Abstract
The emergence and spread of drug resistant malaria parasites are an important factor contributing to the global resurgence of malaria, demonstrating the essence of drug resistance surveillance in endemic areas. In the malarious border regions of Yunnan Province, China, we have selected three study sites to monitor in vitro and in vivo resistance of Plasmodium falciparum parasites to chloroquine (CQ) from 1981 to 2006. In vitro studies using the microtest clearly showed high-degree of CQ resistance in the early 1980s, when CQ was replaced by artemether monotherapy for falciparum malaria. In subsequent in vitro surveys performed in the early 1990s and 2003–2004, we found reductions in both the concentrations inhibiting 50% parasite growth (IC50s) and the percentage of resistant parasites at all study sites, although the degrees of the reduction varied among sites. Even though amodiaquine has never been used in this area, there were consistently high levels of resistance to this drug, confirming cross resistance between CQ and amodiaquine. In vivo clinical studies were consistent with the results of the in vitro assays. The overall rate of resistant clinical cases decreased from 97% in 1981–1983 to 40% in 2005–2006. Collectively, whereas a general trend of reduction in CQ resistance was observed in Yunnan, variations among sites existed in this relatively small area, probably as the result of both geographical heterogeneity of malaria epidemiology in Yunnan and different levels of CQ resistance in neighboring countries.
Keywords: drug resistance, chloroquine, microtest, clinical monitoring, longitudinal surveillance
1. Introduction
With 300–500 million cases and more than one million deaths per year, malaria remains one of the most important public health problems in the world, especially in tropical and subtropical regions (Snow et al., 2005). The worldwide emergence and spread of multidrug resistant (MDR) Plasmodium falciparum parasites have become a pressing issue in malaria control. Currently, artemisinins are the only group of antimalarials without evident clinical resistance, but poor clinical responses and reduced in vitro drug sensitivity have already been reported in some malarious areas (Luxemburger et al., 1998; Gogtay et al., 2000; Sahr et al., 2001; Yang et al., 2003). To help prevent and delay resistance development, artemisinin-based combinatory therapy (ACT) is being adopted globally for malaria chemotherapy (Ashley and White, 2005). In order to prolong the life span of available antimalarial drugs, regional malaria drug policies should be based on comprehensive knowledge of drug sensitivity, which requires close surveillance on drug resistance.
In vivo and in vitro methods are the two basic ways of drug resistance detection, which ideally should be performed in parallel. In vivo studies provide essential information regarding the clinical efficacy of antimalarial drugs in a population, while in vitro tests determine the intrinsic drug resistance properties of the parasite populations. Of the traditional in vitro radioisotope assay and the World Health Organization (WHO) schizont maturation assay, the latter is probably the most widely-used for in vitro studies of fresh clinical samples without requiring special equipment (Rieckmann et al., 1978; Desjardins et al., 1979). Recently, the elucidation of the molecular mechanism of drug resistance has provided another convenient tool for epidemiological surveys of drug resistance in large parasite populations. For example, a single mutation K76T in the P. falciparum chloroquine (CQ) resistance transporter gene (pfcrt) is widely used as a reliable marker for CQ-resistance in epidemiological studies (Djimde et al., 2001; Wongsrichanalai et al., 2002). Taken together, an integrated scheme to precisely determine the in vivo treatment response parameters after drug treatment and in vitro dose responses of fresh parasite isolates may best depict the drug resistance scenario of a parasite population and detect resistant parasites (Noedl, 2005).
In the Greater Mekong Sub-Region, which includes Thailand, Laos, Cambodia, Vietnam, Myanmar, and Yunnan Province of China, malaria transmission occurs mainly in forested and hilly areas, and is closely linked to social, environmental and political problems. MDR P. falciparum is found in all these countries and associated with high mortality rate and treatment costs. In China, falciparum malaria is concentrated in two subtropical provinces, Yunnan and Hainan. Widespread appearance of CQ resistance in P. falciparum has resulted in its withdrawal from treating falciparum malaria in China in the early 1980s. Since then, sporadic surveys of CQ resistance carried out in these two malarious provinces noticed declines in P. falciparum CQ resistance (Liu et al., 2005), which is consistent with findings in other P. falciparum-endemic areas of the world (Kublin et al., 2003; Mita et al., 2003, 2004). Malaria in Yunnan is perennial and mainly distributed in six districts (Dehong, Baoshan, Lincang, Xishuangbanna, Pu’er/Simao, and Honghe), mostly near areas bordering three malaria-endemic countries, Myanmar, Laos, and Vietnam. Since malaria incidence is heterogeneous and drug policies are not uniform in these neighboring countries, the scenario of CQ resistance in Yunnan might be quite different. Indeed, our recent molecular survey indicated that the PfCRT K76T mutation still had over 90% prevalence in Yunnan (Yang et al., 2007), much higher than in Hainan province despite almost simultaneous withdrawals of CQ in these two provinces (Wang et al., 2005), suggesting that resistance surveillance in these border areas requires special attention. Furthermore, with suspected cross resistance between CQ and piperaquine, a drug commonly used in ACT, a more comprehensive knowledge of CQ resistance is needed to guide the local drug policy. Here, we have conducted longitudinal surveillance in three malarious sites (five counties) in Yunnan using both in vitro and in vivo tests to determine the dynamics of CQ resistance.
