Within the normal range, higher serum phosphate concentrations are associated with cardiovascular events and mortality in individuals with chronic kidney disease (CKD) and in those with normal kidney function. Experimental models suggest that phosphate has a direct calcifying effect on vascular smooth muscle. We examined associations of serum phosphate concentrations with vascular and valvular calcification in 439 participants from the Multi-Ethnic Study of Atherosclerosis who had moderate CKD and no clinical cardiovascular disease. Serum phosphate concentrations were within the normal range (2.5 to 4.5 mg/dl) in 95% of study participants. The prevalence of calcification in the coronary arteries, descending thoracic aorta, aortic valve, and mitral valve was 67, 49, 25, and 20%, respectively, measured by electron-beam or multi-detector row computed tomography. After adjustment for demographics and estimated GFR, each 1-mg/dl increment in serum phosphate concentration was associated with a 21% (P = 0.002), 33% (P = 0.001), 25% (P = 0.16), and 62% (P = 0.007) greater prevalence of coronary artery, thoracic, aortic valve, and mitral valve calcification, respectively. Adjustment for traditional risk factors for atherosclerosis, parathyroid hormone, or 1,25-dihydroxyvitamin D levels did not alter these associations. In conclusion, higher serum phosphate concentrations, although still within the normal range, are associated with a greater prevalence of vascular and valvular calcification in people with moderate CKD. It remains to be determined whether lowering phosphate concentrations will impact calcification risk in the setting of kidney disease.
Within the normal laboratory range, higher serum phosphate concentrations have been associated with cardiovascular events and mortality in prospective studies of individuals with chronic kidney disease (CKD) and those with normal kidney function.1–4 Vascular calcification represents an intriguing candidate mechanism connecting phosphate excess with cardiovascular risk. In vitro, phosphate interacts with cultured smooth muscle cells to initiate phenotype transformation, characterized by expression of bone lineage markers and mineralization of extracellular matrix proteins.5,6 Phosphate concentrations >6 mg/dl have been used to promote robust tissue calcification in cell culture models; however, lower phosphate concentrations may be able to initiate calcification in vivo in the presence of other synergistic factors.7 Higher serum phosphate concentrations also stimulate parathyroid hormone (PTH) release and suppress vitamin D activation.8–10 These hormonal disturbances have been associated with cardiovascular risk factors, such as hypertension, inflammation, and glucose intolerance,11–13 suggesting alternative mechanisms to explain observed associations between phosphate levels and cardiovascular events.
In a community-based cohort of adults with moderate CKD and no known clinical cardiovascular disease, we examined associations of serum phosphate concentrations with the presence and extent of calcification at four anatomic sites: Coronary arteries, descending thoracic aorta, mitral valve, and aortic valve. We then explored whether serum PTH and activated vitamin D levels could partially explain potential associations of serum phosphate concentrations with calcification.
RESULTS
We identified 641 Multi-Ethnic Study of Atherosclerosis (MESA) participants (9.4% of original MESA cohort) with an estimated GFR (eGFR) <60 ml/min per 1.73 m2. We excluded 101 participants with a reported history of cancer, 100 with inadequate serum volume for the proposed mineral metabolism measurements, and one participant who was taking sevelamer, leaving 439 participants for analysis. Included participants were similar to those excluded with respect to age and the prevalence of coronary artery calcification (CAC; 67 and 73% among included and excluded participants, respectively). The average age of the study cohort was 70 yr; 62% were female, and 22% had diabetes. The mean eGFR was 50.6 ml/min per 1.73 m2; 97% of participants had stage 3 CKD. Serum phosphate concentrations were ≤4.5 mg/dl in 96% of participants (mean 3.6 mg/dl; SD 0.6).
