Abstract
Phosphorus levels correlate with atherosclerosis in both animal models and humans with advanced chronic kidney disease, but whether this relationship exists among individuals with normal kidney function is unknown. This study aimed to determine whether an association exists between phosphorus levels and coronary artery calcium levels in a community-based cohort of 3015 healthy young adults in the prospective Coronary Artery Risk Development in Young Adults (CARDIA) study. Phosphorus levels were measured at baseline, and presence of coronary artery calcium was assessed by computed tomography 15 yr later. Mean age at study inception was 25.2 yr, and the mean levels of phosphorus and calcium were 3.6 and 9.5 mg/dl, respectively. Only 0.2% of participants had estimated GFR <60 ml/min per 1.73 m2. Phosphorus levels were associated with coronary artery calcium in unadjusted models. In multivariate models, however, phosphorus levels were significantly associated with the category of coronary artery calcium level. In conclusion, higher serum phosphorus levels, even within the normal range, may be a risk factor for coronary artery atherosclerosis in healthy young adults.
Experimental studies have suggested that higher phosphorus levels can cause vascular disease. For example, hyperphosphatemia, arteriosclerosis, and vascular calcification are cardinal features of fibroblast growth factor 23 gene 2 and Klotho gene 3 knockout models, phenotypic characteristics that can be altered in Klotho null mice with a low-phosphorus diet.1–3 Current knowledge remains incomplete, but, clearly, prevention of vascular calcification is a dynamic, multifaceted process. Endogenous inhibitors of crystal formation and of osteogenic differentiation of vascular smooth muscle cells have been identified, including matrix Gla protein and fetuin-A; experimental studies have shown that exposure of experimental animals to high ambient phosphorus is followed by expression of an osteogenic phenotype in vascular smooth muscle cells and by vascular calcification.4–8 Other potential mechanisms linking rising phosphorus levels to vascular disease include inhibition of 1,25-dihydroxyvitamin D synthesis and increased parathyroid hormone (PTH) production.9–11
Several observational studies of dialysis populations have shown that high serum phosphorus levels are antecedent associations of mortality and cardiovascular events, independent of calcium and PTH levels.12–15 If high phosphorus levels truly cause vascular disease, then it seems natural to hypothesize that this relationship also applies within the normal range of phosphorus levels, even in the presence of normal kidney function. Coronary artery calcium levels are believed to reflect accurately the overall burden of atherosclerosis and to exhibit dosage–response relationships with the incidence of future cardiovascular events.16–26 Studying phosphorous levels and coronary artery calcification can potentially reveal the mechanisms by which serum phosphorous may lead to cardiovascular disease and, perhaps, suggest the existence of novel mechanisms for developing atherosclerosis. The major objective of this study was to determine whether an association exists between phosphorus levels and coronary artery calcium levels in community-dwelling young adults.
RESULTS
The Coronary Artery Risk Development in Young Adults (CARDIA) study is a prospective, multicenter, observational study of cardiovascular disease in young adults.27 Initially, 5115 participants, aged 18 to 30 yr, were studied in 1985 and 1986 in Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA. Of these, 3671 (71.8%) returned for the year 15 examination; 3042 (of 3671) participants had coronary artery calcium estimated by computer tomography (CT), and serum phosphorus levels were available for 3015 (of 3042) participants. Table 1 shows the baseline characteristics of these 3015 participants at the first study visit. The mean age of the population was 25.2 yr, 54.4% were women, and 45.0% were black. Mean phosphorus level was 3.6 mg/dl, calcium level was 9.5 mg/dl, and calcium-phosphorus product was 26.3 mg2/dl2. Mean estimated GFR (eGFR) was 116.6 ml/min per 1.73 m2, and values for 0.2% of participants were <60 ml/min per 1.73 m2.
Table 1.
