t-test, t(12) = 2.08, P = 0.06; 50 mM, from 8.93 ± 0.96 to 8.53 ± 1.0 ms, n = 11, paired t-test, t(10) = 1.43, P = 0.18) in adult animals. However, EtOH (30 and 50 mM) decreased the AHP τslow decay time in CA1 interneurons from animals of both age groups, and the results are illustrated in Fig. 4. As demonstrated in Fig. 4A, b and c, bath application of 50 mM EtOH accelerated the frequency of sAPs recorded from an interneuron from an adolescent rat. The AHP slow decay time was 31.07 ms at baseline and 15.80 ms after bath application of 50 mM EtOH (Fig. 4Ac). In cells from adolescent rats, the average AHP τslow decay time (10–90%) at baseline was 28.90 ± 2.73 ms, and it was significantly reduced to 18.8 ± 2.07 ms by 30 mM EtOH (n = 12, paired t-test, t(11) = 6.50, P = 4.44E-05), and 50 mM EtOH reduced τslow from 30.61 ± 2.61 to 14.67 ± 1.66 ms (n = 11, paired t-test, t(10) = 4.27, P = 0.0016). Figure 4B shows that bath application of 50 mM EtOH also accelerated the frequency of sAPs isolated from an interneuron from an adult rat (Fig. 4Bb). Typical voltage traces are shown in Fig. 4B. b and c). In this interneuron, the AHP decay time was 29.08 ms at baseline and 21.07 ms after bath application of 50 mM EtOH (Fig. 4Bc). In cells from adults, the average AHP τslow decay time was significantly reduced to 23.10 ± 3.48 ms from 29.23 ± 3.98 ms after application of 30 mM EtOH (n = 13, paired t-test, t(12) = 5.48, P = 1.41E-04), and from 31.43 ± 2.62 to 24.88 ± 2.86 ms after bath applied 50 mM EtOH (n = 11, paired t-test, t(10) = 5.18, P = 4.12E-04). We compared the effects of EtOH on the τslow decay time of AHPs in interneurons from adolescent and adult rats, and the results are plotted in Fig. 4C. Both 30 and 50 mM EtOH decreased AHP τslow decay time more powerfully in cells from adolescent rats compared with those from adults (unpaired t-test, t(23) = −2.12, P = 0.044 at 30 mM; t(20) = 2.86, P = 0.0095 at 50 mM), suggesting that the EtOH sensitivity of AHP decay time constants may be a mechanism underlying the developmental sensitivity of interneurons to EtOH.
FIG. 4.
Effects of EtOH on AHPs following each sAP, in interneurons from adolescent and adult rats. A, a: an interneuron identified by basic morphological and physiological properties from an adolescent rat. Photomicrography is fluorescence image of an interneuron (scale bar = 20 μm); the current trace is evoked by injection of depolarizing (100 pA) and hyperpolarizing (−200 pA) current for 600 ms. b: in this interneuron, the traces of sAPs were obtained before (control) and after application of EtOH 50 mM. c: the traces of single sAP AHP before and during 50 mM EtOH application were superimposed and show that EtOH decreased in AHP decay time τslow (1 and 2), the dashed lines represent fits to a 2-exponential function (see methods). B: same experimental protocol as A, the results were observed in a neuron from an adult rat. C: the summarized data shows mean percentage change in AHP decay time τslow after the application of EtOH 30 and 50 mM to slices from adolescent and adult rats. EtOH reduced AHP decay time, and its effect is greater in interneurons from adolescent animals compared with adult animals (*P < 0.05, **P < 0.01, unpaired t-test).
EtOH enhanced sAP firing via Ih in interneurons
Previous studies have shown that Ih contributes to resting membrane potential and spontaneous pace-making activity in hippocampal interneurons (Lupica et al. 2001; Maccaferri and McBain 1996). However, the role of Ih in mediating EtOH–induced enhancement of neuronal firing rates remains unclear (Appel et al. 2003; Okamoto et al. 2006; see also Lupica and Brodie 2006 for review). To better understand the potential contribution of Ih to EtOH-induced increases in interneurons firing, we investigated the effects of Ih channel antagonists on EtOH-enhanced sAP firing in hippocampal interneurons from adolescent rats.
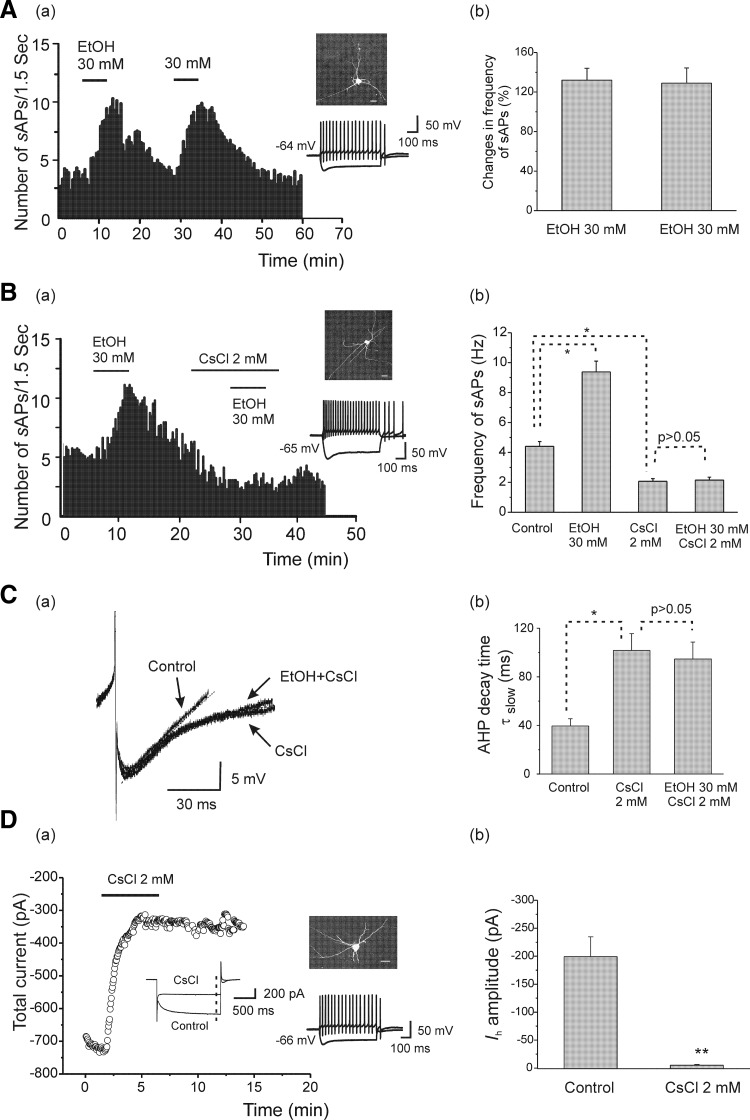
First, to determine that repeated exposures to EtOH in our experimental protocol did not induce rapid tolerance, we applied EtOH (30 mM) to the same slices twice at an interval of 15–18 min. Typical responses are shown in Fig. 5 Aa. On average, the firing rates of the interneurons were significantly increased from 3.80 ± 0.38 to 8.74 ± 0.78 Hz (n = 6, 132.03 ± 12.08%) after the first bath application of EtOH (30 mM). The firing rates returned to baseline after the 15-min washout period and when the same concentration of EtOH was re-applied. The average sAP firing rate was again increased significantly to 8.45 ± 0.84 from 3.75 ± 0.40 Hz (n = 6, 128.99 ± 15.40%; Fig. 5Ab) following bath-applied EtOH for the second time. There was no significant difference in the increase of firing rates between the first and second EtOH applications (n = 6, paired t-test, t(5) = 0.29, P = 0.78), indicating that repeated exposures using this protocol did not result in rapid tolerance.
FIG. 5.
