Abstract
Loss of a critical number of podocytes from the glomerular tuft leads to glomerulosclerosis. Even in health, some podocytes are lost into the urine. Because podocytes themselves cannot regenerate, we postulated that glomerular parietal epithelial cells (PECs), which proliferate throughout life and adjoin podocytes, may migrate to the glomerular tuft and differentiate into podocytes. Here, we describe transitional cells at the glomerular vascular stalk that exhibit features of both PECs and podocytes. Metabolic labeling in juvenile rats suggested that PECs migrate to become podocytes. To prove this, we generated triple-transgenic mice that allowed specific and irreversible labeling of PECs upon administration of doxycycline. PECs were followed in juvenile mice beginning from either postnatal day 5 or after nephrogenesis had ceased at postnatal day 10. In both cases, the number of genetically labeled cells increased over time. All genetically labeled cells coexpressed podocyte marker proteins. In conclusion, we demonstrate for the first time recruitment of podocytes from PECs in juvenile mice. Unraveling the mechanisms of PEC recruitment onto the glomerular tuft may lead to novel therapeutic approaches to renal injury.
Chronic kidney disease, resulting in renal failure and the need for lifelong renal replacement therapy, has become a significant problem worldwide. In the United States, approximately 7% of the total Medicare budget is spent on the treatment of ESRD, and projections suggest that the amount spent will increase by another 50% by 2020.1
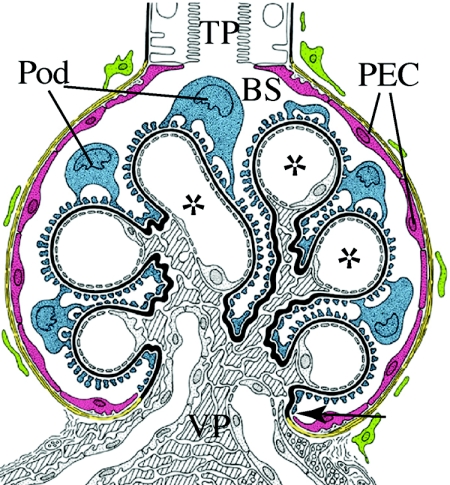
Most renal pathologies that ultimately lead to ESRD originate within the glomerulus. It has now been established that a depletion of podocytes, the visceral epithelium of the capillary convolute (Figure 1), is central in this process. As soon as damage to the glomerular podocytes exceeds a certain threshold (approximately 30%), glomerulosclerosis ensues.2 Indeed, in patients with a surgical reduction of ≥75% of renal mass, a relative lack of podocytes (podocytopenia) and subsequent FSGS in the originally healthy remnant kidney can lead to renal failure.3 Glomerulosclerosis is also the common final pathway of all glomerular diseases leading to ESRD.4 In glomerular diseases such as diabetic nephropathy, glomerulonephritides, or preeclampsia, significant numbers of podocytes are lost as a result of apoptosis, necrosis or excretion of living cells into the urine. Even in normal individuals, low numbers of living podocytes are continuously shed into the urine.5–7 These numbers are too high to be compatible with renal survival for 80 yr, suggesting the existence of a regenerative mechanism. Also, the reversal of early glomerular damage in animal models and humans8–10 argues for the existence of such a mechanism; however, podocytes are postmitotic cells that cannot undergo complete cell divisions and are therefore unable to regenerate themselves.8–10 A potential mechanism for podocyte replacement from bone marrow–derived stem cells has been described in the Alport mouse model as well as in kidney transplants.11–13 Nevertheless, most studies concluded that regeneration occurs predominantly from an as-yet-unknown source of resident renal cells.12,14–16
Figure 1.
Renal glomerulus. The glomerular epithelium consists of PECs (red) and podocytes (Pod; blue), which reside on the capillary convolute. Both epithelia adjoin directly at the vascular pole (VP; arrow). At the tubular pole (TP), the parietal epithelium is connected to the epithelium of the proximal tubule. In male mice, this transition from PECs to proximal tubular cells often occurs within the glomerulus. The glomerular basement membrane (black) forms a continuous barrier between the glomerular epithelium and the endocapillary compartment that contains mesangial cells (shaded) and endothelial cells of the glomerular capillaries (*). Primary urine is filtered across the three-layered filtration barrier (endothelial cells, glomerular basement membrane, and Pod) into Bowman's space (BS).
In this study, we tested the hypothesis that glomerular parietal epithelial cells (PECs) lining the inner aspect of Bowman's capsule migrate onto the glomerular tuft and differentiate into podocytes. Several arguments support this hypothesis. PECs are present in all species whose kidneys contain glomeruli. They are located within the same compartment and are in direct continuity with podocytes at the glomerular vascular stalk, so PECs do not have to cross an anatomic barrier such as the glomerular basement membrane, as was suggested for bone marrow–derived stem cells.11–13 PECs proliferate lifelong at a relatively low frequency,17 express several stem cell marker proteins, and could be transdifferentiated in vitro into other cell types such as adipocytes or neuronal cells, suggesting that these cells retain multipotency.9,18,19 In rodents, PECs do not express any known specific marker protein, which has so far precluded a detailed analysis of the function of these cells.
In this work, we provide the first evidence that PECs possess the capability to migrate onto the glomerular tuft via the vascular stalk, where they differentiate into podocytes. This establishes that PECs represent an intrinsic cell population from which podocytes can be recruited.
RESULTS
Transitional Cells at the PEC/Podocyte Interface
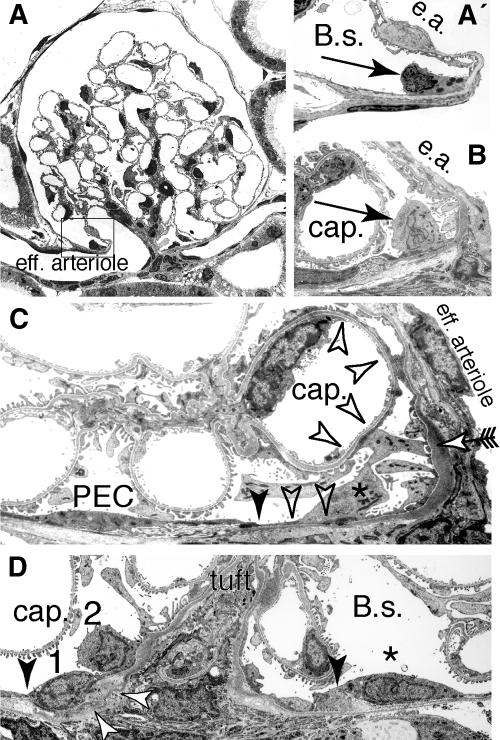
To test our hypothesis that PECs migrate onto the glomerular tuft, we analyzed the vascular stalk of healthy Sprague-Dawley rats by transmission electron microscopy. At this site, cells with features of PECs as well as podocytes could be observed (Figure 2). The phenotype of such transitional cells varied from cells with a flat and condensed oval nucleus and a flat cytoplasm (Figure 2, A′ and D [1]) toward cells with an upright cell body sitting on the basement membrane without foot processes and a large lobulated nucleus (Figure 2, B and D [2]). The last feature was the formation of foot processes. Within a single cell, cellular processes with and without foot processes—including a slit diaphragm—could be observed simultaneously (Figure 2C). The transition from a multilayered electron-lucent parietal basement membrane to a glomerular homogeneous electron-dense basement membrane was abrupt and was always associated with transitional cells (Figure 2, C and D, arrowhead).