Baseline characteristics at study inception (n = 3015)a
| Characteristic | Value |
|---|---|
| Phosphorus (mg/dl) | |
| mean (SD) | 3.6 (0.5) |
| range | 1.3 to 5.7 |
| Calcium (mg/dl) | |
| mean (SD) | 9.5 (0.5) |
| range | 7.1 to 13.2 |
| Calcium-phosphorus product (mg2/dl2) | |
| mean (SD) | 26.3 (14.5) |
| range | 3.0 to 55.1 |
| Age (yr) | |
| mean (SD) | 25.2 (3.6) |
| range | 18.0 to 30.0 |
| Women (%) | 54.4 |
| Race (%) | |
| white | 55.0 |
| black | 45.0 |
| Education <12 yr (%) | 7.2 |
| Cigarette smoker (%) | 26.5 |
| Hypertension (%)b | 8.9 |
| Diabetes (%)b | 0.8 |
| Family history of MI (%) | 14.1 |
| BMI (kg/m2) | |
| mean (SD) | 24.3 (4.4) |
| range | 17.0 to 45.8 |
| LDL cholesterol (mg/dl) | |
| mean (SD) | 109.9 (30.8) |
| range | 20.0 to 260.0 |
| HDL cholesterol (mg/dl) | |
| mean (SD) | 53.3 (13.0) |
| range | 22.0 to 142.0 |
| Triglycerides (mg/dl) | |
| mean (SD) | 72.3 (48.7) |
| range | 16.0 to 932.0 |
| Glucose (mg/dl) | |
| mean (SD) | 82.3 (13.4) |
| range | 37.0 to 457.0 |
| SBP (mmHg) | |
| mean (SD) | 110.2 (10.8) |
| range | 75.0 to 161.0 |
| DBP (mmHg) | |
| mean (SD) | 68.5 (9.6) |
| range | 6.0 to 113.0 |
| eGFR (ml/min per 1.73 m2) | |
| mean (SD) | 116.6 (30.7) |
| range | 3.9 to 521.9 |
| Exercise intensity score | |
| mean (SD) | 422.1 (303.0) |
| range | 0.0 to 2126.0 |
| Phosphorus intake (mg/d) | |
| mean (SD) | 1766.0 (1007.0) |
| range | 202.5 to 16,083.0 |
| Calcium intake (mg/d) | |
| mean (SD) | 1235.7 (825.4) |
| range | 101.1 to 11,685.0 |
| Calorie intake (kcal/d) | |
| mean (SD) | 2813.2 (1570.0) |
| range | 533.2 to 28,385.1 |
| Carbohydrate intake (g/d) | |
| mean (SD) | 324.5 (179.5) |
| range | 63.4 to 2720.9 |
| Fat intake (g/d) | |
| mean (SD) | 117.4 (71.5) |
| range | 11.5 to 1226.6 |
| Protein intake (g/d) | |
| mean (SD) | 102.7 (57.1) |
| range | 12.3 to 964.2 |
| Alcohol intake (g/d) | |
| mean (SD) | 11.3 (20.5) |
| range | 0.0 to 371.3 |
| Medications (%) | |
| asthma | 1.6 |
| hypertension | 1.3 |
| cardiac | 0.8 |
| hormonal | 1.8 |
Missing data: BMI for nine participants; LDL and HDL cholesterol for six participants; triglycerides for seven participants, glucose for 19 participants.
Self-reported.
Multivariate associations of serum phosphorus, calcium, and calcium-phosphorus product are shown in Table 2. The associations of serum phosphorus included younger age, female gender, black race, family history of myocardial infarction (MI), lower body mass index (BMI), HDL cholesterol, triglycerides, lower systolic BP (SBP), diastolic BP (DBP), exercise intensity score, lower carbohydrate intake, and use of cardiac medications. Calcium levels were associated with younger age, male gender, black race, <12 yr of education, self-reported hypertension, lower BMI, LDL cholesterol, HDL cholesterol, triglycerides, glucose, DBP, and lower eGFR values. Calcium-phosphorus product levels were associated with younger age, black race, lower SBP, and higher DBP.
Table 2.
Associations of phosphorus, calcium, and calcium-phosphorus product levels at study inceptiona
| Characteristic | Phosphorus (mg/dl) | P | Calcium (mg/dl) | P | Calcium-Phosphorus Product (mg2/dl2) | P |
|---|---|---|---|---|---|---|
| Age, per 3.6 yr | −0.08 (0.01) | <0.0001 | −0.06 (0.01) | <0.0001 | −0.84 (0.29) | 0.0038 |
| Female gender | 0.07 (0.02) | 0.0007 | −0.26 (0.02) | <0.0001 | −0.47 (0.67) | 0.4835 |
| Black race | 0.13 (0.02) | <0.0001 | 0.06 (0.02) | 0.0019 | 1.54 (0.65) | 0.0175 |
| Education <12 yr | −0.05 (0.03) | 0.1072 | 0.08 (0.03) | 0.0130 | 0.07 (1.06) | 0.9497 |
| Cigarette smoker | 0.03 (0.02) | 0.0923 | <0.01 (0.02) | 0.8159 | 0.61 (0.64) | 0.3426 |
| Hypertension | 0.04 (0.03) | 0.1913 | 0.08 (0.03) | 0.0055 | −0.83 (1.04) | 0.4247 |