Effects of CsCl on sAP firing frequency and Ih in hippocampal interneurons from adolescent rats. A, a: the time course of sAP firing following bath application of 2 separate applications of EtOH (30 mM) at an interval of 15 min. The data are from an interneuron identified by basic morphological (fluorescence image, scale bar = 20 μm) and physiological properties (injected currents 100 pA, −200 pA, 600 ms). b: averaged data illustrating that there were no significant changes in the frequency of sAP firing between the 1st and 2nd application of EtOH (n = 6, paired t-test, P > 0.05). B, a: the time course of responses of sAP firing after bath application of EtOH (30 mM), then CsCl (2 mM) followed by 30 mM EtOH. CsCl decreased sAP firing frequency and blocked the effects of EtOH. b: bar graph summarizing the effects of EtOH, CsCl, and the subsequent addition of EtOH after CsCl, on the frequency of sAP firing (n = 8, 1-way ANOVA, *P < 0.05). C,a: traces of AHPs under control conditions, in the presence of CsCl, and after the subsequent addition of EtOH. - - -, fits with a 2-exponential function. b: averaged data (+SE) show the effects of CsCl alone, and after subsequent application of EtOH, on the slow component of AHP decay time. Note that CsCl (2 mM) significantly extended AHP τslow, and AHP τslow was subsequently unchanged by the addition of EtOH (30 mM; n = 8, 1-way ANOVA *P < 0.05). D: time course of the effect of CsCl on total current in an interneuron from an adolescent rat. Total currents were evoked by a 1.2-s hyperpolarizing voltage step from a holding potential of −50 to −130 mV and were plotted against time at the end of test pulse (under - - -). Inset: representative traces under control conditions and after application of CsCl. b: CsCl significantly reduced Ih amplitude (n = 6, paired t-test, **P < 0.01).
We also found that CsCl, an Ih blocker, significantly decreased the EtOH-induced enhancement of sAP firing rates. This illustrated in Fig. 5Ba. In this interneuron, bath application of 30 mM EtOH increased sAP frequency from 4.93 to 10.08 Hz. After spontaneous activity returned to the baseline following the EtOH washout, 2 mM CsCl was added to the bath and decreased the sAP firing rate to 2.13 from 5.8 Hz. Re-application of 30 mM EtOH, in the presence of CsCl, did not increase the frequency of sAP firing. The averaged results of these experiments are shown in Fig. 5Bb. The mean sAP frequency from eight cells was significantly increased to 9.38 ± 0.71 Hz from 4.40 ± 0.32 Hz after the initial bath application of EtOH (30 mM) but was reduced to 2.07 ± 0.19 Hz by CsCl 2 mM [n = 8, 1-way ANOVA, F(3, 28) = 68.21, P = 5.47E-13]. Re-application of EtOH in the presence of CsCl did not significantly raise the sAP firing frequency, raising it only to 2.15 ± 0.19 Hz. CsCl also induced a small, nonsignificant hyperpolarization of 1.83 ± 0.53 mV (n = 8, −66.15 ± 1.53 to −67.98 ± 1.90 mV). We also assessed the effects of CsCl on the decay time of AHPs that followed sAPs. Typical traces of AHPs from an interneuron from an adolescent rat are shown in Fig. 5Ca. In this interneuron (the same neuron as in Fig. 5Ba), the AHP decay time τslow was extended after bath application of CsCl 2 mM, and τslow was not changed by subsequent application of 30 mM EtOH. In addition, CsCl had no effect on the fast component of AHP decay time (from 9.21 ± 0.86 to 9.65 ± 0.97 ms, n = 8). The averaged effect of CsCl on AHP τslow and its responsiveness to EtOH are shown in Fig. 5Cb, indicating that CsCl significantly prolonged AHP slow decay time to 101.77 ± 13.77 ms from 39.46 ± 6.04 ms of control [n = 8, 1-way ANOVA, F(2, 21) = 7.28, P = 0.0039], and this prolongation was not changed by EtOH (30 mM, 94.67 ± 13.91 ms, n = 8).
We also found that CsCl significantly reduced Ih amplitude. After obtaining stable Ih currents by applying hyperpolarizing voltage steps (1.2-s duration) from holding potentials of −50 to −130 mV under voltage-clamp condition, CsCl (2 mM) was applied in the bath and blocked the recorded Ih currents. Representative traces recorded from an interneuron from an adolescent rat are shown in Fig. 5Da. Each data point was obtained from plotting the total current at the end of the test pulse (- - -, inset) before and after CsCl application. The results obtained from six interneurons are illustrated in Fig. 5Db. CsCl reduced Ih amplitude from −199.17 ± 35.58 to −5.30 ± 1.19 pA (n = 6, paired t-test, t(5) = −4.32, P = 0.0042). This suggests that cesium may have blocked EtOH-induced increases in sAP firing rates due to its suppression of Ih.
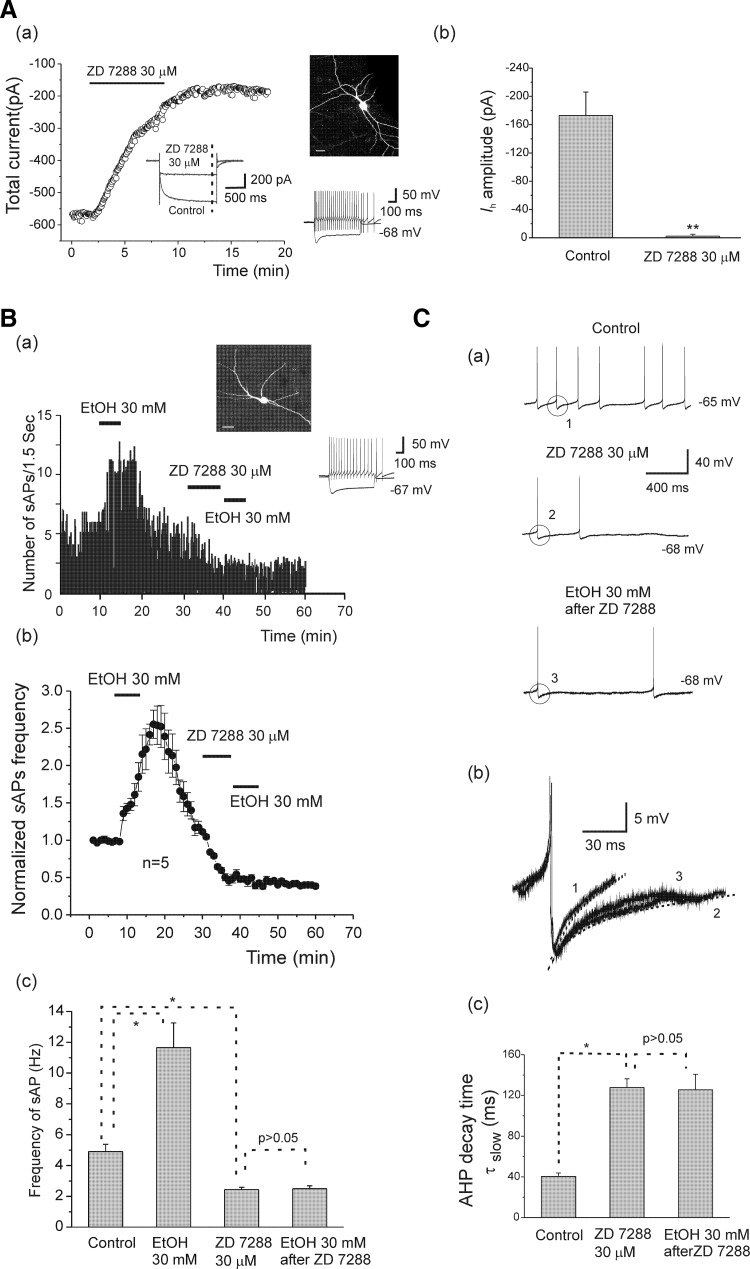
In similar experiments, we replaced cesium with another Ih channel blocker, ZD7288, which is widely used although its selectivity on Ih channels has been questioned in at least one report (Chevaleyre and Castillo 2002). As shown in Fig. 6 Aa, Ih was blocked by ZD7288 (30 μM) in an interneuron from an adolescent rat, and the total current at the end of the test pulse (- - -, inset) was plotted before and during ZD7288 application. Figure 6Ab shows that ZD7288 significantly reduced Ih amplitude from −172.91 ± 33.23 to −2.35 ± 2.70 pA (n = 7, paired t-test, t(6) = −4.89, P = 0.0027), and this effect was irreversible. Bath application of ZD7288 (30 μM) for 7 min also irreversibly decreased sAP firing frequency in five of eight cells tested from adolescent rats and completely blocked sAP firing in three of eight cells (these cells were excluded from analysis). Similar to cesium, blockade of Ih by ZD7288 also significantly reduces the EtOH-induced enhancement of sAP frequency, and a typical example of EtOH (30 mM) on sAP firing is shown in Fig. 6Ba. In this interneuron, bath application of 30 mM EtOH increased sAP frequency from 5.08 to 11.78 Hz. After spontaneous activity returned to the baseline following the EtOH washout, ZD7288 (30 μM) was applied in the bath for 7 min, followed by a re-application of 30 mM EtOH. EtOH failed to increase the frequency of sAPs after the Ih was blocked by ZD7288. The time course for normalized sAP frequency is illustrated in Fig. 6Bb. The summarized data are shown in Fig. 6Bc, indicating that EtOH (30 mM) significantly increased sAP frequency to 11.65 ± 1.35 from 4.90 ± 0.47 Hz, and it was reduced to 2.44 ± 0.15 Hz after application of ZD7288 [n = 5, 1-way ANOVA, F(3, 16) = 53.99, P = 1.367E-8]. After blockade of Ih channels with ZD7288, re-application of EtOH (30 mM) to the slice did not affect frequency of sAPs (2.49 ± 0.19 Hz). ZD7288 (30 μM) also induced a small hyperpolarization of 3.42 ± 0.43 mV (n = 5, −66.8 ± 1.43 to −70.22 ± 1.32 mV). For comparative purposes, we tested the effects of ZD 7288 and CsCl on the resting membrane potential of interneurons from adolescent rats that did not fire spontaneous action potentials. There were no significant changes in the resting membrane potentials by application of CsCl (2 mM) and ZD 7288 (30 μM) [from −66.15 ± 1.76 to −67.98 ± 2.20 mV (n = 6) after application of CsCl; from −66.40 ± 1.67 to −68.20 ± 1.92 mV (n = 5) after application of ZD7288 30 μM].