Serum phosphate concentrations were inconsistently related to traditional cardiovascular risk factors (Table 1). On one hand, higher serum phosphate concentrations were associated with decreased kidney function and higher LDL cholesterol levels. Moreover, participants with phosphate levels >4.0 mg/dl had greater body mass index and a greater prevalence of diabetes and microalbuminuria. On the other hand, higher serum phosphate concentrations were associated with female gender, a lower prevalence of smoking, and lower diastolic BP. We could not detect associations of the serum phosphate concentration with the estimated dietary intake of phosphorous or protein, although higher serum phosphate concentrations were modestly associated with a greater intake of calcium. Among study participants without diabetes, serum phosphate concentrations were unrelated to fasting glucose levels.
Table 1.
Baseline characteristics according to serum phosphate concentrationa
| Characteristic | Serum Phosphate Concentration (mg/dl)
|
P | |||
|---|---|---|---|---|---|
| <3.0(n = 64) | 3.0 to 3.5(n = 157) | 3.6 to 4.0(n = 141) | >4.0(n = 77) | ||
| Demographics | |||||
| age (mean [SD]) | 68.7 (8.5) | 71.0 (8.8) | 69.5 (8.0) | 68.4 (8.1) | 0.0800 |
| race (n [%]) | |||||
| white | 27 (42) | 66 (42) | 64 (45) | 32 (41) | |
| Chinese | 10 (16) | 22 (14) | 23 (16) | 12 (16) | |
| black | 16 (25) | 43 (27) | 28 (20) | 17 (22) | |
| Hispanic | 11 (17) | 26 (17) | 26 (18) | 16 (21) | 0.9600 |
| male gender (n [%]) | 39 (61) | 71 (45) | 38 (27) | 17 (22) | <0.0010b |
| Cardiovascular risk factors | |||||
| BMI (mean [SD]) | 28.9 (5.5) | 28.3 (5.3) | 28.2 (5.2) | 29.7 (6.2) | 0.2100 |
| ever smoked (n [%]) | 38 (60) | 62 (40) | 71 (51) | 33 (43) | 0.0600 |
| diabetes (n [%]) | |||||
| none | 30 (47) | 87 (55) | 71 (50) | 32 (41) | |
| impaired fasting glucose | 20 (31) | 42 (27) | 39 (28) | 21 (27) | |
| diabetes | 14 (22) | 28 (18) | 31 (22) | 24 (31) | 0.3500 |
| SBP (mmHg; mean [SD]) | 137 (27) | 134 (23) | 135 (22) | 134 (25) | 0.8900 |
| DBP (mmHg; mean [SD]) | 76 (13) | 71 (10) | 70 (9) | 70 (12) | 0.0040 |
| LDL cholesterol (mg/dl; mean [SD]) | 111 (32) | 116 (30) | 118 (35) | 125 (41) | 0.1000 |
| HDL cholesterol (mg/dl; mean [SD]) | 50 (16) | 51 (15) | 52 (15) | 53 (16) | 0.7700 |
| triglycerides (mg/dl; mean [SD]) | 161 (195) | 134 (63) | 151 (79) | 148 (71) | 0.2300 |
| C-reactive protein (mg/L; mean [SD]) | 4.0 (4.6) | 4.1 (5.3) | 4.2 (5.7) | 5.1 (8.5) | 0.6100 |
| eGFR (ml/min per 1.73 m2; mean [SD]) | 51.0 (6.4) | 50.8 (8.0) | 51.7 (7.7) | 47.7 (12.8) | 0.0100 |
| cystatin C (mg/L; mean [SD]) | 1.17 (0.25) | 1.24 (0.36) | 1.21 (0.35) | 1.46 (0.86) | <0.0010b |
| microalbuminuria (n [%]) | 13 (20) | 31 (20) | 35 (25) | 23 (30) | 0.2900 |