| Diabetes | 0.16 (0.11) | 0.1198 | −0.09 (0.10) | 0.3227 | 3.06 (3.34) | 0.3584 |
| Family history of MI | 0.08 (0.02) | 0.0015 | 0.01 (0.02) | 0.6178 | 0.94 (0.78) | 0.2275 |
| BMI, per 4.4 kg/m2 | −0.04 (0.01) | <0.0001 | −0.04 (0.01) | <0.0001 | −0.31 (0.30) | 0.3037 |
| LDL cholesterol, per 30.8 mg/dl | −0.02 (0.01) | 0.0548 | 0.05 (0.01) | <0.0001 | 0.17 (0.28) | 0.5512 |
| HDL cholesterol, per 13.0 mg/dl | 0.04 (0.01) | 0.0004 | 0.05 (0.01) | <0.0001 | −0.10 (0.33) | 0.7617 |
| Triglycerides, per 48.7 mg/dl | 0.03 (0.01) | 0.0011 | 0.03 (0.01) | 0.0014 | 0.07 (0.30) | 0.8168 |
| Glucose, per 13.4 mg/dl | >−0.01 (0.01) | 0.7199 | 0.02 (0.01) | 0.0257 | −0.15 (0.30) | 0.6112 |
| SBP, per 10.8 mmHg | −0.09 (0.01) | <0.0001 | 0.01 (0.01) | 0.2413 | −0.81 (0.36) | 0.0245 |
| DBP, per 9.6 mmHg | 0.04 (0.01) | <0.0001 | 0.03 (0.01) | 0.0004 | 0.95 (0.33) | 0.0046 |
| eGFR, per 30.7 units | −0.01 (0.01) | 0.3203 | −0.06 (0.01) | <0.0001 | −0.22 (0.29) | 0.4450 |
| Exercise intensity score, per 303.0 units | 0.02 (0.01) | 0.0157 | 0.01 (0.01) | 0.1278 | 0.25 (0.29) | 0.3826 |
| Phosphorus intake, per 989.1 mg/d | 0.02 (0.06) | 0.7503 | −0.01 (0.05) | 0.7795 | −0.20 (1.77) | 0.9082 |
| Calcium intake, per 817.4 mg/d | 0.02 (0.03) | 0.5837 | 0.05 (0.03) | 0.0783 | −1.08 (0.95) | 0.2552 |
| Carbohydrate intake, per 177.8 g/d | −0.04 (0.02) | 0.0143 | 0.01 (0.02) | 0.4769 | −0.01 (0.57) | 0.9810 |
| Fat intake, per 70.3 g/d | 0.03 (0.02) | 0.1835 | −0.02 (0.02) | 0.4268 | −1.44 (0.78) | 0.0645 |
| Protein intake, per 55.9 g/d | −0.04 (0.04) | 0.3168 | −0.01 (0.03) | 0.7620 | 2.29 (1.21) | 0.0594 |
| Alcohol intake, per 20.2 g/d | 0.01 (0.01) | 0.4190 | −0.01 (0.01) | 0.1940 | −0.43 (0.32) | 0.1772 |
| Medications (%) | ||||||
| asthma | <0.01 (0.01) | 0.9998 | 0.04 (0.06) | 0.4984 | −3.37 (2.16) | 0.1194 |
| hypertension | −0.05 (0.08) | 0.5180 | 0.03 (0.07) | 0.6485 | 2.52 (2.55) | 0.3221 |
| cardiac | 0.26 (0.10) | 0.0061 | 0.02 (0.09) | 0.8185 | 3.72 (3.06) | 0.2237 |
| hormonal | 0.11 (0.06) | 0.0965 | 0.05 (0.06) | 0.4308 | −1.23 (2.02) | 0.5441 |
Linear regression analysis was used. Reference categories for categorical variables were individuals for whom the characteristic was absent. Parameter estimates for continuous variables were computed for intervals corresponding to 1 SD of the study population. Adjustment was made for all of the variables shown in the first column. Data are expressed as parameter estimate (SE).
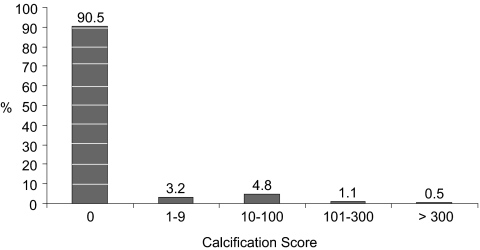
At year 15, 3.2% of the study population had minimal coronary artery calcification, 4.8% had mild calcification, 1.1% had moderate calcification, and 0.5% had severe calcification (Figure 1). Table 3 summarizes associations between phosphorus levels at baseline and coronary artery calcium categorized as 0, >0 to <10, 10 to 100, 101 to 300, or >300 units. In unadjusted models, higher phosphorus levels, as a continuous variable, were associated with lower likelihood of coronary artery calcification (odds ratio [OR] 0.87 per 0.5 mg/dl; P = 0.0332). In multivariate models, higher phosphorus levels were associated with greater likelihood of higher calcium level categories (adjusted OR 1.17 per 0.5 mg/dl; P = 0.0331); considered as quartiles, phosphorus values in the fourth quartile (>3.9 mg/dl) were associated with an adjusted OR of 1.52 (95% confidence interval 1.04 to 2.22) compared with values in the first quartile (≤3.3 mg/dl). Table 3 shows that findings were broadly similar when coronary artery calcium was analyzed as binary variables, defined by calcium scores >0, 10, and 100 units.
Figure 1.
Coronary calcification scores by computer tomography at year 15 (n = 3015).
Table 3.