FIG. 6.
Ih is involved in EtOH enhancement of sAP firing. A, a: time course of the effect of ZD7288 (30 μM) on total current in an interneuron identified by basic morphological (fluorescence image, scale bar = 20 μm) and physiological properties (injected currents 100 pA, −200 pA, 600 ms) from an adolescent rat. Inset: representative traces under control conditions and after application of ZD7288. b: ZD7288 significantly reduced Ih amplitude (n = 7, **P < 0.01, paired t-test). B, a: the time course of responses of sAP firing after bath application of EtOH (30 mM), then ZD7288 (30 μM) followed by 30 mM EtOH in an interneuron from an adolescent rat. ZD7288 (30 μM) irreversibly decreased sAP firing frequency and blocked the effects of EtOH. b: the averaged time course graph for normalized sAP frequency (sAPs/min ±S, n = 5) from 5 cells. c: bar graph summarizing the effects of EtOH alone, ZD7288 alone, and application of EtOH after ZD7288 on the frequency of sAP firing (n = 5, 1-way ANOVA *P < 0.05). C, a: sAP traces were obtained under control, ZD7288 (30 μM), and EtOH (30 mM) after ZD7288. b: the traces of a single AHP in control (1), ZD7288 (2), and EtOH after ZD7288 (3) were superimposed. - - -, fits with a 2-exponential function. c: the summarized data show the effects of ZD7288 alone and application of EtOH after ZD7288 treatment on the slow component of AHP decay time. Note that ZD7288 significantly increased AHP τslow, and AHP τslow was unchanged by EtOH 30 mM after application of ZD7288 (n = 5, 1-way ANOVA *P < 0.05).
We also assessed the effects of ZD7288 on sAP AHP decay time. Typical traces from an interneuron from an adolescent rat are shown in Fig. 6C, a and b. In this interneuron (the same neuron as in Fig. 6Ba), the AHP decay time τslow was 43.9 ms at baseline, 140 ms after bath application of ZD7288 30 μM, and 136.4 ms after bath application of EtOH (Fig. 6Cb). Furthermore, ZD7288 30 μM had no effect on the fast component of AHP decay time (from 9.72 ± 0.67 to 10.56 ± 1.23 ms, n = 5). The averaged data representing the effect of ZD7288 on AHP τslow are shown in Fig. 6Cc, indicating that ZD7288 significantly prolonged AHP decay time slow components (τslow) to 127.73 ± 8.55 from 40.41 ± 3.37 ms of control [n = 5, 1 way ANOVA, F(2, 12) = 18.80, P = 2.01E-04], and τslow remained unchanged in the subsequent application of EtOH (30 mM, 125.57 ± 15.24 ms, n = 5). Altogether these results indicate that Ih participates in the mediation of EtOH-induced increases of sAP firing in hippocampal interneurons.
EtOH effects on Ih in interneurons from adolescent and adult rats
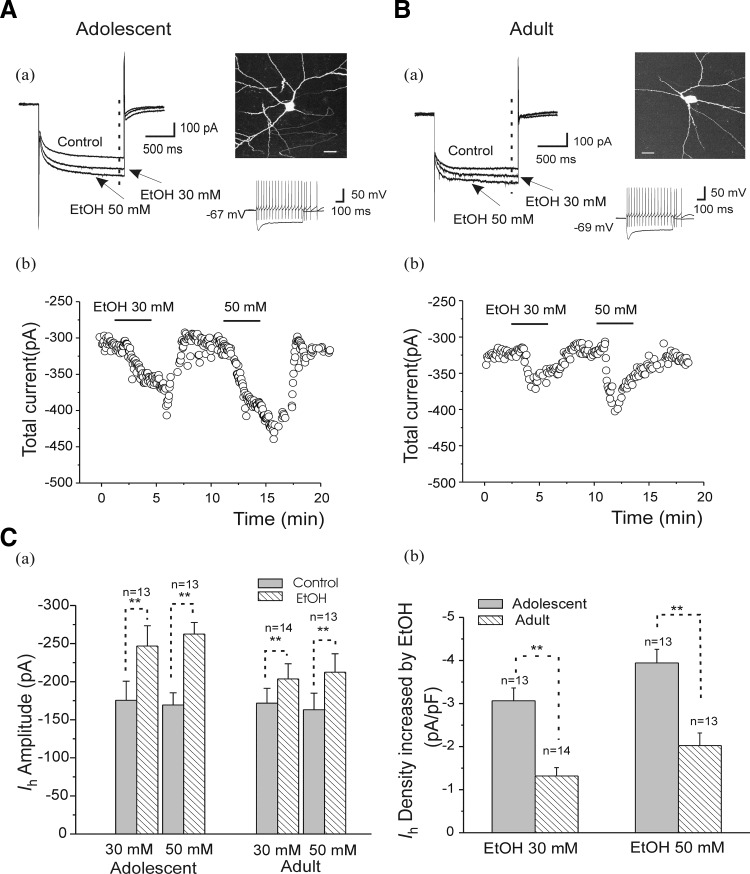
The preceding finding, that Ih mediates EtOH-induced increases in sAP firing, prompted us to explore if it could be of developmental significance. To pursue this, we assessed the effects of ethanol on Ih currents recorded in interneurons from animals in both age groups. We evoked Ih by using 1.2-s hyperpolarizing voltage steps to −130 mV from the −50 mV holding potential. The average Ih amplitude was −180.57 ± 21.14 pA (n = 21) in interneurons from adolescent animals and −163.89 ± 17.89 pA (n = 19) in interneurons from adults. This difference, however, was not statistically significant. Bath application of EtOH (30 and 50 mM) reversibly increased Ih amplitude in cells from animals in both age groups (Fig. 7, A and B). Typical current traces of Ih in the presence of EtOH (30 and 50 mM) of interneurons recorded from adolescents and adults are shown in Fig. 7, A and B, a and b. The total currents corresponding to the - - - in Fig. 7, Aa and Ba, were calculated and plotted before and during EtOH application. EtOH (30, 50 mM) increased Ih amplitude in interneurons from both age groups (Fig. 7Ca). In interneurons from adolescent animals, EtOH (30 mM) significantly enhanced Ih amplitude from −175.61 ± 25.02 to −246.76 ± 26.67 pA (n = 13, paired t-test, t(12) = 11.28, P = 9.60E-08) and 50 mM EtOH further increased Ih amplitudes to −262.42 ± 15.38 from −169.35 ± 15.94 pA (n = 13, paired t-test, t(12) = 26.83, P = 4.41E-12). In interneurons from adult rats, EtOH also increased Ih amplitudes from −171.78 ± 19.56 and −163.15 ± 21.73 to −203.57 ± 19.99 pA (EtOH 30 mM, n = 14, paired t-test, t(13) = 7.79, P = 2.98E-06) and −212.38 ± 24.30 pA (EtOH 50 mM, n = 13, paired t-test, t(12) = 6.81, P = 1.86E-05), respectively (Fig. 7Ca). Because EtOH concentrations <30 or 50 mM had affected sAP firing, we also tested the effect of 10 mM EtOH on Ih and found that Ih amplitude was increased to −200.17 ± 16.50 pA from −170.0 ± 14.50 pA (n = 6, ∼30 pA) in slices from adolescent rats (t[5] = 4.6056, P = 0.0058).
