Abstract
Although metabolic derangement plays a central role in diabetic nephropathy, a better understanding of secondary mediators of injury may lead to new therapeutic strategies. Expression of macrophage migration inhibitory factor (MIF) is increased in experimental diabetic nephropathy, and increased tubulointerstitial mRNA expression of its receptor, CD74, has been observed in human diabetic nephropathy. Whether CD74 transduces MIF signals in podocytes, however, is unknown. Here, we found glomerular and tubulointerstitial CD74 mRNA expression to be increased in Pima Indians with type 2 diabetes and diabetic nephropathy. Immunohistochemistry confirmed the increased glomerular and tubular expression of CD74 in clinical and experimental diabetic nephropathy and localized glomerular CD74 to podocytes. In cultured human podocytes, CD74 was expressed at the cell surface, was upregulated by high concentrations of glucose and TNF-α, and was activated by MIF, leading to phosphorylation of extracellular signal–regulated kinase 1/2 and p38. High glucose also induced CD74 expression in a human proximal tubule cell line (HK2). In addition, MIF induced the expression of the inflammatory mediators TRAIL and monocyte chemoattractant protein 1 in podocytes and HK2 cells in a p38-dependent manner. These data suggest that CD74 acts as a receptor for MIF in podocytes and may play a role in the pathogenesis of diabetic nephropathy.
Diabetic nephropathy (DN) is one of the major complications of diabetes and the most common cause of ESRD. Hyperglycemia activates secondary mediators that lead to DN. In this regard, inflammatory cytokines may be critical in the development of microvascular diabetic complications and nephropathy.1 Monocyte chemoattractant protein 1 (MCP-1) is considered a diagnostic marker and therapeutic target in DN.2 TNF-related apoptosis inducing ligand (TRAIL) expression is increased in diabetic nephropathy and induces tubular cell apoptosis.3 A better understanding of secondary mediators of injury may lead to the design of new therapeutic strategies.
Macrophage migration inhibitory factor (MIF) is a widely expressed pleiotropic cytokine, exhibiting a broad range of immune and inflammatory activities.4 MIF has been implicated in renal injury. A marked increase in MIF was noted in glomeruli and tubules in proliferative forms of human glomerulonephritis, where de novo MIF expression was localized to glomerular endothelial and mesangial cells.5 Cultured tubular and mesangial cells produce MIF in response to inflammatory stimuli.6,7 Enhanced podocyte expression of a MIF transgene induces podocyte injury in mice, independent of leukocyte infiltration, suggesting autocrine effects of MIF on podocytes.8 Urine MIF concentration is significantly increased in proliferative forms of glomerulonephritis and correlates with the degree of renal injury.9
Evidence has also linked MIF to DN. MIF expression is increased in experimental DN.10 Serum MIF concentration are elevated in individuals with type 2 diabetes11 and also locally at sites injured by diabetes in patients with proliferative diabetic retinopathy.12
Although MIF was identified as a soluble, T cell–derived factor in 1966,13 the nature of its membrane receptor was unknown until 2003, when CD74 was shown to be a receptor for MIF.14 CD74 antigen (invariant polypeptide of MHC, HLA-DR γ) is a type II transmembrane protein that plays a critical role in class II MHC antigen processing.15 In addition, CD74 may transduce MIF-induced activation of the extracellular signal–regulated kinase 1/2 (ERK1/2) mitogen-activated protein kinase (MAPK) cascade and promotes cell proliferation and prostaglandin E2 production in leukocytes and fibroblasts.14 On the basis of the observation that CD74 expression was increased in transcriptome studies of human DN, we investigated the role of CD74 in transducing MIF signals in human podocytes. CD74 was found to be increased in podocytes and tubular cells from humans and animals with DN, and cultured podocytes expressed CD74 that mediated MIF activation of MAPK as shown by knockdown experiments. Furthermore, MIF promoted MCP-1 and TRAIL expression in podocytes and tubular cells.
RESULTS
Identification of CD74 as an Overexpressed Gene in Human DN
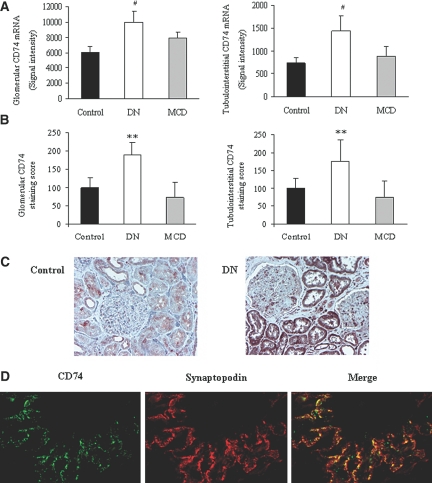
Increased renal interstitial CD74 mRNA expression (a 1.92-fold increase over living kidney donor controls; P = 0.0015) had been noted by transcriptomic analysis of human DN biopsy tubulointerstitium.3,16 We now report similar findings in the renal tubulointerstitium of a different cohort of DN patients: Pima Indians with DN, on whom CD74 mRNA was increased 1.95-fold over living kidney donor control subjects (P < 0.0001; Figure 1A). In addition, a 1.64-fold increase in CD74 mRNA was noted in the glomeruli from Pima Indians with diabetes (P < 0.0001; Figure 1A). By contrast, changes in minimal-change disease (MCD) were <1.5-fold in both compartments (Figure 1A).
