ABSTRACT
Objectives: To analyze our own functional results to delineate a critical vestibular schwannoma size for middle cranial fossa (MCF) surgery with the best possible outcome. Study Design: Retrospective chart review. Setting: Academic tertiary referral center. Methods: Tumors were divided into intracanalicular, tumors 1 to 5, 6 to 10, and 11 to 15 mm in the cerebellopontine angle (CPA). Patients were evaluated at 2 months, 1 year, and 5 years after surgery. Results: At 1 year, House-Brackmann score of I or II was obtained in 100% of intracanalicular and in 96%, 86%, and 85% with tumors up to 5, 10, and 15 mm in the CPA, respectively. Class I hearing was postoperatively preserved in 61%, 41%, 29%, and 20%, and measurable word recognition in 67%, 51%, 35%, and 21% of patients, respectively. Conclusion: The outcome is predominantly a function of tumor size, and these changes influence MCF surgery at an earlier stage than in the translabyrinthine or retrosigmoid approach. For the facial nerve, there is a cutoff at 5-mm extracanalicular extension. Also, chances for successful hearing preservation decrease rapidly with size, and in tumors beyond 1.5 cm are below 20%. Consequently, although an expectant policy with small tumors may be reasonable in some instances, it is not so for MCF candidates.
Keywords: Vestibular schwannoma, hearing preservation, tumor size, facial nerve, middle cranial fossa, long-term results
Surgery remains the only curative treatment for growing vestibular schwannoma (VS) of all sizes. The most important predictive factor for successful surgery is tumor size, and patients with smaller tumors have significantly better chances for a satisfactory preservation of their functional integrity, including facial nerve function and hearing. In view of the ongoing argument that a significant proportion of VS may exhibit a rather slow growth or no growth at a certain point in the lifetime of the patient, some have adopted an expectant policy with small tumors.1 The argument is that postponing surgery until the tumor possibly reaches the certain cerebellopontine angle (CPA) extension would eliminate unnecessary surgery in patients with slowly or nongrowing tumors. Also, if surgery may become necessary at the later point, it would not jeopardize otherwise good surgical results. This critical tumor size is argued at ~1.5- to 2-cm extracanalicular extension based mainly on results of translabyrinthine (TL) and retrosigmoid (RS) surgery.2,3,4 However, the watch-and-see policy neglects the fact that hearing loss is probable and unpredictable in nongrowing tumors as well, and that by postponing surgery, the optimal time for function preservation may be missed.
In our experience, the cutoff point of tumor size at which the results of facial nerve function and hearing preservation start to decline is approach dependent and differs for middle cranial fossa (MCF) as compared with TL and RS. The MCF, with enlarged exposure of the porus and the CPA, is favored for small and medium-sized tumors in patients with residual hearing. With this approach, normal postoperative facial nerve function is reported in at least 90% and hearing preservation in up to 85% of patients.5,6,7 Because of the demanding surgical expertise, it has remained a domain of distinct surgical centers with adequate case load and experience. For this reason, our intention is to analyze our own functional results to detect the subtle and critical changes in the outcome by the tumor size. By meticulous stratification of extracanalicular size in 5-mm steps, we may be able to delineate a critical tumor size for MCF with the best possible functional outcome. In addition, long-term results will be brought on the basis of 5-year postoperative magnetic resonance imaging (MRI) and audiological testing as a further argument in favor of function preservation efforts.
PATIENTS AND METHODS
From a consecutive series of 205 patients undergoing MCF excision of unilateral VS, data of 197 patients were the basis for this retrospective review. The remaining eight patients were either lost to follow-up (five patients) or their tumors did not meet the criteria of maximal size in the CPA not larger than 15 mm (three patients). The tumors were divided in subcategories according to the extracanalicular extension (in the line of the internal auditory canal axis) as measured from the T1-weighted MRI scan as follows: purely intracanalicular tumors (67 patients), tumors 1 to 5 mm in the CPA (56 patients), tumors 6 to 10 mm (42 patients), and tumors 11 to 15 mm in the CPA (32 patients). The patients' ages at the time of surgery ranged from 21 to 78 years (mean 51 years).
