Abstract
Central pattern generators (CPGs) are neuronal networks that control vitally important rhythmic behaviors including breathing, heartbeat, and digestion. Understanding how CPGs recover activity after their rhythmic activity is disrupted has important theoretical and practical implications. Previous experimental and modeling studies indicated that rhythm recovery after central neuromodulatory input loss (decentralization) could be based entirely on activity-dependent mechanisms, but recent evidence of long-term conductance regulation by neuromodulators suggest that neuromodulator-dependent mechanisms may also be involved. Here we examined the effects of altering activity and the neuromodulatory environment before decentralization of the pyloric CPG in Cancer borealis on the initial phase of rhythmic activity recovery after decentralization. We found that pretreatments altering the network activity through shifting the ionic balance or the membrane potential of pyloric pacemaker neurons reduced the delay of recovery initiation after decentralization, consistent with the recovery process being triggered already during the pretreatment period through an activity-dependent mechanism. However, we observed that pretreatment with neuromodulators GABA and proctolin, acting via metabotropic receptors, also affected the initial phase of the recovery of pyloric activity after decentralization. Their distinct effects appear to result from interactions of their metabotropic effects with their effects on neuronal activity. Thus we show that the initial phase of the recovery process can be accounted for by the existence of distinct activity-and neuromodulator-dependent pathways. We propose a computational model that includes activity- and neuromodulator-dependent mechanisms of the activity recovery process, which successfully explains the experimental observations and predicts the results of key biological experiments.
INTRODUCTION
Central pattern generators (CPGs) are neuronal networks that control rhythmic behaviors such as breathing, heartbeat, swimming, feeding, walking, and flying without requiring patterned sensory feedback (Marder and Bucher 2001). Studies in both vertebrates as well as invertebrates have shown that these networks remain functional even when they are isolated from the organism and receive no rhythmic neuronal input from the environment (Marder and Bucher 2001). The vital nature of many of the behaviors governed by CPGs suggests that evolutionary pressures may have led to the emergence of safeguard mechanisms that enable the recovery of the patterned activity after its disruption by trauma or disease. Indeed it has previously been shown that the pyloric network, located in the stomatogastric ganglion (STG) of decapod crustaceans, recovers its rhythmic activity after it is disrupted by the removal of all central neuromodulatory inputs to the network, a process we refer to as decentralization (Golowasch et al. 1999b; Khorkova and Golowasch 2007; Luther et al. 2003; Thoby-Brisson and Simmers 1998). The recovery of activity in the crab occurs within hours to days after decentralization and achieves levels and characteristics similar to those observed in control preparations (Golowasch et al. 1999b; Luther et al. 2003). Based on evidence that neurons of the pyloric network respond to changes in activity with changes in their intrinsic properties (Haedo and Golowasch 2006; Turrigiano et al. 1994) and in their ionic currents (Golowasch et al. 1999a; Haedo and Golowasch 2006; Khorkova and Golowasch 2007), previous modeling work has shown that such recovery of activity can be based entirely on activity-dependent mechanisms (Golowasch et al. 1999b; Zhang and Golowasch 2007). However, new experimental evidence that shows that neuromodulators have long-term effects on ionic conductances in members of the pyloric CPG (that are different from their short-term effects) suggests that neuromodulators also play a role in the slow recovery process (Golowasch et al. 1999a; Haedo and Golowasch 2006; Khorkova and Golowasch 2007).
Here we examined the contributions of activity-dependent and neuromodulator input-dependent mechanisms on the process of recovery of the rhythmic activity of the crab pyloric network after decentralization. We have done this by studying the time course of network activity changes, namely the termination of rhythmic pyloric activity immediately following decentralization and the time course of the beginning of its subsequent recovery, in response to manipulations of the activity or neuromodulator environment prior to decentralization. Using a computer model of this process, we show that the recovery process can be explained by activity- and neuromodulator-dependent mechanisms acting via independent pathways. Finally, we used the model neuron to predict the results of biological experiments and experimentally confirm the prediction.
METHODS
Stomatogastric nervous system preparation
Jonah crabs, Cancer borealis, were obtained from local suppliers in Newark (NJ) and kept in seawater tanks at 10–13°C. Before dissection, crabs were anesthetized by cooling in ice for 20–30 min. The stomatogastric nervous system, including the esophageal ganglion (OG), commissural ganglia (CoGs, which contain neuromodulator-releasing projection neurons), the stomatogastric ganglion (STG), their interconnecting nerves as well as the lateral ventricular (lvn), pyloric dilator (pdn), and pyloric constrictor (pyn) motor nerves, was dissected and pinned down in silicone elastomer-lined Petri dishes (Sylgard 182, Fisher Scientific) in normal chilled C. borealis physiological saline. Normal saline had the following composition (in mM):440.0 NaCl, 11.0 KCl, 13.0 CaCl2, 26.0 MgCl2, 5.0 maleic acid, and 11.0 Trizma base; pH 7.4–7.5. The STG was desheathed before experiments began, even when neurons were not impaled. Neuronal cell types were identified as described before (Harris-Warrick 1992; Selverston et al. 1976).
Organotypic cultures of the stomatogastric nervous system (STNS) were recorded continuously for ≤2 days on an electrophysiology setup at 11–13°C in normal or modified saline (see following text) supplemented with 1 g/l dextrose, 35 u/ml penicillin and 50 u/ml streptomycin. To experimentally modify the activity of the pyloric network, we used several pharmacological methods: 1) low-Na+ saline, 2) GABA, 3) muscimol, 4) baclofen, and 5) proctolin bath applications or hyperpolarizing current injection into the pyloric dilator (PD) neurons. When low external Na+ solutions were used, we replaced the Na+ in the normal saline with equimolar amounts of N-methyl-d-glucamine (Acros Organics). GABA, muscimol, baclofen, and proctolin were dissolved immediately prior to use or kept as stock solutions at 100–1,000 × concentration at 4°C for ≤2 wk, then diluted in saline immediately prior to use. Drugs or low-Na+ solutions were either bath-applied to the entire STNS or restricted to the STG and motor nerves (and excluding the ganglia containing neuromodulator-releasing neurons) by building a wall with petroleum jelly (Vaseline) separating the STG from the anterior part of the STNS and perfusing both sides independently.
Electrophysiology
Extracellular recordings from pyloric network motor nerves were obtained with stainless steel pin electrodes inserted into the Sylgard lining inside Vaseline wells built around the nerves. Reference leads of each electrode were pinned down directly in the bath outside the wells. Signals were amplified using differential amplifiers (A-M Systems amplifier 1700, Carlsborg, WA). Intracellular current injections were performed in current clamp after impaling the PD neurons, each with one electrode filled with a 0.6 M K2SO4 + 20 mM KCl solution. Electrodes were pulled with a Flaming-Brown puller (P97, Sutter Instruments, CA) and had resistances of 12–25 MΩ.
After dissection of the STNS, and desheathing of the STG, the pyloric rhythm was recorded for ∼15–30 min in normal physiological saline before any treatment was started. The bathing solution of the whole preparation or only the STG and motor nerves (but not the OG or CoGs) was then replaced with the substance of choice dissolved in crab saline. The conduction of action potentials along the input nerve stn was blocked in one of two ways: transection of the nerve near its entrance to the STG or by replacing the saline in a well built with Vaseline along the length of the desheathed stn with an isotonic sucrose solution (750 mM sucrose). For this, the crab saline solution inside the well was replaced four or five times with the sucrose solution to ensure complete removal of ions.
To reduce pyloric network activity, we used the injection of hyperpolarizing current (−2 to −6 nA) simultaneously into the two PD neurons found in the STG, each impaled with a single microelectrode. These two neurons are electrically coupled to the pacemaker AB neuron (coupling coefficient ∼0.2) (Rabbah et al. 2005) and are consequently considered to be part of the pyloric pacemaker. Consistent with this, their hyperpolarization effectively reduced or eliminated rhythmic pyloric activity. When proctolin was applied together with PD neuron hyperpolarization, the PD neurons were first hyperpolarized until an approximately stable steady state of very slow (<0.1 Hz) or no pyloric activity was obtained for 5–10 min. Proctolin was then bath-applied to the STG and motor nerves only.
Pyloric frequency was measured cycle by cycle using software developed in our laboratory (Datamaster) on a LabWindows platform, which determines bursts of action potentials from extracellular recordings by detecting threshold crossings by the action potentials. Frequency data for each 5 or 10 min of pyloric activity was averaged and plotted as one bar with variance indicated by a capped line whose height corresponds to the SD of this mean.
Statistical analysis was performed using nonpaired Student's t-test or one-way ANOVAs with Tukey post hoc tests (SigmaStat 2.03, Aspire Software International, Leesburg, VA). All data are presented as averages ±SD.
Mathematical model of a single pyloric neuron
For simplicity and as a model of the pyloric network pacemaker AB neuron, a single two-compartment neuron was built. Although simplified compared with previous AB neuron models (Soto-Treviño et al. 2005), this model expressed endogenous oscillatory activity resembling that of the AB neuron (Figs. 5B and 7B, top traces). Because our goal was to reproduce and provide a mechanistic understanding of the essential aspects of the process of pyloric activity recovery after decentralization, rather than the details of AB neuron bursting activity, we have not attempted to perfectly capture all details of the AB neuron activity. Instead the ionic currents were modeled using a modified version of previous models of decentralization (Golowasch et al. 1999b; Zhang and Golowasch 2007)
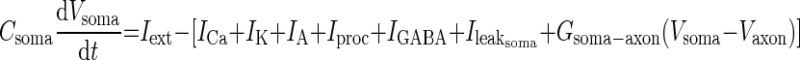 |
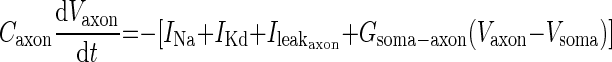 |
(1) |
Each ionic current is described by Hodgkin-Huxley-type equations as described before (Zhang and Golowasch 2007), and identical parameter values were used in this study, including Csoma and Caxon, except where indicated. The differential equations in the model were numerically solved with the software XPP using the default Runge-Kutta integration method (Ermentrout 2002).