Figure 1.
Increased CD74 expression in human DN biopsies. (A) Transcriptomic analysis of CD74 mRNA in glomerular and tubulointerstitial compartments of living kidney donors (control, n = 6) and Pima Indians with diabetes (DN, n = 20) and MCD (n = 4). Data are means ± SD; #P < 0.0001 versus control. (B) Semiquantification of CD74 immunoreactivity within the glomerular and tubular compartments in the kidney biopsies from control subjects (n = 5) and patients with DN (n = 5) and MCD (n = 4). **P = 0.01 versus control. (C) Representative image of a control and a DN sample. A marked increase in CD74 staining in DN is visible in both glomerular and tubular cells. (D) Co-localization of CD74 and synaptopodin in podocytes in a glomerulus from DN biopsy.
In an independent second group of patients with DN, immunohistochemistry demonstrated increased expression of CD74 protein in both the glomerular and tubular compartments (Figure 1, B and C). By contrast, no significant changes in protein expression were observed in MCD (Figure 1B). Confocal microscopy co-localized CD74 and synaptopodin, indicating that podocytes contribute to glomerular CD74 expression in DN (Figure 1D).
Increased CD74 Expression in Experimental DN
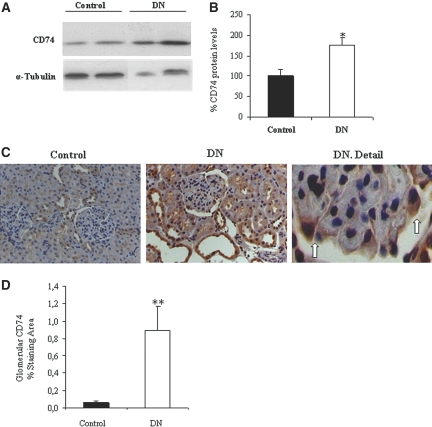
In a chronic rat model of DN induced by a single injection of streptozotocin (STZ) and characterized by the development of albuminuria at 7 mo of follow-up (1071 ± 247 versus 390 ± 70 μg/24 h; P < 0.02; Supplemental Table 1), CD74 was increased in whole kidney by Western blot (Figure 2, A and B). Immunohistochemistry localized the increased CD74 expression to both glomeruli and tubules of DN rats (Figure 2C). In glomeruli, CD74 was localized to podocytes (Figure 2C). Quantification of immunohistochemistry preparations showed a significant increase in glomerular CD74 staining (Figure 2D).
Figure 2.
Expression of CD74 protein in experimental DN. (A) Western blot of whole kidney. Representative image. (B) Western blot quantification expressed as percentage increase over control. Data are means ± SD of 10 rats per group. *P < 0.05 versus control. (C) Immunohistochemistry, representative images of control kidney, DN, and a detail of DN in which podocyte staining for CD74 is observed (arrows). (D) Quantification of glomerular CD74 expression by immunohistochemistry. **P < 0.01 versus control. Magnification, ×200.
Expression of CD74 in Cultured Human Podocytes
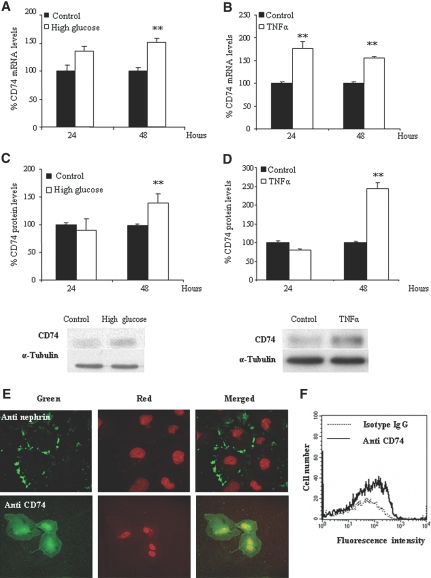
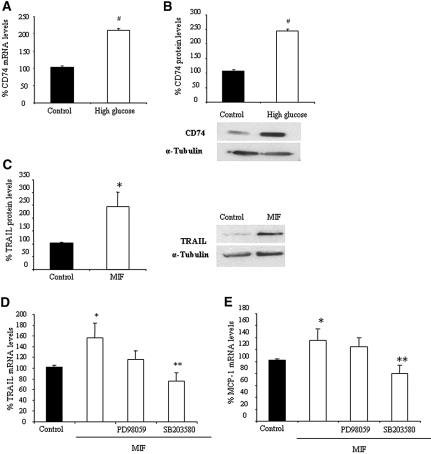
We found differentiated cultured human podocytes to express CD74 mRNA and protein (Figure 3). Either a high-glucose medium or the inflammatory cytokine TNF-α increased CD74 mRNA (Figure 3, A and B) and protein expression (Figure 3, C and D). We observed no changes (<7% variation at 24 or 48 h) in CD74 expression with mannitol, used as an osmolarity control. The delayed increase in protein is consistent with findings in other cell types stimulated with IFN-γ.17 Confocal microscopy showed that the bulk of CD74 localizes to the perinuclear region (Figure 3E). This is consistent with findings in other cell types.18 We also found CD74 in the nucleus. This is also consistent with the fact that CD74 may undergo regulated intramembrane proteolysis and migrate to the nucleus.18 Of more relevance to the present research, we also observed CD74 at the cell membrane. Staining for nephrin confirmed the differentiated state of podocytes (Figure 3E). Flow cytometry confirmed the presence of CD74 at the cell surface (Figure 3F), supporting its possible role as a receptor.
