Abstract
Proteinuria and increased renal reabsorption of NaCl characterize the nephrotic syndrome. Here, we show that protein-rich urine from nephrotic rats and from patients with nephrotic syndrome activate the epithelial sodium channel (ENaC) in cultured M-1 mouse collecting duct cells and in Xenopus laevis oocytes heterologously expressing ENaC. The activation depended on urinary serine protease activity. We identified plasmin as a urinary serine protease by matrix-assisted laser desorption/ionization time of-flight mass spectrometry. Purified plasmin activated ENaC currents, and inhibitors of plasmin abolished urinary protease activity and the ability to activate ENaC. In nephrotic syndrome, tubular urokinase-type plasminogen activator likely converts filtered plasminogen to plasmin. Consistent with this, the combined application of urokinase-type plasminogen activator and plasminogen stimulated amiloride-sensitive transepithelial sodium transport in M-1 cells and increased amiloride-sensitive whole-cell currents in Xenopus laevis oocytes heterologously expressing ENaC. Activation of ENaC by plasmin involved cleavage and release of an inhibitory peptide from the ENaC γ subunit ectodomain. These data suggest that a defective glomerular filtration barrier allows passage of proteolytic enzymes that have the ability to activate ENaC.
Nephrotic syndrome is characterized by proteinuria, sodium retention, and edema. Increased renal sodium reabsorption occurs in the cortical collecting duct (CCD),1,2 where a rate-limiting step in transepithelial sodium transport is the epithelial sodium channel (ENaC), which is composed of the three homologous subunits: α, β, γ.3
ENaC activity is regulated by hormones, such as aldosterone and vasopressin (AVP)4,5; however, adrenalectomized rats and AVP-deficient Brattleboro rats are capable of developing nephrotic syndrome,1,6 and nephrotic patients do not consistently display elevated levels of sodium-retaining hormones,7,8 suggesting that renal sodium hyper-reabsorption is independent of systemic factors. Consistent with this, sodium retention is confined to the proteinuric kidney in the unilateral puromycin aminonucleoside (PAN) nephrotic model.2,9,10
There is evidence that proteases contribute to ENaC activation by cleaving the extracellular loops of the α- and γ-subunits.11–13 Proteolytic activation of ENaC by extracellular proteases critically involves the cleavage of the γ subunit,14–16 which probably leads to the release of a 43-residue inhibitory peptide from the ectodomain.17 Both cleaved and noncleaved channels are present in the plasma membrane,18,19 allowing proteases such as channel activating protease 1 (CAP1/prostasin),20 trypsin,20 chymotrypsin,21 and neutrophil elastase22 to activate noncleaved channels from the extracellular side.23,24 We hypothesized that the defective glomerular filtration barrier in nephrotic syndrome allows the filtration of ENaC-activating proteins into the tubular fluid, leading to stimulation of ENaC. The hypothesis was tested in the PAN nephrotic model in rats and with urine from patients with nephrotic syndrome.
RESULTS
Sodium Retention in PAN Nephrotic Syndrome Involves a Primary Increase in Renal ENaC Activity
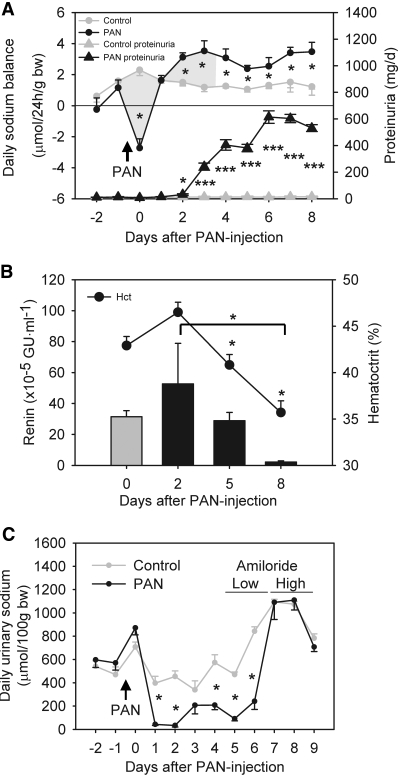
The first 24 h after PAN injection were characterized by net loss of sodium and water, an increase in hematocrit, and increased plasma renin (Figure 1, A and B, and Supplemental Figure 1). This was followed by net sodium retention and a positive cumulative sodium balance from day 3.6 ± 0.8 and onward (Figure 1A) and suppression of plasma renin concentrations in parallel to a fall in hematocrit consistent with expansion of extracellular fluid volume (Figure 1B, Supplemental Figure 1). Plasma aldosterone concentration was maximal at day 5 and was suppressed to 1% of this level at day 8 (P < 0.01; Supplemental Figure 2A). Urinary protein excretion increased from day 3 and coincided with the transition from negative to positive sodium balance (Figure 1A). There was no significant change in AVP concentration 8 d after PAN treatment (Supplemental Table 1). Sodium accumulation in PAN-treated rats was resistant to combined treatment with the AT1 receptor antagonist candesartan (1 mg/kg per d) and the mineralocorticoid receptor antagonist potassium canrenoate (100 mg/kg per d subcutaneously; n = 8; Supplemental Figure 2B).
Figure 1.
Sodium retention in PAN nephrotic syndrome in rats involves a primary increase in renal ENaC activity. (A) Daily sodium balance and urinary protein excretion after PAN injection based on measurements of daily sodium intake, fecal sodium output, and urinary sodium output of rats in metabolic cages (Supplemental Figure 1). Nephrotic rats display negative cumulative sodium balance (shaded area) from days 0 to 3.6 after PAN injection and positive sodium balance thereafter. Nephrotic rats accumulated 2217 ± 167 μmol/100 g body wt sodium (n = 8) from days 0 through 8 compared with 1096 ± 70 μmol/100 g body wt in controls (n = 11; P < 0.0005). The proteinuria in nephrotic rats was significant from days 2 through 8. Arrow indicates time of PAN injection. *P < 0.05; ***P < 0.001. (B) Parallel changes in plasma renin concentrations and hematocrit values of nephrotic rats indicate a shift from volume underfilling to overfilling from days 2 through 5. Renin: *P < 0.05 between days 2 and 8 (t test). Hematocrit: *P < 0.05 versus control (Dunnett test). GU, Goldblatt units. (C) Effect of amiloride treatment on daily urinary sodium output. In PAN nephrotic rats, amiloride (2 mg/d per kg body wt) increases daily urinary sodium excretion more than in controls (n = 6). Arrow indicates time of PAN injection. *P < 0.05.
Treatment of PAN nephrotic rats with amiloride in a step-up protocol with 100 and 500 μg/d (2074 ± 88 μg/kg body wt) increased daily urinary sodium excretion (Figure 1C). Moreover, amiloride had a beneficial effect on ascitic volume, which decreased from 3.5 ± 0.5 ml/100 g body wt (n = 16) to 1.7 ± 0.6 ml/100 g body wt (n = 6; P < 0.05). These results indicate that ENaC activity is enhanced in the PAN-treated animals, whereas ENaC mRNA and protein levels were not changed (data not shown).
