Abstract
Neural responses throughout the sensory system are affected by stimulus history. In the inferotemporal cortex (IT)—an area important for processing information about object shape—there is a substantially reduced response to the second presentation of an image. Understanding the mechanisms underlying repetition suppression may provide important insights into the circuitry that generates responses in IT. In addition, repetition suppression may have important perceptual consequences. The characteristics of repetition suppression in IT are poorly understood, and the details, including the interaction between the content of the first and second stimulus and the time course of suppression, are not clear. Here, we examined the time course of suppression in IT by varying both the duration and stimulus content of two stimuli presented in sequence. The data show that the degree of suppression does not depend directly on the response evoked by the first stimulus in the recorded neuron. Repetition suppression was also limited in duration, peaking at ≈ 200 ms after the onset of the second (test) image and disappearing before the end of the response. Neural selectivity to a continuum of related images was enhanced if the first stimulus produced a weak response in the cell. The dynamics of the response suggests that different parts of the input and recurrent circuitry that gives rise to neural responses in IT are differentially modulated by repetition suppression. The selectivity of the sustained response was preserved in spite of substantial suppression of the early part of the response. The data suggest that suppression in IT is a property of the input and recurrent circuitry in IT and is not directly related to the degree of response in the recorded neuron itself.
INTRODUCTION
Under natural viewing conditions, all objects are observed within the context of a stimulus history. We repetitively observe the same object across multiple time scales (seconds, minutes, hours, days, and weeks). Concomitantly, neural responses in the visual system are strongly modulated by recent stimulus history (Clifford et al. 2007; Grill-Spector et al. 2006; Kohn 2007; Krekelberg et al. 2006a; Wark et al. 2007). For example, in the inferotemporal cortex (IT) of the monkey—an area important for processing information about object shape—presenting two identical stimuli in sequence results in a substantially (≈50%) reduced overall response to the second stimulus. (Li et al. 1993; Lueschow et al. 1994; McMahon and Olson 2007; Miller et al. 1991, 1993b; Sawamura et al. 2006; Xiang and Brown 1998).
There are a variety of potential influences of stimulus history that could be associated with repetition-induced suppression in IT. First, simple adaptation, narrowly defined as an adjustment in sensitivity in a neuron as a result of its recent response, could underlie repetition-induced suppression. An overall reduction in the firing rate to the second stimulus might arise through any number of intracellular processes that simply result from recent excitation of the cell—a form of fatigue of the mechanisms in the cell that give rise to its spiking output (Carandini 2000; Clifford et al. 2007). If suppression in IT results from fatigue as a result of responses to briefly presented images, the reduction in response to a second stimulus should be proportional to the magnitude of the response to the initial stimulus. In addition, any stimulus that produces an equivalent response in the neuron should produce an equivalent degree of adaptation. Alternatively, repetition-induced suppression might be associated with behavioral phenomena, such as priming (McMahon and Olson 2007; Pineda and Nava 1993; Xiang and Brown 1998), short-term memory (Li et al. 1993; Lueschow et al. 1994; Miller et al. 1991, 1993b; Xiang and Brown 1998), changes in classification biases with stimulus adaptation (Ng et al. 2008; Webster et al. 2004; Yamashita et al. 2005), and stimulus expectation (Summerfield and Koechlin 2008). Finally, the overall reduction in response could be the result of changes in the population response in earlier processing stages or in the recurrent networks that produce responses within IT, a mechanistic phenomenon that is only peripherally related to the behavioral phenomenon that co-occur with stimulus repetition. This explanation might suggest that the time course of repetition-induced suppression might not be uniform as the different dynamic components of the circuit adapt independently. In addition, a circuit based explanation for suppression does not predict a simple relationship between the response of the recorded neuron and the degree of suppression.
Sawamura et al. (2006) included a test of whether response fatigue, a narrowly defined form of adaptation, was sufficient to produce repetition suppression in IT cortex. Repetition suppression was examined with the presentation of two different sequential stimuli that produced similarly strong responses. When the equally strong stimuli were physically different, less response suppression was evoked. Only identical stimuli that evoked strong responses produced repetition suppression, providing strong evidence against a simple fatigue model of response suppression (Sawamura et al. 2006). However, do stimuli that produce relatively weak responses in a neuron also produce suppression equal to that produced by stimuli that produce stronger responses? Is the degree of repetition suppression correlated with the response to the initial stimulus? Systematic examination of the relationship between the response to the first and second stimulus in a sequence is difficult in IT, because the tuning dimensions of IT neurons are not known; neurons in IT show selectivity for complex objects that vary among many different dimensions (Allred et al. 2005; Desimone et al. 1984; Hung et al. 2005; Kiani et al. 2007; Kobatake and Tanaka 1994).
To examine this relationship, the response to the first stimulus in a sequence should be systematically varied, and the effect on the second stimulus should be examined. To further address the relationship between stimulus response and repetition suppression, we studied the interaction between pairs of stimuli using morphed images (Akrami et al. 2008; Liu and Jagadeesh 2008b). Morphed images produce predictably different levels of responses in individual IT neurons, as can other systematic manipulations of stimulus features (De Baene et al. 2007; Kayaert et al. 2003; Verhoef et al. 2008). Although this pattern of responses is not necessarily tuning, precisely, morphed stimuli do allow for a systematic examination of the effect of stimulus response on repetition suppression. Furthermore, we aimed to characterize the time course of response suppression because it may systematically differ across brain areas (Krekelberg et al. 2006a). In particular, duration of the stimuli might affect the degree of suppression, and the time course of suppression in single units might not be uniform (Boynton and Finney 2003; Fang et al. 2005; Kourtzi and Huberle 2005; Kourtzi et al. 2003; Krekelberg et al. 2005, 2006a). Therefore we characterized the time course of suppression and its dependence on stimulus duration.
We collected data from neurons in IT cortex while manipulating both the stimulus duration and the stimulus content of pairs of images presented in succession while the monkeys performed a fixation task. Stimulus content was manipulated by choosing two photographic images, one of which produced high firing rates (Eff) and the other did not (Ineff), and morphing between the two images, resulting in stimuli that produced intermediate responses between the two original images (Liu and Jagadeesh 2008b). Our results confirm (Sawamura et al. 2006) that suppression does not depend on the response level of the cell: stimuli that evoked low firing rates resulted in suppression, just as stimuli that produced high firing rates. In addition, we characterized a distinctive time course for repetition suppression; suppression was limited in duration and dynamic. Our data suggest that suppression effects in IT are complex, making the interpretation of functional MRI (fMRI) adaptation (Grill-Spector 2006) and behavioral results complicated (Clifford et al. 2007). In addition, the time course and recovery of responses suggest that suppression might result from circuitry that differs from the circuitry that generates the sustained period of response in IT (Akrami et al. 2008; Brincat and Connor 2006; Sugase et al. 1999).
METHODS
We recorded from 129 IT neurons in two adult rhesus macaques (monkey G: 38 neurons; monkey L: 91 neurons) in the morph stimulus repetition experiment and 67 neurons (monkey G: 22; monkey L: 45) in the standard stimulus repetition experiment. We used our standard recording techniques (Allred et al. 2005; Liu and Jagadeesh 2008b). In 103 morph experiments we used a 500-ms stimulus duration, and an overlapping subset of these units were also tested with the 160- (n = 31) and 80-ms (n = 28) stimulus durations. In the standard stimulus experiments, we used 300-ms stimulus durations and standard, nonmorphed images.
Experimental procedure
Briefly, surgery on each animal was performed to implant a head restraint, a cylinder to allow neural recording, and a scleral search coil to monitor eye position (Judge et al. 1980). Materials for these procedures were obtained from Crist Instruments (Hagerstown, MD) or produced in-house at the University of Washington. Responses of single IT neurons were collected while monkeys performed a fixation task. Spikes were recorded using the Alpha-Omega spike sorter (Nazareth, Israel). Coded spikes were stored on a PC at a rate of 1,000 Hz using CORTEX, a program for neural data collection and analysis developed at the NIH (Bethesda, MD). Eye movements were monitored and recorded (at 500 Hz) using an eye coil based system from DNI (Newark, DE). All animal handling, care, and surgical procedures were performed in accordance with guidelines established by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at the University of Washington.
Chamber placement
Chambers were placed over the right hemisphere, using stereotaxic coordinates. Neural recordings were targeted near the center of the chamber (monkey L: 17 L, 17.5 A; monkey G: 16 L, 17.5A); this location is between the perirhinal sulcus and the anterior middle temporal sulcus in reference to reconstructions from the structural MRI. Recording depths ranged from 27 to 32 mm for monkey L and 30 to 33 mm for monkey G. Depth measurements are from the dural surface, measured during an early recording session. The location of these recording sites could include both IT and perirhinal cortex, although anatomy is currently unavailable because the animals are still participating in other experiments. The recording locations are identical to those in Liu and Jagadeesh (2008b).
Recording procedures
To isolate neurons, we moved the electrode while monkeys performed the passive fixation task with sets of 24 images arranged in 12 pairs (Fig. 2 of Liu and Jagadeesh 2008b), as a well as a set of less familiar images, also arranged in 12 pairs of two images. When the experimenter judged that a neuron responded better to one of the two images in the pairs of images, she recorded from that neuron while the monkey performed the fixation task with that stimulus pair.
We repeatedly sampled a single location until we could no longer isolate cells with selectivity for one of the photographic images used our experiments. We moved the electrode location only when selectivity was not detectable over 2–3 days of recording and moved only slightly across the surface (<1 mm). The range of sampled sites spanned a 4-mm-diam circle centered on the stereotaxic locations above. Using this procedure, we found potential selectivity for one of the image pairs in ∼75% of the attempted sessions after sampling one to three sites along the track; thus the cells included in this sample were found frequently.
Stimuli
Images consisted of photographs of people, animals, natural and man-made scenes, and objects. All images were 90 × 90 pixels and were drawn from a variety of sources, including the world wide web, image databases, and personal photo libraries. Image pairs were organized before recording sessions into pairs of stimuli. From these predefined lists of image pairs, selective neurons were found (see Recording procedures) for a total of 26 unique image pairs used in the analysis. Twelve of these image pairs are shown in Fig. 2 of Liu and Jagadeesh (2008b). Stimuli were presented on a computer monitor with 800 × 600 resolution (refresh rate, 100 Hz). At the viewing distance used, images subtended 4°. In addition to the morphed images, two unrelated sets of 24 images were also tested. These images were only tested in the identical image repetition condition.
Effective and ineffective images
Based on the average response between 80 and 580 ms after the first presentation of a stimulus, we assigned the image in the pair that provided a stronger response to be the Eff image, whereas the other was assigned to be the Ineff image. Because we recorded from multiple neurons with the same stimulus sets, either of the two images in a pair could serve as the Eff image during a particular recording session.