Surgery was performed by the first author, and details of the surgical procedure were reported elsewhere.7,8 Patients were routinely scheduled for evaluation at 2 months, 1 year, and 5 years after surgery. Evaluation consisted of an MRI scan, facial nerve exam with electromyography and neuronography when needed, and audiometric and vestibular testing. Facial nerve function was documented using the House-Brackmann (H-B) scale,9 and the basis for the classification of hearing status was the patient's monosyllable word recognition score (WRS; class I = 100 to 70% WRS; class II = 69 to 50% WRS; class III = 49 to 1% WRS; class IV = 0% WRS) as proposed by Meyer et al10 and shown in Table 1. The American Academy of Otolaryngology—Head and Neck Surgery (AAO-HNS) scale was brought for comparison.11
Table 1.
Hearing Classification Based on Word Recognition Score
| Class | Word Recognition Score (%) |
|---|---|
| I | 70–100 |
| II | 50–69 |
| III | 1–49 |
| IV | 0 |
In addition to 1-year postoperative data of all 197 patients, 5-year postoperative MRI scans were available for 103 (52%) and audiometric tests for 137 (70%) of these patients. Five-year results were used to report the long-term cure and hearing.
RESULTS
Facial Nerve Function
All patients presented with clinically normal facial nerve function before surgery. In the course of surgery, the facial nerve was anatomically preserved in all 197 patients. At 2 months, 134 patients (68%) were H-B grade I, 23 (12%) were H-B II, 12 (6%) were H-B III, 6 (3%) H-B IV, 16 (8%) H-B V, and 6 (3%) were H-B VI. After 1 year, 153 patients (77.7%) were H-B grade I, 31 (15.7%) were H-B II, 11 (5.6%) were H-B III, 1 (0.5%) was H-B IV, and 1 (0.5%) was H-B VI. The only patient with definite paralysis did not agree to further surgical rehabilitation and received intensive eye care. Also, when split by tumor size, notable improvement of the facial function in all subcategories was observed from the 2-month to the 1-year deadline (Table 2). At 1 year, normal and near-normal postoperative facial function (H-B score I or II) was obtained in 100% of patients with intracanalicular tumors, and in 96%, 86%, and 85% of patients with extracanalicular tumors up to 5, 10, and 15 mm in the CPA, respectively.
Table 2.
Facial Nerve Function at 2 Months and 1 Year Postoperatively, Split by Tumor Size
| H-B Index | IC (n = 67) |
1–5 mm (n = 56) |
6–10 mm (n = 42) |
11–15 mm (n = 32) |
||||
|---|---|---|---|---|---|---|---|---|
| 2 mo | 1 y | 2 mo | 1 y | 2 mo | 1 y | 2 mo | 1 y | |
| H-B, House-Brackmann; IC, intracanalicular. | ||||||||
| I | 60 (90%) | 63 (94%) | 38 (68%) | 42 (75%) | 26 (62%) | 28 (67%) | 10 (31%) | 20 (63%) |
| II | 3 (4%) | 4 (6%) | 4 (7%) | 12 (21%) | 6 (14%) | 8 (19%) | 10 (31%) | 7 (22%) |
| III | 2 (3%) | — | 4 (7%) | 2 (4%) | 2 (5%) | 5 (12%) | 4 (12.5%) | 4 (13%) |
| IV | — | — | 3 (5%) | — | 2 (5%) | — | 1 (3%) | 1 (3%) |
| V | 1 (1.5%) | — | 7 (13%) | — | 2 (5%) | — | 6 (19%) | — |
| VI | 1 (1.5%) | — | — | — | 4 (10%) | 1 (2%) | 1 (3%) | — |
Eight patients (4%) developed delayed facial palsy after leaving the hospital, and the occurrence peaked at 10 days postoperatively. All of them were treated with systemic steroids, some also received antiviral medication, and all recovered uneventfully.
Hearing
At the last preoperative hearing evaluation that was used as the basis for further comparison, 188 (95%) patients had measurable speech recognition (classes I to III); of these, 152 (77%) had class I hearing (≥ 70% WRS), and 16 (8%) patients had class II hearing (Tables 3–6). Preservation of the cochlear nerve was attempted in all patients. Anatomic preservation was successful in 142 (72%) patients and failed in others because of tumor infiltration of the nerve. Of the latter, 32 patients had class I hearing preoperatively, but still the cochlear nerve was sacrificed to allow complete tumor removal. After 1 year, overall measurable speech recognition (classes I to III) was maintained in 48% (90/188) of patients who had measurable hearing preoperatively. Good postoperative hearing, defined as > 70% discrimination, was preserved in 46% (70/152) of patients with good preoperative hearing (Table 7).