Our previous modeling studies have shown that the regulation of an intracellular activity-dependent Ca2+ sequestration mechanism as well as slow activity-dependent K+ and Ca2+ conductance regulation are required to produce the intermittent on and off pattern of pyloric activity generation that characterizes the initial phase of the recovery process of the crab pyloric network following decentralization (Zhang and Golowasch 2007), an activity pattern we call “bouting” (Fig. 2, B–D). A similar activity-dependent feedback mechanism, using an intracellular Ca2+ sensor to regulate the maximal Ca2+ conductance, ḠCa, and the transport rate of an internal calcium pump putatively localized on the endoplasmic reticulum (ER) membrane, Rpump, was used. The intracellular cytoplasmic Ca2+ concentration, [Ca]cyt, was treated with a first-order diffusion term responsible for clearing Ca2+ from the cytoplasm and an IP3 and Ca2+-dependent Ca2+ channel responsible for extrusion of Ca2+ from the ER, exactly as described in Zhang and Golowasch (2007). This model is schematically illustrated in Fig. 1, which for simplicity depicts only the soma compartment and only a subset of the ionic currents included. The previous model (Zhang and Golowasch 2007) required three different activity sensors to generate the recovery process after decentralization. Here we found only one sensor to be necessary and sufficient to regulate both the Ca2+ pump rate (Rpump) and ionic conductances. Furthermore, we found that regulation of ḠCa is sufficient to reproduce the phenomenon and have thus simplified the original model further by excluding activity dependence of regulation of ḠCa. Other currents may certainly be regulated by activity in the biological pyloric network, but we have not included them here. We have therefore simplified the model of the ḠCa dynamics to make it dependent on a single activity sensor, SA
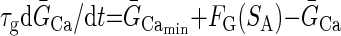 |
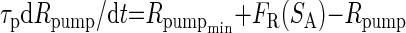 |
(2) |
τg is the time constant of Ca2+ conductance regulation and τp is the time constant of ER pump regulation. ḠCa_min and Rpump_min are constants representing minimal values of ḠCa and Rpump. The activity sensor, SA, has a direct sigmoidal dependence on [Ca]cyt
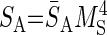 |
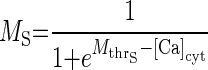 |
(3) |
S̄A represents the maximal value of SA, and MS is the activation variable of SA and is a growing function of intracellular [Ca]cyt. Mthr_S represents the value of [Ca]cyt when MS = 0.5.
FIG. 1.
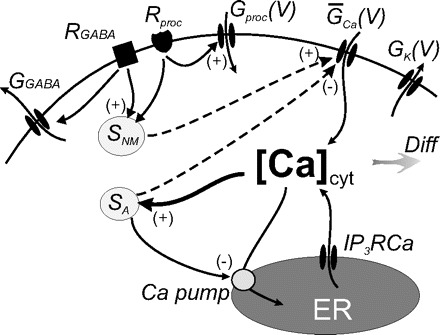
Schematic diagram of intracellular activity- and neuromodulator-dependent regulation. GK(V), GCa(V), and Gproc(V) are the voltage dependent conductances of IK, ICa, and Iproc, respectively. ER here represents the endoplasmic reticulum or any intracellular Ca2+ store. IP3RCa is the activated form of the IP3-sensitive Ca2+ receptor/channel on the ER membrane, and Ca pump on the ER membrane represents the intracellular Ca2+ uptake process. The shaded arrow labeled Diff stands for intracellular Ca2+ diffusion and passive buffering. SA is the activity-dependent Ca2+ sensor. SA detects changes of [Ca]cyt, and in turn regulates both GCa and Ca pump. SNM is the neuromodulator sensor that regulates GCa and no other conductance. SNM is triggered directly by activation of neuromodulator receptors, in particular type-B GABA receptors (RGABA, ▪) and proctolin and other receptors (Rproc, ♥), putatively via G proteins or other activated signaling molecules downstream of the receptors. GABA also activates a voltage-independent hyperpolarizing (or shunting) conductance via GABAA-like receptors (not shown). Proctolin and other non-GABA neuromodulators activate the neuromodulator-sensitive conductance Gproc(V).
The activity sensor in turn regulates both the maximal Ca2+ conductance, ḠCa, and the maximal intracellular Ca2+ pump activity, Rpump, via decreasing sigmoid functions of SA
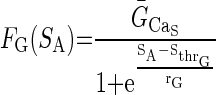 |
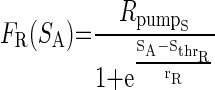 |
(4) |
ḠCa_S and Rpump_S are constants representing the maximal values of ḠCa and Rpump generated via activity-dependent (i.e., Ca2+-dependent) regulation. FG(SA) and FR(SA) are both decreasing functions of SA, and Sthr_G represents the value of SA when FG(SA) = ḠCa_S/2, and Sthr_R represents the value of SA when FR(SA) = Rpump_S/2. The parameters rG and rR represent the steepness of FG(SA) and FR(SA) regulation, respectively, as a function of SA.
GABA activates two types of receptors: GABAA-like, also activated by muscimol and evoking a current with a reversal potential near −70 mV in PD neurons and a GABAB-like receptor, also activated by β-guanidinopropionic acid and baclofen and evoking a current with a similar hyperpolarized reversal potential (Fig. 1) (Parnas et al. 1999; Swensen et al. 2000). To implement both GABAergic effects on pyloric activity in the preceding model, a GABA-activated current, IGABA, was included in the somatic current balance equation [1]. IGABA is a voltage-independent current, but its conductance is dependent on GABA concentration, [GABA], with a maximum conductance at saturation, ḠGABA, and reversal potential, EGABA (Table 2) taken from Swensen et al. (2000)
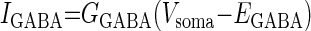 |
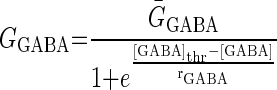 |
(5) |
[GABA]thr represents the value of [GABA] when GGABA = ḠGABA/2.
To model the GABAergic metabotropic effects, we build a neuromodulator-dependent sensor SNM sensitive to the neuromodulator concentration [NM]. For simplicity, we assume this same sensor to depend on the levels of all metabotropic neuromodulators known to be released by stn axon terminals. Thus SNM is sensitive to GABA (consequently also to GABAB) receptor agonists and to proctolin and represents some intracellular signaling pathway likely activated by a G protein coupled to the neuromodulator-activated receptors (Parnas et al. 1999; Swensen et al. 2000). The introduction of a slow-acting neuromodulator-dependent sensor (or signaling pathway) is consistent with the recently discovered existence of long-term neuromodulator effects, including those of proctolin, on voltage-gated conductances in this system (Khorkova and Golowasch 2007). These effects are distinct from the acute (i.e., fast) activation of the neuromodulator-gated current Iproc (Buchholtz et al. 1992; Golowasch and Marder 1992; Swensen and Marder 2000) also included in this model. The sensor SNM activates with time constant τNM toward a steady-state value that depends on the neuromodulator concentration, [NM]. [NM]thr represents the [NM] needed to reach half the maximal S̄NM value
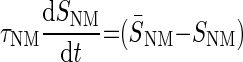 |
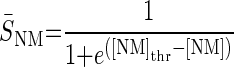 |
(6) |
and
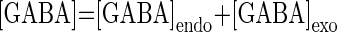 |
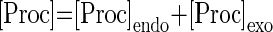 |
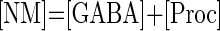 |
The biological concentrations of different neuromodulators are assumed to be at nonsaturation levels. Thus to simplify the model the concentration of any neuromodulator was normalized to levels considered to be the endogenous levels (i.e., levels released during the ongoing activity before decentralization), hence [GABA]endo = 1, [Proc]endo = 1, thus [NM]endo = 2. For the application of an exogenous neuromodulator, its concentration was increased ≥2 times the endogenous concentration. Thus [NM]exo = 2 (NM can be either GABA or proctolin), and [NM] = [NM]endo + [NM]exo.
The concentration of neuromodulators other than GABA (proctolin in our experiments) is represented by [proc] and activation of the conductances Gproc depends on voltage via mprocc(V) and on the neuromodulator concentration [proc]
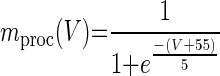 |
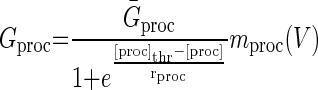 |
(7) |
[proc]thr represents the value of [proc] when Gproc = Ḡproc/2. mproc(V) is identical to what was used in Zhang and Golowasch (2007). In this model, all the time constants of the slow dynamical variables (τg, τp, and τNM) have been scaled down ∼10-fold from values that would produce a realistic time course of recovery solely for the purpose of speeding the computations. When using 10× longer time constants, no qualitative differences from the results shown here are observed. As described before (Zhang and Golowasch 2007), the activity dependent sensor SA needs to be much faster than the other variables and is thus assumed to be instantaneous (see Eq. 3).
To introduce neuromodulator-dependence in the model, Eq. 2 was modified to include a dependence of ḠCa on a neuromodulator sensor SNM via the function FN(SNM)
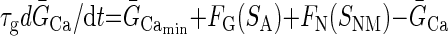 |
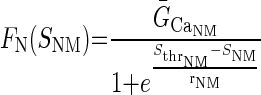 |
(8) |
FN(SNM) is an increasing sigmoid function of SNM, ḠCa_NM is the maximal value of ḡCa that can be generated by neuromodulator-dependent regulation, Sthr_NM is the half-maximal value of SNM, and the parameter rNM represents the sensitivity of FN(SNM) regulation. Without exogenous neuromodulators, FN(SNM) is much smaller than FG(SA), and ḠCa is mainly determined by FG(SA).
