Abstract
A salient peripheral cue can capture attention, influencing subsequent responses to a target. Attentional cueing effects have been studied for head-restrained saccades; however, under natural conditions, the head contributes to gaze shifts. We asked whether attention influences head movements in combined eye–head gaze shifts and, if so, whether this influence is different for the eye and head components. Subjects made combined eye–head gaze shifts to horizontal visual targets. Prior to target onset, a behaviorally irrelevant cue was flashed at the same (congruent) or opposite (incongruent) location at various stimulus-onset asynchrony (SOA) times. We measured eye and head movements and neck muscle electromyographic signals. Reaction times for the eye and head were highly correlated; both showed significantly shorter latencies (attentional facilitation) for congruent compared with incongruent cues at the two shortest SOAs and the opposite pattern (inhibition of return) at the longer SOAs, consistent with attentional modulation of a common eye–head gaze drive. Interestingly, we also found that the head latency relative to saccade onset was significantly shorter for congruent than that for incongruent cues. This suggests an effect of attention on the head separate from that on the eyes.
INTRODUCTION
Under natural, head-unrestrained viewing conditions, saccades are typically composed of a combination of eye and head movements resulting in an overall gaze shift. The role of attention in saccadic eye movements has been studied extensively in head-restrained conditions. It has been established that saccade execution requires a shift of attention to the saccade goal (e.g., Deubel and Schneider 1996; Hoffman and Subramaniam 1995; Kowler et al. 1995; McPeek et al. 1999), indicating a close linkage between eye movements and attention. Such a linkage is also supported by a number of studies showing shared neural substrates for saccadic eye movement planning and attentional processing (e.g., Beauchamp et al. 2001; Cavanaugh and Wurtz 2004; Corbetta et al. 1998; Goldberg et al. 2006; Ignashchenkova et al. 2004; Kastner and Ungerleider 2000; Moore and Fallah 2001; Muller et al. 2005; Thompson et al. 2005). In addition, exogenous attentional cueing of a saccade target position before target presentation can facilitate or inhibit responses to the target, resulting in modulations of saccade latencies (e.g., Dorris et al. 2002; Fecteau and Munoz 2006; Posner et al. 1982).
Considerably less is known about the role of attention in combined eye–head gaze shifts (Cicchini et al. 2008; Corneil and Munoz 1999; Corneil et al. 2004, 2008). Corneil and Munoz (1999) showed that the onset of a distractor in another sensory modality—believed to attract attention—can elicit head movement responses that precede the saccade by >50 ms. Despite this early head movement onset, the subsequent gaze saccades were accurate, indicating that the gaze control system has on-line information about ongoing head movements (Corneil et al. 1999; Vliegen et al. 2004, 2005). Furthermore, in monkeys Corneil et al. (2008) showed that exogenous attentional cues produce changes in electromyographic (EMG) activity that are correlated with attentional facilitation and inhibition of return, as well as with saccade latencies. However, they did not directly compare the effects of exogenous cues on eye and head movement latencies. Since the head movement system is not gated by omnipause neurons (OPNs) (Gandhi and Sparks 2007), as is the case for saccades (Keller 1974, 1977; Luschei and Fuchs 1972), this neck muscle response is believed to reflect the attentional cueing effect of the exogenous cue. The lack of inhibition by OPNs might also cause the observation that movement decisions are generally reflected first in neck muscle activity, followed later by eye movements, as has been observed in a saccade countermanding paradigm (Corneil and Elsley 2005) or with frontal eye field (FEF) or superior colliculus (SC) microstimulation (Chen 2006; Elsley et al. 2007; Tu and Keating 2000). Despite these recent electrophysiological insights into the role of attention on head movements in gaze saccades, it remains largely unexplored how attention influences the timing of the head drive during combined eye–head gaze shifts.
We used a Posner cueing paradigm (e.g., Posner 1980) to specifically examine the influence of exogenous attention on head movements in combined eye–head gaze shifts. We sought to elucidate whether attention influences the latency of head movements and, if so, whether the head is differentially influenced by attention or whether this influence is the same as for saccades. We asked subjects to make combined eye–head gaze shifts to eccentric target positions after presenting a spatially congruent or incongruent, behaviorally irrelevant cue at different times before target onset. By analyzing eye and head movement latencies, we show evidence for a tight coupling between eye and head movements with attention exerting a common influence on both. However, we also find cue-dependent modulations of the head latencies that are different from those of saccade latencies.
METHODS
Subjects
Seven healthy human subjects (ages 23–31 yr) participated in this experiment, of which five were naïve to the goals of this study. All subjects had normal or corrected-to-normal vision and did not have any known neurological disorders. Experiments were approved by the Univerisité catholique de Louvain Ethics Committee in accordance with the Declaration of Helsinki.
Apparatus
Subjects sat in a chair in front of a 90-cm-distant tangential screen and viewed targets located at eye level. Green (fixation) and red (saccade targets) laser spots were back-projected onto the translucent screen by means of M3ST and M2 mirror galvanometers (GSI Lumonics, Billerica, MA).
Movements of the right eye were recorded at 400 Hz using the Chronos video head-mounted eye tracker (Chronos Vision, Berlin). Head movements were recorded through the use of active infrared markers mounted on the eye tracker helmet. The three-dimensional positions of these markers were recorded using a Codamotion system (Codamotion, Leicestershire, UK) at 200 Hz. Muscle activity of the sternocleido-mastoid (SCM) and the trapezius (TR) muscles of the neck were recorded bilaterally. The SCM and TR are two of many muscles involved in rotating the head. Other muscles involved include the splenii and the obliquus capitus inferior, all of which are in the deep muscle layers and whose activity cannot be recorded using surface electrodes. Therefore it should be noted that we did not record from the complete group of muscles involved in head rotation and we may be recording from muscles that are secondary to earlier recruitment in the deeper neck muscles. The EMG signals were measured at a sampling rate of 1 kHz using a NeuroLog EMG system (Digitimer, Hertfordshire, UK). Skin was prepared using isopropyl alcohol and Neuroline 710-15-K–wired electrodes (Ambu, Ballerup, Denmark) were attached onto the left and right SCM and TR muscles (Gray 1977) at two locations about 4 cm apart for each muscle. A ninth electrode was placed on the skin on top of the C7 vertebra (vertebra prominens) as the reference. Each pair of electrodes corresponding to one muscle was connected to a NeuroLog NL844 preamplifier (Digitimer) in a differential setup. The resulting four preamplified signals (highpass filter = 10 Hz, amplification = 1K) were isolated using a NeuroLog NL820 isolator (Digitimer). A real-time computer (PXI-8186; National Instruments, Austin, TX) using LabVIEW (National Instruments) controlled the presentation of the targets, synchronized the recording of the Codamotion and Chronos devices, and recorded the EMG signal at 1 kHz using PXI-6025E (National Instruments) multipurpose data acquisition boards.
Procedure
Each experiment began with a calibration sequence where subjects were required to fixate a series of 17 targets at different two-dimensional positions on the screen while keeping the head still. These data were used to calibrate the eye position traces and to provide a straight-ahead head position reference for the experimental conditions that followed.
