Abstract
Depletion of podocytes, common to glomerular diseases in general, plays a role in the pathogenesis of glomerulosclerosis. Whether podocyte injury in adulthood can be repaired has not been established. Here, we demonstrate that in the adult human kidney, CD133+CD24+ cells consist of a hierarchical population of progenitors that are arranged in a precise sequence within Bowman's capsule and exhibit heterogeneous potential for differentiation and regeneration. Cells localized to the urinary pole that expressed CD133 and CD24, but not podocyte markers (CD133+CD24+PDX− cells), could regenerate both tubular cells and podocytes. In contrast, cells localized between the urinary pole and vascular pole that expressed both progenitor and podocytes markers (CD133+CD24+PDX+) could regenerate only podocytes. Finally, cells localized to the vascular pole did not exhibit progenitor markers, but displayed phenotypic features of differentiated podocytes (CD133−CD24−PDX+ cells). Injection of CD133+CD24+PDX− cells, but not CD133+CD24+PDX+ or CD133-CD24− cells, into mice with adriamycin-induced nephropathy reduced proteinuria and improved chronic glomerular damage, suggesting that CD133+CD24+PDX− cells could potentially treat glomerular disorders characterized by podocyte injury, proteinuria, and progressive glomerulosclerosis.
Glomerular diseases account for 90% of ESRD at a cost of $20 billion/yr in the United States.1 The traditional glomerular disease classification encompasses a bewildering array of descriptive pathologic entities and their clinical counterparts. However, converging evidence suggests that podocyte depletion, secondary to toxic, genetic, immune, infectious, oxidant, metabolic, hemodynamic, and other mechanisms, is a common determining factor that can result in a broad spectrum of clinical syndromes. As long as the podocyte loss is limited, restitution or repair is possible. By contrast, 20 to 40% podocyte loss results in a scarring response, until at greater than 60% podocyte loss glomeruli become globally sclerotic and nonfiltering. These stages of podocyte depletion are accompanied by corresponding degrees of proteinuria and, as an increasing proportion of glomeruli become involved, by measurable reduction in the clearance function of the kidney.1–3 To what extent podocytes can be replaced at all during adult life, and if so, how and at what rate, is still unclear. Indeed, mature podocytes have limited capacity to divide in situ and display all phenotypic and functional features of highly specialized, terminally differentiated cells.4,5 A potential mechanism for podocyte replacement might be stem cell migration from the bone marrow, as described in some models.6–8 However, it has been established that the developmental source of podocytes are resident renal progenitors.4,5,9,10
Recently, on the basis of CD24 expression, a surface molecule that has been used to identify different types of human stem cells,11–13 CD133 (a marker of hematopoietic and other types of adult tissue stem cells13,14), and the polycomb group protein Bmi-1 (a transcription factor that is critical for maintenance of stem cell self renewal15,16), we have identified a subset of cells in adult human kidneys that exhibit stem cell phenotypic and functional features and can regenerate tubular cells of different portions of the nephron, in vitro and in vivo.17–19 These CD133+CD24+ renal progenitors are physically located within Bowman's capsule, the only place in the kidney that appears to be contiguous with both tubular cells as well as glomerular podocytes. In this study, we investigated whether CD133+CD24+ renal progenitors can also regenerate podocytes through their division and migration along Bowman's capsule toward the glomerular tuft. We demonstrate here that CD133+CD24+ renal progenitors are a heterogeneous and hierarchical population of progenitor cells arranged in a precise sequence within the Bowman's capsule of adult human kidneys, which exhibit a heterogeneous differentiative and regenerative potential for either tubular renal cells or podocytes.
RESULTS
Heterogeneous Expression of Renal Progenitors and Podocyte Markers among Cells of Bowman's Capsule
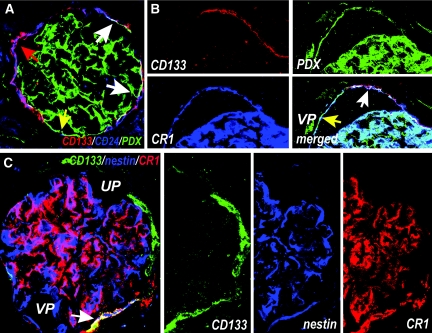
To evaluate the possible existence of a hierarchical relationship between CD133+CD24+ renal progenitors and podocytes, we first analyzed the expression of the renal progenitor markers CD133 and CD24; markers that specifically stain podocytes in the context of adult healthy human glomeruli, such as nestin;20 and complement receptor-1 (CR1)21 or podocalyxin (PDX), which stains podocytes and endothelial cells,22 in glomerular structures of adult human kidneys by using laser confocal microscopy. On the basis of the expression of CD133, CD24, PDX, nestin, and CR1, we demonstrated by confocal microscopy that CD133+CD24+ renal progenitors are a heterogeneous and hierarchical population of undifferentiated and more differentiated cells that are arranged in a precise sequence within Bowman's capsule (Figure 1). Three distinct populations of cells were identified. A subset of more undifferentiated cells expressing CD133 and CD24 in the absence of CR1, nestin, and PDX localized at the urinary pole of Bowman's capsule (Figure 1). A transition population expressing CD133 and CD24, as well as nestin, CR1, and PDX, usually localized between the urinary and the vascular pole (Figure 1). Finally, more differentiated cells expressing neither CD133 nor CD24, but exhibiting the podocyte markers, localized at the vascular pole of Bowman's capsule and were contiguous with fully differentiated podocytes (Figure 1). A list of the different markers of renal cells used in the study is given in Table 1.
Figure 1.
Heterogeneous expression of renal progenitors and podocytes markers by cells of Bowman's capsule in adult human kidney. (A) Triple-label immunofluorescence for CD133 (red), CD24 (blue), and PDX (green) showing co-expression of CD133 and CD24 on one subset (red arrow) and co-expression of CD133, CD24, and PDX on another subset of parietal epithelial cells in Bowman's capsule of a human kidney (white arrows). CD133−CD24−PDX+ cells are also visible (yellow arrow). Sectioning of the glomerulus does not allow its polarity to be established. Objective 20×, zoom 1.7. (B) Triple-label immunofluorescence for CD133, PDX, and CR1 showing a subset of CD133+ cells that co-express PDX and CR1 (white arrow), and more differentiated cells that co-express PDX and CR1 but lack CD133 (yellow arrow) and localize at the vascular pole (VP). Objective 20×, zoom 2.4. (C) Triple-label immunofluorescence for CD133 (green), nestin (blue), and CR1 (red) demonstrating the existence of cells expressing CD133 in absence of the podocyte markers nestin and CR1, which localize to the urinary pole (UP) of Bowman's capsule, and cells expressing CD133, nestin, and CR1 (white arrow), which localize between the urinary and the vascular pole (VP) of Bowman's capsule. Glomerular podocytes also appear as CD133−nestin+CR1+. Objective 20×, zoom 2.8.
Table 1.