Plasmin in Nephrotic Urine Stimulates ENaC Activity
We hypothesized that a serine protease may be present in nephrotic urine and may directly activate ENaC in the plasma membrane. Whole-cell current did not change in single M-1 cells when exposed to urine from control rats, whereas urine collected on day 5 after PAN injection yielded a 5.1 ± 2.0-fold increase in the observed inward current that was prevented in the presence of 2 μM amiloride (control urine −55 ± 12 pA versus nephrotic urine −231 ± 53 pA at −150 mV holding potential; n = 4; P < 0.05; Figure 2, A and B). This stimulatory effect was observed within 30 to 60 s after exposure of the M-1 cells to nephrotic urine, and it was prevented by aprotinin, an inhibitor of serine proteases (Figure 2B). Xenopus laevis oocytes heterologously expressing ENaC confirmed the stimulatory effect of nephrotic urine samples on amiloride-sensitive ENaC whole-cell currents (ΔIami; Figure 2C) compared with control oocytes preincubated in ND96 bath solution or compared with oocytes preincubated in heat-inactivated nephrotic urine samples. The stimulatory effect of a 30-min exposure to nephrotic urine was similar to that of a 5-min exposure to chymotrypsin.21 These findings suggest that nephrotic urine contains a serine protease, which can activate ENaC.
Figure 2.
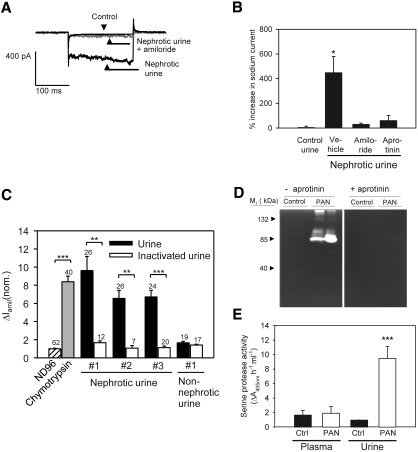
Serine proteases in nephrotic urine stimulates ENaC activity. (A) Traces obtained with whole-cell patch-clamp technique on a single M-1 cell showing baseline current (black) before addition of nephrotic urine (day 5) in the presence of 2 μM amiloride (gray). Subsequently, amiloride was washed away and the same cell was stimulated with the corresponding urine sample (black). The voltage was clamped to −60 mV and then stepped to −150 mV for 200 ms. The traces shown are recorded approximately 30 to 60 s after addition of the various substances. (B) Mean values from four different patch-clamp experiments showing that addition of aprotinin (700 μg/ml) to nephrotic urine samples or the presence of amiloride (2 μM) prevents the activation of currents in single M-1 cells. *P < 0.05 versus control (n = 4, Dunnett test). (C) Preincubation of ENaC-expressing Xenopus laevis oocytes with nephrotic urine samples increased the amiloride-sensitive ENaC whole-cell current to a similar extent as preincubation with chymotrypsin. Control oocytes were preincubated in standard ND96 solution, which was also used as bath solution for all other whole-cell current measurements. The stimulatory effect of nephrotic urine samples was essentially abolished by heat-inactivating the urine samples. The nephrotic urine samples were from three different rats; the non-nephrotic urine sample was from a rat that was given PAN injection and did not develop nephrotic syndrome probably because of an insufficient injection. Numbers of individual oocytes measured in each group are shown above each column. Columns represent normalized mean values ± SEM from six different batches of oocytes (**P < 0.01; ***P < 0.001). (D) Gelatinase activity of PAN nephrotic urine is abolished when zymograms are developed in the presence of aprotinin (1 mg/ml). (E) Serine protease activity in nephrotic urine is high compared with urine from control rats. Activity in plasma is low in both. ***P < 0.001.
Zymography of nephrotic urine showed aprotinin-sensitive protease activity with a molecular size of approximately 75 kD (Figure 2D). In a serine protease assay with a synthetic substrate, nephrotic urine exhibited 10-fold increased activity compared with control (Figure 2E), whereas no difference in plasma serine protease activity was detected between control and nephrotic rats.
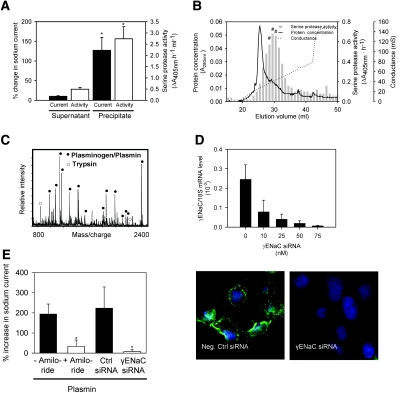
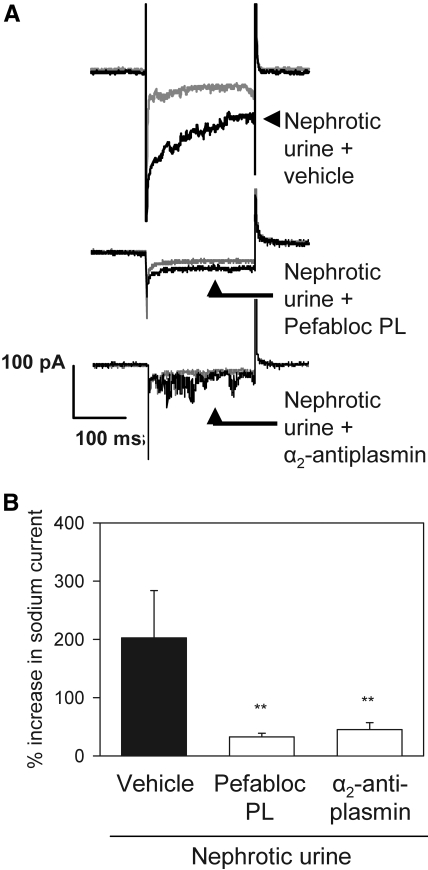
Aprotinin-affinity precipitation of nephrotic urine effectively removed serine protease activity and abolished the ability to activate ENaC current (Figure 3A), whereas total protein concentration was not changed (35.8 versus 34.0 mg/ml for nephrotic urine and aprotinin-affinity supernatant, respectively). The aprotinin-affinity precipitate was highly enriched in serine protease activity compared with nephrotic urine (30.80 ± 0.60 versus 0.20 ± 0.08 ΔA405 nm/h per mg protein, respectively) and stimulated ENaC activity (Figure 3A). Ion exchange chromatography of the precipitate followed by protease activity screening identified fractions with high activity (Figure 3B). Peak fractions corresponding to elution volume 29 to 31 ml were pooled and subjected to repeated aprotinin-affinity precipitation. The precipitate was separated by gel electrophoresis, which yielded two distinct bands with a molecular weight of 40 and 80 kD (Supplemental Figure 3A). Matrix-assisted laser desorption/ionization time of-flight (MALDI-TOF) mass spectrometry identified the proteins as plasmin fragments and plasmin, respectively (Figure 3C, Supplemental Table 2). Zymography using plasmin but not plasminogen exhibited a pattern similar to the proteolytic activity observed with nephrotic urine (Supplemental Figure 3B). Pure plasmin activated a sodium current in M-1 cells that was inhibited by amiloride and by knockdown of the γENaC subunit using small interfering RNA (siRNA; Figure 3, D and E). The protease activity of nephrotic urine was inhibited by the serine protease inhibitor aprotinin, the specific plasmin inhibitors Pefabloc PL, and α2-antiplasmin (Supplemental Figure 4A). The selective plasmin inhibitors prevented the ability of nephrotic urine to activate ENaC currents in single M-1 cells when they were added to the urine samples before exposing the cells to the samples (Figure 4). Thus, plasmin is the dominant protease in nephrotic urine responsible for ENaC activation.
Figure 3.