Image morphing and ranking
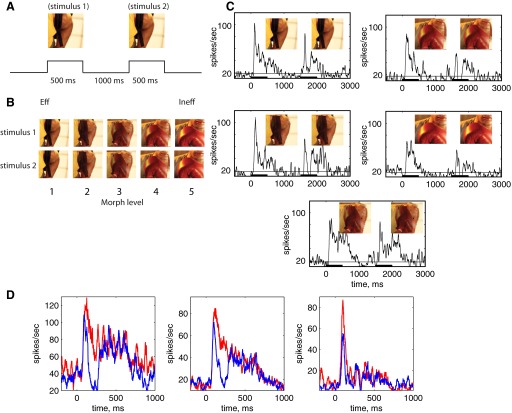
Each of the pairs of images was morphed using MorphX (http://www.norrkross.com/software/morphx/MorphX.php), a freeware, open source program for morphing between two photographic images. We constructed nine intermediate images in between the two original images, as described in Liu and Jagadeesh (2008b); examples of images and their morph variants are presented in Fig. 2 of Liu and Jagadeesh (2008b). Five of these 11 images (the 2 originals, the intermediate image, and 2 images near the endpoints) were used in our experiment. In this study, we term the original Eff image “morph level 5,” the original Ineff image “morph level 1,” and the image putatively containing 50% of each image “morph level 3,” “Morph level 4” and “morph level 2” round out the set of five images. These morph levels cannot be presumed to correspond to percent or proportions of either image (because the morphing algorithm is not guaranteed to be linear). However, putatively, morph levels 1–5 correspond to 0, 20, 50, 80, and 100% of the Eff image. The particular pair used in a recording session depended on observing selectivity for one of the images in the pair.
Fixation task
Suppression of neural responses during stimulus repetition was measured during a fixation task. Two stimuli were presented in sequence, during a single trial, and the monkey's task was to maintain fixation throughout the trial to receive a reward at the end of the trial. The five different images described above were presented in all possible combinations, including five different possible images first in the sequence and each of the five different possible images second in the sequence, yielding 25 different repetition conditions, ranging from the repetition of an identical image to the Eff followed by the Ineff image and the Ineff image followed by the Eff. The 25 conditions were presented in random order.
Each trial began with the appearance of a fixation spot. The monkey was required to maintain fixation within a 4° diam fixation window. After a 500- to 800-ms delay period, the first image was presented, followed by an interstimulus delay period. The second image was presented for the same duration as the first image. The image durations were 80, 160, and 500 ms, with an interstimulus delay of 1,000 ms. After the presentation of the second image, another 1,000-ms delay period occurred, and the animal was rewarded with a drop or drops of juice or water. The intertrial interval was a minimum of 3,500 ms, although it could be longer, depending on how long it took the animal to re-initiate a trial. The monkey was required to maintain fixation through the stimulus presentation period until reward was delivered. Small saccades contained within the 4° fixation window during the fixation period did occur during this period. To test whether these saccades explained the pattern of response suppression, we looked for differences in both saccade frequency and magnitude during the presentation of stimulus 1 and stimulus 2. The analysis included the entire presentation duration of both images. No significant differences were observed.
The set of nonmorphed images was tested with the same task, with different stimulus timing. The stimulus duration was 300 ms, the interstimulus interval was 300 ms (instead of 1,000 ms), and only repetitions of identical stimuli were presented.
Analysis of neural data
Neurons were included in the population for analysis if there was a qualitative assessment of selectivity (n = 129, morph experiment; n = 67, standard stimulus experiment). Most of these cells showed a significant difference in selectivity for the first presentation of the two photographic images (89/129, 69%, morph experiment; 50/67, 75%, standard stimulus experiment; unpaired t-test for an Eff and Ineff image, P < 0.05). Choosing different populations of cells, for example, only cells that pass a selectivity criterion (P < 0.05, t-test), did not change the overall pattern of results. The results shown were similar in the two monkeys.
Average spike rates were calculated (as presented in most figures) by aligning action potentials to the onset of the first stimulus and analyzing the data from before the onset of the first image until after the offset of the second (test) image. The peristimulus time histogram (PSTH) for each cell was calculated by averaging the rate functions across the repeated trials of presentation of the same stimulus. The population PSTH was calculated by averaging the PSTHs across all cells. All completed trials were included in the analyses; trials were excluded if the monkey made a saccade away from the fixation window during the trial. The PSTHs were smoothed by convolving with a Gaussian kernel (window = 20 ms) or calculating a running average of firing rates as described below. Average PSTHs were constructed by first averaging across individual cells for the appropriate stimulus conditions and averaging across the cells (for the relevant conditions). PSTHs were smoothed after averaging across cells.
The running average of firing rate, FR(t), was calculated for each neuron, for cell and condition, by averaging firing rate across multiple presentations of each first and second (test) stimulus in overlapping time bins (also called epochs) of 25 ms, shifted in time steps of 1 ms (Zoccolan et al. 2007). This calculation of average firing rate smoothes the data. The average FR(t) was plotted at the left of the 25-ms bin. Therefore average responses at time 0 consist of the average of responses from 0 to 25 ms after stimulus onset, the point at 1 ms, to the response 1–26 ms after stimulus onset, and so on.
We calculated a suppression ratio (SR) by dividing the FRstimulus2(t)/FRstimulus1(t) for each relevant stimulus condition (i.e., suppression condition). The suppression ratio was only calculated for identical stimulus 1 and stimulus 2 conditions, that is, the 5/25 conditions in the morph data set and all 24/24 conditions in the alternative independent stimulus set. The firing rate was first averaged across the relevant conditions for each cell and then averaged across cell populations by calculating the geometric mean. This ratio is 1 if the two responses are equal, <1 if the response to the second stimulus is less than that to the first stimulus, and >1 if the response to the second stimulus is greater than that to the first stimulus. The suppression ratio describes the relationship between the response to identical second and first images in a sequence.
We calculated a transience index (TI) (Tamura and Tanaka 2001) to compare response suppression to the dynamics of the response to stimulus 1: TI = [FRstimulus1 (transient epoch) − FRstimulus1 (sustained epoch)]/[FRstimulus1 (transient epoch) + FRstimulus1 (sustained epoch)], where the transient epoch was 80–180 ms after stimulus onset and the sustained epoch was 100–80 ms after stimulus offset.
We calculated a stimulus index (SI) that contrasts response during the presentation of stimulus 1 with baseline as [FRstimulus1 (stimulus epoch) − FR (baseline epoch)]/[FRstimulus1 (stimulus epoch) + FR (baseline epoch)]. This index was used to select a subgroup of cells with low response to one image and a high response to the other.
RESULTS
We recorded from 129 single IT units in two macaque monkeys. Cells were chosen based on a qualitative finding of response differences between two different photographic images during the experiment. For each cell, we chose an “effective” image that evoked a stronger response in the neuron and in “ineffective” image that evoked a smaller response. We interpolated between these images to produce intermediate stimuli (Fig. 1B). The Intermediate stimuli usually produced responses intermediate to the two original stimuli from which they were interpolated (Liu and Jagadeesh 2008a,b). Thus we were able to test the response with stimuli that evoked different response levels in the neuron. Stimuli were presented at the fovea, and the content and timing of the first and second stimulus in a sequence were manipulated. The monkeys’ task was to maintain fixation throughout the stimulus presentation period.
FIG. 1.
Repetition suppression paradigm and single cell examples. A: in a single trial, 2 images were presented in sequence. The monkey was required to maintain fixation throughout the stimulus presentation period. B: images were chosen so that 1 image activates the cell being studied (Eff) better than the other one (Ineff) using a larger set of 24 unrelated images. The 5 images, numbered 1–5, putatively correspond to 0, 20, 50, 80, and 100% of the Eff image, but the morphing algorithm cannot be presumed to be linear. All possible combinations of stimulus 1 and stimulus 2 were used in the experiment, yielding 25 different conditions. C: peristimulus time histograms (PSTHs) showing neural response averaged over repeated trials of stimulus presentation to the subset of sequences (5 conditions) of 2 identical stimuli of different morph levels. PSTHs are Gaussian smoothed (kernel = 40 ms). D: average PSTHs for cell shown in C (middle) and 2 additional cells for all repetitions of identical stimuli. PSTHs are constructed by averaging the response in 25-ms bins and stepping each bin by 1 ms. Red line shows response to stimulus 1 and blue to stimulus 2.
We measured the effect of both stimulus duration and stimulus content of stimulus 1 on the response suppression to stimulus 2. Below, the time course of repetition suppression is described first. The relationship between the response level of stimulus 1 and the degree of response suppression is examined. We conclude by looking at the effect of stimulus 1 on tuning of responses to stimulus 2 over the morph stimulus space.