Associations between serum phosphorus at baseline and coronary artery calcium at year 15a
| Parameter | CAC Categoriesb | P | CAC >0 (versus 0) | P | CAC ≥10 (versus <10) | P | CAC ≥100 (versus <100) | P |
|---|---|---|---|---|---|---|---|---|
| Unadjusted | ||||||||
| Phosphorus, per 0.5 mg/dl | 0.87 (0.77 to 0.99) | 0.0332 | 0.87 (0.77 to 0.99) | 0.0309 | 0.89 (0.77 to 1.04) | 0.1356 | 1.03 (0.76 to 1.39) | 0.8360 |
| Phosphorus quartiles | ||||||||
| ≤3.3 (reference) | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| >3.3 to ≤3.6 | 0.72 (0.52 to 1.01) | 0.3353 | 0.72 (0.52 to 1.02) | 0.3519 | 0.73 (0.48 to 1.10) | 0.3014 | 0.56 (0.24 to 1.32) | 0.3502 |
| >3.6 to ≤3.9 | 0.79 (0.57 to 1.10) | 0.8508 | 0.79 (0.57 to 1.09) | 0.8345 | 0.92 (0.63 to 1.36) | 0.4691 | 0.49 (0.20 to 1.20) | 0.1827 |
| >3.9 | 0.74 (0.53 to 1.03) | 0.4410 | 0.74 (0.53 to 1.03) | 0.4226 | 0.74 (0.49 to 1.11) | 0.3552 | 1.10 (0.55 to 2.22) | 0.0986 |
| Adjustedc | ||||||||
| Phosphorus, per 0.5 mg/dl | 1.17 (1.01 to 1.34) | 0.0331 | 1.13 (0.98 to 1.31) | 0.0875 | 1.20 (1.01 to 1.43) | 0.0363 | 1.27 (0.91 to 1.77) | 0.1559 |
| Phosphorus quartiles | ||||||||
| ≤3.3 (reference) | 1.00 | – | 1.00 | – | 1.00 | – | 1.00 | – |
| >3.3 to ≤3.6 | 0.91 (0.63 to 1.31) | 0.0593 | 0.88 (0.61 to 1.28) | 0.0591 | 0.88 (0.56 to 1.38) | 0.0370 | 0.67 (0.26 to 1.71) | 0.2387 |
| >3.6 to ≤3.9 | 1.25 (0.87 to 1.80) | 0.4519 | 1.20 (0.83 to 1.73) | 0.5433 | 1.48 (0.96 to 2.27) | 0.1367 | 0.64 (0.23 to 1.80) | 0.2252 |
| >3.9 | 1.52 (1.04 to 2.22) | 0.0257 | 1.45 (0.99 to 2.14) | 0.0358 | 1.60 (1.01 to 2.55) | 0.0598 | 2.25 (0.97 to 5.21) | 0.0045 |
Numbers included in quartiles 1 through 4 of serum phosphorus were 820, 723, 725, and 747, respectively. Ordinal and binary logistic regression models were used to calculate OR and 95% confidence intervals. The SD for phosphorus levels in the study population was 0.5 mg/dl. CAC, coronary artery calcium.
0, >0 to <10, 10 to 100, 101 to 300, or >300 units.
Adjusted for all variables in the first column of Table 2. P > 0.10 for age–phosphorus, gender–phosphorus, and race–phosphorus interactions.
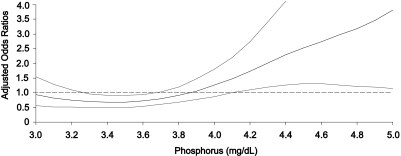
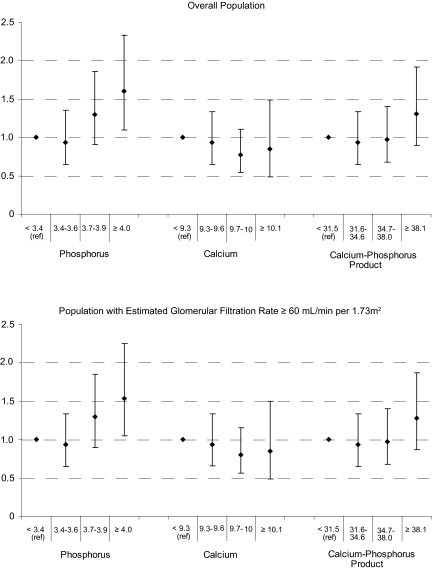
Multivariate P-spline plot analysis (Figure 2) suggested that phosphorus levels >3.9 mg/dl were associated with greater likelihood of coronary artery calcium ≥100. As shown in Figure 3, serum calcium and calcium-phosphorus product levels had no association with coronary artery calcification. Figure 3 also shows that associations between coronary artery calcium and phosphorus, calcium, and calcium-phosphorus product were very similar when individual with eGFR <60 ml/min per 1.73 m2 were excluded.
Figure 2.
