Abstract
Leukocyte 8-hydroxy-2′-deoxyguanosine (8-OHdG) is a surrogate marker of oxidant-induced DNA damage in patients undergoing maintenance hemodialysis (MHD). Glutathione S-transferase M1 (GST M1) is a member of the GST family of proteins, which protect cellular DNA against oxidative damage. This study tested the association of a common GST M1 gene polymorphism [GST M1(−)], known to produce a dysfunctional enzyme, with levels of 8-OHdG in peripheral blood leukocytes and all-cause mortality among MHD patients. Among 488 MHD patients and 372 gender-matched healthy subjects, the frequency of the GST M1(−) genotype was 63.1 and 60.2%, respectively. The GST M1(−) genotype was associated with significantly higher levels of leukocyte 8-OHdG compared with the GST M1(+) genotype, even after adjustment for potential confounders (P < 0.001). Moreover, GST M1(−) patients who also had a common polymorphism in the DNA repair enzyme 8-oxoguanine DNA glycosylase 1 or who underwent dialysis with a bioincompatible cellulose membrane had the highest median levels of leukocyte 8-OHdG. Multivariate Cox regression revealed that among MHD patients, GST M1(−) genotype approximately doubled the risk for all-cause mortality (hazard ratio 2.24; 95% confidence interval 1.30 to 4.51) during the mean follow-up of 34 mo. In conclusion, patients without GST M1 activity are more vulnerable to oxidative stress and are at greater risk for death compared with those who possess GST M1 activity.
Oxidative stress has been implicated in the pathogenesis of inflammatory disease, atherosclerosis, cancer, and aging.1–3 There is mounting evidence for the presence of disordered oxidative and glycoxidative chemistry in patients who undergo maintenance hemodialysis (MHD)4–9 that may contribute to poor cardiovascular and global outcome.10 DNA, in particular, is more susceptible to attack by reactive oxygen species (ROS) than proteins and membrane lipids, which are protected against oxidation by low molecular weight antioxidants as well as antioxidant enzymes. Among the many types of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine (8-OHdG) is one of the most abundant oxidative products of cellular DNA.2 Our previous work demonstrated that peripheral blood leukocytes of MHD patients are suitable for monitoring 8-OHdG in cellular DNA because they are not only the source but also the target of endogenous ROS.4,5 We further found that leukocyte 8-OHdG levels are highest among MHD patients, which is followed by patients who have chronic kidney disease and have not yet received dialysis and then by healthy subjects.4 Furthermore, leukocyte 8-OHdG levels are also higher in patients who undergo dialysis using cellulose membranes compared with those ho undergo dialysis using synthetic membranes or vitamin E–bonded membranes4,5; therefore, leukocyte 8-OHdG seems to act as a surrogate marker of oxidant-induced DNA damage among MHD patients.
A number of mechanisms have evolved in various organisms to minimize the accumulation of 8-OHdG.5,11 Once formed, 8-OHdG lesions are subject to DNA repair primarily through the base excision repair pathway.12 Oxoguanine-DNA glycosylase 1 (OGG1), encoded by human OGG1 gene, is the representative repair enzyme that acts on 8-OHdG lesions.13,14 Genetic factors that modulate the effectiveness of DNA repair in MHD patients were extensively investigated in our previous study,15 and the results showed that there was an association with the hOGG1 gene polymorphisms, particularly the C→G transition at nucleotide 1245. Glutathione S-transferase (GST) M1 is a member of the GST family, which are proteins involved in the detoxification of several chemical carcinogens; this involves conjugating glutathione or binding to them directly.16 In addition to the base excision repair pathway for oxidized DNA lesions, the safe elimination of toxins via GST pathways has been shown to protect cellular DNA against ROS-induced damage.17 The GST M1 gene is located on chromosome 1p13, a region showing frequent loss of heterozygosity in human lung and bladder cancers. Approximately half of all people from different racial groups lack GST enzyme activity, which has a polymorphic expression, and deficiency is due to an homozygous deletion for a 15-kb allele of GST M1 gene.18 The GST M1 null allele [GST M1(−)] has attracted much attention in the epidemiologic studies as a result of risk linkage with lung and bladder cancer19,20 and of increased susceptibility to coronary heart disease among current smokers.21,22 An association of GST M1(−) genotype with a shorter survival among patients with lung cancer was also investigated by Sweeney et al.23 Up to the present, only overexpression of GST in red blood cells has been noted among MHD patients.24 Because GST are able to detoxify numerous toxic compounds and reduce ROS, it is possible that the GST M1 genotype may modify during dialysis the capacity to invoke a response to oxidative stress; however, the role of GST M1(−) genotype in increased oxidant-induced DNA damage and the outcome among MHD patients has not been well established. This has prompted us to assess whether the GST M1 polymorphism is predictive of 8-OHdG levels in leukocyte DNA and all-cause mortality among the MHD patients. On the basis of our previous findings,4,5,15 we further appraised the effects of interaction between the genetic variants of GST M1 and hOGG1 1245C→G gene polymorphism as well as with dialyzer membrane type used on the leukocyte 8-OHdG level.
RESULTS
GST M1 and hOGG1 Gene Polymorphisms
The distributions of genotype for the GST M1 and hOGG1 gene polymorphisms are summarized in Table 1. The frequency of GST M1(−) in MHD patients (63.1%) was similar to that of the healthy control subjects (60.2%; P > 0.05, χ2 test). Similarly, the distribution of the hOGG1 genotypes, namely CC, CG, and GG, was not significantly different between the two groups (P > 0.05, χ2 test). The allelic frequency of hOGG1 1245G was 63.9% among MHD patients and 65.7% among healthy individuals. The hOGG1 variant was in Hardy-Weinberg equilibrium; this was not assessed for the GST M1 variant because the frequency of the heterozygous deletion could not be determined by the current genotyping techniques. There were no differences in the genotype distributions of the GST M1 and hOGG1 polymorphisms with regard to the dialyzer membrane type (P > 0.05, χ2 test) or renal failure cause (P > 0.05, χ2 test) among the MHD patients.
Table 1.
Genotypes of the GST M1 and hOGG1 1245C→G polymorphisms and characteristics of the MHD patients and healthy individualsa
| Parameter | HD Patients(n = 488) | Healthy Subjects(n = 372) |
|---|---|---|
| GST M1 genotypes | ||
| GST M1(+) (n [%]) | 180 (36.9) | 148 (39.8) |
| GST M1(−) (n [%]) | 308 (63.1) | 224 (60.2) |
| hOGG1 1245C→G genotypes | ||
| 1245CC (n [%]) | 68 (13.9) | 48 (12.9) |
| 1245CG (n [%]) | 216 (44.3) | 179 (48.1) |
| 1245GG (n [%]) | 204 (41.8) | 155 (41.7) |
| 1245G allelic frequency | 0.639 | 0.657 |
| Age (yr; mean ± SD) | 60 ± 13 | 61 ± 15 |
| Male (n [%]) | 228 (46.7) | 185 (49.7) |
| 8-OHdG/106 dG (median [IQR]) | 19.2 (13.5 to 23.7) | 7.8 (5.6 to 9.3)b |
| Ascorbate (mg/L; mean ± SD) | 15.7 ± 8.1 | 18.3 ± 4.9c |
| a-Tocopherol/cholesterol (mg/g; mean ± SD) | 11.8 ± 5.4 | 14.5 ± 3.7c |
| WB GSH (μmol/L/% hematocrit; mean ± SD) | 23.6 ± 5.7 | 26.7 ± 3.2c |
| WB GSSG (μmol/L/% hematocrit; mean ± SD) | 3.3 ± 1.5 | 1.6 ± 0.7c |
| Albumin (g/dl; mean ± SD) | 3.8 ± 0.3 | 4.6 ± 0.4c |
| Hemoglobin (g/dl; mean ± SD) | 10.1 ± 1.4 | 14.5 ± 2.0c |
| Ferritin (μg/L; median [IQR]) | 572 (360 to 952) | 55 (46 to 90)b |
| Transferrin saturation (%; mean ± SD) | 41 ± 19 | 20 ± 8c |
IQR, interquartile range; WB, whole-blood.