2. Materials and Methods
2.1. Surveillance sites and time
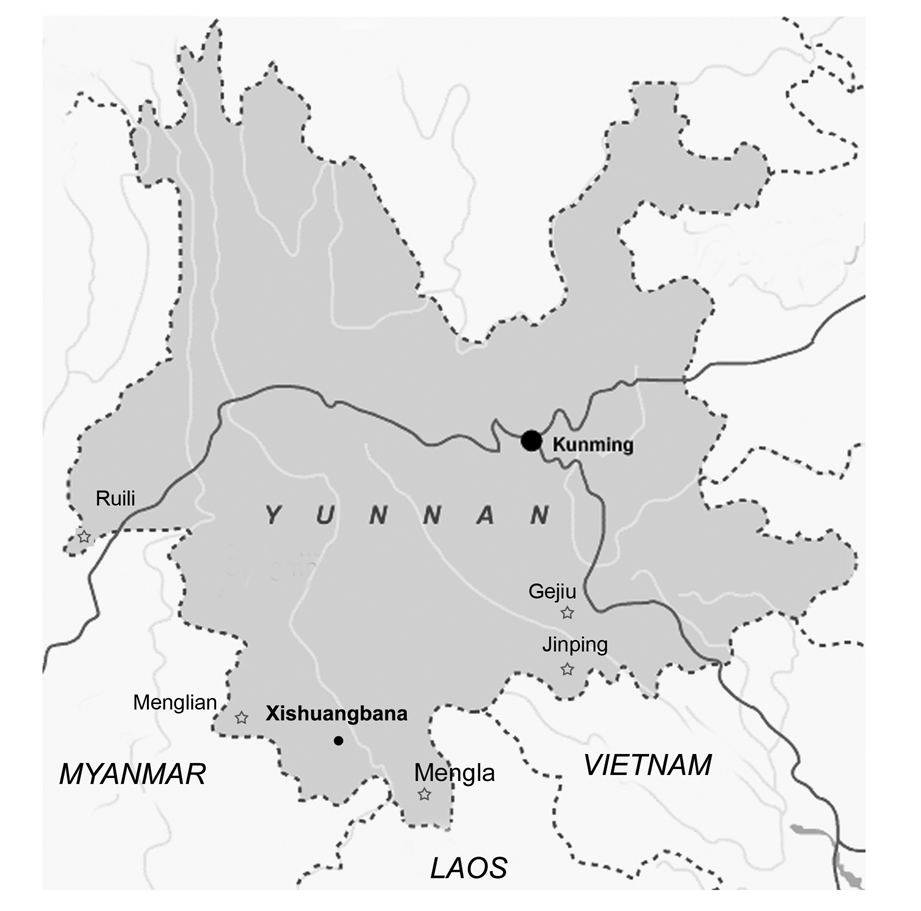
We have selected five counties (Ruili, Mengla, Menglian, Gejiu, and Jinping) as our surveillance sites to represent geographical distributions of the major malaria-endemic districts (Fig. 1). These sites have remained as the strategic drug monitoring sites for the Yunnan Institute of Parasitic Diseases (YIPD). Surveillance at the western site (Ruili) was conducted in 1982, 1992, and 2003, and malaria cases were from Ruili and five surrounding counties or cities. In addition, some imported cases from a neighboring district of Myanmar were also used for in vitro assays of CQ resistance. At the southern site (Mengla and Menglian), located at the China-Lao and China-Myanmar borders, surveillance was conducted in 1981, 1991, 2002, and 2004, and the malaria cases also included those from two surrounding areas (Jinghong city and Jiangcheng county). The southeastern site (Gejiu and Jinping) are located near the China-Vietnam border where surveillance was carried out in 1993, 2003, 2005 and 2006. Malaria cases at the southeastern sites also included those from Honghe and Hekou counties.
Fig. 1.
The map of Yunnan Province with the stars indicating the locations of the western (Ruili), southern (Menglian and Mengla) and southeastern (Gejiu and Jinping) sites.
