Abstract
Systemic simvastatin is known to reduce cholesterol and stimulate modest bone formation, but local surgical placement in polylactic acid domes causes robust bone formation and local swelling. A less invasive and more flexible injection protocol was studied to evaluate the bone-inducing effects compared to surgical implantation. Bone formation rate, short- and long-term bone augmentation histology, and mechanical properties were evaluated to characterize the new bone in a rat bilateral mandible model (test and control sides in same animal). Results demonstrated that multiple (3) injections of 0.5 mg simvastatin effectively reduced soft tissue swelling while preserving bone growth (60% increase of bone width at 24 days) compared to simvastatin dome placement (43% increase at 24 days). Compared to controls, bone formation rate was significantly higher on the simvastatin side, especially in the dome. Three point bending tests revealed higher maximum force to fracture and stiffness at 24 days with simvastatin injections. Long-term evaluation showed that 55% of maximum new bone formed 24 days post-injection was retained at 90 days.
1. Introduction
Inadequate jaw bone thickness may lead to subsequent osseous defects, such as fenestration around roots and dental implants. Ridge-augmentation procedures are complex and often unpredictable. Simplified, safe and reliable methods for adding thickness to maxillary/mandibular bone would be an important step in augmentation of the jaw for dental implants or regeneration of periodontal or peri-implant defects. Even minor augmentations of ridge contours require surgical interventions. Previous animal studies have used surgically-implanted polypeptide growth factors with graft materials; like bone morphogenic proteins (BMPs), platelet-derived growth hormone, insulin-like growth factor, and fibroblast growth factor [1–4]. These approaches have resulted in variable bone formation, usually require high doses and expensive grafting composites, and may induce adverse reactions in the host.
Since locally applied statins (specific inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A [HMG-CoA] reductase in the production of cholesterol) [5] were discovered to be potent stimulators of bone formation [6], the possibility of using these compounds as practical bone anabolic agents has been postulated. There is evidence that statins promote osteoblast differentiation and enhance BMPs in mouse bone marrow stromal cells [7], and simvastatin increased alkaline phosphatase activity and osteocalcin expression levels in human bone marrow stem cells [8]. In the ovariectomized rat model more new bone formation in mandibular defects and skeletal bone was seen when statins were given orally [9,10], and systemic statin-treated humans showed an increase in bone mineral density, bone-specific alkaline phosphatase activity and osteocalcin after 3 months [11]. However, the effect of statins when administered systemically appears to be minimized by clearance by the liver [12], and systemic side-effects of high doses are significant [13].
Local application of statins in healing sites or defects has been shown effective in new bone formation. Statin/collagen matrix grafts applied to the rabbit’s calvaria caused expression of BMP-2, vascular endothelial growth factor and core binding factor 1 in healing bone within 5 days, and 308% more bone than collagen matrix controls [14–16]. We previously have shown that surgical application of low dose (0.5 mg) simvastatin (semisynthetic 2,2-dimethy butyrate analogue of lovastatin) in methylcellulose gel under polylactic acid dome membranes implanted in a bilateral rat mandible model was effective in inducing new bone comparable to > 1.0 mg doses, but doses ≤ 1.0 mg caused less surrounding inflammation than the higher doses [17]. However, some soft tissue swelling occurred even in the control gel/dome membrane side, presumably due to the surgical trauma. For a more flexible approach to augment jaw width, we hypothesized that drug delivery by multiple injections should allow efficient applications to initiate and augment oral bone growth accompanied by minimal tissue damage and inflammation. Furthermore, regenerated bone should provide acceptable mechanical properties and function as normal bone, with significant amounts retained over extended periods of time. Our studies are based on multiple injections of simvastatin to enhance new bone formation in rat mandibles added onto existing bone in vivo. We used low dose simvastatin suspended in methylcellulose gel without combining any bone graft or scaffold materials. We evaluated and characterized bone-inducing capacity, histologic cellular and tissue populations, bone formation rate and mechanical properties comparing surgical dome implants and single and multiple injections. We also investigated histological bony change and mechanical properties after 90 days to explore long-term stability of new bone.
2. Materials and methods
2.1. Animal procedures
All animal procedures were approved by the Institutional Animal Care & Use Committee at the University of Nebraska in accordance with National Institutes of Health Guidelines. A bilateral mandible model using mature female Sprague Dawley rats (Harlan Teklad, Madison, WI) was used for these experiments. The rats were weighed at the day of simvastatin application and again the day of euthanasia. Prior to applying simvastatin all rats were sedated by intraperitoneal injection of 2 parts ketamine (100mg/ml) and 1 part xylazine (20 μl/ml) at a dosage of 0.1 ml/100 g (Phoenix Pharmaceutical, St. Joseph, MO) supplemented with local 0.2 ml 3% mepivacaine without vasoconstrictor (Cook Waite, Chicago, IL) as necessary.
0.1, 0.5, or 1.0 mg simvastatin in 30 μl methylcellulose gel under a polylactic acid dome membrane (SIM-DOME) were surgically implanted supraperiosteally on one side of the mandible, and gel alone in dome membrane (GEL-DOME) was applied on the other, as described previously (Fig. 1) [17]. The previous study showed that the 0.5 mg dose in domes produced the best bone growth/inflammation ratio. Therefore, a single injection of 0.5 mg simvastatin, or three weekly injections of 0.1 mg (suboptimal dose for DOME) or 0.5 mg simvastatin in 50 μl methylcellulose gel (SIM-INJ) were applied to separate rats with gel alone (GEL-INJ) on the contralateral side (Fig. 1). These experimental conditions are outlined in Table 1. Active drug treatments were randomized to right and left sides, and placed over the lateral aspect near the inferior angle of the mandible. Injections were done using a 20 gauge and 1 cm length needle at a marked mandible angle 6 mm deep into the tissue. Muscle was penetrated and a space was created supraperiosteally by moving the needle side-to-side at the same location used in other animals for dome implants. After healing times (3, 7, 24 or 90 days) were attained, the animals were euthanized by CO2 asphyxiation.