Identification of plasmin as an ENaC-activating protease in nephrotic urine. (A) After aprotinin affinity precipitation of nephrotic urine, there is reduced ability of the supernatant to stimulate sodium currents in M-1 cells and serine protease activity (▪), whereas the ability of the precipitate to stimulate sodium current and serine protease activity is increased (□). *P < 0.05 (n = 4). (B) Chromatogram of ion exchange chromatography of the aprotinin-affinity precipitate and corresponding serine protease activities (solid line, A280nm; dotted line, conductance of the elution buffer; ▪, serine protease activity). #Fractions that were pooled, precipitated with aprotinin, and separated by gel electrophoresis to give bands with sizes of 80 and 40 kD (Supplemental Figure 3A). (C) MALDI-TOF mass spectrometry identified the 80-kD band resulting from the procedure in B as plasmin(ogen) (•). Trypsin autolysis peptides (○) were used for internal calibration. (D) Knockdown of γENaC mRNA and protein in M-1 cells. Cells were transfected with γENaC siRNA, and γENaC mRNA level was assessed the next day using reverse transcriptase-quantitative PCR (upper panel) or after 2 d at the protein level using immunofluorescence (lower panels, n = 4). (E) Stimulation of whole-cell inward currents in M-1 cells by native rat plasmin (10 μg/ml). The stimulatory effect of plasmin was prevented when plasmin was applied in the presence of amiloride (2 μM) or by knockdown of γENaC by siRNA (n = 4). *P < 0.05 versus control (t test).
Figure 4.
Plasmin is the dominant ENaC-activating protease in PAN nephrotic urine. (A) Whole-cell current traces recorded from single M-1 cells showing baseline current (gray traces) before and after (black traces) exposure to nephrotic urine containing vehicle, Pefabloc PL (10 μM), or α2-antiplasmin (5 μM). The stimulatory effect of nephrotic urine (top traces) on the sodium inward current was prevented by the selective plasmin inhibitors Pefabloc PL (middle traces) and by α2-antiplasmin (bottom traces). (B) Mean values from similar experiments as shown in A. **P < 0.01 versus control (t test, n = 4).
Plasminogen is Activated by Tubular Urokinase-Type Plasminogen Activator Activity
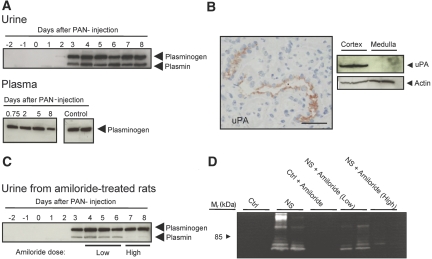
Before PAN injection, plasmin or plasminogen was not detected in urine (Figure 5A). Coincident with the appearance of proteinuria and positive sodium balance at days 3 to 4 (Figure 1A), two bands appear in urine with the expected sizes of plasminogen and active plasmin (Figure 5A). The antibody recognized pure plasminogen and plasmin with similar molecular weights as observed in nephrotic urine (data not shown). Plasminogen and plasmin were present in nephrotic urine from days 3 to 4 to 8. Plasmin was not detectable in rat plasma by Western blotting (Figure 5A). Plasminogen may be converted to plasmin by urokinase-type plasminogen activator (uPA), and immunolabeling of nephrotic rat kidney cryosections for uPA showed immunoreactivity associated with the apical surface of CCD (Figure 5B). Western blotting confirmed expression of uPA in rat kidney cortex (Figure 5B).
Figure 5.
Tubular activation of filtered plasminogen to plasmin by uPA. (A, top) Western blot showing appearance of plasminogen and plasmin in urine at day 3 after induction of PAN nephrosis. (Bottom) Western blot on plasma demonstrating presence of plasminogen but not plasmin in plasma from control and nephrotic rats. Plasminogen and Plasmin bands are detected at approximately 90 and 80 kD, respectively. (B, left) Sections of rat kidney demonstrating uPA immunoreactivity in CCD. (Right) Western blots of control and PAN nephrotic rat kidney tissue demonstrate presence of uPA in the renal cortex. Bar = 50 μm. (C) Western blot showing inhibition of conversion of plasminogen to plasmin after treatment with amiloride. Low dosage: 400 μg/d per kg body wt; high dosage: 2 mg/d per kg body wt. (D) Zymogram of urine from control and nephrotic (NS) rats showing reduced gelatinase activity with high-dosage amiloride.
To test whether uPA converts plasminogen to plasmin in PAN nephrotic rats, we examined urine from amiloride-treated rats, because amiloride, besides being an inhibitor of ENaC, potently inhibits uPA activity.25 The high dosage of amiloride lowered the plasmin/plasminogen ratio in nephrotic urine and abolished immunoreactive plasmin in urine (Figure 5C, Supplemental Figure 4B). Zymography of urine showed an attenuation of protease activity in nephrotic rats treated with amiloride (Figure 5D). Amiloride, 50 μmol/L, had no direct effect on serine protease activity (26.8 ± 1.1 versus 25.1 ± 0.7 ΔA405nm/h/ml without and with amiloride). Thus, amiloride did not inhibit plasmin per se, whereas it inhibited urinary formation of plasmin from plasminogen.
uPA-Mediated Conversion of Plasminogen to Plasmin Is Required for ENaC Activation
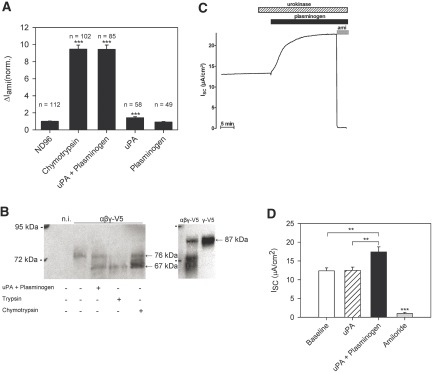
In Xenopus laevis oocytes heterologously expressing rat ENaC, preincubation of the oocytes with uPA alone had only a marginal stimulatory effect on the ΔIami, and exposure to plasminogen had no significant effect. In contrast, the combination of plasminogen and uPA resulted in a significant increase in ΔIami, similar to the increase observed with chymotrypsin (Figure 6A). This indicates that uPA-mediated generation of plasmin is required for ENaC activation. To investigate whether ENaC activation is associated with γENaC cleavage at the cell surface, we used a γENaC construct with a C-terminal V5 tag to detect biotinylated cell surface γENaC fragments by Western blot. Exposure of the oocytes to a combination of uPA and plasminogen yielded a γENaC fragment with a molecular weight of approximately 67 kD (Figure 6B). We observed similar fragments when trypsin or chymotrypsin was used. As previously shown,15,16,26 the 76-kD band corresponds to a cleavage product that arises from γENaC cleavage at its putative furin cleavage site when γENaC is coexpressed with the α- and β-subunits. Full-length γENaC migrates at approximately 87 kD and is readily detectable in whole-cell lysate but not at the cell surface (Figure 6B). Full-length γENaC is the predominant band detected in oocytes expressing γENaC in the absence of the α- and β-subunits (Figure 6B).
Figure 6.