Figure 2.
Transitional cells at the rat parietal cell–Pod interface. (A) At the VP, epithelial cells with features of parietal cells and Pod can be regularly observed. (A′) Early transitional stage (characteristics: glomerular basement membrane, cytoplasmic vesicles; higher magnification of box in A); e.a., efferent arteriole. (B) Late transitional stage (characteristics: upright cell body, large lobulated nucleus); cap., capillary lumen. (C) Partial formation of foot processes within a “late” transitional cell (*) at the vascular stalk. The intercellular junction between the last parietal cell (PEC) and the transitional cell is marked by a filled arrowhead. The transition from a parietal cell basement membrane to a glomerular basement membrane also occurs at this site. The transitional cell projects extensions onto the base of Bowman's capsule as well as onto a capillary (cap.) without forming foot processes (open arrowheads). A third projection extends onto the vascular stalk and forms typical foot processes with a slit diaphragm (arrow). (D) Grazing section along the vascular stalk. Sequential stages of transitional cells at the parietal/Pod interface (1, early stage; 2, later stage). The intercellular junction toward the last PEC (*) is marked by arrowheads, the transition from a parietal to a glomerular basement membrane is marked by open arrows (A through C, transmission electron micrographs of adult Sprague-Dawley rats).
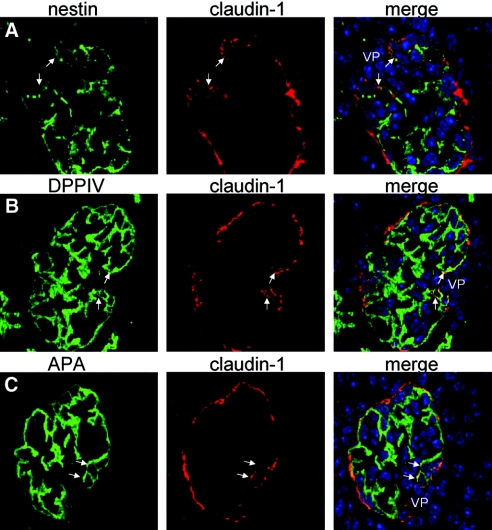
To verify whether transitional cells bear an intermediate phenotype between PECs and podocytes, we determined the expression of marker proteins by triple immunofluorescent labeling and confocal microscopy (Figure 3). Frozen sections of 10-day-old mice were stained with an antibody specific for claudin-1, a marker for PECs within the renal cortex that localizes to intercellular light junctions.20 Apart from the Bowman's capsule, claudin-1–positive cells were regularly present along the vascular stalk at the base of the glomerular tuft, where transitional cells were observed (see previous paragraph; Figure 3, arrows). In a co-staining experiment for the podocyte marker proteins nestin, dipeptidyl peptidase IV, and aminopeptidase A,21–24 the cells at the vascular stalk coexpressed PEC markers as well as the podocyte marker proteins.
Figure 3.
Transitional cells coexpress PEC and Pod marker proteins. (A through C) Normal mouse frozen kidney sections from 10-d-old mice were co-stained with an antiserum specific for claudin-1 (PECs, red) and the Pod markers (green) nestin (A), dipeptidyl peptidase 4 (DPPIV; B), and aminopeptidase A (APA; C). At the VP, claudin-1–positive cells could regularly be observed. These cells at the vascular stalk coexpressed Pod marker proteins (arrows, nuclei are stained blue; immunofluorescent labelings of 2-μm sections analyzed by confocal microscopy).
Lineage Tracing of PECs Using Metabolic Labeling
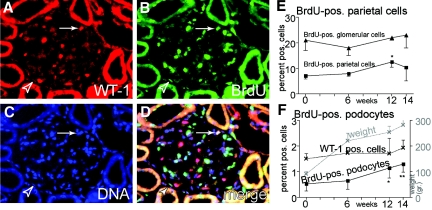
To test our hypothesis that PECs contribute to podocyte turnover, we performed metabolic labeling in adolescent female Sprague-Dawley rats using bromodeoxyuridine (BrdU) for 14 d. We then analyzed BrdU labeling of glomerular cells by triple-immunofluorescent staining immediately after the labeling period (t = 0) and 6, 12, and 14 wk later (Figure 4). Immediately after the labeling, 7.1 ± 1.2% of the PECs were BrdU positive in adolescent rats (versus 1% labeling in adult rats; data not shown), indicating increased PEC proliferation in adolescence. BrdU labeling of PECs was independent of their anatomic location relative to the vascular stalk. Only 0.53 ± 0.28% of all Wilms’ tumor (WT-1)-positive podocytes had incorporated BrdU immediately after BrdU labeling (Figure 4F), confirming that podocytes essentially do not proliferate.
Figure 4.
Metabolic labeling of rat PECs. After weaning, 75-g female Sprague-Dawley rats were labeled with BrdU over 14 d and followed up to 14 wk. (A through D) Representative triple-immunofluorescent staining for the nuclear Pod marker protein WT-1 (red; A), BrdU (green; B), and DNA (blue; C) 12 wk after BrdU labeling. (D) Merged image (phase contrast image not shown). Arrowhead, BrdU-labeled PEC along the inner aspect of Bowman's capsule; arrow, BrdU-labeled WT-1–positive Pod nucleus on the glomerular tuft. (E) BrdU labeling persisted in glomerular cells (mainly mesangial and endothelial cells) without a significant increase over time (▴). Shortly after weaning, 7% of all PECs were metabolically labeled with BrdU. Over time, BrdU labeling of PECs increased significantly, most likely as a consequence of ongoing proliferation and self-regeneration of this cell population (*P = 0.02, one-sided ANOVA test). (F) BrdU-positive Pod were detected significantly more often at 12 or 14 wk after metabolic labeling (*P = 0.03, **P < 0.01, one-sided ANOVA test; n = 3 animals per time point, 300 glomeruli per animal). During the observation period, adolescent rats more than quadrupled their body weights (gray line). No significant increase in Pod numbers (WT-1–positive cells) relative to all glomerular cells was observed.
