ABSTRACT
Objectives: To determine if a relationship exists between the presence of estrogen receptors (ER), progesterone receptors (PR), or vascular endothelial growth factor (VEGF) and the size, growth rate, and behavior of vestibular schwannoma tumors. Design: Nine tumor samples from young female patients with large vestibular schwannoma tumors were preselected because they were presumed to be faster growing, more aggressive tumors. Immunohistochemical staining was performed using monoclonal mouse antibodies to ER, PR, and VEGF. Results: The mean age of the study sample was 32.3 years, mean tumor size was 3.2 cm, and the average growth rate was 0.4 cm per 2 months. The results of immunohistochemical staining for ER and PR in all nine samples were unequivocally negative. Eight of nine tumor samples stained positive for VEGF, with five demonstrating low intensity and three demonstrating moderate intensity staining. Conclusions: There is histopathological evidence for the expression of VEGF in vestibular schwannomas but not for ER and PR. Further studies are necessary to determine the role of VEGF and other molecular pathways in the growth of vestibular schwannomas and the application of anti-VEGF therapy as a potential treatment option in the future.
Keywords: Vestibular schwannoma, acoustic neuroma, estrogen receptor, progesterone receptor, vascular endothelial growth factor, immunohistochemistry
The management of vestibular schwannoma (VS) has become more patient-focused and less aggressive in the past decade thanks to the diagnostic accuracy of magnetic resonance imaging (MRI) and a better understanding of the natural history and behavior of this neoplasm. The options of surgery, radiotherapy, and no treatment (expectant management with serial scanning) are usually offered leaving the decision of treatment to the patient; how the decision is arrived at reflects the patient's understanding of benefits and risks, and through whom information is obtained. Tumor size and patient's age are the two dominant parameters in the decision-making process. What is missing is a reliable way of demonstrating growth potential and tumor aggressiveness; presently, this is only possible through watchful waiting.
The growth of VS is difficult to determine clinically as it is not directly correlated to tumor size, symptoms, the duration of symptoms, or patient's age.1 Many studies evaluating the general growth rate of VS with serial MRI scans agree on the presence of three different growth patterns when tumors were followed expectantly2,3,4,5,6,7,8; 60% showed very slow to no growth, 30% had an approximate growth rate of 0.2 cm per year, and 10% grew at a rapid rate of 1 cm or more per year.8 The rate of growth of an individual tumor was relatively constant.6,9 It is generally believed that in solid tumors, early determination of growth rate with sequential radiological studies may be predictive of future tumor behavior.6,9 By and large, VS grow slowly regardless of the size at presentation. With this understanding, in the past decade, the pendulum has swung toward more conservative management with “watchful waiting” particularly in those with small- to medium-sized tumors.
There are several clinical scenarios that are considered less desirable for conservative management, or for radiotherapy. In the case of a cystic VS, rapid tumor growth can occur without warning, resulting in compressive symptoms of the posterior fossa with sudden neurological deterioration. Histopathological and imaging studies have shown that this increase in volume is due to the rapid expansion of the cystic element of the tumor and not by an actual increase in cellular growth.10,11 The diagnosis of a VS during pregnancy or in a female who is actively seeking pregnancy is also very disconcerting, since rapid cellular proliferation and growth can occur. Early surgical intervention is usually recommended.
In spite of the recognition of VS tumor growth and pregnancy, there is insufficient evidence to link this phenomenon to hormonal influence. The role of steroid hormones has been demonstrated in neoplasms such as breast cancer to stimulate growth both in vivo and in vitro.12,13 This knowledge has resulted in several therapeutic measures aimed at estrogen ablation (ovariectomy, adrenalectomy, or hypophysectomy) and antiestrogen therapy (tamoxifen) to inhibit tumor growth. The absolute amount of estrogen receptor (ER) found in the tumor can be utilized to predict the response to these therapies.12,13 Patients with ER-negative tumors (ER–) have earlier recurrence and poorer prognosis for survival than patients with ER-positive tumors (ER +). The progesterone receptor (PR) level appears to indicate the level of functional estrogenic stimulus.12 The successful application of these concepts to the treatment of breast cancer has led to a search for other hormone-responsive neoplasms.