| Mineral metabolism measurements | |||||
| phosphate (mg/dl; mean [SD]) | 2.8 (0.2) | 3.3 (0.2) | 3.8 (0.1) | 4.4 (0.4) | <0.0001b |
| calcium (mg/dl; mean [SD]) | 10.0 (0.4) | 10.1 (0.5) | 10.1 (0.4) | 10.3 (0.6) | <0.0010b |
| PTH (pg/ml; mean [SD]) | 44 (27) | 37 (18) | 34 (29) | 37 (33) | 0.1400 |
| vitamin 25-D (ng/ml; mean [SD]) | 23 (18) | 24 (15) | 24 (13) | 18 (12) | 0.0100 |
| vitamin 1,25-D (pg/ml; mean [SD]) | 36 (14) | 36 (14) | 37 (15) | 32 (16) | 0.1100 |
| any calcium supplement (n [%]) | 27 (42) | 71 (45) | 66 (47) | 38 (49) | 0.8800 |
| Dietary intake measurements (mean [SD]) | |||||
| calories (kcal/d) | 1614 (975) | 1388 (602) | 1430 (712) | 1555 (713) | 0.1400 |
| calcium (mg/d) | 920 (535) | 936 (684) | 1145 (963) | 1164 (749) | 0.0400 |
| phosphorus (mg/d) | 1068 (579) | 914 (452) | 951 (561) | 1061 (552) | 0.1200 |
| protein (g/d) | 63 (33) | 56 (26) | 56 (30) | 64 (30) | 0.1100 |
| fat (g/d) | 65 (45) | 52 (26) | 55 (31) | 60 (34) | 0.0400 |
BMI, body mass index; DBP, diastolic BP; SBP, systolic BP.
Statistically significant after Bonferroni correction for multiple comparisons (P < 0.002).
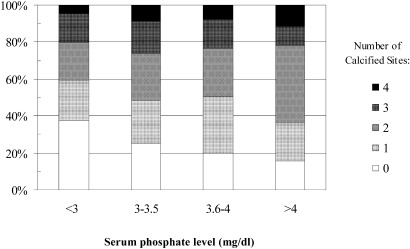
The prevalence of any calcification in the coronary arteries, descending thoracic aorta, aortic valve, and mitral valve was 67, 49, 25, and 20%, respectively. The median CAC score in participants with nonzero baseline CAC scores was 125 Agatston units (interquartile range 35 to 413 Agatston units). A total of 103 (23%) participants had no detectable calcification at any of the four anatomic sites. The number of calcified sites increased with greater serum phosphate concentrations (P = 0.04; Figure 1).
Figure 1.
Proportion of calcified sites by serum phosphate group.
We observed a graded association of higher serum phosphate concentrations with greater calcification prevalence for all four anatomic sites (Table 2). After adjustment for age, race, and gender, participants in the highest phosphate group had a 54% greater prevalence of CAC, and a 69, 68, and 93% greater prevalence of descending thoracic aorta, aortic valve, and mitral valve calcification, compared with participants in the lowest serum phosphate group. Associations of phosphate with calcification prevalence were approximately linear throughout the measured range of phosphate. Adjustment for traditional cardiovascular risk factors did not materially alter the strength of any of the prevalence ratio (PR) estimates (Table 2, model 2). Moreover, PR estimates remained consistent when models were further adjusted for dietary intake (all estimates changed by ≤11%). Results did not change when analyses were restricted to participants with serum phosphate levels within the normal laboratory range (2.5 to 4.5 mg/dl).
Table 2.