Spline plot relating adjusted OR (with 95% confidence intervals) of coronary artery calcium ≥100 and serum phosphorus levels. Adjusted for age, gender, race, hypertension, diabetes, education, smoking, family history of MI, BMI, LDL cholesterol, HDL cholesterol, triglycerides, glucose, SBP, and eGFR.
Figure 3.
Multivariate associations of coronary artery calcification severity and quartiles of phosphorus, calcium, and calcium-phosphorus product. (Top) Overall population. (Bottom) Population with eGFR ≥60 ml/min per 1.73 m2. Adjusted for age, gender, race, hypertension, diabetes, education, smoking, family history of MI, BMI, LDL cholesterol, HDL cholesterol, triglycerides, glucose, SBP, and eGFR.
DISCUSSION
We found that serum phosphorus levels in young adults showed a complex relationship with classic cardiovascular risk factors, including associations with younger age, female gender, black race, family history of MI, low BMI, HDL cholesterol, triglycerides, and lower SBP. Although phosphorus levels, especially levels >3.9 mg/dl, were associated with coronary artery calcium levels, this association was evident only in multivariate models.
To date, most observational data examining the association between phosphorus levels and cardiovascular disease have come from populations with advanced chronic kidney disease (CKD). These studies have shown consistent associations between abnormal phosphorus levels and cardiovascular outcomes; in contrast, associations with calcium levels and PTH levels have been inconsistent in the same studies.12–15 These findings have been extended to phosphorus levels within the normal range by a retrospective analysis of the Cholesterol and Recurrent Events (CARE) trial among individuals with previous MI.28
Few studies have examined associations between phosphorus levels and cardiovascular disease in community-dwelling adults without overt kidney disease. In this regard, a recent report from participants in the Framingham Offspring Study was noteworthy. In this prospective, observational study, the average age of the study population was approximately 20 yr older than in the CARDIA study. Several associations of higher phosphorus levels mirrored the findings in our study. For example, associations of higher phosphorus levels included female gender, lower BP, and lower BMI. During a mean follow-up of 16.1 yr, 524 incident cardiovascular events were observed; when adjustment was made for classic cardiovascular risk factors, GFR, hemoglobin, serum albumin, proteinuria, and C-reactive protein levels, serum phosphorus was associated with these events in a continuous manner, and phosphorus levels ≥3.5 mg/dl were associated with an adjusted hazard ratio 1.55 times levels <2.9 mg/dl.29
Because ours was a nonexperimental study, determining whether the association between phosphorus levels and coronary calcification is a true phenomenon or the magnitude of residual confounding is impossible. Similarly, the relative contributions of genetic and environmental factors to the associations remain speculative. The associations between phosphorus levels and other cardiovascular risk factors were notable and seemed to be qualitatively different from classical cardiovascular risk factors, in the sense that higher phosphorus levels were associated with some seemingly protective factors (younger age, female gender, lower BMI, higher HDL cholesterol, and lower SBP) and other factors that might be considered to increase cardiovascular risk (black race, family history of MI, and higher triglycerides). This duality may account for the observation that associations between phosphorus levels and coronary artery calcification were more apparent in adjusted than unadjusted analyses. Interestingly, a report from the Framingham Offspring Study showed a similar pattern of risk factor duality; this unusual risk factor profile suggests novel mechanisms for cardiovascular disease that may be worth exploring using experimental methods.29
Our study has several strengths. The study population was relatively large, and the interval between putative risk factor and outcome assessments was long. A population of young adults was studied, which should be useful for identifying earlier stage, subclinical coronary artery disease. The candidate risk factor seems to be novel, has little overlap with classic treatable risk factors, and should be modifiable. Intervention trials targeting phosphorus, vitamin D metabolism, and PTH levels are already under way among patients with CKD.30
Unlike many cardiovascular risk factors uncovered in observational studies, potential interventions that can address some of the hypotheses suggested by this study may already exist. Our findings suggest that extending these trials to high-risk individuals without CKD in the general population has the potential to improve public health. Quite apart from the underlying determinants, the associations seen in this study suggest that high phosphorus levels might help to identify young adults for whom modifiable risk factors might be screened and managed more aggressively.
The limitations of our study include its nonexperimental design. In addition, PTH and vitamin D levels were not available, so the hypothesis that our findings reflect phosphorus-induced abnormalities of these axes cannot be refuted. Although inflammatory markers and urinary albumin-creatinine ratios were not available at baseline, adjusting for C-reactive protein and urinary albumin-creatinine ratios at year 15 had no effect on the associations between serum phosphorus and coronary calcium levels. Sample size issues may also be relevant. Approximately 10% of the study population had coronary calcification. To detect a 20% difference between two groups with α of 0.05 and power of 0.90, the total sample size requirement would exceed 10,468. Finally, whereas the design of CARDIA enables enrollment of young adults representative of the overall population, our study was somewhat self-selected, because it was limited to participants who attended the year 15 examination.