The values of all parameters are listed in Table 1.
TABLE 1.
Parameter values of pacemaker model
| Name | Value |
|---|---|
| ḠCa_min | 0.12 μS |
| Rpump_min | 0.002 μMms−1 |
| τg | 300 s |
| τp | 4500 s |
| S̄A | 8 |
| Mthr_S | 0.9 μM |
| ḠCa_S | 0.039 μS |
| Rpump_S | 0.006 μMms−1 |
| Sthr_G | 0.17 |
| Sthr_R | 0.17 |
| rG | 0.012 |
| rR | 0.012 |
| EGABA | −66 mV |
| ḠGABA_max | 0.5 μS |
| [GABA]thr | 2 |
| γGABA | 0.05 |
| τNM | 500 s |
| [NM]thr | 3 |
| rNM | 0.1 |
| ḠCa_NM | 0.012 μS |
| Sthr_NM | 0.6 |
| rS_NM | 0.05 |
| Ḡproc | 0.01 μS |
| [proc]thr | 0.998 |
| rproc | 0.005 |
RESULTS
Animal model
The STNS of crustaceans, such as the crab C. borealis, controls the ingestion and movements involved in food processing in the foregut (Selverston et al. 1976). The movements of one of the forgut chambers, the pylorus, are driven by the pyloric network, located in the STG. The pyloric rhythm is composed of alternating bursts of activity of several neuronal groups (see Fig. 2, B and C. top). This bursting activity is the result of the interactions of reciprocal inhibitory synaptic inputs between the neurons in the circuit and the neuronal intrinsic properties, all driven by the pacemaker activity of the anterior burster neuron (AB) (Nusbaum and Beenhakker 2002). This activity can be recorded both extracellularly from motor nerves (Fig. 2, B and C) as well as intracellularly. We have limited our recordings to extracellular nerve recordings and used intracellular impalements for current injection only.
FIG. 2.
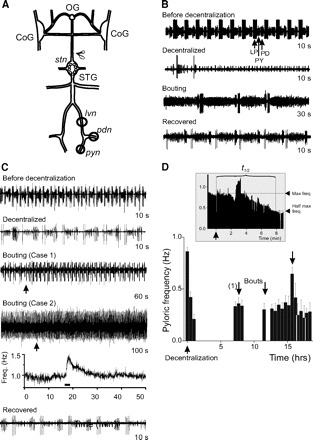
Time course of pyloric network activity recovery after decentralization under normal conditions. A: schematic diagram of the stomatogastric nervous system (STNS) showing the 4 ganglia [esophageal ganglion (OG), commissural ganglia (CoGs), CoGs, and stomatogastric ganglion (STG)], connecting nerves, and motor nerves. Motor nerves used for recording pyloric network activity are indicated [○ representing a petroleum jelly (Vaseline) recording well]. The input stn nerve was cut to decentralize the STG (indicated by scissors). B and C: extracellular lvn recordings of pyloric network activity before (before decentralization), immediately after (decentralized), during (bouting, showing 1 bout of pyloric activity in each case), and after activity has stabilized (recovered). B and C are examples to illustrate 2 extremes of the response to decentralization with B showing complete shutdown of pyloric activity and C showing only a slowing down. Individual bout duration in B was also very brief, whereas that in C was long and appears as an acceleration of the rhythm. Duration of each trace is indicated on the right. D: pyloric rhythm frequency changes as a function of time. Pyloric frequency in a representative preparation was measured every 30 min and the averages (±SD) of 10 min of bursting are plotted against time. The pyloric rhythm stopped completely after decentralization (↑), which corresponds to time t = 0. The 1st bout (1) ↓ occurred ∼7 h after decentralization and 2 other bouts (↓) followed before activity stabilized after ∼16 h. Inset: at an expanded time scale, the instantaneous pyloric frequency (i.e., cycle-by-cycle frequency) around the time of decentralization and the measurement of t1/2.
Neuromodulatory input to the STG comes from the anterior OG and CoGs via a single input nerve, the stomatogastric nerve (stn, Fig. 2A). When the STG was isolated from the rest of the STNS by blocking action potential transmission along the stn, activity of the pyloric network decreased considerably or stopped completely (Fig. 2, B and C, 2nd trace) (see also Golowasch et al. 1999b; Luther et al. 2003; Thoby-Brisson and Simmers 1998; Zhang and Golowasch 2007). This is thought to be due to the cessation of neuromodulator release from axonal terminals of the stn (Marder and Bucher 2007) and the consequent deactivation of Iproc. Over time, the rhythmic activity of the crab and lobster pyloric networks resumes and stabilizes (Golowasch et al. 1999b; Thoby-Brisson and Simmers 1998). In crabs, this occurs after a period characterized by the repeated turning on and off of the rhythm, called bouting (Luther et al. 2003; Zhang and Golowasch 2007). The third trace in Fig. 2, B and C, illustrates two examples of a bout of pyloric activity: in the first, a very brief bout is observed with three bursts of pyloric activity in a single bout (Fig. 2B); otherwise bouts are long-lasting and sometimes appear as an acceleration of a slow ongoing pyloric rhythm (Fig. 2C, cases 1 and 2). We defined a bout as an increase of the pyloric frequency for at least three pyloric cycles and by ≥40% above the background frequency (see examples labeled bouting in Fig. 2, B and C). Case 2 in Fig. 2C shows an extreme example in which this acceleration is not easily distinguished by eye yet is clearly distinguished as a rapid increase in a frequency-versus-time graph followed by an exponential return to baseline (Fig. 2C, 5th trace). Figure 2D shows a bar graph summarizing the changes in pyloric rhythm frequency over several hours after decentralization (Fig. 2D, ↑). Figure 2D also illustrates the rate of pyloric activity deceleration, i.e., rapid pyloric frequency decrease immediately after decentralization (Fig. 2D, inset) and the variability of the pyloric frequency during the bouting period (bouts in Fig. 2D, ↓).
In this study, we examined the hypothesis that the process of recovery of the pyloric network activity can be altered by changes in network activity and the neuromodulator environment preceding decentralization. We experimentally induced activity changes using GABA, GABA agonists, hyperpolarizing current injections into the pacemaker neurons and low external Na+ solutions. All experiments described in the following text followed the same protocol: after recording, the normal pyloric network activity for ∼30 min, a pharmacological agent or a manipulation of the membrane potential of identified pyloric neurons was applied for a defined period. The pharmacological agents were then washed out rapidly with normal saline (or the external current injection discontinued) until stable pyloric network activity was recovered (typically within 5–10 min). The STG was then decentralized by blocking action potential conduction along the input stn nerve.
We used two parameters to characterize the recovery process: the rate of rhythmic activity deceleration starting immediately after decentralization and the time to the first bout. We define the rate of activity deceleration as the time needed for the pyloric rhythm frequency to reach for the first time half of its maximum predecentralization value (t1/2) at the time immediately before decentralization (Fig. 2D, inset). The time to the first bout (tbout) of pyloric activity is the time from the moment of decentralization to the beginning of the first observed bout (Fig. 2D). We chose tbout rather than the time to stable recovery as our landmark to estimate the rate of recovery because, although quite variable, tbout is significantly shorter than the time to stable recovery of activity (Luther et al. 2003). Bouts are a clear sign of the impending recovery of pyloric activity after decentralization and are observed in every preparation examined (see also Luther et al. 2003). Thus even if other processes may be activated after bouting starts that are required for full and stable recovery of activity, bouting seems to be a hallmark of the overall process. We thus refer to tbout as a measure of the delay of the initiation of pyloric rhythm recovery. This choice thus maximizes the number of successful experiments. In control preparations, the average time to half-maximal pyloric rhythm frequency was t1/2 = 0.25 ± 0.26 h, and the average time to first bout was tbout = 6.19 ± 4.76 h (Table 2, n = 18).
TABLE 2.
Recovery variables t1/2 and tbout under different pre-decentralization treatments
| Treatment Before Decentralization | Experimental Results |
n | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model Results |
||||||||||
| Time to Half Maximal Reduction (t1/2), h | Statistical Analysis | Time to First Bout (tbout), h | Statistical Analysis | t1/2, h | tbout, h | |||||
| Control | 0.25 ± 0.26 | 6.19 ± 4.76 | 18 | ≈0 | 0.31 | |||||
| Hyper-polarization | 0.21 ± 0.18 | 1.60 ± 1.72** | 12 | ≈0 | 0.18 | |||||
| Low-Na+ saline | 0.33 ± 0.35 | ANOVA P = 0.56 | 1.22 ± 0.94* | ANOVA P = 0.0004 | 14 | ≈0 | 0.18 | |||
| Muscimol | 0.38 ± 0.27 | 2.05 ± 1.60* | 6 | |||||||
| GABA overnight (12–18 h) | 2.27 ± 3.21* | ANOVA P = 0.023 | 3.91 ± 4.60 | ANOVA P = 0.015 | 10 | 0.18 | 0.28 | |||
| GABA for 5 h | 1.08 ± 1.05 | 1.98 ± 1.30* | 10 | |||||||
| GABA for 1 h | 0.72 ± 0.85 | 1.90 ± 0.84* | 9 | |||||||
| Baclofen | 5.89 ± 8.64** | t-test (vs Control) P = 0.004 | 6.74 ± 8.18 | t-test P = 0.58 | 7 | |||||
| Proctolin | 0.16 ± 0.14 | t-test (vs Control) P = 0.79 | 2.34 ± 1.88* | t-test P = 0.048 | 7 | ≈0 | 0.23 | |||
| Proctolin+Hyper-polarization | 0.11 ± 0.11 | t-test (vs Hyperpol.) P = 0.112 | 0.22 ± 0.17* | t-test P = 0.049 | 5 | |||||
Values represent averages ± SD. ANOVA was followed by post hoc Tukey tests, and the significance of the individual treatments obtained from post hoc tests is indicated (
if significantly different from control at P < 0.05 or
if significantly different from control at P < 0.01.).