Figure 3.
Expression of CD74 by human cultured podocytes. (A) High glucose increases CD74 mRNA. Real-time reverse transcription–PCR (RT-PCR). Data are means ± SD of three independent experiments. **P < 0.01 versus control 48 h. (B) TNF increases CD74 mRNA. Real-time RT-PCR. Data are means ± SD of three independent experiments. **P < 0.01 versus control. (C) High glucose increases CD74 protein expression. Representative Western blot and quantification. Data are means ± SD of three independent experiments. **P < 0.01 versus control 48 h. (D) TNF increases CD74 protein. Data are means ± SD of three independent experiments. **P < 0.01 versus control 48 h. (E) Confocal microscopy localized CD74 to the perinuclear region and the cell membrane. Nephrin staining used the same secondary antibody and confirmed the differentiated status of podocytes. (F) Flow cytometry of nonpermeabilized cells showed that CD74 is expressed in the cell surface.
Actions of MIF in Cultured Podocytes Are Mediated by Engagement of CD74
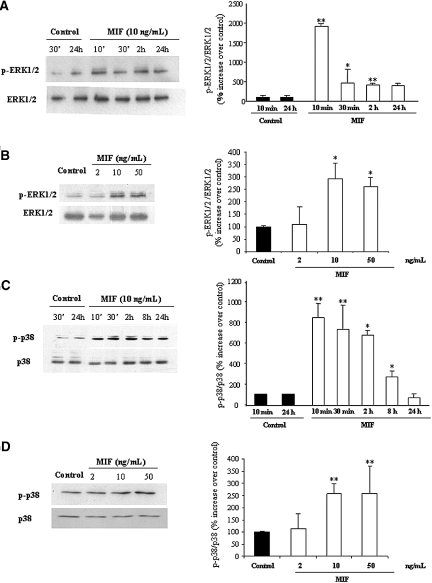
We investigated the sensitivity of podocytes to the CD74 ligand MIF. MIF was previously linked to glomerular injury and the complications of diabetes,10–12 but the MIF receptor(s) in the kidney had not been characterized. Phosphorylation of ERK1/2 was recently found to be a CD74-mediated MIF effect in extrarenal cells.14 In podocytes, MIF induced phosphorylation of ERK1/2 and p38 MAPK in a time- and dosage-dependent manner (Figure 4). As previously reported for CD74-mediated responses in extrarenal cells, ERK1/2 phosphorylation in response to MIF persisted for up to 24 h (Figure 4A). We observed similar results for p38 MAPK (Figure 4, C and D).
Figure 4.
MIF activates MAPK in cultured podocytes. (A through D) MIF stimulation results in ERK1/2 (A and B) and p38 (C and D) phosphorylation. Time course (A, **P < 0.01 versus control *P < 0.05 versus control; C, **P < 0.01 versus control *P < 0.05 versus control) and dose-response at 30 min (B, *P < 0.05 versus control; D, **P < 0.01 versus control). Representative Western blots and quantification (means ± SD) of three independent experiments.
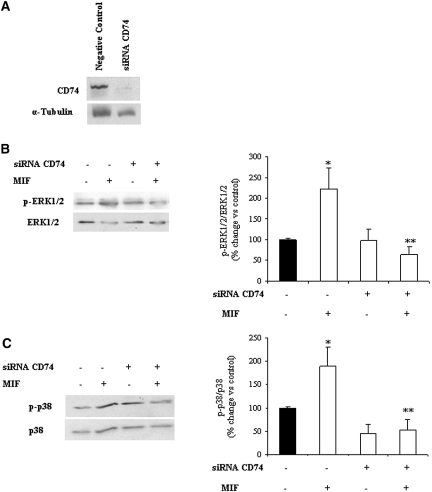
To confirm that MIF actions on podocytes were mediated by CD74, we knocked down CD74 by specific small interfering RNA (siRNA; Figure 5A). As a result, podocyte ERK1/2 and p38 phosphorylation responses to MIF were lost (Figure 5, B and C).
Figure 5.
Downregulation of CD74 prevents MIF actions on podocytes. siRNA downregulation of CD74 protein (Western blot; A) prevents ERK1/2 (B) and p38 (C) phosphorylation induced by 50 ng/ml MIF for 30 min. Data are means ± SD of three independent experiments. (B) *P < 0.05 versus control; **P < 0.01 versus MIF alone. (C) *P < 0.05 versus control; **P < 0.01 versus MIF alone.