Different markers of renal cells used in the study and their localization in adult healthy human kidneys
| Marker | Abbreviation | Cell Type(s) (Healthy Kidney) | Reference(s) |
|---|---|---|---|
| CD133 | CD133 | Renal progenitors, interstitial cells | 17,18 |
| CD24 | CD24 | Renal progenitors, distal tubules | 17,18 |
| Polycomb group protein | Bmi-1 | Renal progenitors, distal tubules | 16–18 |
| Podocalyxin | PDX | Podocytes, endothelial cells | 23 |
| Podocyte-specific PTPase | GLEPP-1 | Podocytes | 23 |
| Nestin | Nestin | Podocytes | 21,23 |
| Complement receptor-1 | CR1 | Podocytes, leukocytes | 22,23 |
| CD45 | CD45 | Leukocytes | 17,18 |
| CD31 | CD31 | Endothelial cells | 17,18 |
| Wilms’ tumor antigen | WT-1 | Podocytes | 17,18,23 |
| Lotus tetragonolobus | LTA | Proximal Tubules | 17,18 |
| Aminopeptidase A | APA | Proximal tubules | 17,18 |
| Na/Gluc1 cotransporter | NaGluc1 | Proximal tubules | 17,18 |
| γ-glutamyltransferase | γ-GT | Proximal convoluted tubules | 17,18 |
| Na/H exchanger | Na/H | Proximal tubule and apical membrane of thin and thick limbs of the loop of Henle | 17,18 |
| Aquaporin-1 | AQ1 | Proximal tubule and descending thin-limb of the loop of Henle | 17,18 |
| Aquaporin-3 | AQ3 | Connecting tubule, collecting ducts | 17,18 |
| Thiazide-sensitive Na/Cl transporter | NaCl | Distal convoluted tubule | 17,18 |
| Synaptopodin | SYN | Podocytes | 23 |
| Nephrin | nephrin | Podocytes | 23 |
| Podocin | podocin | Podocytes | 23 |
| HLA-1 | HLA-1 | All cells of human origin | 17–19 |
Identification of CD133+CD24+PDX−, CD133+CD24+PDX+, and CD133−CD24−PDX+ Cells in Adult Human Kidneys
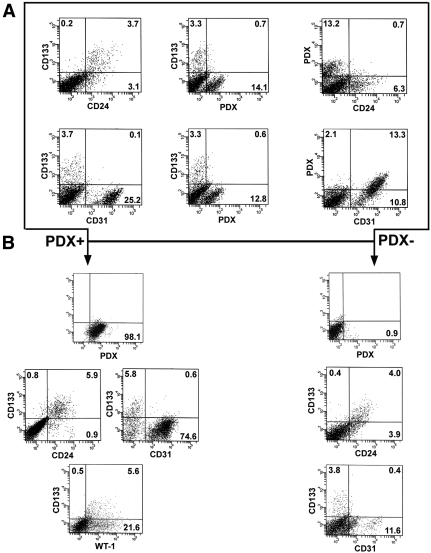
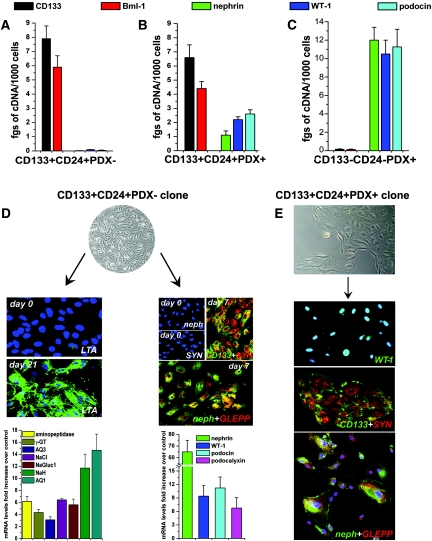
To recover each of the three populations, we took advantage of the surface expression by these cells of CD133 and/or PDX. PDX is considered a podocyte marker but, at least in human kidney, its expression has also been described in some parietal cells of Bowman's capsule,23 and on a subset of endothelial cells.22 Therefore, total renal cells were first depleted of CD45+ cells (leukocytes)17,18 and then analyzed for the contemporaneous expression of CD133, CD24, PDX, and CD31 (an endothelial cell marker) by triple-label immunofluorescence. FACS analysis demonstrated that CD133+CD24+ cells were 1 to 4% of total renal cells, as previously reported,17–19 whereas CD133+CD24+PDX+ cells were 0.2 to 0.7% of total renal cells. In addition, none of these two populations expressed the endothelial cell marker CD31.17,18 These results suggest that CD133+CD24+ cells contain a transition population co-expressing markers of both renal progenitors and podocytes (CD133+CD24+PDX+) and a more undifferentiated population that does not express PDX, in agreement with the findings obtained by confocal microscopy (Figure 2A). To provide further support to this possibility, we separated PDX+ and PDX− cells by immunomagnetic cell sorting, which allowed recovery of the two populations with a purity of more than 98% (Figure 2B). The assessment of renal progenitor markers on PDX+ cells revealed that a relevant percentage (4 to 8%) of these cells co-expressed CD133 and CD24. Moreover, CD133+CD24+PDX+ cells did not express CD31, whereas they consistently co-expressed the podocyte marker WT-1 (Figure 2B, left). These findings provide additional evidence for the existence of a rare, distinct population characterized by an intermediate phenotype between renal progenitors and differentiated podocytes. Of note, CD133+CD24+ cells also represented 1 to 5% of PDX- cells (Figure 2B, right). In previous studies, we demonstrated that the combined surface expression of CD133 and CD24 in healthy adult human kidneys is a selective property of renal progenitor cells of Bowman's capsule.17–19 Thus, CD133+CD24+PDX+, CD133+CD24+PDX−, and CD133−CD24−PDX+ populations were directly purified from total renal cell suspensions by means of immunomagnetic techniques and analyzed by real-time quantitative reverse transcription PCR (RT-PCR). CD133+CD24+PDX− cells expressed the renal progenitor markers CD133 and Bmi-1,17–19 but not the podocyte-specific markers nephrin, podocin, and Wilms’ tumor antigen-1 (WT-1). CD133+CD24+PDX+ cells expressed both renal progenitor and podocyte markers. CD133−CD24−PDX+ cells did not express renal progenitor markers but high levels of podocyte-specific transcripts (Figure 3, A through C). Taken together, these results suggest that CD133+CD24+ renal progenitors represent a heterogeneous population, including a subset of cells (CD133+CD24+PDX+) that displays initial signs of podocyte commitment.
Figure 2.