P < 0.001 by Mann-Whitney U test.
P < 0.001 by ttest.
Baseline Characteristics of Patients Stratified by GST M1 Genotype
Compared with the age-, gender-, and genotype-matched healthy individuals, the MHD patients showed increased oxidative DNA damage in their leukocytes, an impaired antioxidant defense, an increased iron storage and saturation, and decreased hemoglobin and albumin values (Table 1). Among the MHD patients, we observed that the two groups of patients, GST M1(+) and GST M1(−), were similar with respect to gender distribution, the percentage of patients with diabetes, current smoker status, dialysis vintage, lipid profile, and iron status (P > 0.05; Table 2); however, GST M1(−) patients were characterized by being older and having an impaired antioxidant capacity with a lower serum ascorbate level and a whole-blood reduced glutathione (GSH) level; furthermore, they had a lower hemoglobin concentration and needed administration of a higher dosage of epoetin. Serum albumin was modestly lower among the GST M1(−) group, but this did not reach statistical significance (P = 0.052). Leukocyte 8-OHdG content was higher among GST M1(−) patients than GST M1(+) patients (P < 0.001; Table 2); however, no similar significant difference was found between the healthy subjects with the two genotypes (7.9 [6.2 to 10.5]/106 dG for GST M1(−) versus 6.8 [4.5 to 8.1]/106 dG for GST M1(+); P > 0.05).
Table 2.
Characteristics of patients on MHD in terms of GST M1 genotypes
| Characteristic |
GST M1 Genotype
|
P | |
|---|---|---|---|
| GST M1(+)(n = 180) | GST M1(−)(n = 308) | ||
| Age (yr; mean ± SD) | 58 ± 13 | 61 ± 15 | 0.037a |
| Male (n [%]) | 76 (42.2) | 152 (49.3) | 0.128b |
| Diabetes (n [%]) | 68 (37.8) | 104 (33.8) | 0.371b |
| Current smoker (n [%]) | 32 (17.8) | 60 (19.4) | 0.643b |
| Time on hemodialysis (mo; mean ± SD) | 53 ± 57 | 40 ± 32 | 0.609a |
| 8-OHdG/106 dG (median [IQR]) | 14.5 (10.6 to 19.4) | 21.1 (16.0 to 23.4) | <0.001c |
| Albumin (g/dl; mean ± SD) | 3.9 ± 0.3 | 3.8 ± 0.2 | 0.052a |
| Ascorbate (mg/L; mean ± SD) | 18.2 ± 8.7 | 12.4 ± 7.9 | 0.005a |
| α-Tocopherol/cholesterol (mg/g; mean ± SD) | 11.8 ± 5.2 | 11.7 ± 5.4 | 0.888a |
| Cholesterol (mg/dl; mean ± SD) | 176 ± 31 | 179 ± 47 | 0.420a |
| Triglyceride (mg/dl; mean ± SD) | 187 ± 141 | 179 ± 153 | 0.605a |
| WB GSH (μmol/L; mean ± SD) | 740 ± 197 | 693 ± 193 | 0.028a |
| WB GSSG (μmol/L; mean ± SD) | 101 ± 39 | 96 ± 65 | 0.212a |
| WB GSH (μmol/L per % hematocrit; mean ± SD) | 23.9 ± 5.4 | 23.4 ± 6.0 | 0.419a |
| WB GSSG (μmol/L per % hematocrit; mean ± SD) | 3.4 ± 1.6 | 3.3 ± 1.5 | 0.611a |
| Hematocrit (%; mean ± SD) | 30.5 ± 4.2 | 29.6 ± 3.9 | 0.013a |
| Hemoglobin (g/dl; mean ± SD) | 10.3 ± 1.6 | 10.0 ± 1.4 | 0.034a |
| Epoetin dose (U/kg per wk; mean ± SD) | 72 ± 34 | 78 ± 34 | 0.048a |
| Ferritin (μg/L; median [IQR]) | 548 (401 to 768) | 539 (354 to 1003) | 0.811c |
| Serum iron (μg/dl; mean ± SD) | 100 ± 51 | 99 ± 57 | 0.904a |
| Transferrin saturation (%; mean ± SD) | 39 ± 17 | 40 ± 17 | 0.654a |
Comparison between the two patient groups was carried out by
t test,
Pearson χ2 test, and
Mann-Whitney U test.
Interaction between the GST M1 and hOGG1 Gene Polymorphisms
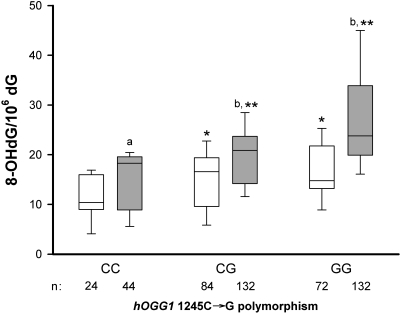
Figure 1 demonstrates a gene–gene interaction in MHD patients, as shown by an additive effect for the GST M1(−) on the hOGG1 1245C→G polymorphism in terms of modulation of the amount of leukocyte 8-OHdG. The median leukocyte 8-OHdG level in patients with the 1245GG or CG genotype was significantly higher than among patients with the 1245CC genotype. GST M1(−) patients had a higher leukocyte 8-OHdG level than GST M1(+) patients for each corresponding hOGG1 genotype (P < 0.001). Moreover, the median leukocyte 8-OHdG level among patients with the GST M1(−) and 1245GG genotypes was the highest among the six groups made up by combinations of the GST M1 and hOGG1 genotypes (F = 18.2, P < 0.001 by ANOVA assessing GST M1×hOGG1 genotype interaction).
Figure 1.
Whisker plots showing the 10th, 25th, 50th, 75th, and 90th percentile distribution of the leukocyte DNA 8-OHdG levels. Differences among hemodialysis patients with the hOGG1 1245CC, CG, and GG genotypes and between patients with GST M1(+) (□) and GST M1(−) (  ) in each hOGG1 genotypes. aP < 0.05 and bP < 0.001 GST M1 (−) versus GST M1 (+) with each corresponding hOGG1 genotype; *P < 0.005 and **P < 0.001 hOGG1 1245 CG or GG genotype versus CC genotype in patients with GST M1(+) or GST M1(−). n, number of patients in each category.
) in each hOGG1 genotypes. aP < 0.05 and bP < 0.001 GST M1 (−) versus GST M1 (+) with each corresponding hOGG1 genotype; *P < 0.005 and **P < 0.001 hOGG1 1245 CG or GG genotype versus CC genotype in patients with GST M1(+) or GST M1(−). n, number of patients in each category.