Cross-sectional associations of serum phosphate concentration with prevalent calcificationa
| Site | % Prevalent Calcification | Model 1b
|
Model 2c
|
||||
|---|---|---|---|---|---|---|---|
| PR | 95% CI | PR | 95% CI | ||||
| Coronary artery (mg/dl) | |||||||
| <3.0 | 58 | 1.00 | Reference | 1.00 | Reference | ||
| 3.0 to 3.5 | 66 | 1.11 | 0.90 to 1.38 | 1.10 | 0.89 to 1.38 | ||
| 3.6 to 4.0 | 67 | 1.31 | 1.04 to 1.65 | 1.27 | 1.00 to 1.61 | ||
| >4.0 | 75 | 1.54 | 1.21 to 1.94 | 1.42 | 1.11 to 1.82 | ||
| P for trend | <0.001 | 0.001 | |||||
| Descending thoracic aorta (mg/dl) | |||||||
| <3.0 | 34 | 1.00 | Reference | 1.00 | Reference | ||
| 3.0 to 3.5 | 49 | 1.22 | 0.89 to 1.69 | 1.32 | 0.95 to 1.83 | ||
| 3.6 to 4.0 | 51 | 1.42 | 1.02 to 2.00 | 1.46 | 1.03 to 2.07 | ||
| >4.0 | 57 | 1.69 | 1.22 to 2.45 | 1.80 | 1.25 to 2.61 | ||
| P for trend | <0.001 | <0.001 | |||||
| Aortic valve (mg/dl) | |||||||
| <3.0 | 20 | 1.00 | Reference | 1.00 | Reference | ||
| 3.0 to 3.5 | 28 | 1.28 | 0.77 to 2.14 | 1.31 | 0.77 to 2.23 | ||
| 3.6 to 4.0 | 23 | 1.37 | 0.80 to 2.37 | 1.40 | 0.79 to 2.47 | ||
| >4.0 | 26 | 1.68 | 0.93 to 3.03 | 1.65 | 0.90 to 3.06 | ||
| P for trend | 0.050 | 0.240 | |||||
| Mitral valve (mg/dl) | |||||||
| <3.0 | 16 | 1.00 | Reference | 1.00 | Reference | ||
| 3.0 to 3.5 | 19 | 1.09 | 0.59 to 2.01 | 1.34 | 0.70 to 2.56 | ||
| 3.6 to 4.0 | 21 | 1.54 | 0.83 to 2.87 | 1.75 | 0.92 to 3.35 | ||
| >4.0 | 23 | 1.93 | 1.00 to 3.73 | 2.01 | 1.00 to 4.07 | ||
| P for trend | <0.001 | 0.030 | |||||
CI, confidence interval.
Model 1 adjusted for age, race, and gender.
Model 2 adds cystatin C, BMI, smoking (ever versus never), diabetes, DBP, LDL cholesterol, ln(C-reactive protein), ln(urinary albumin to creatinine ratio), serum calcium, and serum 1,25-(OH)2D levels.
The strength of association between phosphate concentration and CAC prevalence did not statistically differ by race, gender, or diabetes (all P > 0.1); however, associations of phosphate concentrations with CAC prevalence were significantly stronger among younger participants (PR 1.76 in participants ≤65 yr old versus PR 1.10 in participants ≥75 yr old, P = 0.04 for interaction) and among participants with better kidney function (PR 1.72 in lowest cystatin C quartile versus PR 1.22 in highest cystatin C quartile, P = 0.008 for interaction).
We explored whether associations of phosphate concentrations with calcification might be partially mediated via serum levels of PTH or 1,25-dihydroxyvitamin D [1,25-(OH)2D]. Adjustment for serum levels of PTH and 1,25-(OH)2D did not alter the strength of any estimated PR (Table 3). Among participants with prevalent site-specific calcification, greater serum phosphate concentrations were associated with higher CAC scores but not with the extent of calcification in the thoracic aorta or cardiac valves (Table 4).
Table 3.
Associations of 1-mg/dl greater serum phosphate concentrations with prevalent calcification after adjustment for potential mediators
| Site | Adjusted for Age, Race, Gender, and Kidney Function
|
Add Serum PTH Levels
|
Add Serum 1,25-(OH)2D Levels
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| PR | 95% CI | Pa | PR | 95% CI | Pa | PR | 95% CI | Pa | |
| Coronary artery | 1.22 | 1.08 to 1.37 | 0.001 | 1.19 | 1.05 to 1.35 | 0.005 | 1.19 | 1.05 to 1.35 | 0.005 |
| Descending aorta | 1.33 | 1.13 to 1.58 | 0.001 | 1.32 | 1.11 to 1.57 | 0.002 | 1.33 | 1.12 to 1.58 | 0.001 |
| Aortic valve | 1.25 | 0.93 to 1.70 | 0.140 | 1.27 | 0.93 to 1.73 | 0.130 | 1.24 | 0.90 to 1.70 | 0.190 |
| Mitral valve | 1.61 | 1.14 to 2.31 | 0.008 | 1.67 | 1.17 to 2.38 | 0.005 | 1.69 | 1.18 to 2.41 | 0.004 |
P values not adjusted for multiple comparisons.