Hormonal triggers in the growth of VS are unclear. Certain clinical features of VS have suggested a possible association between the growth of these tumors and sex hormone status. The potential of a hormone-responsive Schwann cell tumor is based on the following observations: (1) this neoplasm tends to occur more frequently in women14; (2) the course of growth is more rapid during periods of hormonal change such as the end of the menstrual cycle or during pregnancy15; and (3) these neoplasms tend to be larger and more vascular in females compared with males.16 Other investigators have focused on proangiogenic factors that influence tumor growth. The expansion of any solid tumor with a volume greater than 2 or 3 mm3 requires angiogenesis to meet the oxygen and nutrient demands of the enlarging tissue.17,18 Vascular endothelial growth factor (VEGF) induces angiogenesis through endothelial cell proliferation and migration. It can be found in circulating thrombocytes and is secreted by several cell types such as macrophages, leukocytes, and smooth muscle cells. Although VEGF has been correlated with several types of tumors and is considered one of the most potent proangiogenic factors, its role in VS has not been clarified. Growth rate and size have been shown to correlate significantly with the total number of vessels.19 It has also been shown that the region of neovascularization tends to be at the expanding surface zone of the tumor.20 This knowledge has led to the search for a probable role of angiogenic growth factors in VS.
The aim of this study is to determine whether relationships exist between the presence of ER, PR, or VEGF and the size, growth rate, and behavior of VS tumors.
METHODS
Study Group
Nine women with unilateral VS were selected as the primary study group. Patient selection was based on the sex, age, and tumor size at the time of surgery. None of the selected patients had evidence of neurofibromatosis. All tumors were surgically removed by the translabyrinthine approach at Sunnybrook Health Sciences Centre between April 2003 and February 2006. All were confirmed as schwannomas histologically.
Immunohistochemical Analysis: Estrogen and Progesterone Receptors, VEGF
Immunoperoxidase staining for estrogen and progesterone receptors and VEGF was performed on formalin-fixed, paraffin-embedded tissue sections, using the avidin-biotin peroxidase complex (ABC) method. The blocks of tissue were obtained from intracapsular tumor biopsies and were histologically confirmed to be schwannomas. They were cut into 4 μm sections and placed on a glass slide. The section was deparaffinized and pretreated with hydrogen peroxide to remove any endogenous peroxidase enzyme activity inherent in the tissue that might produce nonspecific staining. The samples were then incubated with primary mouse monoclonal antibodies to ER (1:100; 6F11, Novocastra, Newcastle, England), PR (1:100; 312, Novocastra), and VEGF (1:200; Clone VG1, MS-1467, Laboratory Vision, Fremont, CA). The sections were washed and a “biotinylated” secondary antibody directed against the Fc portion of the primary antibody was applied to the section. The section was incubated then washed to remove any excess secondary antibody. An ABC was then added. The section was washed again to remove any excess ABC complexes and incubated with diaminobenzidine. The sections were counterstained with hematoxylin. Breast tissue was used as an appropriate positive and negative control for estrogen and progesterone receptors and skin angiosarcoma was used as a positive VEGF control.
Estrogen and progesterone receptor reactions were quantified based on the suggested level of estradiol binding that will correlate with response to hormonal therapy in breast cancer. A strong nuclear staining in greater than 10% of the cells indicated a positive receptor value. The staining intensity of VEGF within the schwannoma cells was classified as “0” (no staining), “+” (weak staining/staining in a few cells), “++” (moderate staining), “+++” (strong staining), and “++++” (very strong staining). Two neuropathologists viewed all nine stained sections individually to control for interobserver variation.
RESULTS
The nine women selected for this study ranged in age from 20 to 42 years (mean, 32.3 years) at the time of tumor surgery. Tumor within the cerebellopontine angle was measured by MRI, in three dimensions: transverse, anteroposterior, and height. Tumor size ranged from 1.3 to 4.3 cm in maximal dimensions within the cerebellopontine angle (mean, 3.2 cm).
The most common presenting symptoms for these patients were unilateral hearing loss, tinnitus, and imbalance. Three of the nine women had follow-up imaging ranging from 2 to 5 months' duration with either MRI or CT scan. Expectant management with serial scanning was not a treatment option in the remaining six patients due to either the large tumor size at diagnosis or the management preference of the patient. The average growth rate of the tumors in this study was 0.4 cm per 2 months (ranging from 0.3 to 0.45 cm per 2 months). These results are summarized in Table 1.
Table 1.