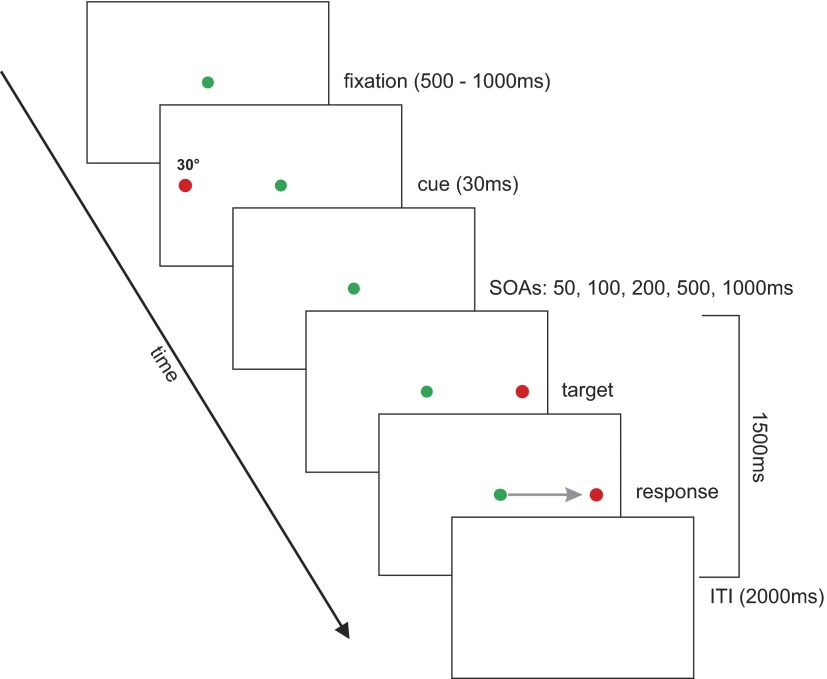
Each test trial began with the presentation of a green fixation spot, 0.1° in diameter, at eye level for a random duration between 500 and 1,000 ms (Fig. 1). Subjects were asked to fixate the fixation spot and maintain an upright, straight-ahead head position. Next, a cue (red laser spot, 0.1° in diameter) was flashed at eye level 30° to the left or right of fixation for a duration of 30 ms. After a variable delay (stimulus-onset asynchrony [SOA] = 50, 100, 200, 500, and 1,000 ms), a target (red laser spot, 0.1° in diameter) was presented for 1.5 s at 30° left or right until the end of the trial. Subjects were asked to ignore the cue and to make a rapid gaze shift (combined eye–head movement) toward the target as soon as it appeared; they were instructed to differentiate between the cue and the target by ignoring the first, flashed red target and making a gaze shift to the second, sustained red target. In addition to the instructions, the eccentricity of the target ensured that subjects recruited the head, and not just the eye, to perform the movement. An intertrial interval (ITI) with no target presented lasted about 2 s, during which subjects were asked to return their gaze and head to the central position. Each subject completed a total of 400 trials.
FIG. 1.
Task setup. Targets were back-projected onto a translucent screen. Each trial began with the presentation of a green fixation spot at center that randomly lasted for 500–1,000 ms. Next, a red cue was flashed for 30 ms either 30° left or right of fixation, aligned vertically with the fixation spot. After a variable delay (corresponding to stimulus-onset asynchronies [SOAs] of 50, 100, 200, 500, and 1,000 ms), a red target was presented at the congruent or incongruent position (randomly determined), also 30° left or right of fixation for 1.5 s. Subjects were asked to ignore the noninformative cue and to make a rapid combined head–eye gaze movement to the target as soon as they saw it. A black screen signaled the end of the trial. The intertrial interval (ITI) lasted 2 s.
Data analysis
Data were analyzed off-line using Matlab (The MathWorks, Natick, MA). Eye position was calibrated using the calibration sequence (offset and amplitude adjustment) and then low-pass filtered (autoregressive forward–backward filter, cutoff frequency = 50 Hz) and differentiated twice (central difference algorithm) to obtain eye velocity and acceleration. Saccades were then detected based on a 1,000°/s2 absolute acceleration threshold (de Brouwer et al. 2001, 2002). We measured the first saccade made after target onset.
Head orientation was computed based on three infrared markers placed on the Chronos helmet. The position of the three markers for the straight-ahead head position (from the calibration sequence) was converted into a reference position quaternion. Head position was then computed as the rotational quaternion between current orientation of the helmet as defined by the three infrared markers and the reference position. Head orientation was also low-pass filtered (autoregressive forward–backward filter, cutoff frequency = 50 Hz) and differentiated twice (central difference algorithm). Head movement onset was detected based on a 200°/s2 absolute acceleration threshold.
We also used a second measure to determine head movement onset, based on the raw recorded EMG activity. EMG onset was detected using a variable-threshold algorithm. This procedure used the resting signal noise of the rectified EMG signal at the beginning of each trial to estimate the noise amplitude. Muscle activity onset was defined as the moment when the rectified EMG signal rose consistently (for ≥30 ms) above mean + 3SD. All trials were visually inspected and EMG onset corrected, if necessary.
We collected a total of 2,800 trials. Of these 93 (3.3%) were removed because EMG signals were not clear enough to allow for movement onset detection. A further 157 (5.6%) were removed because of unclear eye position signals due to the Chronos iris-detection method. We also removed trials in which saccade latency was <80 or >500 ms (Carpenter 1988; Fischer et al. 1993). Such trials came to a total of 331 (11.8%). Also trials in which saccade or head amplitude was >40° or <10° (82 trials = 2.9%) were removed. Errors in which subjects made either saccades or head movements away from instead of toward the target direction were removed (72 trials = 2.6%). Within the data analysis, we also excluded a total of 393 (14%) trials that had saccade latencies outside of 3SD of the mean latency for each subject. This resulted in 1,672 (59.7%) trials retained for further analysis.
RESULTS
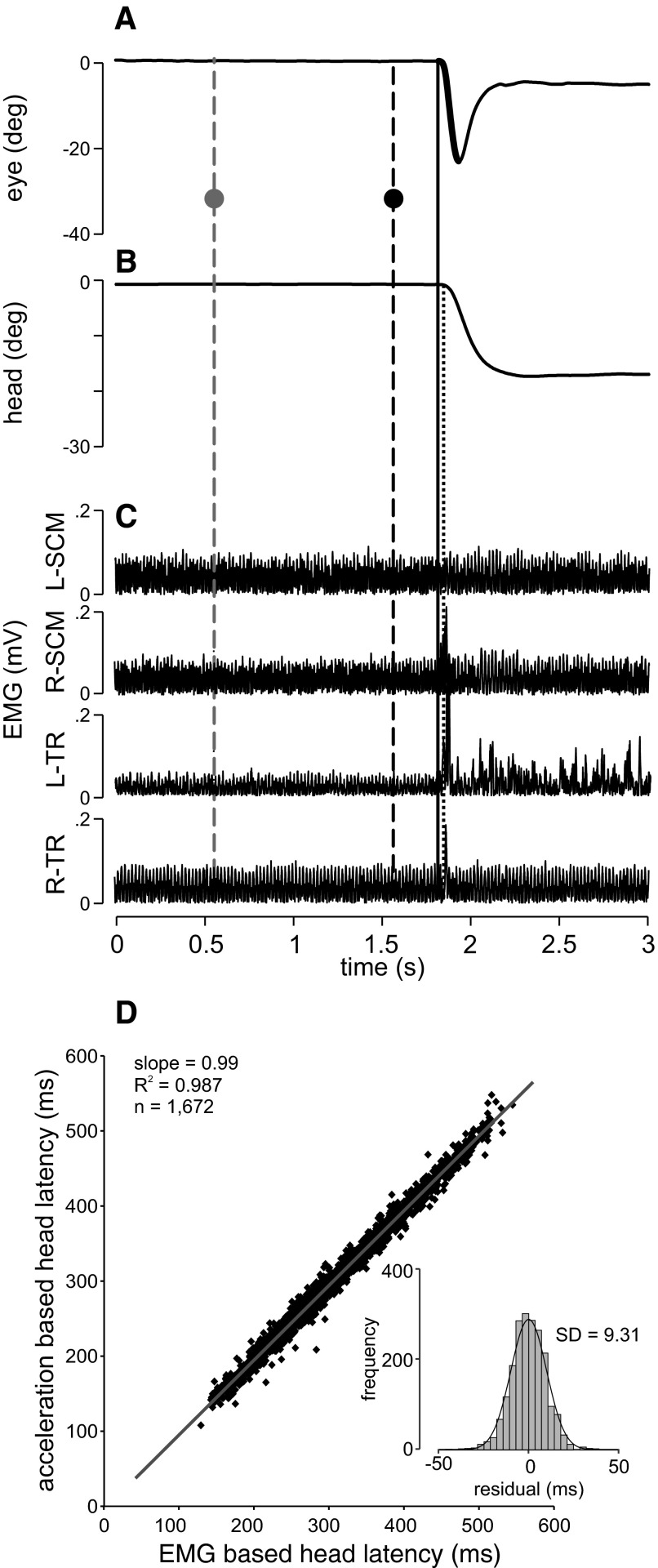
Figure 2, A–C shows a typical congruent test trial for the 1,000-ms SOA condition. The saccade (bold line in Fig. 2A) began before the head movement (vertical solid line). The head movement onset, based on the head acceleration threshold, is shown in Fig. 2B (dotted vertical line) and the onset of the head movement, based on EMG activity, in Fig. 2C. For this leftward movement, mainly the left trapezius (L-TR) and right sternocleido-mastoid (R-SCM) muscles were active. Since the head movement had slower dynamics, there was a vestibuloocular reflex (VOR) period after the saccade ended.