FIG. 7.
Effects of EtOH on Ih recorded in CA1 interneurons from adolescent and adult rats. A, a: representative traces of current obtained before (control) and after application of EtOH 30 and 50 mM in an interneuron identified by basic morphological (fluorescence image, scale bar = 20 μm) and physiological properties (injected currents 100 pA, −200 pA, 600 ms) from an adolescent rat. The current traces are evoked by a 1.2-s hyperpolarizing voltage step from a holding potential of −50 to −130 mV. b: time course of the effects of EtOH (30 and 50 mM) on currents of the same interneuron. Total current (see a) measured at - - - was plotted before and during EtOH application. B: the same experimental protocol as A in an interneuron from an adult rat. C, a: summarized results show that EtOH (30, 50 mM) increased Ih amplitudes in interneurons from adolescent and adult rats (**P < 0.01, paired t-test). b: summarized bar graph shows the effects of EtOH (30 and 50 mM) on Ih density, which was obtained from EtOH-induced increase in the net Ih amplitude divided by each cell is capacitance. EtOH enhanced Ih density more powerfully in neurons from adolescent animals than from adult animals (**P < 0.01, unpaired t-test).
Furthermore, Ih density calculated by normalizing the EtOH-increased net current to the cell capacitance in each cell shows that EtOH increased Ih densities by −3.06 ± 0.29 pA/pF (23.25 ± 1.04 pF cell capacitance, n = 13, EtOH 30 mM) and by −3.93 ± 0.15 pA/pF (23.68 ± 1.16 pF cell capacitance, n = 13, EtOH 50 mM) in interneurons from adolescent animals (Fig. 7Cb). In interneurons from adult animals, Ih densities were increased by −1.32 ± 0.19 pA/pF (25.03 ± 0.86 pF cell capacitance, n = 14) and by −2.02 ± 0.29 pA/pF (24.99 ± 0.89 pF cell capacitance, n = 13) after application of EtOH at 30 mM and 50 mM concentration, respectively (Fig. 7Cb). The effects of EtOH on Ih density were greater in interneurons from adolescent rats than in those from adult rats (Fig. 7, Cb, unpaired t-test, t(25) = −4.95 and P = 4.19E-05 at 30 mM; t(24) = −4.30 and P = 2.4E-04 at 50 mM). These findings are consistent with the developmental differences that we observed with respect to the effects of EtOH on sAPs (Fig. 3).
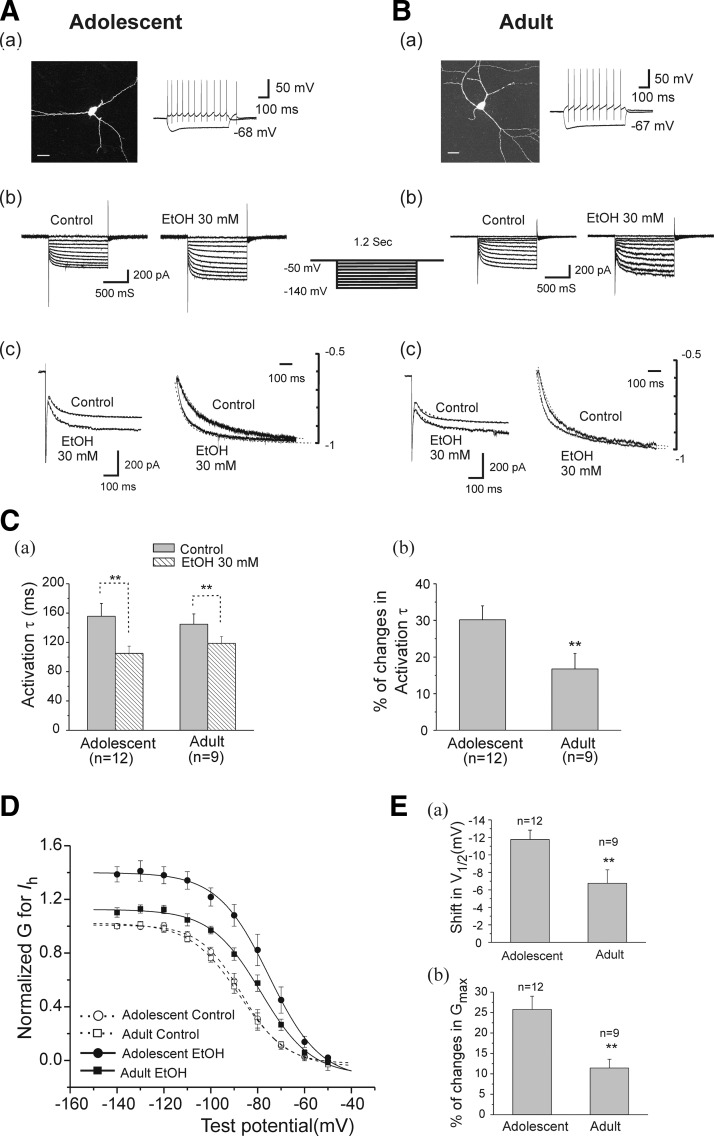
We next assessed the effect of EtOH on voltage-dependent Ih activation by constructing conductance-voltage (G-V) relation curves. Test pulses to potentials between −60 and −140 mV were applied from a holding potential of −50 mV (Fig. 8). Figure 8, A and B, shows that typical Ih voltage-dependent response traces and fitted activation time course of current traces (at −140 mV) in interneurons from adolescent (A) and adult rats (B) after application of 30 mM EtOH. The activation time courses were analyzed by fitting the activating phase of the current trace at the hyperpolarizing step pulse of −140 mV with a single-exponential function. EtOH (30 mM) significantly decreased the activation time constant of Ih from 155.55 ± 17.56 ms in control to 105.03 ± 9.80 ms (n = 12, paired t-test, t(11) = −4.54 and P = 8.50E-04) in neurons from adolescent rats (Fig. 8Ca) and from 144.75 ± 14.04 ms in control to 118.66 ± 9.65 (n = 9, paired t-test, t(8) = −2.97 and P = 0.018) in neurons from adults. This effect was more pronounced in neurons from adolescent rats (30.16 ± 3.81%, n = 12), compared with those from adults (16.74 ± 4.24%, n = 9, unpaired t-test, t(19) = 2.97, P = 0.03, Fig. 8 Cb). Voltage-dependent Ih activation curves were also analyzed before and after application of EtOH (30 mM). Each activation curve was plotted by calculating Ih amplitude at each of the test pulses, and converting into conductance (see methods). For each cell, the calculated conductance was normalized by the control maximal conductance in the absence of EtOH, which was estimated from the Boltzmann equation (Okamoto et al. 2006). We tested 12 neurons from adolescent and 9 neurons from adult animals. The basalV1/2 value and maximal Ih conductance were not significantly different in interneurons from adolescent animals compared with those from adult animals. Addition of EtOH (30 mM) shifted Ih activation curves to more depolarized potentials and also caused an increase in the maximal Ih conductance in interneurons from animals in both age groups. As demonstrated in Fig. 8D, in adolescent animals, fitted data show that 30 mM EtOH shifted V1/2 from −84.95 ± 2.44 to −73.20 ± 2.19 mV (n = 12, paired t-test, t(11) = −10.54, P = 4.41E-07). It also increased maximal Ih Gmax from 2.02 ± 0.23 to 2.74 ± 0.29 nS (n = 12, paired t-test, t(11) = −4.83, P = 5.24E-04) but did not change the k value. In adult animals, 30 mM EtOH also induced a depolarizing shift of V1/2 (from −83.85 ± 1.11 to −77.09 ± 1.19 mV, n = 9, paired t-test, t(8) = −4.68, P = 0.0016), enhanced Gmax (from 2.13 ± 0.18 to 2.38 ± 0.21 nS, n = 9, paired t-test, t(8) = −4.80, P = 0.0013), and failed to alter k values.
