Abstract
Measurement of urinary albumin excretion (UAE) in a 24-h collection is the gold standard method to determine the presence of microalbuminuria. We sought to compare more practical alternatives—measurement of urinary albumin concentration (UAC) or albumin:creatinine ratio (ACR)—in a first morning void or in a spot urine sample with this gold standard. We asked 241 participants of a prospective cohort study to make three 24-h urine collections, a first morning void, and a spot urine sample. Regression analysis showed that the ACR in a first morning void best agreed with 24-h UAE. The prevalence of microalbuminuria determined by data from a first morning void (7.5%, whether by UAC or ACR) nearly equaled the prevalence of microalbuminuria determined by 24-h UAE (10.0%), whereas the prevalence was higher when determined by spot urine samples (25.4% for UAC and 22.4% for ACR; both P < 0.001 versus 24-h UAE). The intraindividual coefficients of variation of the ACR in a first morning void and 24-h UAE were similar (19%). Intraindividual coefficients of variations of all other measurements of albuminuria were significantly greater. In conclusion, measurement of albuminuria in a first morning void, preferably as the ACR, is more reliable than a spot urine sample to diagnose and monitor microalbuminuria.
Microalbuminuria has been established as a valuable risk marker for renal and cardiovascular complications. Consequently, treatment guidelines from various medical societies have recommended assessment of urinary albumin in patients with hypertension or diabetes and even in the general population to identify individuals at increased risk for renal and cardiovascular disease.1–4
Various methods for urine collection are used in clinical practice to determine presence of microalbuminuria. Because urinary albumin excretion (UAE) follows a circadian rhythm, the amount of albumin excreted in urine during a 24-h period has been considered the “gold standard”; however, 24-h urine collection is a cumbersome procedure. More practical and easier alternatives are collection of a first morning void or a spot (random) urine sample. It has been suggested that a first morning void (collection of the first urine void after the individual awakes from sleep) is to be preferred over a spot urine sample (daytime random sample), because the former is less influenced by factors such as hydration status and physical activity, reducing the variability that is caused by these factors.5 From a practical point of view, however, spot urine samples are preferred because they can be collected during consultation at the doctor's office and therefore pose the least inconvenience for individuals.
As yet, only one study has compared the validity of using a first morning void or a spot urine sample as a substitute for a 24-h urine collection to diagnose microalbuminuria and to monitor albuminuria over time6; however, this study included only 11 patients, limiting the validity of the results. The aim of this study, therefore, was to investigate whether albuminuria measures derived from a first morning void or a spot urine sample could be used as an alternative to a 24-h UAE to diagnose microalbuminuria and to monitor albuminuria over time.
RESULTS
Study Population
A total of 241 individuals were included in the study. Table 1 describes the characteristics of the overall study population and stratified by gender. In the overall population, the median urinary albumin concentration (UAC) derived from a spot urine sample was almost twice as high as UAC in a 24-h collection (11.5 versus 6.5 mg/L, respectively; P < 0.001), whereas UAC in a first morning void was numerically nearly similar to the value in 24-h urine collections, although this latter difference also reached statistical significance (6.1 versus 6.5 mg/L; P = 0.013). The median albumin:creatinine ratio (ACR) in a spot urine sample and in a first morning void were 10.5 and 5.7 mg/g, respectively, and were different from the median ACR in a 24-h urine collection (8.3 mg/g; both P < 0.001).
Table 1.