Interaction between the GST M1 Polymorphism and Dialyzer Membrane
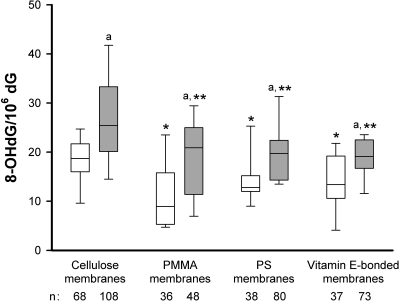
In Figure 2, analysis of GST M1 is extended to include the types of dialysis membrane used. Post hoc analysis demonstrated that the median leukocyte 8-OHdG level in patients treated with cellulose membranes were significantly higher than in patients undergoing dialysis with polymethylmethacrylate (PMMA), polysulfone (PS), or vitamin E–bonded membranes. Furthermore, GST M1(−) patients had a significant higher median leukocyte 8-OHdG content than those with the GST M1(+) genotype for each corresponding dialyzer membrane group (P < 0.001). Median leukocyte 8-OHdG level in the GST M1(−) patients dialyzed with cellulose membranes was the highest among the eight groups made up by combinations of GST M1 genotype and dialyzer membrane type (F = 11.4, P < 0.001 by ANOVA assessing the GST M1×dialyzer membrane interaction).
Figure 2.
Whisker plots of leukocyte DNA 8-OHdG levels in HD patients using cellulose, PMMA, PS, and vitamin E–bonded membranes. Differences between patients with GST M1(+) (□) and GST M1(−) (  ) for each dialyzer membrane. aP < 0.001 GST M1(−) versus GST M1(+) patients with each corresponding dialyzer membrane; *P < 0.001 and **P < 0.001 PMMA, PS, or vitamin E–bonded membranes versus cellulose membranes for patients with GST M1(+) and GST M1(−), respectively. n, number of patients in each category.
) for each dialyzer membrane. aP < 0.001 GST M1(−) versus GST M1(+) patients with each corresponding dialyzer membrane; *P < 0.001 and **P < 0.001 PMMA, PS, or vitamin E–bonded membranes versus cellulose membranes for patients with GST M1(+) and GST M1(−), respectively. n, number of patients in each category.
Predictors of Leukocyte 8-OHdG Level
Stepwise multiple regression analysis revealed a distinct effect of the GST M1 and hOGG1 gene polymorphisms together with dialyzer membrane type on the leukocyte 8-OHdG levels among the 488 MHD patients while adjusting simultaneously for clinically significant variables (Table 3). In addition to the genotype and dialyzer membrane type, the following variables were found to be independent predictors of leukocyte 8-OHdG level: Plasma ascorbate; whole-blood GSH adjusted for hematocrit, age, current smoker status, and diabetes status; and α-tocopherol level adjusted for cholesterol level and serum ferritin (Table 3). This model accounts for approximately 49% of the variation in 8-OHdG content in leukocyte DNA among all MHD patients (R2 = 0.494, P < 0.001). The findings indicate that lower levels of plasma vitamins C and E and whole-blood GSH and higher levels of serum ferritin are associated with greater oxidative damage to leukocyte DNA.
Table 3.
Stepwise multiple regression analysis of the major determinants of leukocyte DNA 8-OHdG level in 488 hemodialysis patientsa
| Variable | Regression Coefficient | 95% CI | P |
|---|---|---|---|
| GST M1 genotype | |||
| GST M1(−) versus GST M1(+) | 0.325 | 0.238 to 0.411 | <0.001 |
| hOGG1 1245C→G genotypeb | |||
| 1245CG | 0.388 | 0.264 to 0.511 | <0.001 |
| 1245GG | 0.763 | 0.634 to 0.891 | <0.001 |
| Dialyzer membrane typec | |||
| PMMA membrane | −0.226 | −0.349 to −0.103 | <0.001 |
| PS membrane | −0.445 | −0.560 to −0.330 | <0.001 |
| vitamin E–bonded membrane | −0.864 | −1.502 to −0.677 | <0.001 |
| Ascorbate per 1 mg/L | −0.201 | −0.304 to −0.140 | <0.001 (1) |
| WB GSH/hematocrit | |||
| per 1 μmol/L per % hematocrit | −0.190 | −0.231 to −0.075 | <0.001 (2) |
| Age per 1 yr | 0.004 | 0.001 to 0.007 | <0.001 (3) |
| Current smoking status | |||
| smoker versus nonsmoker | 0.195 | 0.084 to 0.310 | 0.009 (4) |
| Diabetes | |||
| presence versus absence | 0.114 | 0.045 to 0.279 | 0.017 (5) |
| α-Tocopherol/cholesterol per 1 mg/g | −0.083 | −0.113 to −0.052 | 0.034 (6) |
| ln-ferritin per ln-1 μg/L | 0.075 | 0.046 to 0.105 | 0.045 (7) |
The dependent variable is ln leukocyte DNA 8-OHdG level. Order of appearance in the equation in parentheses.
The 1245CC genotype was the reference category for the 1245CG and 1245GG genotypes.
The cellulose membrane was the reference category for the PMMA, PS, and vitamin E–bonded membranes.
Patient Survival in Relation to GST M1 Gene Polymorphism
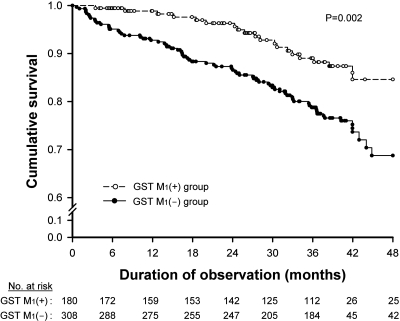
There were 92 deaths during the follow-up. The causes of death among our study cohort included cardiovascular disease (CVD; n = 49), infection and sepsis (n = 24), neoplasm (n = 5), gastrointestinal bleeding (n = 5), hepatic failure (n = 4), chronic obstructive lung disease (n = 3), and cachexia (n = 2). The mortality of the GST M1(+) and GST M1(−) patients was 11.1 and 23.4%, respectively. Survival was compared between the two groups using Kaplan-Meier analysis (Figure 3). The GST M1(−) group had a significantly higher risk for death than the GST M1(+) group (P = 0.002). Multivariate Cox regression analysis was used to determine the independent effect of GST M1 gene polymorphism on predicting all-cause mortality and included eight other variables (Table 4). The presence of the GST M1(−) genotype was an independent predictor of a higher risk for overall mortality among MHD patients with a hazard ratio of 2.24 (95% confidence interval [CI] 1.30 to 4.51; P < 0.001). Significant interactive effects existed between GST M1(−) and the hOGG1 genotype as well as with the dialysis membrane type used, suggesting that the last two variables may have a confounding influence on the association between GST M1(−) genotype and mortality; however, hOGG1 genotype and dialysis membrane type were NS predictors of all-cause mortality in the multivariate model, mainly because of a lack of a significant colinear relationship with GST M1 genotype. Other significant and independent predictors for mortality included leukocyte 8-OHdG, age, the presence of diabetes, plasma ascorbate level, and serum albumin level. Every 10/106-dG increase in leukocyte 8-OHdG level was independently predictive of an 88% increase in all-cause mortality (95% CI 1.15 to 3.08; P < 0.001) among MHD patients.
Figure 3.
Kaplan-Meier analysis of the overall survival among hemodialysis patients in relation to the GST M1 gene polymorphism.
Table 4.