Time course response suppression
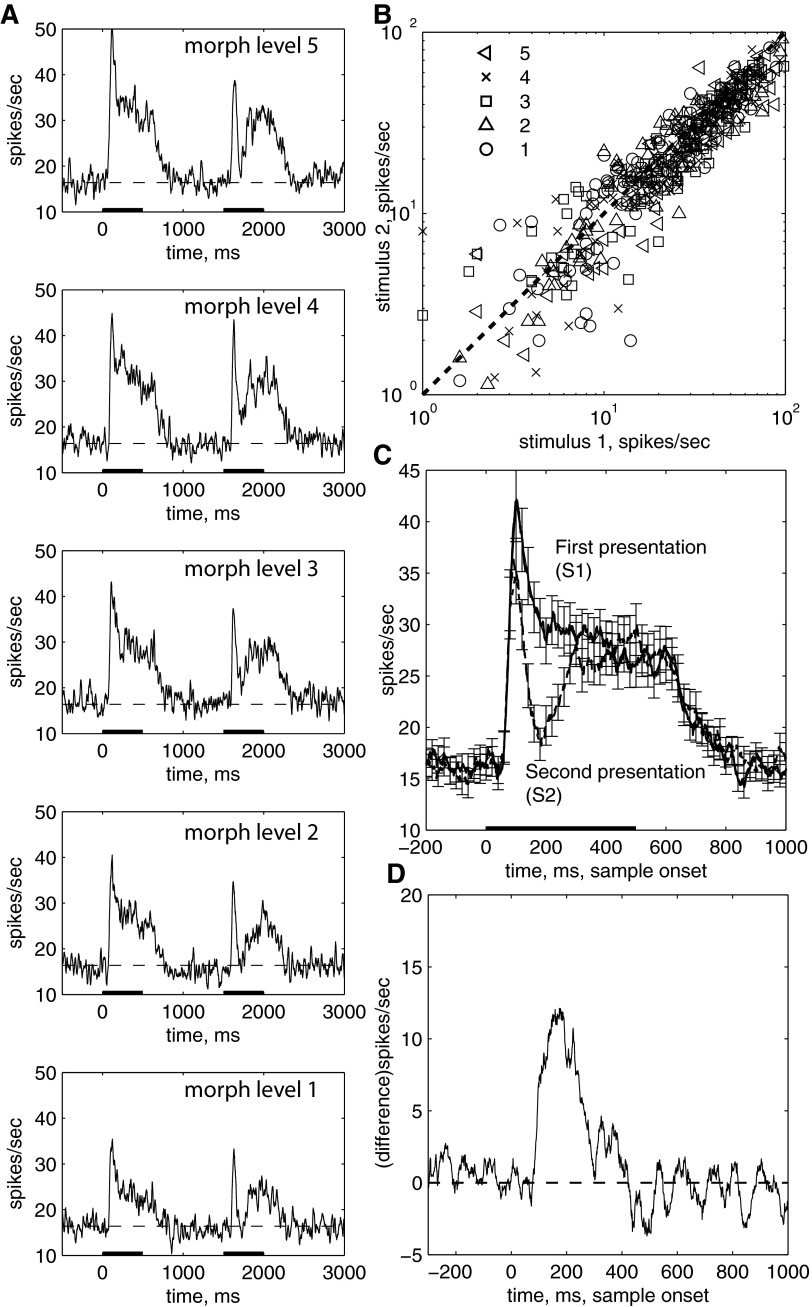
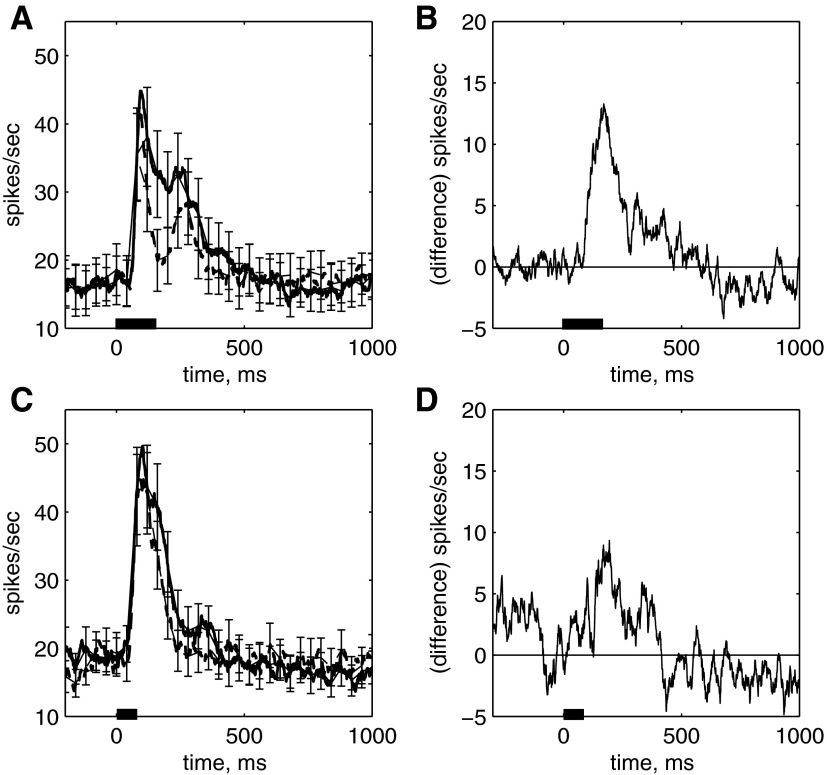
The time course of response suppression was examined in trials when stimulus 1 and stimulus 2 were identical. The first striking observation was that the repetition suppression was clearly limited in duration when stimuli were presented for stimulus durations of 500 ms, separated by 1,000 ms. As seen in the single cell example in Fig. 1C, the response to stimulus 2 was suppressed for a limited duration, with the peak suppression occurring at ∼200 ms after stimulus onset. The response increased and returned to the same level as stimulus 1. This pattern was observed for stimuli that produced different stimulus 1 response levels (Fig. 1C). The time course can be observed in this cell and two other example cells when collapsed across each of the different stimuli (Fig. 1D). Each of these cells produced different sustained and transient response levels (and had different ratios between the transient and sustained response). However, for each cell, the response to stimulus 2 was suppressed at stimulus onset and shortly after, reaching a minimum response at ≈200 ms after stimulus onset. The response recovered to match the sustained response to stimulus 1.
The time course of suppression seen in these example cells was observed in the population response (Fig. 2A). Across the population of neurons, the response to stimulus 2 was suppressed compared with the response to stimulus 1. Significant suppression was seen for all levels of response, and the suppression dipped below baseline for the stimulus that produced the smallest response (Fig. 2A, bottom). When averaged across the entire stimulus duration, the response to stimulus 2 was significantly suppressed for the stimulus epoch (80–580 ms after test onset) for all of the different morph levels (Fig. 2B, morph levels 1–5, different symbols correspond to different images, P ≪ 0.0001). Although the overall response to stimulus 2 is suppressed, as seen in many other examinations of IT, it is the dynamic nature of the suppression that is noteworthy (Fig. 2C). As seen in the average time course for stimulus 1 and stimulus 2 (averaged across all of the different stimulus strengths or morph level and across the population of cells), the average peak suppression occurred at ≈160 ms after the onset of stimulus 2 (paired t-test, 160- to 260-ms epoch, P < 0.00001). The response to stimulus 2 increased, until it equaled the response to stimulus 1 at ≈400 ms (paired t-test, P = 0.73, 400- to 500-ms epoch). Both responses returned to baseline at 840 ms after stimulus onset (P < 0.05, bins from 0–100 to 740–840 ms). The time course of the suppression is further characterized in the average of the response differences to stimulus 1 and stimulus 2 (Fig. 2D).
FIG. 2.
Population average of response to the repetition of an identical stimulus. n = 103 experiments. A: top to bottom: Eff morphed to Ineff image (morph level 5, 4, 3, 2, 1). Data are smoothed with a Gaussian with kernel = 20 ms. B: mean response to presentation of test vs. adapt stimulus, 80- to 580-ms epoch. Legend refers to morph level as in A. The response to stimulus 2 is significantly smaller than the response to the 1st for all morph levels (P < 0.001). C: mean response to stimulus 1 (solid) and stimulus 2 (dashed) stimulus as a function of time following image onset averaged across all morph levels. The time course of response suppression peaks at ∼160 ms after stimulus onset. D: difference between response to stimulus 1 and stimulus 2 as a function of time. The response difference peaks at 160–260 ms and disappears by 400–500 ms. Average population histogram: each point represents mean response in 25-ms bin, stepped 1 ms for each successive point. Error bars are SE, drawn on every 20th point.
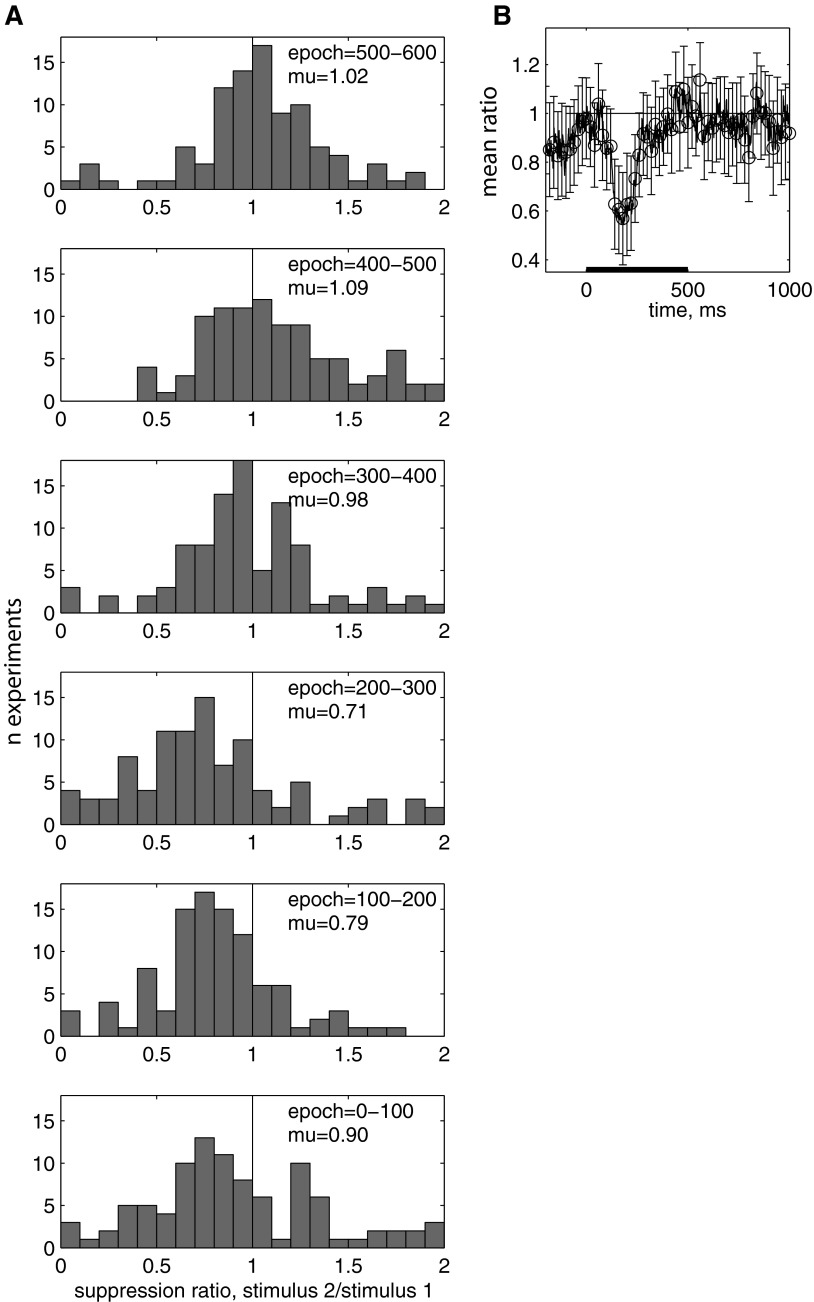
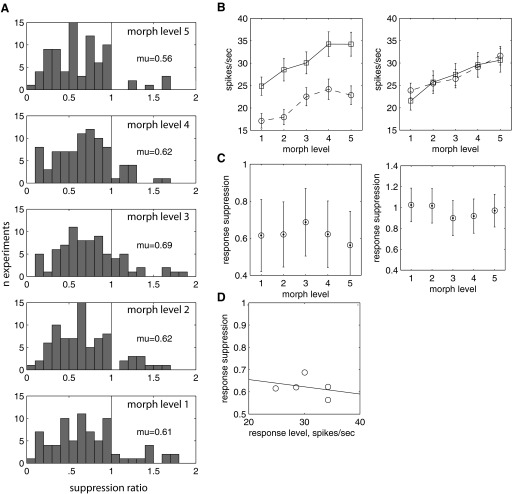
The average time course across the entire population shows that the suppression was limited in duration. This property was consistently found in individual cells in the population. To quantify suppression in each cell, we examined the distribution of the response suppression ratio (stimulus 2/stimulus 1; Fig. 3A). This was calculated by first finding the ratio for the mean response to stimulus 1 and stimulus 2 for each condition in which an identical stimulus was repeated and averaging across the ratio for individual morph levels. The ratio was significantly different from 1 at 0–300 ms after stimulus onset (Fig. 3A, bottom 3 panels; P < 0.05, sign test). By 300–400 ms after stimulus onset, the ratio was no longer significantly different from 1 (Fig. 3, top 2 panels; geometric mean = 0.98; sign test, P = 0.23). The maximum suppression, a ratio of 0.65, occurred at 160–260 ms after stimulus onset. For periods during the 300- to 800-ms epoch, the ratio is significantly >1, suggesting that the response to the second image rebounds above the response to the stimulus 1 during those epochs. The time course shown in finer detail shows a significant suppression of the response that lasts for a narrow window after the onset of the stimulus (Fig. 3B). The suppression ratio is contiguously significantly <1 (P < 0.01) for 107–259 ms after onset of stimulus 2 (in 25-ms epochs).