MIF Increases TRAIL and MCP-1 Expression in Podocytes and Tubular Cells
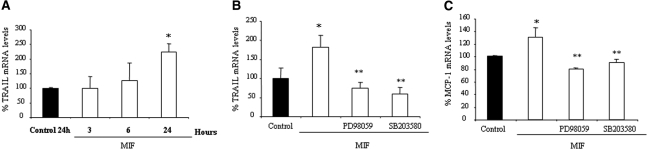
MCP-1 and TRAIL are mediators of renal injury in diabetes.2,3 MIF increased TRAIL mRNA in podocytes in a time-dependent manner (Figure 6A). Inhibitors of ERK1/2 and p38 prevented the upregulation of TRAIL and MCP-1 expression (Figure 6, B and C), suggesting the need for the combined participation of both pathways.
Figure 6.
MIF increases TRAIL and MCP-1 expression in podocytes. (A) Time course of TRAIL mRNA expression in podocytes treated with 10 ng/ml MIF. Real-time RT-PCR. Data are means ± SD of three independent experiments. *P < 0.05 versus control. (B and C) In cells stimulated with 10 ng/ml MIF for 24 h, pretreatment with inhibitors of ERK1/2 (20 μM PD98059) and p38 MAPK (5 μM SB203580) for 1 h prevented the induction of TRAIL mRNA (B, *P < 0.05 versus control, **P < 0.01 versus MIF alone) and MCP-1 mRNA (C, *P < 0.05 versus control, **P < 0.01 versus MIF alone). Data are means ± SD of three independent experiments.
High glucose also upregulated CD74 expression in cultured HK2 human proximal tubular cells (Figure 7, A and B), and MIF also increased TRAIL and MCP-1 expression in these cells (Figure 7, C through E).
Figure 7.
CD74 expression and MIF actions in cultured tubular cells. (A) High glucose increases CD74 mRNA expression at 24 h. Real-time RT-PCR. Data are means ± SD of three independent experiments. #P < 0.0001 versus control. (B) High glucose increases CD74 protein expression at 24 h. Representative Western blot and quantification. Data are means ± SD of three independent experiments. #P < 0.0001 versus low-glucose control. (C) Exposure to 10 ng/ml MIF for 24 h increases TRAIL expression in tubular cells. Representative Western blot and quantification. Data are means ± SD of three independent experiments. *P < 0.05 versus control. (D and E) Cells were pretreated with inhibitors of ERK1/2 (20 μM PD98059) and p38 MAPK (5 μM SB203580) for 1 h and stimulated with 10 ng/ml MIF for 24 h: Effect on TRAIL mRNA (D, *P < 0.05 versus control, **P < 0.01 versus MIF alone) and MCP-1 mRNA expression (E, *P < 0.05 versus control, **P < 0.01 versus MIF alone). Data are means ± SD of three independent experiments.
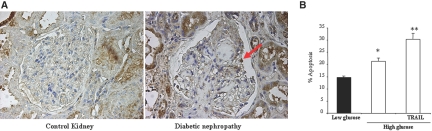
We previously observed de novo TRAIL expression in diabetic glomeruli.3 We now report that podocytes are sites of TRAIL expression in human DN (Figure 8A) and that, in addition to tubular cells,3 TRAIL kills podocytes cultured in a diabetic milieu (Figure 8B).
Figure 8.
TRAIL is expressed by podocytes in diabetic kidney injury and is lethal for cultured podocytes. (A) Representative TRAIL immunohistochemistry of a human glomerulus from a patient with DN. The arrow points a stained podocyte. De novo TRAIL expression in glomeruli was found in 13 of 17 biopsies from patients with DN by our group, whereas no glomerular staining for TRAIL was observed in control glomeruli.3 (B) Culture for 24 h in the presence of 100 ng/ml TRAIL increases the apoptosis rate in podocytes cultured in a high-glucose milieu. *P < 0.05 versus low glucose; **P < 0.01 versus high glucose. Data are means ± SD of three independent experiments.
DISCUSSION
MIF is a pleiotropic cytokine that has been implicated in the development of renal injury5–8,10; however, to date, the receptor mediating MIF actions on kidney cells had not been characterized. We now show (1) that the expression of CD74, a receptor for MIF, is increased in human DN as well as in experimental DN; (2) that podocytes and tubular epithelial cells express CD74 both in culture and in vivo; and (3) that CD74 mediates signal transduction initiated by MIF in these cells, increasing the expression of TRAIL and MCP-1. These results identify CD74 as a potential therapeutic target in DN.
