Abstract
The extant developmental literature investigating age-related differences in the execution of aiming movements has predominantly focused on visuomotor coordination, despite the fact that additional sensory modalities, such as audition and somatosensation, may contribute to motor planning, execution, and learning. The current study investigated the execution of aiming movements toward both visual and acoustic stimuli. In addition, we examined the interaction between visuomotor and auditory-motor coordination as 5- to 10-yr-old participants executed aiming movements to visual and acoustic stimuli before and after exposure to a visuomotor rotation. Children in all age groups demonstrated significant improvement in performance under the visuomotor perturbation, as indicated by decreased initial directional and root mean squared errors. Moreover, children in all age groups demonstrated significant visual aftereffects during the postexposure phase, suggesting a successful update of their spatial-to-motor transformations. Interestingly, these updated spatial-to-motor transformations also influenced auditory-motor performance, as indicated by distorted movement trajectories during the auditory postexposure phase. The distorted trajectories were present during auditory postexposure even though the auditory-motor relationship was not manipulated. Results suggest that by the age of 5 yr, children have developed a multisensory spatial-to-motor transformation for the execution of aiming movements toward both visual and acoustic targets.
INTRODUCTION
Research investigating the planning and control of discrete aiming movements in children has consistently reported age-related differences in movement speed, movement straightness and smoothness, and spatial and temporal variability (Bo et al. 2006; Contreras-Vidal et al. 2005; Hay 1979; Jansen-Osmann et al. 2002; Yan et al. 2000, 2003). These findings have come primarily from visuomotor coordination tasks. However, information relevant for target-directed movements can be provided not only by the visual system, but also by the auditory and somatosensory systems. To better characterize sensory-motor development of limb control, we examined auditory-motor coordination in 5- to 10-yr-old children. Further, we investigated the interaction between visuomotor and auditory-motor coordination by extending the visuomotor adaptation paradigm during center-out hand movements. Specifically, children executed aiming movements toward acoustic targets before and after exposure to a visuomotor perturbation. The interaction between visuomotor adaptation and auditory-motor performance may provide insights into development of the intersensory and sensory-motor properties of aiming movements in children.
Evidence from both behavioral experiments as well as computational modeling has led to the development of a detailed conceptual framework for reaching/aiming movements. This approach conceptualizes the planning and execution of such movements as a series of complex sensory-motor transformations (Bullock and Grossberg 1988; Shadmehr and Wise 2005; Wolpert and Kawato 1998). For example, current theoretical and experimental findings suggest that the task of moving toward a target is solved by computing a spatial difference vector, subtracting the current position of the hand (estimated from sensory modalities such as vision and/or proprioception) from the spatial estimate of the target's location (Bullock and Grossberg 1988; Bullock et al. 1993; Shadmehr and Wise 2005). Subsequently, this spatial difference vector is transformed into motor coordinates, a computation referred to as a “spatial-to-motor transformation” that maps changes in end-effector position to changes in limb configuration (Bullock et al. 1993). These changes in joint angles serve as the basis for the descending commands that move the hand to the target. The accuracy of such transformations is thought to be based on an adaptive internal representation acquired over time, as the relationships between the external environment and the intrinsic characteristics of the arm are learned (Buch et al. 2003; Kagerer et al. 1997; Shadmehr and Mussa-Ivaldi 1994; Wolpert and Kawato 1998).
To investigate the acquisition of such spatial-to-motor transformations, researchers manipulate the sensory environment in which participants move. In the context of the present study, this is accomplished by exposing participants to a visuomotor distortion, in which a conflict is created between the visual feedback of hand position and actual hand position (i.e., the screen cursor–hand relationship). With practice, participants are able to adapt to the perturbation by updating a spatial-to-motor transformation that is appropriate for the novel visuomotor environment (Kagerer et al. 1997; Krakauer et al. 2000). Although these visuomotor paradigms have been extended to investigate age-related differences in school-aged children (Bo et al. 2006; Contreras-Vidal et al. 2005; Ferrel-Chapus et al. 2002), existing developmental research has yet to explore the multisensory properties of these spatial-to-motor transformations. Specifically, it is unknown whether children use shared spatial-to-motor transformations for movements toward targets perceived by different sensory modalities (i.e., both vision and audition).
Existing research in adult humans and monkeys suggests that estimates of target location are mapped to a reference coordinate frame, independent of the modality of the target (Cohen and Andersen 2000; Pouget et al. 2002). If target estimates are mapped to a reference coordinate frame, it should follow that the transformation from spatial to motor coordinates will be consistent across target modalities. Indeed, in a recent study in our laboratory, adaptation to a visuomotor distortion influenced auditory-motor performance in adult participants, suggesting that spatial-to-motor transformations in adults are multisensory (Kagerer and Contreras-Vidal 2008). Although audiovisual interactions are evident very early in development in a variety of contexts (Bahrick and Lickliter 2000; Neil et al. 2006; Rosenblum et al. 1997), it is not clear that visuomotor adaptation will have similar effects on auditory-motor performance in 5- to 10-yr-old children. In a visuomotor adaptation task performed by 4-, 6-, and 8-yr-old children, only the 8-yr-olds successfully updated their spatial-to-motor transformations to be appropriate for the novel environment (Contreras-Vidal et al. 2005). Moreover, recent research in postural control demonstrated that intermodality reweighting was evident in 10-yr-olds but not in 4-yr-olds (Bair et al. 2007), providing further evidence for a change in sensory-motor development around 8 yr of age.
The current study used a crossmodal adaptation paradigm during which 5- to 10-yr-old children executed aiming movements toward acoustic stimuli before and after exposure to a visuomotor perturbation. If spatial-to-motor transformations are multisensory, then it would be predicted that adaptation to the visuomotor distortion will influence auditory-motor performance, as demonstrated by a systematic distortion of the movement trajectories during the auditory postexposure phase (i.e., intersensory effects). Also, if there is a change in multisensory-motor development around 8 yr of age, then such intersensory effects would be present only in the older children.
METHODS
Participants
Forty-one typically developing, right-handed children, divided by age into three groups, 5–6, 7–8, and 9–10 yr, participated in this study (Table 1). All participants were screened with the Movement Assessment Battery for Children (MABC; Henderson and Sugden 1992) and scored at or above the 20th percentile. Handedness was defined based on everyday activities, such as eating and handwriting, and was confirmed by the MABC handedness criteria. Averaged MABC scores, as well as additional participant information such as gender and mean age, are shown for the three groups of children in Table 1. A parent or legal guardian of each child gave informed consent prior to participation in the study. All procedures were approved by the Institutional Review Board at the University of Maryland, College Park. Children received a toy prize as well as a small compensation for their participation.