Identification of CD133+CD24+PDX−, CD133+CD24+PDX+, and CD133−CD24−PDX+ cells in adult human kidney cell suspensions. (A) After depletion of CD45+ cells, total renal cells were analyzed for the contemporaneous expression of CD133, CD24, PDX, and CD31 by triple-label immunofluorescence using FACS analysis, demonstrating that CD133+CD24+ cells represent 3.7%, whereas CD133+CD24+PDX+ cells represent 0.6 to 0.7% of total renal cells. Neither CD133+CD24+ cells nor CD133+CD24+PDX+ cells express the endothelial cell marker CD31. (B) Expression of CD133, CD24, CD31, and WT-1, as assessed by flow cytometry on PDX+ cells (left) and on PDX− cells (right). Purification of PDX+ cells confirms the existence of a population of PDX+ cells that co-express CD133 and CD24 but do not express CD31 and represent a subset of WT-1-expressing podocytes.
Figure 3.
Phenotypic characterization and distinct differentiative properties of clonal progenies of CD133+CD24+PDX− and CD133+CD24+PDX+ cells. Assessment of mRNA levels for CD133, Bmi-1, nephrin, WT-1, and podocin by real-time quantitative RT-PCR on (A) freshly isolated CD133+CD24+PDX− cells, (B) freshly isolated CD133+CD24+PDX+ cells, and (C) freshly isolated CD133−CD24−PDX+ cells. Data are mean ± SEM of triplicate assessment of one representative from independent experiments performed on sorted cells obtained from four different donors; fgs = femtograms. (D) Representative micrograph of clonally expanded CD133+CD24+PDX− cells, objective 10×. Left: Expression of tubular markers before (day 0) and after (day 21) culture in tubular differentiation medium was assessed by confocal microscopy analysis for LTA and by real-time quantitative RT-PCR comparison of multiple tubular specific markers before and after differentiation. Data are mean ± SEM of values obtained in 20 different clones. TO-PRO-3 counterstains nuclei (blue). Objective 20×, zoom 1.8. Right: Expression of podocyte markers before (day 0) and after (day 7) culture of CD133+CD24+PDX− clones in VRAD medium was assessed by confocal microscopy for CD133 (green), synaptopodin (red), nephrin (green), GLEPP (red), and by real-time quantitative RT-PCR comparison of nephrin, WT-1, podocin, and PDX mRNA levels before and after differentiation. TO-PRO-3 counterstains nuclei (blue). Objective 20×, zoom 0.9. (E) Representative micrograph of CD133+CD24+PDX+ cells (objective 20×) and staining for CD133 (green), synaptopodin (red), WT-1 (green), nephrin (green), and GLEPP-1 (red) as assessed by confocal microscopy. One representative of 20 distinct clones analyzed is shown. TO-PRO-3 counterstains nuclei (blue). Objective 20×, zoom 0.9.
Clonally Expanded CD133+CD24+PDX− Cells Generate In Vitro Tubular Cells and Podocytes, whereas CD133+CD24+PDX+ Cells Can Generate Only Podocytes
The functional characterization of the three populations provided definitive evidence that CD133+CD24+PDX− cells represent a multipotent population, whereas CD133+CD24+PDX+ cells represent transition cells showing features of podocyte progenitors and the CD133−CD24−PDX+ cells are fully differentiated podocytes. Indeed, CD133+CD24+PDX− cells could be cloned, maintained in culture, and expanded in a medium that allows the growth of undifferentiated renal progenitors [microvascular endothelial growth medium (EGM-MV)].17–19 More importantly, clonally expanded CD133+CD24+PDX− cells could not only be differentiated toward the tubular lineage if cultured in the tubular differentiating medium [renal epithelial cell growth medium (REGM+HGF)],17,18 but also toward the podocyte lineage if cultured in the podocyte-maintaining medium, VRAD (Vitamin D3, retinoic-acid-supplemented DMEM)24 (Figure 3D). Differentiation toward tubular cells resulted in the acquisition of binding properties of Lotus tetragonolobus agglutinin (LTA), a specific property of proximal tubular epithelia (Figure 3D). Real-time RT-PCR demonstrated strong upregulation of other markers of different portions of the nephron, such as aminopeptidase A and the Na/Gluc1 cotransporter, γ-glutamyltransferase, the Na/H exchanger, aquaporin-1, aquaporin-3, or the thiazide-sensitive Na/Cl transporter (Figure 3D). By contrast, when the same clones of CD133+CD24+PDX− cells were cultured in VRAD, they began to express the podocyte markers nephrin, WT-1, synaptopodin, podocin, PDX, and anti-glomerular epithelial protein 1 (GLEPP-1) at both mRNA and protein levels, as demonstrated by real-time RT-PCR and confocal microscopy, respectively. Differentiation of CD133+CD24+PDX− cells associated with a progressive downregulation of CD133 expression, as already reported.17 Of note, when single cell suspensions obtained from ten clones were recloned by limiting dilution, the resulting second-round subclones displayed the same capacity for multidifferentiation as the original clone, providing additional evidence that CD133+CD24+PDX− cells are indeed multipotent and exhibit self renewal in culture. On the other hand, CD133+CD24+PDX+ cells could not be expanded or cloned in EGM-MV, whereas if plated in VRAD medium they generated clones comprised of a maximum of 800 to 1000 cells that could never be subcloned, suggesting that these cells did not display self-renewal capacities. Confocal microscopy and real-time RT-PCR demonstrated that CD133+CD24+PDX+ clones showed the phenotype of transition cells co-expressing CD133, CD24, WT-1, synaptopodin, GLEPP-1, and nephrin (Figure 3E). Accordingly, clones could never be obtained from CD133+CD24+PDX+ cells in the presence of REGM+HGF medium, suggesting that these cells cannot generate tubular cells. Finally, CD133−CD24−PDX+ were unable to generate clones even in VRAD medium and survived in culture for only a few days, further confirming their nature as terminally differentiated cells. These findings suggest that CD133+CD24+PDX− cells in Bowman's capsule represent an uncommitted population of cells with extensive self-renewal potential that can generate both tubular cells and podocytes. By contrast, CD133+CD24+PDX+ cells do not display the potential to differentiate in tubular cells, suggesting that they represent a progenitor already committed toward the podocyte lineage.
