Abstract
Renal morphogenesis requires a balance between positive and negative signals, which are provided in part by the receptor tyrosine kinase Ret and the putative tumor suppressor Sprouty1, respectively. Tyrosine 1062 of Ret is a binding site for several adaptor and effector proteins, such as Grb2/Sos/Ras, which activate the ERK pathway. Mice lacking Ret tyrosine 1062 nearly mimic the phenotype of Ret-knockout mice, which includes renal agenesis. Sprouty1 regulates Ret activity by modulating the ERK pathway, but the mechanism by which this occurs is uncertain. Here, we show that loss of Sprouty1 rescues the renal agenesis and early postnatal lethality caused by lack of Ret tyrosine 1062. The kidneys and lower urinary tracts of double-mutant mice developed normally. This effect was specific to the urinary system, because loss of Sprouty1 did not rescue the defects in the enteric nervous system characteristic of animals lacking Ret tyrosine 1062. These results suggest that Sprouty1 can modulate ERK signaling downstream of Ret, independent of Grb2/Sos/Ras, during renal morphogenesis.
Development of the metanephric kidney begins when an outgrowth of the caudal Wolffian duct, the ureteric bud (UB), invades the surrounding metanephric mesenchyme (MM).1 Subsequently, the UB branches repeatedly into a tree-like structure to form ultimately the collecting system of the kidney, whereas some cells of the MM generate the nephron epithelia. Both UB outgrowth and branching morphogenesis are regulated by positive and negative signals. Thus, GDNF secreted by the MM induces the UB to form from the caudal portion of the Wolffian duct, which expresses its receptor Ret. Similarly, branching of the UB is promoted by GDNF expressed by the condensing MM at the tips of the UB branches. Conversely, UB outgrowth and branching are negatively regulated by genes that anteriorly restrict GDNF expression in the MM, such as Foxc12 or Slit2/Robo2,3 or by negative regulators that modulate GDNF signaling downstream of Ret, such as Sprouty1.4–7
The GDNF family ligands are a group of neurotrophic factors (GDNF, NRTN, PSPN, and ARTN) required not only for kidney morphogenesis but also for nervous system development and spermatogonia maturation.8 GDNF family ligands signal through a common tyrosine kinase receptor (Ret) and one of the four co-receptors known as GFRα1 through α4. Ret is alternatively spliced to produce two variants with unique C-terminal sequences (Ret9 and Ret51).9 Mice deficient in Ret die within hours after birth as a result of renal agenesis, owing to failure of UB to form. In addition, enteric neuronal precursors fail to colonize the caudal digestive tract.8,10 To date, five autophosphorylation residues have been identified in Ret. Among them, Tyr1062 is a multidocking site that binds to adaptors such as Shc or FRS-2,8 which in turn recruit Grb2/Sos complexes to the plasma membrane. Once at the plasma membrane, Sos activates Ras, triggering the activation of the extracellular signal–regulated kinase (ERK) mitogen-activated protein kinase (MAPK) pathway. We and others have shown that knockin mice lacking Ret tyrosine 1062 develop many defects found in Ret knockout animals, including kidney agenesis and defective migration of enteric neuroblasts, indicating that this tyrosine is critical for many of the receptor's functions.11–13
Sprouty1 (Spry1) belongs to a gene family composed of four members in mammals (Spry1 through 4). Sprouty proteins are inhibitors of receptor tyrosine kinase signaling, more specifically of the ERK MAPK pathway but not other MAPK pathways such as p38 or JNK.14–16 In mice, Sprouty1 antagonizes GDNF signaling during kidney morphogenesis.4,5 Targeted mutation of Spry1 causes hyperactivation of the ERK MAPK pathway in the Wolffian duct, leading to ectopic, supernumerary UB and increased branching morphogenesis. These defects ultimately result in malformations such as multiple ureters; hydroureter; blind ureter; or supernumerary, cystic kidneys, reminiscent of human CAKUT (congenital anomalies of the kidney and urinary tract).4 Interestingly, reducing GDNF gene dosage in Spry1 null mice rescues these defects, suggesting that signaling by GDNF/Ret is the major if not the only target of Sprouty1 during early kidney morphogenesis. The point at which mammalian Sprouty proteins interfere with the ERK MAPK pathway is controversial. In vitro experiments have led to the proposal of two main mechanisms. The first points to regulation of the pathway by Sprouty upstream of Ras, probably at the level of Grb2.17–19 The second indicates that Sprouty proteins regulate the pathway downstream of or independent of Ras by interacting with Raf kinases.20,21 Although these differences may reflect multiple mechanisms of action depending on the cellular context, in vivo evidence for such mechanisms in mammals is lacking.