TABLE 1.
Participant information
| Age Group, yr | Mean Age, yr | Gender | Mean MABC* %ile |
|---|---|---|---|
| 5–6 | 6.1 ± 0.6 | 8 M; 6 F | 69.6 ± 19.9 |
| 7–8 | 7.9 ± 0.5 | 8 M; 5 F | 59.1 ± 23.6 |
| 9–10 | 10.0 ± 0.6 | 10 M; 4 F | 56.6 ± 27.5 |
Values are means ± SD
Apparatus
Participants were seated comfortably in an adjustable chair in front of a computer monitor positioned horizontally on an elevated board that occluded vision of the participant's hand during the task performance (Fig. 1). The participants were asked to use a digital pen to move to targets on a tablet located below the board and the monitor. The monitor provided real-time visual feedback of target position and movement path as data were collected using a digitizing tablet (12 × 12 in.; Wacom Intuos) that recorded pen position at a sampling rate of 99 Hz. OASIS software (Kikosoft, Nijmegen, The Netherlands) was used for the stimulus presentation and data acquisition. During the experimental session, the children's posture was monitored continuously to ensure they were sitting upright and their head was centered with respect to the display monitor.
FIG. 1.
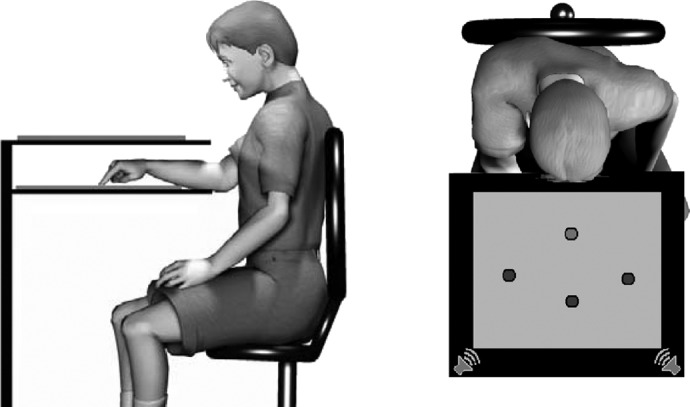
Lateral (left) and overhead (right) views of experimental setup. Start circle and visual targets are depicted by the light and dark circles, respectively, in the overhead view. Auditory target locations are depicted by the speakers.
Procedures
Participants were instructed to use the digitizing pen to move their dominant hand on the tablet from a centrally located home position to peripherally located targets. To control for varying levels of experience using a writing implement, the pen was taped to the back of each participant's index finger such that the pen tip was in contact with the tablet as long as his/her index fingertip lightly touched its surface. There were two experimental conditions.
1) The visual condition required participants to place the pen inside the home position (red circle; 1-cm diameter). Following a 500-ms hold period in the home circle, one of three peripheral targets (blue circle, 1-cm diameter) located 9 cm from the home position appeared. Targets were presented in a randomized order and were positioned in a Cartesian coordinate system at 24, 90, or 156° from the home circle. Participants were instructed to move as quickly and as accurately as possible to the presented target. Once the digitizing pen remained motionless for 1,500 ms, the target circle disappeared and participants were instructed to return to the home circle to begin the next trial.
2) For the auditory condition, participants were instructed to move on the tablet toward one of two acoustic targets while wearing opaque goggles that occluded vision of the target and the movement path. Similar to the visual condition, participants were instructed to move the digitizing pen as quickly and accurately as possible toward the appropriate acoustic target. The participant's hand was returned to the home position by the experimenter to begin the next trial. Targets were represented by an intermittent tone generated by piezo-electric buzzers (4.1 ± 0.5 kHz, 67.5 dB at 30 cm) positioned in the monitor plane. The acoustic targets were presented in a randomized order and were located at either 45 or 135° from the home position.
For both conditions, visual and auditory, trials in which participants removed the pen from the tablet or failed to initiate movement toward the target were recorded but not included in the data analysis. In total, 3.6 and 22.1% of the visual and auditory trials were omitted, respectively. The high number of trials rejected in the auditory condition was the result of the participant's failure to move toward the acoustic stimuli in an appropriate amount of time. Importantly, there were no differences among the three age groups in the percentage of excluded auditory trials. Approximately 15 practice trials per condition were given to each participant before data collection to adjust to the experimental setup and to make sure the children understood the task.
The experiment consisted of five phases: Participants started with a visual baseline phase (preexposure; 24 trials, 8 per target) in which accurate real-time visual feedback of the movement path was provided via the computer monitor. The children then completed an auditory baseline phase (preexposure; 24 trials, 12 per target) without vision. Following these two baselines, participants were exposed to a visuomotor feedback rotation where the pen trace of the movement path was rotated 60° clockwise (CW; visual exposure; 126 trials, 42 per target) with respect to the actual movement. To successfully reach the target, participants had to compensate and move in a direction 60° counterclockwise (CCW) of the desired target. After this adaptation phase, the auditory baseline (postexposure; 9 trials) was reintroduced to determine whether the visuomotor rotation affected auditory-motor performance. This was followed by a visual baseline phase (postexposure; 9 trials, 3 per target) to assess visuomotor aftereffects, the presence of which would indicate successful adaptation to the visual feedback rotation. The data collection session consisted of a total of 192 trials.
Data analysis
Time series of each trial was dual-pass filtered (eighth-order Butterworth, 10-Hz cutoff). Movement onset and offset were determined by an interactive algorithm used in previous research (Contreras-Vidal et al. 2005). Performance during the task was assessed using root mean squared error (RMSE), normalized jerk (NJ), initial directional error (IDE), the variability of initial directional error (IDE-V), and movement time (MT). RMSE (cm) was calculated as a spatial error between the participants' pen position and the ideal trajectory vector, expressed as
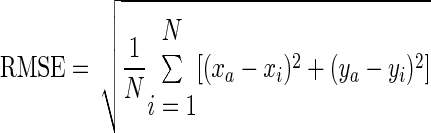 |
(1) |
where (xa, ya) and (xi, yi) are corresponding points of the actual and ideal trajectories, respectively (Contreras-Vidal et al. 2005). NJ (unit free), was considered indicative of the smoothness of the movement
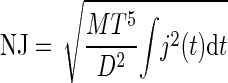 |
(2) |
where j(t) is the rate of change of acceleration, MT is the movement time, and D is the total distance of the movement. IDE was computed as the directional error (measured in degrees) between the participants' pen location and an “ideal” trajectory vector between the home position and the desired target at 80 ms following movement onset. IDE provided a window into movement planning because it was calculated prior to any error correction triggered by visual feedback. IDE-V was the variability (units of SD) around the individual's average IDE value and was computed to assess the directional variability during the baseline conditions.
