Abstract
Normal human urine contains large numbers of exosomes, which are 40- to 100-nm vesicles that originate as the internal vesicles in multivesicular bodies from every renal epithelial cell type facing the urinary space. Here, we used LC-MS/MS to profile the proteome of human urinary exosomes. Overall, the analysis identified 1132 proteins unambiguously, including 177 that are represented on the Online Mendelian Inheritance in Man database of disease-related genes, suggesting that exosome analysis is a potential approach to discover urinary biomarkers. We extended the proteomic analysis to phosphoproteomic profiling using neutral loss scanning, and this yielded multiple novel phosphorylation sites, including serine-811 in the thiazide-sensitive Na-Cl co-transporter, NCC. To demonstrate the potential use of exosome analysis to identify a genetic renal disease, we carried out immunoblotting of exosomes from urine samples of patients with a clinical diagnosis of Bartter syndrome type I, showing an absence of the sodium-potassium-chloride co-transporter 2, NKCC2. The proteomic data are publicly accessible at http://dir.nhlbi.nih.gov/papers/lkem/exosome/.
Urinary exosomes are small extracellular vesicles (<100 nm in diameter) that originate from the internal vesicles of multivesicular bodies (MVB) in renal epithelial cells, including glomerular podocytes, renal tubule cells, and the cells lining the urinary drainage system.1 Exosomes are released into the urine when the outer membrane of the MVB fuses with the apical plasma membrane of the epithelial cell.
Exosomes can be recovered from the urine by differential centrifugation as a low-density membrane fraction. Exosome isolation can result in marked enrichment of low-abundance urinary proteins that have potential pathophysiologic significance. As a consequence, we and others have been working to define optimal conditions for their isolation and purification as a prelude to their use in biomarker discovery studies.1–3
In this study, we thoroughly expanded the known proteome of human urinary exosomes by using a highly sensitive LC-MS/MS system, improved software for identification of peptide ions and a more elaborate data analysis strategy than in our previous study. In addition, we used a neutral loss scanning approach4 to investigate the phosphoproteome of human urinary exosomes. The study identified 1412 proteins including 14 phosphoproteins in human urinary exosomes. Overall, there are 177 proteins that are associated with diseases as judged by their presence on the Online Mendelian Inheritance in Man (OMIM) database, 34 of which are known to be associated with renal diseases. The potential clinical usefulness of urinary exosomes was demonstrated using the well-defined renal tubulopathy, Bartter syndrome type I, as an example. The rich information from the proteomic analysis also provides further insight into the biogenesis of urinary exosomes.
RESULTS
Large-Scale Proteomic Profiling of Human Urinary Exosomes
In this study, we carried out proteomic profiling of a low-density membrane fraction from human urine consisting chiefly of exosomes, using a highly sensitive LC-MS/MS system, based on an ion trap mass spectrometer (LTQ; Thermo-Finnigan; Thermo Electron, San Jose, CA). We unambiguously identified 1132 proteins including 205 proteins seen in our previous study and 927 proteins not seen in our previous study of human urinary exosomes.1 The full list (ambiguous and unambiguous identifications) contains 1412 proteins and can be viewed in Supplemental Table 1, and the list of proteins that were unambiguously identified in both studies can be viewed at http://dir.nhlbi.nih.gov/papers/lkem/exosome/. The expanded list of exosomal proteins includes 177 proteins that are disease related, on the basis of their presence in the OMIM database (Table 1).
Table 1.
Disease-related proteins in human urinary exosomesa
| Gene | Protein Name | Pep | ID | Related to Disease [OMIM] |
|---|---|---|---|---|
| ABCB1 | ATP-binding cassette subfamily B, member 1 | 23 | 61 | Colchicine resistance [MIM: 120080] Crohn disease [MIM: 266600] |
| ABCC9 | ATP-binding cassette, subfamily C, member 9 isoform SUR2A-δ-14 | 1 | 2 | Cardiomyopathy [MIM: 608569] |
| ABCB11 | ATP-binding cassette, subfamily B (MDR/TAP), member 11 | 1 | 3 | Cholestasis, progressive familial intrahepatic 2 [MIM: 601847] |
| Cholestasis, benign recurrent intrahepatic 2 [MIM: 605479] | ||||
| ACAT1 | Acetyl-CoA acetyltransferase 1 precursor | 1 | 2 | α-Methylacetoacetic aciduria [MIM: 203750] |
| ACE | Angiotensin I–converting enzyme isoform 1 precursor | 23 | 96 | Hypertension [MIM: 106180] |
| ACE | Angiotensin I–converting enzyme isoform 2 precurs | 12 | 61 | Renal tubular dysgenesis [267430] |
| ACE2 | Angiotensin I–converting enzyme 2 precursor | 8 | 17 | Hypertension [MIM: 300335] |
| ACOT7 | Acyl-CoA thioesterase 7 isoform hBACHd | 1 | 1 | Mesial temporal lobe epilepsy [MIM: 608096] |
| ACSL4 | Acyl-CoA synthetase long-chain family member 4 isoform 2 | 1 | 2 | Mental retardation, X-linked 63, MRX 63 [MIM: 300387] |
| ACY1 | Aminoacylase 1 | 15 | 43 | Aminoacylase 1 deficiency [MIM: 609924] |
| AHCY | S-adenosylhomocysteine hydrolase | 10 | 28 | Hypermethioninemia [MIM: 180960] |
| AK1 | Adenylate kinase 1 | 4 | 4 | Hemolytic anemia due to AK1 deficiency [MIM: 103000] |
| ALAD | δ-Aminolevulinic acid dehydratase isoform a | 1 | 1 | Acute hepatic porphyria [MIM: 125270] |
| ALB | Albumin precursor | 36 | 139 | Dysalbuminemic hyperthyroxinemia Hyperthyroxinemia, dysalbuminemic analbuminemia bisalbuminemia [MIM: 103600] |
| ALDOA | Aldolase A | 7 | 14 | Aldolase deficiency of red cells Myopathy and hemolytic anemia [MIM: 103850] |
| ALPL | Tissue nonspecific alkaline phosphatase precursor | 3 | 4 | Hypophostasia [MIM: 241500] |
| AMN | Amnionless protein precursor | 1 | 1 | Megaloblastic anemia 1 [MIM: 261100] |
| ANPEP | Membrane alanine aminopeptidase precursor | 69 | 412 | Hypertension [MIM: 151530] |
| APOA1 | Apolipoprotein A-I preproprotein | 6 | 17 | Primary hypoalphalipoproteinemia [MIM: 604091] |
| APOA2 | Apolipoprotein A-II preproprotein | 1 | 1 | Apolipoprotein A-II deficiency, familial |
| Hypercholesterolemia, familial [MIM: 143890] | ||||
| APRT | Adenine phosphoribosyltransferase isoform a | 2 | 2 | 2,8-Dihydroxyadenine urolithiasis [MIM: 102600] |
| APRT | Adenine phosphoribosyltransferase isoform b | 3 | 10 | 2,8-Dihydroxyadenine urolithiasis [MIM: 102600] |
| AQP1 | Aquaporin 1 | 3 | 35 | Aquaporin 1 deficiency, Colton-Null [MIM: 110450] |
| AQP2 | Aquaporin 2 | 7 | 36 | Autosomal recessive nephrogenic diabetes insipidus, type 1 [MIM: 222000]Autosomal dominant nephrogenic diabetes insipidus, type 1 [MIM: 125800] |
| ARL6 | ADP-ribosylation factor–like 6 | 4 | 7 | Bardet-Biedl syndrome 3 [MIM: 209900] |
| ARSE | Arylsulfatase E precursor | 1 | 2 | Chondrodysplasia punctata 1, X-linked recessive [MIM: 302950] |
| ASAH1 | N-acylsphingosine amidohydrolase (acid ceramidase) 1 preproprotein isoform a | 7 | 16 | Farber disease [MIM: 228000] |
| ASAH1 | N-acylsphingosine amidohydrolase (acid ceramidase) 1 isoform b | 9 | 34 | Farber disease [MIM: 228000] |
| ASL | Argininosuccinate lyase isoform 3 | 1 | 1 | Argoninosuccinic aciduria [MIM: 207900] |
| ASS1 | Argininosuccinate synthetase 1 | 20 | 59 | Citrullinemia [MIM: 215700] |
| ATIC | 5-Aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase | 1 | 1 | Aica-ribosiduria due to ATIC deficiency [MIM: 608688] |
| ATP6V0A4 | ATPase, H+ transporting, lysosomal V0 subunit a4 | 1 | 2 | Renal tubular acidosis, distal, autosomal recessive [MIM: 602722] |
| ATP6V1B1 | ATPase, H+ transporting, lysosomal 56/58-kD, V1 subunit B1 | 8 | 17 | Renal tubular acidosis, distal, with progressive deafness [MIM: 267300] |
| B2M | β2-Microglobulin precursor | 1 | 1 | Hypercatabolic hypoproteinemia [MIM: 241600] |
| B4GALT1 | UDP-Gal:βGlcNAc β 1,4- galactosyltransferase 1, membrane-bound form | 1 | 1 | Congenital disorder of glycosylation type IId [MIM: 607091] |