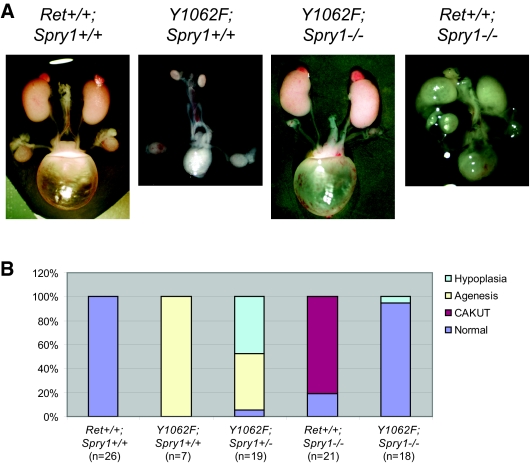
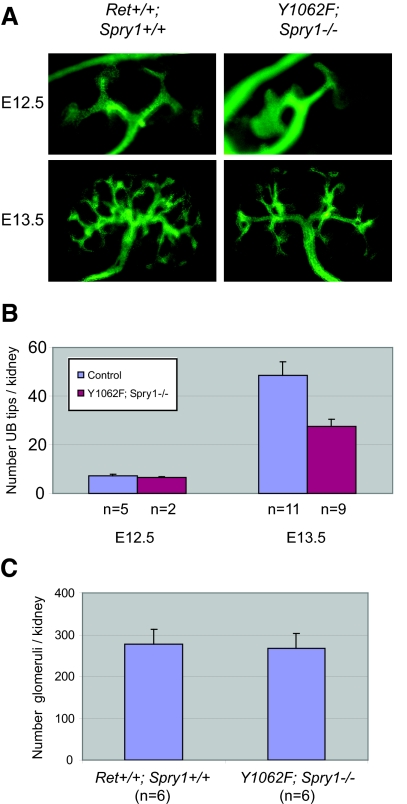
To ascertain whether regulation of Ret signaling by Sprouty1 was dependent on Ret Tyr1062, we crossed mice heterozygous for the targeted mutations to obtain double-heterozygous mice, which were viable and fertile. These mice were then intercrossed and offspring analyzed at birth. Surprisingly, double-mutant mice developed macroscopically normal-sized and -shaped kidneys and ureters and presented urine-filled bladder, indicative of functional kidneys, as well as proper connection between kidneys and ureters (Figure 1A). This phenotype was observed in 17 (95%) of 18 double-mutant mice. Loss of a single Sprouty1 allele modestly rescued renal deficits observed in RetRET(Y1062F/Y1062F) mice (Figure 1B). When analyzed microscopically, kidneys from double-mutant mice presented normal architecture, with well-organized cortex and medulla, and a prominent nephrogenic zone in the periphery that is typically seen at this age (Figure 2, A and C). Closer histologic examination revealed only mild cystic dilation in 50% of the double-mutant mice, whereas the remaining were histologically normal (Figure 2B). In contrast, kidneys from Spry1−/− mice had disorganized, often supernumerary kidneys with cysts that originated from the collecting duct system (Figure 2A and reference 4). We next analyzed the pattern of UB branching by anti-cytokeratin staining of embryonic day 12.5 (E12.5) and E13.5 kidneys. As shown in Figure 3, whereas initial invasion into the MM and number of UB tips at E12.5 were comparable in wild-type (WT) and double-mutant mice, UB tips were less abundant in double-mutant mice at E13.5, although kidney size was similar. Such decrease seemed to reflect a delay in the branching rate, because the number of glomeruli of double-mutant mice at birth was similar to that of WT littermates (Figure 3C).