An examination of the movement trajectories' during auditory baseline indicated that participants consistently executed their movements to directions that were oriented more horizontally than an ideal trajectory between the start and target positions (see Fig. 2). For example, the movement trajectories of the participants were oriented at approximately 25–30° (for the 45° target) and 150–155° (for the 135° target). The computation of IDE
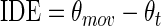 |
(3) |
where θmov is the angular direction of the actual movement and θt is the direction of the target, resulted in predominantly positive IDE values for movements to the 135° target and negative IDE values for movements to the 45° target. Therefore, the visual and auditory conditions were analyzed independently and all auditory analyses were separated by target position, with Bonferroni adjustments.
FIG. 2.
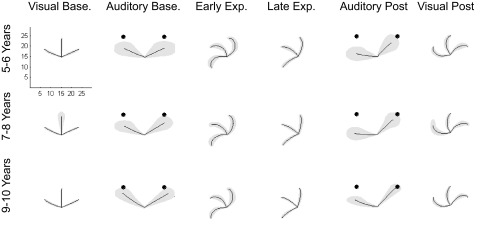
Movement trajectories. Black lines indicate averaged group trajectories and the gray-shaded regions depict 1SD. Black circles depict auditory target locations. Units are in centimeters.
Group differences during visual baseline were tested by separate one-way ANOVAs for the following dependent measures: IDE, NJ, RMSE, IDE-V, and MT. Group differences during auditory baseline were tested by one-way ANOVAs as well, but only for IDE and IDE-V. As stated earlier, separate ANOVAs were conducted for each target position (Bonferroni adjusted) for the auditory condition. Exposure performance was analyzed with two-way ANOVAs on IDE and RMSE, with age-group (herein referred to as GROUP) as a between-subjects factor and BLOCK (two levels: early/late exposure) as the within-subjects factor. Early and late exposure blocks consisted of the first and last 21 trials of exposure to the visuomotor rotation, respectively. To assess auditory aftereffects, GROUP × BLOCK (two levels: baseline/postexposure) ANOVAs were conducted on IDE for each target position (Bonferroni adjusted). Visual aftereffects were investigated with two separate GROUP × BLOCK ANOVAs on IDE and RMSE. Auditory postexposure means were based on the first four movements to each auditory target. The use of four trials per target provided a stable estimate of the magnitude of the aftereffects. Additionally, auditory aftereffects were not expected to decrease during postexposure because participants had not yet been provided the visual error signal necessary to trigger the deadaptation process (Scheidt et al. 2000). Individual means during visual postexposure were computed using the first six trials (two per target) of the postexposure phase. The inclusion of six trials provided a stable estimate and data analysis revealed no GROUP differences in deadaptation during postexposure.
RESULTS
Movement paths from five experimental phases are depicted in Fig. 2. Participants from all three age groups demonstrated accurate movements to each target during visual baseline. Movements during auditory baseline, especially for the 5- to 6-yr-old children, were directed more horizontal with respect to each target position (see Fig. 2, “auditory baseline”), creating a systematic bias toward the horizontal. During early exposure, movement trajectories were characterized by CCW “spiral” patterns, indicative of the feedback-dependent, corrective movements generated in response to the CW visuomotor rotation. These patterns disappeared over the course of the exposure phase as participants gradually directed their movements CCW of the desired visual target to compensate for the rotation. Trajectories during late exposure were nearly as straight as those during the visual baseline phase. Following the removal of the visuomotor rotation (i.e., postexposure), movements to the acoustic targets were rotated CCW relative to the auditory baseline phase, indicative of intersensory effects. During visual postexposure, participants demonstrated spiral patterns that were opposite to those evident during early exposure (i.e., aftereffects).
Baseline phase
VISUAL CONDITION.
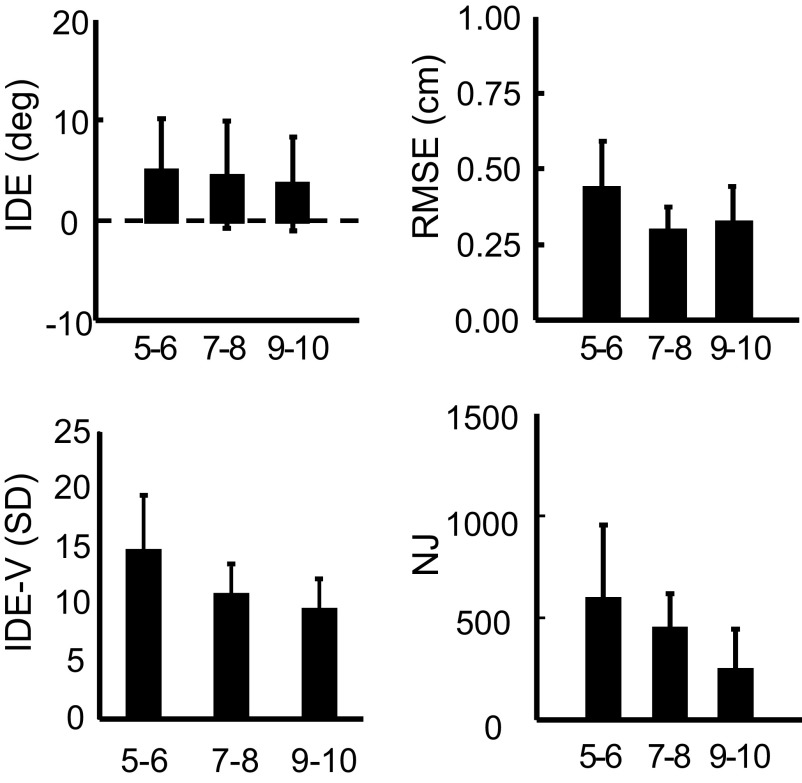
A significant main effect of GROUP was found for NJ [F(2,38) = 7.096, P = 0.002], RMSE [F(2,38) = 5.651, P = 0.007], and IDE-V [F(2,38) = 9.583, P < 0.001] during visual baseline (Fig. 3). Scheffé's post hoc tests revealed that the movements of the 9- to 10-yr-old children were significantly less jerky compared with those of the 5- to 6-yr-olds (P = 0.003). The 5- to 6-yr-old children also had significantly higher RMSE and IDE-V scores than those of the 7- to 8- (RMSE: P = 0.013; IDE-V: P = 0.013) and the 9- to 10-yr-old children (RMSE: P = 0.045; IDE-V: P = 0.001). A significant main effect of GROUP was found for MT [F(2,38) = 8.620, P = 0.001] because the 9- to 10-yr-old children moved significantly faster than the 5- to 6- (P = 0.002) and the 7- to 8-yr-old children (P = 0.008). No age-related differences were found for IDE, suggesting that on average the spatial-to-motor transformations were well tuned to the intended target location across all age groups.