Patient Demographics
| Patient Age | Tumor Size (cm) | Growth Rate (cm/2 months) | GPN Status | Pregnancy History† | ER/PR Staining | VEGF Staining |
|---|---|---|---|---|---|---|
| Tumor size is based on maximal tumor dimension. | ||||||
| GPN, gravapara number; n/a, not applicable; –, negative; +, weak; + +, moderate. | ||||||
| 32 | 3.65 | 0.34 | G1P1 | During third trimester | – | ++ |
| 29 | 3.8 | 0.4 | G1P0 | No | – | + |
| 35 | 3.5 | n/a | G0P0 | No | – | ++ |
| 27 | 4.3 | 0.3 | G0P0 | No | – | + |
| 31 | 3.0 | n/a* | G1P1 | During second trimester | – | + |
| 42 | 1.7 | n/a* | G2P2 | No | – | + |
| 38 | 3.6 | n/a* | G1P1 | Within 6 months postpartum | – | + |
| 37 | 4.0 | n/a* | G2P2 | 2 years postpartum | – | ++ |
| 20 | 1.3 | n/a* | G0P0 | No | – | – |
Cannot calculate growth rate in cases where observation with serial scanning was not performed prior to surgery.
Pregnancy status at time of diagnosis or presentation of initial symptoms.
The results of the immunohistochemical staining for ER and PR in all nine cases were unequivocally negative. Immunohistochemical staining for VEGF was positive in eight out of nine cases. Positive expression of VEGF in the tumor samples was a finely granular cytoplasmic staining of the cells with intensified focal staining in the perinuclear region (Fig. 1). Of the tumor samples demonstrating VEGF expression, five revealed low intensity (+) and three revealed moderate (++) intensity. There was only slight variation of staining within the tissue of each individual tumor. The grading of VEGF stain reaction was performed on the basis of the whole section and in all nine cases was the same for both investigators. Staining also occurred in the cytoplasm of endothelial cells within vessel lumen as expected.
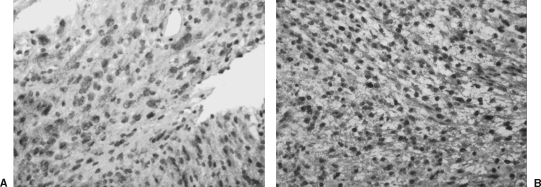
Figure 1.
(A) A vestibular schwannoma displays weak (+) expression of vascular endothelial growth factor (VEGF) (immunohistochemistry and hematoxylin; original magnification 200 ×). (B) A vestibular schwannoma displays moderate ( + +) expression of VEGF in the cytoplasm and especially in the perinuclear region of many of the tumor cells (immunohistochemistry and hematoxylin; original magnification 200 ×).
DISCUSSION
It is generally agreed that a diagnosis of a VS during pregnancy is a source of great concern to the treating physician. For these patients, an expectant approach to treatment or radiotherapy may not be an option. Following a VS with sequential radiographic imaging runs the risk of losing a window of opportunity to operate on a smaller tumor with relatively less morbidity from surgery. Should a tumor grow rapidly, the risks of surgery become greater, particularly with regard to the facial nerve.
Since operative risks and complications are often a function of tumor size,21 attempts at pharmacological manipulation to induce tumor regression have been made. The theoretical potential for treating VS with hormonal manipulation or anti-VEGF antibodies to induce tumor regression preoperatively is to improve surgical outcome for patients with large tumors. This therapy could also be applied to eradicate residual tumor postoperatively or those with unresectable tumors. Despite this potential application, several clinical studies have used antiestrogen therapy empirically in the management of inoperable or recurrent meningiomas with negative results.22,23 Sequential CT scanning following tamoxifen and medroxyprogesterone acetate treatment have been conducted for periods up to 2 years with no objective response obtained.22,23 However, in only 2 of the 11 cases examined in these studies was the sex hormone receptor profile of the tumor known; in both cases, only low levels of PR were noted. Currently, there have not been similar studies applying anti-VEGF therapy empirically to this patient population.
The observation that VS growth may be under the influence of hormonal factors has been reported in the past. In a study by Beatty and colleagues, unilateral VS were removed from six women during pregnancy or within 10 months postpartum (mean, 5 months). Using immunohistochemical analysis there was no correlation between the quantity of ER or PR and pregnancy.24 Flow cytometry used to assess the proliferative potential of these tumors also showed no correlation to pregnancy. The proliferative potential of a tumor cell is most appreciated if cells in all phases of the growth cycle are identified.25 Flow cytometry, however, only identifies tumor cells in S-phase and is not representative of the overall proliferative potential of a tumor. More recent studies have performed immunostains that label for Ki-67 nuclear antigen because it is expressed in the G1, G2, S, and M phases of the cell cycle. The results of these studies show no correlation between tumor size,25,26 growth rate26,27,28 and Ki-67. Szeremeta and associates29 studied 10 cases of VS with Ki-67 immunostain and noted variations of proliferative indices.
