ABSTRACT
Inflammatory myofibroblastic tumor (IMT) is a rare lesion of unknown etiology and difficult diagnosis. The treatment of IMT is controversial. We report a case of IMT of the temporal bone in a young man presenting with a progressive hearing loss. Three years after diagnosis, partial hearing improvement has been documented.
Keywords: Inflammatory pseudotumor, skull base, temporal bone, hearing loss, inflammatory myofibroblastic tumor
Inflammatory myofibroblastic tumor (IMT) is rare.1 The cause of IMT is not known, and no local or systemic causes have been identified. First described in the lung, it has been found in every part of the body.2 In the head and neck, the orbit is the most common site. Involvement of the skull base is rare, with fewer than 15 cases described in the literature. Histologically, IMT consists of an acute and chronic inflammatory cell infiltrate that stimulates a fibrotic reaction. The diagnosis is based on the histopathologic appearances and is confirmed by inmunohistochemical techniques. IMT often has aggressive features that mimic malignant tumors, and it can be difficult to make the diagnosis.3 The treatment of IMT is controversial. Radical surgery, high-dose intravenous steroids, and radiation therapy have been employed, with the decision on treatment modality based on tumor location, size, and behavior. We report a case of IMT of the temporal bone in a young man, which had aggressive clinical features. The patient presented with a progressive hearing loss. Three years after diagnosis, partial hearing improvement has been documented.
CASE REPORT
A 28-year-old man was admitted with a 2-month history of left-sided hearing loss, headache, and otalgia. His hearing loss was not associated with discharge, tinnitus, or vertigo. He had not had previous ear infections. His past medical history was notable only for epilepsy that was controlled by medication. On otoscopy, there appeared to be a grayish mass within the middle ear, which distorted tympanic membrane. Audiometry revealed a severe sensoneural hearing loss on the left side with 0% discrimination at 100 dB. His hearing acuity on the contralateral hearing was normal (Fig. 1). High-resolution computed tomography (CT) scans of the temporal bone (Fig. 2) showed a lytic mass lesion that involved the petrous apex, retropharyngeal space, and middle ear. On magnetic resonance (MR) imaging, the lesion was characterized by heterogeneous signal in T2-weighted images and by intermediate signal intensity in T1-weighted images, with irregular enhancement after the administration of contrast (Fig. 3). The transverse and left sigmoid sinuses were shown to be patent by MR venography, but the internal jugular vein was not visible. Routine blood tests were normal.
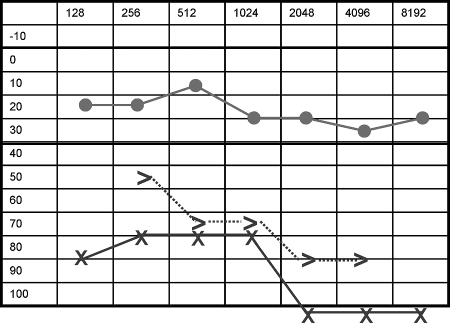
Figure 1.
Pure tone audiometry (PTA) showing sensoneural hearing loss in the left ear.
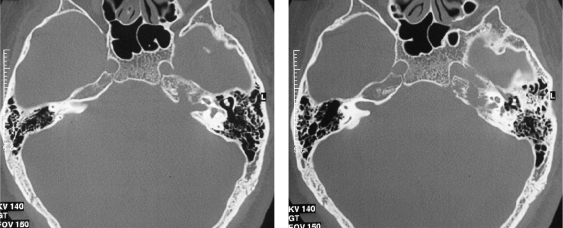
Figure 2.
Axial computed tomography scan showing bone changes affecting the left petrous apex, the lateral aspect of the clivus, and the jugular foramen.
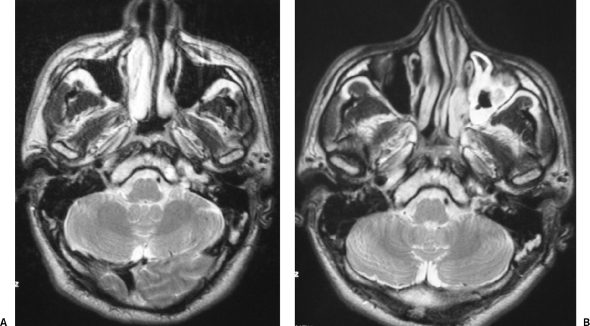
Figure 3.
(A) Preoperative axial T2-weighted magnetic resonance (MR) image reveals a heterogeneous high-intensity lesion affecting the petrous apex and the left aspect of the clivus. (B) Postoperative axial T2-weighted MR image demonstrates a slight reduction in size of the lesion.