FIG. 3.
Repetition suppression follows a limited time course. n = 103 experiments. A: distribution of stimulus 2/stimulus 1 ratio for 6 different epochs. B: geometric mean suppression ratio as a function of time, 25-ms bins, stepped 1 ms. Error bars are SE, drawn on every 20th point.
Response suppression as a function of response to stimulus 1
Qualitative examination of Fig. 2A suggested that the suppression seen did not depend on the morph level of the image (and hence the response level evoked by the stimulus). This impression was confirmed by examining the mean response to stimulus 1 and stimulus 2 as a function of time for each morph level independently. The mean response suppression was similar for both the Eff image, which produces a large response, and the Ineff image, which produces a weak response (Fig. 4A). The difference in response to the two stimulus presentations was not proportional to the morph level or the response evoked by each stimulus (Fig. 4B). The mean response difference during the suppression epoch (160–260 ms) between the first and second stimulus presentation was smallest for morph level 3, which produced an intermediate level of response to the first stimulus. The mean response difference was not significantly different for morph level 1 and 5, which produced the biggest difference in response to stimulus 1 (paired t-test, mean response difference, 160–260 ms after stimulus onset, P = 0.72). If the response to stimulus 2 is reduced by a constant factor at all response levels, the ratio of stimulus 2 to stimulus 1 should be constant, even if the raw difference in response varies. Therefore we also plotted the ratio of stimulus 2/stimulus 1 as a function of time (Fig. 4C). Again, the ratio of suppression is not proportional to response level. Instead, the ratio is similar for all morph levels.
FIG. 4.
Response suppression is independent of the level of response. A: response to stimulus 1 (solid) and stimulus 2 (dashed) image as a function of time for 5 different morph levels that produce different responses to the adapting stimulus, n = 103. B: response difference between stimulus 1 and stimulus 2 as a function of time; 25-ms bins, stepped 1 ms. C: response ratio: stimulus 2/stimulus 1 as a function of time; 25-ms bins, stepped 1 ms.
This relationship can be quantitatively examined by looking at the suppression ratio in the maximum suppression epoch (160–260 ms after the onset of the 2nd stimulus) as a function of the morph level. The distribution of suppression was similar for all morph levels (Fig. 5A). Furthermore, all suppression indexes were significantly different from 1 (sign test, P < 0.001, all morph levels). The geometric mean of the ratios fluctuated between 0.56 (i.e., stimulus 2 nearly 1/2 the response to stimulus 1) to 0.69 (Fig. 5B).
FIG. 5.
Response suppression is independent of the level of response. A: distribution of response suppression for each morph level, 160- to 260-ms epoch. Suppression is significantly different in the 160- to 260-ms epoch for all morph levels (sign test, P < 0.0001). n = 103. B: response mean for adapting stimulus, 160- to 260-ms epoch (left); 300- to 400-ms epoch (right). C: response suppression as a function of morph level; 160- to 260-ms epoch (left); 300- to 400-ms epoch (right). D: suppression ratio as a function of response mean (r = −0.297, P = 0.628).
The morph level of the stimulus did not affect the suppression of response to stimulus 2, suggesting that the mean response level to stimulus 1 did not affect the degree of suppression. This was verified by calculating the mean response of stimulus 1 as a function of morph level. As seen previously (Liu and Jagadeesh 2008b), the mean response to the morphed images (stimulus 1) increased as a function of similarity to the Eff image (i.e., with morph level; Fig. 5B, 160- to 260-ms epoch, left, 300- to 400-ms epoch, right). The degree of suppression was independent of the morph level of the stimulus (Fig. 5C, 160- to 260-ms epoch, left, 300- to 400-ms epoch, right). Many cells in IT show a monotonic change in response as a function of morph level (Liu and Jagadeesh 2008b). However, to test the relationship to response level directly, the suppression ratio was plotted against the mean response level: the suppression index was uncorrelated with the mean response level (Fig. 5D, r = −0.2967, P = 0.6279, 160- to 260-ms epoch).
The magnitude of the response to stimulus 1 does not seem to impact the degree of suppression in response to stimulus 2 when response levels are modulated by changing the stimulus. Neural responses also vary randomly over different trials of stimulus presentation. Does the response level on individual trials to stimulus 1 modulate the response to stimulus 2? To address this question for each cell, we picked the response to the Eff image, presented as stimulus 1, and divided the trials into two groups, based on the mean response (across the entire stimulus presentation period of stimulus 1). Group 1 contained all the trials that produced responses lower than the mean response, whereas group 2 contained all the trials that produced responses greater than the mean response. We examined the degree of suppression found in response to stimulus 2 for both groups (Fig. 6). Because of the way the groups were selected, there was a large difference in response to stimulus 1 between the two groups (Fig. 6A). However, the responses to stimulus 2 were indistinguishable for the two populations during the suppression epoch. The responses to stimulus 2 in the two groups showed a similar time course and similar amplitude of response decrease (Fig. 6B). The mean response at the peak suppression epoch (160–260 ms after test stimulus onset) for stimulus 2 was not different between the groups (Fig. 6C; unpaired t-test, P = 0.3590). For a period toward the end of the stimulus presentation, ∼400–600 ms after stimulus onset, the response to stimulus 2 was significantly lower when the response to stimulus 1 was significantly lower (in the opposite direction predicted by a fatigue hypothesis for stimulus suppression). This difference might be attributed to intratrial correlations of response, resulting from slow variations in the probability of firing across trials.
FIG. 6.
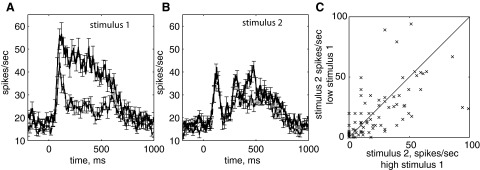
Response suppression of stimulus 2 as a function of variability in response to stimulus 1. A: stimulus 1 response: trials with response greater than mean (solid) and trials with response less than mean (dashed). B: stimulus 2 response; stimulus 1 on trials with adapt response greater than mean (solid) or less than mean (dashed); bin width 25 ms, stepped 1 ms. Error bars are SE, plotted on every 20th point. C: mean response to stimulus 1 at 160- to 260-ms epoch (maximum suppression epoch) for trials with high adapting response (x-axis) and low adapting response (y-axis). Each point is a cell, n = 103.
Repetition suppression as a function of the dynamics of response to stimulus 1 and the range of response to stimulus 1
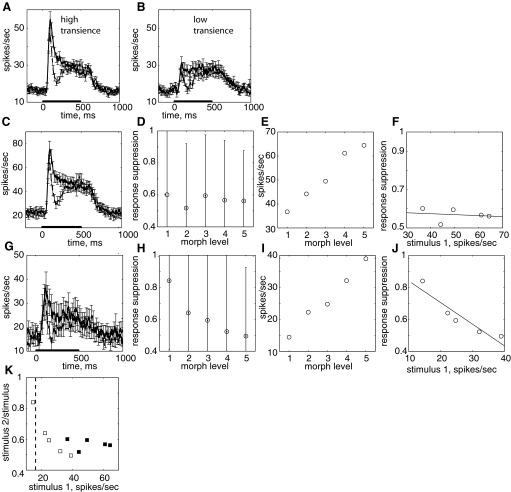
Across the entire population of cells, repetition suppression was largely independent of the response level to stimulus 1 (Figs. 4–6). Did this “response-invariance” of suppression depend on the dynamics of the response to stimulus 1? Cells in IT show a characteristic dynamic response: a short onset transient followed by a longer sustained response (Tamura and Tanaka 2001). The degree of suppression might differ among different cells that show different degrees of transience. To examine this relationship to transience, we calculated a transience index, which averaged 0.12 across the population of cells. We separately examined repetition suppression in two subgroups of cells with high and low transience (greater or less than the mean transience index). The two groups of cells differ dramatically in their response to stimulus 1 (Fig. 7, A and B). However, the degree of stimulus suppression seen in the suppression epoch during repetitions of identical stimuli was not significantly different between the two populations of cells (high transience, mean ratio = 0.56; low transience, mean ratio = 0.65; rank sum, P = 0.36).
FIG. 7.
Dynamics of stimulus suppression in different cell populations. A and B: PSTH, 25-ms bins, 1-ms steps, Solid line, stimulus 1; dashed line, stimulus 2. A: cells with high transience index, n = 49, transience index >0.12. B: cells with low transience index, n = 52, transience index <0.12; suppression ratio in suppression epoch (160–260 ms) is not significantly different, P > 0.50. C–F: cells with high response range, n = 26, responses to morph level 1 and 5 > 15 spikes/s. C: PSTH, 25-ms bins, 1-ms steps, Solid line, stimulus 1; dashed line, stimulus 2. D: suppression ratio as a function of morph level for cells with high response range. E: response as a function of morph level. F: suppression ratio as a function of response. G–J: cells with low response to morph level 1 (contrast with baseline < 0.09, n = 16). G: PSTH, 25-ms bins, 1-ms steps, Solid line, stimulus 1; dashed line, stimulus 2. H: suppression ratio as a function of morph level for cells with high response range. I: response as a function of morph level. J: suppression ratio as a function of response. K: suppression ratio as a function of response for 2 pooled populations. Closed squares from C--F; open squares from G–J. Dashed line is baseline response for population in G–J.
Another possible heterogeneity in the population is the overall response range of the cells. Averaged across all the cells, the mean response to the Eff image was 10 spikes/s higher than the response to the Ineff image, yielding a relatively narrow response range. A possible hypothesis is that response-invariance of suppression is only present when the response range is narrow. To test this possibility, we examined the neural responses for subgroups of cells with different response ranges. The first group included those cells with a large difference in response to the Eff and Ineff images of >15 spikes/s. Picking the cells this way resulted in an average of 28 spikes/s difference between responses to the Eff and Ineff images (Fig. 7C). In this subgroup of cells with 3 times the response range of the population (Fig. 7E), the ratio of suppression was independent of both the morph level (Fig. 7D) and response level (Fig. 7F). Although this subgroup of cells has a high response range across the morph continuum, the response to the weakest stimulus is large (>30 spikes/s). Therefore we also examined repetition suppression in a set of cells chosen based on a small response to the Ineff image [morph level 1, response index compared with baseline, SI (see methods) > 0.09] and a strong response to the effective image (morph level 5, SI > 0.25, n = 16). In this subgroup of cells (Fig. 7G), the response range is also large (25 spikes/s, Fig. 7I), and the response to morph level 1 is near the average baseline firing rate of 16 spikes/s. In this subgroup of cells, the suppression depends on morph level (Fig. 7H) and is significantly correlated with the response level (Fig. 7J; r = 0.93, P < 0.01). Combining these two data sets together and plotting the response suppression as a function of mean response suggests that there is a mean response level below which response suppression does not occur (Fig. 7K). This response level is near the baseline level for the cells (Fig. 7K, dashed line).