Significant predictors for all-cause mortality among 488 MHD patients after a mean follow-up of 34 mo by Cox proportional hazards regression analysisa
| Parameter | All-Cause Mortality
|
||
|---|---|---|---|
| Unit of Increase | Hazard Ratio (95% CI) | P | |
| GST M1(−) genotype | Reference = GST M1(+) | 2.24 (1.30 to 4.51) | <0.001 |
| Leukocyte 8-OHdG | 10/106 dG | 1.88 (1.15 to 3.08) | <0.001 |
| Age | 1 yr | 1.05 (1.03 to 1.08) | 0.001 |
| Diabetes | Reference = no diabetes | 1.56 (1.06 to 2.50) | 0.041 |
| Ascorbate | 10 mg/L | 0.66 (0.42 to 0.81) | 0.009 |
| Albumin | 1 g/dl | 0.84 (0.54 to 0.95) | 0.039 |
The Cox proportional hazards model was used to evaluate the association of all-cause mortality with GST M1 gene polymorphism and included terms for age, gender, presence of diabetes, current smoking status, leukocyte 8-OHdG level, albumin level, hemoglobin level, ascorbate level, and WB GSH level.
DISCUSSION
The GST M1 locus has been found to be entirely deleted in 30 to 50% of white individuals18,25; however, a degree of variation in the prevalence of the GST M1(−) genotype has been reported among various ethnic groups, especially a lower frequency among black individuals (17.5%).22 In this study, the prevalence (60 to 63%) of the GST M1(−) genotype among MHD patients and healthy subjects is close to that of a Chinese population (58.7%)26 and slightly higher than the upper limits of the range for white individuals. One study identified an association of GST M1(−) homozygotes with Balkan endemic nephropathy27; however, no similar relationship between GST M1(−) genotype and the cause of renal failure was observed in our patients.
Previous studies demonstrated that vitamin C in plasma and GSH in whole blood are good markers for evaluating oxidative stress in patients undergoing MHD.4,5,28 In this study, we found that, among MHD patients, the GST M1(−) genotype was associated with a significantly lower antioxidant capacity in terms of plasma ascorbate and whole-blood GSH compared with GST M1(+) genotype. Glutathione is a major intracellular antioxidant, and a decrease in the whole-blood level provides insight into a defective cellular redox status. In MHD patients, plasma ascorbate level is reduced in part as a result of uremia-associated metabolic derangement and a loss of ascorbate during hemodialysis. In addition, we infer that the lack of GST enzyme activity contributes to the augmented oxidative stress and that this subsequently leads to a further decrease in the levels of plasma ascorbate and whole-blood GSH among MHD patients.
GST M1, which participates in the metabolism of a number of genotoxic epoxides, is particularly involved in the protection of DNA from oxidative damage.29 This study provides evidence for the first time that leukocyte DNA damage, when evaluated in terms of 8-OHdG levels, was more intense in MHD patients having the GST M1(−) genotype (Table 2). The observed difference cannot be completely accounted for by the differences in the major confounders that may affect the levels of 8-OHdG, such as age, smoking status, diabetes status, and iron overload.2,30,31 Moreover, multivariate regression analysis validated the independent effect of the GST M1(−) genotype on leukocyte 8-OHdG content (Table 3). Our findings confirm similar results presented in the previous reports in which, in human leukocytes and lung tissue, GST M1 null individuals showed detectable DNA adducts, whereas GST M1 active individuals did not.32–34 Our study is also consistent with those of Chen et al.35 and Izzotti et al.,36 who showed a positive association between GST M1(−) genotype and 8-OHdG levels in the DNA of sperm of varicocele patients or smooth muscle cells in atherosclerotic lesions of human abdominal aorta.
This study provides an opportunity to explore the possible effects of gene–gene or gene–environment interaction on oxidative DNA damage for MHD patients. With GST M1 enzyme deficiency and impairment of DNA repair activity, leukocyte 8-OHdG levels were the highest in the GST M1(−) patients who had the hOGG1 1245GG genotype compared with other combinations. The results indicate that GST M1(−) and hOGG1 1245GG genotypes seem to be host factors that can modulate 8-OHdG levels in leukocyte DNA. The blood–membrane interaction during hemodialysis triggers circulating neutrophils to produce significant ROS, which is subject to GST M1 metabolism.29 Our previous work demonstrated that the production of ROS is further exaggerated by dialysis with cellulose membranes compared with the use of biocompatible membranes such as PMMA, PS, and vitamin E–bonded membranes.4,5 Increased ROS in conjunction with reduced GST activity may lead to more intense oxidative DNA damage in the peripheral blood leukocytes. This increase is greater when GST M1(−) patients undergo dialysis with cellulose membranes, indicating that membrane bioincompatibility, as an environmental factor, synergistically contributes to a higher oxidant-induced DNA damage in MHD patients.
As compared with the general population, patients undergoing MHD are known to have a higher annual mortality rate from ischemic heart disease and cancer; these diseases account for >40% of mortality.37,38 Oxidative stress–mediated carcinogenesis and atherosclerosis are extremely complex; however, this approach may be one way of investigating the gene's moderating effect on such disorders in the context that there is direct or indirect metabolic activity by the gene. Several lines of evidence indicate an association between the GST M1 polymorphism and certain cancers as well as CVD among current smokers, suggesting that the null allele confers increased risk.19–22 A prospective Netherlands study confirmed an increased rate in the progression of atherosclerosis among smokers lacking the detoxifying enzyme GST M1.39 Specifically, de Waart et al.39 demonstrated that male smokers with the GST M1(−) genotype had a higher mean 2-yr progression of the common carotid artery intima-media thickness compared with those with GST M1(+). An observational cohort study showed that the GST M1(−) genotype is associated with a shorter survival for patients with lung cancer.23 We have demonstrated for the first time that, in this large MHD population, the GST M1(−) genotype has a powerful impact on dialysis-related death risk. Patients homozygous for the GST M1 null allele are at a two-fold greater risk for death from any cause compared with those who possess GST M1 activity. This common variant of the GST M1 gene may therefore have health implications for MHD patients because of their higher exposure to dialysis-related oxidant stress.
Because of the limitation with the genotyping assay, in which only the presence or absence of the GST M1 gene was detected, gene dosage effects cannot be assessed in this study. Another possible limitation is that other oxidative stress and inflammatory markers were not measured in our study. The GST M1(−) genotype is predictive of leukocyte 8-OHdG, a biomarker of oxidative stress, and both are independent predictors of mortality in the multivariate model (Table 4). It might be inferred that GST M1(−) could be a surrogate for unmeasured oxidative stress markers, or it may act through nonoxidative stress–related mechanisms, such as inflammation.40 A recent study demonstrated an association between the GST M1(−) genotype and increased plasma malondialdehyde levels in patients with epilepsy.41 Oxidized lipids are GST substrates that are thought to play a role in both atherosclerosis and tumorigenesis.42 Miller et al.21 observed that heavy smokers with the GST M1(−) genotype had increased levels of C-reactive protein, intercellular adhesion molecule 1, and vascular cellular adhesion molecule 1 and decreased levels of albumin. The results provide evidence that the GST M1 polymorphism does seem to modify inflammation and endothelial function among individuals who smoke in addition to oxidative stress.