2.2. Patients and parasite samples
Patients with acute, uncomplicated P. falciparum malaria were recruited to this study after obtaining full informed consent/assent. The human subject protocol involved in this project was reviewed and approved by the institution review board at YIPD before the year 1999 and Yunnan Provincial Bureau of Public Health after year 2000. Infection by P. falciparum was diagnosed based on microscopic examinations of thick and thin blood smears, and only those samples with >2000 asexual parasites/µl of blood were used for in vitro drug assays. After verifying that the patients had not taken antimalarial drugs or antibiotics within the preceding two weeks and lacked detectable levels of 4-aminoquinolines in their urine samples using an earlier reported detection method (Wilson and Edeson, 1954), 1 ml of venous blood was drawn for in vitro drug assay. For consistency, the patient recruitment criteria remained the same throughout the duration of the study.
2.3. In vitro drug test
The microtiter plates for CQ and amodiaquine were provided by WHO, which included two-fold serial dilutions of the drugs ranging from 1.0 to 64 pmol/well for CQ and from 0.25 to 16 pmol/well for amodiaquine. In vitro drug assays were performed using the Rieckmann’s microtest (1978) according to the instructions by WHO (http://www.who.int/drugresistance /malaria/en/markiii.pdf). Blood from patients with >80,000 parasites/µl was diluted with fresh type O blood cells in a complete medium to <40,000 parasites/µl, and 50 µl of the diluted blood were dispensed into each well. Parasites were cultured for 24–36 h at 37°C and thin smears were examined. Only samples with >20% parasite matured to schizonts in the control wells were used for data analysis. According to the standard from WHO, parasites able to grow at ≥8 pmol of CQ or ≥4 pmol of amodiaquine were considered resistant to these drugs, respectively.
2.4. Clinical monitoring of in vivo CQ resistance
Clinical monitoring of in vivo CQ resistance was carried out at two sites in 1981–1983 and at three sites in 2005–2006. For patients participating in in vivo studies, clinical examinations were done on admission and every 12 h while the patients stayed in hospital for the next 7 days. CQ (Shanghai Pharmaceutical Company, Shanghai, China, 150 mg base per tablet) was given to adult patients at a total dose of 1500 mg over 3 days (600 mg on day 0, followed by 300 mg daily for two days). Children were treated with reduced doses following a general guideline of total doses of 25 mg/kg body weight (10 mg/kg on day 0 and 1, 5 mg/kg on day 3). Blood samples for parasitological examination and parasite counts were taken every 12 h until two sequential slides were negative, then daily until day 7. Then patients were discharged from the hospital and further parasitological examinations were performed on days 14, 21, and 28.
In addition, the treatment response was assessed on the basis of parasite clearance from the blood as assessed by the microscopic examination of 100 fields of the thick blood film. The primary endpoint was the 28-day cure rate. For the study performed in 2005–2006, treatment outcome was also categorized according to the latest WHO definitions for late parasitological failure, late clinical failure, and adequate clinical and parasitological response (ACPR) (WHO, 2003). The clinical sensitivity of the parasites to CQ was categorized based on the WHO classification system (WHO, 1973). Susceptible parasites (S) were defined if asexual stage parasites had a ≥75% reduction within 48 h after drug treatment and slides were negative for two consecutive days within 7 days, and no recrudescence occurred within the 28 day follow-up period. Resistant responses (RI–RIII) were categorized according to the WHO criteria: RI = ≥75% reduction in parasitemia within 48 h after initiation of therapy and parasite clearance within seven days but recrudescence within 28 days; RII = ≥75% reduction of initial parasitemia within 48 h of drug treatment, but without parasite clearance within 7 days; RIII = parasitemia ≥ 25% of initial parasite count at 48 h after initiation of therapy. Patients with resistant parasites (RI–RIII) were subsequently given a standard dosage of intramuscular artemether injections following the Ministry of Health recommended five-day treatment regimen (160 mg on day 0, and 80 mg daily for four days).
2.5. Statistical analysis
The 50% inhibitory concentrations (IC50) and concentrations completely inhibitory for parasite maturation (CMIC) were calculated by using the linear regression analysis and compared by Student’s t test.
3. Results
3.1. Surveillance of CQ resistance using the WHO in vitro microtest
We systematically monitored the susceptibility of clinical samples of P. falciparum to CQ using the WHO in vitro microtest at three study sites representing the major malaria endemic regions in Yunnan province for over 20 years. A total of 435 clinical samples were collected during the nine studies and 73% (319) were successfully assayed for CQ resistance (Table 1). Whereas there was a general trend of reduction in IC50 and CMIC at the three study sites, the levels of CQ-sensitivity among these parasite populations were different. In western Yunnan, we have carried out three surveys with a time interval of ~10 years. Compared with each previous survey, statistically significant decreases in the IC50 and CMIC values were observed (P < 0.05). The IC50 and CMIC values in 1993 had a 27 and 29% reduction from 1982, respectively, while these values in 2003 were reduced from 1993 by 30 and 61%, respectively (Table 1). The parasite population in 1982 was very resistant to CQ with a mean IC50 of 480 nM. Despite the significant drop in resistance, parasites still had a mean IC50 of 246 nM in 2003. However, the proportions of resistant parasites were not significantly different among these three surveys. At the southern and southeastern sites, CQ resistance followed a similar trend of decrease. During the last survey at each site, the resistant parasites accounted for 83, 69, and 53% of the samples from the western, southern, and southeastern sites, respectively (Table 1).