Table 4.
Associations of serum phosphate concentration with the severity of calcification among participants with site-specific prevalent calcification
| Site | N | Model 1a
|
Model 2b
|
||||
|---|---|---|---|---|---|---|---|
| Exp(β)c | 95% CI | Pd | Exp(β)c | 95% CI | Pd | ||
| Coronary artery | 293 | 1.64 | 1.16 to 2.32 | 0.005 | 1.70 | 1.12 to 2.56 | 0.010 |
| Descending aorta | 215 | 1.06 | 0.70 to 1.61 | 0.770 | 1.19 | 0.72 to 1.98 | 0.500 |
| Aortic valve | 109 | 0.89 | 0.55 to 1.42 | 0.610 | 0.76 | 0.40 to 1.44 | 0.400 |
| Mitral valve | 87 | 0.89 | 0.45 to 1.74 | 0.720 | 1.60 | 0.53 to 4.83 | 0.400 |
Model 1 adjusted for age, race, and gender.
Model 2 adds cystatin C, BMI, smoking (ever versus never), diabetes, DBP, LDL cholesterol, ln(C-reactive protein), ln(urinary albumin to creatinine ratio), serum calcium, and serum 1,25-(OH)2D levels.
Exp(β) interpreted as relative difference in geometric mean CAC score per each 1-mg/dl higher serum phosphate level.
P values not adjusted for multiple comparisons.
DISCUSSION
In a community-based cohort of individuals with CKD and no clinically apparent cardiovascular disease, higher serum phosphate concentrations within the normal laboratory range were associated with a statistically greater prevalence of coronary artery, descending thoracic aorta, and mitral valve calcification. Associations were independent of traditional atherosclerotic risk factors, estimated kidney function, and measured dietary variables. Adjustment for serum PTH and 1,25-(OH)2D levels, which may be influenced by abnormal phosphate metabolism, did not alter associations of phosphate concentrations with calcification. These data suggest that mild disturbances in phosphate homeostasis might be involved in dystrophic calcification among individuals with moderate CKD.
Four prospective cohort studies have examined associations of serum phosphate concentrations with cardiovascular events and mortality in individuals with CKD and normal kidney function.1–4 In a study of 3490 older, predominately male veterans with stages 3 to 4 CKD, each 1-mg/dl higher serum phosphate concentration was associated with a statistically significant 23% greater adjusted risk for all-cause mortality.2 In the Modification of Diet in Renal Disease (MDRD) study of 839 younger individuals without diabetes and with advanced CKD, each 1-mg/dl higher serum phosphate concentration was associated with 10 and 27% greater risks of death and cardiovascular specific death, respectively; however, neither result was statistically significant.3 In a cholesterol-lowering trial that included 4127 participants without known kidney disease, each 1-mg/dl higher serum phosphate concentration was associated with a 22% greater adjusted risk for all-cause mortality and a 20% greater risk for recurrent myocardial infarction.4 Finally, among 3368 middle-aged participants in the Framingham Offspring Study with normal estimated kidney function and no clinical cardiovascular disease, each 1-mg/dl higher serum phosphate concentration was associated with a 31% greater adjusted risk for the composite outcome, defined by fatal or nonfatal myocardial infarction, angina, heart failure, stroke, transient ischemic attack, or peripheral vascular disease.1 In each of these studies and in our study, nearly all of the measured serum phosphate levels were within the normal laboratory range.