We confirmed increased CD74 expression in human DN at the mRNA and protein levels in two different cohorts of patients. Findings in the rat model corroborated those in humans. Although it is conceivable that CD74 plays a role in other forms of renal disease in which MIF has been found to be increased, data from patients with MCD suggest that it is not upregulated in all forms of glomerular injury. In this regard, high glucose levels could contribute to the increased podocyte and tubular cell CD74 expression in patients with diabetes; however, increased glomerular CD74 may not be specific from DN. In fact, preliminary studies showed that CD74 mRNA expression is increased in hypertensive nephropathy (P.I. et al., unpublished observation). In this regard, podocyte CD74 was also upregulated by inflammatory cytokines, such as TNF-α, that are present in a number of glomerular pathologies, including DN.19
MIF was previously implicated in renal injury. Increased glomerular and tubular MIF expression as well as urinary excretion was noted in human and experimental glomerular injury, including DN.5,9,10 Despite this, the cellular targets of MIF in glomerular injury had not been studied in detail and the receptor mediating MIF actions had not been identified. We now show that the expression of the MIF receptor CD74 is increased in epithelial tubular and glomerular cells in DN, thereby placing cytokine and receptor in the same compartments. The contribution of MIF to renal injury could involve promoting inflammatory mediator expression and modulation of apoptosis and cell proliferation.4 In this regard, there is in vivo evidence of a direct injurious role for MIF on glomerular cells: Transgenic expression of MIF in podocytes led to proteinuria, azotemia, and podocyte injury in the absence of glomerular hypercellularity, suggesting that podocytes are direct targets for MIF.8
MIF activated ERK1/2 and p38 MAPK in podocytes. ERK1/2 and p38 MAPK have been implicated in the progression of various glomerulopathies.20 They are phosphorylated in podocytes from patients with DN.21 MAPK pathways mediate different signaling events either alone or in concert with other pathways.22 p38 MAPK play a critical role in the regulation of proinflammatory cytokine production, cell survival and apoptosis, and the stability of the cytoskeleton.23–26 Phosphorylation of p38 MAPK is increased in injured podocytes, and p38 MAPK inhibition suppressed podocyte injury in experimental nephrotic syndrome.20 Increased glomerular ERK1/2 phosphorylation was also observed in these models.20 The ERK cascade transmits signals from many extracellular agents to regulate cellular processes through actions on more than 160 substrates.20 ERK1/2 and p38 activation were required for MIF-induced induction of TRAIL and MCP-1 in podocytes and tubular cells. TRAIL is a lethal cytokine whose expression increases in tubular cells and appears de novo in podocytes in DN.3 TRAIL induces tubular cell apoptosis in a diabetic milieu and is also lethal for podocytes.
In summary, we have identified a novel role of CD74 in glomerular injury. As a receptor for MIF, CD74 could contribute to podocyte injury in susceptible patients with diabetes by activating MAPK cascades and increasing TRAIL and MCP-1 expression.
CONCISE METHODS
Transcriptomic Analysis
Human renal biopsies were collected in a multicenter study, the European Renal cDNA Bank (ERCB).3,16 The protocol was approved by the local ethical committees. We obtained informed consent from patients according to local guidelines, and we processed samples according to the ERCB protocol. For oligonucleotide array–based gene expression profiling, we included a total of 30 kidney biopsies from individual patients: Pretransplantation kidney biopsies from living donors used as control (n = 6) and protocol biopsies from Pima Indians with histologic diagnosis of DN (n = 20) and from patients with MCD as a proteinuric nephropathy control (n = 4; Supplemental Table 2).
We manually microdissected tubulointerstitial and glomerular compartments from cortical tissue segments. We isolated total RNA using a commercially available isolation protocol. For probe labeling, we used a modification of the Eberwein protocol. Affymetrix microarray analysis following the manufacturer's protocol has been described. We initially obtained image files through Affymetrix GeneChip software (MAS5).16 Subsequently, we performed robust multichip analysis using RMAexpress (http://statwww.berkeley.edu/users/bolstad/RMAExpress). Starting from the normalized robust multichip analysis, the Significance Analysis of Microarrays (SAM; version 1.21, http://www-stat.stanford.edu/∼tibs/SAM/) software was applied using a false discovery rate of 1% to identify genes that were significantly differentially regulated between the analyzed groups.3,16 We evaluated changes in expression of ≥1.5-fold for significance. On the basis of this analysis, we found CD74 to be significantly upregulated in the glomerular and interstitial compartments of patients with DN.
Cell Culture and Reagents
Human podocytes are an immortalized cell line, as described previously,27 transfected with a temperature-sensitive SV40 gene construct and a gene encoding the catalytic domain of human telomerase.28 At a permissive temperature of 33°C, the cells remain in an undifferentiated proliferative state, whereas raising the temperature to 37°C results in growth arrest and differentiation to the parental podocyte phenotype. Undifferentiated podocyte cultures were maintained at 33°C in RPMI 1640 medium with penicillin; streptomycin; insulin, transferrin, and selenite; and 10% FCS. Once cells had reached 70 to 80% confluence, they were cultured at 37°C for at least 14 d before use, when full differentiation had taken place. For experiments, cells were cultured in serum-free medium 24 h before the addition of the stimuli and throughout the experiment. For high-glucose experiment, glucose was added in the medium to reach a final concentration of 700 mg/dl versus control medium with 200 mg/dl glucose. The same amount of mannitol was added as an osmolarity control. TNF-α (30 ng/ml) was from Immugenex (Los Angeles, CA), and TRAIL (100 ng/ml) was from Alexis (Läufelfingen, Switzerland).3 Culture of HK2 human proximal tubular epithelial cells (ATCC, Rockville, MD) and the assessment of apoptosis as hypodiploid cells by flow cytometry have been described.3,29 PD98059 and SB203580 (Stressgen Bioreagent, Ann Arbor, MI) were dissolved in DMSO.