Table 3.
Pre- and Postoperative Hearing Results for Intracanalicular Tumors
| AAO-HNS Scale | Total | Postoperative |
|||
|---|---|---|---|---|---|
| A | B | C | D | ||
| AAO-HNS, American Academy of Otolaryngology—Head and Neck Surgery; WRS, word recognition score. | |||||
| Preoperative | |||||
| A | 50 | 26 | 7 | 0 | 17 |
| B | 11 | 0 | 5 | 0 | 6 |
| C | 2 | 0 | 0 | 0 | 2 |
| D | 4 | 0 | 0 | 2 | 2 |
| Total | 67 | 26 | 12 | 2 | 27 |
| WRS Scale | Total | Postoperative | |||
| I | II | III | IV | ||
| Preoperative | |||||
| I | 62 | 38 | 0 | 2 | 22 |
| II | 1 | 0 | 0 | 1 | 0 |
| III | 3 | 0 | 1 | 2 | 0 |
| IV | 1 | 0 | 0 | 0 | 1 |
| Total | 67 | 38 | 1 | 5 | 23 |
Table 4.
Pre- and Postoperative Hearing Results for Tumors 1–5 mm in the CPA
| AAO-HNS Scale | Total | Postoperative |
|||
|---|---|---|---|---|---|
| A | B | C | D | ||
| AAO-HNS, American Academy of Otolaryngology—Head and Neck Surgery; CPA, cerebellopontine angle; WRS, word recognition score. | |||||
| Preoperative | |||||
| A | 28 | 11 | 0 | 1 | 16 |
| B | 16 | 0 | 8 | 1 | 7 |
| C | 2 | 0 | 0 | 1 | 1 |
| D | 10 | 0 | 1 | 0 | 9 |
| Total | 56 | 11 | 9 | 3 | 33 |
| WRS Scale | Total | Postoperative | |||
| I | II | III | IV | ||
| Preoperative | |||||
| I | 39 | 16 | 2 | 2 | 19 |
| II | 7 | 2 | 1 | 1 | 3 |
| III | 5 | 1 | 0 | 0 | 4 |
| IV | 5 | 0 | 0 | 1 | 4 |
| Total | 56 | 19 | 3 | 4 | 30 |
Table 5.
Pre- and Postoperative Hearing Results for Tumors 6–10 mm in the CPA
| AAO-HNS Scale | Total | Postoperative |
|||
|---|---|---|---|---|---|
| A | B | C | D | ||
| AAO-HNS, American Academy of Otolaryngology—Head and Neck Surgery; CPA, cerebellopontine angle; WRS, word recognition score. | |||||
| Preoperative | |||||
| A | 16 | 4 | 2 | 0 | 10 |
| B | 12 | 0 | 2 | 1 | 9 |
| C | 6 | 0 | 0 | 2 | 4 |
| D | 8 | 0 | 0 | 0 | 8 |
| Total | 42 | 4 | 4 | 3 | 31 |
| WRS Scale | Total | Postoperative | |||
| I | II | III | IV | ||
| Preoperative | |||||
| I | 31 | 9 | 1 | 2 | 19 |
| II | 3 | 0 | 0 | 1 | 2 |
| III | 6 | 0 | 0 | 1 | 5 |
| IV | 2 | 0 | 0 | 0 | 2 |
| Total | 42 | 9 | 1 | 4 | 28 |
Table 6.
Pre- and Postoperative Hearing Results for Tumors 11–15 mm in the CPA
| AAO-HNS Scale | Total | Postoperative |
|||
|---|---|---|---|---|---|
| A | B | C | D | ||
| AAO-HNS, American Academy of Otolaryngology—Head and Neck Surgery; CPA, cerebellopontine angle; WRS, word recognition score. | |||||
| Preoperative | |||||
| A | 11 | 4 | 0 | 0 | 7 |
| B | 13 | 0 | 0 | 0 | 13 |
| C | 3 | 0 | 0 | 0 | 3 |
| D | 5 | 0 | 0 | 0 | 5 |
| Total | 32 | 4 | 0 | 0 | 28 |
| WRS Scale | Total | Postoperative | |||
| I | II | III | IV | ||
| Preoperative | |||||
| I | 20 | 4 | 0 | 1 | 15 |
| II | 5 | 0 | 0 | 0 | 5 |
| III | 6 | 0 | 0 | 1 | 5 |
| IV | 1 | 0 | 0 | 0 | 1 |
| Total | 32 | 4 | 0 | 2 | 26 |
Table 7.