A combination of uPA and plasminogen stimulates ENaC in Xenopus laevis oocytes and in confluent polarized M-1 collecting duct cells. (A) ENaC-expressing oocytes were preincubated for 5 min in control ND96 solution, in ND96 solution containing chymotrypsin (2 μg/ml), in a combination of plasminogen (1 mg/ml) and uPA (150 U/ml), in uPA (150 U/ml) alone, or in plasminogen (1 mg/ml) alone. Preincubation was followed by the assessment of ΔIami at −60 mV by two-electrode voltage clamp. Numbers of individual oocytes measured in each group are given above each column. Columns represent normalized means ± SEM from 10 different batches of oocytes (***P < 0.001 versus control group preincubated in ND96). (B) C-terminally V5-tagged wild-type γENaC was coexpressed with wild-type α and βENaC in X laevis oocytes (αβγ-V5). Oocytes were treated for 5 min with chymotrypsin, trypsin, or urokinase/plasminogen as indicated. The γENaC subunit of biotinylated cell surface ENaC was detected with a monoclonal V5 antibody. Exposure to a combination of uPA and plasminogen results in the appearance of a 67-kD band similar to that observed after exposure to trypsin or chymotrypsin, which is not seen in the nontreated oocytes where the major γENaC band is detected at approximately 76 kD. A band of approximately 87 kD corresponding to full-length ENaC can be seen in the control blot on the right side using membrane fractions from whole-cell lysate of oocytes coexpressing αβγENaC or γENaC alone. As expected, the full-length band is the predominant band in oocytes expressing γENaC alone (γ-V5), whereas cleavage products can be detected in addition to full-length ENaC in oocytes coexpressing all three ENaC subunits (αβγ-V5). The positions of the 72- and 95-kD size markers are indicated. n.i., noninjected oocytes. (C) Representative equivalent short-circuit current (ISC) recording is shown from confluent M-1 cells grown on permeable support. Urokinase (uPA) in a concentration of 150 U/ml and plasminogen in a concentration of 1 mg/ml were added sequentially to the apical bath solution as indicated by the  and ▪, respectively. At the end of the experiment, amiloride (ami; 100 μM) was added apically to confirm that the stimulated ISC was mediated by ENaC. (D) Summary of results from 12 similar experiments as shown in B. Columns represent mean ISC values (±SEM) measured under baseline conditions before the apical addition of uPA (□), 5 min after application of uPA (
and ▪, respectively. At the end of the experiment, amiloride (ami; 100 μM) was added apically to confirm that the stimulated ISC was mediated by ENaC. (D) Summary of results from 12 similar experiments as shown in B. Columns represent mean ISC values (±SEM) measured under baseline conditions before the apical addition of uPA (□), 5 min after application of uPA ( ), 30 min after subsequent application of plasminogen (▪), and in the presence of amiloride (
), 30 min after subsequent application of plasminogen (▪), and in the presence of amiloride ( ). **P < 0.01, ***P < 0.001.
). **P < 0.01, ***P < 0.001.
Similar to the findings in the oocyte system, uPA alone had no effect on the equivalent short circuit current (ISC) from confluent M-1 cells grown on filters (Figure 6, C and D). Subsequent apical application of plasminogen in the presence of uPA resulted in a significant increase in ISC. The stimulated ISC was abolished by apical application of amiloride, which confirms that the ISC is mediated by ENaC expressed in the apical membrane of confluent M-1 cells.27,28 These results confirm that plasmin but not plasminogen or uPA stimulate ENaC activity. In addition to blocking ENaC, amiloride may prevent the conversion of urinary plasminogen to plasmin in vivo.
Plasmin Stimulation Leads to Release of a Peptide from the Extracellular Domain of γENaC
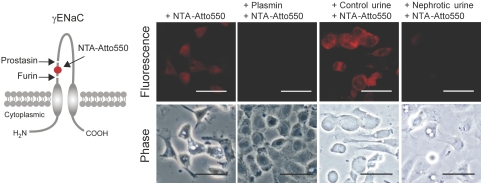
Proteolytic activation of ENaC is thought to involve dual cleavage of its γ-subunit, which results in the release of a 43-residue inhibitory peptide from its extracellular domain.17 To examine this in live cells, we inserted a hexahistidine tag in a γENaC construct expression vector between the cleavage sites for furin and prostasin (Figure 7). The tag binds the extracellular fluorophore NTA-Atto550. M-1 cells transfected with the tagged construct display strong fluorescence when exposed to NTA-Atto550, whereas wild-type cells do not (Figure 7). Pretreatment of transfected cells with plasmin or nephrotic urine abolished the ability of cells to respond to NTA-Atto550 exposure. This indicates effective removal of inhibitory peptide from ectodomain γENaC by plasmin in live cells (Figure 7).
Figure 7.
Plasmin stimulation leads to release of a peptide from the extracellular domain of γENaC. (Left) Schematic diagram of the γENaC subunit showing the putative cleavage sites for furin and prostasin and the localization of the inserted hexahistidine tag, which can be visualized with the fluorophore NTA-Atto550. (Right) M-1 cells expressing the hexahistidine-tagged γENaC subunit are labeled by NTA-Atto550 when treated with vehicle or control urine, whereas cells treated with plasmin or nephrotic urine are unlabeled (n = 4).
Nephrotic Urine from Human Patients Stimulates ENaC Activity
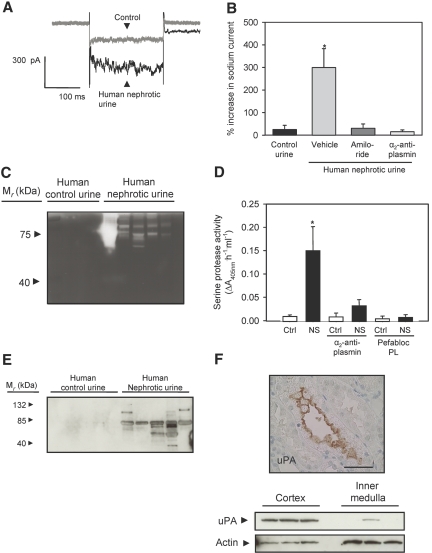
Urine from five patients with nephrotic syndrome (Supplemental Table 3) augmented sodium currents in M-1 cells (Figure 8, A and B). Addition of α2-antiplasmin (5 μM) or amiloride (2 μM) abolished the ability of nephrotic urine to stimulate whole-cell currents in M-1 cells (Figure 8B). Urine zymograms displayed intense proteolytic activity with apparent molecular weight of 75 to 80 kD (Figure 8C). Serine protease activity sensitive to plasmin inhibitors was present in patient urine samples but not in urine from four age- and gender-matched control subjects (Figure 8D). Western blots for plasminogen and plasmin displayed several bands in nephrotic but not in control urine (Figure 8E). Immunohistochemical staining of human nephrectomy specimens showed uPA predominantly in the CCD (Figure 8F). Western blotting with human kidney homogenates confirmed expression of uPA in kidney cortex (Figure 8F).
Figure 8.
Urine from patients with nephrotic syndrome confirms critical findings from the animal study. (A) Whole-cell current traces recorded before (gray) and after (black) exposure of a single M-1 cell to human nephrotic urine. (B) Control urine fails to stimulate M-1 whole-cell currents, and the stimulatory effect of human nephrotic urine is prevented by amiloride and α2-antiplasmin added to the nephrotic urine before flushing the cells with the urine sample (n = 4 in each group). Data are means ± SEM. *P < 0.05. See Supplemental Figure 5 for values from each patient. (C) Zymogram of human urine showing gelatinase activity in urine from patients with nephrotic syndrome but not in urine controls. (D) Inhibition of serine protease activity in human nephrotic urine by α2-antiplasmin and by the plasmin inhibitor Pefabloc PL (n = 4). *P < 0.05. (E) Western blot showing plasmin immunoreactivity in urine from patients with nephrotic syndrome but not in urine controls. (F) Immunohistochemical staining of uPA in sections of human nephrectomy specimens showing uPA immunoreactivity in CCD (top). Western blot of human nephrectomy specimens demonstrating presence of uPA in the renal cortex (bottom). Bar = 50 μm.