FIG. 8.
The effect of EtOH on Ih activation curves in interneurons from adolescent and adult rats. A, a: an interneuron identified by morphological (fluorescence image, scale bar = 20 μm) and physiological properties (injected currents 100 pA, −200 pA, 600 ms) from an adolescent rat. b: the Ih current traces evoked by a series of voltage steps were obtained from the same interneuron (a) before (control) and after application of 30 mM EtOH. c: the left overlapped traces of Ih were obtained at a test potential of −140 mV and were further scaled by amplitude at the end of a 1.2-s hyperpolarizing step command (right overlapped traces) before and after application of 30 mM EtOH. ···, fits by a single-exponential function, and the activation τ was obtained from fitted results. B: the same experimental protocol as A was employed in an interneuron from adult rat. C, a: he summarized Ih activation time course (see overlapping traces, Fig. 9A,c and B,c) at a hyperpolarizing pulse of −140 mV was shown, and EtOH significantly decreased the activation τ in both age groups (**P < 0.01, paired t-test). b: the bar graph shows EtOH induced percentage changes in Ih activation τ in interneurons from adolescent and adult animals (**P < 0.01, unpaired t-test). D: the summary of the effects of EtOH on Ih activation curves in cells recorded from adolescent (n = 12) and adult animals (n = 9). Ih amplitude from each neuron was converted to conductance (G, see methods). The conductance G for the same cell was normalized to the control maximal Ih G the value of which was measured at −140-mV test potential before application of EtOH. The normalized data points were fitted with a Boltzman function. EtOH shifted the curves to the right by altering V1/2 and increased Gmax in both age groups. E: bar graphs of summarized data showing change in V1/2 and Gmax during EtOH application. EtOH shifts V1/2 and increases maximal Ih conductance more in interneurons from adolescent compared with adult rats (**P < 0.01, unpaired t-test).
The comparison of the effects of EtOH on the shift in V1/2 and the increasing percentage of Gmax in interneurons from each age group is shown in Fig. 8E. EtOH more powerfully shifted V1/2 values to depolarization in interneurons from adolescent rats (−11.76 ± 1.07 mV, n = 12) than in those from adults (−6.75 ± 1.07 mV, n = 9, unpaired t-test, t(19) = −2.93, P = 0.008, Fig. 8Ea). Moreover, EtOH also increased maximal Ih conductance more powerfully in cells from adolescent rats (25.76 ± 3.24%, n = 12), compared with those from adults (11.44 ± 2.14%, n = 9, unpaired t-test, t(19) = 3.25, P = 0.004, Fig. 8Eb).
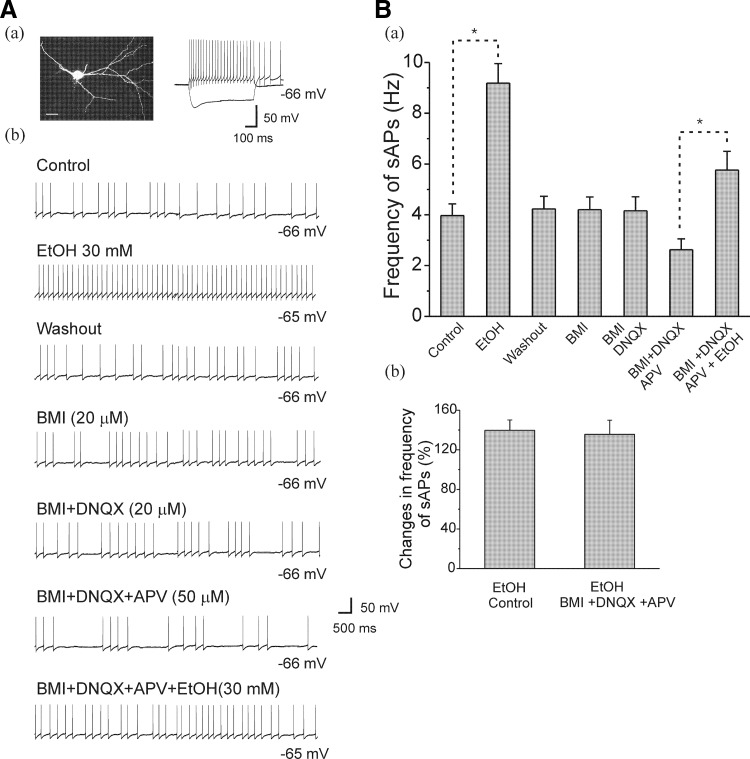
Effects of EtOH on sAPs in interneurons in the presence of glutamatergic and GABAergic blockers
By using acutely isolated VTA neurons in which all synaptic inputs were absent, it has been determined that EtOH excites VAT neurons directly (Brodie et al. 1999; Koyama et al. 2007). Although our preceding results confirmed the involvement of Ih currents in mediating EtOH-induced enhancement of firing rates, these experiments were performed in the presence of all synaptic inputs. Thus it was not clear whether the effect of EtOH on interneuron firing may have been mediated by alterations of excitatory and/or inhibitory synaptic inputs onto the recorded interneurons. We therefore assessed the effects of EtOH on spontaneous firing in 10 interneurons from PD 30–40 rats, after inhibitory and excitatory synaptic inputs were sequentially blocked by BMI (GABAA receptor antagonist), DNQX (AMPA receptor antagonist), and APV (N-methyl-d-aspartate receptor antagonist), respectively. As demonstrated in Fig. 9, the sAPs were recorded from morphologically and physiologically identified interneurons (Fig. 9Aa), and typical traces under each treatment are shown in Fig. 9Ab. In the baseline condition, EtOH (30 mM) increased sAP firing frequency to 9.18 ± 0.77 Hz from 3.97 ± 0.46 Hz (n = 10) in the absence of excitatory or inhibitory antagonists. After washout, the sAP firing frequency returned to baseline (4.23 ± 0.50 Hz, n = 10), and application of BMI (20 μM) did not change the frequency of sAP firing. Similarly, the addition of DNQX (20 μM) did not significantly change the sAP firing frequency (4.16 ± 0.55 Hz, n = 10). However, the addition of APV (50 μM) reduced sAP firing frequency to 2.62 ± 0.43 Hz (n = 10). Under these conditions, i.e., the block of excitatory and inhibitory neurotransmission, EtOH (30 mM) significantly increased sAP firing frequency [5.76 ± 0.82 Hz, n = 10, 1-way ANOVA, F(6, 63) = 11.92, P = 6.84E-9]. The summarized sAP firing frequency from 10 cells under this treatment sequence are illustrated in Fig. 9Ba. The averaged percent change in sAP firing frequency following administration of EtOH (30 mM) in the absence (139.76 ± 10.42%, n = 10) and presence (135.66 ± 14.30%, n = 10) of excitatory and inhibitory antagonists are shown in Fig. 9Bb. There was no significant difference between those two conditions (n = 10, paired t-test, t(9) = 0.33, P = 0.74). Thus it seems unlikely that the effects of EtOH on firing rates in interneurons that we have described in the preceding text are mediated indirectly, through actions on excitatory or inhibitory synaptic activity.