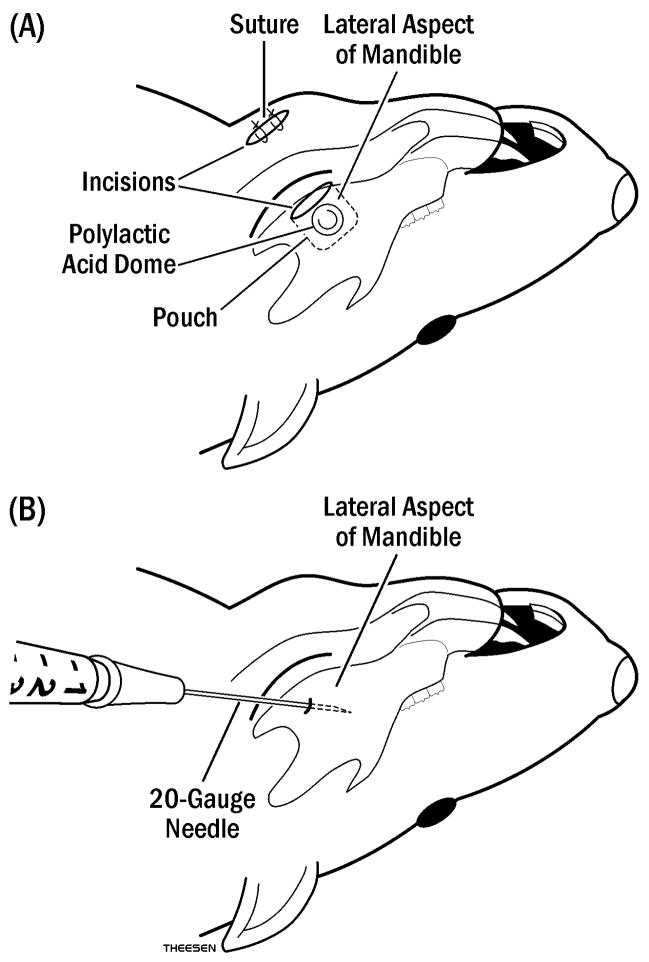
Fig. 1.
Diagram of simvastatin application to rat mandible: (A) Polylactic acid domes carrying simvastatin (SIM-DOME) in methylcellulose gel or gel alone (GEL-DOME) were inserted through an incision into the pouch (dotted line) made on lateral aspect of the mandible, then closed with suture. (B) Injections of simvastatin in 50 μl methylcellulose gel (SIM-INJ) or gel alone (GEL-INJ) were applied using a 20 gauge and 1 cm length needle in a marked mandible angle 6 mm deep into the tissue.
Table 1.
Experimental Groups and Healing Times
| Decalcified Sections* |
Undecalcified Sections** |
Raw sections*** |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Doses SIM/GEL |
Delivery Type |
Healing Time (Day) |
N | Doses SIM/GEL |
Delivery Type |
Healing Time (Day) |
N | Doses SIM/GEL |
Delivery Type |
Healing Time (Day) |
N |
| 0.1 mg/0 | Dome | 24 | 5 | ||||||||
| 0.1 mg/0 | Multiple Injections (3) | 24 | 5 | ||||||||
| 0.5 mg/0 | Dome | 3, 7, 24, 90 | 20 | 0.5 mg/0 | Dome | 25 | 10 | 0.5 mg/0 | Dome | 24, 90 | 10 |
| 0.5 mg/0 | Multiple Injections (3) | 3, 7, 24, 90 | 20 | 0.5 mg/0 | Single Injection | 25 | 10 | 0.5 mg/0 | Multiple Injections (3) | 24, 90 | 10 |
| 1.0 mg/0 | Dome | 24 | 5 | 1.0 mg/0 | Dome | 25 | 10 | ||||
Decalcified sections to determine bone growth and inflammation
Undecalcified sections to determine bone growth and bone formation rate
Raw sections to determine bone mechanical properties
2.2. Sample preparation
To determine parameters of bone growth and inflammation, samples were prepared for decalcified sections to include both sides of the mandible and overlying soft tissue [17]. The specimens were embedded in paraffin and subsequent cross-sections of 5 μm thickness were cut at 200 μm intervals to give a three-dimensional evaluation of the mandibular bone surface, and stained with hematoxylin-eosin.
To determine bone formation rates, undecalcified specimens were created in separate animals. Fifteen and 22 days following simvastatin application, all rats were injected subcutaneously with 0.3 ml of 8 mg/kg calcein (10 days and 3 days before euthanizing). Immediately after euthanasia, the mandibles were removed and placed in 70% alcohol for a minimum of 48 hours. After initial fixation, mandibles were dissected free of soft tissue. The bone posterior to the implanted membrane or injection site was cut away perpendicular to the occlusal plane for making subsequent transverse serial sections. Samples were placed into Villanueva stain for 72 hours and returned to 70% alcohol. During the next 14 days, the specimens were dehydrated in graded ethanol and acetone, and then embedded individually in modified methyl methacrylate. The embedded bones were sectioned at 80 μm thickness with a saw microtome (Model 1600, Leica, Germany) in cross-section.
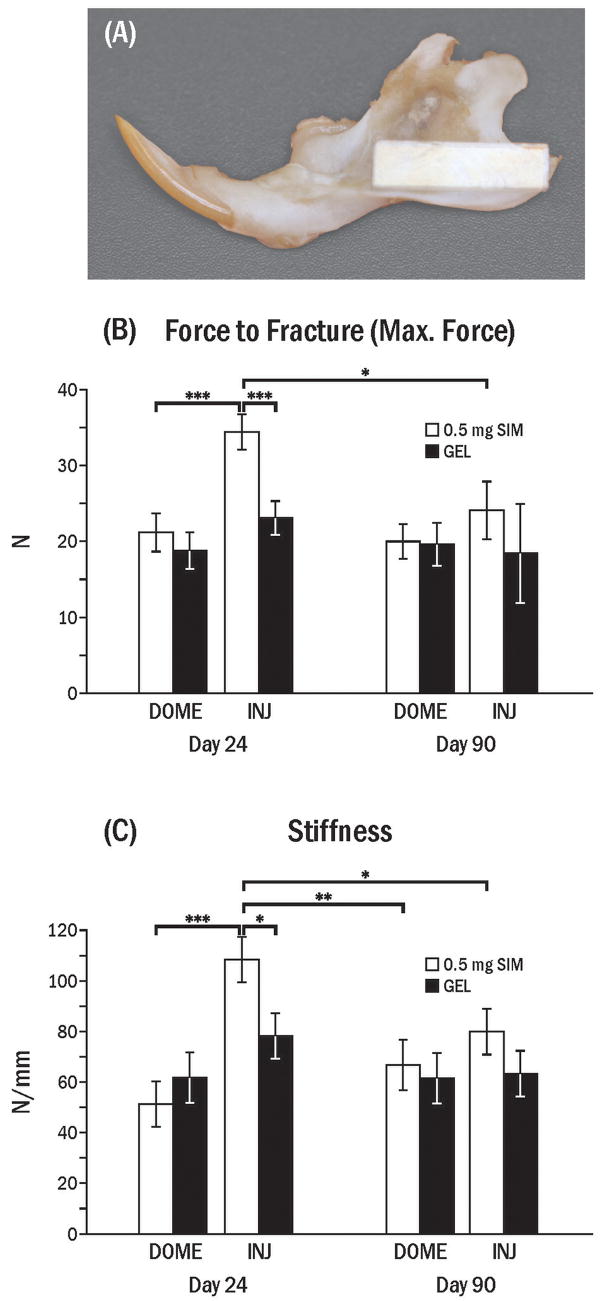
To test mechanical properties, mandibles dissected free of soft tissue were kept in phosphate buffered saline (PBS) at 4 °C until analysis. Rectangular sections (13 ×4 mm) were cut from the posterior - inferior area of mandible where simvastatin was applied by dome placement or injection (Fig. 10A), using a low speed diamond blade saw under PBS coolant immediately before mechanical testing. The specimens did not include alveolar bone where the thickness of bone is increased abruptly around the posterior teeth.