DISCUSSION
Our findings suggest that induction of nephrotic syndrome in rats leads to filtration of plasminogen into the urine, which is activated to plasmin by renal tubular uPA activity. Active plasmin in urine stimulates ENaC in vitro by cleavage of the γENaC subunit. Urine from patients with nephrotic syndrome contains active plasmin, which is the dominant serine protease in nephrotic urine able to activate ENaC. On the basis of the temporal association between appearance of plasmin in urine and primary, renal ENaC-mediated sodium hyper-reabsorption, the findings provide a putative novel mechanistic link between damage to the glomerular filtration barrier, proteinuria, and stimulation of sodium reabsorption in distal tubules and collecting ducts in nephrotic syndrome.
Simultaneous with the onset of proteinuria at day 3 after PAN injection, the cumulated sodium balance changed from negative to positive, and the renin-angiotensin-aldosterone system was suppressed. In this phase, mineralocorticoid receptor blockade, adrenalectomy, angiotensin II receptor blockade, or angiotensin converting enzyme blockade had no effect on renal sodium output or development of ascites.2,29 In agreement with previous data,29 treatment of nephrotic rats with amiloride in the period with positive sodium balance increased sodium excretion and normalized sodium balance, suggesting that the increased sodium reabsorption occurs through ENaC in the aldosterone-sensitive part of the distal nephron. Consistent with this, there is increased expression and activity of the Na,K-ATPase in isolated CCD from rats with nephrotic syndrome.29
The onset of proteinuria in PAN nephrotic syndrome coincided with the shift from a negative to a positive sodium balance. A correlation between proteinuria and an increase in sodium reabsorption has also been observed in the HgCl2 nephropathy model in rats.1 Taken together with the reports of extracellular activation of ENaC by proteases,11–13,20 these observations led us to hypothesize that nephrotic urine contains an ENaC-activating factor. We found that PAN nephrotic urine stimulated amiloride-sensitive sodium currents dependent on serine protease activity that could be heat inactivated.18,20 We identified plasmin in nephrotic urine as the dominant serine protease responsible for activation of sodium currents. Only the combination of plasminogen and uPA, which allows formation of plasmin, stimulates ENaC-mediated short circuit current in M-1 cell monolayers and amiloride-sensitive whole-cell current in ENaC-expressing Xenopus laevis oocytes. The large plasmin-induced increase in ENaC currents in Xenopus laevis oocytes was similar to that induced by chymotrypsin, trypsin, or neutrophil elastase.15,21 Moreover, the combined application of uPA and plasminogen led to the appearance of a γENaC cleavage product at the plasma membrane, which was of a similar size as the fragment observed with trypsin, chymotrypsin, and human neutrophil elastase.16 Consistent with this, plasmin removed a labeled inhibitory peptide region from the extracellular domain of the γENaC subunit in live cells. These findings indicate that ENaC activation by plasmin involves proteolytic cleavage in the extracellular loop of the channel's γ-subunit.
Plasmin and plasminogen were present in nephrotic urine, and the nephrotic urine displayed significantly increased serine protease activity compared with control urine; however, plasma serine protease activity was not different between control and nephrotic rats, and only plasminogen was detected by Western blotting in plasma from control rats. This indicates that plasmin is unlikely to be filtered in large amounts and that the plasminogen-plasmin conversion occurs after glomerular filtration. A candidate for renal tubular plasminogen activation is uPA.30,31 In agreement with previous findings,30 we found uPA expressed at the apical surface of CCD. Western blotting confirmed expression of uPA in rat kidney cortex. Amiloride, in addition to being a potent inhibitor of ENaC, is known to inhibit uPA effectively,25 and treatment of nephrotic rats with amiloride abolished the activation of plasminogen. Tubular uPA is a likely candidate to convert plasminogen to plasmin in nephrotic syndrome.
Human urine samples obtained from patients with nephrotic urine displayed plasmin(ogen) immunoreactivity in Western blots, gelatinase activity in zymograms, and serine protease activity by enzyme assay, whereas control urine samples all were negative. Human nephrotic urine stimulated sodium currents in single M-1 cells, and the stimulation was sensitive to inhibitors of plasmin, which indicates that plasmin is the main ENaC activator also in human nephrotic urine. Furthermore, uPA immunoreactivity was found predominantly in the cortex of human kidney samples, consistent with previous findings.32 The presence of active plasmin in urine and the ability of nephrotic urine to activate ENaC are, thus, not specific for the animal model, and the established beneficial effect of amiloride in nephrotic syndrome may include a hitherto unrecognized target, uPA. Thus, our results derived from the rat model of nephrotic syndrome may be clinically relevant, and it can be speculated that the proposed mechanism may promote sodium retention in pathologic conditions with proteinuria. It is not known to what extent endogenous proteases constitutively activate ENaC in vivo under various physiologic and pathologic conditions. The activity of endogenous proteases and protease inhibitors may be hormonally regulated. In humans, states of low plasma aldosterone seem to be associated with decreased urinary prostasin excretion,33 whereas high concentrations of aldosterone have been shown to increase the expression of prostasin34 and to downregulate the expression of the endogenous serine protease inhibitor, nexin 1 (PN-1), in cultured M-1 cells.35 It was recently shown that rats fed a 1% sodium diet expressed both full-length and cleaved γENaC at the plasma membrane, whereas Na-depleted or aldosterone-treated rats mainly expressed the cleaved subunit.36 In agreement, we37 and others36 recently demonstrated that trypsin can activate ENaC in microdissected mouse and rat distal nephron and that the stimulatory effect of trypsin was more pronounced in tubules from animals maintained on a standard-sodium diet than animals maintained on a low-sodium diet. These findings demonstrate that ENaC can be activated by extracellular proteases in the native renal tubule under normal physiologic conditions and that the degree of proteolytic preactivation of ENaC by endogenous proteases will determine the size of the additional stimulatory effect of proteases such as plasmin occurring in the urine under pathophysiologic conditions.
In summary, our study introduces the novel concept that a leaky glomerular filtration barrier allows filtration of proteases or precursors of proteases with the ability to activate ENaC. Plasmin is generated in tubular fluid from filtered plasminogen by amiloride-sensitive uPA and is the dominant serine protease in nephrotic urine. Plasmin activates ENaC in vitro by proteolytic cleavage of the γ-subunit. We do not know whether plasmin-induced cleavage of γENaC is a direct effect of plasmin on the channel or the result of plasmin-mediated activation of another membrane-anchored protease or protease cascade. It may be speculated that ENaC stimulation by plasmin contributes to hitherto unexplained primary renal NaCl reabsorption in nephrotic syndrome.
CONCISE METHODS
Experimental Protocol
PAN nephrosis in rats was induced as described previously.38 To evaluate the impact of the renin-angiotensin-aldosterone system on sodium balance, we treated PAN rats with subcutaneous injections once daily of antagonists to both angiotensin II receptor and aldosterone receptor, candesartan approximately 1 mg/kg39,40 and canrenoate approximately 100 mg/kg,41,42 or vehicle. To compare ENaC activity in vivo, we treated control and PAN rats with a blocker of ENaC, amiloride, once daily subcutaneously. Days 1 to 3 were a run-in period without amiloride; on days 4 through 6, a dosage of 100 μg/rat was given; and on days 7 through 9, the dosage was increased to 500 μg/rat. These experiments were approved by the Danish Animal Experiments Inspectorate under the Department of Justice (171001-096).
Food, Urine, and Fecal Analysis
Determination of sodium content in food, urine, and feces was as described previously.38 Daily sodium balance was calculated as intake minus fecal and urinary output. The magnitude of accumulated sodium balance was calculated as area under the curve from days 0 through 8.