The typical characteristics of this population are racial homogeneity (all were Chinese), an older age (mean 60 yr), a high prevalence of diabetes (35%), a decreased plasma ascorbate, and a high median leukocyte 8-OHdG. In our statistical analysis, age, presence of diabetes, low plasma ascorbate, and low serum albumin were the independent predictors of mortality in MHD patients. These findings are in general agreement with other reports, which have analyzed risk factors and causes of death among MHD patients.43,44 We have shown that a single time point measurement of leukocyte 8-OHdG is predictive of overall mortality even after a prolonged period of follow-up. Our recent study31 reported that the biomarker is relatively stable in MHD patients because there is high concordance for leukocyte 8-OHdG when measured at baseline and 3 mo later. This may explain in part the intriguing finding that a single measurement of 8-OHdG in leukocyte DNA carries long-term outcome information. CVD (53%) is the main cause of death in our study. Some lines of evidence have shown that the levels of 8-OHdG36 or DNA adducts45 found in atherosclerotic lesions are exceptionally high, which supports the view that part of atherogenic lesions may be initiated by mutational events in arterial smooth muscle cells and the plaques may progress through an initiation-promotion process, rather like a benign tumor.46 Accordingly, Cox regression analysis in this study showed that leukocyte 8-OHdG was a significant independent predictor of mortality, suggesting that 8-OHdG is not simply an innocent bystander but rather may directly contribute to a fatal outcome, perhaps by aggravating the adverse effect of accelerated CVD in MHD patients.
DNA damage and clinical outcome among MHD patients are affected by the interplay between the polymorphic status and environmental exposure. Among these factors, genetic aspects, specifically in the case that is the GST M1 polymorphism, are permanent and irreversible. Other contributors, such as membrane bioincompatibility and decreased antioxidant capacity, are remediable. The use of antioxidants might be of value only to patients who have a particular genotype and are under a high oxidant load; however, interventional antioxidant trials of patients at the highest risk for developing premature CVD and mortality are rare. A randomized, placebo-controlled study showed that GST M1(−) smokers who took vitamin E (400 IU/d) for 2 yr had half the rate of increase in common carotid artery intima-media thickness compared with GST M1(−) smokers who did not taking vitamin E.39 The Secondary Prevention with Antioxidants of Cardiovascular Disease in End-stage Renal Disease (SPACE) study included patients who had ESRD with apparent CVD and demonstrated a significant reduction in the primary composite CVD end point and a decreased rate of myocardial infarction with vitamin E (800 IU/d).47 Albeit the fact that antioxidant supplementation has not been borne out by large trials, for patients with the GST M1(−) genotype, supplementation with vitamins C and E or glutathione,21,48,49 and dialysis with a more biocompatible membrane4,5 might be treatment options that are able to attenuate oxidant-induced DNA damage and improve outcomes closely related to oxidant stress.
In conclusion, our study demonstrates the association of the GST M1 polymorphism with oxidative DNA damage and all-cause mortality among MHD patients. MHD patients with the GST M1(−) genotype are more vulnerable to oxidative stress and have higher 8-OHdG levels in their peripheral blood leukocytes. Patients homozygous for the GST M1 null allele are at a greater risk for death from any cause compared with those who possess GST M1 activity. Further prospective, randomized, controlled studies are needed to determine the beneficial effects of antioxidant therapy, if any, in the reduction of overall mortality among MHD patients. Furthermore, research on this gene–environment interaction might help to improve targeting of therapy to individuals who are more susceptible to disease and mortality.
CONCISE METHODS
Study Population
For determination of the genotype frequency of GST M1(−) and its effect on the 8-OHdG level of leukocyte DNA, a total of 612 patients undergoing MHD at five dialysis facilities in the Taipei metropolitan area, between January 2003 and December 2005, were eligible to participate in the case-control study. Only clinically stable patients with age >20 yr and HD vintage >3 mo before the study were included. Exclusion criteria were weekly dialysis time of <12 h, urea Kt/V of <1.2, comorbidity with malignancy or inflammatory or infectious diseases, supplementation with vitamin C or E, and medications such as oral or intravenous iron supplements and angiotensin converting enzyme inhibitors up to 3 mo before enrollment. Finally, the study population consisted of 488 patients (228 men and 260 women; mean age 60 yr). The causes of ESRD were diabetic nephropathy (n = 164), glomerulonephritis (n = 168), interstitial nephritis (n = 45), nephrosclerosis (n = 31), polycystic kidney disease (n = 18), miscellaneous nephropathies (n = 25), and shrunken kidneys resulting from unknown causes (n = 37). The patients were treated with single-use dialyzers equipped with one of four types of membranes with a membrane surface area of 1.6 to 1.7 m2, namely cellulose (Terumo, Tokyo, Japan) for 176 patients; PS (Fresenius, Borkenberg, Germany) for 118 patients; PMMA (Toray, Tokyo, Japan) for 84 patients; and high-flux, vitamin E-modified, multilayer cellulose (Terumo) for 110 patients. The dialyzer membranes used for a given patient had to have remained constant for at least 6 mo before enrollment in this study. Dialysis machines were sterilized daily, and water treatment circuits and tanks were sterilized weekly. Microorganism colony counts in the water used to prepare the dialysis fluid did not exceed 200 colonies/ml. Endotoxin levels in dialysates, as assessed weekly using the amoebocyte lysate test (Chromogenix, Charleston, SC), were <0.01 EU/ml. A total of 372 nonsmoking individuals without diabetes (185 men and 187 women; mean age 61 yr) and with normal renal function, as defined on the basis of creatinine clearance values of >100 ml/min per 1.73 m2 were enrolled for GST M1 and hOGG1 genotyping as control subjects. The protocol was approved by the Committee on Human Research of Taipei Veterans General Hospital. Informed consent was obtained from each of the study patients.
GST M1 Genotyping
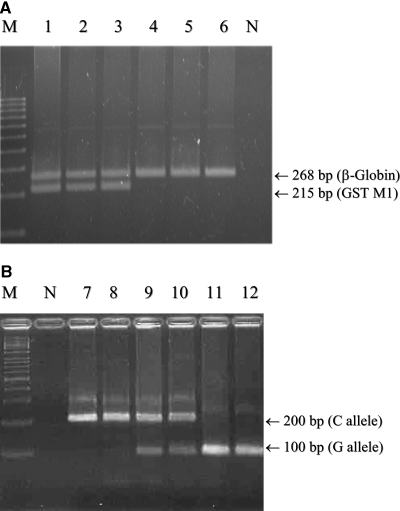
Genomic DNA was isolated from 1 ml of peripheral blood from the patients and healthy individuals by standard procedures using proteinase K digestion and phenol/chloroform extraction. GST M1 gene deletion was detected by multiplex PCR25 using two primers for the GST M1 gene (G5: 5′-GAACTCCCTGAAAAGCTAAAGC-3′; G6: 5′-GTTGGGCTCAAATATACGGTGG-3′) and two primers for the β-globin gene (PC04: 5-CAACTTCATCCACGTTCACC-3′; GH20: 5′-GAAGAGCCAAGGACAGGTAC-3′). PCR was carried out for 35 cycles in a DNA thermal cycler (Perkin-Elmer/Cetus, Norwalk, CT) using a thermal profile of denaturation at 94°C for 40 s, annealing at 55°C for 40 s, and primer extension at 72°C for 40 s. The PCR products were then separated on a 3% agarose/synergel gel (5:1, wt/wt; Diversified Biotech, Newton Center, MA) at 150 V for 1.5 h and stained with 1 μg/ml ethidium bromide at 25°C for 10 min. The GST M1 DNA fragments that were amplified by PCR were 268 and 215 bp in size, but only the 268-bp band was observed in patients with the GST M1(−) genotype (Figure 4A).