FIG. 3.
Visual baseline performance. Initial directional error (IDE, top left), root mean squared error (RMSE, top right), normalized jerk (NJ, bottom right), and variability of initial directional error (IDE-V, bottom left) are shown for the 3 age groups during the visual baseline phase. Error bars represent 1SD.
AUDITORY CONDITION.
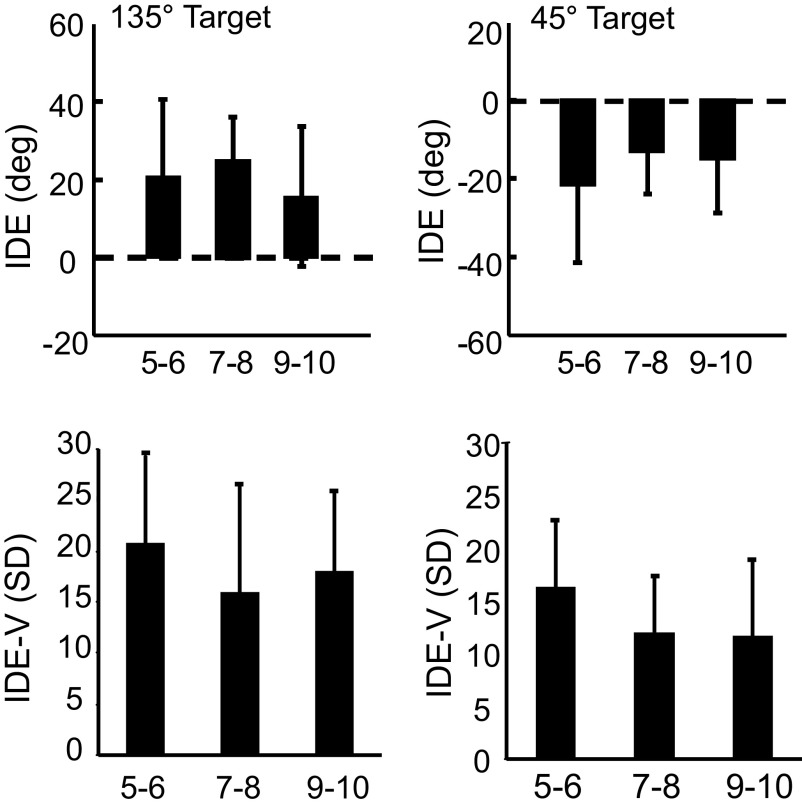
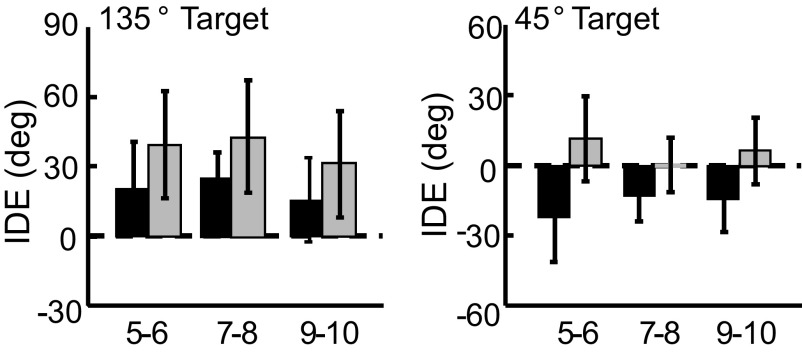
During the auditory baseline phase, the movement trajectories were tilted toward a horizontal axis that passed through the initial position, thus separate statistical analyses were conducted for the two acoustic targets. No significant differences in IDE or IDE-V between the three age groups during auditory baseline for either of the target positions were found (Fig. 4).
FIG. 4.
Auditory baseline. IDE (top panels) and IDE-V (bottom panels) are shown for the 135° target (left) and the 45° target (right) during auditory baseline (error bars = 1SD).
Exposure phase
RMSE and IDE during the exposure phase were best fit with single (Eq. 4) and double exponentials (Eq. 5), respectively
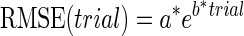 |
(4) |
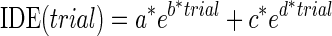 |
(5) |
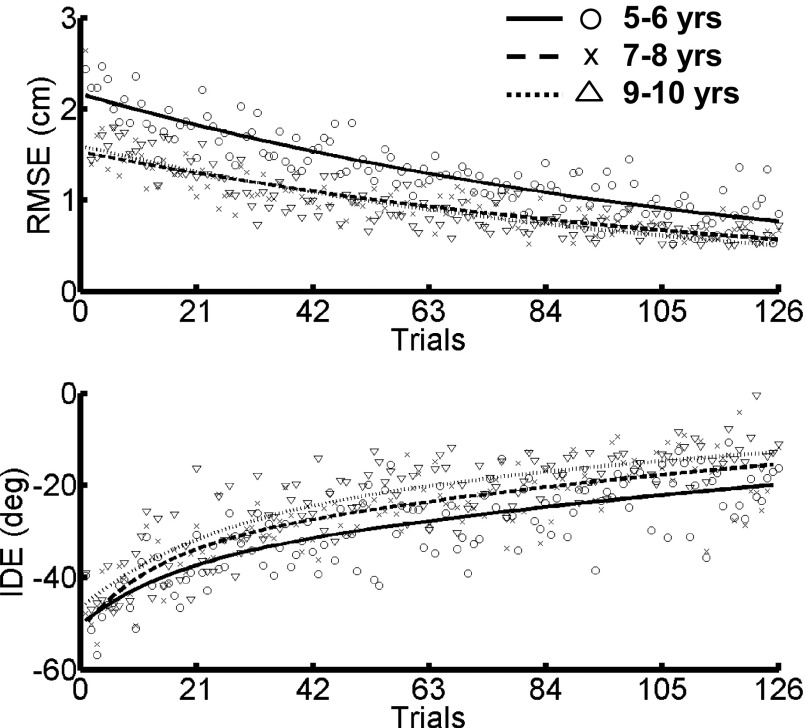
Figure 5 depicts group means and fitted exponential trajectories of RMSE and IDE during the exposure phase. Table 2 contains parameters for Eqs. 4 and 5 for the three age groups. RMSE was largest for the 5- to 6-yr-old children throughout the exposure phase, a result that can be at least partially attributed to the baseline differences illustrated in Fig. 3. Age-related differences in IDE appear to be evident because the magnitude of the directional errors decreased as a function of age. All three age groups were able to adapt to the visuomotor rotation, as indicated by the improvement in RMSE and IDE during the exposure phase.