Postoperative Hearing as Function of Tumor Size
| Tumor Size | Measurable Speech Recognition (Class I–III), % | Good Hearing (Class I), % |
|---|---|---|
| EC, extra-canalicular. | ||
| Intracanalicular, n = 67 | 67 | 61 |
| 1–5 mm EC, n = 56 | 51 | 41 |
| 6–10 mm EC, n = 42 | 35 | 29 |
| 11–15 mm EC, n = 32 | 21 | 20 |
| All tumors, n = 197 | 48 | 46 |
Intracanalicular tumors (67 patients) accounted for 34% of the whole series (Tables 3, 7). Of 62 patients (93%) having class I hearing preoperatively, 38 (61%) maintained such good hearing. One patient improved from class III to class II. Measurable hearing was preserved in 44 of 66 patients (67%).
Fifty-six patients with extracanalicular tumors measuring up to 5 mm in the CPA comprised 28% of the whole group (Tables 4, 7). Five of them had 0% discrimination preoperatively. Class I hearing was maintained in 41% (16/39) of patients, and any hearing was preserved in 51% (26/51). In three patients, hearing improved postoperatively. They all moved to class I, two from class II and one from class III.
Tumors with 6- to 10-mm extension in the CPA were found in 42 patients (21%). Measurable hearing remained in 35% (14/40), and class I hearing in 29% (9/31) of patients (Tables 5, 7). No patients were seen with postoperative hearing improvement.
Larger tumors, 11 to 15 mm in the CPA angle, accounted for 16% (32/197) of patients (Tables 6, 7). Good postoperative hearing (class I) could be preserved in 20% (4/20) of patients, and measurable hearing in 21% (6/29) of patients with such hearing prior to surgery.
Nine patients had 0% discrimination before surgery and were still operated via MCF. Some of them had preoperative pure-tone average within 56 and 60 dB, but none of them improved postoperatively. All of them had their tumors removed completely and with good facial nerve function preservation.
Long-Term Results
Gross total tumor resection was accomplished in all but one patient (99.5%). In this patient with tumor growing after previous gamma-knife irradiation, severe fibrosis of the tumor remnant was the reason to leave a small piece of tumor over the facial nerve in order not to compromise facial nerve function. Postoperative MRI scans with gadolinium obtained 1 and 5 years after surgery detected two residual tumors. Both were in patients with intracanalicular tumors growing deep into the fundus, with residuals detected at the meatal foramen. Five years after surgery seems sufficient time for detection of possible recurrence, figuring cure rate in our series at 98%.
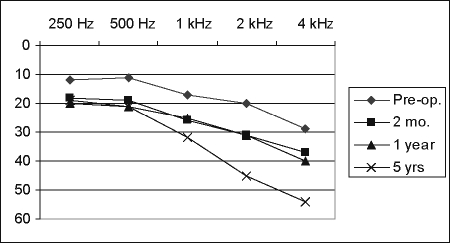
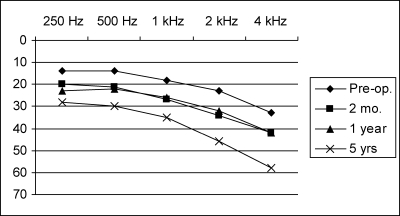
At 2 months and 1 year, pure-tone thresholds were almost identical and showed an average deterioration of 8.4 dB, as compared with preoperative hearing (Figs. 1, 2). At 5 years after surgery, hearing remained preserved in all patients in whom it had been preserved in the early postoperative period. However, hearing thresholds continued to deteriorate further with time, especially in higher frequencies above 1 kHz. At 4 kHz, there was an additional loss of up to 15 dB, which is more than the hearing loss on the healthy, nonoperated side. Thirty-seven percent of these patients demonstrated either a significant (at least 15 dB) increase in pure-tone average or a significant decrease in speech recognition 5 years after surgery.
Figure 1.
Time course of postoperative hearing threshold (38 patients with class I postoperative hearing, intracanalicular tumors).
Figure 2.
Time course of postoperative hearing threshold (71 patients with class I postoperative hearing, all tumor sizes).