Characteristics for the overall study population and men and women separatelya
| Characteristic | Overall (n = 241) | Men (n = 124) | Women (n = 117) |
|---|---|---|---|
| White (%) | 96 | 98 | 94 |
| Age (yr; mean ± SD) | 58.7 ± 11.3 | 61.2 ± 12.1 | 56.0 ± 9.8 |
| Weight (kg; mean ± SD) | 79.6 ± 15.2 | 85.5 ± 14.0 | 73.3 ± 13.8 |
| Use of BP-lowering drugs (%) | 32.4 | 39.5 | 24.8 |
| DBP (mmHg; mean ± SD) | 73.8 ± 8.4 | 76.7 ± 7.4 | 70.8 ± 8.3 |
| SBP (mmHg; mean ± SD) | 128.1 ± 18.4 | 131.7 ± 17.9 | 124.2 ± 18.3 |
| Use of lipid-lowering drugs (%) | 22.0 | 28.2 | 15.4 |
| Cholesterol (mmol/L; mean ± SD) | 4.9 ± 1.0 | 4.7 ± 1.0 | 5.1 ± 1.0 |
| Use of glucose-lowering drugs (%) | 2.5 | 4.8 | 0.0 |
| Glucose (mmol/L; mean ± SD) | 5.2 ± 0.9 | 5.4 ± 1.0 | 5.1 ± 0.6 |
| Serum creatinine (μmol/L; mean ± SD) | 78.1 ± 17.1 | 86.7 ± 16.7 | 69.1 ± 12.1 |
| 24-h urine collection (median [IQR]) | |||
| UAE (mg/24 h) | 11.5 (7.2 to 17.8) | 13.4 (8.7 to 22.8) | 8.7 (6.5 to 14.3) |
| UAC (mg/L) | 6.5 (4.0 to 11.4) | 8.6 (4.9 to 13.5) | 5.1 (3.3 to 8.7) |
| ACR (mg/g) | 8.3 (5.4 to 12.7) | 8.4 (5.3 to 12.7) | 7.9 (5.5 to 12.7) |
| creatinine (g/24 h) | 1.4 (1.1 to 1.7) | 1.6 (1.3 to 1.9) | 1.1 (1.0 to 1.4) |
| creatinine (g/L) | 0.8 (0.6 to 1.1) | 0.9 (0.7 to 1.3) | 0.7 (0.5 to 0.9) |
| First morning void collection (median [IQR]) | |||
| UAC (mg/L) | 6.1 (3.8 to 10.9) | 7.7 (4.6 to 12.4) | 4.8 (3.1 to 8.2) |
| ACR (mg/g) | 5.7 (4.2 to 9.3) | 5.6 (4.0 to 10.2) | 5.7 (4.4 to 8.9) |
| creatinine (g/L) | 1.0 (0.6 to 1.5) | 1.1 (0.7 to 1.7) | 0.8 (0.6 to 1.3) |
| Spot urine sample (median [IQR]) | |||
| UAC (mg/L) | 11.5 (7.4 to 24.9) | 12.1 (7.6 to 28.7) | 11.2 (6.4 to 18.2) |
| ACR (mg/g) | 10.5 (5.7 to 18.9) | 10.2 (5.3 to 17.0) | 11.2 (6.1 to 21.6) |
| creatinine (g/L) | 1.3 (0.74 to 1.8) | 1.5 (1.0 to 2.1) | 1.0 (0.5 to 1.7) |
Data are means ± SD, with the exception of albuminuria and urinary creatinine values, which are given as medians with interquartile range because of skewed data distribution. DBP; diastolic BP; IQR, interquartile range; SBP, systolic BP.
Correlation between Albuminuria Measures
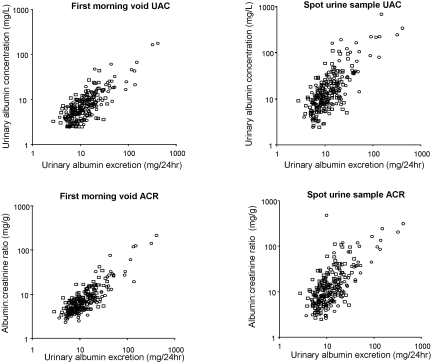
Regression analysis was used to evaluate which method of urine collection and which albuminuria measure shows the best correlation with 24-h UAE (Figure 1). Over the whole range of 24-h UAE, the first morning void showed a lower value and the spot urine sample a higher value of UAC. The same was observed for the ACR. Agreement with 24-h UAE, assessed as the r2 value of the regression analysis, was better for the first morning void than the spot urine sample, although the difference did not reach statistical significance (Table 2). The proportion of measurements within 30% of the value for 24-h UAE, as calculated with the regression equation, was statistically significantly higher when albuminuria measures were derived from first morning voids compared with spot urine samples (Table 2). Finally, the Bland-Altman analysis indicated that the mean difference (bias) with 24-h urine collections was lower when first morning void samples were collected. The lower SD of the mean difference of first morning void collections furthermore indicated that, for an individual, the first morning void sample was more likely to agree with 24-h collection than a spot urine sample. The ACR scored better than UAC except for the bias in the Bland-Altman plot, which was lower for the UAC derived from a first morning void. Essentially similar results were obtained in gender-specific subgroups (Table 2).
Figure 1.