FIG. 5.
Exposure phase. RMSE (top) and IDE (bottom) shown as a function of trials during exposure to the visuomotor rotation. Individual data points represent the group mean, whereas the lines depict double- and single-exponential fits for IDE and RMSE, respectively.
TABLE 2.
Parameters for exponential trajectories
| Age Group, yr | IDE |
RMSE | ||||
|---|---|---|---|---|---|---|
| a | b | c | d | a | b | |
| 5–6 | −12.2 (−22,7, −1.8) | −0.06 (−0.18, 0.05) | −38.1 (−49.5, −26.6) | −0.005 (−0.008, −0.002) | 2.2 (2.1, 2.3) | −0.008 (−0.009, −0.007) |
| 7–8 | −15.9 (−24.5, −7.4) | −0.08 (−0.17, 0.01) | −35.5 (−43.9, −27.2) | −0.007 (−0.009, −0.004) | 1.5 (1.4, 1.6) | −0.008 (−0.009, −0.007) |
| 9–10 | −17.7 (−36.3, 0.9) | −0.05 (−0.12, 0.02) | −29.1 (−49.9, −8.2) | −0.007 (−0.013, 0.000) | 1.6 (1.5, 1.7) | −0.009 (−0.01, −0.008) |
Values in parentheses are 95% confidence bounds.
Analysis of the first and last blocks of the exposure phase revealed a significant BLOCK main effect for both IDE [F(1,38) = 334.33, P < 0.001] and RMSE [F(1,38) = 259.77, P < 0.001], indicating that both dependent measures significantly improved from early to late exposure (Fig. 6). Additionally, significant GROUP main effects were found for IDE [F(2,38) = 5.110, P = 0.011] and RMSE [F(2,38) = 6.550, P = 0.004], indicating that there were age-related differences, collapsed across BLOCK, during the exposure phase. Scheffé's post hoc analysis revealed that the 5- to 6-yr-old children had significantly “larger” (i.e., more negative) IDE scores than those of the 9- to 10-yr-old children (P = 0.011) and significantly higher RMSE scores than the 7- to 8- (P = 0.008) and the 9- to 10-yr-old children (P = 0.021). Collectively, these data indicate that all three age groups significantly improved performance during the exposure phase; however, the 5- to 6-yr-old children demonstrated significantly larger movement errors than the 7- to 8- and 9- to 10-yr-old children.
FIG. 6.
Exposure phase. IDE (left) and RMSE (right) during early exposure (black bars) and late exposure (gray bars) for the 3 age groups (error bars = 1SD). Early and late exposures consist of the first and last 21 trials with the visual feedback rotation, respectively.
Postexposure phase
AUDITORY CONDITION.
For the 135° target, a significant main effect of BLOCK [F(1,37) = 26.943, P < 0.001] was revealed because IDE was larger during auditory postexposure than during baseline (Fig. 7). For movements to the 45° target, a significant BLOCK × GROUP interaction was found [F(2,38) = 6.543, P = 0.004]. Scheffé's post hoc analysis evaluating differential effects of BLOCK on GROUP (Marascuilo and Levin 1970) indicated that the difference between auditory baseline and postexposure for the 5- to 6-yr-old children was significantly greater than the differences between baseline and postexposure for both the 7- to 8- and the 9- to 10-yr-old children (P < 0.05) for the 45° target. The presence of significant intersensory effects demonstrates that exposure to the visuomotor rotation systematically affected reaching performance to acoustic targets.
FIG. 7.
Intersensory effects. IDE during auditory preexposure (black bars) and postexposure (gray bars) for the 45 and 135° targets (error bars = 1SD). The intersensory effects evident during postexposure were computed using the first 4 trials to each auditory target after the visual feedback rotation was removed.
VISUAL CONDITION.
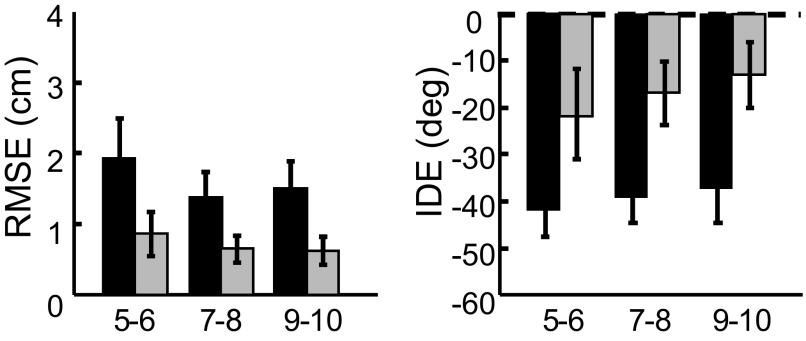
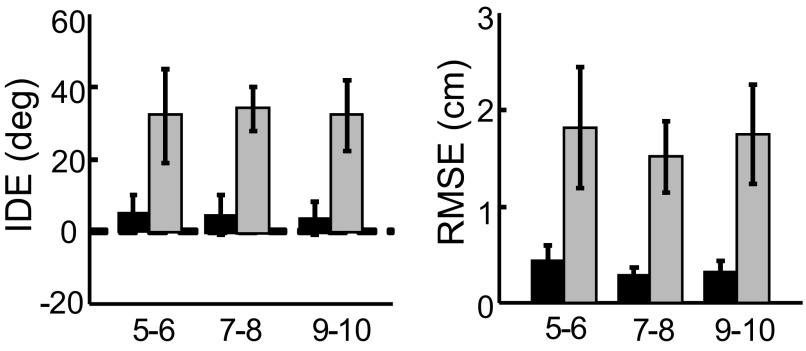
Separate repeated-measures ANOVAs on the visual condition revealed a main effect of BLOCK for both IDE [F(1,38) = 276.63, P < 0.001] and RMSE [F(1,38) = 344.27, P < 0.001], indicating that movement errors during postexposure were significantly larger than those during visual baseline (Fig. 8). There were no significant GROUP main effects or a BLOCK × GROUP interaction. The existence of BLOCK effects for both IDE and RMSE suggests that participants successfully adapted to the imposed visuomotor rotation. Furthermore, the lack of GROUP effects indicated that the three groups of children adapted similarly.