Stimulus latency for stimulus 1 and stimulus 2
One possible prediction of a priming based explanation for repetition suppression is that the latency for the second stimulus in a series might be shorter than that for the first. To examine whether this effect was present in our data, we examined the latency of the response to the first and second stimulus, for the Eff image, by calculating a PSTH averaged over all trials and cells. Across the population, no difference in latency was observed in the pooled population averages. Latency was also calculated for individual cells by calculating the 25-ms time bin, advanced in 1-ms steps, (Li and DiCarlo 2008) at which the response first differed significantly from the baseline response (P < 0.01, paired t-test with baseline). The response to stimulus 1, averaged across cells, was first significantly different from baseline at 90–115 ms after stimulus onset. For stimulus 2, this value occurred in the 100- to 125-ms epoch, later than for the first stimulus presentation. This difference was not significantly different across the population of cells (P = 0.36).
Effect of stimulus characteristics and prior stimulus experience on time course of repetition suppression
In the data shown in Figs. 1–6, the stimuli used in a single session were morphed variants of two images. Therefore the images seen during a single session shared similarities; they constituted a continuous set of stimuli that may influence repetition effects differently than less related stimuli. In addition, the monkeys were highly familiar with many of the images and had been trained extensively in a delayed match to sample task with the same images (Akrami et al. 2008; Liu and Jagadeesh 2008a,b). These characteristics of the stimulus and the extensive training with stimuli might influence the time course of repetition effects. To examine whether the distinctive suppression time course we observed generalizes across image experience and stimulus type, we examined the effect to repetition suppression in two additional data sets consisting of random sets of 24 images. One set consisted of the 24 images from which the morphed exemplars were chosen for the main experiment (Fig. 8A; n = 32). These stimuli were highly familiar to the animals and had also been highly trained in the delayed match to sample task. The fixation task was similar to that in the main experiment, except that each of the 24 images was followed by the identical stimulus (stimulus 1 and stimulus 2 were always identical). The stimulus timing was different (300-ms stimulus durations and an interstimulus interval of 300 ms). The other set of stimuli was a set of 24 images with which the monkeys had not performed the delayed match to sample task (Fig. 8B; n = 35). These images were also familiar to the animal (they were used over the course of a 1-mo recording session) but were not as overfamiliar as the original images, and the monkey did not have experience in performing the DMS task with them. For both sets of data, the mean response to stimulus 2 was significantly smaller than the response to stimulus 1 over the stimulus epoch (80–380 ms after stimulus onset; Fig. 8, B–C). For the data set shown in Fig. 8B, the response to stimulus 1 was 34 spikes/s; the response to stimulus 2 was 21 spikes/s (P ≪ 0.0001; Fig. 8B). For the data set shown in Fig. 8B, the response to stimulus 1 was 27 spikes/s; the response to stimulus 2 was 20 spikes/s (P ≪ 0.0001). The time course seen with these two stimulus sets also resembled the time course seen with the morphed stimulus data. The peak suppression occurred at ∼160–260 ms after stimulus onset (Fig. 8A: suppression ratio, 0.47; P ≪ 0.0001; Fig. 8B: suppression ratio, 0.64; P < 0.001). The suppression disappeared by 400–500 ms after stimulus onset on the falling phase of the response to the stimulus (Fig. 8A: suppression ratio, 0.94; P = 0.22; Fig. 8B: suppression ratio, 0.102; P = 0.50). The time course was similar in this alternative set of data, but the mean suppression was greater (distribution of suppression ratio, 160- to 260-ms epoch for 3 different stimulus sets; Fig. 8E). The suppression seen in the data collected with unrelated (not morphed) stimulus sets, in which every stimulus was repeated, was significantly higher than that seen in the morphed stimulus set (rank sum, P < 0.001).
FIG. 8.
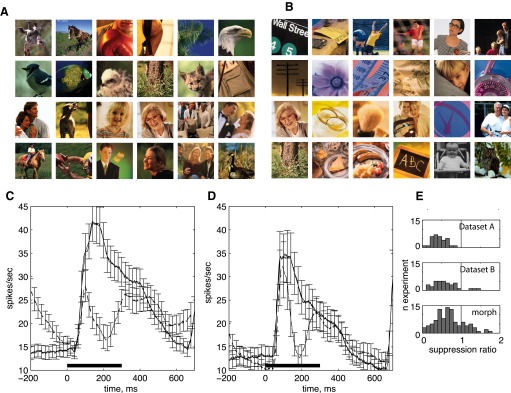
Response suppression for identical stimulus repetitions of nonmorphed stimuli. A: familiar set, also trained in task. B: familiar stimulus set, before training in task with these images. (Two images are in both sets but were learned after the collection of data in D). C and D: repetition of identical stimuli; 300-ms stimulus duration, 300-ms interstimulus interval. C: PSTHs, time-locked to onset of image. Solid line, adapt; dashed line, test. n = 32. A: set of 24 images used in a delayed match to sample task. D: set of 24 images not used in any task but fixation: n = 35. E: comparison of suppression ratio distribution for 3 data sets (images in A, images in B, and the main morph data set in this study); 160- to 260-ms epoch; datasets A and B significantly different from morph data set; rank sum, P < 0.001.
Effect of stimulus duration on magnitude and time course of suppression
Our results thus far have shown that repetition suppression follows a specific time course over the relatively long (500 ms) duration of the stimulus. Next, we examine how repetition suppression was affected by stimulus duration. Two additional stimulus durations were used for a subset of cells (160 ms, n = 31; 80 ms, n = 28). The temporal pattern of suppression was affected by the presentation duration of stimulus 1 and stimulus 2. Because the overall responses were shorter, less time was available for the suppressed response of stimulus 2 to return the response of stimulus 1 (Fig. 9, A and B). The amount of suppression observed for the 160-ms stimulus duration was similar to that seen for the 500-ms stimulus duration (suppression ratio, peaks at 0.65, at 180 ms after test stimulus onset, P > 0.10 comparison to 500 ms). The suppression was also independent of morph level (P < 0.05 for all morph levels) and recovered at about the same point of the response (300–400 ms, suppression ratio no longer significantly different from 1). However, at that point, the response to the stimulus itself was already returning to baseline for both stimulus 1 and stimulus 2 (Fig. 9A). The amount of suppression seen for the 80-ms stimulus presentation was smaller, peaking at a suppression ratio of 0.76, but earlier in time, at 20–120 ms after the onset of the second stimulus. The peak suppression ratio was not significantly less than the peak suppression ratio at 500 ms in the 180- to 260-ms epoch (P > 0.10), but the suppression at 180–260 ms was (P < 0.001) significantly less than that found at 500 ms (Fig. 9C). Furthermore, at 80-ms stimulus duration, the response was significantly suppressed across the population of cells only for the two highest morph levels, which produced the maximum responses in the neuron (P < 0.05). Although the suppression peaked earlier, it remained significantly differently from baseline for a longer duration (400–500 ms after test stimulus onset), and therefore the difference remains significantly different from 0 until the end of the response (Fig. 9B). The time course of the difference in response to stimulus 1 and stimulus 2 were surprisingly similar for the 160- and 500-ms stimulus durations (Figs. 2D vs. 9D, right). The suppression peaks earlier and returns to baseline at about the same point for 80-ms stimulus duration.
FIG. 9.
Response suppression for stimuli of different durations. A and B: 160-ms stimulus duration, n = 31. C and D: 80-ms stimulus duration; n = 28. A and C: mean response to stimulus 1 (solid) and stimulus 2 (dashed) stimulus. B and D: mean response difference to stimulus 1 and stimulus 2 image, 25-ms bins, offset by 1-ms steps between successive points. Horizontal line shows stimulus duration.
The relationship between trial by trial variations in response and the degree of suppression (Fig. 6) could also be examined for the two additional stimulus durations. When trials were selected based on the response to stimulus 1, in either the stimulus or suppression epoch, for the 160-ms stimulus duration, the responses to stimulus 2 were not significantly different in any epoch, including the suppression epoch (P = 0.21, select on stimulus epoch; P = 0.65, select on peak epoch; P = 0.40, select on suppression epoch). The same result was found for the 80-ms stimulus duration (P = 0.90, peak and stimulus epoch; P = 0.77, suppression epoch). For the 80-ms stimulus duration, the response was significantly lower during the late stimulus epoch (400+ ms after stimulus onset) when the response to stimulus 1 was lower.
Effect of stimulus 1 content on stimulus tuning for stimulus 2
The results show that the amount of repetition suppression is independent of the response magnitude. However, did the amount of repetition suppression depend on the stimulus relationship between the two stimuli in the sequence? We have thus far only considered trials in which stimulus 1 and stimulus 2 were identical. Here we examine the effect of different stimulus 1 images on the response to different stimulus 2 images by considering all possible combinations of the five different morph levels. There are a total of 25 different stimulus pairings, whose PSTHs are shown in Fig. 10. The histograms shown along the diagonal represent the population average of response to identical image pairs (Fig. 10, dashed boxes, also shown in Fig. 2A). Each row depicts the response to each of the five different morph levels of stimulus 2 preceded by a specific stimulus 1; stimulus 1 in the top row is the Eff image and stimulus 1 in the bottom row is the Ineff image. The maximum suppression occurred when the stimuli were identical (on the diagonal in the set of graphs), suggesting that the content of stimulus 1 affected the degree of suppression observed. Although the maximum suppression was observed when the stimuli were identical, suppression was also present when stimulus 1 and stimulus 2 were similar (Fig. 10, 2nd row, 1st column and 4th row, last column).
FIG. 10.
Population average response to stimulus 1 and stimulus 2 for all possible sequences of stimulus 1 and stimulus 2; n = 103. The numbers above each response shows the morph level of stimulus 1 and stimulus 2. Each row depicts the identical stimulus 1 paired with each possible stimulus 2. Each column depicts the response to an identical stimulus 2 preceded by a different stimulus 1. Dashed boxes show repetitions of identical test and adapting images. PSTHs, locked to the onset of the adapting image and convolved with a Gaussian for smoothing (kernel = 20 ms).