Blood Analysis
The plasma concentration of sodium and urea and the plasma osmolality were determined on the day of decapitation (day 8 after PAN or vehicle injection). Plasma renin, aldosterone, and AVP concentrations were determined as described previously.38,43,44
Purification of ENaC-Activating Proteins
Aprotinin (USB, Cleveland, OH) was coupled to CNBr-activated Sepharose 4B (Amersham Bioscience, Hillerod, Denmark) according to the manufacturer's protocol and added to the nephrotic urine. The beads were pelleted and washed thoroughly. Bound proteins were eluted and loaded onto a Resource Q column (Amersham Bioscience) equilibrated with 20 mM Tris (pH 7.4). The column was eluted with a linear gradient from 0 to 1 M NaCl. Fractions were collected and assayed for serine protease activity. Fractions with high serine protease activity were subjected to aprotinin-affinity precipitation.
Serine Protease Assay and Zymography
We measured serine protease activity with the chromogenic substrate S-2222 (Chromogenix, Frederiksberg, Denmark) by spectrophotometrically (VersaMax microplate reader; Molecular Devices, Sunnyvale, CA) assessing the change in absorbance at 405 nm. Urinary protease activities were examined by zymography (Invitrogen, Tåstrup, Denmark) according to the manufacturer's protocol.
Mass Spectrometry
SDS-PAGE, silver staining, and tryptic in-gel digestion were done as described previously.45 Mass spectra of the tryptic peptides were obtained with the Ultraflex II TOF/TOF system from Bruker (Bremen, Germany). The proteins were identified by searching against the NCBI nonredundant protein database using Mascot (Matrix Science, http://www.matrixscience.com).
Whole-Cell Patch Clamp of Single M-1 Cells
M-1 cells were obtained from ATCC (Boras, Sweden) and maintained in DMEM:F12 (Life Technologies, Tåstrup, Denmark) supplemented with 5% FCS (Life Technologies) and 5 μM dexamethasone (Sigma, St. Louis, MO) at 37°C/5% CO2. For patch-clamp experiments, M-1 cells grown to confluence in 25-cm2 flasks (Nunc, Roskilde, Denmark) were trypsinized and seeded onto coverslips in DMEM:F12 (Life Technologies) supplemented with 5 μM dexamethasone (Sigma). The cells were incubated at 37°C/5% CO2. Patch-clamp experiments were conducted on single M-1 cells 24 to 48 h after seeding the cells. Details regarding the patch-clamp experiments are given in the online supplemental material. For transepithelial studies, M-1 cells were seeded onto permeable Millicell-HA culture plate inserts (Millipore GmbH, Schwalbach, Germany) and were grown to confluence in PC1 culture medium (Lonza, Verviers, Belgium) as described previously.27
Ussing Chamber
Experiments were essentially performed as described previously27 but with slightly modified Ussing chambers to reduce the volume needed for apical solution exchanges. In confluent M-1 cells, application of trypsin to the apical side had little effect on ENaC-mediated short-circuit current,46 probably because the activity of endogenous proteases is high under our culture conditions, resulting in a high baseline activity of ENaC close to that observed in fully stimulated collecting duct principal cells in native tissue.28 As recently reported, pretreatment of M-1 cells with a furin inhibitor largely augmented the stimulatory effect of trypsin on ENaC currents, whereas pretreatment with aprotinin had little effect.37 Thus, to minimize ENaC activation by endogenous proteases, the confluent M-1 cells grown on filters were preincubated for 6 to 8 h before the experiment with the furin inhibitor dec-RVKR-cmk (decanoyl-Arg-Val-Lys-Arg-chloromethyl ketone from Calbiochem/Merck Biosciences GmbH, Schwalbach, Germany) which was added to the apical bath solution. A 10 mM stock solution of dec-RVKR-cmk was prepared in DMSO and was diluted to a final concentration of 40 μM.
Identification of ENaC Transcripts in M-1 Cells
RNA was extracted from M-1 cells in accordance with the manufacturer's protocol (Qiagen Mini Kit; Qiagen, Ballerup, Denmark) and reverse-transcribed using iScript cDNA synthesis kit (Bio-Rad, Copenhagen, Denmark). ENaC subunit cDNA were amplified with rat ENaC primers that also annealed to mouse sequence (Supplemental Table 4).
Western Blotting
Kidneys were homogenized as described previously.44 Plasma, urine, or homogenate samples were subjected to SDS-PAGE (Bio-Rad) and subsequently transferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore, Copenhagen, Denmark). Blots were probed with polyclonal goat anti-human plasminogen (Abcam, Cambridge, MA), polyclonal rabbit anti-human uPA (Abcam), or polyclonal rabbit anti-actin (Abcam). Primary antibodies were detected with horseradish peroxidase (HRP)-coupled antibodies (Dako, Glostrup, Denmark) and ECL system (Amersham Biosciences).
Cloning and siRNA Experiments
Full-length γENaC subunit cDNA was amplified by PCR using specific primers (Supplemental Table 4) and cloned into pcDNA6.2 (Invitrogen). The hexahistidine tag was inserted between residues 148 and 153 (mouse γENaC numbering) by using PCR. All constructs were verified by sequencing (MWG Biotech, Martinsreid, Germany). Subconfluent M-1 cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. Transfection efficiency was 70 to 75% (Supplemental Figure 6). For NTA-Atto550 (Sigma) labeling, cells expressing the hexahistidine-tagged γENaC subunit were seeded onto coverslips and incubated at 37°C/5% CO2 in culture medium. Twenty four hours after seeding, cells were stimulated with or without plasmin or urine for 5 min, followed by incubation with 10 μM NTA-550 dissolved in DMEM:F12 for 1 min. Cells were washed once with DMEM:F12 and fixed with 4% formaldehyde/PBS. M-1 cells were transfected with siRNA using DharmaFECT1 (Dharmacon, Herlev, Denmark) according to the manufacturer's protocol. The γENaC subunit was knocked down using 75 nM of sequence specific siRNA (siGENOME, cat. no. D-043105-02 and cat. no. D-043105-04; Dharmacon). As a negative control, cells were transfected with Silencer Negative Control (cat. no. AM4611; Ambion, Naerum, Denmark). Knockdown was assessed by reverse transcriptase-quantitative PCR 24 h after transfection, at the protein level by immunofluorescence or functionally after a minimum of 2 d. M-1 cells used for immunofluorescence were transfected with siRNA and seeded onto coverslips and incubated for 48 h at 37°C/5% CO2 in culture medium. After fixation in 4% formaldehyde/PBS, the M-1 cells were incubated with rabbit γENaC antibody (1:200; Sigma) diluted in PBS and 0.5% Tween-20. Subsequently, cells were washed and incubated with Alexa-488 goat anti-rabbit antibody (1:200; Molecular Probes, Tåstrup, Denmark). Cells were counterstained with DAPI, and fluorescence was visualized using appropriate filters.
Immunohistochemistry
Human kidney samples were obtained from randomly selected patients who underwent unilateral nephrectomy for renal cancer at the Department of Urology, Odense University Hospital. All patients gave informed written consent to the use of tissue from the extirpated kidney. The use of tissue was approved by the public regional ethics Committee (File 20010035). Human nephrectomy specimens and cryosections of rat kidneys were fixed in acetone and immunostained with polyclonal rabbit uPA antibody (Abcam) followed by HRP-coupled antibody (Dako). Sections were treated with 0.01% diaminobenzidine (Dako) and 0.02% H2O2 and counterstained with hematoxylin.
Isolation of Oocytes and Two-Electrode Voltage-Clamp Experiments
Isolation of Xenopus laevis oocytes, injection of cRNA, and two-electrode voltage-clamp experiments were performed essentially as described previously.47,48 Details are given in the online supplemental materials.