Scatterplots showing the relationship between 24-h UAE and other albuminuria measures. (Top) Twenty-four-hour UAE versus first morning void and spot urine sample UAC, respectively. (Bottom) Twenty-four-hour UAE versus first morning void and spot urine sample ACR, respectively. •, Male; □, female.
Table 2.
Agreement between 24-h UAE (reference) and other albuminuria measures in the overall population and gender-specific subgroupsa
| Parameter | UAC
|
ACR
|
||
|---|---|---|---|---|
| FMV | SUS | FMV | SUS | |
| Overall (n = 241) | ||||
| r2 | 0.85 | 0.71 | 0.87 | 0.83 |
| P30 | 48.1 | 35.3b | 67.6 | 46.5b |
| Bland-Altman agreement mg/L (SD) | −1.9 (11.0) | 10.1 (19.6)b | −3.1 (8.0) | 4.8 (11.8)b |
| Men (n = 124) | ||||
| r2 | 0.87 | 0.75 | 0.88 | 0.86 |
| P30 | 51.6 | 41.9 | 65.3 | 51.6b |
| Bland-Altman agreement mg/L (SD) | −3.6 (14.4) | 11.8 (24.1)b | −3.6 (10.5) | 4.8 (14.2)b |
| Women (n = 117) | ||||
| r2 | 0.49 | 0.24 | 0.65 | 0.42 |
| P30 | 47.4 | 25.9b | 70.7 | 41.0b |
| Bland-Altman agreement mg/L (SD) | −0.1 (4.5) | 8.4 (13.0)b | −2.6 (3.9) | 4.7 (8.4)b |
FMV, first morning void; P30, proportion of measurements within 30% of the value of 24-h UAE, determined by the regression equation; SUS, spot urine sample.
P ≤ 0.05 spot urine sample versus first morning void.
We used the Passing and Bablok regression equation to calculate values for UAC and the ACR that correspond with a UAE of 30 mg (Table 3). These data indicate that the values obtained in first morning voids were closer to the lower boundary indicating microalbuminuria than values obtained in spot urine samples.
Table 3.
Values of the various albuminuria measures corresponding to 30-mg/24 h UAE calculated with the Passing and Bablok regression equation and according literaturea
| Parameter | Cutoff Value
|
|
|---|---|---|
| Corresponding to 30-mg/24 h UAE | Microalbuminuria | |
| FMV | ||
| UAC (overall; mg/L) | 15.6 | 20 |
| ACR, men (mg/g) | 13.3 | 17 |
| ACR, women (mg/g) | 18.9 | 25 |
| SUS | ||
| UAC (overall; mg/L) | 41.5 | 20 |
| ACR, men (mg/g) | 23.7 | 17 |
| ACR, women (mg/g) | 52.7 | 25 |
Gender-specific cutoff values for ACR are shown.
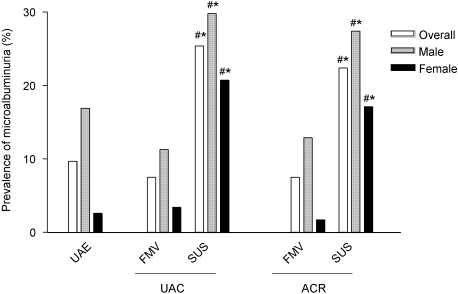
We calculated the prevalence of microalbuminuria using the cutoff values indicating microalbuminuria that are presently advocated in literature.7 The prevalence of microalbuminuria based on 24-h UAE, the reference value, was 10.0% (Figure 2). The prevalence of microalbuminuria using data from first morning voids nearly equaled the prevalence of microalbuminuria on the basis of 24-h urine collections (7.5% based on UAC and 7.5% based on ACR; P = 0.18 and P = 0.07, respectively). The prevalence of microalbuminuria was considerably higher when data from spot urine samples were used (25.4% based on UAC and 22.4% based on ACR; both P < 0.001). When the gender-independent Kidney Disease Outcomes Quality Initiative (KDOQI) cutoff values for the ACR indicating microalbuminuria were used (30 mg/g), 14.9 and 4.7% of all patients received the diagnosis of microalbuminuria on the basis, respectively, of a spot urine sample (P = 0.24 versus 24-h UAE) and first morning void (P = 0.01 versus 24-h UAE).
Figure 2.