Complications
There were no fatalities in this series. Minor epidural hemorrhage was surgically revised in one patient, and two temporalis muscle hematomas required evacuation. Two patients (1%) developed meningitis and were treated successfully with antibiotics, and 3 (1.5%) required revision for continuous cerebrospinal fluid leakage. Dysphasia of several hours duration spontaneously resolved in four patients (2%), and all of them had surgery on the left dominant side. Other transient deficits, like diplopia and arm paresis, were rare (1.5%). Three patients (1.5%) showed signs of pneumatocephalus with minimal compression of the temporal horn in the postoperative computed tomography and were managed conservatively. No patients developed episodes of seizures or aphasia at any time.
DISCUSSION
The outcome after VS surgery is predominantly a function of tumor size. Other factors, such as tumor biology, presenting symptoms, and surgical expertise, are important but difficult to quantify, and thus subordinate to tumor size. Although treatment of large tumors is clearly the domain of surgery, the present challenge is selection and timing of the best treatment for small tumors. Observation, though justified in the elderly because of phases of slow or no growth, will result in tumor growth with subsequent hearing loss in young and middle-aged patients. Radiation may prevent most tumors from growing, but more data are needed to determine long-term tumor control and function preservation rates. Surgical dilemmas include timing of surgery and the choice between hearing preservation approaches (e.g., MCF and RS versus TL surgery). The main arguments are facial nerve function and hearing preservation, which are both functions of tumor size, but not to the same extent, and are also approach dependent.
Inconsistencies in material inclusion and reporting criteria are responsible for confusing statements in the literature regarding the risk to the facial nerve with the three approaches. H-B grade at 1 year postoperatively is accepted as an adequate parameter of success as there is continuous improvement of function during the first postoperative year in at least 17 to 37% of patients. Also, H-B grades should not be added up, and grade III should not be included as favorable outcome. Although some report comparable results with the three approaches for tumors up to 10 mm, 15 mm, or 20 mm,12,13,14,15 others have found that with the MCF the facial nerve is put at increased risk.16,17,18,19 There is huge variation in size distribution within each size group in different studies. For the TL and the RS, critical tumor size after which the results decline is quoted at ~15- to 25-mm extracanalicular extension. In the Tos et al report, the results were as follows: tumors with extrameatal size up to 25 mm had 89% H-B I-II, tumors with size 26 to 40 mm had 71% H-B I-II, and tumors larger than 40 mm had 41% H-B I-II.2 In another large series, Sterkers et al achieved grade I or II with TL approach at 1 month in 52% of patients for large tumors (larger than 3 cm), in 81% for medium tumors (2 to 3 cm), and in 92% for small tumors (up to and including 2-cm extracanalicular).3 They concluded that the facial nerve was at greater risk using RS or MCF approaches than by the TL route. Reviewing the results of the keyhole RS surgery, Magnan et al reported H-B I in 95% and H-B II in 5% of 20 patients with intracanalicular tumors.4 The rates for 71 patients with tumor less than 10 mm in the CPA were H-B I 93%, H-B II 3%, H-B III 3%, and H-B IV 1%, and for tumors less than 25 mm were H-B I 93%, H-B II 7%.
For the MCF, H-B grade I for intracanalicular tumors was reported in 63 to 90% of patients, and grade II in 9 to 14%.7,14,16,20 Results for extracanalicular tumors are more difficult to extract from the literature. Reporting the results for tumors less than 10 mm and tumors 10 to 18 mm separately (greatest diameter, intracanalicular extension included), Isaacson et al noted a deterioration of facial outcome with larger tumors from grade I (83 to 77%) and grade II (11 to 15%).14 In other studies, the intracanalicular and small extracanalicular tumors of differing sizes were reported together, figuring grade I function in 82 to 86.4% of patients and grade II function in 7 to 13%.5,13,15,21 In their study, Arriaga and Chen made an additional attempt to analyze the results for two size classifications, 1.0 cm or smaller and 1.1 to 1.5 cm (intrameatal portion included), displaying them in a figure, but, unfortunately, without numerical values.13 Still, a difference could be noted with better results in 1.0 cm or less tumors, but further information is missing. Weber and Gantz achieved H-B I-II for the MCF approach in 93% of patients 1 year following surgery.6 Eighty-three percent of patients had a tumor confined to the internal auditory canal (IAC) or with less than 5 mm extending into the CPA. The remaining patients had tumors extending 5 to 15 mm into the CPA. In our present study, meticulous stratification in 5-mm steps proved important to show subtle changes in the facial outcome with increasing tumor size. In the whole series of 197 tumors and the CPA extension of 15 mm at maximum, H-B grade I was achieved in 77.7%, and H-B I-II in 93.4% of patients. However, when split into subcategories, and irrespective of the definition of success, whether it is only grade I or grade I and II, there is a clear cutoff at 5-mm extracanalicular extension, after which the otherwise excellent results with the MCF start to decline. This is in accordance with the aforementioned studies also using the MCF approach, in which this information was rather undetailed.