| CA2 | Carbonic anhydrase II | 9 | 25 | Autosomal recessive syndrome of osteopetrosis with renal tubular acidosis [MIM: 259730] |
| CA4 | Carbonic anhydrase IV precursor | 2 | 2 | Proximal renal tubular acidosis [MIM: 114760] |
| CC2D1A | Coiled-coil and C2 domain containing 1A | 6 | 6 | Mental retardation autosomal recessive 3 [MIM: 608443] |
| CD2AP | CD2-associated protein | 14 | 21 | Focal segmental glomerulosclerosis FSGS3 [MIM: 607832] |
| CETP | Cholesteryl ester transfer protein, plasma precursor | 7 | 14 | Cholesterol ester transfer protein deficiency [MIM: 607322] |
| CFH | Complement factor H isoform b precursor | 1 | 1 | Hemolytic uremic syndrome, atypical [MIM: 235400] |
| CFI | Complement factor I | 1 | 1 | Complement factor I deficiency [MIM: 610984] |
| CHMP2B | Chromatin modifying protein 2B | 4 | 15 | Frontotemporal dementia, chromosome 3-linked [MIM: 6000795] |
| CLTC | Clathrin heavy chain 1 | 12 | 24 | Renal cell carcinoma [MIM: 118955] |
| COL18A1 | α 1 type XVIII collagen isoform 1 precursor | 1 | 1 | Knobloch syndrome [MIM: 267750] |
| COL6A1 | Collagen, type VI, α 1 precursor | 6 | 21 | Bethlem myopathy [MIM: 158810] Ullrich congenital muscular dystrophy, autosomal dominant [MIM: 254090] |
| COL6A3 | α 3 type VI collagen isoform 5 precursor | 1 | 2 | Ullrich congenital muscular dystrophy [MIM: 254090] |
| CP | Ceruloplasmin precursor | 6 | 15 | Aceruloplasminemia [MIM: 604290] |
| CRYAB | Crystallin, α B | 7 | 12 | α-B crystallinopathy [MIM: 608810] |
| CRYM | Crystallin, μ isoform 1 | 1 | 3 | Autosomal dominant nonsyndromic deafness [MIM: 123740] |
| CST3 | Cystatin C precursor | 1 | 3 | Icelandic-type cerebroarterial amyloidosis [MIM: 105150] |
| CSTB | Cystatin B | 2 | 10 | Myoclonic epilepsy of Unverricht and Lundborg [MIM: 254800] |
| CTSC | Cathepsin C isoform b precursor | 1 | 1 | Papillo-LeFevre syndrome [MIM: 245000] |
| CTH | Cystathionase isoform 2 | 1 | 1 | Cystathioninuria [MIM: 219500] |
| CTSA | Cathepsin A precursor | 3 | 15 | Galactosialidosis [MIM: 256540] |
| CTSC | Cathepsin C isoform a preproprotein | 1 | 2 | Papillon-Lefevre syndrome [MIM: 245000] |
| CTSD | Cathepsin D preproprotein | 1 | 2 | Neuronal ceroid lipofuscinosis [MIM: 610127] |
| CTNS | Cystinosis, nephropathic isoform 1 | 1 | 3 | Nephropathic cystinosis [MIM: 219800] |
| CUBN | Cubilin | 104 | 672 | Megaloblastic anemia 1, Finnish type [MIM: 261100] |
| CUL4B | Cullin 4B | 1 | 1 | Cabezas syndrome [MIM: 300354] |
| Mental retardation-hypotonic facies syndrome [MIM: 300639] | ||||
| DDAH1 | Dimethylarginine dimethylaminohydrolase 1 | 1 | 5 | Hypertension [MIM: 604743] |
| DDC | Dopa decarboxylase (aromatic l-amino acid decarboxylase) | 11 | 31 | Aromatic L-amino acid decarboxylase deficiency [MIM: 608643] |
| DNM2 | Dynamin 2 isoform 3 | 1 | 1 | Charcot-Marie-Tooth disease, |
| Dominant intermediate B [MIM: 606482] | ||||
| DNM2 | Dynamin 2 isoform 4 | 1 | 1 | Charcot-Marie-Tooth neuropathy, |
| Dominant intermediate B [MIM: 606482] | ||||
| DYSF | Dysferlin | 1 | 1 | Miyoshi myopathy [MIM: 254130] |
| DPYS | Dihydropyrimidinase | 5 | 6 | Dihydropyrimidinuria [MIM: 222748] |
| DSC2 | Desmocollin 2 isoform Dsc2b preproprotein | 1 | 3 | Arrhythmogenic right ventricular dysplasia-11 [MIM: 610476] |
| DSP | Desmoplakin isoform II | 10 | 16 | Keratosis palmoplantaris striata II dilated cardiomyopathy with woolly hair and keratoderma [MIM: 605676] |
| ECE1 | Endothelin-converting enzyme 1 | 1 | 1 | Hirschsprung disease [MIM: 142623] |
| EFEMP1 | EGF-containing fibulin–like extracellular matrix protein 1 precursor | 1 | 3 | Doyne Honeycomb retinal dystrophy [MIM: 126600] |
| ELA2 | Elastase 2, neutrophil preproprotein | 1 | 1 | Cyclic hematopoiesis [MIM: 162800] |
| ENPEP | Glutamyl aminopeptidase (aminopeptidase A) | 25 | 100 | Hypertension [MIM: 138297] |
| FAH | Fumarylacetoacetate hydrolase (fumarylacetoacetase) | 2 | 2 | Tyrosinemia type I [MIM: 276700] |
| FLNB | Filamin B, β (actin-binding protein 278) | 1 | 1 | Spondylocarpotarsal synostosis syndrome [MIM: 272460] |
| FBP1 | Fructose-1,6-bisphosphatase 1 | 7 | 16 | Fructose-1,6-bisphosphatase deficiency [MIM: 229700] |
| FGA | Fibrinogen, α polypeptide isoform α-E preproprotein | 5 | 17 | Renal amyloidosis [MIM: 105200] Dysfibrinogenemia [MIM: 134820] |
| FGG | Fibrinogen, γ chain isoform γ-A precursor | 1 | 1 | Dysfibrinogenemia[MIM: 134850] |
| FTCD | Formiminotransferase cyclodeaminase | 4 | 7 | Glutamate formiminotransferase deficiency [MIM: 229100] |
| FTH1 | Ferritin, heavy polypeptide 1 | 1 | 7 | Iron overload, autosomal dominant [MIM: 134770] |
| FTL | Ferritin, light polypeptide | 5 | 14 | Hyperferritinemia-cataract syndrome [MIM: 600886] |
| FUCA1 | Fucosidase, α-L-1, tissue | 1 | 1 | Fucosidosis [MIM: 230000] |
| FXYD2 | FXYD domain–containing ion transport regulator 2 isoform 1 | 1 | 7 | Hypomagnesemia 2, renal [MIM: 154020] |
| G6PD | Glucose-6-phosphate dehydrogenase isoform a | 1 | 1 | Nonspherocytic hemolytic anemia due to G6PD deficiency [MIM: 305900] |
| GAA | Acid α-glucosidase preproprotein | 4 | 8 | Infantile-onset glycogen storage disease Type II [MIM: 232300] |
| GALK1 | Galactokinase 1 | 1 | 1 | Galactokinase deficiency [MIM: 230200] |
| GBE1 | Glucan (1,4-α-), branching enzyme 1 | 1 | 2 | Type IV glycogen storage disease [MIM: 232500] |
| GCS1 | Mannosyl-oligosaccharide glucosidase | 1 | 1 | Congenital disorder of glycosylation [MIM: 606056] |
| GK | Glycerol kinase isoform a | 1 | 1 | Glycerol kinase deficiency [MIM: 307030] |
| GLB1 | Galactosidase, β 1 isoform a | 16 | 70 | Gangliosidosis GM1 [MIM: 230500] |
| GLUL | Glutamine synthetase | 2 | 2 | Congenital glutamine deficiency [MIM: 610015] |
| GM2A | GM2 ganglioside activator precursor | 2 | 3 | Gangliosidosis GM2 AB variant Tay-Sachs disease [MIM: 272750] |
| GPI | Glucose phosphate isomerase | 9 | 19 | Chronic hemolytic anemia duet to GPI deficiency [MIM: 172400] |
| GPR98 | G protein–coupled receptor 98 precursor | 1 | 1 | Familial febrile seizures [MIM: 604352] Usher syndrome type IIC [MIM: 605472] |
| GSN | Gelsolin isoform b | 10 | 21 | Finnish type familial amyloidosis [MIM: 105120] |
| GSS | Glutathione synthetase | 1 | 3 | Glutathione synthetase deficiency [MIM: 266130] |
| HNMT | Histamine N-methyltransferase isoform 1 | 1 | 1 | Susceptibility to asthma [MIM: 600807] |
| HPD | 4-Hydroxyphenylpyruvate dioxygenase | 1 | 1 | Tyrosinemia type III [MIM: 276710] |
| HPGD | Hydroxyprostaglandin dehydrogenase 15-(NAD) | 6 | 21 | Hypertension [MIM: 601688] |
| HSPG2 | Heparan sulfate proteoglycan 2 | 18 | 41 | Schwartz-Jampel syndrome type 1 [MIM: 255800] |
| HSPB1 | Heat-shock 27-kD protein 1 | 8 | 30 | Charcot-Marie-Tooth disease, type 2F [MIM: 606595] Distal hereditary motor neuropathy IIB [MIM: 608634] |
| ICAM1 | Intercellular adhesion molecule 1 precursor | 1 | 1 | Graves disease [MIM: 275000] |
| IL1RN | Interleukin 1 receptor antagonist isoform 1 precursor | 2 | 2 | Gastric cancer risk [MIM: 137215] |
| IRF6 | Interferon regulatory factor 6 | 1 | 1 | Van der Woude syndrome [MIM: 119300] Popliteal pterygium syndrome [MIM: 119500] |
| ITM2B | Integral membrane protein 2B | 5 | 21 | Familial dementia [MIM: 176500] |
| JUP | Junction plakoglobin | 9 | 15 | Naxos disease [MIM: 601214] |
| KALRN | Kalirin, RhoGEF kinase isoform 3 | 1 | 1 | Coronary heart disease [MIM: 608901] |
| KHK | Ketohexokinase isoform a | 2 | 4 | Essential fructosuria [MIM: 229800] |
| KL | Klotho | 1 | 1 | Hyperphosphatemic tumoral calcinosis [MIM: 211900] |
| KLK1 | Kallikrein 1 preproprotein | 1 | 1 | Decreased urinary activity of kallikrein [MIM: 147910] |
| LGALS3 | Galectin 3 | 1 | 1 | Lymphocyte function–associated antigen 1 [MIM: 116920] |
| LAMP2 | Lysosomal-associated membrane protein 2 precursor | 4 | 26 | Danon disease [MIM: 300257] |
| LRRK2 | Leucine-rich repeat kinase 2 | 4 | 5 | Parkinson disease [MIM: 607060] |
| LYZ | Lysozyme precursor | 1 | 3 | Familial visceral amyloidosis [MIM: 105200] |
| MIF | Macrophage migration inhibitory factor (glycosylation-inhibiting factor) | 1 | 6 | Rheumatoid arthritis [MIM: 604302] |