Figure 1.
Loss of Sprouty1 rescues renal defects found in mice expressing Ret9 Y1062F. (A) Whole mount of genitourinary tracts from newborn mice of the indicated genotypes. (B) Quantification of the renal phenotype of mice with the indicated genotypes. “Agenesis” refers to either unilateral or bilateral agenesis.
Figure 2.
(A) Hematoxylin-eosin staining of paraffin sections of kidneys from newborn mice of the indicated genotypes. Note essentially normal architecture in the double-mutant mice. (B) High-magnification pictures of kidneys from newborn mice of the indicated genotypes. Representative sections are shown from mice with normal (center) as well as mild cystic dilation (right). For comparison, a WT mouse is shown on the left. (C) Periodic acid-Schiff staining of medulla of kidneys from newborn mice of the indicated genotypes shows essentially normal architecture in the double-mutant mice.
Figure 3.
Cytokeratin staining of developing kidneys from E12.5 and E13.5 embryos. (A) Representative pictures of the indicated genotypes at different embryonic ages. (B) Quantification of the UB tips reveals delayed branching in double-mutant mice. At E13.5, significant differences between control and double-mutant mice were observed (P = 0.007 by two-tailed t test). Because no differences between WT and heterozygous mice were observed, Ret+/?;Spry1+/? kidneys were pooled and labeled as “control.” (C) The number of glomeruli at birth of double-mutant mice was similar to that of WT littermates (P = 0.84 by two-tailed t test).
These data indicate that the UB can grow out normally from the Wolffian duct in the absence of Ret Tyr1062 if Sprouty1 is absent, indicating that loss of Sprouty1 de-represses a pathway that is able to compensate the otherwise obligatory role of Tyr1062 in UB outgrowth. Although technical limitations as a result of small embryonic kidney size preclude a direct biochemical analysis of the pathways responsible for UB formation in double-mutant mice, our data shed light on the mechanisms by which Sprouty1 regulate ERK MAPK activity. Extensive analysis performed in different cell types show that in the absence of Tyr1062, Ret9 does not bind either Shc or FRS2, Grb2 is not recruited to the activated signaling complex, and therefore Ras is not activated upon Ret engagement.22–28 Thus, it is unlikely that the loss of Sprouty1 in the double-mutant mice results in ERK activation as a result of de-repression of Grb2 and/or activation of Ras. A more likely explanation is that Sprouty1 regulates the ERK MAPK pathway through modulation of Raf signaling independent of Ras, via a phospholipase C (PLC)γ–protein kinase C–Raf pathway, as is the case for vascular endothelial growth factor–mediated activation of ERK MAPK.20 Favoring this hypothesis, we previously showed that PLCγ activity is necessary for correct UB branching.12 Moreover, in sympathetic neurons lacking Ret Tyr1015, the binding site for PLCγ, phosphorylation of ERK1/2 is reduced by approximately 50%.29
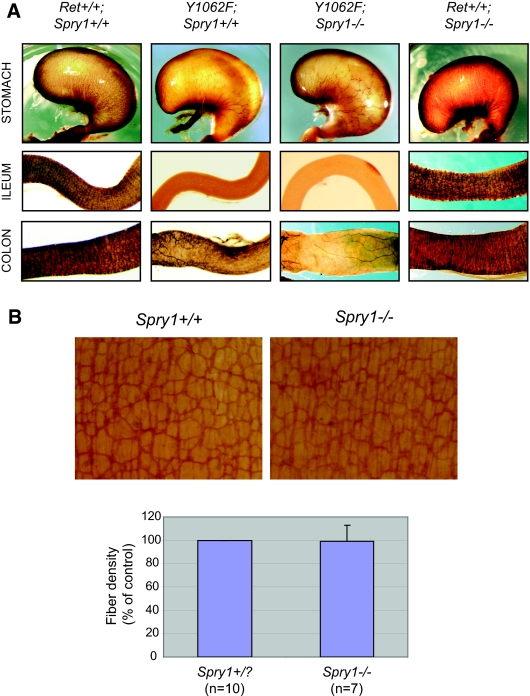