An exploratory tympanotomy and examination of the nasopharynx under anesthetic were undertaken together with biopsies. These investigations were nondiagnostic. Subsequently, an infracochlear approach was performed and the petrous apex opened. This contained hypertrophic mucosa, and there was clear evidence of bone erosion. Biopsies were taken. The patient's postoperative course was uneventful. Histopathologic examination of the biopsy material showed spindle-shaped cells with very low mitotic activity, loosely arranged in a background of inflammatory cells. The spindle cells stained with smooth muscle actin but were negative for ALK (Fig. 4). Plasma cells predominated over lymphocytes, which were found in small aggregates (Fig. 5). Staining with kappa and lambda antibodies demonstrated the polyclonal nature of the plasma cells. The histopathologic diagnosis was inflammatory myofibroblastic tumor. After a period of recovery, CT and MR scans were acquired to act as baseline studies for the future. Over a 3-year period, the lesion showed no progression. Repeated audiometry throughout this period has documented partial hearing recovery such that the patient has 50% discrimination at 85 dB on speech audiometry.
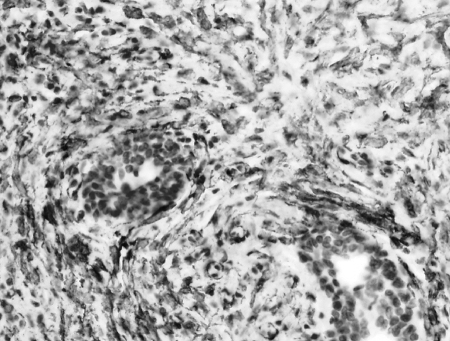
Figure 4.
Spindle-shaped cells with very low mitotic activity, loosely arranged in a background of inflammatory cells. The spindle cells stained with smooth muscle actin but were negative for ALK.
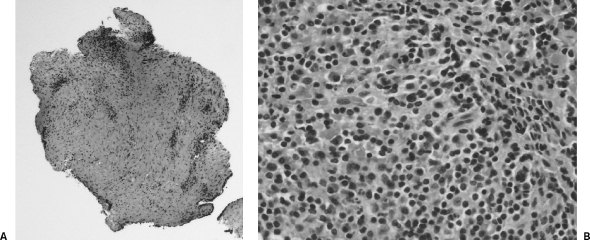
Figure 5.
(A) Low-power view of connective tissue with a chronic inflammatory infiltration. (B) High-power view of a chronic inflammatory infiltration of lymphocytes and plasma cells.
DISCUSSION
IMT is a distinct lesion composed of myofibroblastic cells mixed with inflammatory cells.4,5 It rarely involves the temporal bone. In this location, it usually shows a more aggressive behavior compared with those arising in the orbit. Its histological appearance can show marked variation, and, as a result, it has acquired several names that include plasma cell granuloma, xanthomatous pseudotumor, pseudosarcomatous myofibroblastic proliferation, myofibroblastoma, inflammatory myofibrohistiocytic proliferation, and, most commonly, inflammatory pseudotumor. In our case, the lesion showed a prominent inflammatory infiltrate with bland spindle cells. The cause and pathogenesis of IMT are not known. According to Williamson et al, whatever the cause, a localized derangement of the immune response after an insult may be an underlying mechanism.6 Surgery and trauma have been suggested the progenitors of this altered immune response.7 Routine bacterial and fungal cultures are usually negative. However, IMT has also been proposed to be an altered response to an unknown infectious pathogen.8 Clinically, IMT of the head and neck most frequently presents with swelling, pain, and obstruction of the involved tract, such as the pharynx,9 larynx, and paranasal sinuses.2,8 IMT arising in the ear or the temporal bone may cause otalgia, otorrhea, vertigo, hearing loss, and even complete deafness.10 CT and MR imaging are central to the evaluation and subsequent treatment of IMT of the temporal bone. CT shows a soft tissue mass, usually circumscribed, with strong enhancement after contrast administration. CT is particularly useful when assessing bone erosion and response to treatment. Mulder et al found destruction of the inner ear and variable degrees of erosion of semicircular canals and cochlea in their three cases.10 Chen et al reported three cases of IMT arising in the petrous apex. Aggressive features with bone erosion were found in every case.11 MR imaging characteristically show a soft tissue mass with low signal intensity on T2-weighted images and homogeneous contrast enhancement.12 Han et al13 suggested that this hypointensity may be explained by the relative lack of mobile protons within the fibrotic lesion. Low signal intensity is also characteristic of malignant lesions, which must be included in the differential diagnosis. In our patient, the lesion gave heterogeneous but predominantly high signal intensity on T2-weighted images and had heterogeneous contrast enhancement, especially peripherally. The radiological appearances were atypical, and this made diagnosis more difficult. It is also very unusual to document spontaneous radiological improvement of IMT as in our patient, with bone regeneration observed on CT and diminution in size and enhancement observed with MR.