| MME | Membrane metallo-endopeptidase neprilysin | 48 | 311 | HypertensionImportant cell surface marker in the diagnostic of human acute lymphocytic leukemia [MIM: 120520] |
| MPO | Myeloperoxidase | 7 | 32 | Myeloperoxidase deficiency [MIM: 254600] |
| MTHFD1 | Methylenetetrahydrofolate dehydrogenase 1 | 4 | 5 | Spina bifida [MIM: 601634] |
| MYH14 | Myosin, heavy chain 14 isoform 1 | 1 | 1 | Autosomal dominant nonsyndromic sensorineural deafness [MIM: 600652] |
| MYH3 | Myosin, heavy chain 3, skeletal muscle, embryonic | 1 | 1 | Freeman-Sheldon syndrome [MIM: 193700] |
| MYH9 | Myosin, heavy polypeptide 9, nonmuscle | 19 | 51 | Fechtner syndrome [MIM: 153640]Epstein syndrome [MIM: 153650] |
| MYO15A | Myosin XV | 1 | 3 | Recessive congenital deafness [MIM: 600316] |
| MYO6 | Myosin VI | 7 | 21 | Autosomal recessive congenital sensorineural deafness [MIM: 607821] Autosomal dominant nonsyndromic sensorineural deafness [MIM: 606346] |
| NAGLU | α-N-acetylglucosaminidase precursor | 21 | 63 | Mucopolysaccharidosis type IIIB [MIM: 252920] |
| NDRG1 | N-myc downstream regulated gene 1 | 2 | 5 | Charcot-Marie-Tooth disease type 4D [MIM: 601455] |
| NEB | Nebulin | 2 | 4 | Nemaline myopathy [MIM: 256030] |
| NPHS2 | Podocin | 6 | 9 | Autosomal recessive steroid-resistant nephrotic syndrome [MIM: 600995] |
| PAFAH1B1 | Platelet-activating factor acetylhydrolase, isoform Ib, α subunit (45 kD) | 1 | 1 | Miller-Dieker lissencephaly syndrome [MIM: 607432] |
| PARK7 | DJ-1 protein | 1 | 1 | Parkinson disease 7, autosomal recessive [MIM: 606324] |
| PCBD1 | Pterin-4 α-carbinolamine dehydratase precursor | 1 | 1 | Hyperphenylalaninemia [MIM: 264070] |
| PDCD10 | Programmed cell death 10 | 2 | 3 | Cerebral cavernous malformations [MIM: 603285] |
| PHGDH | Phosphoglycerate dehydrogenase | 2 | 2 | Phosphoglycerate dehydrogenase deficiency [MIM: 601815] |
| PKD1 | Polycystin 1 | 1 | 1 | Polycystic kidney disease, adult, type I [MIM: 601313] |
| PKD2 | Polycystin 2 | 1 | 2 | Polycystic kidney disease, adult, type II [MIM: 173910] |
| PKHD1 | Polyductin isoform 2 | 6 | 9 | Autosomal recessive polycystic kidney disease [MIM: 263200] |
| PKLR | Pyruvate kinase, liver, and RBC isoform 1 | 1 | 1 | Pyruvate kinase deficiency [MIM: 266200] |
| PLOD1 | Lysyl hydroxylase precursor | 1 | 1 | Ehlers-Danlos syndrome, type VIA [MIM: 225400] |
| PRKCH | Protein kinase C, η | 1 | 2 | Cerebral infarction [MIM: 601367] |
| PROM1 | Prominin 1 | 23 | 174 | Autosomal recessive retinal degeneration [MIM: 604365] |
| PRNP | Prion protein preproprotein | 1 | 1 | Creutzfeldt-Jakob disease [MIM: 123400] |
| PSAP | Prosaposin isoform a preproprotein | 3 | 6 | Metachromatic leukodystrophy due to SAP1 deficiency [MIM: 249900] |
| Gaucher disease, atypical due to SAP2 deficiency [MIM: 610539] | ||||
| PSAP | Prosaposin isoform c preproprotein | 1 | 4 | Metachromatic leukodystrophy [MIM: 249900] |
| PSAT1 | Phosphoserine aminotransferase isoform 1 | 2 | 4 | Phosphoserine aminotransferase deficiency [MIM: 610992] |
| PTPRJ | Protein tyrosine phosphatase, receptor type, J precursor | 1 | 1 | Somatic colon cancer [MIM: 114500] |
| RAB3GAP1 | RAB3 GTPase-activating protein | 1 | 1 | Warburg micro syndrome [MIM: 600118] |
| RBP4 | Retinol-binding protein 4, plasma precursor | 2 | 3 | Retinol-binding protein deficiency [MIM: 180250] |
| RDX | Radixin | 16 | 23 | Autosomal recessive deafness 24 [MIM: 611022] |
| ROBO2 | Roundabout, axon guidance receptor, homolog 2 | 1 | 1 | Vesicoureteral reflux 2 [MIM: 610878] |
| RP2 | XRP2 protein | 3 | 5 | X-linked retinitis pigmentosa 2 [MIM: 312600] |
| RYR1 | Skeletal muscle ryanodine receptor isoform 1 | 1 | 1 | Malignant hyperthermia [MIM: 145600] Central core disease [MIM: 117000] Minicore myopathy with external ophthalmoplegia [MIM: 255320] |
| SERPING1 | Complement component 1 inhibitor precursor | 7 | 14 | Hereditary angioedema type I [MIM: 106100] |
| SLC3A1 | Solute carrier family 3, member 1 | 14 | 25 | Cystinuria [MIM: 220100] |
| SLC4A1 | Solute carrier family 4, anion exchanger, member 1 [kAE1] | 2 | 2 | Defective kidney acid secretion leading to distal renal tubular acidosis [MIM: 179800] |
| SLC4A4 | Solute carrier family 4, sodium bicarbonate co-transporter, member 4 [NBC1] | 2 | 3 | Renal tubular acidosis, proximal, with ocular abnormalities [MIM: 604278] |
| SLC5A1 | Solute carrier family 5 (sodium/glucose co-transporter), member 1 [SGLT1] | 2 | 3 | Glucose/galactose malabsorption [MIM: 606824] |
| SLC5A2 | Solute carrier family 5 (sodium/glucose co-transporter), member 2 [SGLT2] | 4 | 9 | Renal glucosuria [MIM: 233100] |
| SLC6A19 | Solute carrier family 6, member 19 | 4 | 8 | Hartnup disorder [MIM: 234500] |
| SLC12A1 | Sodium potassium chloride co-transporter 2 [NKCC2] | 25 | 94 | Bartter syndrome, antenatal, type 1 [MIM: 601678] |
| SLC12A3 | Solute carrier family 12 (sodium/chloride transporters), member 3 [NCC] | 28 | 102 | Gitelman syndrome [MIM: 263800] |
| SLC22A12 | Urate anion exchanger 1 isoform a [URAT1] | 1 | 2 | Renal hypouricemia [MIM: 220150] |
| SLC25A3 | Solute carrier family 25 member 3 isoform b precursor | 1 | 5 | Mitochondrial phosphate carrier deficiency [MIM: 610773] |
| SLC26A4 | Pendrin | 2 | 4 | Pendred syndrome [MIM: 274600]Deafness, autosomal recessive 4 [MIM: 600791] |
| SLC44A4 | NG22 protein isoform 1 | 6 | 59 | Sialidosis 1 [MIM: 606107] |
| SPR | Sepiapterin reductase (7,8-dihydrobiopterin:NADP + oxidoreductase) | 1 | 2 | Dystonia, dopa-responsive, due to sepiapterin reductase deficiency [MIM: 251120] |
| SQSTM1 | Sequestosome 1 | 1 | 2 | Paget disease of bone [MIM: 602080] |
| SUCLA2 | Succinate-CoA ligase, ADP-forming, β subunit | 1 | 1 | Mitochondrial DNA depletion syndrome [MIM: 609560] |
| TECTA | Tectorin α precursor | 1 | 1 | Autosomal dominant nonsyndromic sensorineural hearing loss [MIM: 601842] |
| TF | Transferrin | 12 | 20 | Alzheimer disease [MIM: 104300] |
| TPP1 | Tripeptidyl-peptidase I preproprotein | 8 | 42 | Ceroid lipofuscinosis neuronal 2 [MIM: 204500] |
| TSG101 | Tumor susceptibility gene 101 | 17 | 66 | Breast cancer [MIM: 176960] |
| TTN | Titin isoform novex 1 | 4 | 5 | Cardiomyopathy [MIM: 188840] |
| UMOD | Uromodulin precursor | 35 | 1278 | Medullary cystic kidney disease-2 (MCKD2) [MIM: 603860]Familial juvenile hyperuricemic nephropathy (FJHN) [MIM: 16200] |
| VCP | Valosin-containing protein | 2 | 2 | Inclusion body myopathy with early-onset Paget disease and frontotemporal dementia [MIM: 167320] |
| VAMP7 | Vesicle-associated membrane protein 7 | 1 | 1 | β-Ureidopropionase deficiency [MIM: 606673] |
| VCL | Vinculin isoform meta-VCL | 3 | 5 | Cardiomyopathy, dilated [MIM: 611407] |
| VWF | Von Willebrand factor preproprotein | 1 | 4 | Von Willebrand disease [MIM: 193400] |
| ZMPSTE24 | Zinc metalloproteinase STE24 | 1 | 1 | Mandibuloacral dysplasia [MIM: 608612] |
Information for each protein include “Gene” name, “Protein Name”, “Pep” refers to the number of unique peptides identified in LC-MS/MS, “ID” refers to the number of spectra and “Related to Disease [OMIM]” refers to the disease with which the protein is related according to OMIM. The 34 proteins associated with kidney diseases are presented in italics.
Predictably, a large number of proteins that were identified were integral membrane proteins involved in solute and water transport (Table 2). As seen in our previous study,1 these proteins predominantly represent apical transporters present in every renal tubule segment, including the proximal tubule (sodium-hydrogen exchanger 3, sodium-glucose co-transporter 1 and 2, and aquaporin-1 [AQP1]), the thick ascending limb (sodium-potassium-chloride co-transporter 2 [NKCC2]), the distal convoluted tubule (thiazide-sensitive Na-Cl co-transporter [NCC]), and connecting tubule/collecting duct (AQP2, rhesus blood group C glycoprotein [RhCG, an ammonia channel], B1 subunit of vacuolar H+-ATPase, and pendrin). Note that both polycystin-1 and polycystin-2 were detected in human urinary exosomes.
Table 2.