Table 1.
Longitudinal monitoring of CQ sensitivity of P. falciparum strains in three regions of Yunnan Province using the in vitro microtest
| Study sites | Year | Case No. | IC50 (nM) | CMIC (nM)* | Resistance rate (%) |
|---|---|---|---|---|---|
| Western | 1982 | 22 | 480±252a | 2858±1210a | 100a |
| 1993 | 52 | 352±186b | 1660±1032b | 96a | |
| 2003 | 36 | 246±132c | 652±410c | 83a | |
| Southern | 1981 | 39 | 340±201a | 2240±1151a | 97a |
| 1992# | 63 | 220±123b | 1416±987b | 94a | |
| 2002 | 26 | 183±0.81c | 678±421c | 82a | |
| 2004 | 29 | 117±0.31c | 625±411c | 69b | |
| 1993 | 38 | 272±110a | 904±538a | 79a | |
| Southeastern | 2003 | 14 | 151±43b | 476±206b | 53b |
CMIC - concentration completely inhibiting parasite maturation.
Values followed by a different letter in the same column within each study site were statistically different at P = 0.05 level.
In 1992 and 2004, we tested 30 P. falciparum clinical samples each at the southern site for the sensitivity of the parasites to amodiaquine, an aminoquinoline drug structurally similar to CQ (Table 2). The results showed that in the early 1990s, P. falciparum parasites were highly resistant to amodiaquine, while the survey performed in 2004 showed a similar trend of decrease in resistance, reflected in the reduced IC50 values and rate of resistant isolates. However, 77% of the parasites remained resistant to this drug in 2004, suggesting that amodiaquine should not be deployed in this area for treating falciparum malaria.
Table 2.
Monitoring of amodiaquine sensitivity of P. falciparum strains at the southern site of Yunnan Province using the in vitro microtest
| Year | Case # | IC50 (nM) | CMIC (nM) | Resistance rate (%) |
|---|---|---|---|---|
| 1992 | 30 | 104±42a | 584±271a | 100a |
| 2004 | 30 | 88±38b | 392±134 b | 77 b |
Values followed by a different letter in the same column within each study site were statistically different at P = 0.05 level.
3.2. In vivo efficacy of CQ treatment of uncomplicated P. falciparum cases
In 1981–1983, we completed in vivo efficacy studies of CQ in 50 and 28 patients with uncomplicated P. falciparum malaria at the southern and western sites, respectively. The results showed that 97% of the parasites were CQ-resistant. CQ treatment only cured 2 of the 78 cases, whereas 34, 12 and 30 cases belonged to CQ resistance categories RI, RII, and RIII, respectively. Consistent with the high in vitro IC50s of parasites from these two study sites, 38% of the clinical cases displayed RIII resistance, which required immediate treatment with artemether after 48 h of CQ treatment. All CQ resistant parasites responded well to artemether treatment with no detectable resistance. About 25 years had passed since the withdrawal of CQ from treating falciparum malaria, we re-evaluated the clinical efficacy of CQ in 2005–2006. A total of 32 uncomplicated P. falciparum cases were recruited from these three sites. Almost 60% (19/32) of the cases were susceptible to CQ treatment without recrudescence within 28 days of drug administration. Among the CQ-resistant parasites, 69% had clinical clearance of parasitemia and fever within 7 days of drug treatment, but recrudescence was detected within the 28-day observation period. A total of nine cases were considered late parasitological failures and three cases late clinical failures. The crude ACPR rate was 28/32 (88%) by day 7 but decreased to 20/32 (63%) by day 28. While a similar trend of decline of CQ resistance was observed for the three study sites, the degrees of decline were slightly different among the sites (P>0.05).
4. Discussion
The pace of development and spread of drug resistance in P. falciparum, depending on numerous factors such as drug selective pressure, endemicity, and host immunity, varies from region to region. Yunnan is one of the most malarious regions in China, and connects with several malarious countries of the Greater Mekong Sub-Region. Complex geographic, demographic, and climatic conditions are responsible for the continuous presence of malaria in some places and focal outbreaks in others. For effective chemotherapy, the drug policy has been changing in response to the development of drug resistance, but could not be fully executed in border areas. Especially, malaria occurs in poor, remote, minority communities of Yunnan, which are difficult to carry out drug resistance surveillance. Therefore, we used the WHO microtest for in vitro drug assays in representative malarious areas in Yunnan under the field conditions and obtained satisfactory results with a >70% success rate. Two independent in vivo efficacy studies further corroborated the results from the in vitro assays. Collectively, these results showed that CQ resistance in P. falciparum populations in Yunnan has undergone significant reductions, but large proportions of the parasites remained CQ resistant.