Fig. 10.
Mechanical tests at 24 and 90 days following application of simvastatin. (A) A 4 mm wide × 13 mm long polycarbonate template was placed on the inferior-posterior border of mandible (where simvastatin and/or gel was applied) to guide in specimen cutting. (B) Maximum force to fracture (± SEM) of the rectangular specimen in dome (DOME) and injection (INJ; 3 times) groups (INJ) 24 and 90 days after last simvastatin (SIM) application. (C) Stiffness (± SEM) in DOME and INJ groups 24 and 90 days after SIM application. Stiffness was calculated from the slope of the linear portion of the load-displacement graph. Brackets indicate significant differences at * p < 0.05, ** p < 0.01, *** p < 0.001.
2.3. Evaluation of soft tissue swelling
Swelling of mandibular soft tissue was determined at autopsy by palpating the outside of the cheek and recording the following Swelling Index: 0 = no swelling; 1 = < 1 cm diameter; 2 = 1 to < 1.5 cm diameter; 3 = 1.5 to < 2 cm diameter; 4 = ≥ 2 cm diameter.
2.4. Histomorphometric analysis of decalcified specimens
Three sections for each animal (both sides in the same section) were analyzed without knowledge of group or day using a light microscope and a digital camera interfaced with Sigma Scan Pro 5 software (SPSS Inc., Chicago, IL). Mandibular width of old and new bone was calculated as the average of three levels measured one mm apart (mandible base, middle and upper) and mandibular area of old and new bone was measured under 20× power. Length of periosteal bone surface was measured, then percent osteoblast surface, flat cell surface and osteoclast surface occupying that length were measured under 20× power after confirmation of cell type under 400×; and morphometric analysis of inflammatory cell types was accomplished by grid intersection point counting at 400× power; all as described previously [17]. The percent of plasma cells, lymphocytes, neutrophils, macrophages, monocytes, muscle, fibroblasts, collagen and blood vessels was calculated. The distance between the bone and the cellular infiltration nearest to bone was measured under 40× power.
2.5. Bone formation rate measurement from undecalcified specimens
Two coded sections of each side per animal were measured for fluorescent labels. Slides were viewed using an ultraviolet light microscope equipped with a video camera, and the image was transferred to the computer for BIOQUANT software analysis (R & M Biometrics, Nashville, TN). A horizontal line, parallel to and 2 mm from the bottom of the mandible, was drawn to standardize area of evaluation. Mandibular width was measured under 20× magnification, and length of double labeling, single labeling, and woven bone surface were measured under 200× magnification. The width between the double labels was measured under 400× magnification. Bone formation rate (BFR) was calculated by multiplying mineral apposition rate (MAR: width of double labels divided by 7 days) by bone forming surface as described by Recker et al. [18] and Foldes et al. [19]. For woven bone, an MAR of 5 μm/d was used, the maximum MAR of the lamellar bone that was measured in bone forming areas.
2.6. Three point bending test
The 4 mm wide ×13 mm long mandibular beam specimens were confirmed with a micrometer and the beam was loaded in three point bending at a rate of 10 mm/min to failure. Since the average mandible beam thickness was around 0.9 mm and the distance between supports was 9 mm, this produced a support length - to - diameter (thickness) ratio of approximately 10 as specified in standardized animal bone testing [20]. Two parameters were measured, namely, the maximum force at fracture and the slope of the load-deflection curve, which represented bone stiffness.
2.7. Statistical analyses
Data were not normally distributed (not symmetric). Intragroup (SIM vs. GEL) and intergroup differences among doses and application types, as well as days of healing, were compared using analysis of variance (ANOVA). Pearson Correlation Coefficients were used to compare infiltrated cell and tissue types with bone formation parameters. Comparisons of bone formation surface and BFR between the doses and types of simvastatin application were analyzed using the General Linear Model.
3. Results
3.1. Physical evaluation
Change of rat weight across the experimental period was minimal (≤ 5%), the greatest loss happening 3 days post-dose. There was no significant difference between multiple injections and dome applications.
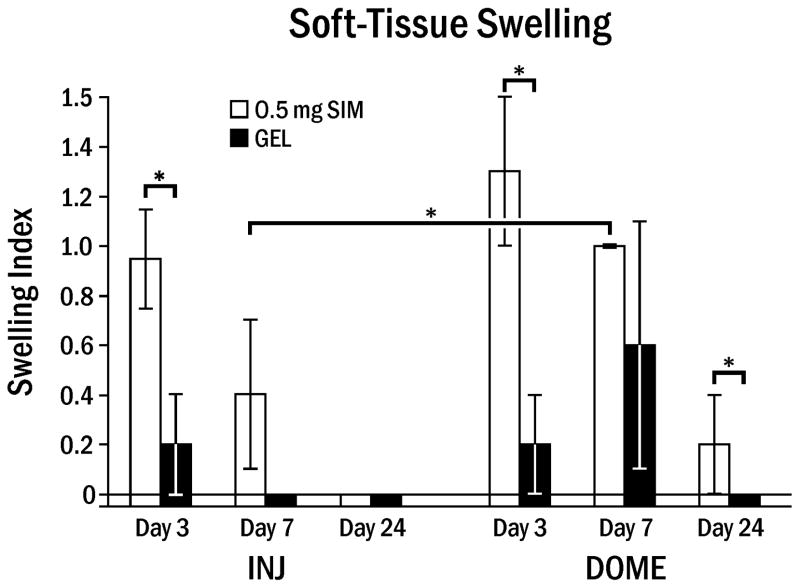
The average swelling index in the 0.5 mg SIM–INJ animals (0.2) was significantly less than for the SIM–DOME (0.5; p = 0.0065). This was most notable at day 7 (Fig. 2). The swelling noted at 3 and 7 days had subsided at 24 days in both the injection and dome groups.
Fig. 2.
Soft-tissue swelling around rat mandible following simvastatin or gel application. Swelling Index (± standard error on the mean) at various days following 0.5 mg simvastatin (SIM) or gel alone (GEL) applied by 3 weekly injections (INJ) or single dome implantation (DOME). Brackets indicate significant differences at * p < 0.05.