FIG. 2.
Typical trial and head movement latencies. Positional traces for the eye (A) and head (B) as well as electromyographic (EMG) muscle activity (C) are shown as a function of time for one typical trial. C: EMG muscle activity is shown for the left (L) and right (R) sternocleido-mastoid (SCM) and trapezius (TR) muscles. The x-axis depicts time beginning from trial onset. The y-axis in A and B depicts positions in degrees from 0 toward the left (signed negative). The y-axis in C depicts EMG activity in millivolts (mV). The first vertical dashed line (gray) across all traces represents the cue onset and the second vertical dashed line (black) represents target onset (SOA = 1,000 ms). The gray and black dots intersecting these lines in A depict the positions of the cue and the target during this trial (i.e., a congruent trial where both cue and target were presented at 30° left). The solid vertical line represents saccade onset (A) and the dotted vertical line depicts head movement onset based on the acceleration criterion (B). The bold segment of the position trace in A represents the saccade. D: correlation between head movement latencies as detected using a 200°/s2 acceleration threshold (y-axis) or using the EMG onset time (x-axis). EMG onset time was calculated as the point in time when the rectified EMG signal rose consistently (for 30 ms) above mean + 3SD. The inset shows the histogram of residuals to the linear regression (gray line, y = 8.63 + 0.99x), i.e., by how much the 2 head movement latency measurements differed.
We measured head movement latencies using two different independent techniques: an acceleration criterion and a threshold criterion (signal rise over mean + 3SD for 30 ms) on the EMG neck muscle activity. To determine the consistency of these two measures of head latency, we plotted them against each other in Fig. 2D. There was a highly significant correlation between the two measures, with a slope of 0.99 and an R2 value of 0.987 (P < 0.01). The inset in the graph depicts the range of residuals with a small SD of 9.31 ms, reflecting the tight correlation. The mean latency difference between the two head onset detection methods revealed that the acceleration-based latency lags behind the EMG latency by 6.02 ms on average. We chose to use the EMG-based head movement latencies for all subsequent analyses for two reasons. First, we believe that EMG-based latencies are more precise than head-acceleration latencies. This is because head-acceleration latencies were calculated by double differentiating the head position signals (see methods). Each differentiation introduces numerical smoothing that makes the precise detection of the movement onset difficult. The use of an acceleration threshold could also be problematic in cases where subjects move more or less fast (and thus need more or less acceleration); in this case the detected onset would change only because of the fixed acceleration threshold. This is not the case when considering EMG activity where the first rapid rise in signal indicates the onset of muscle activity regardless of the level of acceleration. Also, we acquired head position at only 200 Hz (compared with 1 kHz for EMG), which adds to the fact that overall head kinematics provide a much less precise estimation of head movement onset than EMG.
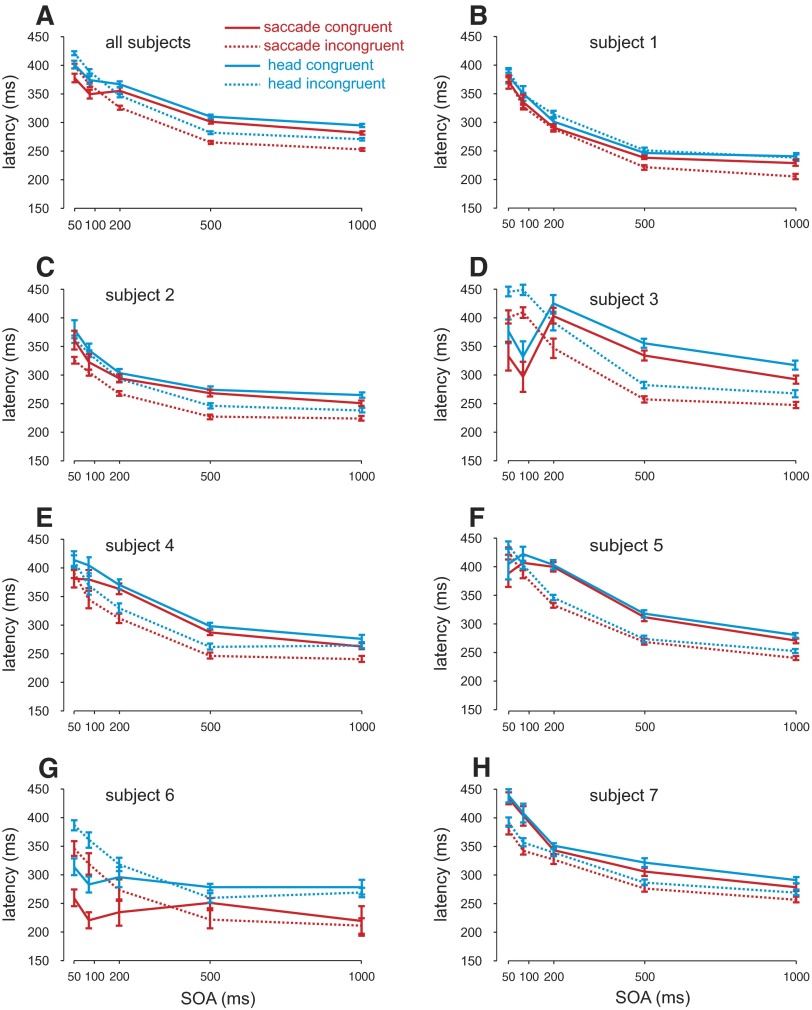
First, we investigated whether attention influenced the eye and head movement latencies differently or in the same manner. Figure 3 A depicts both saccade (red lines) and head movement (blue) latencies plotted as a function of SOA separately for the congruent (cue and target positions are the same, solid lines) and incongruent (opposite cue and target positions, dotted lines) conditions across all subjects. For the two shortest SOAs, saccade latencies were shorter in the congruent condition compared with the incongruent condition. For the longer SOAs, the opposite pattern occurred. The influence of a behaviorally irrelevant cue on subsequent eye movements to a target presented in the congruent compared with other locations has been previously shown to depend on the SOA (e.g., Posner and Cohen 1984); typically, at shorter SOAs, saccades are faster for the congruent condition compared with the incongruent condition (attentional facilitation). At longer SOAs (∼200–300 ms) this pattern reverses (known as inhibition of return [IOR]). An ANOVA with SOA and condition (congruent and incongruent) revealed significant differences between the conditions [F(1,1,662) = 232.22, P < 0.001] and a significant decrease in latencies with greater SOAs [F(4,1,662) = 15.23, P < 0.001], as well as a significant interaction effect between the two [F(4,1,662) = 17.55, P < 0.001]. Bonferroni-corrected t-tests confirmed that latencies were faster in the congruent condition compared with the incongruent condition for the 50- and 100-ms SOAs (P < 0.001), whereas the opposite pattern occurred for longer SOAs (P < 0.001).