Since the report of CQ-resistance almost everywhere in the world, CQ has been generally abandoned in treating malaria. The alleviation of the drug selective pressure has resulted in partial restoration of CQ sensitivity (Kublin et al., 2003; Mita et al., 2003; Wang et al., 2005; Liu et al., 2005), but this process appears to vary significantly between parasite populations. It is considered that the mutations in pfcrt incur a fitness cost to the parasites, and the recent recovery of CQ sensitivity in Malawi is likely due to expansion of wild type allele rather than the back mutation in pfcrt (Mita et al., 2004). In spite of the close geographical proximity and similar drug policies between the two most malarious provinces in China, the falciparum populations in Hainan and Yunnan were quite different. Surveys done in the early 1980s have detected high levels of CQ resistance in these two provinces. In the Hainan island, in vitro assays demonstrated a drop in the rate of CQ-resistant P. falciparum from 98% in 1981 to 61% in 1991 and 26.7% in 1997, which corresponded to a decrease of in vivo resistance rate from 84% in 1981 to 40% in 1991 and 18% in 1997 (Liu et al., 1995, 2005). While CQ resistance has also been declining in Yunnan, >50% of the parasites still showed CQ resistance by in vitro assays in 2003–2004, and ~40% of the parasites displayed in vivo clinical resistance to CQ in 2005–2006 (Table 1, 3). Among the resistant cases, 12% were early clinical failures. This result is further corroborated by our recent molecular analysis, which showed that >78% of P. falciparum populations in southern and western Yunnan still retained the K76T mutation in pfcrt, the major genetic determinant of CQ (Yang et al., 2007). Factors responsible for the maintenance of high-degree CQ-resistance in Yunnan may include sustained CQ pressure from treating sympatric P. vivax malaria and gene flow from neighboring malaria-endemic countries.
Table 3.
Clinical resistance of P. falciparum field isolates to CQ in Yunnan.
| Study sites | Years | Case # | Resistance degree |
Resistance rate (%) | |||
|---|---|---|---|---|---|---|---|
| S | RI | RII | RIII | ||||
| Western | 1981–83 | 28 | 0 | 10 | 5 | 13 | 100 |
| Southern | 50 | 2 | 24 | 7 | 17 | 96 | |
| Total | 78 | 2 | 34 | 12 | 30 | 97 | |
| Western | 2005–06 | 12 | 6 | 5 | 1 | 0 | 50 |
| Southern | 9 | 6 | 3 | 0 | 0 | 33 | |
| Southeastern | 11 | 7 | 1 | 0 | 3 | 36 | |
| Total | 32 | 19 | 9 | 1 | 3 | 41 | |
Within the malaria-endemic areas of Yunnan, the P. falciparum populations are heterogeneous in term of the degree of CQ resistance. The most CQ-resistant P. falciparum population is located in western Yunnan bordering Myanmar, followed by the southern region bordering Myanmar and Laos. The lowest level of CQ resistance was observed in southeastern region bordering Vietnam. To some extent, this reflects well the drug resistance situations in these neighboring countries, which have different malaria drug policies. CQ has been abandoned in treating falciparum malaria in China in late 1970s, but remained as the first-line drug treating uncomplicated falciparum malaria in Laos and Myanmar until the policy changes to ACT in 2005 (Smithuis et al., 2004; Mayxay et al., 2007b). In numerous surveys carried out within the last decade in Laos, significant in vitro resistance to CQ has been observed and levels of treatment failure reached unacceptable levels (Pillai et al., 2001; Guthmann et al., 2002; Berens et al., 2003; Mayxay et al., 2007a). In the area immediately bordering Yunnan, high-grade CQ resistance was also prevalent (Yang et al., 1997; Dittrich et al. 2005). Less frequent surveys done in Myanmar had similar findings (Ejov et al., 1999; Wongsrichanalai et al., 2001). A more recent clinical trial aimed to evaluate the efficacy of CQ in Kachin state bordering western Yunnan showed high levels of CQ resistance with 41% early treatment failures (Smithuis et al., 2004). In contrast, partially due to the earlier withdrawal of CQ from malaria therapy, CQ-resistance was low in Vietnam (Huong et al., 2001). Genetic exchange and spread of resistant genotypes from neighboring countries may be a major factor contributing to the heterogeneity of CQ resistance in different falciparum populations in Yunnan. The increasing human population movement across and along the border areas and escalating numbers of imported malaria cases in Yunnan undoubtedly have enhanced the mixing of parasite populations. In addition, the malaria endemicity is also drastically different between these study sites with the western region being the most malaria-endemic and the southeastern region being the least malaria-endemic. The higher transmission intensity in western Yunnan may have also facilitated genetic mixing of the parasite populations and assisting the maintenance of higher levels of CQ resistance in this region. This suggests that for more effective malaria control and management of multidrug resistance in the Greater Mekong Sub-Region, closer resistance surveillance needs to be carried out at the multinational level and drug policies in different countries must be coordinated.