Percentage of individuals with microalbuminuria. Cutoff values presently advocated in the literature indicating microalbuminuria are used: 24-h UAE 30 mg/24 h, UAC 20 mg/L, and the ACR gender-specific cutoff value (17 mg/g in men and 25 mg/g in women). FMV, first morning void; SUS, spot urine sample. *P ≤ 0.05 versus 24-h urine collection; #P ≤ 0.05 versus FMV.
Intraindividual Coefficient of Variation of Various Albuminuria Measures
To investigate which albuminuria measure can best be used to monitor UAE over time, we calculated intraindividual coefficients of variation (CVs). Because UAE in a 24-h urine collection is considered the “gold standard,” we used intraindividual CV of the 24-h UAE, which was 19.0%, as the reference value for statistical analyses (Table 4). The intraindividual CV of albuminuria measures derived from first morning voids were lower than that of spot urine samples. The intraindividual CV of the ACR in both a first morning void and a spot urine sample was lower than that of UAC. Of note, the intraindividual CV of the ACR derived from a first morning void was the only albuminuria measure that did not differ significantly from 24-h UAE (19.1 and 19.0%, respectively; P = 0.64). When we subdivided the study population into a group with low-normal (0 to 15 mg/24 h), high-normal (15 to 30 mg/24 h), and microalbuminuria (>30 mg/24 h), we obtained similar results, the only difference being that in patients with microalbuminuria, the intraindividual CV for UAC in a first morning void (24.2%) did not differ significantly from the reference value, being 24-h UAE (22.5%; P = 0.74). Assessment of the intraindividual CV in gender-specific subgroups did not make any material differences to the findings (Table 4).
Table 4.
Median (interquartile range) intrapatient CV (%) for the various albuminuria measures in the overall population and in gender-specific subgroups
| Parameter | UAE | UAC | ACR | Creatinine |
|---|---|---|---|---|
| Overall (n = 241) | ||||
| 24-h urine collection | 19.0 (10.9 to 33.2) | 26.2 (17.8 to 42.9) | 19.2 (10.9 to 32.6) | 9.3 (4.8 to 18.4) |
| FMV | 30.9 (19.4 to 47.8)a | 19.1 (11.6 to 28.4) | 23.5 (13.8 to 66.7) | |
| SUS | 40.9 (27.8 to 70.2)a | 35.8 (17.6 to 55.6)a | 31.3 (19.1 to 56.1) | |
| Men (n = 124) | ||||
| 24-h urine collection | 21.4 (11.5 to 33.6) | 30.1 (20.2 to 46.7) | 19.2 (11.1 to 36.0) | 10.0 (4.8 to 20.3) |
| FMV | 29.2 (18.2 to 46.1)a | 18.0 (10.4 to 27.4)a | 22.7 (13.1 to 31.8) | |
| SUS | 38.9 (27.7 to 65.5)a | 30.7 (15.7 to 53.6)a | 31.2 (19.8 to 50.5) | |
| Women (n = 117) | ||||
| 24-h urine collection | 18.2 (9.9 to 31.8) | 22.2 (14.3 to 42.3) | 18.9 (9.8 to 30.4) | 7.7 (4.9 to 14.7) |
| FMV | 32.4 (21.1 to 48.7)a | 19.4 (12.0 to 30.0) | 25.3 (14.2 to 34.9) | |
| SUS | 42.6 (27.8 to 82.1)a | 36.6 (22.2 to 62.2)a | 33.4 (17.2 to 64.4) |
P ≤ 0.05 versus intraindividual CV for 24-h UAE.
Agreement between 24-H UAE and ACR in a First Morning Void and Spot Urine Sample
The data indicate that the ACR in a first morning void shows the best agreement with 24-h UAE. We subsequently investigated whether individuals identified by 24-h UAE as having low-normal (0 to 15 mg/24 h), high-normal (15 to 30 mg/24 h), or microalbuminuria (>30 mg/24 h) are the same as those identified by measuring the ACR in a first morning void using gender-specific cutoff values indicating microalbuminuria (Table 5). These data indicate that 81.8% of all individuals were identified in the same category for UAE when the ACR derived from a first morning void was used. In contrast, when the ACR from spot urine samples was used, 72.1% (P = 0.011 versus first morning void) of all individuals were correctly identified (data not shown).
Table 5.