FIG. 9.
Effects of EtOH on sAP firing frequency of interneurons from adolescent rats in the presence of excitatory and inhibitory antagonists. A, a: an interneuron identified by basic morphological and physiological properties from an adolescent rat. Photomicrography is a fluorescence image of an interneuron (scale bar = 20 μm); the current trace is evoked by injection of depolarizing (100 pA) and hyperpolarizing (-200 pA) current for 600 ms. b: representative traces of firing rates are shown under control and following bath application of inhibitory and excitatory blockers (see labels for each antagonists applied) and EtOH. B, a: the bar graph shows averaged sAP firing frequency under each condition (n = 10, *P < 0.05, 1 1-way ANOVA). b: the summarized data show that EtOH increased the mean percentage of sAP frequency in the absence (control) and presence of inhibitory and excitatory antagonists, there was no significant difference between 2 conditions.
DISCUSSION
We assessed the effects of acute EtOH on the firing characteristics and Ih function of a specific subpopulation of hippocampal interneurons that exhibit intrinsic spontaneous firing properties. Anatomically, the interneurons that we tested were located at the border of SR and S-LM of the CA1 region. We found that 1) acute EtOH had no effect on eAPs elicited by depolarizing current injection in neurons that did not fire spontaneously; 2) in spontaneously firing cells, EtOH increased the action potential firing rate, and decreased the decay time of AHPs; 3) EtOH enhanced Ih amplitude and maximal conductance, shifted Ih voltage-activation to depolarized potentials, and decreased activation time constants in interneurons that fired spontaneously; 4) all of these effects were more pronounced in interneurons from periadolescent rats than in those from adults; and 5) EtOH-induced enhancement of sAP firing rates in interneurons was observed in both the absence and presence of glutamatergic and GABAergic blocking agents.
The effect of EtOH on spontaneous firing in hippocampal CA1 interneurons recorded from periadolescent and adult rats has not been reported previously. In the present study, we chose to record from a subpopulation of spontaneously firing interneurons for this study. Thus these findings represent the first whole cell electrophysiological evidence for developmental modulation of the effects of acute EtOH on spontaneously active hippocampal CA1 interneurons. Consistent with the present findings, we have recently shown that GABAA receptor-mediated tonic currents in dentate granule cells are promoted more efficaciously in slices from periadolescent rats than in those from adults (Fleming et al. 2007). These data suggest that certain GABA receptor-mediated functions in the hippocampal formation are more sensitive to EtOH during adolescence, whereas others are more sensitive during adulthood and underscore the complex nature of the effects of EtOH on inhibitory circuits through which some of these effects may be mediated during development.
Whether acute ethanol enhances or attenuates neuronal firing activity seems to depend on the types of neurons tested. For example, EtOH reduces firing rate of pyramidal cells with regular spiking activity in somatosensory cortex (Sessler et al. 1998) and inhibits persistent activity in prefrontal cortical pyramidal cells (Tu et al. 2007). In contrast, enhancement of neuronal firing by acute EtOH has been demonstrated in dopaminergic cells in VTA (Appel et al. 2003; Brodie and Appel 1998; Koyama et al. 2007; Okamoto et al. 2006), and cerebellar Golgi cells (Carta et al. 2004). Furthermore, in slices from rats that experience withdrawal after repeated intermittent EtOH exposure, VTA dopaminergic cells display enhanced burst firing patterns (Hopf et al. 2007). Thus the present results that EtOH increases sAP firing in this subpopulation of hippocampal GABAergic interneurons is similar to those from dopaminergic cells in the VTA and Golgi cells in cerebellum and provides new evidence for a possible ionic mechanism whereby Ih mediates EtOH-induced increases in hippocampal interneurons. The developmental differences that we have observed underscore the potential importance of the present findings.
However, there is also some controversy in studies of Ih in VTA dopaminergic neurons, and the role of this current in mediating the effects of EtOH. In one recent study, EtOH-induced argumentation of Ih currents in VTA neurons from C57BL/6J mice was attenuated by ZD7288 (Okamoto et al. 2006). Those findings are consistent with the present data from hippocampal interneurons, although a separate study has shown that the stimulatory effect of EtOH on the excitability of VTA neurons from rats was blocked by quinidine but not by cesium or ZD7288 (Appel et al. 2003), suggesting that Ih could be one of many ionic currents responsible for EtOH-induced increase in firing rates VTA cells. Furthermore, it is possible that barium-sensitive currents may also be involved in the effects of EtOH on VTA neurons (McDaid et al. 2008).
The present data also indicate that EtOH-induced enhancement of sAP firing rates in interneurons from periadolescent rats is not dependent on glutamatergic or GABAergic synaptic inputs as this effect persisted even after excitatory and inhibitory synaptic activities mediated by ionotropic neurotransmitter receptors were blocked. A similar finding was also reported in VTA neurons (Okamoto et al. 2006). Given the fact that EtOH-induced increases in firing rates of interneurons were observed in the absence of this synaptic activity, the present data expand current knowledge regarding the role of Ih in the mediation of EtOH effects on nondopaminergic cells and suggest that Ih channels could serve a direct target for EtOH. It should also be noted that the concentration of EtOH that produced the measurable effects of EtOH on the firing rates in our present studies is much lower than those reported in VTA dopaminergic neurons of CB57 mice (Okamoto et al. 2006) or Fisher-334 rats (Appel et al. 2003). As shown in Fig. 3C, EtOH, at concentrations as low as 3 mM, increased in firing rates in neurons from animals of both age groups, suggesting that hippocampal interneurons are very sensitive to EtOH.
It has been shown that the AHP is involved in regulating the firing of s. radiatum hippocampal interneurons (Savic et al. 2001). In the present study, we demonstrated that the decay time (slow component) of AHPs following sAPs was shortened by EtOH. As one of many important conductances governing the excitability of neurons, the hyperpolarization-activated current, Ih, modulates the AHP time course and neurotransmitter release (Aponte et al. 2006; Maccaferri et al. 1993; Stocker et al. 1999). In spontaneously firing neurons, Ih contributes to the pacemaker depolarization that generates rhythmic activity; in nonpacing cells, Ih modulates resting membrane properties and limits the extent of hyperpolarizing and depolarizing responses (Maccaferri and McBain 1996; Pape 1996). The present results show that Ih in interneurons recorded from both age groups was irreversibly blocked by ZD7288 or cesium, and these results are consistent with previous reports (Aponte et al. 2006; Maccaferri and McBain 1996; Lupica et al. 2001). Additionally, the data indicate that Ih is involved in the regulation of spontaneous firing as demonstrated by the suppression of sAP firing by cesium and ZD7288. Ih and apamin-sensitive Ca2+-activated potassium currents are known to contribute to AHPs in hippocampal interneurons and pyramidal neurons (Maccaferri and MacBain 1996; Savic et al. 2001; Stocker et al. 1999) and to regulate their firing patterns, and AHPs consist of fast (fAHP, lasting 2–5 ms), medium (mAHP, lasting 50–100 ms), and slow (sAHP, lasting >1 s) components in hippocampal cells (Storm 1989). Interestingly, we found that the decay time of the AHP slow component (τslow), but not the fast component, was decreased by EtOH. This is consistent with studies of dopaminergic cells and indicates that Ih may also contribute to EtOH enhancement of sAP firing in hippocampal interneurons through limiting the AHP duration and shortening the interval between action potentials to promote multiple discharges during EtOH exposure. Ih is known to be sensitive to the intracellular concentration of Ca2+ in thalamic neurons (Lüthi and MacCormick 1998, 1999), and EtOH-induced increases in intracellular Ca2+ have been detected in neocortical neurons (Allansson et al. 2001) and in cerebellar Purkinje neurons (Gruol et al. 1997). Thus it is possible that EtOH- induced enhancement of Ih could be mediated by internal Ca2+ concentration and thereby contribute to AHP activation.