An important matter to consider in areas of MDR is not to deploy structurally similar drugs, as this often results in fast appearance of resistance due to genetically determined cross resistance. A prominent example is cross resistance between CQ and amodiaquine (Holmgren et al., 2006). Consistently, we have also observed high-grade resistance to amodiaquine in Yunnan even though this drug has never been used in this region for malaria control. In malaria-endemic areas of China, another quinoline drug, piperaquine has been used extensively in the light of CQ resistance and widespread resistance to this drug has been observed (Yang et al., 1995). While in this area resistance to CQ and amodiaquine is highly correlated, cross-resistance between CQ and piperaquine has not been clearly established (Basco and Ringwald, 2003). Piperaquine, in combination with artemisinin-derivatives, is being widely deployed in many areas of CQ resistance (Davis et al., 2005). Though clinical trials have proven that piperaquine-dihydroartemisinin are highly effective for treating uncomplicated falciparum and vivax malaria (Myint et al., 2007), the possibility of cross-resistance between CQ and piperaquine needs to be evaluated so that the full effect of ACT for slowing down and preventing development of resistance to artemisinin-related drugs can be achieved.
Acknowledgements
We would like to thank grant supports from the WHO-TDR (900098) and NIAID, National Institutes of Health (1R01AI075429-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashley EA, White NJ. Artemisinin-based combinations. Curr. Opin. Infect. Dis. 2005;18:531–536. doi: 10.1097/01.qco.0000186848.46417.6c. [DOI] [PubMed] [Google Scholar]
- Basco LK, Ringwald P. In vitro activities of piperaquine and other 4-aminoquinolines against clinical isolates of Plasmodium falciparum in Cameroon. Antimicrob. Agents Chemother. 2003;47:1391–1394. doi: 10.1128/AAC.47.4.1391-1394.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens N, Schwoebel B, Jordan S, Vanisaveth V, Phetsouvanh R, Christophel EM, Phompida S, Jelinek T. Plasmodium falciparum: correlation of in vivo resistance to chloroquine and antifolates with genetic polymorphisms in isolates from the south of Lao PDR. Trop. Med. Int. Health. 2003;8:775–782. doi: 10.1046/j.1365-3156.2003.01099.x. [DOI] [PubMed] [Google Scholar]
- Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine: a resurgent antimalarial drug. Drugs. 2005;65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- Desjardins RE, Canfield CJ, Haynes JD, Chulay JD. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich S, Alifrangis M, Stohrer JM, Thongpaseuth V, Vanisaveth V, Phetsouvanh R, Phompida S, Khalil IF, Jelinek T. Falciparum malaria in the north of Laos: the occurrence and implications of the Plasmodium falciparum chloroquine resistance transporter (pfcrt) gene haplotype SVMNT. Trop. Med. Int. Health. 2005;10:1267–1270. doi: 10.1111/j.1365-3156.2005.01514.x. [DOI] [PubMed] [Google Scholar]
- Djimde A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV, Coulibaly D. A molecular marker for chloroquine-resistant falciparum malaria. N. Engl. J. Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- Gogtay NJ, Kadam VS, Karnad DR, Kanbur A, Kamtekar KD, Kshirsagar NA. Probable resistance to parenteral artemether in Plasmodium falciparum: case reports from Mumbai (Bombay), India. Ann. Trop. Med. Parasitol. 2000;94:519–520. doi: 10.1080/00034983.2000.11813571. [DOI] [PubMed] [Google Scholar]
- Guthmann JP, Kasparian S, Phetsouvanh R, Nathan N, Garcia M, Phompida S, Brockman A, Gastellu M, Legros D. The efficacy of chloroquine for the treatment of acute, uncomplicated, Plasmodium falciparum malaria in Laos. Am. J. Trop. Med. Hyg. 2002;96:553–557. doi: 10.1179/000349802125001654. [DOI] [PubMed] [Google Scholar]
- Ejov MN, Tun T, Aung S, Sein K. Response of falciparum malaria to different antimalarials in Myanmar. Bull. WHO. 1999;77:244–249. [PMC free article] [PubMed] [Google Scholar]