We next assessed whether defective migration of enteric neuroblasts was rescued in double-mutant mice. Whole-mount acetyl cholinesterase staining of stomach, ileum, and colon from RetRET9(Y1062F/Y1062F) mice revealed that, as expected, these mice presented aganglionic intestines and decreased fiber density of the enteric innervation of the stomach. Double-mutant mice presented similar deficits, indicating that loss of Sprouty1 does not compensate for the lack of Ret Tyr1062 in the enteric nervous system (Figure 4A). Intestines from Spry1−/− mice seemed to be normally populated by enteric neurons throughout their entire length. When observed in more detail, enteric plexuses from Spry1−/− mice presented similar fiber density than WT littermates (Figure 4B). Thus, Sprouty1 does not seem to influence GDNF-mediated migration and proliferation of enteric neuroblasts. Rather, such function could be specifically accomplished by Sprouty2,30 indicating that different Sprouty family members have distinct, nonoverlapping roles during mouse development. Supporting this notion, all Spry family members are expressed in the enteric neurons of the myenteric plexus (Supplemental Figure 1).
Figure 4.
(A) Lack of effect of Spry1 deficiency on the enteric innervation defects observed in RetRET9(Y1062F/Y1062F) mice, as revealed by whole-mount acetylcholine esterase activity of digestive tracts from newborn mice. (B) Whole-mount acetylcholine esterase activity of digestive tracts from newborn WT or Spry1−/− mice. Bottom panel shows no difference on plexus density between the two genotypes.
Finally, we wanted to analyze whether loss of Spry1 rescued early postnatal lethality observed in mice lacking Ret tyrosine 1062 in the context of Ret9. To this end, we genotyped offspring of double-heterozygous mice at postnatal day 0.5, that is, the morning these mice were born. Consistent with our previous observations,12 we recovered only 2.53% (versus an expected percentage of 6.25%) of RetRET(Y1062F/Y1062F);Spry1+/+ pups, confirming that these mice die soon after birth. In contrast, mendelian proportions of RetRET(Y1062F/Y1062F);Spry1−/− were obtained (18 of 237, 7.6 versus 6.25%) at that age, indicating that deletion of Sprouty1 rescues early lethality observed in mice lacking Ret tyrosine 1062. When the same analysis was performed 7 d after birth, only one of 148 RetRET(Y1062F/Y1062F);Spry1−/− mice was recovered (0.5 versus 6.25%), indicating that these mice die within the first week after birth, presumably as a result of the absence of enteric innervation.31 In summary, we have found that genetic deletion of Spry1 rescues renal but not enteric defects found in RetRET(Y1062F/Y1062F) mice, suggesting a putative mechanism of regulation of the ERK MAPK pathway by Sprouty1 during early kidney development.
CONCISE METHODS
Histology and Cytokeratin Staining
Kidneys from newborn mice were fixed in 4% paraformaldehyde, sectioned at 5 μm, and processed for hematoxylin-eosin or periodic acid-Schiff staining. Glomeruli counts were performed as described previously.12 The histologic evaluations were done in a blinded manner. For cytokeratin staining, embryonic kidneys were fixed and stained with antibodies to cytokeratin and Alexafluor 488–conjugated secondary antibody as described previously.4 Samples were cleared and mounted in Citifluor mounting medium (Citifluor Ltd, London, UK) and examined with a Nikon 80i epifluorescent microscope. Approximately five digital images were captured of each specimen at different focal planes, and the total number of UB tips were counted using Nikon NIS-Elements software (Nikon, Surrey, UK).