3.2. Effect of single and multiple injections on bone formation
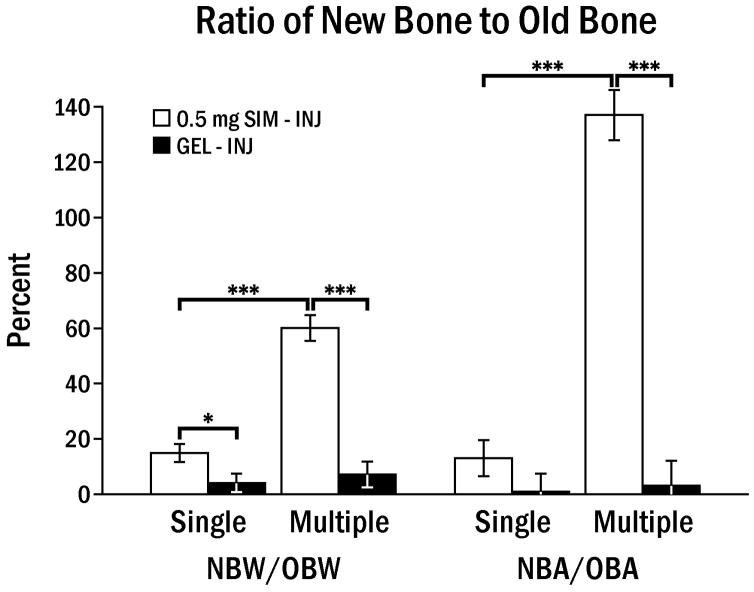
The mean values of new bone width and area with 0.5 mg SIM multiple injections were 1.0 mm and 2.01 mm2, respectively, and this was significantly higher than contralateral control sides or single SIM injections (Fig. 3). However, the mean value of new bone width and area of 0.5 mg single injection or 0.1 mg multiple injections was not statistically different between control and test side (data not shown).
Fig. 3.
Comparison of ratio of new bone to old bone width (NBW/OBW) and area (NBA/OBA) between single (1) and multiple (3) injections of 0.5 mg SIM in 50 μl methylcellulose gel (SIM-INJ) or gel alone (GEL-INJ), 24 days following the last injection. Brackets indicate significant differences at * p < 0.05, *** p < 0.001.
3.3. Histomorphometric comparison of multiple injections and dome implantation
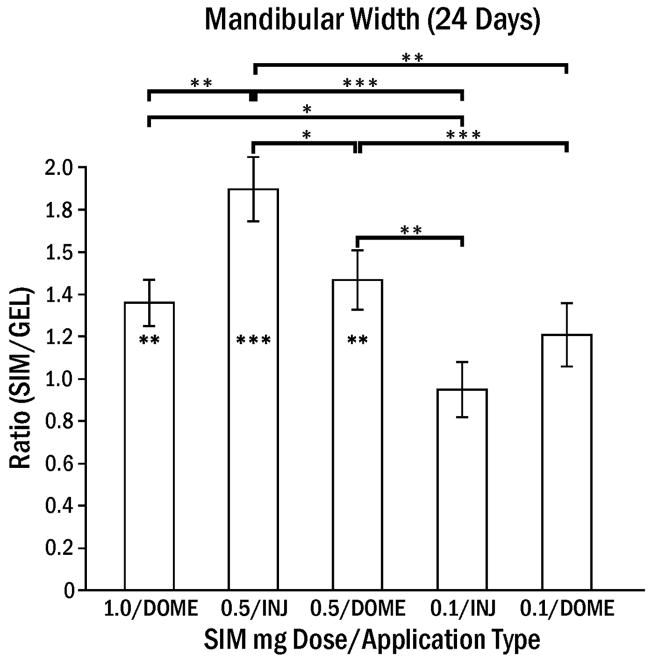
The average length of the osteoblast surface was not significantly different in SIM-DOME (25.1 ± 3.8%) compared to SIM-INJ (15.2 ± 2.8%), although the day 7 peak was higher in SIM-DOME (42.2 ± 5.7%, p < 0.01). 0.1 mg SIM-INJ showed minimal osteoblast activity. The ratio of SIM/GEL mandibular width was highest for multiple 0.5 mg SIM-INJ after 24 days (Fig. 4). The percentage of osteoclast surface at 24 days was 4.8% in 0.5 mg SIM-INJ and 4.0% in 0.5 mg SIM-DOME, which was significantly greater (p < 0.05) compared to that of percentage of osteoclasts at 3 and 7 days (< 0.4%). This suggests that the remodeling process had begun at 24 days.
Fig. 4.
Ratio of rat mandibular width following simvastatin application normalized by the control (GEL) side (± SEM). Since control side widths were approximately one mm, the ratio is equal to approximate mandibular widths on the test side. Simvastatin in gel (SIM) or gel alone (GEL) was applied by 3 weekly injections (INJ) or one dome implant (DOME). Footnote symbol in bar indicates significant difference between SIM and GEL, and brackets indicate significant differences between application types at * p < 0.05, ** p < 0.01, *** p < 0.001.
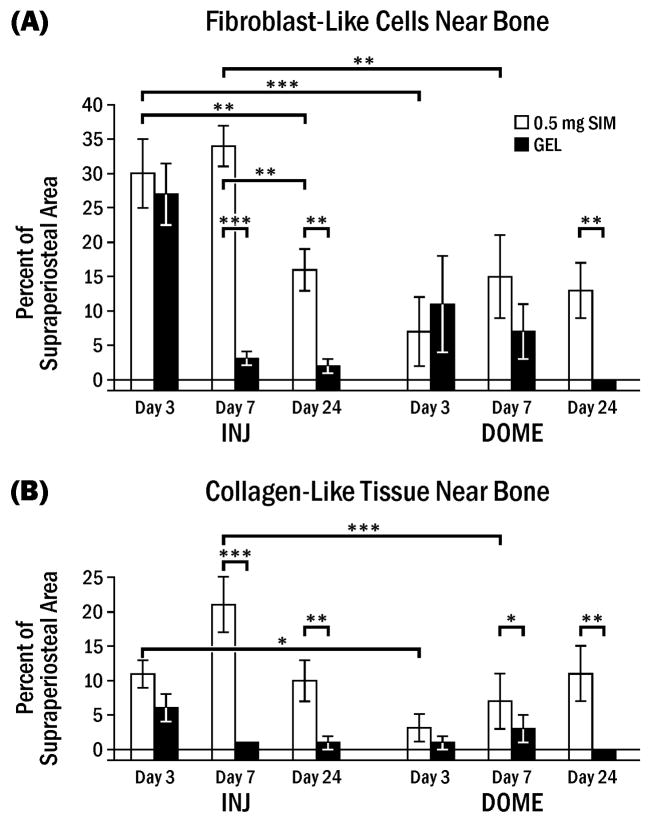
The lateral side of the rat mandible was mainly composed of muscle before treatment. However, 0.5 mg SIM-INJ induced infiltration of other cells and tissues, such as fibroblast-like cells (flat, elongated cells with very little cytoplasm), inflammatory cells, blood vessels and collagen. Fibroblast-like cells and collagen-like tissue predominated peaking at 7 days (Fig. 5, 6). The percentage of these cells/tissues on the SIM–INJ side was generally higher than GEL-INJ or SIM-DOME. Inflammatory cells near bone were very rare, but some neutrophils and lymphocytes (< 1%) were observed around gel apart from bone. The distance between the cellular infiltrate and bone was as follows for the various conditions: 1) multiple injections of 0.5 mg SIM-INJ (0.02 ± 0.27 mm); 2) multiple injections of GEL-INJ (0.42 ± 0.17 mm); 3) 0.5 mg SIM-DOME (0.78 ± 0.17 mm); 4) GEL-DOME (1.00 ± 0.17 mm). These findings showed that the infiltrate was significantly closer to bone following injections of either gel or simvastatin than following dome placement (p < 0.05), and the distance between infiltrate and bone was negatively correlated with bone growth after 7 days (r = −0.52, p < 0.0001).