Solute and water transportersa
| Ref Seq | Gene | Protein Name | Pep | ID |
|---|---|---|---|---|
| NP_000918 | ABCB1 | ATP-binding cassette, subfamily B, member 1 | 23 | 61 |
| NP_003733 | ABCB11 | ATP-binding cassette, subfamily B (MDR/TAP), member 11 | 1 | 3 |
| NP_005680 | ABCB6 | ATP-binding cassette, subfamily B, member 6 | 1 | 1 |
| NP_064694 | ABCC9 | ATP-binding cassette, subfamily C, member 9 isoform SUR2A-δ-14 | 1 | 2 |
| NP_149163 | ABCC11 | ATP-binding cassette, subfamily C, member 11 isoform a | 1 | 1 |
| NP_932766 | AQP1 | Aquaporin 1 | 3 | 35 |
| NP_000477 | AQP2 | Aquaporin 2 | 7 | 36 |
| NP_000692 | ATP1A1 | Na+/K+-ATPase α 1 subunit isoform a proprotein | 19 | 57 |
| NP_001001787 | ATP1B1 | Na+/K+-ATPase β 1 subunit isoform b | 1 | 1 |
| NP_001001937 | ATP5A1 | ATP synthase, H+ transporting, mitochondrial F1 complex, α subunit precursor | 3 | 5 |
| NP_001677 | ATP5B | ATP synthase, H+ transporting, mitochondrial F1 complex, β subunit precursor | 4 | 5 |
| NP_001174 | ATP6AP1 | ATPase, H+ transporting, lysosomal accessory protein 1 precursor | 1 | 1 |
| NP_001685 | ATP6V0C | ATPase, H+ transporting, lysosomal, V0 subunit c | 1 | 9 |
| NP_005168 | ATP6V0A1 | ATPase, H+ transporting, lysosomal V0 subunit a isoform 1 | 1 | 1 |
| NP_065683 | ATP6V0A4 | ATPase, H+ transporting, lysosomal V0 subunit a4 | 1 | 2 |
| NP_004682 | ATP6V0D1 | ATPase, H+ transporting, lysosomal, V0 subunit d1 | 1 | 1 |
| NP_689778 | ATP6V0D2 | ATPase, H+ transporting, lysosomal 38 kD, V0 subunit D2 | 2 | 4 |
| NP_001681 | ATP6V1A | ATPase, H+ transporting, lysosomal 70 kD, V1 subunit A, isoform 1 | 22 | 49 |
| NP_001683 | ATP6V1B1 | ATPase, H+ transporting, lysosomal 56/58 kD, V1 subunit B1 | 8 | 17 |
| NP_001684 | ATP6V1B2 | Vacuolar H+-ATPase B2 | 12 | 27 |
| NP_001686 | ATP6V1C1 | ATPase, H+ transporting, lysosomal 42 kD, V1 subunit C1 isoform A | 1 | 1 |
| NP_001034451 | ATP6V1C2 | Vacuolar H+-ATPase C2 isoform a | 1 | 1 |
| NP_057078 | ATP6V1D | H+-transporting two-sector ATPase | 2 | 3 |
| NP_001687 | ATP6V1E1 | Vacuolar H+-ATPase E1 isoform a | 2 | 2 |
| NP_001034456 | ATP6V1E1 | Vacuolar H+-ATPase E1 isoform c | 1 | 1 |
| NP_001034455 | ATP6V1E1 | Vacuolar H+-ATPase E1 isoform b | 1 | 1 |
| NP_004222 | ATP6V1F | ATPase, H+ transporting, lysosomal 14 kD, V1 subunit F | 1 | 1 |
| NP_004879 | ATP6V1G1 | Vacuolar H+-ATPase G1 | 1 | 2 |
| NP_998784 | ATP6V1H | ATPase, H+ transporting, lysosomal 50/57 kD, V1 subunit H isoform 1 | 8 | 34 |
| NP_036415 | KCNG2 | Potassium voltage-gated channel, subfamily G, member 2 | 1 | 2 |
| NP_853514 | PKD1L3 | Polycystin 1–like 3 | 1 | 1 |
| NP_001009944 | PKD1 | Polycystin 1 isoform 1 precursor | 1 | 1 |
| NP_000288 | PKD2 | Polycystin 2 | 1 | 2 |
| NP_057405 | RHCG | Rhesus blood group, C glycoprotein | 5 | 8 |
| NP_000531 | RYR1 | Skeletal muscle ryanodine receptor isoform 1 | 1 | 1 |
| NP_006505 | SCN10A | Sodium channel, voltage-gated, type X, α | 1 | 11 |
| NP_054858 | SCN11A | Sodium channel, voltage-gated, type XI, α | 1 | 1 |
| NP_000329 | SLC12A1 | Sodium potassium chloride co-transporter 2 | 25 | 94 |
| NP_000330 | SLC12A3 | Solute carrier family 12 (sodium/chloride transporters), member 3 | 28 | 102 |
| NP_064631 | SLC12A9 | Solute carrier family 12 (potassium/chloride transporters), member 9 | 1 | 1 |
| NP_003975 | SLC13A2 | Solute carrier family 13 (sodium-dependent dicarboxylate transporter), member 2 | 5 | 10 |
| NP_073740 | SLC13A3 | Solute carrier family 13 member 3 isoform a | 2 | 2 |
| NP_001011554 | SLC13A3 | Solute carrier family 13 member 3 isoform b | 1 | 3 |
| NP_004161 | SLC1A1 | Solute carrier family 1, member 1 | 3 | 6 |
| NP_066568 | SLC15A2 | Solute carrier family 15 (H+/peptide transporter), member 2 | 1 | 1 |
| NP_060954 | SLC22A11 | Solute carrier family 22 member 11 | 2 | 9 |
| NP_653186 | SLC22A12 | Urate anion exchanger 1 isoform a | 1 | 2 |
| NP_003049 | SLC22A2 | Solute carrier family 22 member 2 isoform a | 2 | 3 |
| NP_003051 | SLC22A5 | Solute carrier family 22 member 5 | 1 | 1 |
| NP_004781 | SLC22A6 | Solute carrier family 22 member 6 isoform a | 1 | 3 |
| NP_695010 | SLC22A6 | Solute carrier family 22 member 6 isoform d | 1 | 1 |
| NP_004245 | SLC22A8 | Solute carrier family 22 member 8 | 1 | 2 |
| NP_005838 | SLC23A1 | Solute carrier family 23 (nucleobase transporters), member 1 isoform a | 4 | 6 |
| NP_689898 | SLC23A1 | Solute carrier family 23 (nucleobase transporters), member 1 isoform b | 6 | 10 |
| NP_005975 | SLC25A1 | Solute carrier family 25 (mitochondrial carrier; citrate transporter), member 1 | 1 | 1 |
| NP_998776 | SLC25A3 | Solute carrier family 25 member 3 isoform b precursor | 1 | 5 |
| NP_775897 | SLC26A11 | Solute carrier family 26, member 11 | 1 | 1 |
| NP_000432 | SLC26A4 | Pendrin | 2 | 4 |
| NP_003030 | SLC2A5 | Solute carrier family 2 (facilitated glucose/fructose transporter), member 5 | 12 | 22 |
| NP_775867 | SLC39A5 | Solute carrier family 39 (metal ion transporter), member 5 | 1 | 1 |
| NP_000332 | SLC3A1 | Solute carrier family 3, member 1 | 14 | 25 |
| NP_001012679 | SLC3A2 | Solute carrier family 3 (activators of dibasic and neutral amino acid transport), member 2 isoform a | 10 | 18 |
| NP_002385 | SLC3A2 | Solute carrier family 3 (activators of dibasic and neutral amino acid transport), member 2 isoform c | 3 | 5 |
| NP_536856 | SLC44A1 | CDW92 antigen | 1 | 1 |
| NP_065161 | SLC44A2 | CTL2 protein | 18 | 70 |
| NP_000333 | SLC4A1 | Solute carrier family 4, anion exchanger, member 1 | 2 | 2 |
| NP_003750 | SLC4A4 | Solute carrier family 4, sodium bicarbonate co-transporter, member 4 | 2 | 3 |
| NP_000334 | SLC5A1 | Solute carrier family 5 (sodium/glucose co-transporter), member 1 | 2 | 3 |
| NP_689564 | SLC5A10 | Solute carrier family 5 (sodium/glucose co-transporter), member 10 isoform 1 | 2 | 2 |
| NP_001035915 | SLC5A10 | Solute carrier family 5 (sodium/glucose co-transporter), member 10 isoform 2 | 1 | 3 |
| NP_848593 | SLC5A12 | Solute carrier family 5 (sodium/glucose co-transporter), member 12 isoform 2 | 2 | 4 |
| NP_003032 | SLC5A2 | Solute carrier family 5 (sodium/glucose co-transporter), member 2 | 4 | 9 |
| NP_666018 | SLC5A8 | Solute carrier family 5 (iodide transporter), member 8 | 1 | 1 |
| NP_001011547 | SLC5A9 | Solute carrier family 5 (sodium/glucose co-transporter), member 9 | 1 | 3 |
| NP_057699 | SLC6A13 | Solute carrier family 6 (neurotransmitter transporter, GABA), member 13 | 1 | 1 |
| NP_001003841 | SLC6A19 | Solute carrier family 6, member 19 | 4 | 8 |
| NP_004165 | SLC9A3 | Solute carrier family 9 (sodium/hydrogen exchanger), isoform 3 | 2 | 3 |
| NP_004776 | SLC9A3R2 | Solute carrier family 9 isoform 3 regulator 2 | 1 | 1 |
| NP_851322 | SLCO4C1 | Solute carrier organic anion transporter family, member 4C1 | 2 | 2 |
| NP_003365 | VDAC1 | Voltage-dependent anion channel 1 | 6 | 43 |
| NP_005653 | VDAC3 | Voltage-dependent anion channel 3 | 1 | 1 |
Table contains all of the proteins that are solute and water transporters.
Exosomes derive from MVB and are delivered to the urine when the outer membranes of MVB fuse with the apical plasma membrane. Interestingly, 22 of the proteins identified in this study are recognized as components of the apparatus responsible for the formation of MVB (Table 3). These 22 proteins account for approximately 75% of the proteins that constitute the ESCRT-0, ESCRT-I, ESCRT-II, and ESCRT-III complexes involved in multivesicular body formation.5
Table 3.