Activity suppression by hyperpolarization or low Na+ advances recovery after decentralization
To determine how the activation of pyloric rhythm recovery is affected by a purely activity-dependent process, we first attempted to reduce or eliminate pyloric activity during several hours preceding decentralization. Reasoning that reduced pyloric activity before decentralization should upregulate any activity-dependent recovery mechanisms available to the neurons at that time, a shorter delay to the initiation of recovery would be expected if measured from the moment of decentralization.
The rhythmic activity of the pyloric network can be inhibited by either hyperpolarization of the PD neurons that are electrically coupled to the pacemaker AB neuron or by reducing the extracellular Na+ concentration (Fig. 3). In preparations in which the PD neurons were hyperpolarized with current injection for ∼5 h prior to decentralization, the average time to half-maximal pyloric rhythm frequency (i.e., of rhythm cessation) was not different from control values (t1/2 = 0.21 ± 0.18 h, n = 12, Fig. 3A). A one-way ANOVA was used to compare control, hyperpolarized, and low-Na+-treated (see following text) preparations, and all these effects were indistinguishable from each other (P = 0.56, Table 2). However, the average time to first bout in hyperpolarized preparations was significantly shorter than in control (tbout = 1.60 ± 1.72 h, n = 12, P = 0.003; post hoc Tukey test from 1-way ANOVA test; Table 2, Fig. 3A).
FIG. 3.
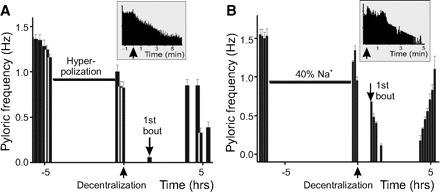
Pyloric activity changes and recovery induced by hyperpolarization and low Na+ application prior to decentralization. A: pyloric frequency changes in a representative preparation hyperpolarized for ∼4.5 h before decentralization. B: pyloric frequency changes in a representative preparation incubated with 40% Na+ solution for ∼6 h before decentralization (↑). Decentralization corresponds to time t = 0. In both preparations, the 1st bout (↓) appeared faster than in control, but there was no delay in rhythmic activity deceleration (t1/2) compared with control. Insets: at an expanded time scale, the instantaneous pyloric frequency around the time of decentralization.
When preparations were incubated in 40–50% Na+ saline for ∼5 h prior to decentralization, results similar to those observed in preparations that were hyperpolarized before decentralization were obtained (Fig. 3B, Table 2). The time to half-maximal frequency was 0.33 ± 0.35 h (n = 14), which was not significantly different from control or from hyperpolarized PD preparations (overall 1-way ANOVA, P = 0.56; Fig. 3B, Table 2). However, the average time required to produce the first bout in these low-Na+-treated preparations (tbout = 1.22 ± 0.94 h; n = 14) was significantly shorter than control (post hoc Tukey test P < 0.001; Table 2, Fig. 3B). The overall one-way ANOVA comparing these effects showed a significant difference between these treatments (P = 0.0004, Table 2).
Treatment with GABA before decentralization advances the recovery of activity but delays the rhythm deceleration after decentralization
Treatment of the nondecentralized pyloric network with 1 mM GABA stopped the pyloric rhythm completely and reversibly (Fig. 4A). For this reason, we initially intended to use GABA as a means of reducing pyloric network activity prior to decentralization. Examples of pyloric activity before GABA application, immediately after washout of GABA but before decentralization and at t1/2 are shown in Fig. 4B. When preparations were pretreated with 1 mM GABA for 0 (control), 1, 5, and 12–18 h, the half-maximal time of pyloric rhythm deceleration showed a time-dependent increase (t1/2 = 0.25 ± 0.26, 0.72 ± 0.85, 1.08 ± 1.05, and 2.27 ± 3.21 h, respectively) which was statistically significant (P = 0.023, 1-way ANOVA, Fig. 4A, Table 2). On the other hand, the time to first bout decreased when GABA was applied before decentralization for 0, 1, 5, or 12–18 h (tbout = 6.19 ± 4.76, 1.90 ± 0.84, 1.98 ± 1.30, and 3.91 ± 4.60 h, respectively). This dependence on GABA incubation time was statistically significant overall (P = 0.015, 1-way ANOVA; Table 2), but a post hoc Tukey test revealed that only preincubation of ≤5 h had significant effects on tbout (P = 0.04). We believe that this reveals a possible interplay between activity-dependent and neuromodulator-dependent mechanisms (see discussion). It is important to note that occasionally the activity never completely stopped after GABA pretreatment. Nevertheless, when this happened, bouts often appeared as transient frequency increases over the background frequency that could be easily distinguished from random frequency variation or transient artifacts by the criteria described before (see Fig. 2C, case 2).
FIG. 4.
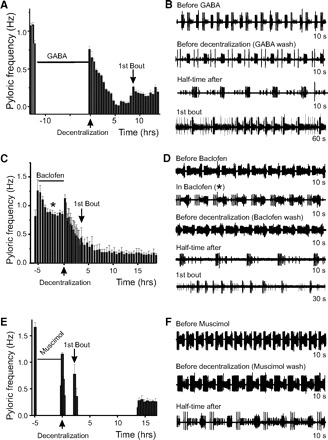
Effect of metabotropic receptor activation on pyloric activity changes and recovery from decentralization. A: pyloric frequency changes in a representative preparation incubated in 1 mM GABA for ∼12 h (horizontal bar) before decentralization. The 1st bout appears as a low-frequency increase. B: extracellular lvn recordings of pyloric network activity of the preparation in A before GABA incubation, after GABA wash off but before decentralization, at half time (t1/2) after decentralization, and during the 1st bout of pyloric activity. As sometimes observed, the pyloric rhythm did not completely turn off in this case. C: pyloric frequency changes in a representative preparation incubated with 0.5 mM baclofen for ∼5 h before decentralization. D: extracellular lvn recordings of pyloric network activity of the preparation shown in C before baclofen incubation, in baclofen (marked by *), after baclofen wash off but before decentralization, at half time (t1/2) after decentralization, and during the 1st bout of pyloric activity. E: pyloric frequency changes in a representative preparation incubated with 0.5 mM muscimol for ∼5 h before decentralization. F: extracellular lvn recordings of pyloric network activity of the preparation in E before muscimol incubation, after muscimol washed off but before decentralization, and at half-time (t1/2) after decentralization. In A, C, and E decentralization corresponds to time t = 0 and is indicated by ↑ along the time axis, and the 1st bout is indicated by ↓. Time under each extracellular trace in B, D, and F indicates duration of the entire trace.
If the recovery process was solely activity dependent, in GABA-pretreated preparations, only the advanced recovery time would be observed (i.e., a reduced tbout), as in hyperpolarized or low-sodium saline-treated preparations with no effect on the rhythm deceleration rate (i.e., t1/2). However, the experimental results also showed a significant reduction in the rate of pyloric activity deceleration after decentralization (i.e., an increase in t1/2). Additionally, short-term (1 h) pretreatment with GABA only reduced tbout, whereas t1/2 was not significantly affected (Table 2). The difference in the kinetics of these two effects suggested to us the possibility that they may be mediated by two different mechanisms.
Because it is known that pyloric network neurons express at least two types of GABA receptors, an ionotropic GABAA-type and a metabotropic GABAB-type (Swensen et al. 2000), we examined whether the two observed effects could be mediated independently by the two different receptor types.
Metabotropic GABAB receptor activation only affects the kinetics of pyloric rhythm deceleration
Pretreatment of the STG with the GABAB receptor agonist baclofen (0.5 mM) for ∼5 h before decentralization slightly (but not significantly) slowed the pyloric rhythmic activity from 1.15 ± 0.27 to 0.98 ± 0.24 Hz (P = 0.163, n = 6, Student's t-test, Fig. 4, C and D). Yet the time course of pyloric rhythm deceleration after decentralization in these preparations was significantly higher than control (t1/2 = 5.89 ± 8.64 h, P = 0.004, n = 7, Student's t-test; Fig. 4C, Table 2). On the other hand, the average time required to produce the first bout was not significantly different from control preparations (tbout = 6.74 ± 8.18 h; P = 0.58, n = 7, Student's t-test; Table 2). Therefore the baclofen pretreatment resembles pretreatment with GABA in its effects on the time course of pyloric activity deceleration after decentralization but not on the delay of pyloric rhythm recovery initiation as indicated by the time to generate the first bout of pyloric activity (Fig. 4C, Table 2).
Ionotropic GABAA receptor agonist only reduces the time to first bout
Pretreatment of the STG with the GABAA receptor agonist muscimol (0.5 mM) for ∼5 h before decentralization completely shut down pyloric activity (Fig. 4E). Decentralization after muscimol treatment resulted in the complete and rapid termination of rhythmic activity (Fig. 4, E and F). The time course of events after decentralization was similar to that observed in preparations whose activity was reduced before decentralization by either hyperpolarization or low-Na+ saline. After muscimol treatment, t1/2 was 0.38 ± 0.27 h (n = 6) with no significant difference from control (a 1-way ANOVA was used to compare control, hyperpolarized, low-Na+-treated, and muscimol-treated preparations, and all these treatments had indistinguishable effects; P = 0.56, Table 2). However, the average time to first bout was significantly shorter than in control preparations (tbout = 2.05 ± 1.60 h, n = 6; overall 1-way ANOVA P = 0.0004; post hoc Tukey test P = 0.04; Table 2). Participation of this ionotropic mechanism in controlling the time course of activity recovery is consistent with the activity-dependent reduction in the delay to the onset of pyloric activity after GABA pretreatment (Table 2).