CONCISE METHODS
Study Population
The CARDIA study used a random sampling scheme to ensure that population-based samples were balanced within centers by age, race, gender, and education level. The Chicago and Minneapolis centers used census tract information to achieve the population balance mandated in the original request for proposals, which required that the study should have approximately equal proportions of men, women, and black and white Americans. The Oakland center selected study participants from the Kaiser-Permanente health plan membership, and the Birmingham center used telephone exchanges to sample the entire city population. One household member per household was randomly selected and assessed for the study eligibility criteria: Aged between 18 and 30 yr with a permanent address in the target area. Follow-up examinations were performed at years 2, 5, 7, 10, and 15.
Measurements
Gender, race, education, cigarette smoking, previous hypertension and diabetes, and parental history of MI were ascertained by structured interviews and self-administered questionnaires. Participants were instructed to fast for 12 h and to avoid heavy physical activity and smoking for 2 h before the examination. Sitting BP was measured in the right arm after 5 min of rest. First- and fifth-phase Korotkoff sounds were recorded three times at 1-min intervals with a random zero sphygmomanometer (WA Baum Company, Copiague, NY), and the average of the second and third measurements was used in the analyses presented here. Phosphorus, calcium, albumin, and creatinine levels were measured with a SMAC 12 continuous-flow analyzer (Technicon Instruments Corp., Tarrytown, NY) at American BioScience Laboratories (now SmithKline Beecham, King of Prussia, PA).
Data from the Third National Health and Nutrition Examination Survey (1988 to 1992) were used to align creatinine levels from the first CARDIA examination to those expected on the basis of the age, gender, and racial characteristics of the CARDIA participants. Thus, 0.23 mg/dl was subtracted from creatinine levels for CARDIA participants, and the reexpressed Modification of Diet in Renal Disease formula was used to estimate GFR. Calcium levels were corrected for the presence of serum albumin levels <4 g/dl with the following formula: Corrected calcium (mg/dl) = observed calcium (mg/dl) + 0.8 (4 − serum albumin [mg/dl]).31 Enzymatic methods were used to measure HDL cholesterol and triglycerides (University of Washington Northwest Lipid Research Clinical Laboratory, Seattle, WA), and the Friedewald equation was used to calculate LDL cholesterol levels. Glucose levels were measured by the hexokinase-ultraviolet method at Linco (now Millipore, Billerica, MA).
The CARDIA diet history was an interviewer-administered instrument based on 24-h recall.32,33 The CARDIA activity instrument was an interviewer-administered self-report that assessed frequency of participation over the previous 12 mo in eight vigorous-intensity and five moderate-intensity leisure activities; a score of 200 exercise units was equivalent to exercise performed at six metabolic equivalents for 2 h/wk for 11 mo of the year.34,35 Both instruments have been found to have good validity and reliability.
An Imatron (South San Francisco, CA) C-150 electron beam scanner, a GE (Fairfield, CT) Lightspeed multidetector scanner, or a Siemens (Berlin, Germany) VZ multidetector scanner was used for CT scanning. Two scans were performed on each participant, with a hydroxyapatite phantom used to standardize image brightness. Scans were electrocardiogram gated at 80% (Imatron) or 50% (GE and Siemens) of the R-R interval, with a slice thickness of 3 mm (Imatron) or 2.5 mm (GE and Siemens), and completed within 100 (Imatron), 520 (GE), or 360 ms (Siemens). Image processing software was used to identify potential calcific foci with at least two adjacent pixels of area ≥1.87 mm2 and density >130 Hounsfield units (HU). Total coronary calcium scores were calculated by multiplying focus area by a coefficient ranging between 1 and 4, based on the peak density in the focus (1 = 131 to 200 HU, 2 = 201 to 300 HU, 3 = 301 to 400 HU, and 4 = ≥401 HU).36 Scan readers were blinded to participant characteristics and to image data from the other CT scan performed on each participant. Reproducibility rates within and between scan readers were high.37
Statistical Analysis
The analysis was restricted to individuals with CT scans at year 15. Participants with CT scans were older than those without scans (25.2 versus 24.3 yr), more likely to be white (54.8 versus 39.1%), and more likely to have completed a high school education (92.8 versus 86.1%). P < 0.05 was considered statistically significant. Linear regression was used to quantify associations of phosphorus, calcium, and calcium-phosphorus product levels. To address the hypothesis that serum phosphorus levels at baseline were associated with coronary artery calcium burden 15 yr later, we treated coronary artery calcium level as an ordinal variable defined by the categories 0 (no calcification), 1 to <10 (minimal calcification), 10 to 100 (mild calcification), 101 to 300 (moderate calcification), and >300 (severe calcification),17,38–40 and we used ordinal logistic regression to quantify parameter estimates. We checked for statistical interactions between phosphorus and age, gender, and race in the multivariate analysis of coronary calcification category and found none (P > 0.1 for all interactions tested). Binary logistic regression models were used to examine associations between phosphorus levels and coronary artery calcium >0 (versus 0), ≥10 (versus <10), and ≥100 (versus <100).40 Penalized smoothing splines (P-splines) were used for graphical depiction of the adjusted association between scores of ≥100 and the range of phosphorus levels observed in this study.41,42 We performed several sensitivity analyses. Thus, phosphorus-related findings were similar when coronary artery calcium was treated as a linear variable and when urinary albumin-creatinine ratios measured at year 15 were included as covariates (C-reactive protein levels and urinary albumin-creatinine ratios were not measured at baseline).