FIG. 3.
Saccade and head latencies. A: saccade (red lines) and head movement latencies (blue lines) in milliseconds are plotted separately for congruent (solid lines: cue and target presented at the same location) and incongruent (dotted lines: cue and target presented at opposite locations) as a function of SOA for all subjects combined. Error bars represent SE. B–F: individual subject data plotted in the same manner as A.
Head movement latencies closely matched the pattern observed for saccade latencies (blue lines). ANOVA analyses revealed significant differences between the congruent and incongruent conditions [F(1,1,662) = 7.56, P < 0.01] and significant decreases in latencies with larger SOAs [F(4,1,662) = 328.54, P < 0.001] as well as a significant interaction effect [F(4,1,662) = 14.436, P < 0.001]. Bonferroni-corrected t-tests confirmed that latencies were faster in the congruent condition compared with the incongruent condition for the 50-ms SOAs (P < 0.01), whereas the opposite pattern occurred for the 200-, 500-, and 1,000-ms SOAs (P < 0.001). There was a nonsignificant difference for the 100-ms condition (P = 0.06).
Figure 3, B–H shows individual subject latencies for the saccade and head movements plotted in the same manner as in Fig. 3A. As is apparent, the patterns of head movement and saccade latencies were very similar within each condition. Four subjects showed both attentional facilitation and IOR (subjects 3, 4, 5, and 6; P < 0.05) and three only showed IOR across all SOAs (subjects 1, 2, and 7; P < 0.05). The effects of attentional facilitation and IOR are both highly dependent on stimulus luminance and can vary across different subjects (unpublished observations). Importantly, for each subject, the latency patterns remain very similar across saccade and head movements (see following text for quantitative analysis).
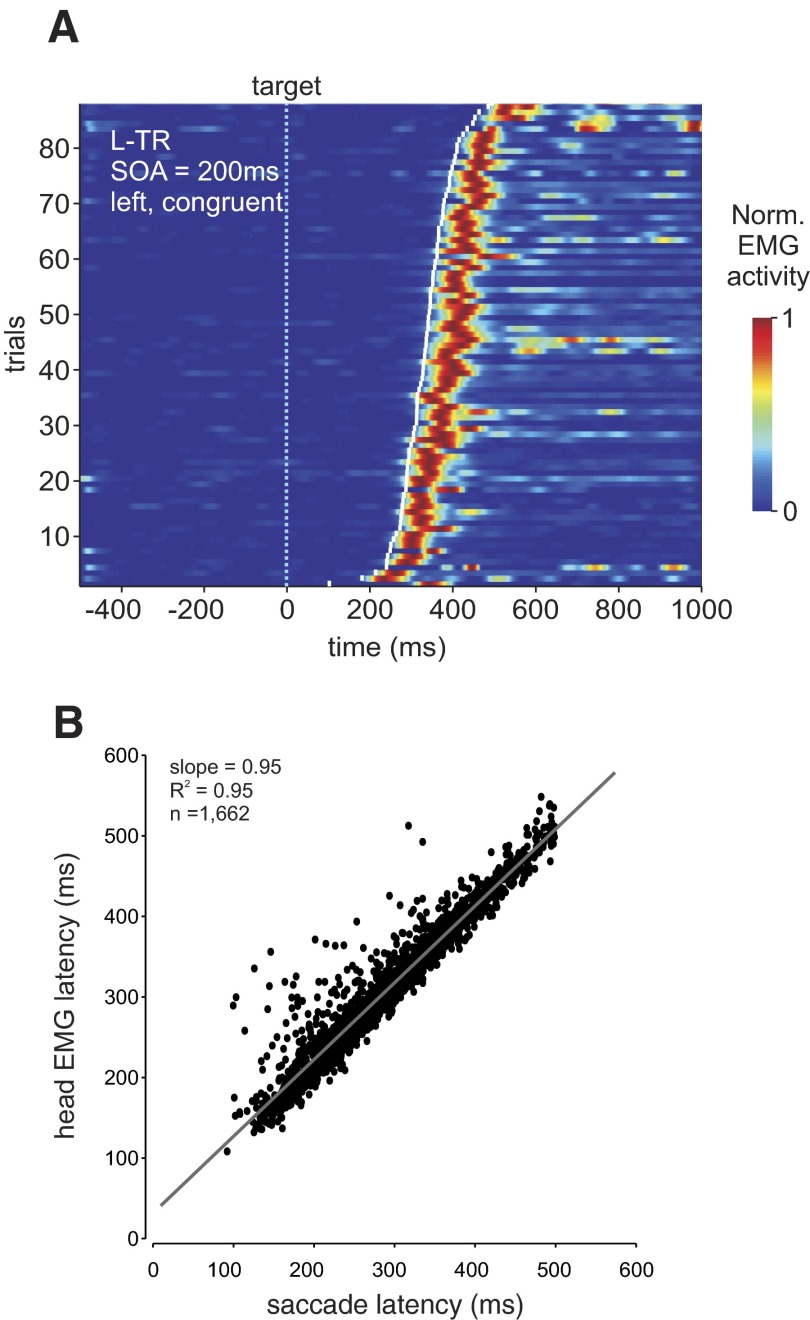
We directly compared saccade latency to head movement onset in Fig. 4. Figure 4A depicts the relationship between saccade onset and EMG activity in an example condition for the left trapezius muscle (SOA = 200 ms, leftward movements, congruent condition) across 86 trials sorted by saccade latency (white tick marks) and aligned on target onset (time 0, dotted vertical line) for a typical subject. The figure shows that for each trial (horizontal raster) the maximum normalized EMG activity (red color) closely follows saccade onset by an approximately constant interval. Figure 4B shows a quantification of the relationship between saccade latency and head movement latency across the two cueing conditions. The correlation between head movement onset and saccade latency was highly significant [R(1,672) = 0.977, P < 0.001] across all subjects. Individual correlations ranged between 0.784 and 0.995 (subject 1 = 0.974, 2 = 0.978, 3 = 0.98, 4 = 0.982, 5 = 0.995, 6 = 0.784, 7 = 0.993).
FIG. 4.
Coupling between saccade and head movement (EMG) latencies. A: example of coupling between saccade onset and normalized EMG activity for the left trapezius muscle (L-TR) for 86 leftward movements sorted by saccade onset relative to target presentation (time 0, light blue vertical dotted line). Trials are from the 200-ms SOA condition. Saccade onset is depicted by the white tick marks for each trial. Muscle activity is normalized with respect to peak amplitude and color coded, with 1 (red) equal to maximum activity. B: correlation between head movement latency (based on EMG, y-axis) and saccade latency (x-axis). The regression was as follows: y = 31.7 + 0.954x (gray line).