Metabolic labeling persisted in PECs throughout the observation period of 14 wk (Figure 4E). After 12 and 14 wk, BrdU-positive podocytes increased to 1.1 ± 0.33 and 1.3 ± 0.32%, respectively (Figure 4F). These results support the existence of a regenerative mechanism for podocytes. To resolve this issue further we performed, genetic cell tracing experiments.
A Fragment of the PODXL1 Promoter Drives Expression in PECs but not in Podocytes
To resolve whether PECs migrate onto the glomerular tuft and differentiate into podocytes, we had to identify a PEC-specific promoter to allow genetic tagging of PECs. Three kb of the human podocalyxin (hPODXL1) 5′ flanking region and 0.3 kb of the rabbit Podxl1 5′ untranslated region (GenBank accession no. EU360962) were used to drive expression of rabbit podocalyxin in transgenic mice (pPEC-cPodxl1; Figure 5). This transgenic mouse was originally designed to reproduce the expression pattern of the endogenous podocalyxin gene within podocytes. Unexpected, transgene expression was detected exclusively within PECs but not in podocytes in six of nine founder lines using a monoclonal anti-serum specific for transgenic rabbit podocalyxin24 (Figure 5, B and C).
Figure 5.
Identification of a PEC-specific promoter. (A) Map of the pPEC-cPodxl1 transgene. A 3-kb hybrid of the human and rabbit podocalyxin (hPODXL1) promoter (parietal cell promoter, pPEC) was used to drive expression of rabbit podocalyxin (cPodxl1) in transgenic mice. BGHpA, bovine growth hormone polyadenylation signal. (B through D) Transgene expression within the renal cortex. (B) In adult pPEC-cPodxl1–transgenic mice, rabbit podocalyxin was expressed exclusively in PECs of the renal cortex (arrowheads). (C) Transgene expression was restricted to PECs (arrowheads) and did not extend into the S1 segment of proximal tubular cells, which in male mice extends into Bowman's capsule (arrows). No labeling of Pod was observed (immunohistologic staining using anti-rabbit podocalyxin mouse mAb 4B3). (D and E) In newborn pPEC-cPodxl1 mice, the parietal promoter was active exclusively in mature PECs of the capillary loop stage or mature glomeruli (arrowheads). No transgene expression was observed in earlier developmental stages (e.g., S-shaped bodies [arrow]).
Nephrogenesis persists in newborn mice, so all developmental stages can be observed within the kidney cortex. Transgene expression was detected exclusively in mature PECs beginning at the capillary loop stage but not in earlier stages of glomerular development or anywhere else within the kidney (Figure 5, D and E).
A Novel Transgenic Mouse Line for Inducible Genetic Tagging of PECs
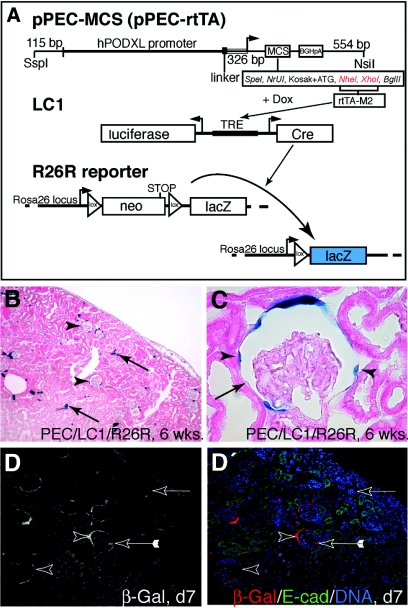
Because of the exceptional specificity of the identified promoter for PECs, we generated a second transgenic mouse to allow inducible genetic tagging of PECs at any desired time point. For this purpose, we placed the enhanced reverse tetracycline transactivator (rtTA-M2)25 under the control of the PEC-specific promoter described in the previous two paragraphs (pPEC-rtTA). Six founders were bred to homozygous LC1/R26R reporter mice for further analysis. Upon administration of doxycycline for 14 d, the LC1 transgene reversibly expressed Cre recombinase under the control of an inducible promoter (tetracycline-responsive element)26 only in PECs. Once Cre excision occurred within the ubiquitously active ROSA26 locus,27 β-galactosidase (β-gal) is constitutively and irreversibly expressed within the cells and all its progeny (“genetic tagging”; Figure 6A).
Figure 6.
Genetic tagging of PECs in a triple-transgenic doxycycline-inducible rtTA mouse line. (A) pPEC-MCS, the rabbit podocalyxin cDNA, was replaced by a multiple cloning site (MCS; available restriction sites indicated). The enhanced tetracycline reverse transactivator (rtTA-M2) was cloned into Nhe1/Xho1 (red). pPEC-rtTA–transgenic mice were generated by pronuclear injection and mated to LC1 mice expressing Cre recombinase and luciferase under the control of tetracycline-responsive elements (TRE), which can be activated by rtTA-M2 in the presence of doxycycline and R26R: This reporter line irreversibly expresses β-gal (LacZ) under the control of the ubiquitous ROSA26 locus only after Cre excision of an interposed floxed neomycin cassette (neo; acting as a stop signal) has occurred. (B) Genetic labeling of PECs using doxycycline in 6-wk-old triple-transgenic pPEC-rtTA mice (pPEC-rtTA/LC1/R26R; 9 wk of age). Sporadic labeling was observed in some tubular cells of the renal cortex (arrows). (C) After induction, Cre recombination occurred in 72% of all PECs (arrowheads, labeled PEC; arrow, unlabeled PEC). (D and D′) Cre recombination was induced in triple-transgenic mice 5 d after birth (d5), when nephrogenesis still persists. Two days after doxycycline administration (d7), specific genetic labeling of PECs was verified by immunofluorescent staining (arrowheads). In 1 to 2% of all glomeruli, labeled cells on the glomerular tuft were observed (arrow with tails, confocal triple-immunofluorescent labeling; red, β-gal [genetic marker for PECs]; green, E-cadherin (proximal tubular cell marker); blue, DNA).