Macroscopically, IMT presents as a solid, rubbery mass. The classic microscopic appearance is that of a mixture of spindle cells with fibroblasts and myofibroblasts and a variable inflammatory infiltrate, with plasma cells predominating over lymphocytes. There is usually minimal mitosis and nuclear pleomorphism. The ratio of cells to stroma is variable. Han et al found no relationship between the duration of signs and symptoms and the degree of fibrosis.13 The diagnosis of IMT is one of exclusion. The differential diagnoses include: Wegener's granulomatosis, chronic osteomyelitis, sarcoidosis, cholesterol granuloma, cholesteatoma, histiocytosis X, eosinophilic granuloma, lymphoproliferative disorders, and other malignancies.3
Management options include high-dose intravenous corticosteroids, subtotal or total surgical excision, and radiotherapy. However, the treatment of choice remains controversial. Some recommend that steroids should be tried in the first instance,8 although others propose surgery.6,14 Radiotherapy is employed when the resection is not possible or the patient has no response to medical treatment. It would seem to have a limited role, as Lee et al15 reported after low-dose radiotherapy in a series of six patients. Our patient recovered without any treatment other than a biopsy and has been observed for 3 years. Repeated CT and MR scans have demonstrated a reduction in size of the mass. The last audiometric examination documented sustained improvement in his hearing, from 0% discrimination at 100 dB to 50% discrimination at 85 dB over the time period.
In conclusion, IMT of the temporal bone is a rare condition. Because of its potentially aggressive behavior, the diagnosis is always a challenge. Treatment of this condition remains controversial and our case fuels the debate.
REFERENCES
- Coffin C M, Watterson J, Priest J R, et al. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and inmunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- De Vuysere S, Hermans R, Sciot R, Crevits I, Marchal G. Extraorbital inflammatory pseudotumor of the head and neck: CT and MR findings in three patients. AJNR Am J Neuroradiol. 1999;20:1133–1139. [PMC free article] [PubMed] [Google Scholar]
- Schönermark M P, Issing P, Stöver T, Ruh S, Lenarz T. Fibroinflammatory pseudotumor of the temporal bone. Skull Base Surg. 1998;8:45–50. doi: 10.1055/s-2008-1058590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S W, Goldblum J R. Enzinger and Weiss Soft Tissue Tumors, 5th ed. Philadelphia: Mosby; 2007. pp. 284–289.
- Barnes L, Eveson JW, Reichart P, Sidransky D, editor. World Health Organization Classification of Tumors. Pathology and Genetics of Head and Neck Tumors. Lyon: IARC Press; 2005. pp. 150–151.
- Williamson R A, Paueksakon P, Coker N J. Inflammatory pseudotumor of the temporal bone. Otol Neurotol. 2003;24:818–822. doi: 10.1097/00129492-200309000-00021. [DOI] [PubMed] [Google Scholar]
- Chan J K. Inflammatory pseudotumor: a family of lesions of diverse nature and etiologies. Adv Anat Pathol. 1996;3:156–169. [Google Scholar]
- Som P M, Brandwein M S, Maldjian C, Reino A J, Lawson W. Inflammatory pseudotumor of the maxillary sinus: CT and MR findings in six cases. AJR Am J Roentgenol. 1994;163:689–692. doi: 10.2214/ajr.163.3.8079869. [DOI] [PubMed] [Google Scholar]
- Hytiroglou P, Brandwein M S, Strauchen J A, Mirante J P, Urken M L, Biller H F. Inflammatory pseudotumor of the parapharyngeal space: case report ad review of the literature. Head Neck. 1992;14:230–234. doi: 10.1002/hed.2880140311. [DOI] [PubMed] [Google Scholar]
- Mulder J J, Cremers W R, Joosten F, Wiersma A, den Broek P van. Fibroinflammatory pseudotumor of the ear: a locally destructive benign lesion. Arch Otolaryngol Head Neck Surg. 1995;121:930–933. doi: 10.1001/archotol.1995.01890080096019. [DOI] [PubMed] [Google Scholar]
- Chen J M, Moll C, Schotton J C, Fisch U. Inflammatory pseudotumors of the skull base. Skull Base Surg. 1994;4:93–98. doi: 10.1055/s-2008-1058977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparotti R, Zanetti D, Bolzoni A, Gamba P, Morassi M L, Ungari M. Inflammatory myofibroblastic tumor of the temporal bone. AJNR Am J Neuroradiol. 2003;24:2092–2096. [PMC free article] [PubMed] [Google Scholar]
- Han M H, Chi J G, Kim M S, et al. Fibrosing inflammatory pseudotumors involving the skull base: MR and CT manifestations with histopathologic comparison. AJNR Am J Neuroradiol. 1996;17:515–521. [PMC free article] [PubMed] [Google Scholar]
- Ruaux C, Noret P, Godey B. Inflammatory pseudotumor of the nasal cavity and sinuses. J Laryngol Otol. 2001;115:563–566. doi: 10.1258/0022215011908234. [DOI] [PubMed] [Google Scholar]
- Lee D K, Cho Y, Hong S H, Chung Q H, Ahn Y C. Inflammatory pseudotumor involving the skull base: response to steroid and radiation therapy. Otolaryngol Head Neck Surg. 2006;135:144–148. doi: 10.1016/j.otohns.2006.01.016. [DOI] [PubMed] [Google Scholar]