Observed associations of higher serum phosphate concentrations with cardiovascular events provoke questions regarding potential mechanisms of association. In vitro, phosphate acts directly on cultured vascular smooth muscle cells to initiate phenotype transformation, characterized by loss of contractility, expression of bone-specific markers, and calcification of matrix proteins.5,6 Recently, the sodium-dependent phosphate transporter PIT-1 was shown to be necessary and sufficient to induce these changes and to activate the osteoblast lineage transcription factor CBFA1.6 Experimental models have used exogenous phosphate concentrations >6 mg/dl to obtain a robust calcification response. It remains possible that lower phosphate concentrations might be sufficient to initiate a calcification response in vivo in the presence of additional synergistic factors. For example, the circulating calcification inhibitor fetuin-A may contribute as much as 50% of serum calcification inhibitory activity and is significantly reduced in patients with CKD.7
Higher serum phosphate concentrations also suppress vitamin D activation and stimulate PTH release.8–10 Experimental and epidemiologic studies have reported associations of lower vitamin D and higher PTH concentrations with cardiovascular risk factors, such as hypertension, inflammatory biomarkers, and glucose intolerance.11–13 For these reasons, alterations in activated vitamin D and/or PTH metabolism have been suggested as potential mechanisms to explain associations of phosphate with cardiovascular risk. In this cohort of participants with moderate CKD, serum phosphate concentrations were weakly associated with 1,25-(OH)2D and PTH levels, if at all, and adjustment for these hormone levels did not alter the strength of association between phosphate concentrations and calcification prevalence. These findings decrease the likelihood that vitamin D or PTH is a central mechanism to explain associations of phosphate with calcification in early kidney disease; however, findings are limited by the measurement of hormone levels on a single occasion and by the potential for laboratory measurement error.
It is possible that higher serum phosphate concentrations are a surrogate marker for other processes that contribute to vascular calcification. In this study, we could not detect meaningful associations of serum phosphate concentrations with traditional cardiovascular risk factors or with measured estimates of dietary intake. Moreover, adjustment for a comprehensive set of traditional risk factors modestly increased the strength of association between phosphate concentrations and calcification. Although it is possible that observed associations are confounded by residual error in estimating kidney function, associations of phosphate concentrations with calcification remained consistent after adjustment for cystatin C, age, race, and gender, which collectively account for nearly 85% of the variation in measured GFR among individuals with CKD.14 Furthermore, associations of phosphate concentrations with cardiovascular events were previously observed in individuals without kidney disease1 and were stronger among participants who had relatively better kidney function in our study.
A second important limitation is cross-sectional ascertainment of serum phosphate concentrations and calcification measurements, leaving open the possibility that phosphate concentrations may increase in response to calcified lesions or in response to other processes linked with arterial calcification. Vascular calcification represents the result of long-standing atherosclerotic and calcification processes. It is unclear whether the steady-state serum phosphate concentration measured in this study accurately represents phosphorous balance that occurred when calcification was developing. As with any CKD study population, it is possible that participants represent healthy survivors with kidney disease and that study findings may not apply equally to sicker individuals with CKD and those with extensive cardiovascular disease.
Although imaging studies were obtained in duplicate at each MESA visit, some imprecision in vascular calcification measurements by computed tomography (CT) scanning is expected. Moreover, mineral metabolism disturbances have been linked specifically with medial rather than intimal vascular calcification in CKD; however, current imaging techniques are unable to distinguish these pathologic processes.
In summary, we observed an association of higher serum phosphate concentrations within the normal laboratory range and the prevalence of vascular and valvular calcification in individuals with moderate CKD and no clinical cardiovascular disease. These observational data do not address the potential effects of lowering serum phosphate concentrations in the setting of early kidney dysfunction. Observed associations were independent of traditional risk factors, severity of kidney dysfunction, dietary intake, and concentrations of PTH and 1,25-(OH)2D. It is possible that even mild elevations in serum phosphate concentrations may contribute to calcification risk in individuals with moderate CKD.