Cre recombination was induced in triple-transgenic pPEC-rtTA/LC1/R26R mice at the age of 6 wk by administration of doxycycline for 14 d (Figure 6, B and C). As judged by enzymatic and immunofluorescent β-gal staining, Cre recombination occurred exclusively within PECs in four transgenic lines. When analyzing the ratio of β-gal–positive to –negative PECs (defined as a cell along Bowman's capsule with a visible nucleus), 72% of all PECs were genetically tagged by Cre recombination. Genetic labeling then persisted in PECs throughout life (1-yr observation period; data not shown), supporting the notion that this cell population regenerates itself. Aberrant Cre recombination in podocytes or other cells in the glomerular tuft was negligible (less than one cell per 100 glomeruli). Similar to pPEC-cPodxl1–transgenic mice, the triple-transgenic lines exhibited sporadic Cre recombination in tubular cells, most notably of collecting ducts (Figure 6B), the thin part of the loop of Henle, and epithelial cells of the pyramid, but not in the urothelial epithelium (see below). In the absence of doxycycline, Cre recombination did not occur even in triple-transgenic pPEC-rtTA/LC1/R26R mice (n = 10; data not shown) older than 1 yr. In summary, the pPEC-rtTA/LC1/R26R mice provide a novel tool to introduce a specific and irreversible genetic label into PECs in vivo and thereby allow for the tracing of these cells for any desired period of time.
Genetic Tagging of PECs in Adolescent Mice
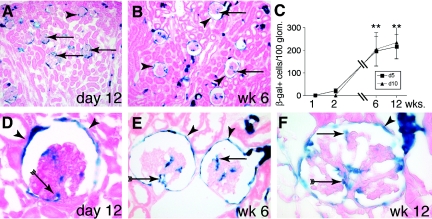
For testing whether PECs contribute to the generation of podocytes, newborn triple-transgenic mice were genetically labeled by doxycycline administration on postnatal day 5. On postnatal day 7, Cre recombination was evident specifically in PECs by their expression of β-gal (Figure 6, D and D′). At this time point, 1.6 labeled cells per 100 glomeruli were detected within the glomerular tuft. Subsequently, a constant increase in genetically labeled glomerular cells was observed over time. On postnatal day 12, 21 ± 10 labeled cells were observed per 100 glomeruli. β-Gal–positive cells increased approximately 10-fold at weeks 6 and 12, respectively (Figure 7). We observed a similar increase of labeled glomerular cells in triple-transgenic mice that received doxycycline 10 d after birth, when nephrogenesis has ceased (Figure 7C). We also noted an increase in genetically labeled tubular epithelial cells.
Figure 7.
Recruitment of PECs onto the glomerular tuft in adolescent pPEC-rtTA/LC1/R26R mice. (A) After genetic tagging of PECs 5 d after birth, β-gal–positive cells (arrows) can be detected on glomerular tufts on day 12. (B) Six weeks after birth, genetically tagged cells are present within most glomeruli (arrows) of the outer cortex as well as close to the medulla. Genetic labeling persists in PECs (arrowheads). (C) Statistical analysis of β-gal–positive cells per 100 glomeruli over time in triple-transgenic PEC-TETon mice induced 5 (d5) or 10 d after birth (d10). A similar increase of β-gal–positive cells over time was observed in both groups (**P < 0.01 ANOVA; n = 5 for each time point). (D through F) Genetic labeling persists in PECs (arrowheads) 12 d (D) and 6 and 12 wk (E and F) after doxycycline administration. β-Gal–positive cells were identified close to the VP (arrow with tails) as well as projecting into the periphery of the glomerulus (arrow). (F) Occasionally, glomeruli with up to 20 β-gal–positive cells were observed at 12 wk of age (arrowheads, labeled PECs; A, B, and D through F, X-gal/eosin staining on 6-μm cryosections).
To test whether genetically labeled cells were fully differentiated podocytes, we analyzed the kidneys of the 6- and 12-wk-old mice by double-immunofluorescent staining and confocal microscopy. Genetically labeled β-gal–positive cells always coexpressed the podocyte marker proteins nephrin, synaptopodin, and WT-1 but not the endothelial marker von Willebrand factor (Figure 8). Of note, genetically labeled transitional cells at the PEC/podocyte interface expressed intermediate levels of the podocyte marker nephrin (Figure 8A, arrowheads).
Figure 8.
β-Gal–positive cells on the glomerular tuft are fully differentiated Pod. (A) Double-immunofluorescent staining for β-gal (red) and the Pod marker protein nephrin (green) in 6-wk-old triple-transgenic PEC-TETon mice induced with doxycycline at the age of 5 d. PECs expressed constitutively β-gal (open arrowheads). β-Gal–positive cells on the vascular tuft were exclusively Pod as demonstrated by nephrin coexpression (green, arrows). Transitional cells, located close to the vascular stalk, were genetically labeled (β-gal positive) and expressed low to intermediate levels of the Pod marker protein nephrin (filled arrowheads). (B) No co-localization of β-gal–positive cells (red, arrow) with the endothelial cell marker vWF (green, arrowheads) was observed in the mice described. Interstitial capillaries are marked in B (vWF) (open arrowhead). (C) β-Gal–positive Pod derived from PECs coexpress WT-1 (arrow, open arrowheads, β-gal positive/WT-1 negative PEC nuclei). Panel shows an enzymatic β-gal staining (in blue)/immunohistochemical 3-amino-9-ethyl-carbazole (AEC) anti–WT-1 (in red) double stainings.
In conclusion, these data show that β-gal–labeled PECs are recruited onto the glomerular tuft during adolescence and that these cells fully differentiate into podocytes.
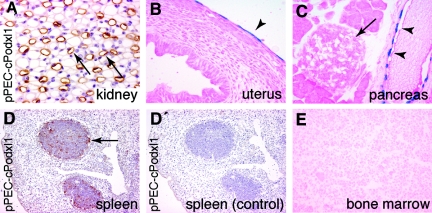
Extrarenal Activity of the PEC-Specific Promoter
The extrarenal activity of the PEC-specific promoter was analyzed by a chemiluminescent assay of total tissue lysates and verified by histology (Table 1, Figure 9). β-Gal expression was noted within the basal layers of the seminiferous epithelium of the testis, the pancreatic ducts, spleen, and thymus follicles (likely MHCII-negative follicular dendritic cells; Figure 9, B through E). Cre recombination was never observed after doxycycline administration within the bone marrow of triple-transgenic mice even when analyzed up to 8 mo (Figure 9E).
Table 1.