SAS 9.1 (SAS Institute, Cary, NC) was used for all analyses with the following exceptions: We adjusted national parameter estimates from the Third National Health and Nutrition Examination Survey for the sampling weights implicit in complex survey designs using SUDAAN software (Research Triangle Institute, Research Triangle Park, NC) for complex sample surveys, and we used S-PLUS 6.1 (Insightful Corp., Seattle, WA) for P-spline analysis.
DISCLOSURES
R.N.F. has received consulting fees from Amgen and Genzyme. A.J.C. has received consulting fees from Amgen. C.A.H. has received consulting fees as a member of the Executive Committee of the EVOLVE trial, an Amgen-sponsored clinical trial of cinacalcet HCl therapy in dialysis patients.
Acknowledgments
This study was performed as a deliverable under contracts HHSN267200715002C and HHSN267200715003C (National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD).
We thank United States Renal Data System colleagues Beth Forrest for regulatory assistance, Shane Nygaard for manuscript preparation, and Nan Booth, MSW, MPH, for manuscript editing.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Sitara D, Razzzaque MS, Hesse M, Yoganathan S, Taguchi T, Erben RG, Juppner H, Lanske B: Homozygous ablation of fibroblast growth factor-23 results in hyperphosphatemia and impaired skeletogenesis, and reverses hypophosphatemia in Phex-deficient mice. Matrix Biol 23: 421–432, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 3.Morishita K, Shirai A, Kubota M, Katakura Y, Nabeshima Y, Takeshige K, Kamiya T: The progression of aging in klotho mutant mice can be modified by dietary phosphorus and zinc. J Nutr 131: 3182–3188, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM: Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: E10–E17, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Steitz SA, Speer MY, Curinga G, Yang HY, Haynes P, Aebersold R, Schinke T, Karsenty G, Giachelli CM: Smooth muscle cell phenotypic transition associated with calcification: upregulation of Cbfa1 and downregulation of smooth muscle lineage markers. Circ Res 89: 1147–1154, 2001 [DOI] [PubMed] [Google Scholar]
- 6.Wada T, McKee MD, Steitz S, Giachelli CM: Calcification of vascular smooth muscle cell cultures: Inhibition by osteopontin. Circ Res 84: 166–178, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Chen NX, O’Neill KD, Duan D, Moe SM: Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int 62: 1724–1731, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G: Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386: 78–81, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Portale AA, Halloran BP, Morris RC Jr: Physiologic regulation of the serum concentration of 1,25-dihydroxyvitamin D by phosphorus in normal men. J Clin Invest 83: 1494–1499, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zittermann A, Schleithoff SS, Tenderich G, Berthold HK, Korfer R, Stehle P: Low vitamin D status: A contributing factor in the pathogenesis of congestive heart failure? J Am Coll Cardiol 41: 105–112, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Smogorzewski M, Zayed M, Zhang YB, Roe J, Massry SG: Parathyroid hormone increases cytosolic calcium concentration in adult rat cardiac myocytes. Am J Physiol 264: H1998–H2006, 1993 [DOI] [PubMed] [Google Scholar]
- 12.Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Stevens LA, Djurdjev O, Cardew S, Cameron EC, Levin A: Calcium, phosphate, and parathyroid hormone levels in combination and as a function of dialysis duration predict mortality: Evidence for the complexity of the association between mineral metabolism and outcomes. J Am Soc Nephrol 15: 770–779, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Slinin Y, Foley RN, Collins AJ: Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: The USRDS waves 1, 3, and 4 study. J Am Soc Nephrol 16: 1788–1793, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Mautner GC, Mautner SL, Froehlich J, Feuerstein IM, Proschan MA, Roberts WC, Doppman JL: Coronary artery calcification: Assessment with electron beam CT and histomorphometric correlation. Radiology 192: 619–623, 1994 [DOI] [PubMed] [Google Scholar]
- 17.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, Shaw LJ, Wiegers SE: Assessment of coronary artery disease by cardiac computed tomography: A scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 114: 1761–1791, 2006 [DOI] [PubMed] [Google Scholar]
- 18.McNamara JJ, Molot MA, Stremple JF, Cutting RT: Coronary artery disease in combat casualties in Vietnam. JAMA 216: 1185–1187, 1971 [PubMed] [Google Scholar]
- 19.Detrano R, Hsiai T, Wang S, Puentes G, Fallavollita J, Shields P, Stanford W, Wolfkiel C, Georgiou D, Budoff M, Reed J: Prognostic value of coronary calcification and angiographic stenoses in patients undergoing coronary angiography. J Am Coll Cardiol 27: 285–290, 1996 [DOI] [PubMed] [Google Scholar]