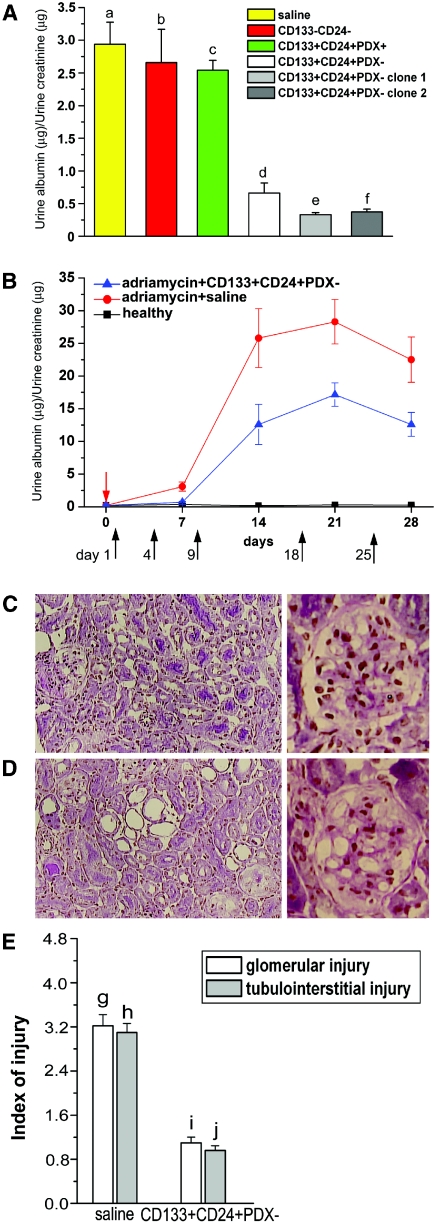
Only CD133+CD24+PDX− Cells Reduce Proteinuria and Improve Glomerular and Tubular Injury in Severe Combined Immunodeficiency Mice Suffering from Nephrotic Syndrome
The ability of CD133+CD24+PDX− and CD133+CD24+PDX+ cells to regenerate injured renal cells was then assessed in a model of adriamycin-induced renal injury, which resembles focal segmental glomerulosclerosis, a human disorder characterized by podocyte depletion and tubular damage.25 To this end, CD133+CD24+PDX−, CD133+CD24+PDX+ cells, or saline were injected into adriamycin-treated Severe Combined Immunodeficiency (SCID) mice. As an additional control, adriamycin-treated SCID mice were injected with a mixture of CD133−CD24− renal cells. Because persistent podocyte depletion induces proteinuria, urinary albumin/creatinine ratio levels were measured in all mice 7 d after adriamycin injection. Mice with adriamycin-induced nephropathy showed high urinary albumin/creatinine ratio levels that were unaffected by injection of saline, CD133−CD24− cells, or CD133+CD24+PDX+ cells (Figure 4A). By contrast, injection of CD133+CD24+PDX− cells strongly reduced proteinuria, as reflected by significantly lower urinary albumin/creatinine ratios (Figure 4A). Similar results were obtained when single clones of CD133+CD24+PDX− cells were used (Figure 4A). Because adriamycin-induced nephropathy is a chronic disorder characterized by persistent proteinuria and progressive glomerulosclerosis with tubulointerstitial injury, we also tested the effect of CD133+CD24+PDX− cell treatment over long periods of time. As shown in Figure 4B, treatment with repeated injections of CD133+CD24+PDX− cells significantly reduced urinary albumin/creatinine ratios at all time points analyzed. In chronically injured kidneys, the improvement of proteinuria induced by injection of CD133+CD24+PDX− cells was also associated with reduced glomerular and tubulointerstitial injury, as demonstrated in periodic acid-Schiff (PAS)-stained sections at day 28 (Figure 4, C through E). In saline-treated mice, glomerulosclerosis was significantly increased in association with reduction of glomerular surface area, whereas relative interstitial volume was expanded. Moreover, the degree of tubular atrophy, as characterized by a decrease in the height of tubular epithelial cells, loss of brush border, and vacuolization, was aggravated in the saline-treated group as compared with CD133+CD24+PDX− cells at day 28 after adriamycin injection (Figure 4, C through E).
Figure 4.
CD133+CD24+PDX− cells, but not CD133+CD24+PDX+ or CD133-CD24− cells, reduce proteinuria and improve glomerular and tubulointerstitial injury in SCID mice affected by adriamycin-induced nephropathy. (A) Albumin/creatinine ratio as measured at day 7 in adriamycin-treated mice that received saline (n = 12), CD133−CD24− (n = 6), CD133+CD24+PDX+ (n = 6), and CD133+CD24+PDX− (n = 12) bulk of cells or two representative clones of CD133+CD24+PDX− cells (n = 3 for each clone). Data are expressed as mean ± SEM. a versus b, a versus c, b versus c, d versus e, d versus f, e versus f, NS; a versus d, b versus d, c versus d, P < 0.001; a versus e, a versus f, P < 0.01; b versus e, b versus f, c versus e, c versus f, P < 0.05. (B) Time course experiments of albumin/creatinine ratio as measured in healthy (black square) or adriamycin-treated mice that received saline (red circle) or CD133+CD24+PDX− cells (blue triangle). Red arrow points to the day of adriamycin injection. Black arrows point to the days of CD133+CD24+PDX− cell injection. Data are expressed as mean ± SEM. (n = 12 mice at each time point for each group of treatment). P < 0.0001 between the two groups of treatment (ANOVA for multiple comparisons). (C) Left: PAS staining of renal cortical sections of mice with adriamycin-induced nephropathy treated with CD133+CD24+PDX− cells; objective 20×. Right: High-power magnification of a glomerulus; objective 40×. (D) Left: PAS staining of renal cortical sections of mice with adriamycin-induced nephropathy treated with saline.; objective 20×. Right: High-power magnification of a glomerulus; objective 40×. (E) Quantitation of glomerular and tubulointerstitial injuries in renal cortical sections of saline-treated and CD133+CD24+PDX− treated mice after the induction of adriamycin nephropathy. Data are expressed as mean ± SEM. g versus i and h versus j, P < 0.001.
CD133+CD24+PDX− and CD133+CD24+PDX+ Cells Exhibit Different Regenerative and Differentiative Potential in SCID Mice Suffering from Nephrotic Syndrome
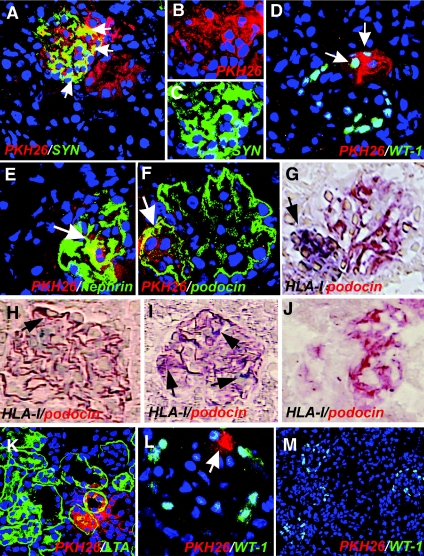
To further investigate their ability to regenerate injured podocytes and tubular cells, CD133+CD24+PDX−, CD133+CD24+PDX+, and CD133−CD24− renal cells were labeled with the red fluorescent dye PKH26 before their injection into adriamycin-treated SCID mice. Labeled CD133+CD24+PDX− cells localized to glomerular structures, where they acquired the podocyte-specific markers synaptopodin, WT-1, nephrin, and podocin (Figure 5, A through F). In addition, double-label immunohistochemistry for human HLA-I antigen and podocin confirmed the engraftment of CD133+CD24+PDX− cells into the glomerular structures (Figure 5, G through I). Furthermore, relevant numbers of labeled CD133+CD24+PDX− cells were also observed in tubular structures labeled with LTA (Figure 5K). Identical results were obtained when single clones of CD133+CD24+PDX− cells were used. By contrast, when labeled CD133+CD24+PDX+ cells were injected, only rare red-labeled podocytes were observed (Figure 5L). Finally, red labeling was never observed in mice injected with CD133−CD24− renal cells (Figure 5M), with saline solution (Figure 5J), or in healthy mice injected with CD133+CD24+PDX− cells (Supplementary Figure 1). Quantitation of the number of PKH26-positive cells expressing markers of differentiated podocytes or tubular cells was performed on sections stained with podocin or LTA, respectively. On day 7 after injury, the number of cells that showed PKH26 labeling was equal to 11.08 ± 3.3% of all podocytes, and to 7.5 ± 1.9% of all proximal tubular cells in mice treated with CD133+CD24+PDX− cells, whereas no tubular cells and only 0.8 ± 0.4% of all podocytes were replaced by CD133+CD24+PDX+ cells. After 45 d the number of CD133+CD24+PDX− cells engrafted in the kidney of mice with adriamycin-induced nephropathy remained similar (Supplementary Figure 2), thus confirming the different regenerative and differentiative potential of CD133+CD24+PDX− and CD133+CD24+PDX+ cells.