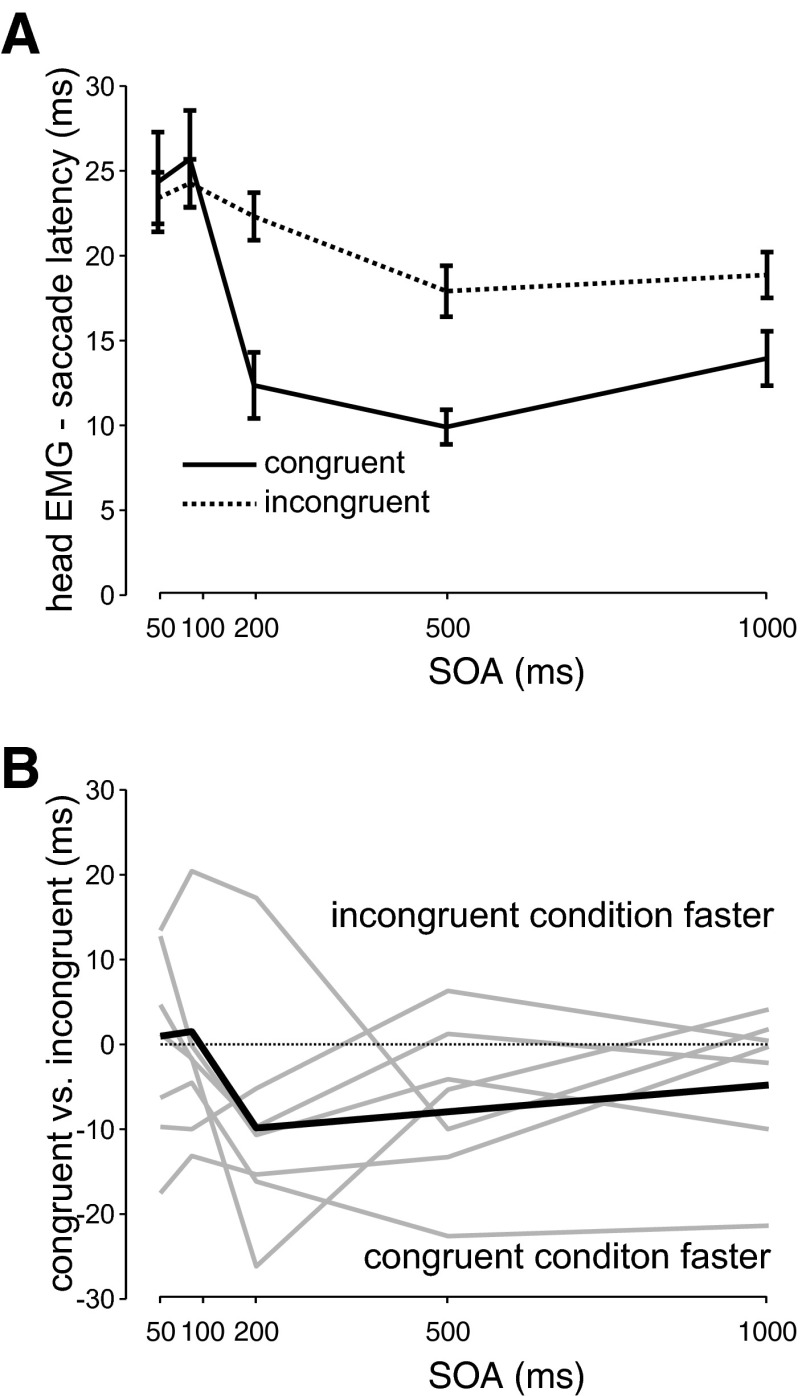
As a further analysis, we compared the relative timing of eye and head movements across the cueing conditions. To do so, we subtracted saccade latency from head EMG latency for each trial, giving us the relative latency of the head and the eye. We then separately examined the relative latency at each SOA. This is shown in Fig. 5 A (all subjects together) and indicates that the head generally lags behind the eye and that the relative latency not only is condition dependent but also is modulated by SOA. A two-way ANOVA analysis with condition and SOA as factors revealed a significant main effect across both conditions [F(1,1,662) = 11.55, P < 0.001, Student–Newman–Keuls [SNK] < 0.05] and SOAs [F(4,1,662) = 14.28, P < 0.001] with significant differences between the two shorter and three longer SOAs (post hoc test, SNK < 0.05). This can also be seen in the individual subject analysis in Fig. 5B, in which the differences between congruent and incongruent relative latencies are plotted as a function of SOA for each subject (light gray lines) and across all subjects (black line). This value was calculated by subtracting the relative latency in the congruent condition from that of the incongruent condition. No differences across conditions for the relative latencies would result in a flat line at 0 (shown by the dotted line) across all SOAs. As can be seen, all subjects showed large differences between conditions that were different across SOAs. Individual separate ANOVA analyses for SOA (short and long) revealed significant differences between conditions (congruent and incongruent). For the short SOAs, three subjects (P < 0.01) had significantly shorter relative latencies in the congruent compared with the incongruent conditions and four subjects had no differences (P > 0.05). For the long SOAs, four subjects had significantly shorter relative latencies during the congruent compared with the incongruent conditions (P < 0.01) and three showed no differences between the two conditions (P > 0.05). In summary, these differences in the relative latency between the eye and head for the congruent and incongruent cueing conditions indicate a separate influence of attention on the head-only component that differs from its influence on the eye component.
FIG. 5.
Difference between saccade and head movement onsets. A: mean difference across all subjects between head and saccade onset plotted as a function of SOA separately for congruent (solid line) and incongruent (dotted line) conditions. Error bars indicate SE. B: difference between congruent and incongruent relative latencies plotted as a function of SOA across all subjects (thick black line) and for individual subjects (thin gray lines).
Taken together, the analysis of the saccade and head movement latencies showed a tight coupling of both systems (Fig. 4) that was similarly modulated by attentional cueing. This is consistent with a trigger for both eye and head movements that is influenced in the same manner by attention. In addition, the relative latencies also revealed an independent effect of attention on the head movement latency compared with saccade latency.
DISCUSSION
To summarize our results, we have shown that head movements are influenced by attentional cueing in a manner very similar to that of saccades. Both eye and head movements showed shorter latencies in the congruent compared with the incongruent condition during the shortest SOA. This effect was previously shown for saccades with the head fixed and is generally referred to as attentional facilitation or capture (Fecteau and Munoz 2005; Jonides and Irwin 1981; Klein 2000; Posner and Cohen 1984). At longer SOAs, we found the opposite pattern, where both head and saccade reaction times were longer for the congruent compared with the incongruent condition. This effect is known as inhibition of return (IOR) and is commonly measured as the relative difference between congruent and incongruent conditions (Abrams and Dobkin 1994a,b; Klein 2000; Maylor and Hockey 1985; Posner and Cohen 1984; Posner et al. 1985; Rafal et al. 1994; Reuter-Lorenz et al. 1996; Tanaka and Shimojo 1996; Taylor and Klein 1998).
Furthermore, our results revealed the presence of an additional modulation of head movement latencies relative to eye latencies across the cueing conditions. Head movements were generally initiated slightly but significantly earlier (relative to saccades) when directed to previously cued locations, particularly for longer SOA conditions. Taken together, this pattern of influence on eye and head movements argues for a dual influence of attention on combined eye–head movements and is consistent with the presence of both common and separate drives for the head and the eyes.
Common effects of attention on the eye and the head
Our results revealed a similar influence of attention on the latency of the eye and head, suggesting that both motor systems receive a shared trigger signal. This is consistent with the findings of Corneil et al. (2008), who first demonstrated that exogenous attentional cues modulate neck EMG activity in monkeys and that this modulation is correlated with saccade latencies. Compared with their study, we did not observe any cue-related modulation of neck EMG activity, likely because our surface electrodes were not sensitive enough to pick up the small cue-related signals and because Corneil et al. (2008) recorded from fifth layer neck muscles compared with surface muscles in our case.
Many regions known to be involved in saccadic eye movements and attention (Cavanaugh and Wurtz 2004; Corbetta et al. 1998; Ignashchenkova et al. 2004; Krauzlis 2004, 2005; McPeek 2006, 2008; Moore and Fallah 2001; Muller et al. 2005; Pierrot-Deseilligny et al. 2004; Thompson et al. 2005; Wardak et al. 2006) are also involved in combined eye–head movements. These include the frontal eye fields (Chen 2006; Elsley et al. 2007; Knight and Fuchs 2007; Monteon et al. 2005; Tu and Keating 2000; van der Steen et al. 1986), the supplementary eye fields (Chen and Walton 2005; Martinez-Trujillo et al. 2003, 2004), and the superior colliculus (Freedman and Sparks 1997a; Freedman et al. 1996; Klier et al. 2001; Martinez-Trujillo et al. 2003; Walton et al. 2007, 2008). Previous studies have provided evidence in support of a single gaze controller that programs both the eye and head components (Galiana and Guitton 1992; Guitton 1992; Guitton et al. 2003; Lefèvre and Galiana 1992; Sparks et al. 2001). Thus our results are consistent with attentional modulation of neural activity within this SEF–FEF–SC network representing the common gaze pathway.