Transcriptional activity of the identified PEC promotera
| Parameter | Tissue
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood | Bone Marrow | CNS
|
Eye | Fatty Tissue | Gut
|
Kidney | Liver | Lung | Muscle (Skeletal) | Myocardium | Pancreas | Reproductive Organs
|
Salivary Glands | Skin | Spleen | Thymus | |||||
| Front | Hind | Stomach | Small Intestine | Large Intestine | Female | Male | |||||||||||||||
| Chemiluminescenceb | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. | Neg. | +++ | Neg. | Neg. | Neg. | Neg. | ++ | (+) | +++ | ++ | Neg. | ++ | ++ |
| Histologyc | Neg. | Neg. | ND | ND | ND | Neg. | Neg. | Neg. | ND | Parietal cells, thin part of loop of Henle, mosaic: collecting duct, epithelial cells of the pyramid | Neg. | ND | ND | Neg. | Pancreatic duct | Uterine glandular cells | Basal layers of the seminiferous epithelium | Salivary ducts | Neg. | Follicular dendritic cells (?) | Follicular dendritic cells (?) |
ND, not done; Neg., no expression of β-gal detected; (+), weak luminescence; ++, significant luminescence; +++, strong luminescense.
The pattern of Cre recombination was evaluated in tissues of triple-transgenic pPEC-rtTA/LC1/R26R mice induced with doxycycline at the age of 6 wk using a sensitive chemiluminescent assay.
β-Gal expression was confirmed by enzymatic stainings with X-Gal on cryosections.
Figure 9.
Activity of the parietal cell promoter. (A) Within the kidney, activity of the PEC promoter was also observed within the thin limb of the loop of Henle (arrows, pPEC-cPodxl1 transgenic mouse; brown, anti-rabbit podocalyxin staining, hematoxylin counter staining). (B) Mesothelial cells lining the peritoneal cavity on the uterus were genetically labeled in a mosaic manner (arrowhead). (C) Cre recombination occurred within the epithelium of the pancreatic ducts (arrowheads) but not within glandular cells or pancreatic islets (arrow). (D and D′) Activity of the parietal cell promoter within follicles of the spleen (arrow) was visualized by immunohistology in pPEC-cPodxl1–transgenic mice (D, anti-rabbit podocalyxin in brown; D′ control using irrelevant primary antibody). (E) No evidence for Cre recombination was observed within cells of the bone marrow of mice more than 8 mo after induction (B, C, and E, pPEC-rtTA/LC1/R26R mouse, X-gal/eosin staining on cryosections).
DISCUSSION
In this study, we provide the first evidence that podocytes are recruited from PECs under physiologic conditions. This concept is based on four major findings.
Our first major finding was that transitional cells with morphologic and immunohistochemical features of both PECs and podocytes could be detected at the glomerular vascular stalk. One feature of transitional cells was the presence of a glomerular basement–type membrane, which was always associated with them but not with PECs. Further features included heterogeneous vacuoles, an upright cell body, and the transition to a large lobulated nucleus. Cells with similar features were first described within the glomeruli of sheep and were subsequently detected in many species.28 Given their location close to the vascular pole, these cells were termed “peripolar cells.” Because of the multiple vacuoles, which were also observed in this study, it was initially speculated that these cells secrete hormones or other mediators29; however, until now, no specific hormone could be detected and the function of these cells remained elusive. Our data suggest that peripolar cells are PECs in the process of differentiating into podocytes. Our findings are also in agreement with Bariety et al.,30 who showed that cells expressing podocyte markers extend along the vascular stalk onto Bowman's capsule at the vascular pole in the majority of glomeruli of normal human kidneys. In this work, we observed that cells expressing the PEC marker claudin-1 extend along the vascular stalk onto the glomerular tuft and coexpress podocyte marker proteins. The region of overlap defines the area of PEC to podocyte differentiation.
Second, in metabolic pulse-chase experiments in juvenile rats, we observed a two- to three-fold increase in BrdU-labeled podocytes after BrdU administration. Because direct labeling of podocytes could no longer contribute to the increase, such an increase could be explained by nuclear divisions of BrdU-labeled podocytes. Although there is no evidence to support the occurrence of significant cellular divisions (cytokinesis) of podocytes in vivo,9 at least nuclear divisions have been observed in podocytes under very particular conditions such as infusion of high dosages of fibroblast growth factor 231; however, no bi-nucleated podocytes were observed in our study.
More likely, the two- to three-fold increase represents podocyte replacement by BrdU-labeled hematopoietic cells11–13 or cells of an intrarenal origin, such as PEC. In the latter case, one can calculate that at a labeling efficiency of 11% of all PECs at 14 wk, between 7.0 ± 0.2 and 12.0 ± 0.3% of all podocytes would have been derived from BrdU-positive PECs, concurring with our results obtained from the genetic tagging experiments.
Our third major finding was the identification of a novel promoter with PEC specificity in the glomerulus, which provided the foundation to trace the fate of PECs in vivo. That the novel PEC-specific promoter identified in this study was derived from a relatively podocyte-specific gene (podocalyxin) further supports a close relationship between these two cell populations. So far, it is unknown why the promoter was active exclusively in PECs and not in podocytes. In development, endogenous podocalyxin is also expressed by PECs within the kidney, albeit at a low level.32 For the purposes of our studies, however, this issue is of little relevance, because genetic labeling using Cre recombination follows an “all or nothing law,” whereby only cells that express sufficient levels of Cre recombinase undergo recombination. These cells (e.g., PECs) will then express maximum levels of the reporter gene β-gal. Consequently, even if there were low-level expression of the construct in podocytes, this would not be detected with our system because the threshold for Cre recombination is obviously not reached in podocytes or any other glomerular cell. Because no genetically marked cells were observed within the kidney in triple-transgenic mice that had not received doxycycline, the use of the doxycycline-inducible system effectively precluded aberrant Cre recombination during the chasing period. Also, Cre recombination did not occur in bone marrow cells. This finding is important given the observation of bone marrow–derived cells in podocyte locations in diseased rodent and human kidneys.11–13 Such cells, therefore, cannot account for the findings of this study. In summary, the pPEC-rtTA/LC1/R26R mice provide a novel tool to introduce a specific and irreversible genetic label into PECs in vivo and thereby allow for the tracing of these cells for any desired period of time. Any increase in labeled podocytes after completion of the genetic labeling with doxycycline could have originated only from PECs migrating onto the glomerular tuft.