Figure 5.
Distinct regenerative potential of CD133+CD24+PDX−, CD133+CD24+PDX+, or CD133−CD24− cells for podocytes and tubular cells in SCID mice affected by adriamycin-induced nephropathy. (A) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with synaptopodin (SYN, green), which demonstrates engraftment of CD133+CD24+PDX− cells in both glomerular and tubular structures and differentiation toward podocytes and tubular cells at day 7. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.4. (B) High magnification of the split image showed in panel a detailing PKH26 labeling in glomerular cells. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.7. (C) High magnification of the split image showed in panel a, detailing synaptopodyn (SYN) labeling in glomerular cells. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.7. (D) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with WT-1 (green), which demonstrates engraftment of CD133+CD24+PDX− cells and tubular structures and differentiation toward podocytes (arrow) and tubular cells at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.2. (E) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with nephrin (green), which demonstrates engraftment of CD133+CD24+PDX− cells in glomerular structures and differentiation toward podocytes (arrow) at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.7. (F) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with podocin (green), which demonstrates engraftment of CD133+CD24+PDX− cells in glomerular structures and differentiation toward podocytes (arrow) at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.8. (G) Double-label immunohistochemistry for podocin (AEC, red) and HLA-I human antigen (vector SG, dark blue) in a section adjacent to panel f from kidneys of SCID mice with adriamycin-induced nephropathy treated with CD133+CD24+PDX− cells, which confirms that the PKH26-labeled cells shown in panel f also exhibit HLA-I immunostaining (arrow) at day 28. Objective 40×. (H) Double-label immunohistochemistry for podocin (AEC, red) and HLA-I human antigen (vector SG, dark blue) in kidneys of SCID mice with adriamycin-induced nephropathy treated with CD133+CD24+PDX− cells, which demonstrates engraftment of a CD133+CD24+PDX− cell in a glomerulus and its differentiation toward a podocyte (arrow) at day 28. Objective 40×. (I) Double-label immunohistochemistry for podocin (AEC, red) and HLA-I human antigen (vector SG, dark blue) in kidneys of SCID mice with adriamycin-induced nephropathy treated with CD133+CD24+PDX− cells, which demonstrates engraftment of several CD133+CD24+PDX− cells and their differentiation toward podocytes (arrows) at day 28. Objective 40×. (J) Double-label immunohistochemistry for podocin (AEC, red) and HLA-I human antigen (vector SG, dark blue) in kidneys of SCID mice with adriamycin-induced nephropathy treated with saline, which shows absence of HLA-I immunostaining at day 28. Objective 40×. (K) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX− cells (red) and stained with LTA (green), which demonstrates engraftment of CD133+CD24+PDX− cells in proximal tubular structures and differentiation toward tubular cells at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×. (L) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133+CD24+PDX+ cells (red) and stained with WT-1 (green), which demonstrates rare CD133+CD24+PDX+ cells in glomerular structures at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 40×, zoom 1.7. (M) Representative micrograph of kidney sections of mice with adriamycin-induced nephropathy treated with PKH26-labeled CD133−CD24− cells (red) and stained with WT-1 (green), which demonstrates the absence of CD133−CD24− cells in glomerular or tubular structures at day 28. TO-PRO-3 counterstains nuclei (blue). Objective 20×.
DISCUSSION
Recent insights have defined a unified concept of glomerular diseases in which podocyte injury or loss is a common determining factor, which suggests the need for rational clinical efforts to allow podocyte preservation.1,3 Mature podocytes are postmitotic cells that can undergo DNA synthesis to a limited degree but do not proliferate because they arrest in the G2/M phase of the cell cycle.1,3 However, in most adult epithelia, replacement of damaged or dead cells is maintained through the presence of stem/progenitor cells.26 Unless the epithelial stem/progenitor cells are permanently damaged, most epithelia are able to repair their tissues after injuries.26 Although glomerular disorders represent the most prominent cause of ESRD, remission of the disease and regression of renal lesions have been observed in experimental animals and even in humans.27 This shows that remodeling of glomerular architecture is possible, which would imply regeneration of the injured podocytes and reconstitution of the glomerular tuft. However, the inability of the podocyte to proliferate and replace injured cells suggests the existence of potential stem/progenitor cells within the adult glomerulus.
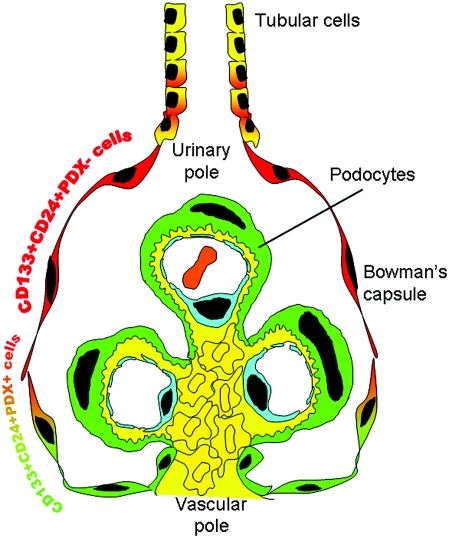
In this study, we provide the first evidence that podocytes can be regenerated from a resident population of renal progenitors localized within the parietal epithelium of Bowman's capsule of the human renal glomerulus. On the basis of expression of the renal progenitor markers CD133 and CD24, as well as of the podocyte markers PDX, nestin, synaptopodin, and CR1, we demonstrated by confocal microscopy and FACS analysis that CD133+CD24+ renal progenitors are a heterogeneous and hierarchical population of undifferentiated and more differentiated cells that are arranged in a precise sequence within Bowman's capsule. A subset of more undifferentiated cells expressing renal progenitor markers in the absence of podocyte markers localized at the urinary pole of Bowman's capsule. As demonstrated by clonal analysis, these cells could act as bipotent progenitors for both tubular cells and podocytes in vitro and in vivo and exhibited self-renewal potential. A transition population expressing both renal progenitors and podocyte markers was localized between the urinary and the vascular pole of Bowman's capsule and exhibited differentiative properties only toward the podocyte lineage and a limited potential of clonogenicity and amplification (Figure 6). Finally, more differentiated cells, that did not express renal progenitor markers but exhibited high levels of the podocyte specific markers localized at the vascular pole of Bowman's capsule, contiguous to podocytes. These cells shared with podocytes all of the properties of postmitotic cells and could not be cloned or amplified in culture, consistent with their nature as terminally differentiated podocytes, thus showing agreement with previous studies that there are capsular parietal cells localized at the vascular pole of the glomerulus analogous in size, shape, and phenotype to visceral podocytes, but whose function and role were unknown.23,28,29 The discovery that CD133+CD24+ renal progenitors represent a potential source for podocyte replacement provides the basis for a novel concept that podocyte injuries can be repaired in principle by a resident stem cell compartment.4 In addition, the results of this study provide an intriguing explanation for the genesis of crescents and pseudocrescents, which are known to reflect uncontrolled proliferation of parietal epithelial cells in response to injury.30,31 Indeed, it is tempting to speculate that CD133+CD24+ renal progenitors proliferate in an attempt to replace injured podocytes, but if regeneration occurs in a dysregulated manner it can generate hyperplastic lesions that can lead to renal progenitor depletion, scar formation, and nephron loss.