Proteins of the ESCRT complex found in human urinary exosomesa
| Gene | Protein Name | Pep | ID | Ref Seq | ESCRT Complex |
|---|---|---|---|---|---|
| HGS | Hepatocyte growth factor–regulated tyrosine kinase substrate | 1 | 1 | NP_004703 | ESCRT-0 |
| TSG101 | Tumor susceptibility gene 101 | 15 | 47 | NP_006283 | ESCRT-I |
| VPS28 | Vacuolar protein sorting 28 isoform 1 | 5 | 8 | NP_057292 | ESCRT-I |
| VPS28 | Vacuolar protein sorting 28 isoform 2 | 2 | 8 | NP_898880 | ESCRT-I |
| VPS37B | Vacuolar protein sorting 37B | 4 | 10 | NP_078943 | ESCRT-I |
| VPS37C | Vacuolar protein sorting 37C | 1 | 1 | NP_060436 | ESCRT-I |
| VPS25 | EAP25 | 4 | 15 | NP_115729 | ESCRT-II |
| VPS36 | EAP45 | 2 | 3 | NP_057159 | ESCRT-II |
| SNF8 | EAP30 | 1 | 1 | NP_009172 | ESCRT-II |
| CHMP2A | CHMP2A | 6 | 40 | NP_055268 | ESCRT-III |
| CHMP2B | CHMP2B | 3 | 11 | NP_054762 | ESCRT-III |
| VPS24 | CHMP3 | 1 | 3 | NP_057163 | ESCRT-III |
| VPS24 | CHMP3 | 1 | 4 | NP_001005753 | ESCRT-III |
| CHMP4B | CHMP4B | 2 | 6 | NP_789782 | ESCRT-III |
| CHMP5 | CHMP5 | 2 | 7 | NP_057494 | ESCRT-III |
| CHMP1A | CHMP1A | 1 | 3 | NP_002759 | ESCRT-III |
| CHMP1B | CHMP1B | 1 | 2 | NP_065145 | ESCRT-III |
| CHMP6 | CHMP6 | 2 | 3 | NP_078867 | ESCRT-III |
| VPS4A | Vacuolar protein sorting factor 4A | 11 | 25 | NP_037377 | ATPase complex |
| VPS4B | Vacuolar protein sorting factor 4B | 11 | 32 | NP_004860 | ATPase complex |
| PDCD6IP | ALIX | 27 | 104 | NP_037506 | Accessory |
| C1orf58 | Hypothetical protein LOC148362 | 11 | 34 | NP_653296 | Accessory |
Table contains all of the proteins that are members of the ESCRT Complex (ESCRT-0, ESCRT-I, ESCRT-II, ESCRT-III, ATPase complex and accessory).
In addition, 17 proteins identified in this study are subunits of the human vacuolar H+-ATPase (Table 4). Vacuolar H+-ATPases are ATP-dependent proton pumps for proton transport into intracellular organelles.6 These proteins also mediate proton transport across the apical plasma membrane of type A intercalated cells and across the basolateral plasma membrane of type B intercalated cells.7 The B1 subunit is selectively expressed in intercalated cells, and its detection in urinary exosomes establish that intercalated cells secrete exosomes as do other types of epithelial cells lining the renal tubule. These proteins constitute 78% of the subunits of the V0 and V1 domains of the vacuolar H+-ATPase.8
Table 4.
Vacuolar H+-ATPase subunits in human urinary exosomesa
| Ref Seq | Gene | Protein Name | Pep | ID |
|---|---|---|---|---|
| NP_005168 | ATP6V0A1 | ATPase, H+ transporting, lysosomal V0 subunit a isoform 1 | 1 | 1 |
| NP_065683 | ATP6V0A4 | ATPase, H+ transporting, lysosomal V0 subunit a4 | 1 | 2 |
| NP_001685 | ATP6V0C | ATPase, H+ transporting, lysosomal, V0 subunit c | 1 | 1 |
| NP_004682 | ATP6V0D1 | ATPase, H+ transporting, lysosomal, V0 subunit d1 | 1 | 1 |
| NP_689778 | ATP6V0D2 | ATPase, H+ transporting, lysosomal 38 kD, V0 subunit D2 | 2 | 4 |
| NP_001681 | ATP6V1A | ATPase, H+ transporting, lysosomal 70 kD, V1 subunit A, isoform 1 | 22 | 49 |
| NP_001683 | ATP6V1B1 | ATPase, H+ transporting, lysosomal 56/58 kD, V1 subunit B1 | 8 | 17 |
| NP_001684 | ATP6V1B2 | vacuolar H+-ATPase B2 | 12 | 27 |
| NP_001686 | ATP6V1C1 | ATPase, H+ transporting, lysosomal 42 kD, V1 subunit C1 isoform A | 1 | 1 |
| NP_001034451 | ATP6V1C2 | vacuolar H+-ATPase C2 isoform a | 1 | 1 |
| NP_057078 | ATP6V1D | H(+)-transporting two-sector ATPase | 2 | 3 |
| NP_001687 | ATP6V1E1 | vacuolar H+-ATPase E1 isoform a | 2 | 2 |
| NP_001034455 | ATP6V1E1 | vacuolar H+-ATPase E1 isoform b | 1 | 1 |
| NP_001034456 | ATP6V1E1 | vacuolar H+-ATPase E1 isoform c | 1 | 1 |
| NP_004222 | ATP6V1F | ATPase, H+ transporting, lysosomal 14 kD, V1 subunit F | 1 | 1 |
| NP_004879 | ATP6V1G1 | vacuolar H+-ATPase G1 | 1 | 2 |
| NP_998784 | ATP6V1H | ATPase, H+ transporting, lysosomal 50/57 kD, V1 subunit H isoform 1 | 8 | 34 |
Table contains proteins that are found in human urinary exosomes and are subunits of the human vacuolar H+-ATPase.
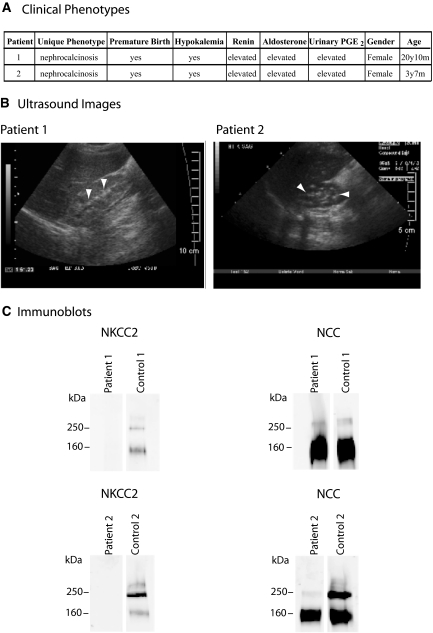
An example of the utility of exosome analysis is shown in Figure 1, describing immunoblotting in patients with Bartter syndrome type I, associated with mutations in the SLC12A1 gene, which encodes for the NKCC2 sodium-potassium-chloride co-transporter protein.9 The NKCC2 protein was found in the proteome of the human urinary exosomes as shown in Table 1. Urine samples were obtained from two patients (patients 1 and 2) with clinical phenotypes consistent with Bartter syndrome type I (Figure 1A).10 The clinical diagnosis for the patients with Bartter syndrome type I was confirmed by the ultrasound images showing deposits of calcium in the kidney also known as nephrocalcinosis9, 10 (Figure 1B) and other typical laboratory findings. The two urinary exosome samples obtained from the patients with Bartter syndrome type I were analyzed by immunoblotting for the presence of the NKCC2 protein (Figure 1C). Compared with the respective control samples, patients 1 and 2 showed an absence of the NKCC2 protein bands, expected at 160 kD for monomeric NKCC2 and 320 kD for dimeric NKCC2. In addition, the samples (patients 1 and 2) were probed for the thiazide-sensitive co-transporter (NCC) protein to ensure that urinary exosomes were successfully isolated and loaded properly. Strong NCC bands were obtained in samples from both patients with Bartter syndrome type I and control samples.
Figure 1.
Disease-related protein: NKCC2 and Bartter syndrome type I. (A) Details of clinical phenotype for patients with Bartter syndrome type I, patient 1 and patient 2. (B) Ultrasound images showing calcium deposits (white arrowheads) in the kidneys of patients 1 and 2. (C) Immunoblot of urinary exosomes samples from patient 1, patient 2, control 1, and control 2 using polyclonal rabbit anti-NKCC2 and NCC antibodies.
Phosphoproteomic Analysis of Human Urinary Exosomes
Protein phosphorylation is a key element of most cell regulatory processes. Recently, technical approaches that allow phosphoproteomic profiling on a large scale have been introduced.4, 11–13 We used neutral loss scanning with high-stringency target-decoy analysis to identify phosphorylation sites present in exosomal proteins from human urine samples.
Nineteen phosphorylation sites corresponding to 14 phosphoproteins were identified (Table 5). These included both newly identified phosphorylation sites and sites that had been previously identified. Two orphan G-protein–coupled receptors are included in the former group, viz. GPRC5B and GPRC5C. In GPRC5B, we identified one new phosphorylation site, T389, and, in GPRC5C, we identified three new phosphorylation sites, T435, S395, and Y426. These proteins are also known as retinoic acid–induced gene 2 (GPRC5B) and retinoic acid–induced gene 3 (GPRC5C).
Table 5.
Human urinary exosome phosphopeptidesa
| Ref Seq | Protein Name, Sequence | Site | Gene | Novel Site | MSn | Motif | GO Function |
|---|---|---|---|---|---|---|---|
| NP_061123 | G protein–coupled receptor family C, group 5, member C isoform b | GPRC5C | Metabotropic glutamate, GABA-B–like receptor activity Protein binding Receptor activity | ||||
| R.AEDMYSAQSHQAA(T*)PPKDGK.N | T435 | Yes | MS2, MS3 | Proline-directed | |||
| K.VP(S*)EGAYDIILPR.A | S395 | Yes | MS2, MS3 | Phosphoserine/threonine binding group | |||
| R.AEDM(Y*)SAQSHQAATPPKDGK.N | Y426 | Yes | MS2 | Tyrosine kinase | |||
| NP_001035149 | Secreted phosphoprotein 1 isoform c | SPP1 | Cytokine activity Growth factor activity Integrin binding Protein binding | ||||
| K.AIPVAQDLNAPSDWD(S*)R.G | S192 | No | MS2 | Miscellaneous | |||
| R.GKD(S*)YETSQLDDQSAETHSHK.Q | S197 | MS2, MS3 | Basophilic | ||||
| R.GKDSYETSQLDDQ(S*)AETHSHK.Q | S207 | MS2, MS3 | Acidophilic | ||||
| NP_057319 | G protein–coupled receptor, family C, group 5, member B precursor | GPRC5B | Metabotropic glutamate, GABA-B–like receptor activity Receptor activity Sevenless binding | ||||
| R.SNVYQPTEMAVVLNGG(T*)IPTAPPSHTGR.H | T389 | Yes | MS2 | Basophilic | |||
| NP_000477 | Aquaporin 2 | AQP2 | Transporter activity Water channel activity | ||||
| R.RQ(S*)VELHSPQSLPR.G | S256 | No | MS2, MS3 | Basophilic | |||
| NP_004860 | Vacuolar protein sorting factor 4B | VPS4B | ATP binding ATPase activity, Coupled nucleotide binding Protein binding | ||||
| K.EGQPSPADEKGND(S*)DGEGESDDPEKKK.L | S102 | Yes | MS2 | Acidophilic | |||
| NP_054762 | Chromatin modifying protein 2B | CHMP2B | Not classified | ||||
| K.ATI(S*)DEEIER.Q | S199 | No | MS2, MS3 (unfiltered) | Acidophilic | |||
| NP_687033 | Proteasome α 3 subunit isoform 2 | PSAM3 | Protein binding Threonine endopeptidase activity | ||||
| K.ESLKEEDE(S*)DDDNM | S243 | No | MS2, MS3 | Acidophilic | |||
| NP_036382 | Related RAS viral (r-ras) oncogene homolog 2 | RRAS2 | GTP binding Nucleotide binding Protein binding | ||||
| R.KFQEQECPP(S*)PEPTRK.E | S186 | Yes | MS2, MS3 | Proline-directed | |||
| NP_031381 | Heat-shock 90-kD protein 1, β | HSP90AB1 | ATP binding Nitric-oxide synthase regulator activity Nucleotide binding TPR domain binding Unfolded protein binding | ||||
| K.IEDVG(S*)DEEDDSGKDKK.K | S255 | No | MS2 | Acidophilic | |||
| NP_612433 | Kinesin family member 12 | KIF12 | ATP binding Microtubule motor activity Nucleotide binding | ||||
| R.VTTRPQAPK(S*)PVAK.Q | S236 | Yes | MS2, MS3 | Proline-directed | |||
| NP_079119 | Cytochrome b reductase 1 | CYBRD1 | Ferric-chelate reductase activity | ||||
| R.NLALDEAGQRS(T*)M. | T285 | Yes | MS2 | Basophilic | |||
| NP_001037857 | Mucin 1 isoform 7 precursor | MUC1 | NF-κB binding protein heterodimerization activity | ||||
| R.DTYHPMSEYPTYH(T*)HGR.Y | T118 | Yes | MS2, MS3 (unfiltered) | Acidophilic | Protein homodimerization activity RNA binding Tat protein binding Unfolded protein binding | ||
| NP_000330 | Solute carrier family 12 (sodium/chloride transporters), member 3 | SLC12A3 | Sodium ion binding Sodium:chloride | ||||
| R.GARP(S*)VSGALDPK.A | S811 | Yes | MS2 | Basophilic | symporter activity Symporter activity Transporter activity | ||
| NP_000329 | Sodium potassium chloride co-transporter 2 | SLC12A1 | Potassium ion binding Sodium ion binding | ||||
| K.IEYYRN(T*)GSISGPK.V | T118 | No | MS2, MS3 | N/A | Sodium:potassium:chloride symporter activity | ||
| K.IEYYRNTG(S*)ISGPK.V | S120 | No | MS2, MS3 | Basophilic | Symporter activity Transporter activity |
Table contains phosphopeptides found in urinary exosomes. MSn refers to spectra for phosphorylation site identification, Motif refers to phosphorylation motif site and GO Function.