Differential effects of attention on the eye and the head
Our data also showed a distinct influence of spatial attention on head latency compared with eye latency. One explanation of these findings is the existence of a twofold influence of attention on head movements, through both a common gaze drive and a separate head drive. Recently, evidence has been presented for an independent head controller in addition to the gaze controller, which can modulate the head component of the gaze shift. This is supported by findings reporting context-dependent head contributions to gaze shifts in addition to a stereotypical close coupling between the eye and the head (Bizzi et al. 1972; Freedman and Sparks 1997b; Hanes and McCollum 2006; Monteon et al. 2005; Oommen and Stahl 2005; Oommen et al. 2004; Zangemeister and Stark 1982). This separate head drive could involve areas such as M1, FEF, and/or SEF and link to brain stem areas controlling the head, bypassing the superior colliculus (SC), which we assume to be part of the neural pathway involved in driving gaze. In this view and consistent with previous studies, a common gaze drive would program the default head contribution to a given gaze shift, whereas the separate head drive could implement a more cognitive control strategy. Our results indicate that both of these drives might be influenced separately by attention.
Since our conclusions for a separate influence of attention on the head and eye latencies are based on a relative latency difference between the eye and the head, we cannot exclude the possibility that attention may influence the eye drive separately in addition to gaze rather than a separate head drive. However, we believe this is not likely the case. There is much independent evidence for a separate pathway for the head that is used during combined eye–head gaze shifts (Bizzi et al. 1972; Freedman and Sparks 1997b; Hanes and McCollum 2006; Oommen and Stahl 2005; Oommen et al. 2004; Zangemeister and Stark 1982). In contrast, although evidence has been shown for a saccade drive pathway from FEF to the brain stem that bypasses the SC, it appears that this pathway is not normally used by the brain, as evidenced by major deficits in saccade production when the SC is lesioned with moderate to little recovery, especially with respect to saccade latency (Albano and Wurtz 1982; Hanes et al. 2005; Mohler and Wurtz 1977; Schiller et al. 1980; Wurtz and Goldberg 1972). Therefore we believe the relative differences between the eye and the head latencies are due to a separate head drive rather than a separate saccade drive.
Alternatively, instead of a separate head drive involved in the cognitive modulation of head latencies, one could also imagine that the eye saccade is delayed through the gating of the OPNs, whereas the head movement is not suppressed by this inhibition and can thus start earlier (Gandhi and Sparks 2007), making use of only the common gaze drive without the need of an additional head drive. However, this does not explain the differences in head latency onset (relative to saccade onset) between the congruent and incongruent conditions. It could be that in addition to bypassing the OPNs, the head latency is shortened in the congruent condition by the previous presence of the cue. Indeed, Corneil et al. (2008) have shown activity related to the cue in neck muscle activity. One could imagine that the cue affects the neural activity in SC differently for different SOAs and that this difference percolates through to the neck muscles (since the head movement is not gated by OPNs), changing the time of movement initiation. The exact nature of this cue–SC interaction remains unknown, but our data indicate that the same stimulus parameters would affect different subjects differently. Given the small size of the differential effect observed, this hypothesis might be a plausible alternative to the above-suggested separate head control.
Functional implications
Our results suggest that attention may have an independent influence on head movements, i.e., separate from that on saccadic eye movements. The reason for this influence, however, remains unknown. One might speculate about the phylogenetic origin of this attentional influence on the head motor system. For example, it could be an evolutionary vestige from older species in which head movements are more prominent (e.g., due to a lower eye-in-head movement range). In this case, the head would act more like an eye in humans and thus attentional modulation of the head drive might be expected.
There may also be a functional role for attentional modulation of head movement in humans. It is well known that head movements are often initiated before eye movements in natural conditions (Land 1992; Pelz et al. 2001). Therefore it makes sense not only to quickly orient our eyes but also to purposefully direct our head to a salient target (i.e., a cued location). This is particularly true for large gaze shifts toward very eccentric targets that cannot be reached by movements of the eye alone. In this case, the head is needed and should be subject to attentional changes in a similar way as the eyes are (consistent with our findings of similar modulation of eye and head with attention). In addition, for larger saccades, volitional control should speed up the head movement to give the slower head motor system some advantage. To do so, the independent head drive has to be differently affected by attention than the gaze drive. This is in accordance with our findings.
Conclusions
We have shown that attention modulates head movements in two ways during combined eye–head gaze shifts. First, eye and head movement latencies were highly correlated, with head movement latencies showing the same pattern on attentional facilitation and IOR as saccade latencies, suggesting a common influence of attention. In addition, head relative to eye latencies revealed an additional influence of attention on the head motor system.
GRANTS
A. Z. Khan was supported by Canadian Institutes of Health Research. G. Blohm held a Marie Curie fellowship (European Union [EU]) and was supported by Fonds National de la Recherche Scientifique (FNRS, Belgium). R. M. McPeek was supported by National Eye Institute Grant EY-014885. This project was supported by the European Space Agency (EU), FNRS (Belgium), Interuniversity Attraction Poles (IAP) and PRODEX (Belgium), Action de Recherche Concertée (UC Louvain, Belgium), and Natural Sciences and Engineering Research Council of Canada.
Acknowledgments
We thank P. Daye for help with the experimental setup.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Abrams and Dobkin 1994a.Abrams RA, Dobkin RS. The gap effect and inhibition of return: interactive effects on eye movement latencies. Exp Brain Res 98: 483–487, 1994a. [DOI] [PubMed] [Google Scholar]
- Abrams and Dobkin 1994b.Abrams RA, Dobkin RS. Inhibition of return: effects of attentional cuing on eye movement latencies. J Exp Psychol Hum Percept Perform 20: 467–477, 1994b. [DOI] [PubMed] [Google Scholar]
- Albano and Wurtz 1982.Albano JE, Wurtz RH. Deficits in eye position following ablation of monkey superior colliculus, pretectum, and posterior-medial thalamus. J Neurophysiol 48: 318–337, 1982. [DOI] [PubMed] [Google Scholar]
- Beauchamp et al. 2001.Beauchamp MS, Petit L, Ellmore TM, Ingeholm J, Haxby JV. A parametric fMRI study of overt and covert shifts of visuospatial attention. Neuroimage 14: 310–321, 2001. [DOI] [PubMed] [Google Scholar]
- Bizzi et al. 1972.Bizzi E, Kalil RE, Morasso P. Two modes of active eye-head coordination in monkeys. Brain Res 40: 45–48, 1972. [DOI] [PubMed] [Google Scholar]
- Carpenter 1988.Carpenter RHS Movements of the Eyes. London: Pion, 1988.