Figure 6.
Schematic representation of the hierarchical distribution of CD133+CD24+PDX− and CD133+CD24+PDX+ cells within human glomeruli. CD133+CD24+PDX− renal progenitors (red) are localized at the urinary pole in close contiguity with tubular renal cells (yellow). A transitional cell population (CD133+CD24+PDX+, red/green) displays features of either renal progenitors (red) or podocytes (green) and localizes between the urinary pole and the vascular pole. At the vascular pole of the glomerulus, the transitional cells are localized in close continuity with cells that lack CD133 and CD24, but exhibit the podocyte markers and the phenotypic features of differentiated podocytes (green).
Finally, the demonstration that CD133+CD24+ renal progenitors can regenerate injured podocytes suggests that these cells might provide a novel tool for cell therapy of proteinuric renal disorders. However, only CD133+CD24+PDX− cells displayed the potential to regenerate podocytes and tubular cells and functionally improve glomerular injury, which suggests that these cells can behave as bipotent progenitors. By contrast, CD133+CD24+PDX+ cells did not induce improvement of glomerular function and rarely generated podocytes, suggesting that these cells display a very limited engraftment capacity in agreement with their lack of self-renewal potential. Finally, CD133−CD24− cells did not engraft within injured human kidneys at all and did not induce functional improvement of glomerular injury, which is consistent with their nature of terminally differentiated cells. Taken together, these results suggest that CD133+CD24+PDX− renal progenitors may be ideal for stem-cell-based kidney regeneration because of their broad differentiation potential, which allows replacement of both podocytes and tubular cells because of their inherent organ-specific identity. Although we cannot exclude the possibility that the engraftment observed might at least in part be related to cell fusion and exchange of PKH26 dye between cells, the observation that CD133+CD24+PDX− renal progenitors reduce proteinuria is of potential clinical utility. Indeed, several studies have examined the possibility that bone-marrow-derived stem cells might be used for renal repair.32–36 However, their beneficial role can be offset by their abnormal local differentiation into adipocytes accompanied by glomerular sclerosis.33 In conclusion, the results of this study provide the first demonstration that glomerular injury can be repaired by using resident renal progenitor cells and suggest that the kidney might contain a “renopoietic system” (Figure 6) with a bipotent progenitor localized at the urinary pole of Bowman's capsule, where it can initiate the replacement and regeneration of glomerular and tubular epithelial cells.
CONCISE METHODS
Antibodies
The following antibodies were used: anti-CD24 mAb (SN3), anti-WT-1 mAb (F6), and anti-nephrin mAb (C17) (Santa Cruz Biotechnology, Santa Cruz, California); anti-human HLA-I mAb (W6/32) (Sigma-Aldrich, Saint Louis, Missouri); anti-CD133/2 mAb (293C3) and PE-conjugated anti CD133/2 mAb (293C3) (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany); anti-synaptopodin mAb (G1D4) (Progen, Heidelberg, Germany); anti-podocin pAb (Alpha-Diagnostic, San Antonio, Texas); anti-nestin pAb (Chemicon, Temecula, California); anti-CD31 mAb (WM-59), anti-CR1 mAb (E11), PE-conjugated mouse anti-IgG2b (MPC-11), and anti-IgG1 (E11) (BD Biosciences, San Diego, California); anti-PDX mAb (222328) and PE-conjugated anti-PDX mAb (222328) (R&D Systems, Minneapolis, Minnesota); anti-GLEPP-1 mAb (5C11) (BioGenex, San Ramon, California); and anti IgG2a (HOPC-1) and PE-conjugated goat anti rat IgG (H+L) (Southern Biotech, Birmingham, Alabama). Alexa Fluor 633-labeled goat anti-mouse IgG1, Alexa Fluor 488-labeled goat anti-mouse IgG2a, Alexa Fluor 488-labeled goat anti-mouse IgG1, Alexa Fluor 488-labeled goat anti-rabbit IgG, Alexa Fluor 546-labeled goat anti-mouse IgG2b, and Alexa Fluor 546-labeled goat anti-mouse IgG2a were from Molecular Probes (Eugene, Oregon).
Tissues
Normal kidney fragments were obtained from 15 patients who underwent nephrectomy because of renal tumors, in accordance with the recommendations of the Ethical Committee of the Azienda Ospedaliero-Universitaria Careggi in Florence, Italy.