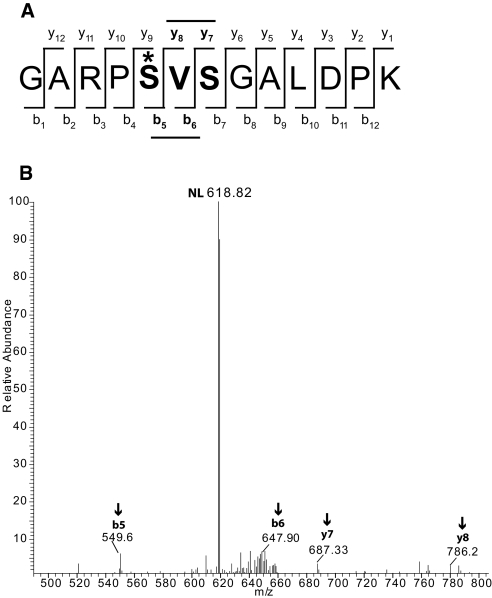
A new phosphorylation site was also identified in the COOH-terminal tail of the thiazide-sensitive co-transporter (NCC) at S811 (Figure 2). This site is distinct from the N-terminal site previously identified14 and may play a role in regulation of transport. This amino acid is conserved in humans, chimpanzees, rhesus monkeys, and horses but not in mice and rats. Simon et al.15 showed that, in rat, the amino acid sequence surrounding this site is absent owing to a difference in exon splicing.
Figure 2.
Novel phosphorylation site in the NCC. The serine-811 on the NCC protein is phosphorylated. (A) The phosphorylation site on the peptide is denoted by an asterisk (*). (B) The neutral loss peak (NL) from the +2 mass spectrum and the site-determining ions b5, b6, y7, and y8.
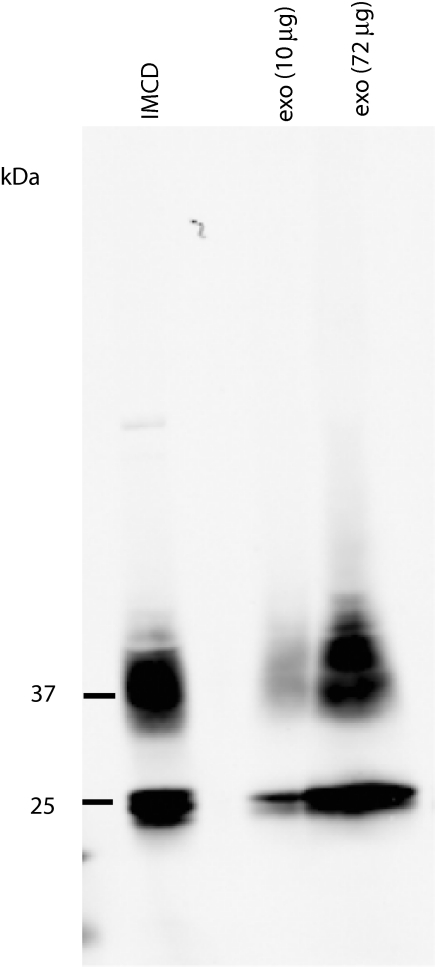
Novel phosphorylation sites were also identified in RRAS2 (TC21), VPS4B (an ESCRT component), cytochrome b reductase, proteasome α 3 subunit, and mucin 1. This study also revealed previously identified phosphorylation sites in AQP2 (S256),16 NKCC2 (T118 and S120),17 CHMP2B (S199),18 HSP90AB1 (S255),19 and SPP1 (S192, S197, and S207).20 Phosphorylation of AQP2 at S256 was confirmed by immunoblotting human urinary exosomes samples with a phospho-specific antibody for this site (Figure 3).
Figure 3.
Detection of AQP2-S256 phosphorylation in urinary exosomes. IMCD, rat inner medullary collecting duct treated with dDAVP (V2R-selective vasopressin analog) for 30 min; exo (10 μg), is human urinary exosomes, 10 μg; exo (72 μg), human urinary exosomes, 72 μg.
DISCUSSION
Large-Scale Proteomic Profiling of Human Urinary Exosomes
One of the objectives of this study was to expand the existing human urinary exosome database by using a higher sensitivity LC-MS/MS mass spectrometer and improved computational tools for matching spectra to proteins in the human proteome. The LTQ mass analyzer has an increased trapping efficiency, ion capacity, and ion ejection rate compared with the LCQ mass analyzer1 used in our previous study. We identified the peptide sequences using the SEQUEST program and analyzed them using the target-decoy database search strategy and the InsPecT tool. The target-decoy database search strategy allows adjustment of SEQUEST search parameters to ensure a given false-discovery rate (FDR).13 The InsPecT tool uses de novo sequencing to generate tag filters, which are then used to search the database to “look for any peptide that matches the tag.”21 The data have been made available to the general public and can be downloaded from our laboratory's website (http://dir.nhlbi.nih.gov/papers/lkem/exosome/). In addition, the database can be searched using the BLAST algorithm.
As illustrated in Figure 1, analysis of human urinary exosomes by mass spectrometry and immunoblotting can provide information with regard to genetic diseases involving apical proteins as shown by the qualitative assessment of urinary exosome samples from patients with Bartter syndrome type I. The urinary exosome patient samples showed a complete absence of NKCC2 protein bands. Mutations in the SLC12A1 gene cause Bartter syndrome type I.9 Many such mutations presumably result in misfolding of the NKCC2 protein, preventing the apical delivery of the protein and therefore preventing incorporation into urinary exosomes.
Barriers to Clinical Use of Human Urinary Exosome Analysis
We previously reviewed the potential of urinary exosome analysis as a route to biomarker discovery in renal diseases and delineated barriers to success with the approach.2, 21, 22 One important barrier is the lack of standard protocols for collection, processing, and storage of urine samples to allow reproducible measurements to be made in any clinical laboratory. We have proposed a set of procedures that can serve as a beginning point in the development of such techniques (http://intramural.niddk.nih.gov/research/uroprot/). Our current approach includes an ultracentrifugation step, which requires expensive instrumentation and long processing times. Filtration methods have been proposed to replace the ultracentrifugation step.3 A particular knotty problem is removal of Tamm-Horsfall protein, an extraordinary abundant urinary protein that interferes with successful mass spectrometry and immunoblotting.23 In the long run, the most important technical challenge may be to develop quantification approaches that allow detection of changes in excretion rates of particular biomarker candidates. Both labeling and nonlabeling methods have been developed to make protein mass spectrometry quantitative24; however, the biggest barrier to quantification lies in development of adequate normalization techniques providing surrogates for timed collections of urine, which are notoriously inaccurate.21 Use of creatinine as a normalizing variable may be inadequate because of high subject-to-subject variability in its rate of excretion.21 Even without quantification, urinary exosome analysis can be valuable in situations such as genetic diseases (e.g., Bartter syndrome type I [Figure 1]), where a protein may be entirely absent from urinary exosomes.
Relevance to Renal Biology
Several of the proteins newly identified in urinary exosomes in this study may have considerable relevance to renal biology and the mechanism of renal disease. Our previous study1 identified proteins that were characteristic of most of the cell types facing the urinary space from podocytes through transitional epithelial cells of the urinary drainage system. In this study, we identified markers of two additional cell types, type A and B intercalated cells. Specifically, the B1 subunit of the H+-ATPase is apically located in type A intercalated cells,25 and the anion transporter pendrin is present in type B intercalated cells.26 Previously, we showed that urinary exosomes are derived from the apical endosomal pathway so that, although the B1 subunit of the H+-ATPase is also expressed in type B intercalated cells, its basolateral location probably precludes delivery to urinary exosomes. Overall, we identified 17 different vacuolar H+-ATPase subunits in urinary exosomes, 78% of the whole V0–V1 complex.8
We identified all of the subunits of the four ESCRT complexes (ESCRT-0 through ESCRT-III) in urinary exosomes in this study. The ESCRT complexes play a central role in the formation of MVB and the secretion of exosomes.5
Four different orphan G-protein–coupled receptors were identified in urinary exosomes in this study, namely GPR98, GPRC5A, GPRC5B, and GPRC5C. These receptors are presumably apically located in one or more renal tubule cells. GPR98 (also known as very large G-protein–coupled receptor 1 or Neurepin) has more than 6000 amino acids. The three GPRC5 proteins are members of the metabotropic glutamate family, but their natural ligands are unknown. It will be of interest in future studies to discover the role of these proteins in renal development and regulation.