Agreement with respect to clinical class (normoalbuminuria, microalbuminuria, and macroalbuminuria) when using cutoff values presently advocated in the literature for 24-h UAE and the ACR in an FMV
| 24-H UAE (mg/24 h) | FMV (n [%])
|
Overall | ||
|---|---|---|---|---|
| Men: 0.0 to 8.5 mg/gWomen: 0.0 to 12.5 mg/g | Men: 8.5 to 17.9 mg/gWomen: 12.5 to 25.0 mg/g | Men: >17.0 mg/gWomen: >25.0 mg/g | ||
| 0 to 15 | 160 (66.4) | 4 (1.7) | 0 (0.0) | 164 (68.0) |
| 15 to 30 | 32 (13.3) | 20 (8.3) | 1 (0.4) | 53 (22.0) |
| >30 | 0 (0.0) | 7 (2.9) | 17 (7.1) | 24 (10.0) |
| Overall | 192 (79.6) | 31 (12.9) | 18 (7.5) | 241 (100.0) |
DISCUSSION
This study was conducted to investigate whether albuminuria measures derived from a first morning void and spot urine samples can replace a 24-h UAE measurement for diagnosing microalbuminuria and for monitoring albuminuria over time. The results indicate that the agreement with 24-h UAE was better for albuminuria measures derived from first morning voids than spot urine samples. Furthermore, when we used for microalbuminuria cutoff values that are advocated in literature, the prevalence of microalbuminuria on the basis of albuminuria measures derived from a first morning void were close to the prevalence of microalbuminuria on the basis of 24-h UAE. Finally, the intraindividual variability of albuminuria measures derived from first morning voids was lower than that of spot urine samples, with the intraindividual variability of the ACR in a first morning void being equal to 24-h UAE.
A number of studies have addressed the issue of which urine collection method (first morning void or spot urine sample) and which albuminuria measure (UAC or the ACR) can be used as alternative to measurement of 24-h UAE.8–13 These studies were based on measurement of the UAC and ACR in either a spot urine sample or a first morning void but did not compare both. Another disadvantage is that most of these studies assessed albuminuria measures at a single occasion but did not assess albuminuria measures at consecutive visits. For determination of which of the albuminuria measures can be used to replace 24-h UAE, it is of importance to determine, first, which of the albuminuria measure shows the best agreement with 24-h UAE and, second, which of the albuminuria measures shows the least intraindividual variability over time. To our knowledge, only one study determined the intraindividual variability of UAC and ACR derived from a first morning void as well as from a spot urine sample and compared these values with the intraindividual variability of 24-h UAE.6 It was reported that the intraindividual CV of the UAC in a first morning void was the lowest; however, that study included only 11 patients and stored samples at −20°C before analysis. This may have introduced measurement error, because it has been reported that frozen storage of urine samples induces considerable variability in albumin concentration.14 Given the limited number of data, the KDOQI recently recommended that additional research on urine collection methods and urinary albumin measurement is warranted.15 This study was designed to provide such data.
Albuminuria measures derived from a first morning void corresponded best with 24-h UAE and showed a lower intraindividual and interindividual variability than albuminuria measures derived from spot urine samples. These results indicate that first morning voids are to be preferred over spot urine samples as an alternative to 24-h urine collections. This conclusion is in contrast with recommendations in present diabetic and hypertensive treatment guidelines. According to these guidelines, the preferred method to assess albuminuria is measurement of the ACR in a spot urine sample.2,4 Our data indicate that spot urine samples should not be used because the cutoff value of the UAC and ACR in a spot urine sample indicating microalbuminuria show only modest agreement with the cutoff values advocated in the literature, resulting in a considerably higher prevalence of individuals being classified as having microalbuminuria. Furthermore, UAC and ACR in a spot urine sample show a high intraindividual variability, making it less reliable to monitor albuminuria in a specific patient. The explanation for why albuminuria measures in a first morning void correspond better with 24-h UAE than albuminuria measures in a spot urine sample may be that the former is less influenced by factors such as physical exercise and diet. Given the aforementioned data, we advocate collection of first morning void urine samples when 24-h urine collection is not feasible.
The ACR showed overall better agreement with 24-h UAE and a lower intraindividual variability than UAC. This may be explained by the fact that measuring only UAC is influenced by intraindividual variations in urinary volume. Creatinine is excreted in urine with relative constancy over time.16 When UAC is divided by creatinine concentration, it will result in a “correction” for intraindividual variation in urinary volume. As a result of parallel changes in urinary creatinine concentration, the ACR is more helpful in diagnosing microalbuminuria and monitoring albuminuria over time. On the basis of this consideration, we recommend measurement of the ACR in a first morning void when determination of 24-h UAE is not feasible.