These experiments demonstrated that when only ionotropic GABAA receptors were activated during pretreatment, as in the case with muscimol, only the initial rate of recovery of the network's activity (tbout) was changed and not the rate of activity deceleration after decentralization (t1/2). On the other hand, if during pretreatment only GABAB metabotropic receptors were activated by baclofen, only the rate of activity deceleration after decentralization (t1/2) was significantly affected but not the delay of initiation of activity recovery (tbout). It appears therefore that two independent signaling pathways control the effects of GABA pretreatment on the recovery after decentralization, one activated via GABAA and another via GABAB receptor. GABAA signaling is mediated by an ionotropic effect and thus results in changes in the ionic balance of the cell. We suggest that GABAA receptor activation launches an activity-dependent mechanism of network activity recovery, identified by a decreased time to first bout, tbout. This mechanism is likely also launched by the other activity-altering treatments tested (hyperpolarization and incubation in low-Na+ saline). We suggest further that GABAB signaling, probably acting through a G protein pathway (Duan and Cooke 2000; Swensen et al. 2000), controls the rate of activity deceleration.
To verify the plausibility of such a mechanism, we adapted a computational model of decentralization, previously developed to account for the process of bouting in an untreated pyloric network (Zhang and Golowasch 2007), to include two independent processes of Ca2+ conductance regulation plus the activity-dependent regulation of intracellular Ca2+ sequestration and applied to the model the same experimental manipulations as were used here on the biological preparations to examine their effects on the initial phase of the recovery from decentralization.
Model of activity- and neuromodulator-dependent recovery of pyloric network activity
The schematic diagram of neuromodulator and activity-dependent feedback mechanisms shown in Fig. 1 illustrates the essential components of our model and the hypothesis of activity regulation it represents. In our previous model (Zhang and Golowasch 2007), we used three Ca2+ sensors that operated in an activity-dependent manner. Here our goal was to develop a model that could provide a plausible explanation for the observed effects of modifying both activity and of neuromodulator application prior to decentralization on the recovery of pyloric activity after decentralization. For this, we first developed a simplified form of our previous model by reducing the three activity-dependent sensors to a single one (SA) regulated only by intracellular calcium ([Ca]cyt, Fig. 1). While SA increases with increasing [Ca]cyt (see Ms in Eq. 3), it has an inverse effect on the maximum Ca2+ conductance, ḠCa, as well as on the intracellular Ca2+ pump activity, Rpump (Eq. 4). In spite of the simplification in the activity-dependent branch of regulation, this model still adequately captured the activity-dependent aspects of the recovery from decentralization, including the generation of bouting and delayed recovery of stable activity [Fig. 5, compare with Fig. 2B of Zhang and Golowasch (2007)]. As with the biological preparations (Fig. 2D), decentralization was rapidly (<1 oscillation period) followed by a cessation of all rhythmic activity, and the first bout appeared with a delay of 0.31 h (Table 2; this is ∼3 h when realistic time constants are used). Bouting activity ensued (Fig. 5, A and B, center) and became regular and sustained after ∼4.5 h (i.e., ∼45 h with realistic time constants, Fig. 5A). Bursting activity after recovery was slower than before decentralization also as observed in the biological preparations (compare Fig. 2, B and C, with 5B). When activity terminates following decentralization, the level of the activity sensor SA drops (Fig. 5A, bottom), which then leads to recovery of activity via enhancement of ḠCa (Eqs. 2–4) ((Zhang and Golowasch 2007).
FIG. 5.
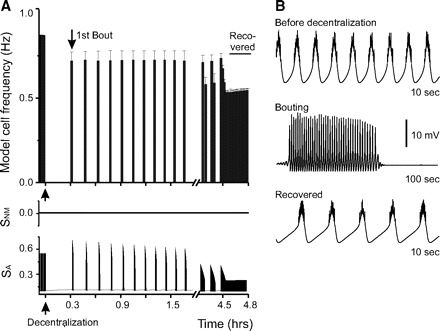
Recovery process after decentralization in the model neuron under control conditions. A: control case. Bursting frequency changes in the model neuron after decentralization. Decentralization corresponds to time t = 0 and is indicated by ↑ under the time axis, and the 1st bout is indicated by ↓. After decentralization, the neuron rapidly stops bursting. Activity becomes stable ∼4.5 h after decentralization (recovered). The bottom 2 traces show the changes of sensors SNM and SA as a function of time. SA tracks the activity changes instantaneously, whereas SNM does not change in response to activity changes. B: magnified traces of model neuron membrane potential in control conditions before decentralization, during bouting, and after activity has stably recovered.
The effect of neuromodulators is here hypothesized to be independent from activity-dependent effects. Neuromodulator-dependent effects are thus represented by a direct activation of the sensor SNM at increasing neuromodulator concentrations (Fig. 1 and Eq. 6). Activation of SNM only affects the maximum calcium conductance, ḠCa (Eq. 8), but does not affect the activity-dependent sensor SA or the intracellular Ca2+ pump activity (Fig. 1). Two different sets of neuromodulator receptors, GABAB receptors and other metabotropic receptors such as those sensitive to proctolin, are modeled (Fig. 1). GABAB is known to weakly activate a K+ conductance in crab STG neurons (Swensen et al. 2000), and this effect is not included in the model, only the slower effect on SNM (Eq. 6). RGABA activation is also assumed to activate IGABA, but for simplicity, the ionotropic GABAA and the metabotropic GABAB receptors are combined together here. Proctolin and other peptidergic neuromodulators are known to activate a distinct conductance (Buchholtz et al. 1992; Golowasch and Marder 1992; Swensen and Marder 2000), and this effect is here represented by the direct activation of GNM. We suggest that activated proctolin receptors also have slow effects on the sensor represented by SNM (Fig. 1, Eq. 6). In fact, in our model, both GABA and proctolin are assumed to have identical effects on this sensor (Eq. 6). However, they have distinct ionotropic effects (Eqs. 5 and 1, respectively). Finally, IP3RCa, represents the Ca2+-dependent release of Ca2+ from intracellular stores via an IP3-dependent mechanism (Zhang and Golowasch 2007). We hypothesize that during the normal, ongoing pyloric rhythm the activity level of the sensor SNM is very low (in fact, with [NM] = 2, SNM = 0.27), and thus a decrease in SNM activity contributes very little to the postdecentralization events (Fig. 5A, middle). Instead before decentralization, the neuromodulator-activated current, Iproc, predominantly drives the generation of oscillatory activity.
We represent the effect of decentralization by setting the neuromodulator proctolin concentration [proc] to zero. This brings Gproc to almost zero, turning Iproc off and inactivating the neuron's pacemaking capability (see Zhang and Golowasch 2007). Activity deceleration after decentralization decreases the value of SA, which initiates the increase in ḠCa that will eventually lead to the recovery of bursting activity (Zhang and Golowasch 2007).
Model neuron hyperpolarization reduces time to first bout with no effect on deceleration rate
Introduction of a small negative current (Iext = −0.5 nA, Eq. 1) to the model neuron caused its hyperpolarization and inhibited bursting activity. In our experiment, the model neuron was hyperpolarized for 45 min before decentralization, then hyperpolarization was stopped, and the model neuron resumed bursting almost immediately. Two minutes later, decentralization was performed. In this experiment, the bursts stopped instantaneously after decentralization (Fig. 6, Table 2), similar to what was seen in the control case. However, it took 0.18 h to produce the first bout, which is almost half as short as the time required in control conditions (Fig. 5, Table 2). As described before, here the activity sensor SA tracked changes in activity (Fig. 6, bottom) while the neuromodulator sensor SNM had no appreciable change. These results matched the results of biological experiments, suggesting that activity-dependent regulation alone can regulate the initial rate of recovery after decentralization as measured by the time to produce the first bout.
FIG. 6.
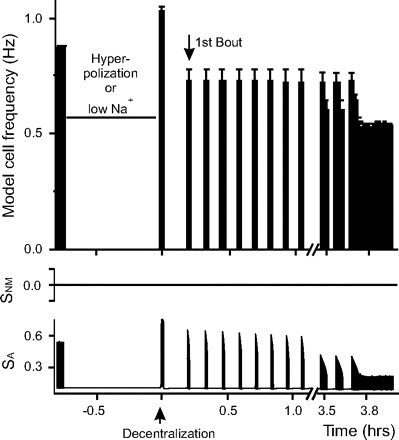
Recovery process in the model neuron hyperpolarized before decentralization. Before decentralization, the model neuron was hyperpolarized or “incubated” with low-Na+ solution for 45 min (horizontal bar). Both treatments have nearly identical effects. Decentralization corresponds to time t = 0 (up arrow). After decentralization the neuron rapidly stops bursting. The 1st bout is indicated by down arrow. The bottom 2 traces show the changes of sensors SNM and SA as a function of time. SA tracks the activity changes instantaneously, whereas SNM does not change during hyperpolarization or decentralization.
Low-Na+ saline incubation (experimental results shown in Fig. 3) reduces the activity of the STG pacemaker neuron in a similar manner as hyperpolarization. Note however, that low-Na+ treatment in the biological system is likely to also hyperpolarize the neuromodulator-containing axonal terminals from the input stn nerve and thus also reduce the release of neuromodulators. Hence, low-Na+ saline incubation was also modeled here by simultaneously setting neuronal activity and both [GABA] and [Proc] to zero. The results from this treatment are virtually indistinguishable from those of hyperpolarization treatment alone (t1/2 ≈ 0, tbout = 0.18 h). When the model cell was only hyperpolarized without affecting the neuromodulator levels, we observed an almost indistinguishable effect to those observed when the neuromodulators are also inactivated.