- Holmgren G, Gil JP, Ferreira PM, Veiga MI, Obonyo CO, Bjorkman A. Amodiaquine resistant Plasmodium falciparum malaria in vivo is associated with selection of pfcrt 76T and pfmdr1 86Y. Infect. Genet. Evol. 2006;6:309–314. doi: 10.1016/j.meegid.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Huong NM, Hewitt S, Davis TM, Dao LD, Toan TQ, Kim TB, Hanh NT, Phuong VN, Nhan DH, Cong LD. Resistance of Plasmodium falciparum to antimalarial drugs in a highly endemic area of southern VietNam: a study in vivo and in vitro. Trans. R.Soc. Trop. Med. Hyg. 2001;95:325–329. doi: 10.1016/s0035-9203(01)90254-8. [DOI] [PubMed] [Google Scholar]
- Kublin JG, Cortese JF, Njunju EM, Mukadam RA, Wirima JJ, Kazembe PN, Djimde AA, Kouriba B, Taylor TE, Plowe CV. Reemergence of chloroquine-sensitive Plasmodium falciparum malaria after cessation of chloroquine use in Malawi. J. Infect. Dis. 2003;187:1870–1875. doi: 10.1086/375419. [DOI] [PubMed] [Google Scholar]
- Liu DQ, Feng XP, Yang HL, Lin SG, Chen WJ, Yang PF. Fluctuation in the resistance of Plasmodium falciparum to chloroquine in China. Chin. J. Parasitol. Parasitic Dis. 2005;23:27–31. [PubMed] [Google Scholar]
- Liu DQ, Liu RJ, Ren DX, Gao DQ, Zhang CY, Qui CP, Cai XZ, Ling CF, Song AH, Tang X. Changes in the resistance of Plasmodium falciparum to chloroquine in Hainan, China. Bull. WHO. 1995;73:483–486. [PMC free article] [PubMed] [Google Scholar]
- Luxemburger C, Brockman A, Silamut K, Nosten F, van Vugt M, Gimenez F, Chongsuphajaisiddhi T, White NJ. Two patients with falciparum malaria and poor in vivo responses to artesunate. Trans. R. Soc. Trop. Med. Hyg. 1998;92:668–669. doi: 10.1016/s0035-9203(98)90807-0. [DOI] [PubMed] [Google Scholar]
- Mayxay M, Nair S, Sudimack D, Imwong M, Tanomsing N, Pongvongsa T, Phompida S, Phetsouvanh R, White NJ, Anderson TJC, Newton PN. Combined Molecular and clinical assessment of Plasmodium falciparum antimalarial drug resistance in the Lao People’s Democratic Republic (Laos) Am. J. Trop. Med. Hyg. 2007a;77:36–43. [PubMed] [Google Scholar]
- Mayxay M, Pongvongsa T, Phompida S, Phetsouvanh R, White NJ, Newton PN. Diagnosis and management of malaria by rural community health providers in the Lao People’s Democratic Republic (Laos) Trop. Med. Int. Health. 2007b;12:540–546. doi: 10.1111/j.1365-3156.2007.01820.x. [DOI] [PubMed] [Google Scholar]
- Mita T, Kaneko A, Lum JK, Bwijo B, Takechi M, Zungu IL, Tsukahara T, Tanabe K, Kobayakawa T, Björkman A. Recovery of chloroquine sensitivity and low prevalence of the Plasmodium falciparum chloroquine resistance transporter gene mutation K76T following the discontinuance of chloroquine use in Malawi. Am. J. Trop. Med. Hyg. 2003;68:413–415. [PubMed] [Google Scholar]
- Mita T, Kaneko A, Lum JK, Zungu IL, Tsukahara T, Eto H, Kobayakawa T, Björkman A, Tanabe K. Expansion of wild type allele rather than back mutation in pfcrt explains the recent recovery of chloroquine sensitivity of Plasmodium falciparum in Malawi. Mol. Biochem. Parasitol. 2004;135:159–163. doi: 10.1016/j.molbiopara.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Myint HY, Ashley EA, Day NP, Nosten F, White NJ. Efficacy and safety of dihydroartemisinin-piperaquine. Trans. R. Soc. Trop. Med. Hyg. 2007;101:858–866. doi: 10.1016/j.trstmh.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Noedl H. Artemisinin resistance: how can we find it? Trends Parasitol. 2005;21:404–405. doi: 10.1016/j.pt.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Pillai DR, Labbe A-C, Vanisaveth V, Hongvangthong B, Pomphida S, Inkathone S, Zhong K, Kain KC. Plasmodium falciparum malaria in Laos: chloroquine treatment outcome and predictive value of molecular markers. J. Infect. Dis. 2001;183:789–795. doi: 10.1086/318836. [DOI] [PubMed] [Google Scholar]