FIG. 8.
Visual aftereffects. IDE (left) and RMSE (right) during visual preexposure (black bars) and postexposure (gray bars) for the 3 age groups (error bars = 1SD). Visual aftereffects were computed using the first 6 trials during the postexposure phase.
DISCUSSION
The findings of this experiment demonstrated: 1) the presence of significant aftereffects and intersensory effects during visual and auditory postexposure in all groups, respectively; and 2) no consistent age-related differences in the magnitude of these effects in the two experimental conditions (visual and auditory). The presence of intersensory effects indicates that a spatial-to-motor transformation acquired during visuomotor adaptation is accessible not only during the visually guided, but also the acoustically guided, aiming tasks. Moreover, in a previous experiment in our laboratory (Kagerer and Contreras-Vidal 2008), adult participants completed a similar paradigm and demonstrated nearly equivalent relative effects, for both the visual and the auditory conditions, as we found in the present study for children. Collectively, the data on adults and our findings indicate that the mechanisms underlying multisensory spatial-to-motor transformations are developed and functional by 5 yr of age.
The results from the current study demonstrating similar intersensory effects in 5- to 10-yr-old children are somewhat surprising considering the existing developmental literature. For example, in a postural control task, Bair et al. (2007) demonstrated that intermodality reweighting of visual and touch information increased linearly with age. Moreover, intermodality reweighting was significant at 10 yr of age, but not at 4 yr. It is possible that a flexible reweighting mechanism demonstrated by the older children in Bair et al. (2007) reflects more complex processes than the intersensory effects examined in the current study. Specifically, in Bair et al. (2007), simultaneous visual and touch information were available to the participants to provide an estimate of the current “state” (i.e., position and velocity) of the multisegmented body. Thus the visual and touch information were dynamically reweighted to maintain postural equilibrium.
Multisensory spatial-to-motor transformations
The results from the current study, as well as those in Kagerer and Contreras-Vidal (2008), clearly demonstrate that adaptation to a visuomotor distortion influences auditory-motor aiming performance. An interpretation of these data posits that sensory estimates of target location, independent of the modality of the targets, are mapped to a common target representation or reference coordinate system. In this interpretation, the interaction between vision and audition occurs during target localization and thus prior to the spatial-to-motor transformation. During adaptation to a visuomotor distortion, an updated spatial-to-motor transformation is acquired and the previously used transformation is suppressed. Although not investigated in the current experiment, this framework can easily account for aiming movements directed toward targets that are simultaneously perceived by multiple sensory systems as the sensory estimates can be integrated to compute a multisensory estimate of target position. Support for such a reference coordinate system comes from recent evidence in adults indicating target positions perceived by different sensory modalities (audition, vision, and proprioception) are specified in gaze- or eye-centered coordinates (Pouget et al. 2002). This finding is consistent with research suggesting that the spatial difference vector is also specified in gaze/eye-centered coordinates (Batista et al. 1999; Buneo et al. 2002; Cohen and Andersen 2000). It should be noted that the existing literature is equivocal with respect to determining which coordinate frame movement trajectories are specified. In contrast to the gaze-centered reference frame, others have posited that the reference coordinate frame is hand-centered (Gordon et al. 1994) or even context-dependent (Battaglia-Mayer et al. 2003). Nonetheless, if sensory information from multiple modalities is specified in a reference coordinate system, independent of the modality of the targets, then an experimentally introduced manipulation of one sensory-motor relationship (i.e., a visuomotor perturbation) should influence other sensory-motor relationships (i.e., auditory-motor) as well. Indeed, this was the case for 5- to 10-yr old children, as reported in the current study, and in adult participants (Kagerer and Contreras-Vidal, 2008).
Neural correlates of multisensory integration
Existing research on animal models demonstrated effects similar to those reported in the current study (King 1999, 2002; Knudsen and Brainard 1991, 1995). Knudsen and colleagues placed prismatic lenses, which displaced the visual field horizontally, over the eyes of juvenile barn owls. Adaptation to the prismatic lenses resulted in a horizontal displacement of sound localization in the same direction as the visual shift induced by the prisms (Knudsen and Knudsen 1989). These data demonstrated the effects of visual experience on auditory localization as related to orientation and attention toward both visual and acoustic stimuli, behaviors thought to be facilitated by the superior colliculus or optic tectum.
Importantly, these data do not necessarily account for the multisensory interactions that facilitate more complex motor tasks, such as the planning and execution of discrete aiming movements in children. The intersensory interactions demonstrated in the current study may be attributed to specific cortical regions such as the posterior parietal (PPC) and premotor cortices (Andersen et al. 1997; Buneo and Andersen 2006; Wise et al. 1997). The PPC receives inputs from the auditory and visual cortices and is thought to be involved in the computation of the spatial difference vector between arm and target positions (Andersen et al. 1997; Buneo and Andersen 2006). Specifically, research on monkeys has shown that the parietal reach region, a division of the superior parietal lobule, is critical for the planning and execution of reaching movements (Andersen et al. 1998; Batista and Andersen 2001). A homologous area is thought to be present in humans (Connolly et al. 2003).
Age-related changes in both gray and white matter in these cortical areas have been reported previously in children (Giedd et al. 1999; Paus et al. 1999; Toga et al. 2006). However, additional research is necessary to better understand the relationship between developmental changes in the brain and functional sensory-motor behavior.
Age-related differences during discrete aiming
Although age-related differences in the visual aftereffects and auditory intersensory effects were not found in the current study, there were significant differences among the three age groups during the baseline phases. Results revealed that the movements of the 5- to 6-yr-old children were significantly more jerky during visual baseline compared with those of the 9- to 10-yr-old children. Moreover, the 5- to 6-yr-old children had significantly larger RMSE scores than those of both the 7- to 8- and the 9- to 10-yr-olds. There were no age-related differences in IDE; however, the 5- to 6-yr-old children had significantly greater IDE-V values than those of both the 7- to 8- and the 9- to 10-yr-old children. These data suggest that although directional planning, on average, was similar between the three age groups, the spatial-to-motor transformations in the younger children were less fine-tuned (Contreras-Vidal et al. 2005). Moreover, the older children demonstrated increased temporal and spatial control, as indicated by the smoother movement trajectories and decreased spatial error. Differences in NJ potentially indicate that the younger children are more reliant on feedback-dependent corrective movements to reach the desired target (Yan et al. 2000, 2003), a result that is consistent with the MT data because the younger children moved significantly more slowly than did the older children. Similar age-related findings with respect to these dependent measures were previously reported (Contreras-Vidal et al. 2005) and indicate that the performance differences among these groups of children appear to be rooted more in control than in planning aspects. Although not compared statistically, directional variability appeared to be larger in auditory, compared with visual, baseline, suggesting that children in all age groups struggled to consistently localize the acoustic stimuli.