“GABA pretreatment” of the model neuron both slows the kinetics of pyloric rhythm deceleration and reduces time to first bout
Activation of the hyperpolarizing GABA current (Eq. 5) in the model neuron for 45 min before decentralization completely inhibited the bursting activity even if all other currents, including Iproc, were left intact (Fig. 7A). After 45 min of exogenous application of GABA at a level two times larger than the endogenous level ([GABA] = 2, as explained in methods; corresponding to simultaneous GABAA and GABAB receptor activation), [GABA] was briefly reduced to control levels ([GABA] = 1) before it was set to zero (together with [proc] = 0) to simulate decentralization. During RGABA activation, the activity of both SA and SNM was affected with SA tracking the neuron's activity and turning off (Fig. 7A, bottom), which increased FG(SA), and with SNM increasing to a high level (Fig. 7A, middle), which increased FN(SNM). On GABA removal, bursting activity rapidly resumed at an increased frequency relative to control, similar to the observations we have made in biological experiments using baclofen or GABA (compare Fig. 7, A and B, with 4, C and D, which shows the metabotropic GABAergic effects; although not shown in Fig. 4A, we have observed this effect in 30% of the experiments with GABA bath applications). While the activity sensor SA rapidly increased, which reduced FG(SA), the SNM sensor only slowly returned to its low baseline level. This kept FN(SNM) high for a relatively longer period than FG(SA; Fig. 7A), thus maintaining ḠCa at an elevated level. When decentralization was performed a moment later, the bursting activity remained high after decentralization due to this increased ḠCa level, only gradually decreasing over the course of ∼20 min (t1/2 = 0.18 h), compared with a nearly instantaneous inactivation in control conditions (Table 2). At the same time, it took a slightly shorter time (0.28 h) for the model cell to produce the first bout compared with 0.31 h in control conditions (Table 2). These results closely matched the effects of equivalent manipulations with GABA observed in biological experiments (Fig. 4A).
FIG. 7.
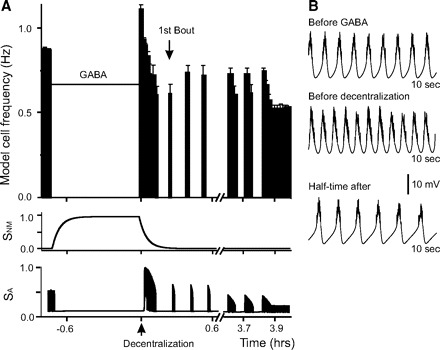
Recovery process in the model neuron treated with GABA before decentralization. A: bursting frequency in the model neuron “pretreated” with GABA for 45 min before decentralization (horizontal bar). Decentralization (up arrow) corresponds to time t = 0. A significant delay in the deceleration of rhythmic activity occurs after decentralization. The 1st bout is marked by down arrow. The bottom 2 traces show the changes of sensors SNM and SA as a function of time. SA tracks the activity changes instantaneously, whereas SNM slowly tracks the changes in GABA concentration. B: membrane potential traces of the model neuron activity before GABA incubation, before decentralization but after GABA was removed, and at half-time (t1/2) after decentralization.
Effects of pretreatment with proctolin are consistent with the activation of SNM and SA
Our decentralization modeling experiments suggest that the application of a neuromodulator, e.g., proctolin, that can enhance activity due to its activation of an inward current (Buchholtz et al. 1992; Golowasch and Marder 1992; Swensen and Marder 2000) and has metabotropic effects (Swensen and Marder 2000), would simultaneously activate SNM and SA. In contrast with the effects of GABA application, the enhanced sensor SA levels would lead to the reduction of ḠCa levels, but the enhanced sensor SNM levels would have a similar effects as GABA with an the opposite effect on ḠCa levels. As a consequence, the delay of initiation of activity recovery (tbout) would be reduced but not the rate of activity deceleration (t1/2) after decentralization.
Thus we tested the effects of proctolin on bursting activity recovery first in our model and then in the biological preparation. In model experiments exogenous proctolin application at levels up to two times larger than the endogenous level (1 unit, as explained in methods) was maintained for ∼45 min before decentralization. After this time, [Proc] was briefly reduced to control levels before it was set to zero to simulate decentralization. Bursting activity terminated almost instantaneously (Table 2). However, it took ∼25% less time (0.23 h) for the first bout to be produced than in control (Fig. 8A, Table 2).
FIG. 8.
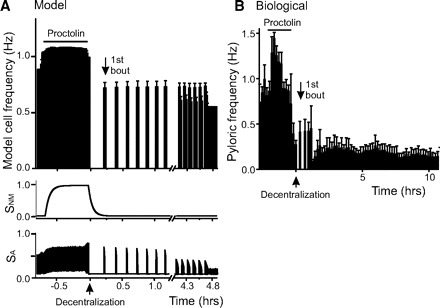
Recovery of pyloric activity after decentralization in the proctolin treated preparations. A: model neuron. Before decentralization, the proctolin concentration was increased for ∼45 min to 3 times the endogenous level present before decentralization. After this, the concentration was reduced back to the standard endogenous level briefly before it was set to 0 to simulate decentralization (↑). The bottom 2 traces show the changes of sensors SNM and SA as a function of time. SA tracks the activity changes instantaneously, whereas SNM slowly tracks the proctolin concentration changes. Decentralization takes place at t = 0. The 1st bout is indicated by ↓. B: biological preparation. Pyloric frequency vs. time of this representative preparation in which proctolin was bath applied at 1 μM for ∼2 h then washed until the pyloric frequency returned to control levels. The preparation was then decentralized at t = 0 (↑) and activity recorded for the next 10 h, during which the 1st bout occurred (↓).
A similar protocol was applied to biological pyloric network preparations: they were incubated in 1 μM proctolin for 1–18 h before decentralization (no difference in effects was observed over this wide range). In all proctolin-pretreated biological preparations, rhythmic activity was slightly (but not statistically significantly) increased on proctolin bath application (from 0.99 ± 0.42 to 1.21 ± 0.42 Hz; P = 0.213, n = 7, Student's t-test, Fig. 8B). On subsequent decentralization, the pyloric rhythm rapidly ceased (t1/2 = 0.16 ± 0.14 h), a delay that was not significant different from control preparations (P = 0.79, n = 7, Student's t-test; Fig. 8B, Table 2). However, the initiation delay of activity recovery was significantly shortened to tbout = 2.34 ± 1.88 h compared with control (P = 0.048, n = 7, Student's t-test; Fig. 8B, Table 2).
We conclude from these modeling and experimental results that although both neuromodulators proctolin and GABA could have identical metabotropic signaling effects, the interactions with their distinct ionotropic effects, result in significant phenotypical differences in their effects on the initial phase of rhythmic activity recovery from decentralization.
Separation of activity-dependent from neuromodulator-dependent pathways
The low-Na+ and muscimol treatments we performed were designed to reduce activity without interfering with the putative neuromodulator-dependent pathways. Because the axons of the projection neurons that release neuromodulators in the STG enter the ganglion, these treatments could reduce the level of release even if the applications were restricted strictly to only the STG. In fact, it has recently been shown that some of these terminals hyperpolarize in response to GABA (Stein et al. 2007). To exclude the confounding possibility that the reduced activity of the neuromodulator-releasing terminals (and thus reduced neuromodulator release) may be responsible for what we call activity-dependent effects, we applied the neuromodulator proctolin (5 × 10−7 M) onto the STG only while simultaneously reducing the pyloric network activity by hyperpolarizing both PD neurons (with −3 to −6nA current injection). After 5 h of simultaneous hyperpolarization and proctolin application, current injection was discontinued (but the cells left impaled with the electrodes for ≥1 additional hour) and proctolin was washed for ∼10 min before the preparations were decentralized. We obtained stable impalements in five of nine preparations. We measured t1/2 and tbout under the hypothesis that the first bout would occur earlier compared with hyperpolarization treatment alone due to the added positive effect of the neuromodulator on the initiation of recovery. According to the predictions of the model, we hypothesized also that an increased delay in the rhythm deceleration after decentralization might be observed. We found that, in fact, the time to first bout occurs significantly faster in proctolin + hyperpolarization treated preparations than in hyperpolarization-only treated preparations (0.22 ± 0.17, n = 5, compared with 1.60 ± 1.72, n = 12; P = 0.049, Student's t-test, Table 2). On the other hand, the time it takes for the preparations to decelerate and turn off after decentralization was not significantly affected relative to hyperpolarization-only treated preparations (Table 2). This lack of effect on t1/2 may be due to the short-duration of the applications, which was obligatory due to the difficulty in maintaining neurons stably impaled for >6–7 h.
DISCUSSION
Many different types of neurons possess activity-dependent homeostatic mechanisms that stabilize or recover their lost activity patterns after a perturbation or disruption (Davis 2006; Davis and Bezprozvanny 2001; Frick and Johnston 2005; Turrigiano 1999; Turrigiano and Nelson 2004; Zhang and Linden 2003), including neurons from the crustacean pyloric network (Golowasch et al. 1999a; Haedo and Golowasch 2006; Turrigiano et al. 1994). Previous experimental and modeling work suggested that the recovery of rhythmic activity of pyloric network neurons after the complete removal of neuromodulatory input by decentralization could be accomplished by the readjustment of intrinsic properties solely mediated by activity-dependent mechanisms (Golowasch et al. 1999b; LeMasson et al. 1993; Liu et al. 1998; Luther et al. 2003; Zhang and Golowasch 2007). Recently, however, Khorkova and Golowasch (2007) showed the existence of slow regulatory effects on conductances by neuromodulators, in particular proctolin. The present study was designed to examine the hypothesis that in the crab pyloric network the process of pyloric activity recovery after decentralization is determined not only by an activity-dependent mechanism but also by a parallel neuromodulator-dependent pathway. For this, we experimentally altered the network activity before decentralization in various ways, which included both specific (ionotropic) activity-reducing methods as well as by (metabotropic) neuromodulator bath applications, and then examined the initial phase of this recovery experimentally and in a model of the pyloric network pacemaker. We measured two hallmark features of this initial phase of recovery: the delay to the beginning of the generation of bouting activity and the rate of deceleration of rhythmic activity after decentralization. The effects of all predecentralization treatments (i.e., hyperpolarization, low-Na+ saline, GABAA and GABAB receptor agonists, and proctolin application) applied to both the biological pyloric network preparations and our simplified model match closely. Therefore we conclude that distinct activity- and neuromodulator-dependent signaling mechanisms may regulate ionic conductances such as GCa in the pacemaker of the pyloric network.