Acetylcholinesterase Histochemistry
Newborn mouse gut was dissected and fixed in 4% paraformaldehyde for 1 to 2 h at 4°C, transferred to saturated sodium sulfate, and stored overnight at 4°C. The gut was then incubated in buffer (ethopropazine HCl [Sigma, St. Louis, MO] 0.2 mM, acetylthiocholine iodide [Sigma] 4 mM, glycine 10 mM, cupric sulfate 2 mM, and sodium acetate 65 mM [pH 5.5]) for 2 to 4 h. Staining for acetylcholinesterase was developed by incubating for 1.5 min in sodium sulfide (1.25%, pH 6). Tissue was rinsed extensively with water before photographing under a dissecting microscope. High-power photomicrographs were obtained by opening the gut along the mesenteric border, flattening the tissue with the serosal side up, and mounting in 50% glycerol before photographing.
DISCLOSURES
None.
Acknowledgments
This work was supported by grants from Ministerio de Educación y Ciencia (BFU2004-03632 and BFU2007-67619) and funding from Suport als Grups de Recerca (Generalitat de Catalunya) to M.E.; O'Brien Center for Kidney Disease Research (P30 DK079333) and HD047396 to S.J.; and grants from the Wellcome Trust (080470) and the Medical Research Council (G0601104) to M.A.B. E.J.R. is recipient of a predoctoral fellowship from Ministerio de Educación y Ciencia. X.D. holds a contract from Fondo de Investigaciones Sanitarias. M.E. holds a contract from the “Ramón y Cajal” program.
We are grateful to Ana Velasco, Amanda Knoten, and Palvinder Kaur for technical assistance. We also thank Graeme R. Guy and Xavier Matias-Guiu for critical reading of the manuscript.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Saxen L. Organogenesis of the Kidney. Cambridge: Cambridge University Press, 1987
- 2.Kume T, Deng K, Hogan BL: Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127: 1387–1395, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Grieshammer U, Le Ma, Plump AS, Wang F, Tessier-Lavigne M, Martin GR: SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell 6: 709–717, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD: Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 8: 229–239, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD: Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol 299: 466–477, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Costantini F, Shakya R: GDNF/Ret signaling and the development of the kidney. Bioessays 28: 117–127, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Airaksinen MS, Saarma M: The GDNF family: Signalling, biological functions and therapeutic value. Nat Rev Neurosci 3: 383–394, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Airaksinen MS, Titievsky A, Saarma M: GDNF family neurotrophic factor signaling: Four masters, one servant? Mol Cell Neurosci 13: 313–325, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Baloh RH, Enomoto H, Johnson EM Jr, Milbrandt J: The GDNF family ligands and receptors: Implications for neural development. Curr Opin Neurobiol 10: 103–110, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Wong A, Bogni S, Kotka P, de Graaff E, D'Agati V, Costantini F, Pachnis V: Phosphotyrosine 1062 is critical for the in vivo activity of the Ret9 receptor tyrosine kinase isoform. Mol Cell Biol 25: 9661–9673, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jain S, Encinas M, Johnson EM Jr, Milbrandt J: Critical and distinct roles for key RET tyrosine docking sites in renal development. Genes Dev 20: 321–333, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jijiwa M, Fukuda T, Kawai K, Nakamura A, Kurokawa K, Murakumo Y, Ichihara M, Takahashi M: A targeting mutation of tyrosine 1062 in Ret causes a marked decrease of enteric neurons and renal hypoplasia. Mol Cell Biol 24: 8026–8036, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy GR, Wong ES, Yusoff P, Chandramouli S, Lo TL, Lim J, Fong CW: Sprouty: How does the branch manager work? J Cell Sci 116: 3061–3068, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Mason JM, Morrison DJ, Basson MA, Licht JD: Sprouty proteins: Multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol 16: 45–54, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Chen