- Cavanaugh and Wurtz 2004.Cavanaugh J, Wurtz RH. Subcortical modulation of attention counters change blindness. J Neurosci 24: 11236–11243, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen 2006.Chen LL Head movements evoked by electrical stimulation in the frontal eye field of the monkey: evidence for independent eye and head control. J Neurophysiol 95: 3528–3542, 2006. [DOI] [PubMed] [Google Scholar]
- Chen and Walton 2005.Chen LL, Walton MM. Head movement evoked by electrical stimulation in the supplementary eye field of the rhesus monkey. J Neurophysiol 94: 4502–4519, 2005. [DOI] [PubMed] [Google Scholar]
- Cicchini et al. 2008.Cicchini GM, Valsecchi M, De'Sperati C. Head movements modulate visual responsiveness in the absence of gaze shifts. Neuroreport 19: 831–834, 2008. [DOI] [PubMed] [Google Scholar]
- Corbetta et al. 1998.Corbetta M, Akbudak E, Conturo TE, Snyder AZ, Ollinger JM, Drury HA, Linenweber MR, Petersen SE, Raichle ME, Van Essen DC, Shulman GL. A common network of functional areas for attention and eye movements. Neuron 21: 761–773, 1998. [DOI] [PubMed] [Google Scholar]
- Corneil and Elsley 2005.Corneil BD, Elsley JK. Countermanding eye-head gaze shifts in humans: marching orders are delivered to the head first. J Neurophysiol 94: 883–895, 2005. [DOI] [PubMed] [Google Scholar]
- Corneil et al. 1999.Corneil BD, Hing CA, Bautista DV, Munoz DP. Human eye-head gaze shifts in a distractor task. I. Truncated gaze shifts. J Neurophysiol 82: 1390–1405, 1999. [DOI] [PubMed] [Google Scholar]
- Corneil and Munoz 1999.Corneil BD, Munoz DP. Human eye-head gaze shifts in a distractor task. II. Reduced threshold for initiation of early head movements. J Neurophysiol 82: 1406–1421, 1999. [DOI] [PubMed] [Google Scholar]
- Corneil et al. 2008.Corneil BD, Munoz DP, Chapman BB, Admans T, Cushing SL. Neuromuscular consequences of reflexive covert orienting. Nat Neurosci 11: 13–15, 2008. [DOI] [PubMed] [Google Scholar]
- Corneil et al. 2004.Corneil BD, Olivier E, Munoz DP. Visual responses on neck muscles reveal selective gating that prevents express saccades. Neuron 42: 831–841, 2004. [DOI] [PubMed] [Google Scholar]
- de Brouwer et al. 2002.de Brouwer S, Missal M, Barnes G, Lefèvre P. Quantitative analysis of catch-up saccades during sustained pursuit. J Neurophysiol 87: 1772–1780, 2002. [DOI] [PubMed] [Google Scholar]
- de Brouwer et al. 2001.de Brouwer S, Missal M, Lefèvre P. Role of retinal slip in the prediction of target motion during smooth and saccadic pursuit. J Neurophysiol 86: 550–558, 2001. [DOI] [PubMed] [Google Scholar]
- Deubel and Schneider 1996.Deubel H, Schneider WX. Saccade target selection and object recognition: evidence for a common attentional mechanism. Vision Res 36: 1827–1837, 1996. [DOI] [PubMed] [Google Scholar]
- Dorris et al. 2002.Dorris MC, Klein RM, Everling S, Munoz DP. Contribution of the primate superior colliculus to inhibition of return. J Cogn Neurosci 14: 1256–1263, 2002. [DOI] [PubMed] [Google Scholar]
- Elsley et al. 2007.Elsley JK, Nagy B, Cushing SL, Corneil BD. Widespread presaccadic recruitment of neck muscles by stimulation of the primate frontal eye fields. J Neurophysiol 98: 1333–1354, 2007. [DOI] [PubMed] [Google Scholar]
- Fecteau and Munoz 2005.Fecteau JH, Munoz DP. Correlates of capture of attention and inhibition of return across stages of visual processing. J Cogn Neurosci 17: 1714–1727, 2005. [DOI] [PubMed] [Google Scholar]
- Fecteau and Munoz 2006.Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci 10: 382–390, 2006. [DOI] [PubMed] [Google Scholar]
- Fischer et al. 1993.Fischer B, Weber H, Biscaldi M, Aiple F, Otto P, Stuhr V. Separate populations of visually guided saccades in humans: reaction times and amplitudes. Exp Brain Res 92: 528–541, 1993. [DOI] [PubMed] [Google Scholar]
- Freedman and Sparks 1997a.Freedman EG, Sparks DL. Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: evidence for a gaze displacement command. J Neurophysiol 78: 1669–1690, 1997a. [DOI] [PubMed] [Google Scholar]
- Freedman and Sparks 1997b.Freedman EG, Sparks DL. Eye–head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol 77: 2328–2348, 1997b. [DOI] [PubMed] [Google Scholar]
- Freedman et al. 1996.Freedman EG, Stanford TR, Sparks DL. Combined eye–head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol 76: 927–952, 1996. [DOI] [PubMed] [Google Scholar]
- Galiana and Guitton 1992.Galiana HL, Guitton D. Central organization and modeling of eye–head coordination during orienting gaze shifts. Ann NY Acad Sci 656: 452–471, 1992. [DOI] [PubMed] [Google Scholar]
- Gandhi and Sparks 2007.Gandhi NJ, Sparks DL. Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region. J Neurophysiol 98: 360–373, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg et al. 2006.Goldberg ME, Bisley JW, Powell KD, Gottlieb J. Saccades, salience and attention: the role of the lateral intraparietal area in visual behavior. Prog Brain Res 155: 157–175, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray 1977.Gray H Anatomy: Descriptive and Surgical. New York: Crown Publishers, 1977.
- Guitton 1992.Guitton D Control of eye–head coordination during orienting gaze shifts. Trends Neurosci 15: 174–179, 1992. [DOI] [PubMed] [Google Scholar]
- Guitton et al. 2003.Guitton D, Bergeron A, Choi WY, Matsuo S. On the feedback control of orienting gaze shifts made with eye and head movements. Prog Brain Res 142: 55–68, 2003. [DOI] [PubMed] [Google Scholar]
- Hanes and McCollum 2006.Hanes DA, McCollum G. Variables contributing to the coordination of rapid eye/head gaze shifts. Biol Cybern 94: 300–324, 2006. [DOI] [PubMed] [Google Scholar]
- Hanes et al. 2005.Hanes DP, Smith MK, Optican LM, Wurtz RH. Recovery of saccadic dysmetria following localized lesions in monkey superior colliculus. Exp Brain Res 160: 312–325, 2005. [DOI] [PubMed] [Google Scholar]
- Hoffman and Subramaniam 1995.Hoffman JE, Subramaniam B. The role of visual attention in saccadic eye movements. Percept Psychophys 57: 787–795, 1995. [DOI] [PubMed] [Google Scholar]
- Ignashchenkova et al. 2004.Ignashchenkova A, Dicke PW, Haarmeier T, Thier P. Neuron-specific contribution of the superior colliculus to overt and covert shifts of attention. Nat Neurosci 7: 56–64, 2004. [DOI] [PubMed] [Google Scholar]
- Jonides and Irwin 1981.Jonides J, Irwin DE. Capturing attention. Cognition 10: 145–150, 1981. [DOI] [PubMed] [Google Scholar]
- Kastner and Ungerleider 2000.Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci 23: 315–341, 2000. [DOI] [PubMed] [Google Scholar]
- Keller 1974.Keller EL Participation of the medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol 37: 316–332, 1974. [DOI] [PubMed] [Google Scholar]
- Keller 1977.Keller EL Control of saccadic eye movements by midline brain stem neurons. In: Control of Gaze by Brain Stem Neurons, edited by Baker R, Berthoz A. Amsterdam: Elsevier, 1977, p. 327–336.