Detection of ENaC Cleavage Products at the Cell Surface
Biotinylation experiments were essentially performed as described by Harris et al.16 using 30 oocytes per group. All biotinylation steps were performed at 4°C. Oocytes were incubated in the biotinylation buffer containing 10 mM triethanolamine (pH 9.5), 150 mM NaCl, 2 mM CaCl2, and 1 mg/ml EZ-link sulfo-NHS-SS-Biotin (Pierce, Rockford, IL) for 15 min with gentle agitation. The biotinylation reaction was stopped by washing the oocytes twice with quench buffer containing 192 mM glycine and 25 mM Tris-Cl (pH 7.5). Oocytes were then washed three times with ND96 solution and lysed by passing them five times through a 27-G needle in lysis buffer containing 1% Triton X-100 and 1% Igepal CA-630 (Sigma), 500 mM NaCl, 5 mM EDTA, and 50 mM Tris-Cl (pH 7.4), supplemented by a protease inhibitor cocktail (Complete Mini EDTA-Free protease inhibitor cocktail tablets; Roche Diagnostics, Mannheim, Germany). The lysates were incubated for 20 min on ice and centrifuged for 10 min at approximately 13,500 × g. Supernatants were transferred to 1.5-ml Eppendorf tubes containing 100 μl of Immunopure immobilized Neutravidin beads (Pierce) equilibrated with lysis buffer. After overnight incubation at 4°C with gentle agitation, the beads were pelleted for 3 min at approximately 13,500 × g. Supernatants were removed, and beads were washed three times with lysis buffer. A total of 100 μl of 2× SDS-PAGE sample buffer (Rotiload 1; Roth, Karlsruhe, Germany) was added to the beads. Samples were boiled for 5 min at 95°C before loading them on the 10% SDS-PAGE. Monoclonal anti-V5 antibody was obtained from Invitrogen (Karlsruhe) and used at a dilution of 1:5000. HRP-labeled secondary sheep anti-mouse antibodies were purchased from Sigma (Taufkirchen, Germany) and used at a dilution of 1:10,000.
Human Urine Samples and Patient Data
Four healthy control subjects and five patients with nephrotic syndrome were enrolled in the study. The study was approved by the public regional ethics committee (file VF 20040231), and oral and written informed consents were obtained from each participant before the study. The urine was aspirated from 24-h urine samples. All analytical procedures were as described for rat urine.
Statistical Analysis
Data are presented as means ± SEM. Data were compared using the unpaired t test, and variance was tested by the F test and Welch correction was used when appropriate. Multiple comparisons were analyzed by ANOVA and post hoc analysis by Dunnett or Bonferroni multiple comparison test. The level of significance was P < 0.05.
NOTE ADDED IN PROOF
While the manuscript was in press, a paper by Passero et al. was published also showing that plasmin activates ENaC by cleaving the gamma subunit (J. Biol. Chem. 2008 Nov 3, PMID: 18981180).
DISCLOSURES
None.
Acknowledgments
This work was supported by the AP Møller Foundation for the Advancement of Medical Science, the Danish Medical Research Council, the Danish Kidney Foundation, the Danish Heart Foundation, the Danish Hypertension Society, the Danish Society of Nephrology, the Danish Medical Association Research Fund/the Hartelius Family Memorial Grant, Institute of Clinical Research (University of Southern Denmark), King Christian the Xth Foundation, the NOVO Nordisk Foundation, the Deutsche Forschungsgemeinschaft (SFB423: Kidney Injury: Pathogenesis and Regenerative Mechanisms; project A12; C.K.), the Johannes and Frieda Marohn Stiftung (C.K.), an Elitenetwork Bavaria fellowship (S.H.), and the BioMedTec International Graduate School “Lead Structures of Cell Function” of the Elitenetwork Bavaria (S.H.).
The seminal observation that nephrotic urine activates ENaC function was reported in abstract form at the annual meeting of the American Society of Nephrology; October 29 through November 1, 2004; St. Louis, MO (J Am Soc Nephrol 15: 2004, 306A; poster no. SA-PO029).
We thank Mette Svendsen and Dina Dræby for assistance with animal procedures and Inge Andersen, Gitte Dybmose, Mette Fredenslund, Bodil Kristensen, Anette Rasmussen, Lis Teusch, Jessica Ott, and Ralf Rinke for skillful technical assistance. In addition, we thank Søren Andersen for skilled technical assistance with MALDI-TOF mass spectrometry. We thank Anthony M. Carter for linguistic advice.
Published online ahead of print. Publication date available at www.jasn.org.
P.S. and C.B. contributed equally to this work.
Supplemental information for this article is available online at http://www.jasn.org/.
See related editorial, “Plasmin and Sodium Retention in Nephrotic Syndrome,” on pages 233–234.
REFERENCES
- 1.Deschenes G, Doucet A: Collecting duct (Na+/K+)-ATPase activity is correlated with urinary sodium excretion in rat nephrotic syndromes. J Am Soc Nephrol 11: 604–615, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa I, Rennke HG, Hoyer JR, Badr KF, Schor N, Troy JL, Lechene CP, Brenner BM: Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest 71: 91–103, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canessa CM, Schild L, Buell G, Thorens B, Gautschi I, Horisberger JD, Rossier BC: Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature 367: 463–467, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA: Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol Renal Physiol 279: F46–F53, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA: Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogt B, Favre H: Na+,K(+)-ATPase activity and hormones in single nephron segments from nephrotic rats. Clin Sci (Lond) 80: 599–604, 1991 [DOI] [PubMed] [Google Scholar]
- 7.Usberti M, Federico S, Meccariello S, Cianciaruso B, Balletta M, Pecoraro C, Sacca L, Ungaro B, Pisanti N, Andreucci VE: Role of plasma vasopressin in the impairment of water excretion in nephrotic syndrome. Kidney Int 25: 422–429, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Vande Walle JG, Donckerwolcke RA, van Isselt JW, Derkx FH, Joles JA, Koomans HA: Volume regulation in children with early relapse of minimal-change nephrosis with or without hypovolaemic symptoms. Lancet 346: 148–152, 1995 [DOI] [PubMed] [Google Scholar]
- 9.Chandra M, Hoyer JR, Lewy JE: Renal function in rats with unilateral proteinuria produced by renal perfusion with aminonucleoside. Pediatr Res 15: 340–344, 1981 [DOI] [PubMed] [Google Scholar]
- 10.Yu Z, Schumacher M, Frey BM, Frey FJ, Vogt B: Regulation of epithelial sodium channel in puromycin aminonucleoside-induced unilateral experimental nephrotic syndrome in normal and analbuminemic Nagase rats. Nephron Physiol 101: 51–62, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Kleyman TR, Myerburg MM, Hughey RP: Regulation of ENaCs by proteases: An increasingly complex story. Kidney Int 70: 1391–1392, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Planes C, Caughey GH: Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol 78: 23–46, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossier BC: The epithelial sodium channel: Activation by membrane-bound serine proteases. Proc Am Thorac Soc 1: 4–9, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Carattino MD, Hughey RP, Kleyman TR: Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem 283, 25290–25295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diakov A, Bera K, Mokrushina M, Krueger B, Korbmacher C: Cleavage in the gamma-subunit of the epithelial sodium channel (ENaC) plays an important role in the proteolytic activation of near-silent channels. J Physiol 586, 4587–4608, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC: A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem 282: 58–64, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Bruns JB, Carattino MD, Sheng S, Maarouf AB, Weisz OA, Pilewski JM, Hughey RP, Kleyman TR: Epithelial Na+ channels are fully activated by furin- and prostasin-dependent release of an inhibitory peptide from the gamma subunit. J Biol Chem 282: 6153–6160, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Caldwell RA, Boucher RC, Stutts MJ: Serine protease activation of near-silent epithelial Na+ channels. Am J Physiol Cell Physiol 286: C190–C194, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hughey RP, Bruns JB, Kinlough CL, Kleyman TR: Distinct pools of epithelial sodium channels are expressed at the plasma membrane. J Biol Chem 279: 48491–48494, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Vallet V, Chraibi A, Gaeggeler HP, Horisberger JD, Rossier BC: An epithelial serine protease activates the amiloride-sensitive sodium channel. Nature 389: 607–610, 1997 [DOI] [PubMed] [Google Scholar]