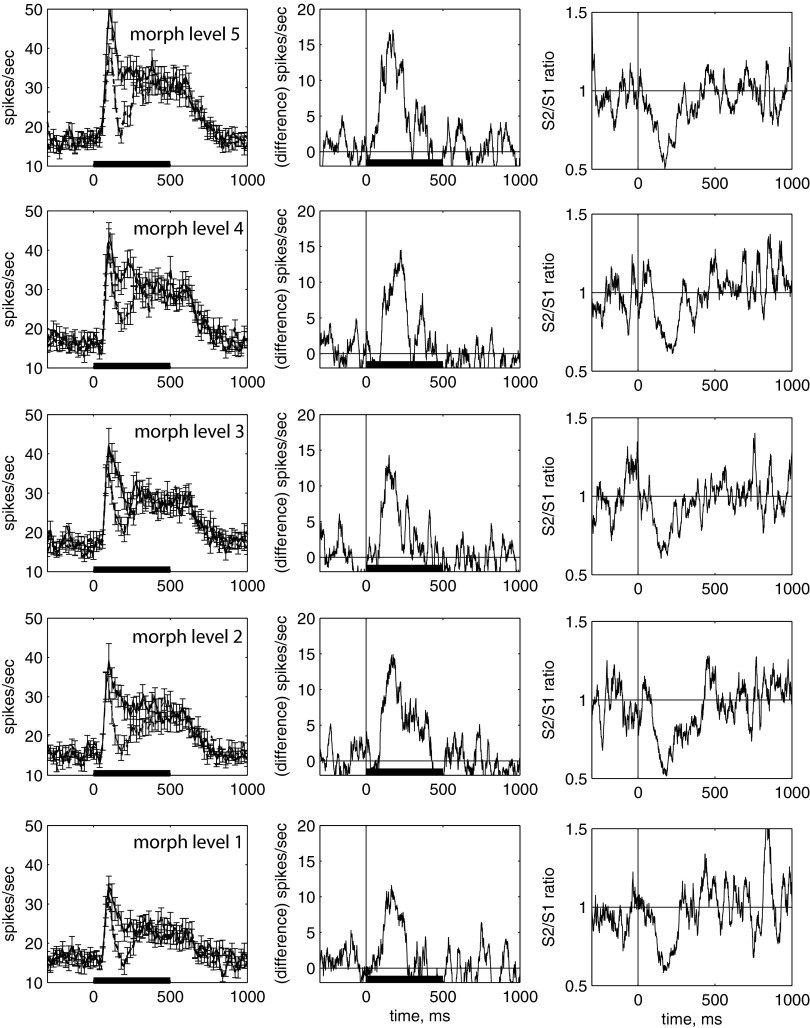
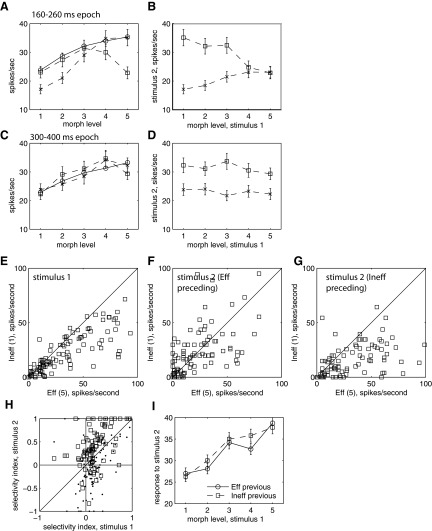
Because of this interaction between the content of the first and second stimulus, stimulus 1 changes the “tuning” of stimulus 2 (Fig. 11). Figure 11A shows the mean response to stimulus 2, in the suppression epoch, for the histograms shown in the top row in Fig. 10 (Fig. 11A, dashed lines, squares) and for histograms in the bottom row (Fig. 11A, dashed lines, crosses). For comparison, the response to stimulus 1 (averaged across all presentations of the same morph level) is also shown (Fig. 11A, solid line, circles). When stimulus 2 was preceded by the Eff image or to a similar morphed image (level 4), the response to the Eff image was suppressed (Fig. 11A, dashed line, squares). When stimulus 2 was preceded by the Ineff image, the response to the Ineff image was suppressed (Fig. 11A, dashed line, crosses).
FIG. 11.
Responses as a function of morph level for stimulus 1 and stimulus 2; n = 103. A and C: Circles, stimulus 1 response as a function of morph level of stimulus 1. Squares, stimulus 2 response as a function of morph level of stimulus 2, preceded by Eff stimulus 1. Crosses, stimulus 2 response as a function of morph level of stimulus 2, preceded by Ineff stimulus 1. A: 160- to 260-ms epoch. C: 300- to 400-ms epoch. B and D: squares, response to Eff stimulus 2 as a function of morph level of stimulus 1. Crosses, response to Ineff stimulus 2 as a function of morph level of stimulus 1. B: 160- to 260-ms epoch. D: 300- to 400-ms epoch. E–G: 160- to 260-ms epoch. E: Ineff stimulus 1 vs. Eff stimulus 1; each point is cell. F: Ineff stimulus 2 vs. Eff stimulus 2 (preceded by Eff stimulus 1). G: Ineff stimulus 2 vs. Eff stimulus 2 (preceded by Ineff stimulus 1). H: selectivity index (Eff − Ineff)/(Eff + Ineff). x-axis, stimulus 1; y-axis, stimulus 2; crosses, preceded by Eff stimulus 1; squares, preceded by Ineff stimulus 1. I: response to stimulus 1 based on whether stimulus 2 on previous trial was the Eff or Ineff image; 160- to 260-ms epoch.
We characterized “selectivity” (or “tuning”) as the difference in response to the “Eff” and “Ineff” stimulus, where high selectivity implies a large difference. Selectivity for the Eff and Ineff image was dramatically decreased (eliminated) when the images were preceded by the Eff image, but selectivity for the Eff and Ineff images was enhanced when preceded by the Ineff image (Fig. 11A). This effect on selectivity appeared during the epoch in which maximum suppression was found (160–260 ms; Fig. 11A) but disappeared before the end of the response to the stimulus (300–400 ms; Fig. 11C).
The response to different stimulus 2 images was modulated by the image that preceded it (Fig. 11A). Therefore the response to a particular stimulus 2 image depended both on the image itself and on the image that preceded it. This relationship can be observed by plotting the response to the Eff and Ineff image as a function of the preceding image (any of the 5 different morphed images appearing as stimulus 1; Fig. 11, B and D). In this figure, the response to the Eff (squares) and Ineff (crosses) stimulus 2 images are shown as a function of the morph level of stimulus 1. For each curve, stimulus 2 is the same; the stimulus 1 that preceded it is different. The response to stimulus 2 was affected by the image that preceded it, with the maximum suppression of response occurring when stimulus 1 and stimulus 2 were identical and with suppression scaling with the similarity of stimulus 1 and stimulus 2. The effect was so robust that the response difference between the Eff stimulus 2 and the Ineff stimulus 2 decreased from 18 spikes/s when preceded by the Ineff image to <1 spike/s when preceded by the Eff image (Fig. 11B; suppression epoch, 160–260 ms). The effect disappears when the suppression recovers (Fig. 11D; recovery epoch, 300–400 ms).
To examine the distribution of these effects across the population of cells, we plotted the mean response to the Eff and Ineff image when they appeared as stimulus 1 (Fig. 11E) and as stimulus 2 preceded by the Eff image (Fig. 11F) and as stimulus 2 preceded by the Ineff image (Fig. 11G) for the 160- to 260-ms epoch where suppression was strongest. The responses for stimulus 1 in the 160- to 260-ms epoch is higher for Eff than Ineff images (Eff mean = 35 spikes/s, Ineff mean = 24 spikes/s, P < 0.0001, 80/103 cells have greater response to Eff) for most cells because the response to stimulus 1 was used to define the Eff and Ineff image. The difference in response between the Eff and Ineff image disappeared when the stimulus 2 was preceded by the Eff image (Fig. 11F; Eff mean = 24 spikes/s Ineff mean = 23 spikes/s, paired t-test, P = 0.9215, 44/103 cells have greater response to Eff). The difference in response between the Eff and Ineff images returned when stimulus 2 was preceded by the Ineff image (Fig. 11G; Eff mean = 35 spikes/s, Ineff mean = 17 spikes/s; paired t-test, P ≪ 0.0001, 77/103 have greater response to Eff). This pattern results in significantly lower selectivity for the Eff and Ineff image when preceded by the Eff image (selectivity index; Fig. 11H; stimulus 2 preceded by Eff, dots; preceded by Ineff, squares; both indexes significantly different from selectivity for stimulus 1, paired t-test, P ≪ 0.0001).
Because the stimulus conditions shown in Fig. 10 were shown in random order, on occasion, each stimulus in an individual trial was preceded by either the Eff or Ineff image, presented at the end of previous trial, as stimulus 2. We examined whether the responses to stimulus 1 were different based on whether they had been preceded by an Eff or Ineff image. If the effect on tuning seen in Fig. 11A was observable across trials, we would expect responses to the different stimulus 2 images to differ, based on whether they were preceded by the Eff or Ineff image. No such effect could be detected (Fig. 11I; comparisons at each morph level not significantly different from each other, P > 0.10).
DISCUSSION
We confirmed that cells in IT show a strong and consistent repetition effect—stimulus responses to an image were suppressed when preceded by an identical image regardless of the response level of the initial stimulus (Sawamura et al. 2006). The suppression followed a striking time course: the responses were suppressed for a short epoch immediately after the onset transient but returned to unsuppressed levels during the sustained period of the response. The result of this suppression was that neural selectivity for images was altered for a short period of time after stimulus onset but returned to normal by the end of the response. These results have implications for understanding the mechanisms underlying repetition effects in IT, for the interpretation of behavioral classification results, and for the interpretation of fMRI adaptation results.
Comparison to other studies of repetition suppression in IT
Repetition suppression has been studied extensively in the inferotemporal cortex (Li et al. 1993; Lueschow et al. 1994; McMahon and Olson 2007; Miller et al. 1991, 1993b; Sawamura et al. 2006; Xiang and Brown 1998). The robustness of the time course seen in the data reported here might reflect specific characteristics of this experimental design. The striking time course seen in Figs. 1–3 is occasionally visible in other studies, but it has not been emphasized or examined systematically (Miller et al. 1993a; Sawamura et al. 2006). In the data reported here, several characteristics of the experimental design might have magnified the dynamics. For example, the high degree of familiarity with these stimuli and extensive training in the sample-to-match task may have induced the dynamics. For example, the creation of recurrent networks in the form of stored attractor nodes might change the dynamics of neural responses in IT (Akrami et al. 2008). Repeated presentation of a consistent set of stimuli (morphed images) that contained related sets of features may have produced unusual dynamics of adaptation in IT. However, the similarity of time course seen in two nonmorph stimulus data sets, containing responses to untrained and nonmorphed stimuli, suggest that these are not likely explanations (Fig. 8). In addition, although the time course of repetition suppression seen here has not been extensively discussed in previous examinations, double-peaked responses during stimulus suppression have been seen (Miller et al. 1993b; Sawamura et al. 2006; Verhoef et al. 2008).