Animal Model
We studied two groups of ten 10-wk-old Wistar Kyoto rats (Criffa, Barcelona, Spain). We induced diabetes by a single intraperitoneal injection of STZ (Sigma, St. Louis, MO), 50 mg/kg in 0.01 M citrate buffer (pH 4.5).30 Control rats received the STZ vehicle. We killed rats at 7 mo after induction of diabetes.31 All studies were performed in accordance with the European Union normative. We administered insulin (1 to 4 IU subcutaneously; Insulatard NPH; Novo Nordisk, Bagsvaerd, Denmark) weekly so as to prevent death but not with the aim of totally correcting hyperglycemia. We initiated insulin administration 7 d after STZ, having checked that the animals had glycemia >400 mg/dl (Glucocard; Menarini, Barcelona, Spain). We measured systolic BP monthly in conscious, restrained rats by the tail-cuff sphygmomanometer (NARCO, Biosystems, Austin, TX). Albuminuria was measured by ELISA (Celltrend, Luckenwalde, Germany; Supplemental Table 1).
Immunofluorescence and Immunohistochemistry Staining
We performed immunohistochemistry for CD74 in human kidney biopsies on formalin-fixed paraffin sections (4 μm) using Histostain SP Kit (Broad Spectrum, AEC, 95–9943; Zymed Labs, San Francisco, CA). Antigen was retrieved by microwave heating (10 min in citric acid, 10 mM [pH 6.0]). Endogenous peroxidase activity was quenched with 3%. Sections were blocked with serum, incubated with anti-CD74 (rabbit polyclonal, sc-20082, 1:30; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C, and stained with biotinylated secondary antibody. For the semiquantification of staining intensity, we visually assessed 10 random views (×200 magnification) in each slide as 0 to 3 (0, no staining; 1, moderate staining; 2, strong staining; 3, very strong staining) in a blinded manner. We calculated the mean value for all of the views in one slide. CD74 expression was expressed as percentage change over control. The nonaffected parts of tumor nephrectomies served as a control group (n = 5), and we studied patients with DN (n = 5) and MCD (n = 4; Supplemental Table 2). TRAIL immunohistochemistry has been described.3
For immunofluorescence, the unfixed renal tissue was embedded in Tissue-Tek (Società Italiana Chimici, Rome, Italy), snap-frozen in a mixture of isopentane and dry-ice, and stored at −80°C. Subsequently, 5-μm sections were fixed in cold acetone and stained with rabbit anti-CD74 (Santa Cruz Biotechnology) followed by AlexaFluor 488 goat anti-rabbit IgG (Molecular Probes, Invitrogen, Milan, Italy). After washing, the procedure was repeated with the second primary antibody (mouse anti-synaptopodin; Progen, Frankfurt, Germany), followed by AlexaFluor 546 goat anti-mouse (Molecular Probes). Sections were mounted with antifading mounting medium (Vectashield; Vector Laboratories, Burlingame, CA). Specificity of labeling was demonstrated by the lack of staining after substituting proper control IgG (rabbit primary antibody isotype control and mouse primary antibody isotype control, both from Zymed, Invitrogen) for the primary antibody.
In rats immunohistochemistry was carried out in paraffin-embedded tissue sections 5 μm thick.31 The primary antibody was goat polyclonal anti-CD74 (1:60, Santa Cruz Biotechnology). Sections were counterstained with Carazzi's hematoxylin. Negative controls included incubation with a nonspecific Ig of the same isotype as the primary antibody. The percentage of positive glomerular surface area was quantified in 20 glomeruli per rat.
Western Blot
Tissue and cell samples were homogenized in lysis buffer31; then separated by 10 or 12% SDS-PAGE under reducing conditions and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA); blocked with 5% skim milk in PBS/0.5% vol/vol Tween 20 for 1 h; washed with PBS/Tween; and incubated with goat polyclonal anti-CD74 (1:500), mouse monoclonal anti–p-ERK (1:500), rabbit polyclonal anti-ERK1/2 (1:2000), mouse monoclonal anti-p-p38 (1:500), or goat polyclonal anti-p38 (1:2500; all from Santa Cruz Biotechnology), and mouse monoclonal anti-TRAIL (1:1000, Pharmingen). Antibodies were diluted in 5% milk PBS/Tween. Blots were washed with PBS/Tween and subsequently incubated with appropriate horseradish peroxidase–conjugated secondary antibody (1:2000; Amersham, Aylesbury, UK). After washing, the blots were developed with the chemiluminescence method (ECL; Amersham). Blots were then probed with mouse monoclonal anti–α-tubulin antibody (1:2000; Sigma) and levels of expression were corrected for minor differences in loading.
Real-Time Reverse Transcription–PCR
RNA was isolated by Trizol (Invitrogen, Paisley, UK).31 One microgram of RNA was reverse-transcribed with High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Real-time PCR reactions were performed on an ABI Prism 7500 sequence detection PCR system (Applied Biosystems) according to the manufacturer's protocol using the ΔΔCt method.31 Expression levels are given as ratios to glyceraldehyde-3-phosphate dehydrogenase. Predeveloped primer and probe assays were obtained for human glyceraldehyde-3-phosphate dehydrogenase, MCP-1, TRAIL, and CD74 from Applied Biosystems.