The growing literature indicates that the effects of EtOH on neurobehavioral function in general are developmentally regulated (Little et al. 1996; Markwiese et al. 1998; Swartzwelder et al. 1995a,b; White and Swartzwelder 2004; White et al. 2000), and particularly for GABAA receptor-mediated inhibitory function in the hippocampus (Li et al. 2003, 2006). We have shown previously that EtOH increases the frequency of sIPSCs in hippocampal pyramidal cells more potently in slices from adult rats than in that from adolescents in the absence of any age-dependent effect on mIPSCs frequency (Li et al. 2003, 2006). Our present data partially agree with our previous findings that the EtOH increases frequency of sIPSCs in pyramidal cells recorded from both age groups. In contrast to more potent EtOH enhancement of sIPSCs observed in CA1 pyramidal cells recorded from adult rats, EtOH enhances action potential frequency by interneurons but its effect is less potent for adults than for adolescents.
There is an apparent discrepancy between the present results from interneurons and our previous reports on data from pyramidal cells. Specifically, our earlier results showing greater enhancement of sIPSC frequency by EtOH in CA1 pyramidal cells from adult animals compared with those from adolescents (Li et al. 2006) would seem to predict that EtOH would increase interneuron firing more rather than less potently in interneurons from adults. However, in this report we found the opposite. EtOH promoted interneuron firing more potently in cells from periadolescents than in those from adults. One possible explanation would be that in the present report we recorded from interneurons that do not directly produce IPSCs in CA1 pyramidal cells. We sampled a small subset of interneurons located within SML-SR subregions. It is possible that these interneurons could have their primary synaptic influence elsewhere, while a distinct set of interneurons contact primarily the apical and basal dendrites of pyramidal cells (Buhl et al. 1994). It is well known that interneurons have diversified morphological and electrophysiological properties (Freund and Buzsaki 1996; Somogyi and Klausberger 2005), and it is likely that interneurons carrying different biomarkers may have different sensitivities to GABAA receptor modulators (Karson et al. 2008; Thomson et al. 2000). Indeed when CA1 pyramidal neurons are activated by either proximal or distal stimulation, the effect of ethanol on the resulting IPSCs is quite different. Proximally evoked IPSCs are far more sensitive to ethanol than those that are evoked by distal stimulation nearer to the region from which we recorded interneurons for the present study (Weiner et al. 1997), and this insensitivity is mediated at least in part by GABAB receptor activation (Wu et al. 2005). Thus any of a number of factors could account for the apparent discrepancy between the present data and those from CA1 pyramidal cells. In the future it will be important to conduct experiments like those reported here on interneurons more proximal to CA1 pyramidal cells.
There could also be differences associated with the Ih channel's physiological properties, developmental regulation of Ih channel subunit expression, or Ih function among special interneuronal populations. For instance, we found that Ih channel physiological function with regard to its sensitivity to EtOH is regulated developmentally as Ih current density was significantly increased by EtOH in cells from periadolescent than adult rats. EtOH (30 mM) caused V1/2 to shift toward more depolarization ∼12 mV in periadolescent rats, whereas there is ∼6 mV shifting in the adults. Furthermore, EtOH increased maximum Ih more powerfully in interneurons from periadolescents than in those from adults. Those results further support the idea that developmental regulation of the EtOH sensitivity of sAP firing could be due to its action on the Ih channel's physiological function. Four Ih channel subunits (HCN1-4) encoding Ih are expressed in axons and presynaptic terminals of GABAergic interneurons in the hippocampus (Notomi and Shigemoto 2004), and differential regulation of HCN isoform expression has been described during hippocampus development (Bender et al. 2001). The progressive expression of HCN1 is confined primarily to parvalbumin-expressing basket-type cells, whereas HCN2 is more prominent in other interneuron populations (Bender et al. 2001), although one study indicates that parvalbumin-expressing fast-spiking interneurons co-express HCN1 and HCN2 (Aponte et al. 2006). Moreover, an age-dependent spatiotemporal evolution of specific HCN expression in distinct hippocampal cell populations has been demonstrated (Brewster et al. 2007), suggesting that these channels co-expressing with unique biomarkers in hippocampal interneurons may have differing functions in mediation of EtOH sensitivity of interneurons in local network activity.
In summary, these results demonstrate for the first time that EtOH regulates spontaneous firing in a subpopulation of hippocampal interneurons, and this effect is mediated by EtOH action on Ih expressed in these interneurons and is developmentally regulated. This finding is of particular interest because it demonstrates the complex nature of the effects of EtOH on inhibitory circuits and the developmental mediation of these effects.
GRANTS
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA-014894 to H. S. Swartzwelder and by Veterans Affairs Senior Research Career Scientist Awards to H. S. Swartzwelder and W. A. Wilson.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Allansson et al. 2001.Allansson L, Khatibi S, Olsson T, Hansson E. Acute ethanol exposure induces transients, cell swelling and transformation of actin cytoskeleton in astroglial primary cultures. J Neurochem 76: 472–479, 2001. [DOI] [PubMed] [Google Scholar]
- Aponte et al. 2006.Aponte Y, Lien C-C, Reisinger E, Jonas P. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol 574: 229–243, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel et al. 2003.Appel SB, Liu Z, McElvain MA, Brodie MS. Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther 306: 437–446, 2003. [DOI] [PubMed] [Google Scholar]
- Ariwodola and Weiner 2004.Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors. J Neurosci 24: 10679–10686, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender et al. 2001.Bender RA, Brewster A, Santoro B, Ludwig A, Hofmann F, Biel M, Baram TZ. Differential and age-dependent expression of hyperpolarization-activated, cyclic nucleotide-gated cation channel isoforms 1-4 suggests evolving roles in the developing rat hippocampus. Neuroscience 106: 689–698, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand and Lacaille 2001.Bertrand S, Lacaille J-C. Unitary synaptic currents between lacunosum-moleculare interneurons and pyramidal cells in rat hippocampus. J Physiol 532: 369–384, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewster et al. 2007.Brewster AL, Chen Y, Bender RA, Yeh A, Shigemoto R, Baram TZ. Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus. Cereb Cortex 17: 702–712, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie and Appel 1998.Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res 22: 236–244, 1998. [PubMed] [Google Scholar]
- Brodie et al. 1999.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res 23: 1848–1852, 1999. [PubMed] [Google Scholar]
- Brown and Difrancesco 1980.Brown H, Difrancesco D. Voltage-clamp investigations of membrane currents underlying pace-maker activity in rabbit sino-atrial node. J Physiol 308: 331–351, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhl et al. 1994.Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature 368: 823–828, 1994. [DOI] [PubMed] [Google Scholar]
- Carta et al. 2003.Carta M, Ariwodola OJ, Weiner JL, Valenzuela CF. Alcohol potently inhibits the kainate receptor-dependent excitatory drive of hippocampal interneurons. Proc Natl Acad Sci USA 100: 6813–6818, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta et al. 2004.Carta M, Mameli M, Valenzuela CF. Alcohol enhances GABAergic transmission to cerebellar granule cells via an increase in Golgi cell excitability. J Neurosci 24: 3746–3751, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre and Castillo 2002.Chevaleyre V, Castillo PE. Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP. Proc Natl Acad Sci USA 99: 9538–9543, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie et al. 2000.Christie BR, Franks KM, Seamans JK, Saga K, Sejnowski TJ. Synaptic plasticity in morphologically identified CA1 stratum radiatum interneurons and giant projection cells. Hippocampus 10: 673–683, 2000. [DOI] [PubMed] [Google Scholar]