- Rieckmann KH, Campbell GH, Sax LJ. Drug sensitivity of Plasmodium falciparum.An in vitro micro technique. Lancet i. 1978:22–23. doi: 10.1016/s0140-6736(78)90365-3. [DOI] [PubMed] [Google Scholar]
- Sahr F, Willoughby VR, Gbakima AA, Bockarie MJ. Apparent drug failure following artesunate treatment of Plasmodium falciparum malaria in Freetown, Sierra Leone: four case reports. Ann. Trop. Med. Parasitol. 2001;95:445–449. doi: 10.1080/00034980120072284. [DOI] [PubMed] [Google Scholar]
- Smithuis F, Shahmanesh M, Kyaw MK, Savran O, Lwin S, White NJ. Comparison of chloroquine, sulfadoxine/pyrimethamine, mefloquine and mefloquine-artesunate for the treatment of falciparum malaria in Kachin State, North Myanmar. Trop.Med. Int. Health. 2004;9:1184–1190. doi: 10.1111/j.1365-3156.2004.01323.x. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–217. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socheat D, Denis MB, Fandeur T, Zhang Z, Yang H, Xu J, Zhou X, Phompida S, Phetsouvanh R, Lwin S, Lin K, Win T, Than SW, Htut Y, Prajakwong S, Rojanawatsirivet C, Tipmontree R, Vijaykadga S, Konchom S, Cong le D, Thien NT, Thuan le K, Ringwald P, Schapira A, Christophel E, Palmer K, Arbani PR, Prasittisuk C, Rastogi R, Monti F, Urbani C, Tsuyuoka R, Hoyer S, Otega L, Thimasarn K, Songcharoen S, Meert JP, Gay F, Crissman L, Cho-Min-Naing Chansuda W, Darasri D, Indaratna K, Singhasivanon P, Chuprapawan S, Looareesuwan S, Supavej S, Kidson C, Baimai V, Yimsamran S, Buchachart K. Mekong malaria. II. Update of malaria, multi-drug resistance and economic development in the Mekong region of Southeast Asia. Southeast Asian J. Trop. Med. Public Health. 2003;34 Suppl. 4:1–102. [PubMed] [Google Scholar]
- Wang X, Mu J, Li G, Chen P, Guo X, Fu L, Chen L, Su X, Wellems TE. Decreased prevalence of the Plasmodium falciparum chloroquine resistance transporter 76T marker associated with cessation of chloroquine use against P. falciparum malaria in Hainan, People's Republic of China. Am. J. Trop. Med. Hyg. 2005;72:410–414. [PubMed] [Google Scholar]
- WHO. Chemotherapy of malaria and resistance to antimalarials, Report of a WHO scientific group. World Health Organ. Tech. Rep. Ser. 1973;529:1–121. [PubMed]
- WHO. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. WHO/HTM/RBM. 2003:1–50.
- Wilson T, Edeson JF. Studies on the chemotherapy of malaria III. The treatment of acute malaria with chloroquine. Med. J. Malaya. 1954;9:115–131. [PubMed] [Google Scholar]
- Wongsrichanalai C, Pickard AL, Wernsdorfer WH, Meshnick SR. Epidemiology of drug-resistant malaria. Lancet Infect. Dis. 2002;2:209–218. doi: 10.1016/s1473-3099(02)00239-6. [DOI] [PubMed] [Google Scholar]
- Wongsrichanalai C, Lin K, Pang LW, Faiz MA, Noedl H, Wimonwattrawatee T, Laoboonchai A, Kawamoto F. In vitro susceptibility of Plasmodium falciparum isolates from Myanmar to antimalarial drugs. Am. J. Trop. Med. Hyg. 2001;65:450–455. doi: 10.4269/ajtmh.2001.65.450. [DOI] [PubMed] [Google Scholar]
- Yang H, Liu D, Dong Y, Yang P, Liu R, Zhan B, Zhang C. Sensitivity of Plasmodium falciparum to seven antimalarials in China-Laos border. Chin. J. Parasitol.Parasitic Dis. 1995;13:111–113. [PubMed] [Google Scholar]
- Yang H, Liu D, Yang Y, Huang K, Dong Y, Yang P, Liao M, Zhang C. In vitro sensitivity of Plasmodium falciparum to eight antimalarias in China-Myanmar and China-Lao PDR border areas. Southeast Asian J. Trop. Med. Public Health. 1997;28:460–464. [PubMed] [Google Scholar]
- Yang H, Liu D, Yang Y, Fan B, Yang P, Li X, Li C, Dong Y, Yang C. Changes in susceptibility of Plasmodium falciparum to artesunate in vitro in Yunnan Province, China. Transact. Royal Soc. Trop. Med. Hyg. 2003;97:226–228. doi: 10.1016/s0035-9203(03)90127-1. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhang Z, Sun X, Wan W, Cui L, Zhang X, Zhong D, Yan G, Cui L. Molecular analysis of chloroquine resistance in Plasmodium falciparum in Yunnan Province, China. Trop. Med. Pub. Health. 2007;12:1051–1060. doi: 10.1111/j.1365-3156.2007.01882.x. [DOI] [PubMed] [Google Scholar]