In our computational model, these two signaling mechanisms were represented by two sensors: SA, the activity-sensitive mechanism already described in previous theoretical (Golowasch et al. 1999b; LeMasson et al. 1993; Liu et al. 1998; Zhang and Golowasch 2007) and experimental work (Golowasch et al. 1999a; Haedo and Golowasch 2006; Turrigiano et al. 1994), and SNM, a neuromodulator-dependent mechanism. The activity-dependent mechanism homeostatically regulates neuronal activity by upregulating activity when it is low and by downregulating it when it is high. This, we have suggested before, takes place via the control of an intracellular signaling pathway activated by cytosolic calcium, which regulates Ca2+ and K+ conductances (Zhang and Golowasch 2007). Our present modeling work (cf. Zhang and Golowasch 2007) shows that the regulation of just one Ca2+ conductance is sufficient to explain the compensatory changes that lead to the recovery of rhythmic activity after decentralization. In fact, we have evidence (not shown in this study) that either the neuromodulator activated current Iproc or IK could each be the sole targets of the activity-dependent regulatory pathway if parameters of this pathway in our model are slightly adjusted and lead to bouting and stable recovery of rhythmic activity. However, as has been shown in other systems (Desai et al. 1999b; Linsdell and Moody 1994; MacLean et al. 2003), more than a single conductance is likely to be simultaneously regulated in biological systems, perhaps leading to a richer and more complex range of activity and activity regulation patterns. Together with this regulation of an ionic conductance, the simultaneous regulation of an intracellular Ca2+ pump by the same SA sensor (see diagram in Fig. 1) accounts for all observed characteristics of this recovery process, including the period and dynamics of bouting activity as well as the delayed recovery of stable but slower pyloric activity. The second long-term mechanism of neuronal activity regulation revealed by our experimental data appears to depend on slow-acting neuromodulatory input. We propose that this mechanism, represented in this model by the SNM sensor, only regulates (positively) the voltage-gated Ca2+ conductance, and, as a consequence, it is not homeostatic in nature.
Neuromodulators in the stomatogastric nervous system have been thought to predominantly have acute effects on network activity. However, some the effects of these neuromodulators and of the activation of neuromodulator-containing projection neurons to the STG can be very long-lasting (Beenhakker and Nusbaum 2004; Beenhakker et al. 2004; Dickinson 2006). Slowly developing neuromodulatory effects in the pyloric network were recently reported in which the slow effects of proctolin were shown to be radically different from those resulting from acute proctolin application (Khorkova and Golowasch 2007). It is possible that acute neuromodulatory effects in this system operate either via different signaling mechanisms [probably a calmodulin-dependent mechanism (Swensen and Marder 2000)] than the slow-acting effects or that the sensitivities to neuromodulator or signaling molecules are very different in both cases. Here we show new slowly developing neuromodulatory effects by GABA (via a GABAB receptor) and proctolin that are unrelated to their acute effects. GABA and proctolin require several hours of incubation to reveal their effects on pyloric activity deceleration or the appearance of the first bout of pyloric activity after decentralization. Whether the signaling pathways that mediate the effects we have here described on the initial phases of the activity recovery after decentralization and those responsible for the long-term effects on ionic currents described by Khorkova and Golowasch (2007) are the same remains to be examined. Nevertheless, our model suggests that they need not be different. Having identical metabotropic effects (as in our model), the different overall effects of GABA and proctolin on the initial phases of the recovery of rhythmic activity after decentralization can be accounted for on the basis of their distinct ionotropic effects: GABA having a strong inhibitory effect on pyloric activity (GABAB agonists having a weak inhibitory effect), and proctolin having a weak excitatory effect on pyloric activity. Under these conditions, the net overall effect of a predecentralization GABA application is a strong enhancement of ḠCa. In contrast, a predecentralization proctolin bath application produces a weaker enhancement of ḠCa.
According to this model, the voltage-dependent and neuromodulator-activated inward current Iproc acts as a pacemaker current, and its removal by decentralization hyperpolarizes the pacemaker cell and consequently deactivates ICa. This leads to the drop in [Ca]cyt and to a rapid decrease of the activity sensor SA. As a consequence, ḠCa increases with time constant τg until ICa is high enough to sustain temporary bursting activity, i.e., bouting activity, and later a new stable bursting pattern. When the neuronal activity is suppressed with hyperpolarizing current, low-Na+ solution or muscimol before decentralization, SA is decreased, which in turn increases FG(SA). Even though FG(SA) begins its return to its control level when the treatment is removed, ḠCa still remains elevated compared with its control level. If decentralization occurs at this time, or shortly after the inhibitory treatment is removed, ḠCa will already be at an elevated level and closer to the level that will sustain bouting activity, reducing the time to generate the first bout.
A similar effect on the time to begin bouting can be obtained with proctolin preincubation. During proctolin pretreatment the neuron becomes depolarized due to the activation of Gproc(V). This leads to the increase of the neuromodulator-sensitive sensor SNM directly and to the increase of the activity sensor SA indirectly due to enhanced Ca2+ influx. The ultimate effect on activity is the result of the opposing effects of these sensors on ḠCa: SA downregulates [via FG(SA), Eq. 2] and SNM upregulates [via FN(SNM), Eq. 8] ḠCa. In contrast, GABA application leads to a hyperpolarization of the neuron via activation of GGABA. This leads to the indirect decrease of SA due to a reduction in Ca2+ influx and an increase of SNM by the activated GABAB receptors and to an upregulation of ḠCa by both mechanisms [via FA(SA) and FN(SNM)]. Due to the dependence of ḠCa on both SA and SNM (Fig. 1), these two cases of neuromodulator action will have different effects on the activity of the neuron. In one case (GABA effect), ḠCa will be sufficiently enhanced to be at a supra-threshold level for the generation of rhythmic activity at the time of decentralization, whereas in the other (proctolin effect), ḠCa will stay below this threshold. As a consequence, GABA (or baclofen) pretreatment leads to a slow deceleration (after decentralization) of a rhythm activated beyond the threshold for stable activity to be produced. The slow deceleration of activity in this case is due to slowly decaying Ca2+ conductance to below the threshold level. Proctolin, however, cannot upregulate ḠCa enough to surpass this threshold and therefore has no effect (compared with control) on the deceleration rate of the rhythm when decentralization occurs. However, both GABA and proctolin will reduce the time to the generation of the first bout.
The operation of the neuromodulator-dependent mechanism (represented by the SNM sensor) was assumed here to only affect one ionic conductance, ḠCa, which was sufficient to explain the observed effects of neuromodulators on the rate of deceleration of activity (t1/2) after decentralization. A direct effect of neuromodulators proctolin and GABA on Ca2+ conductances such as those proposed here remains to be experimentally demonstrated. It is of course possible that the neuromodulator effects that we have described involve a number of additional conductances. In fact, our model can be modified to include the regulation of several conductances by activity and by neuromodulators adding a richer repertoire of activity and its regulation. Indeed the fact that bouting activity can sometimes be observed among a background of nonzero pyloric activity may be due to different signaling pathways, sensitive to different features of activity, or activated by distinct signaling mechanisms that could be responsible on the one hand for regulating the background pyloric activity and on the other underlie bouting activity.
A number of different conductances are often regulated by the same neuromodulator (Khorkova and Golowasch 2007), and there is growing evidence that many conductances may be coordinately regulated within and between network neurons (Khorkova and Golowasch 2007; MacLean et al. 2003; Schulz et al. 2007), suggesting possible interactions between the ion channels directly or regulatory interactions at the transcription or at some posttranscriptional level (Kaczmarek 2006; Schulz et al. 2007). The possibility that activity of the target network regulates the release of neuromodulators, thus making the neuromodulator-dependent regulation indirectly activity-dependent, also needs to be considered and experimentally tested. Wood et al. (2004) have shown the existence of a feedback circuit from the pyloric network to projection neurons. However, in this case, the pyloric network does not appear to be influenced back; instead a different rhythm-generating network in the STG, the gastric mill network, is affected.
The results of our experiments, in which proctolin was applied simultaneously with the reduction of pyloric network activity (via PD neuron hyperpolarization), indicate that these effects can operate independently. However, incontrovertible proof is not easy to obtain in this case because a reliable method to separate activity-dependent from neuromodulator-dependent effects over the very long time that is required for the process of pyloric activity recovery to occur does not exist. Furthermore, we cannot completely exclude the possibility of such interactions because it has been shown before that the growth factor BDNF, for example, is released by neurons in an activity-dependent fashion and then acts on the releasing neuron as well as other adjacent neurons to homeostatically regulate their activity (Desai et al. 1999a). However, in the crustacean stomatogastric system, no growth factors are presently known, and only one peptidergic neuromodulator has been shown to be produced by neurons within the STG. However, this modulator is not produced by neurons of the pyloric network itself (Skiebe et al. 2002), and it is thus unlikely to act as “activity messenger” in the system.
Testing the validity of our model will be complicated by several possible factors that we have, for the sake of simplicity, disregarded in our model. Thus multiple conductances rather than a single one may be regulated, multiple sensors of activity and receptor activation may exist, and interactions between activity- and neuromodulator-dependent mechanisms may be present. Additionally, we have modeled the process of activity recovery of the entire pyloric network assuming that they are generated by the changes triggered in a single pacemaker neuron. It is certainly possible that all neurons and synapses in the pyloric network are potential targets for similar forms of regulation. It is therefore now crucial to experimentally determine the molecular details of the signaling pathways activated by the different neuromodulators in this system, at the very least those activated via GABAB and proctolin receptors. This should allow the testing of our proposed model by separately modifying intracellular Ca2+ and Ca2+ sensor levels, and the levels of the signaling molecules activated by these neuromodulators.