- Cope et al. 2002.Cope DW, Maccaferri G, Márton LF, Roberts JDB, Cobden PM, Somogyi P. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience 109: 63–80, 2002. [DOI] [PubMed] [Google Scholar]
- Fleming et al. 2007.Fleming RL, Wilson WA, Swartzwelder HS. Magnitude and ethanol sensitivity of tonic GABAA receptor-mediated inhibition in dentate gyrus changes from adolescence to adulthood. J Neurophysiol 97: 3806–3811, 2007. [DOI] [PubMed] [Google Scholar]
- Freund and Buzsaki 1996.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus 6: 347–470, 1996. [DOI] [PubMed] [Google Scholar]
- Freund and Gulyas 1997.Freund TF, Gulyas AI. Inhibitory control of GABAergic interneurons in the hippocampus. Can J Physiol Pharmacol 75: 479–487, 1997. [PubMed] [Google Scholar]
- Galindo et al. 2005.Galindo R, Zamudio PA, Valenzuela CF. Alcohol is a potent stimulant of immature neuronal networks: implications for fetal alcohol spectrum disorder. J Neurochem 94: 1500–1511, 2005. [DOI] [PubMed] [Google Scholar]
- Gruol et al. 1997.Gruol DL, Parsons KL, DiJulio N. Acute ethanol alters calcium signals elicited by glutamate receptor agonists and K+ depolarization in cultured cerebellar Purkinje neurons. Brain Res 773: 82–89, 1997. [DOI] [PubMed] [Google Scholar]
- Gulyas et al. 1998.Gulyas AI, Toth K, McBain CJ, Freund TF. Stratum radiatum giant cells: a type of principal cell in the rat hippocampus. Eur J Neurosci 10: 3813–3822, 1998. [DOI] [PubMed] [Google Scholar]
- Hopf et al. 2007.Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol 98: 2297–310, 2007. [DOI] [PubMed] [Google Scholar]
- Karson et al. 2008.Karson MA, Whittington KC, Alger BE. Cholecystokinin inhibits endocannabinoid-sensitive hippocampal IPSPs and stimulates others. Neuropharmacology 54: 117–128, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama et al. 2007.Koyama S, Brodie MS, Appel SB. Ethanol inhibition of M-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J Neurophysiol 97: 1977–1985, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacaille and Schwartzkroin 1988.Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. J Neurosci 8: 1400–1410, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. 2003.Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of hippocampal GABAA receptor-mediated IPSCS to ethanol. Alcohol Clin Exp Res 27: 2017–2022, 2003. [DOI] [PubMed] [Google Scholar]
- Li et al. 2006.Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcohol Clin Exp Res 30: 119–126, 2006. [DOI] [PubMed] [Google Scholar]
- Little et al. 1996.Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res 20: 1346–1351,1996. [DOI] [PubMed] [Google Scholar]
- Lupica et al. 2001.Lupica CR, Bell JA, Hoffman AF, Watson PL. Contribution of the hyperpolarization-activated current (Ih) to membrane potential and GABA release in hippocampal interneurons. J Neurophysiol 86: 261–268, 2001. [DOI] [PubMed] [Google Scholar]
- Lupica and Brodie 2006.Lupica CR, Brodie MS. Queer currents, steady rhythms, and drunken DA neurons.J Neurophysiol 95: 585–586, 2006. [DOI] [PubMed] [Google Scholar]
- Lüthi and McCormick 1998.Lüthi A, McCormick DA. Periodicity of thalamic synchronized oscillations: the role of Ca2+-mediated upregulation of Ih. Neuron 20: 553–563, 1998. [DOI] [PubMed] [Google Scholar]
- Lüthi and McCormick 1999.Lüthi A, McCormick DA. Modulation of a pacemaker current through Ca2+-induced stimulation of cAMP production. Nat Neurosci 2: 634–641, 1999. [DOI] [PubMed] [Google Scholar]
- Maccaferri et al. 1993.Maccaferri G, Mangoni M, Lazzari A, DiFrancesco D. Properties of the hyperpolarization-activated current in rat hippocampal CA1 pyramidal cells. J Neurophysiol 69: 2129–2136, 1993. [DOI] [PubMed] [Google Scholar]
- Maccaferri and McBain 1996.Maccaferri G, McBain CJ. The hyperpolarization-activated current (Ih) and its contribution to pacemaker activity in rat CA1 hippocampal stratum oriens-alveus interneurons. J Neurophysiol 497: 119–130, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid et al. 2008.McDaid J, McElvain MA, Brodie MS. Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J Neurophysiol 100: 1202–1210, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison and Nicoll 1984.Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA 1 pyramidal neurons in vitro. J Physiol 354: 319–331, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee 1998.Magee JC Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese et al. 1998.Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res 22: 416–421, 1998. [PubMed] [Google Scholar]
- Mayer and Westbrook 1983.Mayer ML, Westbrook GL. A voltage-clamp analysis of inward (anomalous) rectification in mouse spinal sensory ganglion neurons. J Physiol 340: 19–45, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi and Shigemoto 2004.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neuro 471: 241–276, 2004. [DOI] [PubMed] [Google Scholar]
- Okamoto et al. 2006.Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol 95: 619–626, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape 1996.Pape HC Queer current and pacemaker: the hyperpolarization-activated cation current in neurons. Annu Rev Physiol 58: 299–327, 1996. [DOI] [PubMed] [Google Scholar]
- Sanna et al. 2004.Sanna E, Talani G, Busonero F, Pisu MG, Purdy RH, Serra M, Biggio G. Brain steroidogenesis mediates ethanol modulation of GABAA receptor activity in rat hippocampus. J Neurosci 24: 6521–6530, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savic et al. 2001.Savic N, Pedarzani P, Sciancalepore M. Medium afterhyperpolarization and firing pattern modulation in interneurons of stratum radiatum in the CA3 hippocampal region. J Neurophysiol 85: 1986–1997, 2001. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin and Mathers 1978.Schwartzkroin PA and Mathers LH. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res 157: 1–10, 1978. [DOI] [PubMed] [Google Scholar]
- Sessler et al. 1998.Sessler FM, Hsu FC, Felder TN, Zhai J, Lin RC, Wieland SJ, Kosobudb AK. Effects of ethanol on rat somatosensory cortical neurons. Brain Res 804: 266–274, 1998. [DOI] [PubMed] [Google Scholar]
- Somogyi and Klausberger 2005.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol 562: 9–26, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear 2000.Spear L The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24: 417–463, 2000. [DOI] [PubMed] [Google Scholar]
- Stocker et al. 1999.Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci USA 96: 4662–4667, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm 1989.Storm JF An after-hyperpolarization of medium duration in rat hippocampal pyramidal cells. J Physiol 409: 171–190, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartzwelder et al. 1995a.Swartzwelder HS, Wilson WA, Tayyeb MI. Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res 19: 1480–1485, 1995a. [DOI] [PubMed] [Google Scholar]
- Swartzwelder et al. 1995b.Swartzwelder HS, Wilson WA, Tayyeb MI. Differential sensitivity of NMDA receptor-mediated synaptic potentials to ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res 19: 320–323, 1995b. [DOI] [PubMed] [Google Scholar]
- Thomson et al. 2000.Thomson AM, Bannister AP, Hughes DI, Pawelzik H. Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur J Neurosci 12: 425–436, 2000. [DOI] [PubMed] [Google Scholar]
- Tu et al. 2007.Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, Chandler LJ, Woodward JJ. Ethanol inhibits persistent activity in prefrontal cortical neurons. J Neurosci 27: 4765–4775, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida et al. 1998.Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol 506: 755–773, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner et al. 1997.Weiner JL, Gu C, Dunwiddie TV. Differential ethanol sensitivity of subpopulations of GABAA synapses onto rat hippocampal CA1 pyramidal neurons. J Neurophysiol. 77: 1306–1312, 1997. [DOI] [PubMed] [Google Scholar]
- White et al. 2000.White AM, Ghia AJ, Levin ED, Swartzwelder HS. Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res. 24: 1251–1256, 2000. [PubMed] [Google Scholar]
- White and Swartzwelder 2004.White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann NY Acad Sci 1021: 206–220, 2004. [DOI] [PubMed] [Google Scholar]
- Wu et al. 2005.Wu PH, Poelchen W, Proctor WR. Differential GABAB receptor modulation of ethanol effects on GABA(A) synaptic activity in hippocampal CA1 neurons. J Pharmacol Exp Ther 312: 1082–1089, 2005. [DOI] [PubMed] [Google Scholar]