Phosphoproteomic Analysis of Human Urinary Exosomes
Posttranslational modifications (PTM) of proteins play an important role in protein function. Among the most important PTM is phosphorylation. Protein phosphorylation regulates cellular signaling processes and may determine protein structure, function, and subcellular localization.12 The ability to detect PTM, such as phosphorylation, in urinary exosomes may provide an additional level of information that could aid in diagnosis and treatment of a variety of renal disorders. Furthermore, discovery of PTM in urinary exosomes can provide clues about physiologic and pathophysiologic mechanism. In this study, we identified 14 phosphoproteins. The specific phosphorylation sites identified included six that were previously identified and eight that had not been previously identified. Among the novel sites was serine-811 in the NCC protein. This amino acid is conserved in humans, chimpanzees, rhesus monkeys, and horses but not in mice and rats. The amino acid sequence surrounding this site is absent in rodents owing to a difference in exon splicing.15 Finally, AQP2 phosphorylated at serine-256 was readily detectable in urinary exosomes. Because this phosphorylation event is increased by vasopressin-stimulated activation of adenylyl cyclase,16 measurements of the amount of serine-256–phosphorylated AQP2 in urine may provide an improved means of assessing the state of vasopressin activation using phospho-specific antibodies.
CONCISE METHODS
Urinary Exosome Isolation
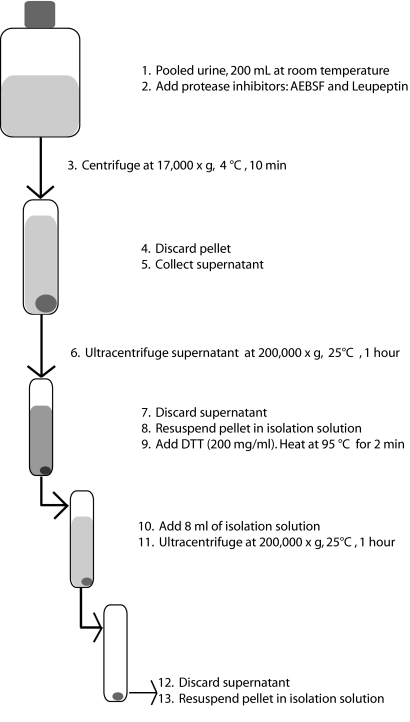
Urine was collected from eight healthy humans: Four men (aged 22 to 33) and four women (aged 24 to 35) (National Institute of Diabetes and Digestive and Kidney Diseases Clinical Research Protocol 00-DK-0107). Fifty milliliters per subject was collected and mixed together. The urinary exosome isolation procedure is shown in Figure 4. Protease inhibitors were added (1.67 ml of 100 mM NaN3, 2.5 ml of 11.5 mM 4-[2-aminoethyl] benzenesulfonyl fluoride, and 50 μl of 1 mM leupeptin). The mixed sample was centrifuged at 17,000 × g for 10 min at 4°C. The 17,000 × g supernatant was ultracentrifuged at 200,000 × g for 1 h at 25°C. The ultracentrifugation step was repeated 3 additional times, adding new 17,000 × g supernatant volume each time to each of the 12 tubes. Each of the 12 pellets was suspended with 50 μl of “isolation solution” (10 mM triethanolamine and 250 mM sucrose). The suspensions were pooled together.
Figure 4.
Differential centrifugation procedure for the isolation of urinary exosomes from urine.
The abundant urinary protein uromodulin or Tamm-Horsfall protein forms very high molecular weight complexes through disulfide linkages. These complexes sediment in the 200,000 × g spin unless denatured. To denature the zona pellucida domains in the Tamm-Horsfall protein, we mixed the resuspended pellet with 200 mg/ml dithiothreitol (DTT) at 95°C for 2 min. The resuspended pellet was added to an ultracentrifuge tube, and isolation solution was added to increase the volume to 8 ml. The sample was centrifuged at 200,000 × g for 1 h at 25°C. The pellet was suspended in 50 μl of isolation solution and frozen at −80°C.
In-Gel Trypsin Digestion
The protein concentration was determined using the Bradford Assay. This sample was solubilized in Laemmli sample buffer (1.5% SDS, 6% glycerol/10 mM Tris HCl, and 60 mg/ml DTT). Proteins in the exosome sample were separated by 1D SDS-PAGE using a Bio-Rad Ready Gel 4 to 15% polyacrylamide gradient gel with 125 μg distributed among two lanes. The gel was stained with Colloidal Coomassie Blue (GelCode Blue Stain Reagent; Pierce, Rockford IL) for 10 min and destained using ddH2O (2 × 30 min). The gel was divided from top to bottom into 40 1-mm strips over the entire molecular weight range of the gel. Each strip was diced into small pieces (1 mm3) and placed into labeled centrifuge tubes.
The gels pieces were destained by adding 100 μl of 25 mM ammonium bicarbonate (NH4HCO3)/50% acetonitrile (ACN) for 10 min and were dried using a SpeedVac. The samples were reduced in a solution of 10 mM DTT and 25 mM NH4HCO3 at 56°C for 1 h. The samples were alkylated in a solution containing 55 mM iodoacetamide and 25 mM NH4HCO3 in the dark at room temperature for 45 min. The gel pieces were washed with 25 mM NH4HCO3 and dehydrated in a solution containing 25 mM NH4HCO3 and 50% ACN. The samples were dried using the SpeedVac. The samples were rehydrated in a solution containing 12.5 ng/μl trypsin (V5113; Promega, Madison, WI) in 25 mM NH4HCO3 and digested overnight at 37°C. Peptides were extracted using 50% ACN/0.1% formic acid (FA). The extracted samples were dried using the SpeedVac to remove ACN and then reconstituted with 0.1% FA. All 40 peptide samples were desalted using C18 Zip Tips (Millipore, Billerica, MA) before analysis by mass spectrometry.
Nanospray LC-MS/MS
A high-sensitivity linear ion trap mass spectrometer, LTQ (Thermo Electron Corp.) equipped with a nanoelectrospray ion source was used to acquire m/z ratios in both precursor ions (MS1) and fragmented ions (MS2) scans. To reduce further the sample complexity before mass analysis, we injected the tryptic peptides extracted from each gel slice using an Agilent 1100 nanoflow system (Agilent Technologies, Palo Alto, CA) into a reversed-phase liquid chromatographic column (PicoFrit, Biobasic C18; New Objective, Woodburn, MA). This LC-MS/MS method allows the acquisition of raw data files that are the MS/MS scans of the five highest intensity peaks after fragmentation with collision-induced dissociation in the LTQ mass analyzer.
Analysis of Data
The raw data files were searched against the NCBI Reference Sequences (RefSeq) human protein database by using BIOWORKS software (Thermo Finnigan). BIOWORKS utilizes SEQUEST, which is a program that “finds database candidate sequences whose theoretical spectra are compared with the experimental spectrum.”27 To identify thoroughly peptide sequences, we searched the raw data files using the target-decoy approach and InsPecT.
In addition, we analyzed the data in a two-step process. The first step was to assess and minimize false-discovery peptide identifications using the target-decoy approach, manual inspection of spectra, and InsPecT. The second step was to assess and eliminate ambiguous protein identifications.
Target-Decoy
To apply the target-decoy database searching strategy,13 we used the NHLBI Proteomics Core Facility in-house software to create a composite database containing the forward and reverse sequences of the nonredundant NCBI Reference Sequences (RefSeq) human protein database released on January 26, 2007. We used the forward sequences as the target database and the reversed sequences as the decoy database. We searched the raw data files against this composite database. After the search, we assessed the FDR by the number of peptides matched from the reversed sequences. The parameters that determine the stringency of the filtering criteria include XCorr, Sp rank, and delta Cn. These parameters were incrementally adjusted, thereby reducing the false-discovery identifications until a target FDR was achieved. In our case, the data were filtered to a target of 2% FDR, and the actual FDR was 1.91%. The filter settings used were min Xcorr rank 1, min Sp rank 10, min delta Cn 0.08, charge + 1 min Xcorr 2.37, charge + 2 min Xcorr 2.87, and charge + 3 min Xcorr 3.37.
InsPecT
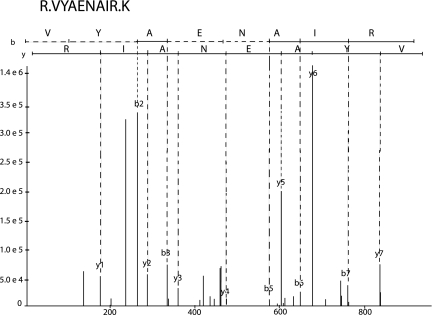
We performed an additional analysis of the tandem mass spectrometry data using the InsPecT tool.28 InsPecT uses de novo sequencing to generate sequence information (tag filters) from the experimental data. The tag filters are used to search the human protein database, nonredundant NCBI Reference Sequences (RefSeq) human protein database released on January 26, 2007, and identify peptide sequences that match with the experimental data. The size of the tag filters are three peptides in length on average. As shown in Figure 5, the tag filter generated for the protein CHMP1A matches the experimental data accurately. The peptide sequences identified using the tag filters are then scored to estimate that the top match is correct.28 The score procedure computes the P value for each peptide sequence by “comparing the match quality score to the distribution of quality scores for incorrect matches.” For these data, we accept only peptide matches with P ≤ 0.05.
Figure 5.
Spectrum generated by InsPecT for CHMP1A protein (NP_002759). The peptide sequence is RVYAENAIRK. The tag region for the b ions and the y ions are shown by the black solid lines.
Minimizing False-Discovery Peptide Identifications
In addition to the target-decoy approach the InsPecT analysis, we validated the quality of proteins identified by manually checking the spectra of those proteins with one unique peptide. We filtered out the proteins that did not have the expected molecular weight that matched to the corresponding regions in the 1-D SDS PAGE.
Elimination of Ambiguous Protein Identifications
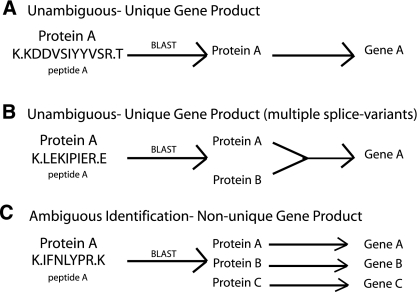
Once proteins were identified using the approaches described, we needed to determine whether all identifications corresponded to unique gene products. An “ambiguous identification” is defined as an identification for which the peptide sequence that is used to determine the protein identity is found in multiple proteins that are not splice variants of the same gene (Figure 6).