- 20.Guerci AD, Arad Y, Agatston A: Predictive value of EBCT scanning. Circulation 97: 2583–2584, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Schmermund A, Denktas AE, Rumberger JA, Christian TF, Sheedy PF, Bailey KR, Schwartz RS: Independent and incremental value of coronary artery calcium for predicting the extent of angiographic coronary artery disease: Comparison with cardiac risk factors and radionuclide perfusion imaging. J Am Coll Cardiol 34: 777–786, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Bielak LF, Rumberger JA, Sheedy PF, Schwartz RS, Peyser PA: Probabilistic model for prediction of angiographically defined obstructive coronary artery disease using electron beam computed tomography calcium score strata. Circulation 102: 380–385, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Arad Y, Spadaro LA, Goodman K, Newstein D, Guerci AD: Prediction of coronary events with electron beam computed tomography. J Am Coll Cardiol 36: 1253–1260, 2000 [DOI] [PubMed] [Google Scholar]
- 24.O’Malley PG, Taylor AJ, Jackson JL, Doherty TM, Detrano RC: Prognostic value of coronary electron-beam computed tomography for coronary heart disease events in asymptomatic populations. Am J Cardiol 85: 945–948, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Wong ND, Hsu JC, Detrano RC, Diamond G, Eisenberg H, Gardin JM: Coronary artery calcium evaluation by electron beam computed tomography and its relation to new cardiovascular events. Am J Cardiol 86: 495–498, 2000 [DOI] [PubMed] [Google Scholar]
- 26.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, O’Leary DH, Tracy R, Watson K, Wong ND, Kronmal RA: Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med 358: 1336–1345, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR Jr, Liu K, Savage PJ: CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol 41: 1105–1116, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G: Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 112: 2627–2633, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D’Agostino RB Sr, Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Qunibi WY: Reducing the burden of cardiovascular calcification in patients with chronic kidney disease. J Am Soc Nephrol 16[Suppl 2]: S95–S102, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Payne RB, Little AJ, Williams RB, Milner JR: Interpretation of serum calcium in patients with abnormal serum proteins. BMJ 4: 643–646, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald A, Van HL, Slattery M, Hilner J, Bragg C, Caan B, Jacobs D Jr, Liu K, Hubert H, Gernhofer N: The CARDIA dietary history: Development, implementation, and evaluation. J Am Diet Assoc 91: 1104–1112, 1991 [PubMed] [Google Scholar]
- 33.Liu K, Slattery M, Jacobs D Jr, Cutter G, McDonald A, Van HL, Hilner JE, Caan B, Bragg C, Dyer A: A study of the reliability and comparative validity of the cardia dietary history. Ethn Dis 4: 15–27, 1994 [PubMed] [Google Scholar]
- 34.Jacobs D, Hahn L, Haskell W, Pirie P, Sideny S: Validity and reliability of short physical activity history: Cardia and the Minnesota Heart Health Program. J Cardiopulm Rehabil 9: 448–459, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sidney S, Jacobs DR Jr, Haskell WL, Armstrong MA, Dimicco A, Oberman A, Savage PJ, Slattery ML, Sternfeld B, Van Horn L: Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol 133: 1231–1245, 1991 [DOI] [PubMed] [Google Scholar]
- 36.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 37.Carr JJ, Nelson JC, Wong ND, McNitt-Gray M, Arad Y, Jacobs DR Jr, Sidney S, Bild DE, Williams OD, Detrano RC: Calcified coronary artery plaque measurement with cardiac CT in population-based studies: Standardized protocol of Multi-Ethnic Study of Atherosclerosis (MESA) and Coronary Artery Risk Development in Young Adults (CARDIA) study. Radiology 234: 35–43, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Rumberger JA, Sheedy PF, Breen JF, Fitzpatrick LA, Schwartz RS: Electron beam computed tomography and coronary artery disease: scanning for coronary artery calcification. Mayo Clin Proc 71: 369–377, 1996 [DOI] [PubMed] [Google Scholar]
- 39.Cheng YJ, Church TS, Kimball TE, Nichaman MZ, Levine BD, McGuire DK, Blair SN: Comparison of coronary artery calcium detected by electron beam tomography in patients with to those without symptomatic coronary heart disease. Am J Cardiol 92: 498–503, 2003 [DOI] [PubMed] [Google Scholar]
- 40.Manson JE, Allison MA, Rossouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Margolis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML: Estrogen therapy and coronary-artery calcification. N Engl J Med 356: 2591–2602, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Eilers P, Marx B: Flexible smoothing with B-splines and penalties. Stat Sci 11: 89–121, 1996 [Google Scholar]
- 42.Eisen EA, Agalliu I, Thurston SW, Coull BA, Checkoway H: Smoothing in occupational cohort studies: an illustration based on penalised splines. Occup Environ Med 61: 854–860, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]