Confocal Microscopy
Confocal microscopy was performed on 5-μm sections of frozen renal tissues, or on cells cultured on chamber slides by using a LSM510 META laser confocal microscope (Carl Zeiss, Jena, Germany), as described.37 Staining with FITC-labeled LTA (Vector Laboratories, Burlingame, California) was performed as described.17
Immunomagnetic Cell Sorting and Flow Cytometry
To obtain PDX+ and PDX− cells, cortex from normal kidney fragments was minced and digested with collagenase IV (750 U/ml; Sigma) for 45 min at 37°C, then depleted of leukocytes using anti-CD45 MicroBeads (Miltenyi), as described previously.17,18 The CD45− fraction was collected and analyzed by flow cytometry. PDX+ and PDX− cells were isolated by incubating the CD45− fraction with anti-PDX mAb (R&D Systems) and using rat anti-mouse IgG2a+b MicroBeads (Miltenyi) as the secondary antibody. The separation was performed by magnetic cell sorting using LS columns (Miltenyi), and the efficiency of separation was evaluated by cell labeling with goat anti-rat IgG (H+L) PE (Southern Biotech). The recovered fractions were used for flow cytometric analysis. Flow cytometric analysis of surface molecules was performed as described.17,18 To assess the expression of cytoplasmic WT-1, after incubation with anti-CD133/2 mAb, cells were fixed for 15 min with formaldehyde (2% in PBS), permeabilized with PBS containing 0.5% BSA and 0.5% saponin, and then incubated with the specific mAbs. Each area of positivity was determined by gating on the same cells stained with isotype-matched mAbs. A total of 104 events for each sample was acquired. Because the combined surface expression of CD133 and CD24 in healthy adult human kidneys is a selective property of renal progenitors cells of Bowman's capsule,17–19 CD133+CD24+PDX+, CD133+CD24+PDX−, and CD133−CD24−PDX+ populations were directly recovered from total renal cell suspensions. To recover CD133+CD24+PDX+, CD133+CD24+PDX−, and CD133−CD24−PDX+ populations, total renal cells were first depleted of CD45 and CD31 and then labeled with PE-conjugated anti-PDX mAb, followed by magnetic labeling with anti-PE Multisort microbeads using the anti-PE Multisort Kit (Miltenyi), according to the manufacturer's instructions. To obtain CD133+CD24+PDX+ and CD133−CD24−PDX+ cells, magnetic beads were removed by using Multisort release reagent, and cells were treated with a second magnetic separation for CD133 (CD133 Cell Isolation Kit, containing the anti-CD133/1 mAb, clone AC133, also used for hematopoietic stem cell sorting). By contrast, to obtain CD133+CD24+PDX− cells, PDX− cells were directly treated with a second magnetic separation for CD133. The purified cell fractions consisted of more than 98% of CD133+CD24+PDX+, CD133+CD24+PDX−, or CD133−CD24−PDX+ cells. To recover CD133−CD24− cells, total renal cells depleted of CD45 and CD31 were also sequentially depleted of CD133 and CD24 by magnetic cell sorting. The purified cell fractions consisted of more than 99% of CD133−CD24− cells.
Cell Cultures and In Vitro Differentiation
Cells were plated in EGM-MV (Lonza Ltd., Basel, Switzerland) with 20% FBS (Hyclone, Logan, Utah) or in VRAD medium24 containing DMEM-F12 (Sigma) supplemented with 10% FBS, vitamin D3 100 nM (Sigma), and all-trans retinoic acid (100 μM; Sigma). Generation of clones was achieved by limiting dilution in 96-well plates and in four-chamber glass slides (VWR International, West Chester, Pennsylvania). Tubulogenic differentiation was induced as described elsewhere with REGM (Lonza Ltd.).17,18 For podocyte differentiation, cells were treated for 3–7 d with VRAD medium.24
Real-Time Quantitative RT-PCR
Taq-Man RT-PCR was performed as described.37,38 CD133, Bmi-1, WT-1, nephrin, podocin, PDX, Na/Cl transporter, Na/Gluc1 cotransporter, aminopeptidase A, γ-glutamyltransferase, Na/H exchanger, aquaporin-1, and aquaporin-3 quantification was performed using Assay on Demand kits (Applied Biosystems, Warrington, United Kingdom).
Immunohistochemistry
Double immunohistochemistry for HLA-I and podocin was performed as detailed elsewhere.17,18,39 Briefly, after a 30-min preincubation with normal horse serum (Vectastain ABC kit), sections were layered for 30 min with anti-human-podocin pAb, followed by biotinylated anti-rabbit IgG horse antibody, and the avidin-biotin-peroxidase complex (Vectastain ABC kit), and 3-amino-9-ethylcarbazole (AEC) (red color) as peroxidase substrate. Sections were subsequently exposed to anti-HLA-I mAb (Sigma), followed by biotinylated anti-mouse IgG horse antibody and the avidin-biotin-peroxidase complex (Vectastain ABC kit). Vector SG (bluish-gray color, Vector Laboratories) was used as a chromogen. Colocalization of AEC and vector SG in the same cell resulted in a final purple/dark blue color.
Xenograft in SCID Mice Model of Adriamycin Nephropathy
Adriamycin nephropathy was induced in female SCID mice (Harlan, Udine, Italy) at the age of 6 wk by a single intravenous injection of adriamycin (doxorubicin hydrochloride, 6 mg/kg in PBS, Sigma) on day 0 in the tail vein. Animal experiments were performed in accordance with institutional, regional, and state guidelines and in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. On day 1, and again on days 4, 9, 18, and 25 after adriamycin injection, two groups of mice received intravenous administration as follows: group 1, saline (n = 60 mice); and group 2, PKH26-labeled CD133+CD24+PDX− cells (n = 60 mice; 0.75 × 106 cells/d). Twelve mice were killed at each time point after adriamycin injection (day 7, 14, 21, 28, and 45) for each group.
Additional groups of mice were treated with saline (n = 12), CD133−CD24− (n = 6), CD133+CD24+PDX+ (n = 6), CD133+CD24+PDX− (n = 12), or with clonally expanded PKH26-labeled CD133+CD24+PDX− cells (n = 3 mice for each clone) (0.75 × 106 cells/d at day 1 and 4 after adriamycin injection). A total number of five distinct clones obtained from three different donors was used. Mice were killed at day 7 after adriamycin injection. All of the organs of the mice were examined for cells trapping or engraftment. After injection, a limited number of cells were entrapped in the lung, whereas no cells were observed in the other organs.
As an additional control, PKH26-labeled CD133+CD24+PDX− cells were injected in healthy mice (n = 6 mice; 0.75 × 106 cells/d on day 1, and again on days 4, 9, 18, and 25). Mice were killed at day 28.
In all mice, urinary albumin and creatinine in 24-h urine were determined with Albuwell M kit (Exocell, Philadelphia, Pennsylvania) and Creatinine Assay kit (Cayman Chemical, Ann Arbor, Michigan). Normal range of urinary albumin or creatinine in our experiments was calculated in eight additional untreated mice per day. Kidneys were collected from all animals.
Analysis of Renal Morphology
For analysis of mouse renal morphology, kidney sections of 5-μm thickness of animals killed at day 28 were fixed in ethanol and stained with PAS reagent (Carlo Erba, Milan, Italy). Twenty high-power fields (400×) of renal cortex were randomly selected for assessing tubular (atrophy, casts, and vacuolization) and interstitial changes (fibrosis and inflammation) and graded from 0 to 5 (tubulointerstitial area in the cortex was graded as follows: 0, normal; 1, area of interstitial inflammation and fibrosis, tubular atrophy, and vacuolization involving <10%; 2, lesion area between 10 and 20%; 3, lesion area between 20 and 30%; 4, lesion area between 30 and 40%; and 5, lesions involving >40% of the field). Fifty randomly selected glomeruli were assessed for glomerular damage (well developed exudative, mesangial proliferation and glomeruli hypertrophy) and graded as follows: 0, normal; 1, slight glomerular damage of the mesangial matrix and/or hyalinosis with focal adhesion involving <10% of the glomerulus; 2, sclerosis of 10 to 20%; 3, sclerosis of 20 to 30%; 4, sclerosis of 30 to 40%; and 5, sclerosis >40% of the glomerulus. All scoring was performed in a blinded manner.