The lack of age-related differences during the visual postexposure phase appears to be inconsistent with the extant literature. Previous research demonstrated improved performance during exposure to a visuomotor rotation in 4-, 6-, and 8-yr-old children; however, only the 8-yr-old children exhibited significant aftereffects following the removal of the distortion (Contreras-Vidal et al. 2005). The authors concluded that the younger children did not acquire an internal representation for the manipulated environment. A key methodological difference between Contreras-Vidal et al. (2005) and the current study was that the former consisted of 60 exposure trials, whereas the experiment reported here required participants to complete 126 trials under the rotated visuomotor environment. By increasing the number of exposure trials, the younger participants in the current study appear to have adapted to the novel visuomotor environment.
Results from the current study revealed a main effect of GROUP during the exposure phase, indicating that the 9- to 10-yr-old children had significantly smaller IDE (i.e., less negative) and RMSE scores compared with those of the 5- to 6-yr-old children. Generally, one would expect that smaller errors during exposure would indicate larger aftereffects during postexposure; however, there were no age-related differences for visual aftereffects. Since auditory postexposure was between the visual exposure and postexposure phases, one could argue that the nine aiming movements toward the auditory targets resulted in differential levels of deadaptation to the visuomotor perturbation. However, since vision was occluded during auditory postexposure, participants did not receive a visual error signal that would trigger such a deadaptation process (Scheidt et al. 2000). Furthermore, an examination of IDE across trials of auditory postexposure revealed no consistent decreases in the magnitude of the aftereffects. Similarly, these data cannot be explained by differential rates of deadaptation across the six trials included in the computation of visual aftereffects. Analyses including only the first visual postexposure trial revealed identical results as when the average of six trials was used.
Results from the auditory postexposure phase revealed that the 5- to 6-yr-old children demonstrated significantly larger intersensory effects for movements to the 45° target compared with those of both the 7- to 8- and the 9- to 10-yr-old children. No such age-related differences were found for movements to the 135° target. An examination of the individual movement trajectories revealed that three of the 5- to 6-yr-old participants initially moved at a direction of 90° in a Cartesian coordinate system, prior to directing their movement toward the 45° target. This secondary movement toward the target could be interpreted as a “corrective” response; however, no on-line error signal was provided to the participants during the auditory condition. Therefore these trajectories were probably the result of the participants initiating movement before the desired auditory target was properly localized. These erroneous trajectories, in addition to the “shallow” movement paths during baseline (i.e., large negative IDE values), resulted in larger-than-normal auditory aftereffects to the 45° target for these participants.
Conclusion
In summary, the current results indicate that adaptation to a visuomotor rotation influenced the execution of aiming movements to acoustic targets in 5- to 10-yr-old children. These findings suggest that by the age of 5–6 yr, children have developed a multisensory spatial-to-motor transformation for both visually guided and acoustically guided aiming movements. To our knowledge, our current study is the first to demonstrate that the execution of discrete aiming movements in young children involves multisensory-motor transformations that are invariant of the target modality. Future studies should continue to explore age-related changes in the intermodal interactions related to the execution of sensory-motor tasks.
GRANTS
This research was supported by National Institute of Child Health and Human Development Grants R01-HD-42527 to J. E. Clark and R03-HD-050372 to F. A. Kagerer.
Acknowledgments
We thank M. M. Pangelinan, M. A. Oliveira, and two anonymous reviewers for insightful comments during preparation of this manuscript and all the children and parents who participated in this study.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Andersen et al. 1998.Andersen RA, Snyder LH, Batista AP, Buneo CA, Cohen YE. Posterior parietal areas specialized for eye movements (LIP) and reach (PRR) using a common coordinate frame. Novartis Found Symp 218: 109–122, 1998. [DOI] [PubMed] [Google Scholar]
- Andersen et al. 1997.Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu Rev Neurosci 20: 303–330, 1997. [DOI] [PubMed] [Google Scholar]
- Bahrick and Lickliter 2000.Bahrick LE, Lickliter R. Intersensory redundancy guides attentional selectivity and perceptual learning in infancy. Dev Psychol 36: 190–201, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bair et al. 2007.Bair WN, Kiemel T, Jeka JJ, Clark JE. Development of multisensory reweighting for posture control in children. Exp Brain Res 183: 435–446, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista and Andersen 2001.Batista AP, Andersen RA. The parietal reach region codes the next planned movement in a sequential reach task. J Neurophysiol 85: 539–544, 2001. [DOI] [PubMed] [Google Scholar]
- Batista et al. 1999.Batista AP, Buneo CA, Snyder LH, Andersen RA. Reach plans in eye-centered coordinates. Science 285: 257–260, 1999. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer et al. 2003.Battaglia-Mayer A, Caminiti R, Lacquaniti F, Zago M. Multiple levels of representation of reaching in the parieto-frontal network. Cereb Cortex 13: 1009–1022, 2003. [DOI] [PubMed] [Google Scholar]
- Bo et al. 2006.Bo J, Contreras-Vidal JL, Kagerer FA, Clark JE. Effects of increased complexity of visuo-motor transformations on children's arm movements. Hum Mov Sci 25: 553–567, 2006. [DOI] [PubMed] [Google Scholar]