We observed a relatively high intraindividual variability of albuminuria measures in healthy individuals in this study, even in 24-h urine collections. This finding is consistent with the literature.17,18 Factors such as dietary nutrients intake, body position, and exercise may influence intraindividual variability.19 The relatively high intraindividual variability of albuminuria measures hampers reliable classification of individuals into normoalbuminuric or microalbuminuric range. This finding underlines the recommendation in treatment guidelines that urinary albumin measurements be repeated several times to evaluate reliably the risk status of a specific individual.3,4
This study indicates that a lower cutoff value for the ACR indicating microalbuminuria may be required. Passing and Bablok regression analysis showed that the value of the ACR in a first morning void corresponding to a UAE of 30 mg/24 h is 13.3 mg/g in men and 18.9 mg/g in women. These values are lower than the gender-specific cutoff values for microalbuminuria presently advocated in the literature, being 17 and 25 mg/g, respectively.7 In addition, the prevalence of microalbuminuria on the basis of 24-h UAE was 10.0%, whereas it was 7.5% on the basis of the ACR in a first morning void. Although the difference in prevalence was not statistically significant, it indicates that 25% of all individuals with microalbuminuric on the basis of 24-h UAE are not classified as having microalbuminuria when the ACR derived from a first morning void is used.
Some issues need to be addressed when interpreting our findings. Creatinine is excreted with relative constancy over time.16 The low intrapatient variability of the 24-h creatinine excretion, being 9.1%, indicates that, in general, individuals did not have problems with 24-h urine collections. Individuals who agreed to participate in this study probably found collecting a 24-h urine sample less aggravating than did individuals who refused to participate and thereby are probably less likely to make collection errors. This may have introduced bias. The consequence could be that, if a random selection of the general population were included, then the results would probably have pointed more in the direction of a first morning void or a spot urine sample because the individuals likely would have made more collection errors in the 24-h urine collection procedure. Furthermore, one should realize that our study was conducted in a predominantly white cohort without diabetes or hypertension. Further research in other populations should corroborate our findings. Finally, the definitive answer to which urine collection and albuminuria measure should be used in clinical practice should come from prospective studies evaluating which method predicts renal and cardiovascular outcomes best. Our data do not allow performance of such analyses because of the short follow-up time. The strength of this study is that both 24-h urines, first morning voids, and spot urine samples were collected at three consecutive occasions. Most studies that compared the value of various albuminuria measures did so by looking only at the correlation between 24-h UAE and either a first morning void or a spot urine sample, using a single measurement per individual.
In conclusion, albuminuria measures derived from first morning voids are a more reliable alternative to 24-h UAE than spot urine samples to diagnose microalbuminuria and to monitor albuminuria over time. If it is decided to collect first morning voids, then measurement of the ACR is to be preferred over UAC.
CONCISE METHODS
Study Population
This study was conducted of individuals who participated in the Prevention of Renal and Vascular End-stage disease (PREVEND) study. The PREVEND study was designed to investigate prospectively the natural course of UAE and its relation to renal and cardiovascular disease. Participants regularly visit an outpatient department once every 3 yr for measurement of health status. Details of the study protocol have been published elsewhere.20 The PREVEND study was approved by the local medical ethics committee and is performed in accordance with the guidelines of the Declaration of Helsinki.
Study Design
The PREVEND study participants were informed about this study during their visit to the outpatient department. To participate in this study, individuals who were using antihypertensive medication had to be on a stable dosage of such medication. When individuals agreed to participate, three additional appointments were made to visit our research unit. The time interval between visits was 3 wk. Before the first visit, written and verbal instructions on how to collect urine samples were given. Individuals were asked to collect a 24-h urine sample from 10:00 p.m. to 10:00 p.m. The next morning, after finishing the 24-h urine collection, participants were asked to collect a midstream first morning void. Participants were advised to store their urine collections at 4°C. After delivering the 24-h urine and first morning void at the research unit that same day, participants were asked to collect a midstream spot urine sample at our research clinic, which mostly took place between 8:00 and 11:00 a.m. At each of the three visits, participants thus delivered a 24-h urine collection, a first morning void, and a spot urine sample.