Statistical Analysis
The results were expressed as mean ± SEM. Comparison between groups was performed by the Mann-Whitney test or by ANOVA for multiple comparisons (ANOVA for repeated measures), as appropriate. P < 0.05 was considered to be statistically significant.
DISCLOSURES
None.
Acknowledgments
The research leading to these results has received funding from the European Community under the European Community's Seventh Framework Programme (FP7/2007-2013), grant number 223007, and from the European Research Council Starting Grant under the European Community's Seventh Framework Programme (FP7/2007-2013), ERC grant number 205027. This study was supported by the Tuscany Ministry of Health, the Ministero dell’Istruzione, dell’Universita′ e della Ricerca (MIUR), the Foundation of Ente Cassa di Risparmio di Firenze, and the Associazione Italiana per la Ricerca sul Cancro. E. Parente is a recipient of a Fondazione Italiana per la Ricerca sul Cancro fellowship. E. Ronconi and C. Sagrinati contributed equally to this work.
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information is available for this article online at http://www.jasn.org/.
See related editorial, “Parietal Epithelial Cells Regenerate Podocytes,” on pages 231–233.
REFERENCES
- 1.Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 [DOI] [PubMed] [Google Scholar]
- 2.D'Agati VD: Podocyte injury in focal segmental glomerulosclerosis: Lessons from animal models (a play in five acts). Kidney Int 73: 399–406, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Hozman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Shankland SJ: The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT: Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells 24: 2448–2455, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto H, Mundel TM, Sund M, Xie L, Cosgrove D, Kalluri R: Bone-marrow-derived stem cells repair basement membrane collagen defects and reverse genetic kidney disease. Proc Natl Acad Sci U S A 103: 7321–7326, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker JU, Hoerning A, Schmid KW, Hoyer PF: Immigrating progenitor cells contribute to human podocyte turnover. Kidney Int 72: 1468–1473, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Kreidberg JA: Podocyte differentiation and glomerulogenesis. J Am Soc Nephrol 14: 806–814, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV: Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell 2: 284–291, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Shackleton M, Vaillant F, Simpson KJ, Stingl J, Smyth GK, Asselin-Labat ML, Wu L, Lindeman GJ, Visvader JE: Generation of a functional mammary gland from a single stem cell. Nature 439: 84–88, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Kubota H, Avarbock MR, Brinster RL: Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A 100: 6487–6492, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, Biancotti JC, Hutnick L, Krueger RC Jr., Fan G, de Vellis J, Sun YE: CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci U S A 105: 1026–1031, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shmelkov SV, St Clair R, Lyden D, Rafii S: AC133/CD133/Prominin-1. Int J Biochem Cell Biol 37: 715–719, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ: BmI-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature 425: 962–967, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozakowski N, Soleiman A, Pammer J: BMI-1 expression is inversely correlated with the grading of renal clear cell carcinoma. Pathol Oncol Res 14: 9–13, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P: Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. J Am Soc Nephrol 17: 2443–2456, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P: Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 18: 3128–3138, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML, Parente E, Mancina R, Netti GS, Becherucci F, Gacci M, Carini M, Gesualdo L, Rotondi M, Maggi E, Lasagni L, Serio M, Romagnani S, Romagnani P: Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med 205: 479–490, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perry J, Ho M, Viero S, Zheng K, Jacobs R, Thorner PS: The intermediate filament nestin is highly expressed in normal human podocytes and podocytes in glomerular disease. Pediatr Dev Pathol 10: 369–382, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Barisoni L, Kriz W, Mundel P, D'Agati V: The dysregulated podocyte phenotype: A novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Horvat R, Hovorka A, Dekan G, Poczewski H, Kerjaschki D: Endothelial cell membranes contain PDX—the major sialoprotein of visceral glomerular epithelial cells. J Cell Biol 102: 484–491, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bariety J, Mandet C, Hill GS, Bruneval P: Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Takano Y, Yamauchi K, Hiramatsu N, Kasai A, Hayakawa K, Yokouchi M, Yao J, Kitamura M: Recovery and maintenance of nephrin expression in cultured podocytes and identification of HGF as a repressor of nephrin. Am J Physiol Renal Physiol 292: F1573–F1582, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases—Insights from animal models. Kidney Int 67: 404–419, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Blanpain C, Horsley V, Fuchs E: Epithelial stem cells: Turning over new leaves. Cell 128: 445–458, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remuzzi G, Benigni A, Remuzzi A: Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest 116: 288–296, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly G, Downie I, Gardiner DS, More IA, Lindop GB: The peripolar cell: A distinctive cell type in the mammalian glomerulus. Morphological evidence from a study of sheep. J Anat 168: 217–227, 1990 [PMC free article] [PubMed] [Google Scholar]
- 29.Thumwood CM, McCausland J, Alcorn D, Ryan GB: Scanning and transmission electron-microscopic study of peripolar cells in the newborn lamb kidney. Cell Tissue Res 274: 597–604, 1993 [DOI] [PubMed] [Google Scholar]
- 30.Smeets B, Dijkman HB, Wetzels JF, Steenbergen EJ: Lessons from studies on focal segmental glomerulosclerosis: An important role for parietal epithelial cells? J Pathol 210: 263–272, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Le Hir M, Keller C, Eschmann V, Hähnel B, Hosser H, Kriz W: Podocyte bridges between the tuft and Bowman's capsule: An early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol 12: 2060–2071, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Humphreys BD, Bonventre JV: Mesenchymal stem cells in acute kidney injury. Annu Rev Med 59: 311–325, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L, Milovanceva-Popovska M, Kerjaschki D, Floege J: Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol 18: 1754–1764, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Sagrinati C, Ronconi E, Lazzeri E, Lasagni L, Romagnani P: Stem-cell approaches for kidney repair: Choosing the right cells. Trends Mol Med 14: 277–285, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Lin F, Moran A, Igarashi P: Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in post ischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 115: 1743–1755, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romagnani P, Annunziato F, Liotta F, Lazzeri E, Mazzinghi B, Frosali F, Cosmi L, Maggi L, Lasagni L, Scheffold A, Kruger M, Dimmeler S, Marra F, Gensini G, Maggi E, Romagnani S: CD14+CD34 low cells with stem cell phenotypic and functional features are the major source of circulating endothelial progenitors. Circ Res 97: 314–322, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Lasagni L, Francalanci M, Annunziato F, Lazzeri E, Giannini S, Cosmi L, Sagrinati C, Mazzinghi B, Orlando C, Maggi E, Marra F, Romagnani S, Serio M, Romagnani P: An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J Exp Med 197: 1537–1549, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Romagnani P, Annunziato F, Lasagni L, Lazzeri E, Beltrame C, Francalanci M, Uguccioni M, Galli G, Cosmi L, Maurenzig L, Baggiolini M, Maggi E, Romagnani S, Serio M: Cell cycle-dependent expression of CXC chemokine receptor 3 by endothelial cells mediates angiostatic activity. J Clin Invest 107: 53–63, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]