Fig. 5.
Fibroblast-like cells and collagen-like tissue near lateral rat mandible following simvastatin or gel application. Percent (± SEM) of supraperiosteal area comprised of fibroblast-like cells (A) or collagen-like tissue (B) at various days following 0.5 mg simvastatin in gel (SIM) or gel alone (GEL) applied by 3 weekly injections (INJ) or one dome implantation (DOME). Brackets indicate significant differences at * p < 0.05, ** p < 0.01, *** p < 0.001.
Fig. 6.
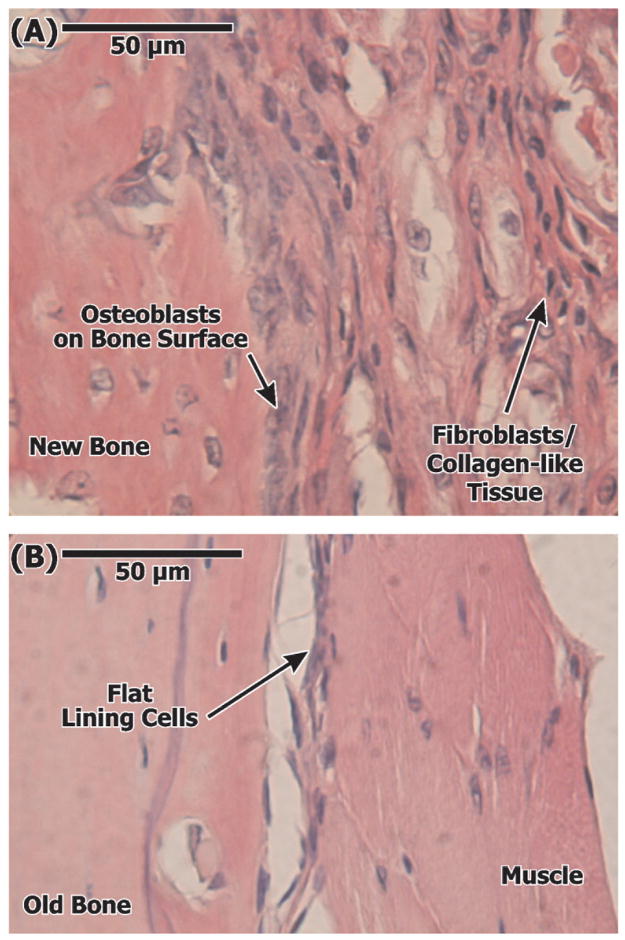
Representative photomicrographs of decalcified sections of soft tissue-bone interface adjacent to 0.5 mg SIM (A) and GEL (B) applications showing increased fibroblasts and collagen-like tissue, along with osteoblast-lined surface, on the SIM side. Stained with hematoxylin and eosin.
Also using Pearson Correlation Coefficients, percentage of fibroblast-like cells near bone at 3 days was positively related with 3 day new bone width (r = 0.91, p = 0.0338) and new bone area (r = 0.91, p = 0.0304) in the injections. At 7 days, there was no correlation between new bone formation parameters and fibroblast- or collagen-like structures. At 24 days, the percentage of the fibroblast-like cells was negatively related with flat cell (resting) bone surface (r = −0.99, p = 0.0017) and positively related with osteoclast bone surface (r = 0.99, p = 0.0017) in the injection group.
3.4. Measurement of the bone type and formation rate
From analysis of the undecalcified specimens, most new bone on the lateral and inferior surface was woven bone (65–70% of forming surface) rather than lamellar bone. Since new bone formation occurred so rapidly with multi-focal events, mineralization was seen in a diffusely-labeled pattern and it was difficult to find double labels in the woven bone area (Fig. 7). 1.0 mg SIM-DOME appeared to cause woven bone formation further from the application site than other doses or application types compared to the gel control, as seen with higher woven bone area on the inferior mandible surface (p < 0.05).
Fig. 7.
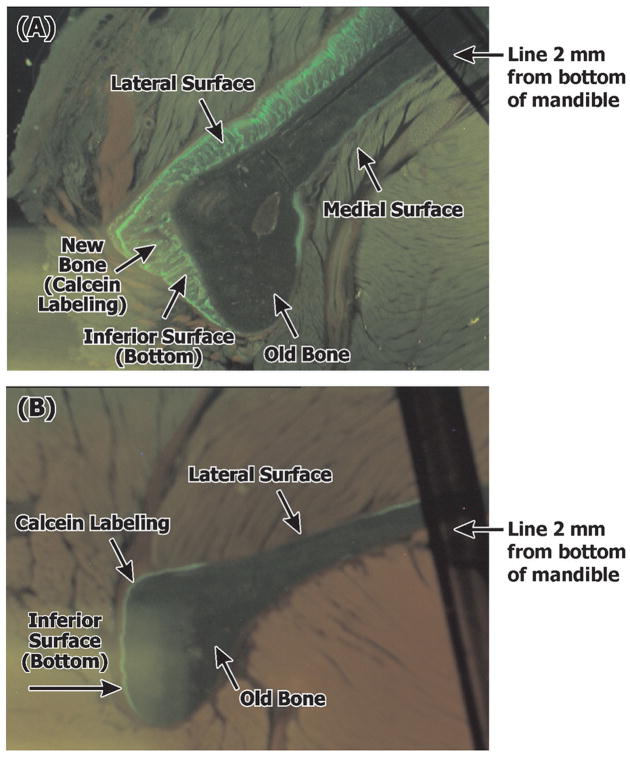
Representative photomicrographs of undecalcified sections of the rat mandible (black line was drawn parallel to and 2 mm from the bottom of the mandible to standardize area of evaluation). Application of 0.5 mg SIM-DOME (A) shows increased new bone on lateral and inferior surfaces (green fluorescence: calcein label) compared to GEL-DOME (B).