A similar observation has been made regarding hearing preservation with different approaches. After surgical removal of VS, hearing preservation rates vary in the literature from 17 to 71%.22,23,24 The reasons for such a wide range of variation are manifold (e.g., divergent considerations of hearing quality, different criteria of patient selection, and individual/institutional surgical expertise). Comparison of results between the MCF and the RS approach shows a tendency for higher preservation rates achieved with the MCF in small tumors.15,20 However, Mangham concluded that the trend toward a better hearing outcome with the MCF may never achieve statistical significance across institutions because of high variability among surgical teams and small numbers of teams reporting results.20 Even if one assumes that there is a real difference of 15% in the success rate between approaches, a sample-size analysis for an unpaired case control study showed that data from 226 middle fossa surgeons and 226 retrosigmoid surgeons were required before one could reliably (90% of the time) detect the 15% difference between groups. Colleti and Fiorino observed that the MF route afforded significantly better results (p < 0.05) than the retrosigmoid transmastoid (RS-TM) route when the distance of the tumor from the IAC fundus was 3 mm or less.16 In contrast, the hearing of their patients was preserved in higher percentages after the RS-TM approach (p < 0.05) when IAC enlargement was greater than 7 mm.
Stratification of hearing outcome, as for facial function outcome, shows a negative impact of increasing tumor size. In the Arts et al report of MCF surgery, the relationship between tumor size and hearing preservation was not as strong as had been previously noted, probably attributable to the relatively uniform size distribution of their tumors ranging from 3 to 18 mm.21 Still, there was a noticeable deterioration in average hearing outcomes as largest tumor dimensions increased beyond 8 mm. By the use of the Mantel-Haenszel χ2 test, Wiet et al revealed that the success of preserving useful hearing with both MCF and RS was strongly correlated with the size of the tumor (p < 0.001).15 They detected a trend toward worsening hearing outcome for VS beyond 1.1 cm, and useful hearing rate (class A and B) dropped from 57% for intracanalicular tumors (in their study defined as tumors with no more than 0.4-cm posterior fossa extension) to 48% for all tumors with ≤ 1.0 cm extension into the CPA, to only 17% of the patients operated on for tumors with < 1.6 cm extension into the CPA. Meyer et al divided 162 tumors of their MCF series in groups of ≤ 1 cm, 1.1 to 1.4 cm, and > 1.5 cm, based on the overall length of the tumor as measured from a T1-weighted MRI scan with gadolinium and included the IAC component as well as the CPA portion of the tumor.10 Class I hearing was postoperatively preserved in 59%, 39%, and 33% of patients, respectively. Measurable word recognition (classes I to III) was preserved in 72%, 42%, 43%, respectively. We have adopted their proposal to use the WRS, because we agree that it is a more sensitive parameter of communication ability than pure-tone thresholds. The patient with good word recognition should benefit significantly from amplification, although a patient with little word recognition and good pure-tone average will benefit less. The results of our present series are in accordance with the Wiet and Meyer studies and demonstrate that chances for successful hearing preservation decrease rapidly with size, and in tumors beyond 1.5-cm extracanalicular extension are below 20%.
Long-Term Outcome
Durability of preserved postoperative hearing is an important issue that justifies the efforts of surgery and puts it into the right perspective. Several studies are concentrated on this topic, and Chee et al summarized the recent reports.12,22 Hearing changes in the operated ear at the preoperative and early postoperative levels were compared with late postoperative hearing, and with hearing on the nonoperated side. In most studies, a significant change was defined as a decline of 15 dB or more on pure tone average (PTA) and/or significant change in speech discrimination as defined in a binomial model by Thorton and Raffin.25 The mean follow-up period in these studies varied between 2.3 and 9.4 years, and the percentage of patients with significant late hearing deterioration on the operated side was between 0 and 56%. Although 29.4% of their patients demonstrated a significant deterioration over the course of time, all of them maintained usable hearing, and Tucci et al concluded that long-term hearing preservation is a realistic goal in selected acoustic neuroma operations.26 Changes in hearing did not correlate with tumor size, preoperative hearing, intraoperative change in hearing, the interval between initial symptoms and surgery, sex, or age in one study,27 and hearing at long-term follow-up was significantly better in patients with excellent preoperative hearing in another study.28 Hearing preservation proved important and realistic in our study, too, as at 5 years after surgery it remained preserved in all patients in whom it had been preserved in the early postoperative period. A significant change was noticed in 37% of patients, and specifically at higher frequencies above 1 kHz. The reason for the delayed hearing loss in patients who undergo VS surgery is still unknown. Histopathologic studies on the temporal bone demonstrated new bone formation within the cochlea suggestive of vascular etiology for the hearing loss,27,29 but this still remains to be further explained.