- Buch et al. 2003.Buch ER, Young S, Contreras-Vidal JL. Visuomotor adaptation in normal aging. Learn Mem 10: 55–63, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock and Grossberg 1988.Bullock D, Grossberg S. Neural dynamics of planned arm movements: emergent invariants and speed-accuracy properties during trajectory formation. Psychol Rev 95: 49–90, 1988. [DOI] [PubMed] [Google Scholar]
- Bullock et al. 1993.Bullock D, Grossberg S, Guenther FH. A self-organizing neural model of motor equivalent reaching and tool use by a multijoint arm. J Cogn Neurosci 5: 408–435, 1993. [DOI] [PubMed] [Google Scholar]
- Buneo and Andersen 2006.Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia 44: 2594–2606, 2006. [DOI] [PubMed] [Google Scholar]
- Buneo et al. 2002.Buneo CA, Jarvis MR, Batista AP, Andersen RA. Direct visuomotor transformations for reaching. Nature 416: 632–636, 2002. [DOI] [PubMed] [Google Scholar]
- Cohen and Andersen 2000.Cohen YE, Andersen RA. Reaches to sounds encoded in an eye-centered reference frame. Neuron 27: 647–652, 2000. [DOI] [PubMed] [Google Scholar]
- Connolly et al. 2003.Connolly JD, Andersen RA, Goodale MA. FMRI evidence for a “parietal reach region” in the human brain. Exp Brain Res 153: 140–145, 2003. [DOI] [PubMed] [Google Scholar]
- Contreras-Vidal et al. 2005.Contreras-Vidal JL, Bo J, Boudreau JP, Clark JE. Development of visuomotor representations for hand movement in young children. Exp Brain Res 162: 155–164, 2005. [DOI] [PubMed] [Google Scholar]
- Ferrel-Chapus et al. 2002.Ferrel-Chapus C, Hay L, Olivier I, Bard C, Fleury M. Visuomanual coordination in childhood: adaptation to visual distortion. Exp Brain Res 144: 506–517, 2002. [DOI] [PubMed] [Google Scholar]
- Giedd et al. 1999.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci 2: 861–863, 1999. [DOI] [PubMed] [Google Scholar]
- Gordon et al. 1994.Gordon J, Ghilardi MF, Ghez C. Accuracy of planar reaching movements. I. Independence of direction and extent variability. Exp Brain Res 99: 97–111, 1994. [DOI] [PubMed] [Google Scholar]
- Hay 1979.Hay L Spatial-temporal analysis of movements in children: motor programs versus feedback in the development of reaching. J Mot Behav 11: 189–200, 1979. [DOI] [PubMed] [Google Scholar]
- Henderson and Sugden 1992.Henderson SE, Sugden DA. Movement Assessment Battery for Children. London: The Psychological Corporation, 1992.
- Jansen-Osmann et al. 2002.Jansen-Osmann P, Richter S, Konczak J, Kalveram KT. Force adaptation transfers to untrained workspace regions in children: evidence for developing inverse dynamic motor models. Exp Brain Res 143: 212–220, 2002. [DOI] [PubMed] [Google Scholar]
- Kagerer and Contreras-Vidal.Kagerer FA, Contreras-Vidal J. Crossmodal motor adaptation. Exp Brain Res doi: 10.1007/s00221-008-1630-3. [DOI] [PubMed]
- Kagerer et al. 1997.Kagerer FA, Contreras-Vidal JL, Stelmach GE. Adaptation to gradual as compared with sudden visuo-motor distortions. Exp Brain Res 115: 557–561, 1997. [DOI] [PubMed] [Google Scholar]
- King 1999.King AJ Sensory experience and the formation of a computational map of auditory space in the brain. Bioessays 21: 900–911, 1999. [DOI] [PubMed] [Google Scholar]
- King 2002.King AJ Neural plasticity: how the eye tells the brain about sound location. Curr Biol 12: R393–R395, 2002. [DOI] [PubMed] [Google Scholar]
- Knudsen and Brainard 1991.Knudsen EI, Brainard MS. Visual instruction of the neural map of auditory space in the developing optic tectum. Science 253: 85–87, 1991. [DOI] [PubMed] [Google Scholar]
- Knudsen and Brainard 1995.Knudsen EI, Brainard MS. Creating a unified representation of visual and auditory space in the brain. Annu Rev Neurosci 18: 19–43, 1995. [DOI] [PubMed] [Google Scholar]
- Knudsen and Knudsen 1989.Knudsen EI, Knudsen PF. Vision calibrates sound localization in developing barn owls. J Neurosci 9: 3306–3313, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakauer et al. 2000.Krakauer JW, Pine ZM, Ghilardi MF, Ghez C. Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20: 8916–8924, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marascuilo and Levin 1970.Marascuilo LA, Levin JR. Appropriate post hoc comparison for interaction and nested hypotheses in analysis of variance designs: the elimination of type IV errors. Am Educ Res J 7: 397–421, 1970. [Google Scholar]
- Neil et al. 2006.Neil PA, Chee-Ruiter C, Scheier C, Lewkowicz DJ, Shimojo S. Development of multisensory spatial integration and perception in humans. Dev Sci 9: 454–464, 2006. [DOI] [PubMed] [Google Scholar]
- Paus et al. 1999.Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science 283: 1908–1911, 1999. [DOI] [PubMed] [Google Scholar]
- Pouget et al. 2002.Pouget A, Ducom JC, Torri J, Bavelier D. Multisensory spatial representations in eye-centered coordinates for reaching. Cognition 83: B1–B11, 2002. [DOI] [PubMed] [Google Scholar]
- Rosenblum et al. 1997.Rosenblum LD, Schmuckler MA, Johnson JA. The McGurk effect in infants. Percept Psychophys 59: 347–357, 1997. [DOI] [PubMed] [Google Scholar]
- Scheidt et al. 2000.Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA. Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol 84: 853–862, 2000. [DOI] [PubMed] [Google Scholar]
- Shadmehr and Mussa-Ivaldi 1994.Shadmehr R, Mussa-Ivaldi FA. Adaptive representation of dynamics during learning of a motor task. J Neurosci 14: 3208–3224, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr and Wise 2005.Shadmehr R, Wise SP. The Computational Neurobiology of Reaching and Pointing. Cambridge, MA: The MIT Press, 2005.
- Toga et al. 2006.Toga AW, Thompson PM, Sowell ER. Mapping brain maturation. Trends Neurosci 29: 148–159, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise et al. 1997.Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci 20: 25–42, 1997. [DOI] [PubMed] [Google Scholar]
- Wolpert and Kawato 1998.Wolpert DM, Kawato M. Multiple paired forward and inverse models for motor control. Neural Networks 11: 1317–1329, 1998. [DOI] [PubMed] [Google Scholar]
- Yan et al. 2000.Yan JH, Thomas JR, Stelmach GE, Thomas KT. Developmental features of rapid aiming arm movements across the lifespan. J Mot Behav 32: 121–140, 2000. [DOI] [PubMed] [Google Scholar]
- Yan et al. 2003.Yan JH, Thomas KT, Stelmach GE, Thomas JR. Developmental differences in children's ballistic aiming movements of the arm. Percept Mot Skills 96: 589–598, 2003. [DOI] [PubMed] [Google Scholar]