Figure 6.
Criteria to disambiguate data set. (A) An unambiguous identification when a peptide sequence was a 100% match without gaps to one and only one protein. (B) An unambiguous identification when a peptide sequence was a 100% match without gaps to more than one protein, but these proteins are splice-variant products of one unique gene. (C) An ambiguous identification when a peptide sequence was a 100% match without gaps to more than one protein deriving from more than one gene, and the identification was based only on that single peptide.
To disambiguate the data set, we generated software that automates the comparison of each peptide sequence to the protein sequences in the RefSeq Human Protein Database using the BLAST algorithm. An identification was considered unambiguous when the sequence was a 100% match without gaps to one and only one protein (Figure 6A). An identification was also considered unambiguous when the sequence was a 100% match without gaps to more than one protein but these proteins are splice-variant products of one unique gene (Figure 6B). An identification was considered ambiguous when a peptide sequence was a 100% match without gaps to more than one protein deriving from more than one gene and the identification was based only on that single peptide (Figure 6C). The proteins identified from at least one unambiguous peptide were considered unambiguous proteins. The proteins that contained only ambiguous peptides were considered ambiguous proteins.
Patients with Bartter Syndrome Type I
We collected spot urine samples from two patients with clinically diagnosed Bartter syndrome type I. The patients were enrolled in the institutional review board–approved protocol 76-HG-0238. We obtained written informed consent from the parents and/or patient. We collected urine samples from healthy humans and used them as controls. We processed all samples using the differential centrifugation method to isolate human urinary exosomes described already. Each sample was prepared for immunoblotting by solubilizing in Laemmli buffer (1.5% SDS, 6% glycerol, 10 mM Tris HCl, and 60 mg/ml DTT). The samples, patient 1 and patient 2, and the control samples, control 1 and control 2, were loaded onto a 1-D SDS-PAGE gel on the basis of time as measured by creatinine excretion. The proteins were transferred to Immobilon-P (Millipore) membranes, blocked, and probed with antigen-specific NKCC2 and NCC primary antibodies. We incubated the blots with species-specific fluorescence secondary antibodies (Alexa 688) and visualized them using the Odyssey Infrared Imaging System (LiCor, Lincoln, NE).
Phosphopeptide Enrichment and LC-MS/MS Analysis
We collected urine specimens (200 ml) from six healthy humans, three men and three women. We processed the specimens 400 ml/d for 3 d and pooled them. The exosome isolation was as described previously except that phosphatase inhibitors 10 mM NaF (Sigma, St. Louis, MO), 20 mM β-glycerol phosphate (Fluka, St. Louis, MO), and 1 mM sodium orthovanadate (Sigma) were added. The pellet was resuspended in 6 M guanidine HCl/50 mM NH4HCO3.
The sample was concentrated using a Centricon tube at 13,500 × g, with a starting volume of 420 μl and a final volume of 55 μl. The sample was reduced with 50 mM DTT for 1 h at 56°C. The sample was alkylated by addition of 100 mM iodoacetamide for 1 h (dark) at room temperature and was digested with trypsin overnight at 37°C. The sample was centrifuged at 16,000 × g for 20 min. The supernatant was kept, and 100% FA was added to inactivate the trypsin. The sample was desalted on a 1-ml HLB column (Waters Oasis, Milford, MA) by positive displacement via a syringe with a luer adapter. The sample was eluted with two elution buffers. Elution buffer 1 contained 50% ACN and 0.1% FA, and elution buffer 2 contained 90% ACN and 0.1% FA. The eluents, 50 and 90%, were dried using the SpeedVac.
Phosphopeptides were enriched from the samples using the Pierce Phosphopeptide Isolation Kit (cat. no. 89853) according to the manufacturer's protocol. Phosphopeptide samples were desalted using C18 ZipTips (Millipore) before analysis by mass spectrometry.
Phosphopeptide samples were analyzed on an Agilent 1100 nanoflow system (Agilent Technologies) LC connection to a Finnigan LTQ FT mass spectrometer (Thermo Electron) equipped with a nanoelectrospray ion source as described previously.11 The five most intense ions were sequentially isolated and fragmented (MS2) in the linear ion trap using collision-induced dissociation. The data-dependent neutral loss algorithm in XCALIBUR software was used to trigger an MS3 scan when a neutral loss of 98.0, 49.0, or 32.7 Da was detected among the two most intense fragment ions in a given MS2 spectrum.
Analysis of Phosphopeptide Data Sets
We searched MS raw data files against a composite database containing the forward and reversed peptide sequence of the Human RefSeq Database from January 26, 2007. Putative phosphopeptides were selected and filtered to produce MS2 and MS3 data sets with target FPR of 2% (high stringency) and 20% (low stringency) via the PhosphoPIC program.4 This software was also used to merge MS2 and MS3 data sets into a single file to facilitate subsequent data analysis. Phosphopeptides identified in MS2 spectra were submitted for automated phosphorylation site assignment using the Ascore algorithm.13 A site with an Ascore ≥19 (>99% confidence) was considered to be unambiguously assigned. Phosphopeptides present only in MS3 spectra were checked manually. We used Scansite (http://scansite.mit.edu/motifscan_seq.phtml) to determine the phosphorylation motif for the identified sites. We searched the PhosphoSite database (http://www.phosphosite.org) to determine whether the sites were novel or previously identified.
Disclosures
None.
Acknowledgments
We thank Brian Ruttenberg for valuable contribution in developing the code/program used to disambiguate the proteomic data.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1.Pisitkun T, Shen RF, Knepper MA: Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A 101: 13368–13373, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou H, Yuen PS, Pisitkun T, Gonzales PA, Yasuda H, Dear JW, Gross P, Knepper P, Star RA: Collection, storage, preservation, and normalization of human urinary exosomes for biomarker discovery. Kidney Int 69: 1471–1476, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheruvanky A, Zhou H, Pisitkun T, Kopp JB, Knepper MA, Yuen PS, Star RA: Rapid isolation of urinary exosomal biomarkers using a nanomembrane ultrafiltration concentrator. Am J Physiol Renal Physiol 292: F1657–F1661, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffert JD, Wang G, Pisitkun T, Shen RF, Knepper MA: An automated platform for analysis of phosphoproteomic datasets: Application to kidney collecting duct phosphoproteins. J Proteome Res 6: 3501–3509, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams RL, Urbé S: The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 8: 355–368, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Forgac M: Vacuolar ATPases: Rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8: 917–929, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Gluck SL, Underhill DM, Iyori M, Holliday LS, Kostrominova TY, Lee BS: Physiology and biochemistry of the kidney vacuolar H+-ATPase. Annu Rev Physiol 58: 427–445, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP: Renal vacuolar H+-ATPase. Physiol Rev 84: 1263–1314, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Simon DB, Karet FE, Hamdan JM, Di Pietro A, Sanjad SA, Lifton RP: Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet 13: 183–188, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Kleta R, Bockenhauer D: Bartter syndromes and other salt-losing tubulopathies. Nephron Physiol 104: 73–80, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Hoffert JD, Pisitkun T, Wang G, Shen RF, Knepper MA: Quantitative phosphoproteomics of vasopressin-sensitive renal cells: Regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci U S A 103: 7159–7164, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen WG, White FM: Proteomic analysis of cellular signaling. Expert Rev Proteomics 1: 343–354, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Beausoleil SA, Villén J, Gerber SA, Rush J, Gygi SP: A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol 24: 1285–1292, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Moriguchi T, Urushiyama S, Hisamoto N, Iemura S, Uchida S, Natsume T, Matsumoto K, Shibuya H: WNK1 regulates phosphorylation of cation-chloride-coupled cotransporters via the STE20-related kinases, SPAK and OSR1. J Biol Chem 280: 42635–42693, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Simon DB, Nelson-Williams C, Bia MJ, Ellison D, Karet FE, Molina AM, Vaara I, Iwata F, Cushner HM, Koolen M, Gainza FJ, Gitleman HJ, Lifton RP: Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet 12: 24–30, 1996 [DOI] [PubMed] [Google Scholar]
- 16.Kuwahara M, Fushimi K, Terada Y, Bai L, Marumo F, Sasaki S: cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenopus oocytes. J Biol Chem 270: 10384–10387, 1995 [DOI] [PubMed] [Google Scholar]
- 17.Gimenez I, Forbush B: Regulatory phosphorylation sites in the N-terminus of the renal Na-K-Cl cotransporter (NKCC2). Am J Physiol Renal Physiol 289: F1341–F1345, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Villén J, Beausoleil SA, Gerber SA, Gygi SP: Large-scale phosphorylation analysis of mouse liver. Proc Natl Acad Sci U S A 104: 1488–1493, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lees-Miller SP, Anderson CW: Two human 90-kDa heat shock proteins are phosphorylated in vivo at conserved serines that are phosphorylated in vitro by casein kinase II. J Biol Chem 264: 2431–2437, 1989 [PubMed] [Google Scholar]
- 20.Christensen B, Nielsen MS, Haselmann KF, Petersen TE, Sorensen ES: Post-translationally modified residues of native human osteopontin are located in clusters: Identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem J 390: 285–292, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoorn EJ, Pisitkun T, Zietse R, Gross P, Frokiaer J, Wang NS, Gonzales PA, Star RA, Knepper MA: Prospects for urinary proteomics: Exosomes as a source of urinary biomarkers. Nephrology (Carlton) 10: 283–290, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Gonzales P, Pisitkun T, Knepper MA: Urinary exosomes: Is there a future? Nephrol Dial Transplant 23: 1799–1801, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Pisitkun T, Johnstone R, Knepper MA: Discovery of urinary biomarkers. Mol Cell Proteomics 5: 1760–1771, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Pisitkun T, Hoffert JD, Yu MJ, Knepper MA: Tandem mass spectrometry in physiology. Physiology (Bethesda) 22: 390–400, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Finberg KE, Wagner CA, Bailey MA, Paunescu TG, Breton S, Brown D, Giebisch G, Geibel JP, Lifton RP: The B1-subunit of the H(+) ATPase is required for maximal urinary acidification. Proc Natl Acad Sci U S A 102: 13616–13621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, Green ED: Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 98: 4221–4226, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sadygov RG, Cociorva D, Yates JR 3rd: Large-scale database searching using tandem mass spectra: Looking up the answer in the back of the book. Nat Methods 1: 195–202, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Tanner S, Shu H, Frank A, Wang LC, Zandi E, Mumby M, Pevzner P, Bafna V: InsPecT: Identification of posttranslationally modified peptides from tandem mass spectra. Anal Chem 77: 4626–4639, 2005 [DOI] [PubMed] [Google Scholar]