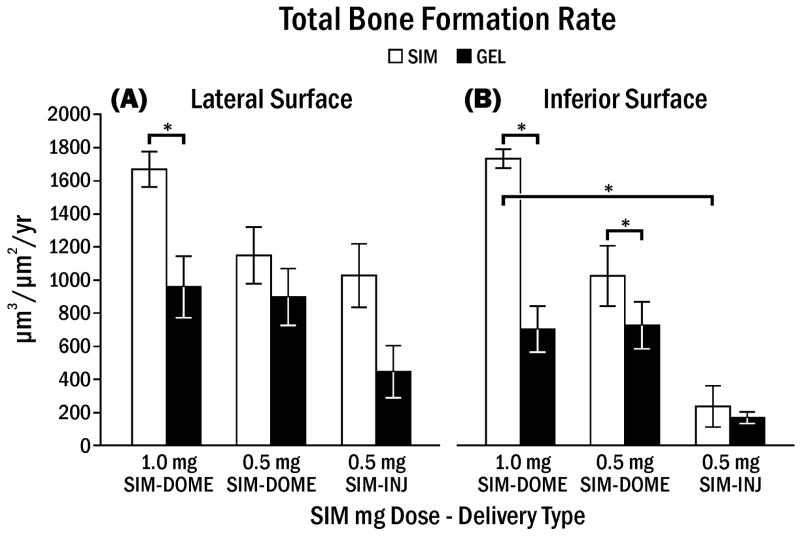
Bone formation rate (woven BFR + lamellar BFR) on the lateral aspect of the mandible showed a significant difference within animal between SIM and GEL, especially in the group treated with 1.0 mg simvastatin (Fig. 8A). However, there was no significant difference between SIM and GEL on the lateral surface treated with dome treatments and a single injection of 0.5 mg SIM. Bone formation rate associated with control injection sites was much lower in contrast to the control side of surgically implanted gel domes, which stimulated considerable bone growth on lateral and inferior surfaces (Fig. 8).
Fig. 8.
Bone formation rate (woven BFR + lamellar BFR) on lateral aspect (A) or inferior aspect (B) of rat mandible following simvastatin or gel application. Bone formation rate (± SEM) following various simvastatin doses in gel (SIM) or gel alone (GEL) applied by single injection (INJ) or dome implant (DOME). Brackets indicate significant differences at * p < 0.05.
3.5. Long-term histologic study
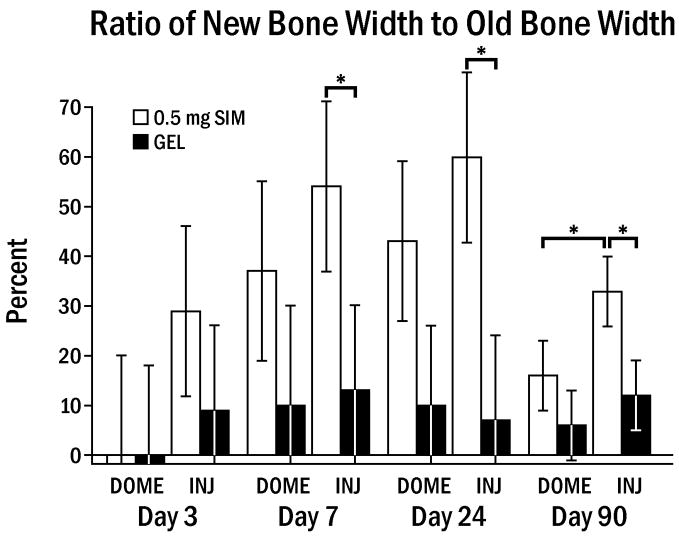
Specimens 90 days from the time of last injection or dome placement showed significantly reduced width and area (45–75%) of new bone compared to the bone parameters at 24 days. The ratio of new bone to old bone in test versus control sides in the dome group at 90 days was no longer significantly different, but multiple injections showed a continuing statistical difference between test and control sides, where 55 % of maximum SIM-induced bone was retained (Fig. 9, p < 0.05). Remaining new bone tended to be denser, the outer surface of the mandible was lined by quiescent flat periosteal cells, and normal muscle tissue and periosteum surrounded the bone.
Fig. 9.
Ratio of new bone to old bone width (± SEM) at 3,7,24 and 90 days. The ratio peaked at 24 days. There were still statistical differences between SIM-INJ and both GEL-INJ and SIM-DOME at 90 days at * p < 0.05.
3.6. Mechanical studies
Multiple injections of simvastatin resulted in significantly higher maximum force to fracture (p < 0.001) and stiffness (p < 0.05) than the control side after 24 days (Fig. 10 B, C). By 90 days these differences were not significant (p > 0.05), although SIM-INJ side remained 1.3 times higher in both stiffness and fracture force than GEL-INJ. The SIM-DOME side was not significantly different from controls for either mechanical property at 24 days and 90 days, and was significantly lower than the SIM-INJ side at 24 days (p < 0.05).
4. Discussion
This study was designed to investigate the impact of the simvastatin delivery system (gel injection or gel/dome implants) on bone growth and inflammation, and the histologic and mechanical characteristics of new bone induced by these applications after various times. This can help determine how topical simvastatin could be applied clinically to increase bone thickness. For this purpose, a bilateral mandible model using mature female Sprague Dawley rats was used and bone surface was stimulated without making a surgical defect, to exclude natural bone healing and to simulate adding to thin bone. Mature rats do not show active bone growth and only represent remodeling similar to adult humans.
Local application has a therapeutic advantage by preventing systemic side-effects and focusing the drug dose. 0.5 mg SIM in rats which weigh approximately 300 g is equal to 1.7 mg/kg. The clinically effective dosage range to treat hypercholesterol in humans by oral administration is up to 1.0 mg/kg/day. However, a topical dose affects a localized area of bone, whether in a 70 kg human or 0.3 kg rat. Even with weekly 0.5 mg injections, the 1.5 mg/kg/week compares favorably with the 7 mg/kg/week in human oral regimens. In addition, simvastatin has a half-life of 1–3 hours [21] and tends to concentrate at very low levels in peripheral tissue within the first two hours after administration [22]. Therefore, local doses in the studied range should be well tolerated in the human. Animals in the current study tolerated the implanted simvastatin dome and injection of simvastatin gel with minimal adverse effects. All animals looked normal and active immediately after recovery from the anesthesia used for the application of drug. Weight loss at 3 days was 14–21 g; however, after 24 days weight loss was reduced significantly to a minimal 7–9 g.