Flow Cytometry Analysis of Cell Surface CD74 Expression
Cells were detached with 2 mM EDTA and 5 × 105 cells were incubated for 30 min at 4°C with 8 μg/ml rabbit anti-CD74 antibody (Santa Cruz Biotechnology) or control IgG followed by a 30-min 4°C incubation with 1:100 FITC secondary antibody (Pharmingen, San Diego, CA).32 Mean cell fluorescence was calculated using Cell Quest Software (Becton Dickinson, Franklin Lakes, NJ).
Confocal Microscopy
Cells plated onto Labtek slides were fixed in 4% paraformaldehyde and permeabilized in 0.2% Triton X-100 in PBS for 10 min each. After washing in PBS, cells were incubated overnight at 4°C with rabbit polyclonal anti-CD74 antibody (1:50; Santa Cruz Biotechnology) or rabbit polyclonal anti-nephrin (1:100),33 followed by incubation with FITC secondary antibody (1:200; Sigma). Cell nuclei were counterstained with propidium iodide. After washing, cells were mounted in 70% glycerol in PBS and analyzed with a DM-IRB confocal microscope (Leica DM, Bannockburn, IL).31
Transfection of siRNA
Cells were grown in six-well plates (Costar, Cambridge, MA) and transfected with a mixture of 25 nmol/ml siRNA (Santa Cruz), Opti-MEM I Reduced Serum Medium and Lipofectamine 2000 (Invitrogen). After 18 h, cells were washed and cultured for 48 h in complete medium and serum-depleted for 24 h before addition of MIF. This time point was selected from a time course of decreasing CD74 protein expression in response to siRNA. A negative control scrambled siRNA provided by the manufacturer did not reduce CD74 protein.
Statistical Analysis
Data are given as means ± SD. Mann-Whitney, two-sided t test or one-way ANOVA was applied to indicate significantly different mean values in comparison with the control group. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
This study was supported by grant FIS 06/0046, SAF03/884, LSHB-CT-2004-6 (ADDNET), LSHB-CT-2007-036644 (DIALOK), Sociedad Española de Nefrología, ISCIII-RETIC REDinREN/RD06/0016, and Comunidad de Madrid/FRACM/S-BIO0283/2006. Salary support was provided by FIS to A.B.S., MEC to M.D.S.N., Programa Intensificación Actividad Investigadora (ISCIII/Agencia Laín-Entralgo/CM) to A.O., and the Finnish Kidney Foundation and the Kyllikki and Uolevi Lehikoinen Foundation for P.I. Fondecyt 1080083 to S.M. This research was supported, in part, by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Anneli von Behr is acknowledged for technical assistance and Mar Gonzalez García-Parreño and Alberto Puime for technical help.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Mora C, Navarro JF: Inflammation and diabetic nephropathy. Curr Diab Rep 6: 463–468, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Tesch GH: MCP-1/CCL2: A new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am J Physiol Renal Physiol 294: F697–F701, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Lorz C, Benito-Martin A, Boucherot A, Ucero AC, Rastaldi MP, Henger A, Armelloni S, Santamaria B, Berthier CC, Kretzler M, Egido J, Ortiz A: The death ligand TRAIL in diabetic nephropathy. J Am Soc Nephrol 19: 904–914, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morand EF, Leech M, Bernhagen J: MIF: A new cytokine link between rheumatoid arthritis and atherosclerosis. Nat Rev Drug Discov 5: 399–410, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Lan HY, Yang N, Nikolic-Paterson DJ, Yu XQ, Mu W, Isbel NM, Metz CN, Bucala R, Atkins RC: Expression of macrophage migration inhibitory factor in human glomerulonephritis. Kidney Int 57: 499–509, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Rice EK, Nikolic-Paterson DJ, Hill PA, Metz CN, Bucala R, Atkins RC, Tesch GH: Interferon-gamma induces macrophage migration inhibitory factor synthesis and secretion by tubular epithelial cells. Nephrology (Carlton) 8: 156–161, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Rice EK, Tesch GH, Cao Z, Cooper ME, Metz CN, Bucala R, Atkins RC, Nikolic-Paterson DJ: Induction of MIF synthesis and secretion by tubular epithelial cells: A novel action of angiotensin II. Kidney Int 63: 1265–1275, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Sasaki S, Nishihira J, Ishibashi T, Yamasaki Y, Obikane K, Echigoya M, Sado Y, Ninomiya Y, Kobayashi K: Transgene of MIF induces podocyte injury and progressive mesangial sclerosis in the mouse kidney. Kidney Int 65: 469–481, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Brown FG, Nikolic-Paterson DJ, Hill PA, Isbel NM, Dowling J, Metz CM, Atkins RC: Urine macrophage migration inhibitory factor reflects the severity of renal injury in human glomerulonephritis. J Am Soc Nephrol 13[Suppl 1]: S7–S13, 2002 [PubMed] [Google Scholar]
- 10.Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH: Macrophages in mouse type 2 diabetic nephropathy: Correlation with diabetic state and progressive renal injury. Kidney Int 65: 116–128, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Herder C, Kolb H, Koenig W, Haastert B, Muller-Scholze S, Rathmann W, Holle R, Thorand B, Wichmann HE: Association of systemic concentrations of macrophage migration inhibitory factor with impaired glucose tolerance and type 2 diabetes: Results from the Cooperative Health Research in the Region of Augsburg, Survey 4 (KORA S4). Diabetes Care 29: 368–371, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Tashimo A, Mitamura Y, Nagai S, Nakamura Y, Ohtsuka K, Mizue Y, Nishihira J: Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabet Med 21: 1292–1297, 2004 [DOI] [PubMed] [Google Scholar]