Analytical Methods
Urine samples were stored in plastic containers at 4°C. UAC and urinary creatinine concentration were determined within 48 h after collection. UAC was measured with a Behring BN II analyzer (Dade Behring, Marburg, Germany) in a central laboratory. The intra-assay and interassay CVs for urinary albumin evaluated in our laboratory were 2.2 and 2.6%, respectively. The lower limit of detection was 2.40 mg/L. Urinary creatinine was determined by an enzymatic method (Modular Analytics SWA, Roche, Tokyo, Japan) with intra-assay and interassay CVs evaluated in our laboratory of 1.9 and 2.1%, respectively.
Statistical Analysis
We drew scatterplots to investigate the association between 24-h UAE and the various albuminuria measures. Each data point in these plots represents the geometric mean of the three values that were obtained at the three visits to our research unit. Agreement between 24-h UAE and the albuminuria measures was assessed, first, by calculating the r2 value of the regression analysis between 24-h UAE and the albuminuria measure under investigation and, second, by calculating the amount of scatter. The amount of scatter was defined as the proportion of measurements within 30% of the value of 24-h UAE, determined by the regression equation. McNemar test was used to test for differences between the amount of scatter in first morning voids versus that of spot urine samples. Because the r2 value of the regression analysis is a measure of the strength of the linear associations rather than a measure of agreement, we also calculated the agreement of the first morning void and spot urine sample with a 24-h collection according to the method of Bland and Altman.21 We used Passing and Bablok regression analysis to calculate the value of the UAC and ACR that corresponds with a 24-h UAE of 30 mg/24 h. This was done for both first morning voids and spot urine samples. Because the creatinine component in the ACR depends on muscle mass, the ACR differs between genders because muscle mass is lower in women than in men; therefore, we used gender-specific regression equations for the ACR.
We calculated the prevalence of microalbuminuria on the basis of the geometric mean value of all albuminuria measures. For this purpose, we used the cutoff values indicating microalbuminuria that are presently advocated in literature (Table 6) and the KDOQI guideline.3 We used gender-specific cutoff values for the ACR indicating microalbuminuria.7 We used McNemar test to test for differences between the prevalence of microalbuminuria on the basis of different urine collection techniques and albuminuria measures.
Table 6.
Cutoff values presently advocated in literature indicating normoalbuminuria, microalbuminuria, and macroalbuminuria
| Parameter | UAE
|
FMV or SUS
|
|||
|---|---|---|---|---|---|
| 24-h Urine (mg/24 h) | Timed Overnight (μg/min) | UAC (mg/L) | ACR
|
||
| (mg/mmol) | (mg/g) | ||||
| Normal | <30 | <20 | <20 | ||
| men | <2.5 | <20 | |||
| women | <3.5 | <30 | |||
| Microalbuminuria | 30 to 300 | 20 to 200 | 20 to 200 | ||
| men | 2.5 to 25 | 20 to 200 | |||
| women | 3.5 to 35 | 30 to 300 | |||
| Macroalbuminuria | >300 | >200 | >200 | ||
| men | >25 | >200 | |||
| women | >35 | >300 | |||
We assessed the intraindividual CV for each albuminuria measure to determine which albuminuria measure shows the least variation over time in an individual and consequently is the best measure to monitor albuminuria over time. We calculated the intraindividual CV per individual as the SD of three measurements divided by their geometric mean value. Intraindividual CVs were not calculated when participants did not deliver three urine collections or when the albumin concentration was below the detection limit. We tested differences between intraindividual CVs for statistical significance by the Wilcoxon signed rank test.
Finally, we investigated whether individuals who were identified by 24-h UAE as having low-normal (0 to 15 mg/24 h), high-normal (15 to 30 mg/24 h), or microalbuminuria (>30 mg/24 h) were the same as those who were identified by measurement of the geometric mean value of the ACR in a first morning void and spot urine sample. We tested differences between the percentage of correctly identified individuals on the basis of first morning void and spot urine sample for significance with McNemar test.
Data are means ± SD or, in the case of non-normal distribution, medians with interquartile range. P ≤ 0.05 (two-sided) was adopted to indicate statistical significance. Statistical analysis was carried out using SPSS 14 (SPSS, Inc., Chicago, IL).
DISCLOSURES
None.
Acknowledgments
We thank Dade Behring (Marburg, Germany) for supplying equipment (Behring Nephelometer II) and reagents for nephelometric measurement of urinary albumin.
Published online ahead of print. Publication date available at www.jasn.org.
E.C.W. and H.J.L.H. contributed equally to this work.