- 21.Chraibi A, Vallet V, Firsov D, Hess SK, Horisberger JD: Protease modulation of the activity of the epithelial sodium channel expressed in Xenopus oocytes. J Gen Physiol 111: 127–138, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caldwell RA, Boucher RC, Stutts MJ: Neutrophil elastase activates near-silent epithelial Na+ channels and increases airway epithelial Na+ transport. Am J Physiol Lung Cell Mol Physiol 288: L813–L819, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Adachi M, Kitamura K, Miyoshi T, Narikiyo T, Iwashita K, Shiraishi N, Nonoguchi H, Tomita K: Activation of epithelial sodium channels by prostasin in Xenopus oocytes. J Am Soc Nephrol 12: 1114–1121, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Vallet V, Horisberger JD, Rossier BC: Epithelial sodium channel regulatory proteins identified by functional expression cloning. Kidney Int Suppl 67: S109–S114, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Vassalli JD, Belin D: Amiloride selectively inhibits the urokinase-type plasminogen activator. FEBS Lett 214: 187–191, 1987 [DOI] [PubMed] [Google Scholar]
- 26.Harris M, Garcia-Caballero A, Stutts MJ, Firsov D, Rossier BC: Preferential assembly of epithelial sodium channel (ENaC) subunits in Xenopus oocytes: Role of furin-mediated endogenous proteolysis. J Biol Chem 283: 7455–7463, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertog M, Letz B, Kong W, Steinhoff M, Higgins MA, Bielfeld-Ackermann A, Fromter E, Bunnett NW, Korbmacher C: Basolateral proteinase-activated receptor (PAR-2) induces chloride secretion in M-1 mouse renal cortical collecting duct cells. J Physiol (Lond) 521: 3–17, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letz B, Ackermann A, Canessa CM, Rossier BC, Korbmacher C: Amiloride-sensitive sodium channels in confluent M-1 mouse cortical collecting duct cells. J Membr Biol 148: 127–141, 1995 [DOI] [PubMed] [Google Scholar]
- 29.Deschenes G, Wittner M, Stefano A, Jounier S, Doucet A: Collecting duct is a site of sodium retention in PAN nephrosis: A rationale for amiloride therapy. J Am Soc Nephrol 12: 598–601, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Piedagnel R, Tiger Y, Lelongt B, Ronco PM: Urokinase (u-PA) is produced by collecting duct principal cells and is post-transcriptionally regulated by SV40 large-T, arginine vasopressin, and epidermal growth factor. J Cell Physiol 206: 394–401, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Wagner SN, Atkinson MJ, Wagner C, Hofler H, Schmitt M, Wilhelm O: Sites of urokinase-type plasminogen activator expression and distribution of its receptor in the normal human kidney. Histochem Cell Biol 105: 53–60, 1996 [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Hagege J, Mougenot B, Sraer JD, Ronne E, Rondeau E: Different expression of the plasminogen activation system in renal thrombotic microangiopathy and the normal human kidney. Kidney Int 50: 2011–2019, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Olivieri O, Castagna A, Guarini P, Chiecchi L, Sabaini G, Pizzolo F, Corrocher R, Righetti PG: Urinary prostasin: A candidate marker of epithelial sodium channel activation in humans. Hypertension 46: 683–688, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Narikiyo T, Kitamura K, Adachi M, Miyoshi T, Iwashita K, Shiraishi N, Nonoguchi H, Chen LM, Chai KX, Chao J, Tomita K: Regulation of prostasin by aldosterone in the kidney. J Clin Invest 109: 401–408, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakida N, Kitamura K, Tuyen DG, Maekawa A, Miyoshi T, Adachi M, Shiraishi N, Ko T, Ha V, Nonoguchi H, Tomita K: Inhibition of prostasin-induced ENaC activities by PN-1 and regulation of PN-1 expression by TGF-[beta]1 and aldosterone. Kidney Int 70: 1432–1438, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Frindt G, Ergonul Z, Palmer LG: Surface expression of epithelial Na channel protein in rat kidney. J Gen Physiol 131: 617–627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nesterov V, Dahlmann A, Bertog M, Korbmacher C: Trypsin can activate the epithelial sodium channel (ENaC) in microdissected mouse distal nephron. Am J Physiol Renal Physiol July 23, 2008 [epub ahead of print] [DOI] [PubMed]
- 38.Bistrup C, Thiesson HC, Jensen BL, Skott O: Reduced activity of 11beta-hydroxysteroid dehydrogenase type 2 is not responsible for sodium retention in nephrotic rats. Acta Physiol Scand 184: 161–169, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Cervenka L, Wang CT, Navar LG: Effects of acute AT1 receptor blockade by candesartan on arterial pressure and renal function in rats. Am J Physiol 274: F940–F945, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Cervenka L, Navar LG: Renal responses of the nonclipped kidney of two-kidney/one-clip Goldblatt hypertensive rats to type 1 angiotensin II receptor blockade with candesartan. J Am Soc Nephrol 10[Suppl 11]: S197–S201, 1999 [PubMed] [Google Scholar]
- 41.Thiesson HC, Jensen BL, Bistrup C, Ottosen PD, McNeilly AD, Andrew R, Seckl J, Skott O: Renal sodium retention in cirrhotic rats depends on glucocorticoid-mediated activation of mineralocorticoid receptor due to decreased renal 11beta-HSD-2 activity. Am J Physiol Regul Integr Comp Physiol 292: R625–R636, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Jonassen TE, Promeneur D, Christensen S, Petersen JS, Nielsen S: Decreased vasopressin-mediated renal water reabsorption in rats with chronic aldosterone-receptor blockade. Am J Physiol Renal Physiol 278: F246–F256, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Bie P, Sandgaard NC: Determinants of the natriuresis after acute, slow sodium loading in conscious dogs. Am J Physiol Regul Integr Comp Physiol 278: R1–R10, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Norregaard R, Uhrenholt TR, Bistrup C, Skott O, Jensen BL: Stimulation of 11-beta-hydroxysteroid dehydrogenase type 2 in rat colon but not in kidney by low dietary NaCl intake. Am J Physiol Renal Physiol 285: F348–F358, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Shevchenko A, Wilm M, Vorm O, Mann M: Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal Chem 68: 850–858, 1996 [DOI] [PubMed] [Google Scholar]
- 46.Bertog M, Foglein A, Korbmacher C: Proteolytic regulation of the epithelial sodium channel (ENaC) in the M-1 mouse collecting duct cell line. Acta Physiol 189: O07–O04, 2007 [Google Scholar]
- 47.Yang LM, Rinke R, Korbmacher C: Stimulation of the epithelial sodium channel (ENaC) by cAMP involves putative ERK phosphorylation sites in the C termini of the channel's beta- and gamma-subunit. J Biol Chem 281: 9859–9868, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Konstans AA, Mavrelos D, Korbmacher C: Conservation of pH sensitivity in the epithelial sodium channel (ENaC) with Liddle's syndrome mutation. Pflugers Arch 441: 341–350, 2000 [DOI] [PubMed] [Google Scholar]