Figure 4.
(A and B) Gel electrophoretogram of PCR products of the GST M1 gene (A) and PCR and restriction fragment-length polymorphism analysis of the 1245C→G polymorphism of the hOGG1 gene (B). The sizes of the DNA fragments of GST M1 gene amplified by PCR were 268 and 215 bp, respectively (lanes 1 through 3), but only 268 bp is amplified from total DNA of the patients with the GST M1(−) genotype (lanes 4 through 6). The C→G substitution at nucleotide 1245 of the hOGG1 gene creates a recognition sequence for Fnu4H I, which digests the 200-bp fragment into two 100-bp fragments. Lanes 7 and 8 are wild type (CC), lanes 9 and 10 are heterozygotes (CG), and lanes 11 and 12 are homozygotes (GG) for the hOGG1 1245C→G polymorphism. M, DNA size marker; N, negative control.
hOGG1 1245 C→G Genotyping
To study the C→G transition at nucleotide 1245 of the hOGG1 gene, we analyzed cellular DNA isolated from peripheral blood by PCR as described previously.15,50 Identification of the 1245C→G transversion was performed by using restriction fragment-length polymorphism analysis. In brief, 10 μl of the 200-bp PCR product was subjected to Fnu4HI digestion (2.5 U of enzyme in a 15-μl digest). The presence of a C→G transversion creates a Fnu4HI recognition site, which leads to digestion of the 200-bp PCR product into two fragments of 100 bp. Heterozygous individuals gave two fragments (200 and 100 bp) and individuals with homozygous C→G transversion gave a single fragment of 100 bp. The Fnu4HI digests of the PCR amplification products were examined by electrophoresis on 3% agarose gels, which was followed by ethidium bromide staining (Figure 4B).
Measurements of 8-OHdG Contents in Leukocyte DNA
Venous blood samples were drawn from fasting healthy individuals or the MHD patients at the start of a dialysis session before heparin administration. Blood (10 ml) was withdrawn into an EDTA-containing Vacutainer tube (Becton Dickinson, Franklin Lakes, NJ) and centrifuged in the same tube at 1300 × g at 4°C for 15 min. The buffy coat fraction was collected and transferred to a 20-ml centrifuge tube on ice. Hypotonic saline solution was added to lyse the residual red blood cells. Leukocytes were collected by centrifugation at 500 × g for 5 min and were frozen at −80°C until determination of the DNA's 8-OHdG content. Total leukocyte DNA was extracted by using the pronase/ethanol method,51 with some modifications.4,5 The amount of 8-OHdG was measured using an HPLC system equipped with an electrochemical detector (Bioanalytical Systems, West Lafayette, IN), as described previously.4,5 dG (Sigma Chemical Co., St. Louis, MO) and 8-OHdG (Cayman, Ann Arbor, MI) were used as standards. The 8-OHdG levels are expressed as the number of 8-OHdG molecules/106 dG molecules. Intra-assay coefficients of variance (CV) ranged from 3 to 7%, and interassay CV ranged from 4 to 9%, where the lower number refers to the CV for the high standard and the higher number refers to the CV for the low standard.
Laboratory Measurements
Immediately after sampling, whole blood (0.5 ml) was deproteinized with an equal volume of 20% TCA for determination of the reduced glutathione (GSH) level. GSH was quantified as described by Beutler et al.52 For GSH derivatization, 0.5 ml of whole blood was treated with an equal volume of 12% perchloric acid containing 40 mM N-ethylmaleimide and 2 mM bathophenanthroline disulfonic acid. Oxidized glutathione (GSSG) levels in the derivatized glutathione samples were determined using an HPLC system similar to that developed by Asensi et al.53 We measured hematocrit levels to adjust the GSH and GSSG values. We determined serum iron using commercial kits and an autoanalyzer (Hitachi 736-60; Naka, Japan). We measured total iron-binding capacity by the TIBC Microtest (Daiichi, Tokyo, Japan), and we determined serum ferritin by a RIA (Incstar, Stillwater, MN). We calculated transferrin saturation as the serum iron concentration/TIBC × 100. Plasma ascorbate was measured by the method described by Kyaw.54 Plasma concentrations of α-tocopherol were determined using the procedure described by Catignani and Bieri,55 with some modifications.4,5 The concentration of α-tocopherol was calculated from a calibration curve constructed using internal standards. Total cholesterol was also measured to adjust the α-tocopherol values. All assays were performed with duplicate samples.
Outcome Data Collection
The cohort was followed up to January 2007. During the follow-up, 72 patients moved away from the dialysis facilities. The outcome data of 34 of these 72 patients could be obtained, whereas the remaining 38 patients, including 20 who received a kidney transplant and eight who were transferred to peritoneal dialysis, were censored. At the end of the follow-up, 368 patients were confirmed to be alive and still on HD treatment and 92 patients had died while being treated. The mean follow-up period was 34 ± 13 mo. We obtained date and cause of death by reviewing the hospital record forms. For patients who were transferred to other dialysis units, we reviewed the questionnaire forms filled out by the attending physicians at those units.
Statistical Analysis
Descriptive statistics included mean values ± SD for continuous data and percentages for categorical data. The values for 8-OHdG content in leukocyte DNA and serum ferritin were not normally distributed and are reported as medians with an interquartile range. Comparison of the genotype frequencies of GST M1 and hOGG1 1245C→G gene polymorphisms between MHD patients and healthy individuals were performed by χ2 test. For between-two-group comparisons, we used the t test for normally distributed data and the Mann-Whitney rank sum test for data with a non-normal distribution. We analyzed comparisons of the leukocyte 8-OHdG levels among the six genotype groups and the eight groups formed by interaction of the two GST M1 genotypes and the four dialyzer membranes using one-way ANOVA for significant differences; we assessed the main effects and the GST M1×hOGG1 genotype and GST M1×dialyzer membrane interaction coefficients. The 8-OHdG content in leukocyte DNA and the serum ferritin values were positively skewed; therefore, we used natural logarithmic transformation to normalize the distribution (i.e., ln leukocyte 8-OHdG and ln-ferritin) before multivariate analysis. We performed stepwise multiple regression analysis using the 8-OHdG content of leukocyte DNA as the dependent variable. We assessed the independent effect of each explanatory variable on the dependent variable among the MHD patients using three indicators, namely the GST M1 genotype, the hOGG1 genotype, and dialyzer membrane type. The GST M1(+) group was the reference category versus the GST M1(−) group. The 1245CC genotype was the reference category versus the 1245CG and 1245GG genotypes, and the cellulose membrane was the reference category versus the PMMA, PS, and vitamin E–bonded membranes. The three indicators were forced into the regression equation before testing other variables and could not be removed. An explanatory variable was considered as having an independent effect on leukocyte 8-OHdG levels when it led to a statistical significance in R2 change statistics. We also performed forward stepwise multiple regression to assess the independent effect of 15 covariables, including age, gender, presence of diabetes, current smoking status, dialysis vintage, urea reduction rate, blood antioxidants (ascorbate, α-tocopherol adjusted for cholesterol, and whole-blood GSH and GSSG adjusted for hematocrit), albumin, hemoglobin, and iron indices (serum ferritin and iron and transferrin saturation), on the leukocyte 8-OHdG contents (dependent variable). We generated survival curves by the Kaplan-Meier method. We compared differences in the survival curves between patients with GST M1(+) and GST M1(−) by the log-rank test. We used the multivariate Cox proportional hazards model to assess the association between GST M1 polymorphism and all-cause mortality, adjusting for potential confounding factors. Any variables showing a difference between the GST M1(+) and GST M1(−) genotypes of P < 0.20,56 as well as established risk factors for a poor outcome, were considered as potential confounders and were adjusted for in the Cox regression analysis. We performed a backward elimination procedure using P > 0.05 to remove any identified independent predictors for all-cause mortality in MHD patients. We performed statistical analysis using the computer software SPSS 12.0 (SPSS Inc., Chicago, IL). P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Acknowledgments
This study was supported by grants from the National Science Council (NSC 92-2314-B010-027, NSC 93-2314-B010-032, and NSC 94-2314-B010-033), and Taipei Veterans General Hospital (V95C1-062, V96S5-004, and V96ER2-012).