- Klein 2000.Klein RM Inhibition of return. Trends Cogn Sci 4: 138–147, 2000. [DOI] [PubMed] [Google Scholar]
- Klier et al. 2001.Klier EM, Wang H, Crawford JD. The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci 4: 627–632, 2001. [DOI] [PubMed] [Google Scholar]
- Knight and Fuchs 2007.Knight TA, Fuchs AF. Contribution of the frontal eye field to gaze shifts in the head-unrestrained monkey: effects of microstimulation. J Neurophysiol 97: 618–634, 2007. [DOI] [PubMed] [Google Scholar]
- Kowler et al. 1995.Kowler E, Anderson E, Dosher B, Blaser E. The role of attention in the programming of saccades. Vision Res 35: 1897–1916, 1995. [DOI] [PubMed] [Google Scholar]
- Krauzlis 2004.Krauzlis RJ Recasting the smooth pursuit eye movement system. J Neurophysiol 91: 591–603, 2004. [DOI] [PubMed] [Google Scholar]
- Krauzlis 2005.Krauzlis RJ The control of voluntary eye movements: new perspectives. Neuroscientist 11: 124–137, 2005. [DOI] [PubMed] [Google Scholar]
- Land 1992.Land MF Predictable eye–head coordination during driving. Nature 359: 318–320, 1992. [DOI] [PubMed] [Google Scholar]
- Lefèvre and Galiana 1992.Lefèvre P, Galiana HL. Dynamic feedback to the superior colliculus in a neural network model of the gaze control-system. Neural Networks 5: 871–890, 1992. [Google Scholar]
- Luschei and Fuchs 1972.Luschei ES, Fuchs AF. Activity of brain stem neurons during eye movements of alert monkeys. J Neurophysiol 35: 445–461, 1972. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo et al. 2003.Martinez-Trujillo JC, Klier EM, Wang H, Crawford JD. Contribution of head movement to gaze command coding in monkey frontal cortex and superior colliculus. J Neurophysiol 90: 2770–2776, 2003. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo et al. 2004.Martinez-Trujillo JC, Medendorp WP, Wang H, Crawford JD. Frames of reference for eye–head gaze commands in primate supplementary eye fields. Neuron 44: 1057–1066, 2004. [DOI] [PubMed] [Google Scholar]
- Maylor and Hockey 1985.Maylor EA, Hockey R. Inhibitory component of externally controlled covert orienting in visual space. J Exp Psychol Hum Percept Perform 11: 777–787, 1985. [DOI] [PubMed] [Google Scholar]
- McPeek 2006.McPeek RM Incomplete suppression of distractor-related activity in the frontal eye field results in curved saccades. J Neurophysiol 96: 2699–2711, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek 2008.McPeek RM Reversal of a distractor effect on saccade target selection after superior colliculus inactivation. J Neurophysiol 99: 2694–2702, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPeek et al. 1999.McPeek RM, Maljkovic V, Nakayama K. Saccades require focal attention and are facilitated by a short-term memory system. Vision Res 39: 1555–1566, 1999. [DOI] [PubMed] [Google Scholar]
- Mohler and Wurtz 1977.Mohler CW, Wurtz RH. Role of striate cortex and superior colliculus in visual guidance of saccadic eye movements in monkeys. J Neurophysiol 40: 74–94, 1977. [DOI] [PubMed] [Google Scholar]
- Monteon et al. 2005.Monteon JA, Martinez-Trujillo JC, Wang H, Crawford JD. Cross-coupled adaptation of eye and head position commands in the primate gaze control system. Neuroreport 16: 1189–1192, 2005. [DOI] [PubMed] [Google Scholar]
- Moore and Fallah 2001.Moore T, Fallah M. Control of eye movements and spatial attention. Proc Natl Acad Sci USA 98: 1273–1276, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller et al. 2005.Muller JR, Philiastides MG, Newsome WT. Microstimulation of the superior colliculus focuses attention without moving the eyes. Proc Natl Acad Sci USA 102: 524–529, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oommen et al. 2004.Oommen BS, Smith RM, Stahl JS. The influence of future gaze orientation upon eye–head coupling during saccades. Exp Brain Res 155: 9–18, 2004. [DOI] [PubMed] [Google Scholar]
- Oommen and Stahl 2005.Oommen BS, Stahl JS. Amplitudes of head movements during putative eye-only saccades. Brain Res 1065: 68–78, 2005. [DOI] [PubMed] [Google Scholar]
- Pelz et al. 2001.Pelz J, Hayhoe M, Loeber R. The coordination of eye, head, and hand movements in a natural task. Exp Brain Res 139: 266–277, 2001. [DOI] [PubMed] [Google Scholar]
- Pierrot-Deseilligny et al. 2004.Pierrot-Deseilligny C, Milea D, Muri RM. Eye movement control by the cerebral cortex. Curr Opin Neurol 17: 17–25, 2004. [DOI] [PubMed] [Google Scholar]
- Posner 1980.Posner MI Orienting of attention. Q J Exp Psychol 32: 3–25, 1980. [DOI] [PubMed] [Google Scholar]
- Posner 1984.Posner MI, Cohen Y. Components of Visual Orienting. Hillsdale, NJ: Erlbaum, 1984.
- Posner et al. 1982.Posner MI, Cohen Y, Rafal RD. Neural systems control of spatial orienting. Philos Trans R Soc Lond B Biol Sci 298: 187–198, 1982. [DOI] [PubMed] [Google Scholar]
- Posner et al. 1985.Posner MI, Rafal RD, Choate LS, Vaughan J. Inhibition of return: neural basis and function. Cogn Neuropsychol 2: 211–228, 1985. [Google Scholar]
- Rafal et al. 1994.Rafal R, Egly R, Rhodes D. Effects of inhibition of return on voluntary and visually guided saccades. Can J Exp Psychol 48: 284–300, 1994. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz et al. 1996.Reuter-Lorenz PA, Jha AP, Rosenquist JN. What is inhibited in inhibition of return? J Exp Psychol Hum Percept Perform 22: 367–378, 1996. [DOI] [PubMed] [Google Scholar]
- Schiller et al. 1980.Schiller PH, True SD, Conway JL. Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol 44: 1175–1189, 1980. [DOI] [PubMed] [Google Scholar]
- Sparks et al. 2001.Sparks DL, Freedman EG, Chen LL, Gandhi NJ. Cortical and subcortical contributions to coordinated eye and head movements. Vision Res 41: 3295–3305, 2001. [DOI] [PubMed] [Google Scholar]
- Tanaka and Shimojo 1996.Tanaka Y, Shimojo S. Location vs feature: reaction time reveals dissociation between two visual functions. Vision Res 36: 2125–2140, 1996. [DOI] [PubMed] [Google Scholar]
- Taylor and Klein 1998.Taylor TL, Klein RM. Inhibition of return to color: a replication and nonextension of Law, Pratt, and Abrams (1995). Percept Psychophys 60: 1452–1456, 1998. [DOI] [PubMed] [Google Scholar]
- Thompson et al. 2005.Thompson KG, Biscoe KL, Sato TR. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci 25: 9479–9487, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu and Keating 2000.Tu TA, Keating EG. Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J Neurophysiol 84: 1103–1106, 2000. [DOI] [PubMed] [Google Scholar]
- van der Steen et al. 1986.van der Steen J, Russell IS, James GO. Effects of unilateral frontal eye-field lesions on eye-head coordination in monkey. J Neurophysiol 55: 696–714, 1986. [DOI] [PubMed] [Google Scholar]
- Vliegen et al. 2004.Vliegen J, Van Grootel TJ, Van Opstal AJ. Dynamic sound localization during rapid eye–head gaze shifts. J Neurosci 24: 9291–9302, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vliegen et al. 2005.Vliegen J, Van Grootel TJ, Van Opstal AJ. Gaze orienting in dynamic visual double steps. J Neurophysiol 94: 4300–4313, 2005. [DOI] [PubMed] [Google Scholar]
- Walton et al. 2007.Walton MM, Bechara B, Gandhi NJ. Role of the primate superior colliculus in the control of head movements. J Neurophysiol 98: 2022–2037, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton et al. 2008.Walton MM, Bechara B, Gandhi NJ. Effect of reversible inactivation of superior colliculus on head movements. J Neurophysiol 99: 2479–2495, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardak et al. 2006.Wardak C, Ibos G, Duhamel JR, Olivier E. Contribution of the monkey frontal eye field to covert visual attention. J Neurosci 26: 4228–4235, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtz and Goldberg 1972.Wurtz RH, Goldberg ME. Activity of superior colliculus in behaving monkey. IV. Effects of lesions on eye movements. J Neurophysiol 35: 587–596, 1972. [DOI] [PubMed] [Google Scholar]
- Zangemeister and Stark 1982.Zangemeister WH, Stark L. Types of gaze movement: variable interactions of eye and head movements. Exp Neurol 77: 563–577, 1982. [DOI] [PubMed] [Google Scholar]