The fourth major finding of this study was that in cell-tracing experiments, podocytes were recruited from PECs. Recruitment of podocytes was high during postnatal glomerulogenesis as well as in juvenile mice, when glomerulogenesis was completed and a rapid, approximately 10-fold increase in size of the kidney occurred. Our data thereby provide the experimental proof for a hypothesis that in parallel was proposed on the basis of findings in human PEC.33 Thus, in the study of Ronconi et al.,33 it was shown that PECs express stem cell marker proteins and gradually lose the expression of these proteins as they approach the glomerular tuft. Furthermore, PECs that express stem cell markers retained multipotency and could engraft and differentiate into podocytes in the glomeruli of developing fetal kidneys. Our observation also provides an explanation for the findings of Bariety et al.30 and Gibson et al.,30,34 who observed that Bowman's capsule can be lined by “parietal podocytes” in atubular glomeruli of damaged human transplant kidneys. Interestingly, parietal podocytes coexpressed PEC and podocyte marker proteins (e.g., Pax2, WT-1), suggesting that these cells resembled transitional cells at the vascular stalk. Our findings, therefore, suggest that parietal podocytes in atubular glomeruli are the result of a premature PEC differentiation triggered by an as-yet-unknown signal.
Our data do not exclude that other mechanisms of podocyte regeneration exist, such as their replacement by bone marrow30,34derived cells11–13; however, our data are more suitable to explain the development of the glomerular tip lesion, which by some authors is considered to represent an early stage in the evolution of FSGS.35 Thus, our findings suggest that within the glomerulus a gradient exists, whereby podocytes at the glomerular tip would represent the “oldest” podocytes, which in turn might be most susceptible to damage.
In summary, using various lines of evidence, we demonstrate that podocytes can be recruited from PECs. Our findings explain how the growing glomerulus is covered with podocytes despite their inability to undergo cell division. Finally, the observation of podocyte recruitment from PECs lays the basis for searching for pharmacologic strategies aimed at accelerating podocyte and thereby glomerular regeneration. This will represent a completely novel approach to treating chronic kidney disease.
CONCISE METHODS
Plasmid Construction and Generation of Transgenic Mice
The human promoter/enhancer of hPODXL1 (-3 kb until 97 bp 5′ of the ATG) was amplified by PCR (Accuprime; Invitrogen, Karlsruhe, Germany) from a BAC clone RP11-180C16 (accession no. AC008264.10, Homo sapiens chromosome 7) using forward primer 5′-AGTAACTAGTCTTCATAGTATTGGCTTCTGT3-′ and reverse primer 5′-AGTAAGATCTTGTGGGTGGCTCCGGAGGC-3′. The resulting promoter fragment was cloned in reverse orientation into pGlowTOPO (Invitrogen) using Spe1/BglII (clone A). Rabbit podocalyxin cDNA, including 326 bp of cPodxl1 5′untranslated region, was released using EcoRI (fragment size 2 kb) from a full-length hPODXL1 clone,24 filled in with Klenow (Promega, Madison, WI) and cloned into clone A using XbaI blunt ended with Klenow (pGlowTopo-cPodxl1). The human promoter region phPODXL1 was reversed in orientation by digesting clone A with Bgl II and SpeI, filled with Klenow and ligated into PGlowTopo-cPodxl1, digested with BglII and Spe1, filled with Klenow (pPEC-cPodxl1). The entire promoter region was sequenced (GenBank accession no. EU360962).
The cPodxl1 cDNA was replaced by a multiple cloning site by PCR mutagenesis using Accuprime and the primers PECMCS.fwd 5′P-ATGGCTAGCCTCGAGATCTGGACAACCTGACCAAGGACG-3′ and PECMCS.rev 5′P-GGTGGTCGCGACTAGTCCTCGCTCCGGGGGCCTGGA-3′ using pPEC-cPodxl1 as template. The template was removed by digestion with DpnI, and the resulting 8-kb product was recircularized using T4 ligase (pPEC-MCS; Fermentas, St. Leon-Rot, Germany). PUHrt62–1, containing the improved reverse tetracycline–inducible transactivator (rtTA-M2),25 was digested with Xba1/BamH1 and cloned into pPEC-MCS digested with Spe1/BglII (pPEC-rtTA-M2). The enhanced transcription factor rtTA-M2 is characterized by lower background activity and a higher sensitivity to doxycycline. The promoter and coding region, including the polyadenylation signal of this clone, was entirely sequenced. For pronuclear injection of pPEC-cPodxl1 and pPEC-rtTA-M2, the prokaryotic sequence was removed using SspI and NsiI. Pronuclear injection was performed according to standard procedures into a 129/SvEv × C57BL/6J genetic background at the Interfakultaere Biomedizinische Forschungseinrichtung (IBF) of the Heidelberg University (Heidelberg, Germany). All three transgenes were heterozygous within the experimental animals. For achieving this, the PEC-rtTA line was mated to homozygous LC1/R26R mice, yielding 50% triple-transgenic offspring.
All animal studies were approved by the University of Michigan Committee on Use and Care of Animals and by the LANUV Cologne 9.32.2.10.35.07.041 and Stuttgart 35-9185.81/G-32/03. All experimental groups contained a similar distribution of males and females unless stated otherwise. Animals received regular feeding and water ad libitum and were kept under specific pathogen–free conditions in a 12-h light cycle (22°C, 50% humidity).
Genotyping
Nine founder mice were identified by PCR from tail biopsies, which were incubated in 190 μl of Viagen DirectPCR lysis reagent (Viagen cat. no. 102-T; Viagen, Los Angeles, CA) and 10 μl of Proteinase K (Sigma cat. no. 6556; Sigma Aldrich, St. Louis, MO) stock concentration of 4 mg/ml at 55°C overnight and heat inactivated at 85°C for 45 min. The following primers were used: Cre forward GCATAACCAGTGAAACAGCATTGCTG and reverse GGACATGTTCAGGGATCGCCAGGCG; LacZ forward TTCACTGGCCGTCGTTTTACAACGTCGTGA and reverse ATGTGAGCGAGTAACAACCCGTCGGATTCT; LC1 forward TTACAGATGCACATATCGAGG and reverse TAACCCAGTAGATCCAGAGG; TetOn forward AATCGAGATGCTGGACAGGCATCATACCCA and reverse GGCATAGAATCGGTGGTAGGTGTCTCTCTT; and ROSA26 forward GCGAAGAGTTTGTCCTCAACC, ROSA26_1 reverse GGAGCGGGAGAAATGGATATG, and ROSA26_2 reverse AAAGTCGCTCTGAGTTGTTAT.