The soft tissue swelling noted in this study was less following injections than dome implantations. The swellings were firm and histologically comprised overwhelmingly of fibroblasts and collagen concentrated around the periosteum with very few inflammatory cells (lymphocytes, neutrophils, macrophages). It is possible that simvastatin particles themselves in gel cause irritation which could stimulate bone formation. This surely has some impact, as the distance between the fibrous response in the soft tissue and the new bone growth (area) at 7 days was negatively correlated (r = −0.52, p < 0.0001).
A single injection of SIM showed limited bone formation, suggesting it was unable to maintain the needed concentration or that multiple pulsing doses give an additive or synergistic effect (Fig. 3). The cumulative and integrated effects of the three injections resulted in equivalent or greater bone growth and periosteal cellular activity compared to surgical dome implantation, but with much less swelling (Fig. 2). In fact, the three-injection SIM-INJ protocol nearly doubled the width of the mandible compared to the GEL-INJ, adding an average of one millimeter of new bone in this small animal. While multiple injections to the rat mandible gave an additive effect on increasing bone thickness, a 58% increase in bone thickness occurred in mouse calvaria after a single injection of 2.2 mg SIM-GEL in a previous study, but the dimension of new bone thickness was much less (< 0.1 mm) and was associated with considerable inflammation [23]. In addition, the amount of new bone thickness appeared to be associated with original bone size, suggesting that the amount of new bone using the same technique on human-sized alveolar bone should be dramatically thicker than one millimeter and therefore clinically relevant.
Significantly higher osteoblast surface at 7 days and BFR between 15 and 22 days (Fig. 8) on the GEL-DOME side compared to the GEL-INJ side (single injection; p = 0.03) indicated that surgical trauma played a role in dome stimulation of bone formation. The possibility that a systemic effect from locally applied simvastatin carried over to the control side was discounted in an earlier study [17], where data used to calculate mandibular area showed that bone growth on the gel alone side was not different in animals treated on the contralateral side with simvastatin versus untreated mandibles. Swelling and infiltration were still present on the gel side only, presumably due to surgical trauma.
Multiple injections of simvastatin caused more fibroblast-like cells and collagen-like tissue near new bone than SIM–DOME, often contiguous with the periosteum (Fig. 6). Statins have been shown to stimulate bone formation in bone marrow-derived mesenchymal stem cells [24], and induced osteoblastic differentiation in bone marrow cells [25, 26]. It was not possible with the histologic approach used in this study to determine how many of the fibroblast-like cells were osteoblast precursors or stem cells, from where the fibroblast-like cells originated, or what is the precise role of these fibroblast-like cells.
Judging from the decalcified bone data, osteoblast activity was on the decline after 7 days. Therefore, the week between calcein incorporation (days 15 and 22) in the undecalcified specimens was catching bone formation on the downswing after a single injection of SIM. It is noted, however, that bone formation was still a predominant activity during that period on the lateral bone surface. There was almost no resorption activity preceding bone formation at day 3 or 7. This result is consistent with in vitro studies showing a decreased measure of osteoclastic potential (RANKL/osteoprotegrin mRNA) caused by simvastatin in mouse bone cell culture [27]. In a recent report, twice weekly injections of simvastatin appeared to reduce bone resorption in a ligature-induced rat periodontitis model [28]. However, in our studies, osteoclast surface was 4% of the total bone surface at 24 days, implying a remodeling process at that later time. Bone modeling can be defined by continuous bone formation without the necessity of a previous resorption phase, so we consider simvastatin-induced bone formation as a modeling, not remodeling process. However, later remodeling resulted in either bone resorption and reshaping of the new bone. This remodeling response and effects were evaluated in long–term studies of the fate of the new bone in a non-defect, non-implant model at 90 days after application of drug. About 45–75% of the ratio of new bone to old bone at 24 days was reduced at 90 days after simvastatin application (Fig. 9). This resorption may be caused by the tension of overlying tissue and the non-physiologic shape without functional loading. This implies that stressing bone with an implant or root structure providing proper loading is an essential requisite for protection of functional bone. In spite of the limitations of the current model, however, 55% of the maximum new bone formed following multiple SIM-INJ was retained at 90 days, significantly greater than controls.
We measured mechanical properties of mandibles at 24 and 90 days to characterize the new bone, which was woven bone at 24 days with a highly increased bone formation rate (Fig. 8). Increased bone volume was expected to increase both fracture force and stiffness. This was observed at 24 days for the injection sites, where these mechanical properties were 1.4–1.5 times higher than controls. Bone remodeling by 90 days reduced bone volume, lowered fracture force by 30% and stiffness by 25%, with values not significantly different than controls. However, the observation that injection sites maintained mechanical properties that were 1.3 times higher that controls implies that new mature bone was present and exerting mechanical advantage to existing bone structure. Dome placement, on the other hand, was ineffective at producing improved mechanical performance at either 24 or 90 days.
5. Conclusions
It can be concluded from the current study that multiple injections of a 0.5 mg dose of simvastatin gel can induce an accumulative effect in new bone formation with minimal soft-tissue swelling and without attenuation of mechanical properties compared to dome implantation at 24 days. However, much of the new bone was remodeled after 90 days without functional loading. The clinical relevance of these findings is that injection delivery systems for simvastatin may provide a comfortable and flexible method not only to initiate bone formation in areas of thin, vulnerable alveolar bone, but also to enhance bone formation during and after traditional bone augmentation procedures. Further investigations to better target the drug and preserve new bone induced by simvastatin are needed.
Acknowledgments
This research was supported by the National Institute of Dental and Craniofacial Research (NIH-DE015096; RAR & DMC) and simvastatin was supplied by Merck Research Laboratories. The authors thank Deb Dalton and Susan McCoy for manuscript preparation and Kim Theesen for graphic design. The authors claim no conflict of interest.