In summary, our results demonstrate that the process of recovery after decentralization is likely governed by complex regulatory mechanisms, integrating both activity- and neuromodulator-dependent inputs. Multiple and redundant regulatory mechanisms are very common in biological systems and could be an evolutionary conserved way to ensure the uninterrupted function of vitally important processes in the face of constantly changing environmental conditions.
GRANTS
This work was supported by National Institute of Mental Health Grant 64711 to J. Golowasch.
Acknowledgments
We thank Drs. Farzan Nadim and Amithaba Bose for comments and suggestions.
Present address of R. Rodriguez: Dept. of Biology, Brooklyn College, Ingersoll Hall, 2900 Bedford Avenue, Brooklyn, New York 11210.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Beenhakker et al. 2004.Beenhakker MP, Blitz DM, Nusbaum MP. Long-lasting activation of rhythmic neuronal activity by a novel mechanosensory system in the crustacean stomatogastric nervous system. J Neurophysiol 91: 78–91, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker and Nusbaum 2004.Beenhakker MP, Nusbaum MP. Mechanosensory activation of a motor circuit by coactivation of two projection neurons. J Neurosci 24: 6741–6750, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholtz et al. 1992.Buchholtz F, Golowasch J, Epstein IR, Marder E. Mathematical model of an identified stomatogastric ganglion neuron. J Neurophysiol 67: 332–340, 1992 [DOI] [PubMed] [Google Scholar]
- Davis 2006.Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci 29: 307–323, 2006 [DOI] [PubMed] [Google Scholar]
- Davis and Bezprozvanny 2001.Davis GW, Bezprozvanny I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu Rev Physiol 63: 847–869, 2001 [DOI] [PubMed] [Google Scholar]
- Desai et al. 1999a.Desai NS, Rutherford LC, Turrigiano GG. BDNF regulates the intrinsic excitability of cortical neurons. Learn Mem 6: 284–291, 1999a [PMC free article] [PubMed] [Google Scholar]
- Desai et al. 1999b.Desai NS, Rutherford LC, Turrigiano GG. Plasticity in the intrinsic excitability of cortical pyramidal neurons. Nat Neurosci 2: 515–520, 1999b [DOI] [PubMed] [Google Scholar]
- Dickinson 2006.Dickinson PS. Neuromodulation of central pattern generators in invertebrates and vertebrates. Curr Opin Neurobiol 16: 604–614, 2006 [DOI] [PubMed] [Google Scholar]
- Duan and Cooke 2000.Duan S, Cooke IM. Glutamate and GABA activate different receptors and Cl(−) conductances in crab peptide-secretory neurons. J Neurophysiol 83: 31–37, 2000 [DOI] [PubMed] [Google Scholar]
- Ermentrout 2002.Ermentrout B. Simulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students. Soc for Industrial and Applied Math, Philadelphia, PA, 2002
- Frick and Johnston 2005.Frick A, Johnston D. Plasticity of dendritic excitability. J Neurobiol 64: 100–115, 2005 [DOI] [PubMed] [Google Scholar]
- Golowasch et al. 1999a.Golowasch J, Abbott LF, Marder E. Activity-dependent regulation of potassium currents in an identified neuron of the stomatogastric ganglion of the crab Cancer borealis. J Neurosci 19: RC33, 1999a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golowasch et al. 1999b.Golowasch J, Casey M, Abbott LF, Marder E. Network stability from activity-dependent regulation of neuronal conductances. Neural Comput 11: 1079–1096, 1999b [DOI] [PubMed] [Google Scholar]
- Golowasch and Marder 1992.Golowasch J, Marder E. Proctolin activates an inward current whose voltage dependence is modified by extracellular Ca2+ J Neurosci 12: 810–817, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haedo and Golowasch 2006.Haedo RJ, Golowasch J. Ionic mechanism underlying recovery of rhythmic activity in adult isolated neurons. J Neurophysiol 96: 1860–1876, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick 1992.Harris-Warrick RM. Dynamic Biological Networks: The Stomatogastric Nervous System. Cambridge, MA: MIT Press, 1992
- Kaczmarek 2006.Kaczmarek LK. Non-conducting functions of voltage-gated ion channels. Nat Rev Neurosci 7: 761–771, 2006 [DOI] [PubMed] [Google Scholar]
- Khorkova and Golowasch 2007.Khorkova O, Golowasch J. Neuromodulators, not activity, control coordinated expression of ionic currents. J Neurosci 27: 8709–8718, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMasson et al. 1993.LeMasson G, Marder E, Abbott LF. Activity-dependent regulation of conductances in model neurons. Science 259: 1915–1917, 1993 [DOI] [PubMed] [Google Scholar]
- Linsdell and Moody 1994.Linsdell P, Moody WJ. Na+ channel mis-expression accelerates K+ channel development in embryonic Xenopus laevis skeletal muscle. J Physiol 480: 405–410, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. 1998.Liu Z, Golowasch J, Marder E, Abbott LF. A model neuron with activity-dependent conductances regulated by multiple calcium sensors. J Neurosci 18, 2309–2320, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther et al. 2003.Luther JA, Robie AA, Yarotsky J, Reina C, Marder E, Golowasch J. Episodic bouts of activity accompany recovery of rhythmic output by a neuromodulator- and activity-deprived adult neural network. J Neurophysiol 90: 2720–2730, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean et al. 2003.MacLean JN, Zhang Y, Johnson BR, Harris-Warrick RM. Activity-independent homeostasis in rhythmically active neurons. Neuron 37: 109–120, 2003 [DOI] [PubMed] [Google Scholar]
- Marder and Bucher 2001.Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Curr Biol 11: R986–996, 2001 [DOI] [PubMed] [Google Scholar]
- Marder and Bucher 2007.Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol 69: 291–316, 2007 [DOI] [PubMed] [Google Scholar]
- Nusbaum and Beenhakker 2002.Nusbaum MP, Beenhakker MP. A small-systems approach to motor pattern generation. Nature 417: 343–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas et al. 1999.Parnas I, Rashkovan G, Ong J, Kerr DI. Tonic activation of presynaptic GABAB receptors in the opener neuromuscular junction of crayfish. J Neurophysiol 81: 1184–1191, 1999 [DOI] [PubMed] [Google Scholar]
- Rabbah et al. 2005.Rabbah P, Golowasch J, Nadim F. Effect of electrical coupling on ionic current and synaptic potential measurements. J Neurophysiol 94: 519–530, 2005 [DOI] [PubMed] [Google Scholar]
- Schulz et al. 2007.Schulz DJ, Goaillard JM, Marder EE. Quantitative expression profiling of identified neurons reveals cell-specific constraints on highly variable levels of gene expression. Proc Natl Acad Sci USA 104: 13187–13191, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selverston et al. 1976.Selverston AI, Russell DF, Miller JP. The stomatogastric nervous system: structure and function of a small neural network. Prog Neurobiol 7: 215–290, 1976 [DOI] [PubMed] [Google Scholar]
- Skiebe et al. 2002.Skiebe P, Dreger M, Meseke M, Evers JF, Hucho F. Identification of orcokinins in single neurons in the stomatogastric nervous system of the crayfish, Cherax destructor J Comp Neurol 444: 245–259, 2002 [DOI] [PubMed] [Google Scholar]
- Soto-Treviño et al. 2005.Soto-Treviño C, Rabbah P, Marder E, Nadim F. Computational model of electrically coupled, intrinsically distinct pacemaker neurons. J Neurophysiol 94: 590–604, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein et al. 2007.Stein W, DeLong ND, Wood DE, Nusbaum MP. Divergent co-transmitter actions underlie motor pattern activation by a modulatory projection neuron. Eur J Neurosci 26: 1148–1165, 2007 [DOI] [PubMed] [Google Scholar]
- Swensen et al. 2000.Swensen AM, Golowasch J, Christie AE, Coleman MJ, Nusbaum MP, Marder E. GABA and responses to GABA in the stomatogastric ganglion of the crab Cancer borealis J Exp Biol 203: 2075–2092, 2000 [DOI] [PubMed] [Google Scholar]
- Swensen and Marder 2000.Swensen AM, Marder E. Multiple peptides converge to activate the same voltage-dependent current in a central pattern-generating circuit. J Neurosci 20: 6752–6759, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoby-Brisson and Simmers 1998.Thoby-Brisson M, Simmers J. Neuromodulatory inputs maintain expression of a lobster motor pattern-generating network in a modulation-dependent state: evidence from long-term decentralization in vitro. J Neurosci 18: 2212–2225, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano 1999.Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci 22: 221–227, 1999 [DOI] [PubMed] [Google Scholar]
- Turrigiano et al. 1994.Turrigiano G, Abbott LF, Marder E. Activity-dependent changes in the intrinsic properties of cultured neurons. Science 264: 974–977, 1994 [DOI] [PubMed] [Google Scholar]
- Turrigiano and Nelson 2004.Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci 5: 97–107, 2004 [DOI] [PubMed] [Google Scholar]
- Wood et al. 2004.Wood DE, Manor Y, Nadim F, Nusbaum MP. Intercircuit control via rhythmic regulation of projection neuron activity. J Neurosci 24: 7455–7463, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and Linden 2003.Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. Nat Rev Neurosci 4: 885–900, 2003 [DOI] [PubMed] [Google Scholar]
- Zhang and Golowasch 2007.Zhang Y, Golowasch J. Modeling recovery of rhythmic activity: hypothesis for the role of a calcium pump. Neurocomputing 70: 1657–1662, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]