Transgene expression was evaluated in pPEC-Podxl1 mice by immunohistology according to standard techniques36 using an antiserum specific for rabbit podocalyxin (4B3).24 Six of nine founder lines expressed transgenic rabbit podocalyxin. Nine founder animals transgenic for pPEC-rtTA were obtained.
Doxycycline Treatment
Double- or triple-transgenic animals received doxycycline hydrochloride via the drinking water for a total of 14 d (5% sucrose and 0.5 mg/ml doxycycline, protected from light), which was exchanged every 2 d. For induction of 5- or 10-d-old animals, 50 ng/g body wt doxycycline dissolved in 0.45% NaCl solution was injected intraperitoneally over a total of 3 d.
Perfusion Fixation and Electron Microscopy
Mice were anesthetized (Avertin, Sigma Aldrich, St. Louis, MO) and ice-cold perfusion solution (3% paraformaldehyde, 0.2% glutaraldehyde, 2.5 mM EGTA, and 4 mM MgCl2 in 0.5 × PBS [pH 7.6]) was perfused into the left ventricle for 3 min followed by 20% sucrose for 1 min. Organs and tissues were immediately recovered and snap-frozen in Tissue-Tek (Miles Inc., Iowa City, IA) or embedded in paraffin. Transmission electron microscopy was performed as described previously.37
β-Gal Assays
β-Gal activity was measured in unfixed snap-frozen tissues using a commercial chemiluminescent assay, Galacto-Star (Tropix, Bedford, MA), as described previously.38 For enzymatic X-Gal staining, 6-μm cryosections were cut and incubated overnight at 35°C in a humidified atmosphere in staining solution (1 mg/ml X-Gal, 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, and 2 mM MgCl2 in PBS [pH 7.8]). On the next day, samples were counterstained with eosin, washed in tap water, and mounted (Immu-Mount; Thermo Scientific, Waltham, MA).
Immunofluorescence
Immunofluorescence was performed as described previously39,40 on 2- and 4-μm cryosections blocked with 10% donkey serum in PBS (017-000-121; Jackson Immunoresearch Laboratories, West Grove, PA) and incubated with the following antibodies: Chicken anti–β-gal polyclonal antibody (1:100, ab9361, lot no. 301516; Abcam, Cambridge, UK), mouse anti-nephrin polyclonal antibody (1:100; a gift of Lawrence B. Holzman, Ann Arbor, MI), mouse anti-synaptopodin polyclonal antibody (1:100, 65294; Progen, Heidelberg, Germany), rabbit anti–claudin-1 (DAKO, Glostrup, Denmark), rabbit anti–von Willebrand factor (A0082; DAKO), chicken anti-nestin (DAKO), rat anti–aminopeptidase A (ASD41), rat anti-DPPIV (ASD36),23 goat anti-rabbit Dylight 549 (1:100, 35557; Pierce Biotechnology, Rockford, IL), Alexa Fluor 488–conjugated goat anti-rat (1:200, A11006; Molecular Probes, Eugene, OR), Cy3-conjugated donkey anti-chicken IgG and Cy2-conjugated rabbit anti-mouse IgG (1:100, 703-225-155 and 715-225-151, respectively; Jackson Immunoresearch Laboratories). Nuclei were stained with TO-PRO-3 (624/661; 1:200, T3605; Molecular Probes). All secondary antibodies, except the anti-mouse antibody, were immunoabsorbed with 4% normal mouse serum. Irrelevant rabbit IgG was used as control. For immunohistochemical stainings, the X-gal–stained cryosections were boiled in citrate buffer, blocked against avidin and biotin, and stained with the anti–WT-1 antiserum (1:400; Santa Cruz Biotechnology, Santa Cruz, CA). Secondary anti-rabbit (BA-1000; Vector Laboratories, Burlingame, CA) was visualized using 3-amino-9-ethyl-carbazole (AEC). Sections were evaluated with an Olympus BX 41 microscope (Hamburg, Germany) and Zeiss LSM 510 Meta laser inverted confocal microscope, (Goettingen, Germany). Images were collected with AnalySIS (Soft Imaging System, Münster, Germany) and prepared for presentation with Adobe Photoshop and Illustrator software (Adobe Systems, Mountain View, CA).
Metabolic Labeling
Female Sprague-Dawley rats weighing 75 g were purchased from Charles River (Charles River Laboratories, Inc., Wilmington, MA). BrdU (B-5002; Sigma) labeling was performed as described previously.17 In brief, rats received a subcutaneous injection of 25 μg of BrdU in 500 μl sterile 0.9% NaCl twice a day for 14 d. Animals were anesthetized with isoflurane and ketamine/rompune and perfusion-fixed through the left ventricle with 3% paraformaldehyde in 0.5% PBS (pH 7.6) for 3 min. Four-micrometer cryosections were boiled in citrate buffer three times for 5 min; blocked with 10% donkey serum; incubated with anti-BrdU antibody (MAB3424, 1:200; Chemicon, Billerica, MA), anti–WT-1 (1:400; Santa Cruz Biotechnology, Santa Cruz, CA), and Hoechst 33342 (0.1 mg/ml; Sigma), counterstained; and mounted as described already.
Statistical Analysis
Three hundred glomeruli were evaluated for each experimental animal on random transverse sections through the middle part of the kidney. Glomeruli that did not have a glomerular tuft or that were sectioned close to the edge were disregarded. Transitional cells were identified on X-gal–stained cryosections as large upright epithelial cells located along the vascular stalk. PECs were counted only when their cellular body, including the nucleus, was seen. Data were analyzed using Prism 4.0 for Macintosh (GraphPad, San Diego, CA) using the one-sided ANOVA test.
DISCLOSURES
None.
Acknowledgments
This work was supported by the German Research Foundation (MO1082/1-1, 1-2, 3-1); the Else-Kröner-Fresenius-Stiftung; the START Program of the Faculty of Medicine, RWTH-Aachen (to M.J.M.); and by the NIH (R01-DK058270 to D.B.K.). M.M. is a member of the DFG research group “Mechanisms of Chronic Renal Failure” and of the Transregio/SFB DFG consortium “Mechanisms of Organ Fibrosis.”
Published online ahead of print. Publication date available at www.jasn.org.
D.A. and D.K. contributed equally to this work.
See related editorial, “Parietal Epithelial Cells Regenerate Podocytes,” on pages 231–233.