CONCLUSION
Our results show that gain in VS size influences the functional results of facial nerve preservation with MCF at an earlier stage than with TL or RS approach. Also, chances for successful hearing preservation decrease rapidly with size, from 67% measurable speech recognition in intracanalicular tumors to 21% in tumors with 1.5-cm extracanalicular extension. Best results, considering both parameters, are achieved in tumors up to 5 mm in the CPA. Consequently, although an expectant policy with small tumors may be reasonable in some instances, it is not so for MCF candidates. In these patients, the decision for surgery should be favored before VS extends beyond 5 mm in the CPA.
REFERENCES
- Stangerup S E, Caye-Thomasen P, Tos M, Thomsen J. The natural history of vestibular schwannoma. Otol Neurotol. 2006;27:547–552. doi: 10.1097/01.mao.0000217356.73463.e7. [DOI] [PubMed] [Google Scholar]
- Tos M, Charabi S, Thomsen J. Clinical experience with vestibular schwannomas: epidemiology, symptomatology, diagnosis, and surgical results. Eur Arch Otorhinolaryngol. 1998;255:1–6. doi: 10.1007/s004050050012. [DOI] [PubMed] [Google Scholar]
- Sterkers J M, Morrison G A, Sterkers O, El-Dine M M. Preservation of facial, cochlear, and other nerve functions in acoustic neuroma treatment. Otolaryngol Head Neck Surg. 1994;110:146–155. doi: 10.1177/019459989411000202. [DOI] [PubMed] [Google Scholar]
- Magnan J, Barbieri M, Mora R, et al. Retrosigmoid approach for small and medium-sized acoustic neuromas. Otol Neurotol. 2002;23:141–145. doi: 10.1097/00129492-200203000-00006. [DOI] [PubMed] [Google Scholar]
- Slattery W H, Brackmann D E, Hitselberger W. Middle fossa approach for hearing preservation with acoustic neuromas. Am J Otol. 1997;18:596–601. [PubMed] [Google Scholar]
- Weber P C, Gantz B J. Results and complications from acoustic neuroma excision via middle cranial fossa approach. Am J Otol. 1996;17:669–675. [PubMed] [Google Scholar]
- Gjuric M, Wigand M E, Wolf S R. Enlarged middle fossa vestibular schwannoma surgery: experience with 735 cases. Otol Neurotol. 2001;22:223–230. doi: 10.1097/00129492-200103000-00019. [DOI] [PubMed] [Google Scholar]
- Gjuric M. Enlarged middle fossa surgery: indications, advantages, and surgical technique. Mediterr J Otol. 2005;1:128–135. [Google Scholar]
- House J W, Brackmann D E. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- Meyer T A, Canty P A, Wilkinson E P, Hansen M R, Rubinstein J T, Gantz B J. Small acoustic neuromas: surgical outcomes versus observation or radiation. Otol Neurotol. 2006;27:380–392. doi: 10.1097/00129492-200604000-00015. [DOI] [PubMed] [Google Scholar]
- Monsell E M, Balkany T A, Gates G A, Goldenberg R A, Meyerlesff W L, House J W. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma (vestibular schwannoma) Otolaryngol Head Neck Surg. 1995;113:179–180. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- Friedman R A, Kesser B, Brackmann D E, Fisher L M, Slattery W H, Hitselberger W E. Long-term hearing preservation after middle fossa removal of vestibular schwannoma. Otolaryngol Head Neck Surg. 2003;129:660–665. doi: 10.1016/j.otohns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Arriaga M A, Chen D A. Facial function in hearing preservation acoustic neuroma surgery. Arch Otolaryngol Head Neck Surg. 2001;127:543–546. doi: 10.1001/archotol.127.5.543. [DOI] [PubMed] [Google Scholar]