REFERENCES
- 1.Keane WF, Eknoyan G: Proteinuria, albuminuria, risk, assessment, detection, elimination (PARADE): A position paper of the National Kidney Foundation. Am J Kidney Dis 33: 1004–1010, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 3.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–266, 2002 [PubMed] [Google Scholar]
- 4.American Diabetes Association: Standards of medical care in diabetes—2007. Diabetes Care 30[Suppl 1]: S4–S41, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Mogensen CE, Vestbo E, Poulsen PL, Christiansen C, Damsgaard EM, Eiskjaer H, Froland A, Hansen KW, Nielsen S, Pedersen MM: Microalbuminuria and potential confounders: A review and some observations on variability of urinary albumin excretion. Diabetes Care 18: 572–581, 1995 [DOI] [PubMed] [Google Scholar]
- 6.Howey JE, Browning MC, Fraser CG: Selecting the optimum specimen for assessing slight albuminuria, and a strategy for clinical investigation: Novel uses of data on biological variation. Clin Chem 33: 2034–2038, 1987 [PubMed] [Google Scholar]
- 7.Warram JH, Gearin G, Laffel L, Krolewski AS: Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol 7: 930–937, 1996 [DOI] [PubMed] [Google Scholar]
- 8.Zelmanovitz T, Gross JL, Oliveira JR, Paggi A, Tatsch M, Azevedo MJ: The receiver operating characteristics curve in the evaluation of a random urine specimen as a screening test for diabetic nephropathy. Diabetes Care 20: 516–519, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Ng WY, Lui KF, Thai AC: Evaluation of a rapid screening test for microalbuminuria with a spot measurement of urine albumin-creatinine ratio. Ann Acad Med Singapore 29: 62–65, 2000 [PubMed] [Google Scholar]
- 10.Incerti J, Zelmanovitz T, Camargo JL, Gross JL, de Azevedo MJ: Evaluation of tests for microalbuminuria screening in patients with diabetes. Nephrol Dial Transplant 20: 2402–2407, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Jermendy G, Farkas K, Nadas J, Daroczy A, Peterfai E: Practical aspects of measuring microalbuminuria in diabetic patients. Diabetes Nutr Metab 14: 195–200, 2001 [PubMed] [Google Scholar]
- 12.Ahn CW, Song YD, Kim JH, Lim SK, Choi KH, Kim KR, Lee HC, Huh KB: The validity of random urine specimen albumin measurement as a screening test for diabetic nephropathy. Yonsei Med J 40: 40–45, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Nathan DM, Rosenbaum C, Protasowicki VD: Single-void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care 10: 414–418, 1987 [DOI] [PubMed] [Google Scholar]
- 14.Brinkman JW, De Zeeuw D, Duker JJ, Gansevoort RT, Kema IP, Hillege HL, de Jong PE, Bakker SJ: Falsely low urinary albumin concentrations after prolonged frozen storage of urine samples. Clin Chem 51: 2181–2183, 2005 [DOI] [PubMed] [Google Scholar]
- 15.National Kidney Foundation: KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 49: S12–154, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Borst JG, de Vries LA: The three types of “natural” diuresis. Lancet 2: 1–6, 1950 [DOI] [PubMed] [Google Scholar]
- 17.Marre M, Claudel JP, Ciret P, Luis N, Suarez L, Passa P: Laser immunonephelometry for routine quantification of urinary albumin excretion. Clin Chem 33: 209–213, 1987 [PubMed] [Google Scholar]
- 18.Justesen TI, Petersen JL, Ekbom P, Damm P, Mathiesen ER: Albumin-to-creatinine ratio in random urine samples might replace 24-h urine collections in screening for micro- and macroalbuminuria in pregnant woman with type 1 diabetes. Diabetes Care 29: 924–925, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Metcalf PA, Baker JR, Scragg RK, Dryson E, Scott AJ, Wild CJ: Dietary nutrient intakes and slight albuminuria in people at least 40 years old. Clin Chem 39: 2191–2198, 1993 [PubMed] [Google Scholar]
- 20.Pinto-Sietsma SJ, Janssen WM, Hillege HL, Navis G, De Zeeuw D, de Jong PE: Urinary albumin excretion is associated with renal functional abnormalities in a nondiabetic population. J Am Soc Nephrol 11: 1882–1888, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Bland JM, Altman DG: Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310, 1986 [PubMed] [Google Scholar]