Aspects of this work were presented at the annual meeting of the American Society of Nephrology; October 31 through November 5, 2007; San Francisco, CA.
We are extremely grateful to Drs. Brian Chen, F.G. Hsieh, K.L. Kuo, and H.H. Liou and N.Y. Hsiao for kind help in the collection of samples at their hemodialysis facilities. We are also deeply indebted to P.C. Lee for expert secretarial assistance and graphic design.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Southorn PA, Powis G: Free radicals in medicine. II. Involvement in human disease. Mayo Clin Proc 63: 390–408, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Ames BN: Endogenous oxidative DNA damage, aging, and cancer. Free Radic Res Commun 7: 121–128, 1989 [DOI] [PubMed] [Google Scholar]
- 3.Floyd RA: Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J 4: 2587–2597, 1990 [PubMed] [Google Scholar]
- 4.Tarng DC, Huang TP, Wei YH, Liu TY, Chen HW, Wen Chen T, Yang WC: 8-Hydroxy-2′-deoxyguanosine of leukocyte DNA as a marker of oxidative stress in chronic hemodialysis patients. Am J Kidney Dis 36: 934–944, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Tarng DC, Huang TP, Liu TY, Chen HW, Sung YJ, Wei YH: Effect of vitamin E-bonded membrane on the 8-hydroxy 2′-deoxyguanosine level in leukocyte DNA of hemodialysis patients. Kidney Int 58: 790–799, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Peuchant E, Carbonneau MA, Dubourg L, Thomas MJ, Perromat A, Vallot C, Clerc M: Lipoperoxidation in plasma and red blood cells of patients undergoing haemodialysis: Vitamins A, E, and iron status. Free Radic Biol Med 16: 339–346, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Maccarrone M, Taccone-Gallucci M, Meloni C, Cococcetta N, Di Villahermosa SM, Casciani CU, Finazzi-Agro A: Activation of 5-lipoxygenase and related cell membrane lipoperoxidation in hemodialysis patients. J Am Soc Nephrol 10: 1991–1996, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B: Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int 49: 1304–1313, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Miyata T, Ueda Y, Yamada Y, Izuhara Y, Wada T, Jadoul M, Saito A, Kurokawa K, van Ypersele de Strihou C: Accumulation of carbonyls accelerates the formation of pentosidine, an advanced glycation end product: Carbonyl stress in uremia. J Am Soc Nephrol 9: 2349–2356, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Locatelli F, Canaud B, Eckardt KU, Stenvinkel P, Wanner C, Zoccali C: Oxidative stress in end-stage renal disease: An emerging threat to patient outcome. Nephrol Dial Transplant 18: 1272–1280, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B: Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 344: 721–724, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Yu Z, Chen J, Ford BN, Brackley ME, Glickman BW: Human DNA repair systems: an overview. Environ Mol Mutagen 33: 3–20, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Aburatani H, Hippo Y, Ishida T, Takashima R, Matsuba C, Kodama T, Takao M, Yasui A, Yamamoto K, Asano M: Cloning and characterization of mammalian 8-hydroxyguanine-specific DNA glycosylase/apurinic, apyrimidinic lyase, a functional mutM homologue. Cancer Res 57: 2151–2156, 1997 [PubMed] [Google Scholar]
- 14.Lu R, Nash HM, Verdine GL: A mammalian DNA repair enzyme that excises oxidatively damaged guanines maps to a locus frequently lost in lung cancer. Curr Biol 7: 397–407, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Tarng DC, Tsai TJ, Chen WT, Liu TY, Wei YH: Effect of human OGG1 1245C->G gene polymorphism on 8-hydroxy-2′-deoxyguanosine levels of leukocyte DNA among patients undergoing chronic hemodialysis. J Am Soc Nephrol 12: 2338–2347, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Hayes JD, Strange RC: Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radic Res 22: 193–207, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Ryberg D, Skaug V, Hewer A, Phillips DH, Harries LW, Wolf CR, Ogreid D, Ulvik A, Vu P, Haugen A: Genotypes of glutathione transferase M1 and P1 and their significance for lung DNA adduct levels and cancer risk. Carcinogenesis 18: 1285–1289, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Board P, Coggan M, Johnston P, Ross V, Suzuki T, Webb G: Genetic heterogeneity of the human glutathione transferases: A complex of gene families. Pharmacol Ther 48: 357–369, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Rebbeck TR: Molecular epidemiology of the human glutathione S-transferase genotypes GSTM1 and GSTT1 in cancer susceptibility. Cancer Epidemiol Biomarkers Prev 6: 733–743, 1997 [PubMed] [Google Scholar]
- 20.Katoh T, Inatomi H, Kim H, Yang M, Matsumoto T, Kawamoto T: Effects of glutathione S-transferase (GST) M1 and GSTT1 genotypes on urothelial cancer risk. Cancer Lett 132: 147–152, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Miller EA, Pankow JS, Millikan RC, Bray MS, Ballantyne CM, Bell DA, Heiss G, Li R: Glutathione-S-transferase genotypes, smoking, and their association with markers of inflammation, hemostasis, and endothelialfunction: the atherosclerosis risk in communities (ARIC) study. Atherosclerosis 171: 265–272, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Li R, Boerwinkle E, Olshan AF, Chambless LE, Pankow JS, Tyroler HA, Bray M, Pittman GS, Bell DA, Heiss G: Glutathione S-transferase genotype as a susceptibility factor in smoking-related coronary heart disease. Atherosclerosis 149: 451–462, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Sweeney C, Nazar-Stewart V, Stapleton PL, Eaton DL, Vaughan TL: Glutathione S-transferase M1, T1, and P1 polymorphisms and survival among lung cancer patients. Cancer Epidemiol Biomarkers Prev 12: 527–533, 2003 [PubMed] [Google Scholar]
- 24.Carmagnol F, Sinet PM, Rapin J, Jerome H: Glutathione-S-transferase of human red blood cells; assay, values in normal subjects and in two pathological circumstances: Hyperbilirubinemia and impaired renal function. Clin Chim Acta 117: 209–217, 1981 [DOI] [PubMed] [Google Scholar]
- 25.Bell DA, Taylor JA, Paulson DF, Robertson CN, Mohler JL, Lucier GW: Genetic risk and carcinogen exposure: A common inherited defect of the carcinogen-metabolism gene glutathione S-transferase M1 (GSTM1) that increases susceptibility to bladder cancer. J Natl Cancer Inst 85: 1159–1164, 1993 [DOI] [PubMed] [Google Scholar]