The stimulus duration, however, did alter the time course of suppression. Short durations meant that the response terminated before the suppressed response to stimulus 2 could recover to stimulus 1 levels (Fig. 9). Thus the limited duration of stimulus suppression may have been less apparent in studies that used shorter stimulus durations (McMahon and Olson 2007). Other studies show recovery of the suppressed response that does not rise to the level of the response to the initial stimulus (Miller et al. 1993b; Sawamura et al. 2006). One possible explanation of this difference might be that suppression results are often reported for a subset of cells that show significant suppression (Miller et al. 1993b; Sawamura et al. 2006). This selection process, which usually uses the entire duration of stimulus presentation to assess whether significant suppression has occurred, would be expected to bias the population toward cells in which the response was more likely to be suppressed throughout the stimulus presentation, rather than limited to a narrow epoch.
In addition, although a similar time course can be observed in data sets that do not use morphed stimuli (Fig. 8A) or have not been trained extensively in a delayed match to sample task (Fig. 8B), the monkeys used in this study were extensively trained in matching tasks before the beginning of recording experiments (Allred and Jagadeesh 2007; Liu and Jagadeesh 2008a,b). This training may have altered the dynamics of responses to matching stimuli or to second stimuli presented in a series.
Implications for mechanism of repetition suppression and sustained neural response in IT
Repetition effects—most notably suppression resulting after the presentation of an image—is a ubiquitous phenomenon in IT. Many interpretations of it implicitly have assumed that suppression is mechanistically some form of adaptation or response fatigue. Other studies of extrastriate cortex argue against a simple model of response fatigue as the mechanism for response suppression (Clifford et al. 2007; Kohn 2007; Kohn and Movshon 2003, 2004; Krekelberg et al. 2006a,b; Priebe and Lisberger 2002; Priebe et al. 2002; Sawamura et al. 2005, 2006). The data presented here extend these results. Neural responses showed a similar degree of suppression following the presentation of stimuli that produced different levels of responses in individual neurons (Fig. 4). In addition, individual cells were suppressed to similar levels on individual trials regardless of the response evoked by the first stimulus (Fig. 6). Therefore firing rate fatigue seems to be an inadequate explanation for the mechanism behind the robust and consistent suppression seen in IT neurons (Figs. 4 and 5). The time course of the response also suggests that firing rate fatigue is an insufficient explanation, because the neural responses recover over the course of the presentation of a static stimulus, returning to the response level evoked on the first presentation. The time course further suggests that part of the response in IT is immune to suppression, because the sustained response level is the same for the first and second stimulus in a sequence (Fig. 3).
Several lines of evidence suggest that the dynamic responses of IT neurons might reflect different circuitry: the initial response reflecting feed-forward responses that evolve over time to reflect recurrent connections in IT, along with integration of the influence of top down connections from prefrontal cortex and elsewhere (Akrami et al. 2008; Bar 2007; Brincat and Connor 2006; Sugase et al. 1999). Our data support the current evidence suggesting that suppression is a circuit property, involving the differential suppression of intracortical circuitry and feed-forward connections, with the end result involving a complex interplay between excitatory, inhibitory, and recurrent connections to the recorded neuron (Boudreau and Ferster 2005). The activation of top down circuits under cognitive control may also play a significant role (Summerfield and Koechlin 2008).
Implications for attentional/expectation based causes for repetition suppression
The monkeys used in this study were extensively trained in a delayed sample to match task before these recordings were made. After this training, many neurons in IT can be selective for the stimuli used in this study (Allred and Jagadeesh 2007; Liu and Jagadeesh 2008b). In addition, the stimuli might be processed by the animal despite the lack of any requirement to do so. Processing of these images, for example, might include implicit expectations about what stimulus will appear next and thus could influence the degree of repetition suppression observed. (Summerfield and Koechlin 2008). In Summerfield and Koechlin, the likelihood that a stimulus would be repeated modulated the degree of repetition suppression seen in the fMRI signal. Our experiment was not designed to test the effect of stimulus repetition frequency, but the likelihood that an identical stimulus would be repeated was 20% in the main dataset (Figs. 1–6). The mean suppression seen with this stimulus repetition probability was less than that seen in two alternative data sets in which the stimuli were repeated 100% of the time (Fig. 8E), raising the possibility that cognitive processing of the stimuli might modulate the degree of repetition suppression seen. To test those possibilities, it would be informative to examine repetition suppression in conditions where the degree of expectation and attention to the two stimuli was directly manipulated, unlike this study, in which other differences between the data sets (including stimulus content and stimulus timing) were present. If such manipulations modulate repetition suppression, top-down models of response modulation will be favored over mechanistic models of adaptation and fatigue in these neurons.
Implications for the effects of repetition suppression on perception
On first glance, the robust decrement in selectivity that follows the presentation of an Eff stimulus in IT (Fig. 11A) might be expected to significantly alter perception. Cells in this sample population were no longer selective for a pair of images during the suppression epoch when preceded by an Eff stimulus (Fig. 11B). If this selectivity were used as the basis for perception, the ability to discriminate the stimulus should be impaired for a short period after the presentation of stimulus 2. One possibility is that the properties of response suppression seen during this task, in which the stimuli require no processing would be different when the stimuli are actively processed, that is, that the effect of repetition suppression would be filtered out by equating the cognitive demands in processing the two stimuli. Assuming this is not the case, absent additional evidence (Liu and Jagadeesh 2008b), the large modulations of stimulus tuning can be translated into predictions of perceptual consequences. The translation depends critically on the presumed readout of responses. If populations of neurons are compared, rather than the single neuron's response to different stimuli, the predictions of a simple read-out mechanism predict no large effects on the discrimination of the stimulus, even as neurons’ individual response are substantially modulated by repetition. One simple method of comparing responses is to simply take the difference in response between the two populations with different stimulus preferences. In such a read out scheme, the differing adaptation in the two populations results in only minor effect on the difference in response (Fig. 11, A and B). The time course, as well, has implications for the perceptual interpretation of the images. Because the response selectivity returns to unsuppressed levels during the end of the neural responses, if neural responses during the end of the response are considered, no effect on classification would be expected. This potential lack of impact of significant suppression observed at the single cell level on plausible population comparisons support the possibility that the repulsion effect commonly seen in psychophysics does not necessarily stem from an alteration in neural selectivity for the images (Ng et al. 2008).
The results further suggest that reverse engineering of the operation of neural populations based on the behavioral changes in classification after adaptation will require more knowledge of neural responses under the same conditions (including the details of timing, constraints on the periods of responses that might be interpreted to produce the behavior, and perhaps measurement during the performance of the relevant behaviors) (Ng et al. 2008). Factors other than the content and timing of the stimulus, including attention, stimulus familiarity, stimulus category and content, and task might also influence suppression and its interpretation (Li et al. 1993; Lueschow et al. 1994; Miller et al. 1991, 1993b; Peissig et al. 2007; Sawamura et al. 2006; Xiang and Brown 1998).
Implications for interpretation of fMRI adaptation results
Following the long tradition of using stimulus adaptation to gain insight into the underlying neural representations, repetition suppression has been used extensively in fMRI to examine neural tuning for stimuli. The signal measured in fMRI, the blood oxygen level–dependent (BOLD) signal sums activity across a relatively large spatial scale of several square millimeter of cortex and across a broad temporal window (Logothetis et al. 1999). The principle underlying the use of adaptation in fMRI is that, if different groups of neurons respond to different categories of stimuli, the responses of only the group that responded to the initial category will be adapted. Thus alternating between the stimuli that produces responses in the two different populations will result in a relatively high BOLD response. On the other hand, if the same population of neurons is activated by the two stimulus categories, the responses will adapt, resulting in a relatively smaller BOLD response (Grill-Spector et al. 2006; Krekelberg et al. 2006a). Thus an adapted BOLD signal has been used to infer that neurons in the voxels are either tuned (respond differently) or un-tuned (respond similarly) for the category in question (Fang et al. 2005). Initially, the assumption has been that single-unit adaptation underlies the adaptation seen in fMRI (Grill-Spector 2006; Krekelberg et al. 2006a; Sawamura et al. 2006), with the caveat that the coupling between the BOLD signal and neural activity might also provide a mechanistic explanation for BOLD adaptation, independent of what is seen in single unit activity (Boynton et al. 1996; Krekelberg et al. 2006a). Assuming that single unit activity is relevant to the interpretation of fMRI adaptation results, what do the current data contribute?
First, the data suggest that the timing of stimuli might be important in the nature and degree of adaptation seen, independent of the tuning seen in the neurons contained within a voxel. The degree of suppression, integrated over many stimulus presentations, might be larger for the 160-ms duration stimulus than for the 500-ms duration stimulus, because the response has more time to recover to its sustained level with longer stimulus presentations. Therefore these data suggest that the time scale of stimulus presentation may play a significant and perhaps not trivially predictable role in the interpretation of fMRI adaptation (Krekelberg et al. 2006a).
Second, the data show that cells may be suppressed by many stimuli to which they respond, even if the responses to the images are much less than their maximum response. Images that drive a cell less well produce equivalent suppression of the response, compared with images that drive the cell well (Figs. 4, 5, and 7), over a fairly large response range. Thus the graded responses of neurons, and the nongraded effect of suppression must be considered in interpreting fMRI adaptation results. The shape of tuning curves derived from fMRI might be substantially different from those of the underlying neural population, because suppression does not seem to be proportional to the response of a cell to a stimulus.
Conclusions
We tested the effect of stimulus repetition–induced suppression on responses in inferotemporal cortex. The degree of suppression measured depended on both the content of the repeated images, with the greatest degree of adaptation when the two images were identical, regardless of the level of response evoked by each. The suppression measured was limited in duration, showing at least two differentially suppressed components of the IT response. These data will assist in interpreting the population models of adaptation used to interpret fMRI and behavioral results during stimulus adaptation and point toward the complexities in such interpretation; they also suggest that the sustained response in IT is protected from the effect of repetition suppression.
GRANTS
This research was supported by the Sloan Foundation, the McKnight Foundation, the Whitehall Foundation, and the NIH National Center for Research Resources.