REFERENCES
- 1.US Renal Data System: USRDS 2007 Annual Report, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007
- 2.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Novick AC, Gephardt G, Guz B, Steinmuller D, Tubbs RR: Long-term follow-up after partial removal of a solitary kidney. N Engl J Med 325: 1058–1062, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Hara M, Yamamoto T, Yanagihara T, Takada T, Itoh M, Adachi Y, Yoshizumi A, Kawasaki K, Kihara I: Urinary excretion of podocalyxin indicates glomerular epithelial cell injuries in glomerulonephritis. Nephron 69: 397–403, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Vogelmann SU, Nelson WJ, Myers BD, Lemley KV: Urinary excretion of viable podocytes in health and renal disease. Am J Physiol Renal Physiol 285: F40–F48, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu D, Petermann A, Kunter U, Rong S, Shankland SJ, Floege J: Urinary podocyte loss is a more specific marker of ongoing glomerular damage than proteinuria. J Am Soc Nephrol 16: 1733–1741, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Fries JW, Sandstrom DJ, Meyer TW, Rennke HG: Glomerular hypertrophy and epithelial cell injury modulate progressive glomerulosclerosis in the rat. Lab Invest 60: 205–218, 1989 [PubMed] [Google Scholar]
- 9.Pabst R, Sterzel RB: Cell renewal of glomerular cell types in normal rats: An autoradiographic analysis. Kidney Int 24: 626–631, 1983 [DOI] [PubMed] [Google Scholar]
- 10.Marshall CB, Shankland SJ: Cell cycle and glomerular disease: A minireview. Nephron Exp Nephrol 102: e39–e48, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT: Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24: 2448–2455, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R: Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci U S A 103: 7321–7326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker JU, Hoerning A, Schmid KW, Hoyer PF: Immigrating progenitor cells contribute to human podocyte turnover. Kidney Int 72: 1468–1473, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ito T, Suzuki A, Imai E, Okabe M, Hori M: Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol 12: 2625–2635, 2001 [DOI] [PubMed] [Google Scholar]
- 15.Imasawa T: Roles of bone marrow cells in glomerular diseases. Clin Exp Nephrol 7: 179–185, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Lin F, Moran A, Igarashi P: Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vogetseder A, Karadeniz A, Kaissling B, Le Hir M: Tubular cell proliferation in the healthy rat kidney. Histochem Cell Biol 124: 97–104, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P: Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P: Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 18: 3128–3138, 2007 [DOI] [PubMed] [Google Scholar]
- 20.Ohse T, Pippin JW, Vaughan MR, Brinkkoetter PT, Krofft RD, Shankland SJ: Establishment of conditionally immortalized mouse glomerular parietal epithelial cells in culture. J Am Soc Nephrol 19: 1879–1890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou J, Yaoita E, Watanabe Y, Yoshida Y, Nameta M, Li H, Qu Z, Yamamoto T: Upregulation of nestin, vimentin, and desmin in rat podocytes in response to injury. Virchows Arch 448: 485–492, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Chen J, Boyle S, Zhao M, Su W, Takahashi K, Davis L, Decaestecker M, Takahashi T, Breyer MD, Hao CM: Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J Am Soc Nephrol 17: 1283–1291, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Mentzel S, Dijkman HB, Van Son JP, Koene RA, Assmann KJ: Organ distribution of aminopeptidase A and dipeptidyl peptidase IV in normal mice. J Histochem Cytochem 44: 445–461, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Kershaw DB, Thomas PE, Wharram BL, Goyal M, Wiggins JE, Whiteside CI, Wiggins RC: Molecular cloning, expression, and characterization of podocalyxin-like protein 1 from rabbit as a transmembrane protein of glomerular podocytes and vascular endothelium. J Biol Chem 270: 29439–29446, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Urlinger S, Baron U, Thellmann M, Hasan MT, Bujard H, Hillen W: Exploring the sequence space for tetracycline-dependent transcriptional activators: Novel mutations yield expanded range and sensitivity. Proc Natl Acad Sci U S A 97: 7963–7968, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schonig K, Schwenk F, Rajewsky K, Bujard H: Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res 30: e134, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soriano P: Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71, 1999 [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi H, Kaku H, Onodera T, Kurokawa R, Morisada M: Peripolar cells in guinea pigs under experimental hyperplasia of juxtaglomerular cells induced by long-term, low-dose calcium condition. Exp Toxicol Pathol 46: 283–286, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Gardiner DS, Downie I, Gibson IW, More IA, Lindop GB: The glomerular peripolar cell: A review. Histol Histopathol 6: 567–573, 1991 [PubMed] [Google Scholar]
- 30.Bariety J, Mandet C, Hill GS, Bruneval P: Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Kriz W, Hahnel B, Rosener S, Elger M: Long-term treatment of rats with FGF-2 results in focal segmental glomerulosclerosis. Kidney Int 48: 1435–1450, 1995 [DOI] [PubMed] [Google Scholar]
- 32.Bariety J, Bruneval P, Hill G, Irinopoulou T, Mandet C, Meyrier A: Posttransplantation relapse of FSGS is characterized by glomerular epithelial cell transdifferentiation. J Am Soc Nephrol 12: 261–274, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P: Regeneration of glomerular podocytes by human renal progenitors. J Am Soc Nephrol 20: 322–332, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gibson IW, Downie TT, More IA, Lindop GB: Atubular glomeruli and glomerular cysts: A possible pathway for nephron loss in the human kidney? J Pathol 179: 421–426, 1996 [DOI] [PubMed] [Google Scholar]
- 35.D'Agati VD, Fogo AB, Bruijn JA, Jennette JC: Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 43: 368–382, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Moeller MJ, Soofi A, Sanden S, Floege J, Kriz W, Holzman LB: An efficient system for tissue-specific overexpression of transgenes in podocytes in vivo. Am J Physiol Renal Physiol 289: F481–F488, 2005 [DOI] [PubMed] [Google Scholar]
- 37.Kriz W, Hahnel B, Hosser H, Ostendorf T, Gaertner S, Kranzlin B, Gretz N, Shimizu F, Floege J: Pathways to recovery and loss of nephrons in anti-Thy-1 nephritis. J Am Soc Nephrol 14: 1904–1926, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Moeller MJ, Kovari IA, Holzman LB: Evaluation of a new tool for exploring podocyte biology: Mouse Nphs1 5′ flanking region drives LacZ expression in podocytes. J Am Soc Nephrol 11: 2306–2314, 2000 [DOI] [PubMed] [Google Scholar]
- 39.Moeller MJ, Soofi A, Braun GS, Li X, Watzl C, Kriz W, Holzman LB: Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. EMBO J 23: 3769–3779, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moeller MJ, Soofi A, Hartmann I, Le Hir M, Wiggins R, Kriz W, Holzman LB: Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol 15: 61–67, 2004 [DOI] [PubMed] [Google Scholar]