- 13.David JR: Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A 56: 72–77, 1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, Delohery T, Chen Y, Mitchell RA, Bucala R: MIF signal transduction initiated by binding to CD74. J Exp Med 197: 1467–1476, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker-Herman S, Arie G, Medvedovsky H, Kerem A, Shachar I: CD74 is a member of the regulated intramembrane proteolysis-processed protein family. Mol Biol Cell 16: 5061–5069, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid H, Boucherot A, Yasuda Y, Henger A, Brunner B, Eichinger F, Nitsche A, Kiss E, Bleich M, Grone HJ, Nelson PJ, Schlondorff D, Cohen CD, Kretzler M: Modular activation of nuclear factor-kappaB transcriptional programs in human diabetic nephropathy. Diabetes 55: 2993–3003, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Houghton AN, Thomson TM, Gross D, Oettgen HF, Old LJ: Surface antigens of melanoma and melanocytes: Specificity of induction of Ia antigens by human gamma-interferon. J Exp Med 160: 255–269, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leng L, Bucala R: Insight into the biology of macrophage migration inhibitory factor (MIF) revealed by the cloning of its cell surface receptor. Cell Res 16: 162–168, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Navarro JF, Mora-Fernandez C: The role of TNF-alpha in diabetic nephropathy: Pathogenic and therapeutic implications. Cytokine Growth Factor Rev 17: 441–450, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Koshikawa M, Mukoyama M, Mori K, Suganami T, Sawai K, Yoshioka T, Nagae T, Yokoi H, Kawachi H, Shimizu F, Sugawara A, Nakao K: Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol 16: 2690–2701, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Sakai N, Wada T, Furuichi K, Iwata Y, Yoshimoto K, Kitagawa K, Kokubo S, Kobayashi M, Hara A, Yamahana J, Okumura T, Takasawa K, Takeda S, Yoshimura M, Kida H, Yokoyama H: Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am J Kidney Dis 45: 54–65, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Roux PP, Blenis J: ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68: 320–344, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams JL, Badger AM, Kumar S, Lee JC: p38 MAP kinase: Molecular target for the inhibition of pro-inflammatory cytokines. Prog Med Chem 38: 1–60, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Kimura C, Zhao QL, Kondo T, Amatsu M, Fujiwara Y: Mechanism of UV-induced apoptosis in human leukemia cells: roles of Ca2+/Mg(2+)-dependent endonuclease, caspase-3, and stress-activated protein kinases. Exp Cell Res 239: 411–422, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Guay J, Lambert H, Gingras-Breton G, Lavoie JN, Huot J, Landry J: Regulation of actin filament dynamics by p38 map kinase-mediated phosphorylation of heat shock protein 27. J Cell Sci 110: 357–368, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Schiffer M, Bitzer M, Roberts IS, Kopp JB, ten Dijke P, Mundel P, Bottinger EP: Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest 108: 807–816, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saleem MA, O'Hare MJ, Reiser J, Coward RJ, Inward CD, Farren T, Xing CY, Ni L, Mathieson PW, Mundel P: A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol 13: 630–638, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Coward RJ, Welsh GI, Koziell A, Hussain S, Lennon R, Ni L, Tavare JM, Mathieson PW, Saleem MA: Nephrin is critical for the action of insulin on human glomerular podocytes. Diabetes 56: 1127–1135, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Justo P, Sanz A, Lorz C, Gomez-Garre D, Mezzano S, Egido J, Ortiz A: Expression of Smac/Diablo in tubular epithelial cells and during acute renal failure. Kidney Int Suppl S52–S56, 2003 [DOI] [PubMed]
- 30.Ortiz A, Ziyadeh FN, Neilson EG: Expression of apoptosis-regulatory genes in renal proximal tubular epithelial cells exposed to high ambient glucose and in diabetic kidneys. J Investig Med 45: 50–56, 1997 [PubMed] [Google Scholar]
- 31.Sanz AB, Justo P, Sanchez-Nino MD, Blanco-Colio LM, Winkles JA, Kreztler M, Jakubowski A, Blanco J, Egido J, Ruiz-Ortega M, Ortiz A: The cytokine TWEAK modulates renal tubulointerstitial inflammation. J Am Soc Nephrol 19: 695–703, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lorz C, Ortiz A, Justo P, Gonzalez-Cuadrado S, Duque N, Gomez-Guerrero C, Egido J: Proapoptotic Fas ligand is expressed by normal kidney tubular epithelium and injured glomeruli. J Am Soc Nephrol 11: 1266–1277, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Ihalmo P, Rinta-Valkama J, Mai P, Astrom E, Palmen T, Pham TT, Floss T, Holthofer H: Molecular cloning and characterization of an endogenous antisense transcript of Nphs1. Genomics 83: 1134–1140, 2004 [DOI] [PubMed] [Google Scholar]