References
- 1.Damien CJ, Christel PS, Benedict JJ, Patat JL, Guillemin G. A composite of natural coral, collagen, bone protein and basic fibroblast growth factor tested in a rat subcutaneous model. Annal Chirurgiae Gynaecol, Suppl. 2002;207:117–128. [PubMed] [Google Scholar]
- 2.Meinel L, Zoidis E, Zapf J, Hassa P, Hottiger MO, Auer JA, et al. Localized insulin-like growth factor I delivery to enhance new bone formation. Bone. 2003;33:660–672. doi: 10.1016/s8756-3282(03)00207-2. [DOI] [PubMed] [Google Scholar]
- 3.Roldan JC, Jepsen S, Miller J, Freitag S, Rueger DC, Açil Y, et al. Bone formation in the presence of platelet-rich plasma vs. bone morphogenetic protein-7. Bone. 2004;34:80–90. doi: 10.1016/j.bone.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Pryor ME, Polimeni G, Koo KT, Hartman MJ, Gross H, April M, et al. Analysis of rat calvaria defects implanted with a platelet-rich plasma preparation: histologic and histometric observations. J Clin Periodontol. 2005;32:966–972. doi: 10.1111/j.1600-051X.2005.00772.x. [DOI] [PubMed] [Google Scholar]
- 5.Tobert JA. New developments in lipid-lowering therapy: the role of inhibitors of hydroxymethylglutaryl-coenzyme A reductase. Circulation. 1987;76:534–538. doi: 10.1161/01.cir.76.3.534. [DOI] [PubMed] [Google Scholar]
- 6.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 7.Song C, Guo Z, Ma Q, Chen Z, Liu Z, Jia H, et al. Simvastatin induces osteoblastic differentiation and inhibits adipocytic differentiation in mouse bone marrow stromal cells. Biochem Biophys Res Commun. 2003;308:458–462. doi: 10.1016/s0006-291x(03)01408-6. [DOI] [PubMed] [Google Scholar]
- 8.Baek KH, Lee WY, Oh KW, Tae HJ, Lee JM, Lee EJ, et al. The effect of simvastatin on the proliferation and differentiation of human bone marrow stromal cells. J Korean Med Sci. 2005;20:438–444. doi: 10.3346/jkms.2005.20.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Junqueira JC, Mancini MN, Carvalho YR, Anbinder AL, Balducci I, Rocha RF. Effects of simvastatin on bone regeneration in the mandibles of ovariectomized rats and on blood cholesterol levels. J Oral Sci. 2002;44:117–124. doi: 10.2334/josnusd.44.117. [DOI] [PubMed] [Google Scholar]
- 10.Pytlik M, Janiec W, Misiarz-Myrta M, Gubala I. Effects of simvastatin on the development of osteopenia caused by ovariectomy in rats. Pol J Pharmacol. 2003;55:63–71. [PubMed] [Google Scholar]
- 11.Tikiz C, Tikiz H, Taneli F, Gumuser G, Tuzun C. Effects of simvastatin on bone mineral density and remodeling parameters in postmenopausal osteopenic subjects: 1-year follow-up study. Clin Rheumatol. 2005;24:447–452. doi: 10.1007/s10067-004-1053-x. [DOI] [PubMed] [Google Scholar]
- 12.Todd PA, Goa KL. Simvastatin: a review of its pharmacological properties and therapeutic potential in hypercholesterolaemia. Drugs. 1990;40:583–607. doi: 10.2165/00003495-199040040-00007. [DOI] [PubMed] [Google Scholar]
- 13.Gage TW, Pickett FA. Mosby’s Dental Reference. 7. St. Louis: Elsevier Mosby; 2005. p. 712. [Google Scholar]
- 14.Wong RW, Rabie AB. Statin collagen grafts used to repair defects in the parietal bone of rabbits. Brit J Oral Maxillofac Surg. 2003;41:244–248. doi: 10.1016/s0266-4356(03)00081-0. [DOI] [PubMed] [Google Scholar]
- 15.Wong RW, Rabie AB. Histologic and ultrastructural study on statin graft in rabbit skulls. J Oral Maxillofac Surg. 2005;63:1515–1521. doi: 10.1016/j.joms.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Wong RW, Rabie AB. Early healing pattern of statin-induced osteogenesis. Brit J Oral Maxillofac Surg. 2005;43:46–50. doi: 10.1016/j.bjoms.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Stein D, Lee Y, Schmid MJ, Killpack B, Genrich MA, Narayana N, et al. Local simvastatin effects on mandibular bone growth and inflammation. J Periodontol. 2005;76:1861–1870. doi: 10.1902/jop.2005.76.11.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Recker RR. Bone Histomorphometry Techniques and Interpretation. Boca Raton: CRC Press; 1983. [Google Scholar]
- 19.Foldes J, Shih MS, Parfitt AM. Frequency distributions of tetracycline-based measurements: implications for the interpretation of bone formation indices in the absence of double-labeled surfaces. J Bone Miner Res. 1990;5:1063–1067. doi: 10.1002/jbmr.5650051010. [DOI] [PubMed] [Google Scholar]
- 20.American Society of Agricultural Engineers Standard. Shear and three-point bending test of animal bone. 2003; 612–614. ASAE Standard S459 DEC 01(Approved Feb, 1993; reaffirmed Mar 1998 by ANSI).
- 21.Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fund Clin Pharmacol. 2005;19:117–125. doi: 10.1111/j.1472-8206.2004.00299.x. [DOI] [PubMed] [Google Scholar]
- 22.Germershausen JI, Hunt VM, Bostedor RG, Bailey PJ, Karkas JD, Alberts AW. Tissue selectivity of the cholesterol-lower agents lovastatin, simvastatin and pravastatin in rats in vivo. Biochem Biophys Res Commun. 1989;158:667–675. doi: 10.1016/0006-291x(89)92773-3. [DOI] [PubMed] [Google Scholar]
- 23.Thylin MR, McConnell JC, Schmid MJ, Reckling RR, Ojha J, Bhattacharyya I, et al. Effects of simvastatin gels on murine calvarial bone. J Periodontol. 2002;73:1141–1148. doi: 10.1902/jop.2002.73.10.1141. [DOI] [PubMed] [Google Scholar]
- 24.Sonobe M, Hattori K, Tomita N, Yoshikawa T, Aoki H, Takakura Y, et al. Stimulatory effects of statins on bone marrow-derived mesenchymal stem cells. Study of a new therapeutic agent for fracture. Biomed Mater Eng. 2005;15:261–267. [PubMed] [Google Scholar]
- 25.Maeda T, Matsunuma A, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun. 2001;280:874–877. doi: 10.1006/bbrc.2000.4232. [DOI] [PubMed] [Google Scholar]
- 26.Maeda T, Matsunuma A, Kurahashi I, Yanagawa T, Yoshida H, Horiuchi N. Induction of osteoblast differentiation indices by statins in MC3T3-E1 cells. J Cell Biochem. 2004;92:458–471. doi: 10.1002/jcb.20074. [DOI] [PubMed] [Google Scholar]
- 27.Kaji H, Kanatani M, Sugimoto T, Chihara K. Statins modulate the levels of osteoprotegerin/receptor activator of NFkappaB ligand mRNA in mouse bone-cell cultures. Horm Metab Res. 2005;37:589–592. doi: 10.1055/s-2005-870538. [DOI] [PubMed] [Google Scholar]
- 28.Vaziri H, Naserhojjati-Roodsari R, Tahsili-Fahadan N, Khojasten A, Mashhadi-Abbas F, Eslami B, et al. Effect of simvastatin administration on periodontitis-associated bone loss in ovariectomized rats. J Periodontol. 2007;78:1561–1567. doi: 10.1902/jop.2007.060480. [DOI] [PubMed] [Google Scholar]