Acknowledgments
We thank K. M. Ahl for technical help and R. Kiani for comments on an earlier version of this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Akrami et al. 2008.Akrami A, Liu Y, Treves A, Jagadeesh B. Converging neuronal activity in inferior temporal cortex during the classification of morphed stimuli. Cereb Cortex 2008. doi: 10.1093/cercor/bhn125. [DOI] [PMC free article] [PubMed]
- Allred et al. 2005.Allred S, Liu Y, Jagadeesh B. Selectivity of inferior temporal neurons for realistic pictures predicted by algorithms for image database navigation. J Neurophysiol 94: 4068–4081, 2005. [DOI] [PubMed] [Google Scholar]
- Allred and Jagadeesh 2007.Allred SR, Jagadeesh B. Quantitative comparison between neural response in macaque inferotemporal cortex and behavioral discrimination of photographic images. J Neurophysiol 98: 1263–1277, 2007. [DOI] [PubMed] [Google Scholar]
- Bar 2007.Bar M The proactive brain: using analogies and associations to generate predictions. Trends Cogn Sci 11: 280–289, 2007. [DOI] [PubMed] [Google Scholar]
- Boudreau and Ferster 2005.Boudreau CE, Ferster D. Short-term depression in thalamocortical synapses of cat primary visual cortex. J Neurosci 25: 7179–7190, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton et al. 1996.Boynton GM, Engel SA, Glover GH, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4221, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton and Finney 2003.Boynton GM, Finney EM. Orientation-specific adaptation in human visual cortex. J Neurosci 23: 8781–8787, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brincat and Connor 2006.Brincat SL, Connor CE. Dynamic shape synthesis in posterior inferotemporal cortex. Neuron 49: 17–24, 2006. [DOI] [PubMed] [Google Scholar]
- Carandini 2000.Carandini M Visual cortex: fatigue and adaptation. Curr Biol 10: R605–R607, 2000. [DOI] [PubMed] [Google Scholar]
- Clifford et al. 2007.Clifford CW, Webster MA, Stanley GB, Stocker AA, Kohn A, Sharpee TO, Schwartz O. Visual adaptation: neural, psychological and computational aspects. Vision Res 47: 3125–3131, 2007. [DOI] [PubMed] [Google Scholar]
- De Baene et al. 2007.De Baene W, Premereur E, Vogels R. Properties of shape tuning of macaque inferior temporal neurons examined using Rapid Serial Visual Presentation. J Neurophysiol, 97: 2900–2916, 2007. [DOI] [PubMed] [Google Scholar]
- Desimone et al. 1984.Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci 4: 2051–2062, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang et al. 2005.Fang F, Murray SO, Kersten D, He S. Orientation-tuned FMRI adaptation in human visual cortex. J Neurophysiol 94: 4188–4195, 2005. [DOI] [PubMed] [Google Scholar]
- Grill-Spector 2006.Grill-Spector K Selectivity of adaptation in single units: implications for FMRI experiments. Neuron 49: 170–171, 2006. [DOI] [PubMed] [Google Scholar]
- Grill-Spector et al. 2006.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci 10: 14–23, 2006. [DOI] [PubMed] [Google Scholar]
- Hung et al. 2005.Hung CP, Kreiman G, Poggio T, DiCarlo JJ. Fast readout of object identity from macaque inferior temporal cortex. Science 310: 863–866, 2005. [DOI] [PubMed] [Google Scholar]
- Judge et al. 1980.Judge SJ, Richmond BJ, Chu FC. Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20: 535–538, 1980. [DOI] [PubMed] [Google Scholar]
- Kayaert et al. 2003.Kayaert G, Biederman I, Vogels R. Shape tuning in macaque inferior temporal cortex. J Neurosci 23: 3016–3027, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiani et al. 2007.Kiani R, Esteky H, Mirpour K, Tanaka K. Object category structure in response patterns of neuronal population in monkey inferior temporal cortex. J Neurophysiol 97: 4296–4309, 2007. [DOI] [PubMed] [Google Scholar]
- Kobatake and Tanaka 1994.Kobatake E, Tanaka K. Neuronal selectivities to complex object features in the ventral visual pathway of the macaque cerebral cortex. J Neurophysiol 71: 856–867, 1994. [DOI] [PubMed] [Google Scholar]
- Kohn 2007.Kohn A Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol 97: 3155–3164, 2007. [DOI] [PubMed] [Google Scholar]
- Kohn and Movshon 2003.Kohn A, Movshon JA. Neuronal adaptation to visual motion in area MT of the macaque. Neuron 39: 681–691, 2003. [DOI] [PubMed] [Google Scholar]
- Kohn and Movshon 2004.Kohn A, Movshon JA. Adaptation changes the direction tuning of macaque MT neurons. Nat Neurosci 7: 764–772, 2004. [DOI] [PubMed] [Google Scholar]
- Kourtzi and Huberle 2005.Kourtzi Z, Huberle E. Spatiotemporal characteristics of form analysis in the human visual cortex revealed by rapid event-related fMRI adaptation. Neuroimage 28: 440–452, 2005. [DOI] [PubMed] [Google Scholar]
- Kourtzi et al. 2003.Kourtzi Z, Tolias AS, Altmann CF, Augath M, Logothetis NK. Integration of local features into global shapes: monkey and human FMRI studies. Neuron 37: 333–346, 2003. [DOI] [PubMed] [Google Scholar]
- Krekelberg et al. 2006a.Krekelberg B, Boynton GM, van Wezel RJ. Adaptation: from single cells to BOLD signals. Trends Neurosci 29: 250–256, 2006a. [DOI] [PubMed] [Google Scholar]
- Krekelberg et al. 2006b.Krekelberg B, van Wezel RJ, Albright TD. Adaptation in macaque MT reduces perceived speed and improves speed discrimination. J Neurophysiol 95: 255–270, 2006b. [DOI] [PubMed] [Google Scholar]
- Krekelberg et al. 2005.Krekelberg B, Vatakis A, Kourtzi Z. Implied motion from form in the human visual cortex. J Neurophysiol 94: 4373–4386, 2005. [DOI] [PubMed] [Google Scholar]
- Li et al. 1993.Li L, Miller EK, Desimone R. The representation of stimulus familiarity in anterior inferior temporal cortex. J Neurophysiol 69: 1918–1929, 1993. [DOI] [PubMed] [Google Scholar]
- Li and DiCarlo 2008.Li N, DiCarlo JJ. Unsupervised natural experience rapidly alters invariant object representation in visual cortex. Science 321: 1502–1507, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu and Jagadeesh 2008a.Liu Y, Jagadeesh B. Modulation of neural responses in inferotemporal cortex during the interpretation of ambiguous photographs. Eur J Neurosci 27: 3059–3073, 2008a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu and Jagadeesh 2008b.Liu Y, Jagadeesh B. Neural selectivity in anterior inferotemporal cortex for morphed photographic images during behavioral classification or fixation. J Neurophysiol 100: 966–982, 2008b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis et al. 1999.Logothetis NK, Guggenberger H, Peled S, Pauls J. Functional imaging of the monkey brain. Nat Neurosci 2: 555–562, 1999. [DOI] [PubMed] [Google Scholar]
- Lueschow et al. 1994.Lueschow A, Miller EK, Desimone R. Inferior temporal mechanisms for invariant object recognition. Cereb Cortex 4: 523–531, 1994. [DOI] [PubMed] [Google Scholar]
- McMahon and Olson 2007.McMahon DB, Olson CR. Repetition suppression in monkey inferotemporal cortex: relation to behavioral priming. J Neurophysiol 97: 3532–3543, 2007. [DOI] [PubMed] [Google Scholar]
- Miller et al. 1993a.Miller EK, Gochin PM, Gross CG. Suppression of visual responses of neurons in inferior temporal cortex of the awake macaque by addition of a second stimulus. Brain Res 616: 25–29, 1993a. [DOI] [PubMed] [Google Scholar]
- Miller et al. 1991.Miller EK, Li L, Desimone R. A neural mechanism for working and recognition memory in inferior temporal cortex. Science 254: 1377–1379, 1991. [DOI] [PubMed] [Google Scholar]
- Miller et al. 1993b.Miller EK, Li L, Desimone R. Activity of neurons in anterior inferior temporal cortex during a short-term memory task. J Neurosci 13: 1460–1478, 1993b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng et al. 2008.Ng M, Boynton GM, Fine I. Face adaptation does not improve performance on search or discrimination tasks. J Vis 8: 11–20, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peissig et al. 2007.Peissig JJ, Singer J, Kawasaki K, Sheinberg DL. Effects of long-term object familiarity on event-related potentials in the monkey. Cereb Cortex 17: 1323–1334, 2007. [DOI] [PubMed] [Google Scholar]
- Pineda and Nava 1993.Pineda JA, Nava C. Event-related potentials in macaque monkey during passive and attentional processing of faces in a priming paradigm. Behav Brain Res 53: 177–187, 1993. [DOI] [PubMed] [Google Scholar]
- Priebe et al. 2002.Priebe NJ, Churchland MM, Lisberger SG. Constraints on the source of short-term motion adaptation in macaque area MT. I. The role of input and intrinsic mechanisms. J Neurophysiol 88: 354–369, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priebe and Lisberger 2002.Priebe NJ, Lisberger SG. Constraints on the source of short-term motion adaptation in macaque area MT. II. Tuning of neural circuit mechanisms. J Neurophysiol 88: 370–382, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura et al. 2005.Sawamura H, Georgieva S, Vogels R, Vanduffel W, Orban GA. Using functional magnetic resonance imaging to assess adaptation and size invariance of shape processing by humans and monkeys. J Neurosci 25: 4294–4306, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamura et al. 2006.Sawamura H, Orban GA, Vogels R. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the FMRI adaptation paradigm. Neuron 49: 307–318, 2006. [DOI] [PubMed] [Google Scholar]
- Sugase et al. 1999.Sugase Y, Yamane S, Ueno S, Kawano K. Global and fine information coded by single neurons in the temporal visual cortex. Nature 400: 869–873, 1999. [DOI] [PubMed] [Google Scholar]
- Summerfield and Koechlin 2008.Summerfield C, Koechlin E. A neural representation of prior information during perceptual inference. Neuron 59: 336–347, 2008. [DOI] [PubMed] [Google Scholar]
- Tamura and Tanaka 2001.Tamura H, Tanaka K. Visual response properties of cells in the ventral and dorsal parts of the macaque inferotemporal cortex. Cereb Cortex 11: 384–399, 2001. [DOI] [PubMed] [Google Scholar]
- Verhoef et al. 2008.Verhoef BE, Kayaert G, Franko E, Vangeneugden J, Vogels R. Stimulus similarity-contingent neural adaptation can be time and cortical area dependent. J Neurosci 28: 10631–10640, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wark et al. 2007.Wark B, Lundstrom BN, Fairhall A. Sensory adaptation. Curr Opin Neurobiol 17: 423–429, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster et al. 2004.Webster MA, Kaping D, Mizokami Y, Duhamel P. Adaptation to natural facial categories. Nature 428: 557–561, 2004. [DOI] [PubMed] [Google Scholar]
- Xiang and Brown 1998.Xiang JZ, Brown MW. Differential neuronal encoding of novelty, familiarity and recency in regions of the anterior temporal lobe. Neuropharmacology 37: 657–676, 1998. [DOI] [PubMed] [Google Scholar]
- Yamashita et al. 2005.Yamashita JA, Hardy JL, De Valois KK, Webster MA. Stimulus selectivity of figural aftereffects for faces. J Exp Psychol Hum Percept Perform 31: 420–437, 2005. [DOI] [PubMed] [Google Scholar]
- Zoccolan et al. 2007.Zoccolan D, Kouh M, Poggio T, DiCarlo JJ. Trade-off between object selectivity and tolerance in monkey inferotemporal cortex. J Neurosci 27: 12292–12307, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]