CONCISE METHODS
Study population
The MESA is a prospective cohort study designed to evaluate the presence, extent, and progression of subclinical cardiovascular disease.15 Briefly, 6814 participants who were aged 45 to 84 yr and identified their race/ethnicity as white/Caucasian, black/African-American, Chinese, or Spanish/Hispanic/Latino were recruited from six US communities between July 2000 and August 2002 (Forsyth County, NC; Northern Manhattan and the Bronx, NY; Baltimore City and Baltimore County, MD; St. Paul, MN; Chicago, IL; and Los Angeles County, CA). Individuals were excluded from MESA when they had any previous diagnosis of cardiovascular disease (physician-diagnosed heart attack, angina, stroke, transient ischemic attack, heart failure, or atrial fibrillation; use of nitroglycerin; or previous angioplasty, coronary artery bypass graft, valve replacement, pacemaker or defibrillator implantation, or any surgery on the heart or arteries). All participants gave informed consent, and institutional review board approval was obtained for each study site.
We selected all MESA study participants with CKD, defined by an eGFR <60 ml/min per 1.73 m2, for this ancillary study. We used the four-variable MDRD equation to estimate GFR.16 We excluded participants with a reported history of cancer, which may alter calcium-phosphate metabolism, and those with inadequate stored sample volume to perform the ancillary measurements.
Mineral Metabolism Measurements
Blood specimens were collected in the morning after an overnight fast. Calcium, phosphate, and PTH levels were measured at the Clinical Chemistry Laboratory at Fletcher Allen Health Care using the Vitros 950IRC instrument. Serum calcium was determined by colorimetric reaction. Serum phosphate concentrations were measured by reflectance spectrophotometry. Bioactive PTH (84–amino acid polypeptide) was measured by ELISA (Human Bioactive Intact PTH ELISA; Alpco Diagnostics, Windham, NH).
Serum 25-hydroxyvitamin D [25(OH)D] and 1,25-(OH)2D levels were measured on two occasions. An original selection of 164 serum samples were measured at the University of Vermont Laboratory for Clinical Biochemistry Research (Colchester, VT). The remaining samples were measured at Mayo Clinic Medical Laboratories (Rochester, MN). 1,25-(OH)2D was measured at both sites using the Diasorin 1,25-Dihydroxyvitamin D 125I RIA Kit (Stillwater, MN). 25(OH)D was measured at the University of Vermont using an ELISA (25-Hydroxyvitamin D ELISA; Alpco Diagnostics) and at Mayo Clinic Medical Laboratories using an RIA (25-Hydroxyvitamin D 125I RIA Kit; Diasorin). We looked for potential differences between the 25(OH)D assays by comparing mean 25(OH)D concentrations from each assay, after adjustment for age, race, gender, and estimated kidney function. No significant difference in mean 25(OH)D concentrations was found.
Calcification Measurements
Coronary artery calcium, descending thoracic aorta calcium, aortic valve calcium, and mitral valve calcium were assessed using either electron-beam CT or multi-detector CT. Each participant was scanned twice over phantoms of known calcium concentration. Calcium scores were quantified using the Agatston scoring method.17 The mean value of two scan results was used for all analyses of CAC, and the value from the first scan was used for analyses of calcification in the descending thoracic aorta and mitral and aortic valves, as described previously.18
Statistical Analysis
Baseline participant characteristics were examined with respect to 0.5-mg/dl categories of serum phosphate concentrations. The presence of any calcification at each site was defined as a mean Agatston score >0; therefore, the absence of CAC required two negative scans.
We used relative risk regression with Poisson error distribution and robust standard error to estimate associations of serum phosphate concentrations with calcification prevalence after adjustment for potential confounding factors. Estimates from these analyses can be interpreted as prevalence ratio. We used linear regression to estimate adjusted cross-sectional associations of serum phosphate concentrations with the extent of calcification among participants with nonzero calcification scores, which were log-transformed to stabilize the variance and moderate the influence of outlying values. Estimates from the linear regression models can be interpreted as the relative difference in the geometric mean calcification score.
Potential confounding factors were selected a priori and are listed in Table 1. Covariate blocks were added to progressive nested multivariable models. Exploratory models included adjustment for serum PTH and 1,25-(OH)2D levels to investigate whether these potential mechanistic factors might alter the strength of associations between phosphate levels and calcification. The Wald test for statistical significance of interaction terms was used to evaluate whether associations differed by age, race, gender, diabetes, or kidney function. Graphical methods were used to explore functional relationships of serum phosphate concentrations with CAC prevalence; these analyses suggested that the log-linear model fit the data as well as more complex models. Analyses were conducted using STATA 9.0 (Stata Corp., College Station, TX).