- 26.Mak JC, Ho SP, Leung HC, Cheung AH, Law BK, So LK, Chan JW, Chau CH, Lam WK, Ip MS, Chan-Yeung M: Relationship between glutathione S-transferase gene polymorphisms and enzyme activity in Hong Kong Chinese asthmatics. Clin Exp Allergy 37: 1150–1157, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Andonova IE, Sarueva RB, Horvath AD, Simeonov VA, Dimitrov PS, Petropoulos EA, Ganev VS: Balkan endemic nephropathy and genetic variants of glutathione S-transferases. J Nephrol 17: 390–398, 2004 [PubMed] [Google Scholar]
- 28.Tarng DC, Liu TY, Huang TP: Protective effect of vitamin C on 8-hydroxy-2′-deoxyguanosine level in peripheral blood lymphocytes of chronic hemodialysis patients. Kidney Int 66: 820–831, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Bolt HM, Thier R: Relevance of the deletion polymorphisms of the glutathione S-transferases GSTT1 and GSTM1 in pharmacology and toxicology. Curr Drug Metab 7: 613–628, 2006 [DOI] [PubMed] [Google Scholar]
- 30.Kasai H: Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res 387: 147–163, 1997 [DOI] [PubMed] [Google Scholar]
- 31.Kuo KL, Hung SC, Wei YH, Tarng DC: Intravenous iron exacerbates oxidative DNA damage in peripheral blood lymphocytes in chronic hemodialysis patients. J Am Soc Nephrol 19: 1817–1826, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kato S, Bowman ED, Harrington AM, Blomeke B, Shields PG: Human lung carcinogen-DNA adduct levels mediated by genetic polymorphisms in vivo. J Natl Cancer Inst 87: 902–907, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Butkiewicz D, Cole KJ, Phillips DH, Harris CC, Chorazy M: GSTM1, GSTP1, CYP1A1 and CYP2D6 polymorphisms in lung cancer patients from an environmentally polluted region of Poland: Correlation with lung DNA adduct levels. Eur J Cancer Prev 8: 315–323, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Rojas M, Cascorbi I, Alexandrov K, Kriek E, Auburtin G, Mayer L, Kopp-Schneider A, Roots I, Bartsch H: Modulation of benzo[a]pyrene diolepoxide-DNA adduct levels in human white blood cells by CYP1A1, GSTM1 and GSTT1 polymorphism. Carcinogenesis 21: 35–41, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Chen SS, Chang LS, Chen HW, Wei YH: Polymorphisms of glutathione S-transferase M1 and male infertility in Taiwanese patients with varicocele. Hum Reprod 17: 718–725, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Izzotti A, Cartiglia C, Lewtas J, De Flora S: Increased DNA alterations in atherosclerotic lesions of individuals lacking the GSTM1 genotype. FASEB J 15: 752–757, 2001 [DOI] [PubMed] [Google Scholar]
- 37.Lindner A, Charra B, Sherrard DJ, Scribner BH: Accelerated atherosclerosis in prolonged maintenance hemodialysis. N Engl J Med 290: 697–701, 1974 [DOI] [PubMed] [Google Scholar]
- 38.Marple JT, MacDougall M: Development of malignancy in the end-stage renal disease patient. Semin Nephrol 13: 306–314, 1993 [PubMed] [Google Scholar]
- 39.de Waart FG, Kok FJ, Smilde TJ, Hijmans A, Wollersheim H, Stalenhoef AF: Effect of glutathione S-transferase M1 genotype on progression of atherosclerosis in lifelong male smokers. Atherosclerosis 158: 227–231, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Danielski M, Ikizler TA, McMonagle E, Kane JC, Pupim L, Morrow J, Himmelfarb J: Linkage of hypoalbuminemia, inflammation, and oxidative stress in patients receiving maintenance hemodialysis therapy. Am J Kidney Dis 42: 286–294, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Liu CS, Tsai CS: Enhanced lipid peroxidation in epileptics with null genotype of glutathione S-transferase M1 and intractable seizure. Jpn J Pharmacol 90: 291–294, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Strange RC, Fryer AA: The glutathione S-transferases: Influence of polymorphism on cancer susceptibility. IARC Sci Publ 148: 231–249, 1999 [PubMed] [Google Scholar]
- 43.Deicher R, Ziai F, Bieglmayer C, Schillinger M, Horl WH: Low total vitamin C plasma level is a risk factor for cardiovascular morbidity and mortality in hemodialysis patients. J Am Soc Nephrol 16: 1811–1818, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Zimmermann J, Herrlinger S, Pruy A, Metzger T, Wanner C: Inflammation enhances cardiovascular risk and mortality in hemodialysis patients. Kidney Int 55: 648–658, 1999 [DOI] [PubMed] [Google Scholar]
- 45.De Flora S, Izzotti A, Walsh D, Degan P, Petrilli GL, Lewtas J: Molecular epidemiology of atherosclerosis. FASEB J 11: 1021–1031, 1997 [PubMed] [Google Scholar]
- 46.International Commission for Protection Against Environmental Mutagens and Carcinogens: The possible involvement of somatic mutations in the development of atherosclerotic plaques: Report of ICPEMC Subcommittee 7/1. Conclusions and recommendations. Mutat Res 239: 143–148, 1990 [PubMed] [Google Scholar]
- 47.Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS: Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomised placebo-controlled trial. Lancet 356: 1213–1218, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Domenici FA, Vannucchi MT, Jordao AA Jr, Meirelles MS, Vannucchi H: DNA oxidative damage in patients with dialysis treatment. Ren Fail 27: 689–694, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Usberti M, Gerardi G, Micheli A, Tira P, Bufano G, Gaggia P, Movilli E, Cancarini GC, De Marinis S, D'Avolio G, Broccoli R, Manganoni A, Albertin A, Di Lorenzo D: Effects of a vitamin E-bonded membrane and of glutathione on anemia and erythropoietin requirements in hemodialysis patients. J Nephrol 15: 558–564, 2002 [PubMed] [Google Scholar]
- 50.Kohno T, Shinmura K, Tosaka M, Tani M, Kim SR, Sugimura H, Nohmi T, Kasai H, Yokota J: Genetic polymorphisms and alternative splicing of the hOGG1 gene, that is involved in the repair of 8-hydroxyguanine in damaged DNA. Oncogene 16: 3219–3225, 1998 [DOI] [PubMed] [Google Scholar]
- 51.Kendall TL, Byerley DJ, Dean R: Isolation of DNA from blood. Anal Biochem 195: 74–76, 1991 [DOI] [PubMed] [Google Scholar]
- 52.Beutler E, Duron O, Kelly BM: Improved method for the determination of blood glutathione. J Lab Clin Med 61: 882–888, 1963 [PubMed] [Google Scholar]
- 53.Asensi M, Sastre J, Pallardo FV, Estrela JM, Vina J: Determination of oxidized glutathione in blood: High-performance liquid chromatography. Methods Enzymol 234: 367–371, 1994 [DOI] [PubMed] [Google Scholar]
- 54.Kyaw A: A simple colorimetric method for ascorbic acid determination in blood plasma. Clin Chim Acta 86: 153–157, 1978 [DOI] [PubMed] [Google Scholar]
- 55.Catignani GL, Bieri JG: Simultaneous determination of retinol and alpha-tocopherol in serum or plasma by liquid chromatography. Clin Chem 29: 708–712, 1983 [PubMed] [Google Scholar]
- 56.Parmar MK, Machin D: Survival Analysis: A Practical Approach, New York, John Wiley & Sons, 1995