- Isaacson B, Telian S A, El-Kashlan H K. Facial nerve outcomes in middle cranial fossa vs translabyrinthine approaches. Otolaryngol Head Neck Surg. 2005;133:906–910. doi: 10.1016/j.otohns.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Wiet R J, Mamikoglu B, Odom L, Hoistad D L. Long-term results of the first 500 cases of acoustic neuroma surgery. Otolaryngol Head Neck Surg. 2001;124:645–651. doi: 10.1177/019459980112400609. [DOI] [PubMed] [Google Scholar]
- Colletti V, Fiorino F. Is the middle fossa approach the treatment of choice for intracanalicular vestibular schwannoma? Otolaryngol Head Neck Surg. 2005;132:459–466. doi: 10.1016/j.otohns.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Wiegand D A, Ojemann R G, Fickel V. Surgical treatment of acoustic neuroma (vestibular schwannoma) in the United States: report from the Acoustic Neuroma Registry. Laryngoscope. 1996;106(1 Pt 1):58–66. doi: 10.1097/00005537-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Driscoll C L, Jackler R K, Pitts L H, Banthia V. Is the entire fundus of the internal auditory canal visible during the middle fossa approach for acoustic neuroma? Am J Otol. 2000;21:382–388. doi: 10.1016/s0196-0709(00)80048-4. [DOI] [PubMed] [Google Scholar]
- Kumon Y, Sasaki S, Kohno K, et al. Selection of surgical approaches for small acoustic neuromas. Surg Neurol. 2000;53:52–60. doi: 10.1016/s0090-3019(99)00199-8. [DOI] [PubMed] [Google Scholar]
- Mangham C A., Jr Retrosigmoid versus middle fossa surgery for small vestibular schwannomas. Laryngoscope. 2004;114:1455–1461. doi: 10.1097/00005537-200408000-00026. [DOI] [PubMed] [Google Scholar]
- Arts H A, Telian S A, El-Kashlan H, Thompson B G. Hearing preservation and facial nerve outcomes in vestibular schwannoma surgery: results using the middle cranial fossa approach. Otol Neurotol. 2006;27:234–241. doi: 10.1097/01.mao.0000185153.54457.16. [DOI] [PubMed] [Google Scholar]
- Chee G H, Nedzelski J M, Rowed D. Acoustic neuroma surgery: the results of long-term hearing preservation. Otol Neurotol. 2003;24:672–676. doi: 10.1097/00129492-200307000-00023. [DOI] [PubMed] [Google Scholar]
- Brackmann D E, House J R, Hitselberger W E. Technical modifications to the middle fossa craniotomy approach in removal of acoustic neuromas. Am J Otol. 1994;15:614–619. [PubMed] [Google Scholar]
- Gjuric M, Zizic Mitrecic M, Greess H, Berg M. Vestibular schwannoma volume as a predictor of hearing outcome after surgery. Otol Neurotol. 2007;28:822–827. doi: 10.1097/MAO.0b013e318068b2b0. [DOI] [PubMed] [Google Scholar]
- Thornton A R, Raffin M J. Speech discrimination scores modeled as a binomial variable. J Speech Hear Res. 1978;21:507–518. doi: 10.1044/jshr.2103.507. [DOI] [PubMed] [Google Scholar]
- Tucci D L, Telian S A, Kileny P R, Hoff J T, Kemink J L. Stability of hearing preservation following acoustic neuroma surgery. Am J Otol. 1994;15:183–188. [PubMed] [Google Scholar]
- McKenna M J, Halpin C, Ojemann R G, et al. Long-term hearing results in patients after surgical removal of acoustic tumors with hearing preservation. Am J Otol. 1992;13:134–136. [PubMed] [Google Scholar]
- Umezu H, Aiba T, Tsuchida S, Seki Y. Early and late postoperative hearing preservation in patients with acoustic neuromas. Neurosurgery. 1996;39:267–271. discussion 271–272. doi: 10.1097/00006123-199608000-00007. [DOI] [PubMed] [Google Scholar]
- Shelton C, Hitselberger W E, House W F, Brackmann D E. Hearing preservation after acoustic tumor removal: long-term results. Laryngoscope. 1990;100(2 Pt 1):115–119. doi: 10.1288/00005537-199002000-00001. [DOI] [PubMed] [Google Scholar]