DISCLOSURES
B.R.K. receives consulting fees from Shire and Abbott, Inc.
Acknowledgments
This research was supported by contracts N01-HC-95159 through N01-HC-95166, N01-HC-95169, R01-HL-071739, and R01-HL-072403 from the National Heart, Lung, and Blood Institute and a National Institutes of Health Career Development Award K23 DK63274-01.
We thank the other investigators, the staff, and the participants of the MESA study for valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB Sr, Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Menon V, Greene T, Pereira AA, Wang X, Beck GJ, Kusek JW, Collins AJ, Levey AS, Sarnak MJ: Relationship of phosphorus and calcium-phosphorus product with mortality in CKD. Am J Kidney Dis 46: 455–463, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Tonelli M, Isles C, Craven T, Tonkin A, Pfeffer MA, Shepherd J, Sacks FM, Furberg C, Cobbe SM, Simes J, West M, Packard C, Curhan GC: Effect of pravastatin on rate of kidney function loss in people with or at risk for coronary disease. Circulation 112: 171–178, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Chen NX, O'Neill KD, Duan D, Moe SM: Phosphorus and uremic serum up-regulate osteopontin expression in vascular smooth muscle cells. Kidney Int 62: 1724–1731, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, Morii H, Giachelli CM: Phosphate regulation of vascular smooth muscle cell calcification. Circ Res 87: E10–E17, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, Metzger T, Wanner C, Jahnen-Dechent W, Floege J: Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: A cross-sectional study. Lancet 361: 827–833, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Almaden Y, Hernandez A, Torregrosa V, Canalejo A, Sabate L, Fernandez Cruz L, Campistol JM, Torres A, Rodriguez M: High phosphate level directly stimulates parathyroid hormone secretion and synthesis by human parathyroid tissue in vitro. J Am Soc Nephrol 9: 1845–1852, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Pitts TO, Piraino BH, Mitro R, Chen TC, Segre GV, Greenberg A, Puschett JB: Hyperparathyroidism and 1,25-dihydroxyvitamin D deficiency in mild, moderate, and severe renal failure. J Clin Endocrinol Metab 67: 876–881, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Slatopolsky E, Finch J, Denda M, Ritter C, Zhong M, Dusso A, MacDonald PN, Brown AJ: Phosphorus restriction prevents parathyroid gland growth: High phosphorus directly stimulates PTH secretion in vitro. J Clin Invest 97: 2534–2540, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristal-Boneh E, Froom P, Harari G, Ribak J: Association of calcitriol and blood pressure in normotensive men. Hypertension 30: 1289–1294, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Saleh FN, Schirmer H, Sundsfjord J, Jorde R: Parathyroid hormone and left ventricular hypertrophy. Eur Heart J 24: 2054–2060, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Wareham NJ, Byrne CD, Carr C, Day NE, Boucher BJ, Hales CN: Glucose intolerance is associated with altered calcium homeostasis: A possible link between increased serum calcium concentration and cardiovascular disease mortality. Metabolism 46: 1171–1177, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Rule AD, Bergstralh EJ, Slezak JM, Bergert J, Larson TS: Glomerular filtration rate estimated by cystatin C among different clinical presentations. Kidney Int 69: 399–405, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP: Multi-ethnic study of atherosclerosis: Objectives and design. Am J Epidemiol 156: 871–881, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 18.Budoff MJ, Katz R, Wong ND, Nasir K, Mao SS, Takasu J, Kronmal R, Detrano RC, Shavelle DM, Blumenthal RS, O'Brien K, D, Carr JJ: Effect of scanner type on the reproducibility of extracoronary measures of calcification: The multi-ethnic study of atherosclerosis. Acad Radiol 14: 1043–1049, 2007 [DOI] [PubMed] [Google Scholar]