R, Kim O, Yang J, Sato K, Eisenmann KM, McCarthy J, Chen H, Qiu Y: Regulation of Akt/PKB activation by tyrosine phosphorylation. J Biol Chem 276: 31858–31862, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Gross I, Bassit B, Benezra M, Licht JD: Mammalian sprouty proteins inhibit cell growth and differentiation by preventing ras activation. J Biol Chem 276: 46460–46468, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Hanafusa H, Torii S, Yasunaga T, Nishida E: Sprouty1 and Sprouty2 provide a control mechanism for the Ras/MAPK signalling pathway. Nat Cell Biol 4: 850–858, 2002 [DOI] [PubMed] [Google Scholar]
- 19.Leeksma OC, Van Achterberg TA, Tsumura Y, Toshima J, Eldering E, Kroes WG, Mellink C, Spaargaren M, Mizuno K, Pannekoek H, de Vries CJ: Human sprouty 4, a new ras antagonist on 5q31, interacts with the dual specificity kinase TESK1. Eur J Biochem 269: 2546–2556, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Sasaki A, Taketomi T, Kato R, Saeki K, Nonami A, Sasaki M, Kuriyama M, Saito N, Shibuya M, Yoshimura A: Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat Cell Biol 5: 427–432, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Yusoff P, Lao DH, Ong SH, Wong ES, Lim J, Lo TL, Leong HF, Fong CW, Guy GR: Sprouty2 inhibits the Ras/MAP kinase pathway by inhibiting the activation of Raf. J Biol Chem 277: 3195–3201, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi H, Ichihara M, Iwashita T, Murakami H, Shimono Y, Kawai K, Kurokawa K, Murakumo Y, Imai T, Funahashi H, Nakao A, Takahashi M: Characterization of intracellular signals via tyrosine 1062 in RET activated by glial cell line-derived neurotrophic factor. Oncogene 19: 4469–4475, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Besset V, Scott RP, Ibanez CF: Signaling complexes and protein-protein interactions involved in the activation of the Ras and phosphatidylinositol 3-kinase pathways by the c-Ret receptor tyrosine kinase. J Biol Chem 275: 39159–39166, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Asai N, Murakami H, Iwashita T, Takahashi M: A mutation at tyrosine 1062 in MEN2A-Ret and MEN2B-Ret impairs their transforming activity and association with shc adaptor proteins. J Biol Chem 271: 17644–17649, 1996 [DOI] [PubMed] [Google Scholar]
- 25.Arighi E, Alberti L, Torriti F, Ghizzoni S, Rizzetti MG, Pelicci G, Pasini B, Bongarzone I, Piutti C, Pierotti MA, Borrello MG: Identification of Shc docking site on Ret tyrosine kinase. Oncogene 14: 773–782, 1997 [DOI] [PubMed] [Google Scholar]
- 26.Alberti L, Borrello MG, Ghizzoni S, Torriti F, Rizzetti MG, Pierotti MA: Grb2 binding to the different isoforms of Ret tyrosine kinase. Oncogene 17: 1079–1087, 1998 [DOI] [PubMed] [Google Scholar]
- 27.De Vita G, Melillo RM, Carlomagno F, Visconti R, Castellone MD, Bellacosa A, Billaud M, Fusco A, Tsichlis PN, Santoro M: Tyrosine 1062 of RET-MEN2A mediates activation of Akt (protein kinase B) and mitogen-activated protein kinase pathways leading to PC12 cell survival. Cancer Res 60: 3727–3731, 2000 [PubMed] [Google Scholar]
- 28.Kurokawa K, Iwashita T, Murakami H, Hayashi H, Kawai K, Takahashi M: Identification of SNT/FRS2 docking site on RET receptor tyrosine kinase and its role for signal transduction. Oncogene 20: 1929–1938, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Encinas M, Rozen EJ, Dolcet X, Jain S, Comella JX, Milbrandt J, Johnson EM Jr: Analysis of Ret knockin mice reveals a critical role for IKKs, but not PI 3-K, in neurotrophic factor-induced survival of sympathetic neurons. Cell Death Differ 15: 1510–1521, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taketomi T, Yoshiga D, Taniguchi K, Kobayashi T, Nonami A, Kato R, Sasaki M, Sasaki A, Ishibashi H, Moriyama M, Nakamura K, Nishimura J, Yoshimura A: Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat Neurosci 8: 855–857, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Stanchina L, Baral V, Robert F, Pingault V, Lemort N, Pachnis V, Goossens M, Bondurand N: Interactions between Sox10, Edn3 and Ednrb during enteric nervous system and melanocyte development. Dev Biol 295: 232–249, 2006 [DOI] [PubMed] [Google Scholar]