Kasantikul and Brown, using a fluorescent steroid histochemical technique, first reported ER in VS.30 This study has often been criticized for using phosphate-buffered saline as a negative control and estradiol concentrations so high that binding to other nonspecific sites could not be excluded.
Martuza and associates used a radioligand binding method with dextran-coated charcoal absorption on unselected tissue samples. Estrogen receptors were observed in 7 of 16 schwannomas using very low estradiol levels, suggesting the presence of high-affinity estrogen-specific type I binding sites.31 However, if the criteria established for breast cancer are applied to this study, most of the Schwann cell tumors fall into the borderline or negative category. In a similar study, Markwalder and colleagues found 4 and 13 of the 21 schwannoma tissue samples to be considered ER- and PR-positive, respectively, according to the criteria established for breast cancer.22 This study has been criticized for the use of a single saturating dose of hormone32 and the fact that almost all of the patients received preoperative prednisone to decrease brain swelling. The effect of glucocorticoid therapy on the receptor assay is unknown. Similarly, Monsell and Wiet examined intracapsular VS tissues and found 19% of the samples were ER+, 17% were PR+, and 8% were positive for both receptors.33 Contrary to the above studies, other authors using the same staining technique have been unable to demonstrate the presence of ER or PR on acoustic neuroma specimens.34,35
To date there have been several studies in the literature applying an immunohistochemical method to stain for ER and PR on VS samples.24,36,37 One of these studies was performed on pregnant female patients24 while the other two were performed on a consecutive series of unselected VS patients.36,37 There is a consistent finding among these studies demonstrating that ER and PR are not represented in any clinically relevant quantities. In our study, a series of nine young female patients with large VS were preselected because they were presumed to have faster-growing, more aggressive tumors. It was felt by the authors that this select study population would have the highest probability of ER + and PR + findings given their shared characteristics. The results of this study revealed no positive staining for either ER or PR. This fails to provide support to the clinical hypothesis that VS have a potential hormone-responsive property. The methods of assay used were both specific and sensitive. Further investigation is required to provide an explanation for the clinical observations of VS growth patterns in pregnancy.
Contradicting results have also been reported for the role of VEGF in the growth of VS. Brieger and coworkers studied VEGF and other proangiogenic growth factors in 34 patients and concluded that tumor angiogenesis is not likely to be a relevant mechanism of VS growth.38 Contrary to this investigation, two studies by Cayé-Thomasen and associates described the expression of VEGF and VEGF receptor-1 (VEGFR-1) in Schwann cell cytoplasm, with a more intense staining of the perinuclear region in all 15 and 27 patients included in the respective studies. The authors reported a significant correlation between the concentration of VEGF and VEGFR-1 expression in VS and tumor growth rate, but not symptom duration or tumor size.17,18 Our findings are supportive of the results of the latter two investigations that VS cells express VEGF. Whether or not VEGF plays a causative role in the growth of this tumor is unknown.
Experiments have shown that intravenous infusion of anti-VEGF monoclonal antibodies can reduce tumor weight by up to 96% in nude mice injected with malignant tumor cells.39 The anti-VEGF treatment used in this study was directed toward the suppression of VEGF directly or at the receptor level. This may prove to be a potential treatment option for fast-growing VS in the future.
CONCLUSION
There is histopathological evidence for the expression of VEGF in VS but not for ER and PR. A causative role of sex hormones in the growth of VS and hormonal manipulation in the treatment of this disease cannot be supported. Nevertheless, it is possible that sex hormones play an important role in tumor growth through a less direct pathway. There is emerging evidence that Merlin, the protein encoded by the NF2 gene, likely interacts with proteins and molecules that would lead to differential levels of cell regulation and tumor suppression.40 Future studies in the role of VEGF and sex hormones will likely be at the molecular level to have any significant impact on therapy.
REFERENCES
- Leeuwen J PPM van, Cremers C WRJ, Thewissen N PMW, Harhangi B S, Meijer E. Acoustic neuroma: correlation among tumour size, symptoms, and patient age. Laryngoscope. 1995;105:701–707. doi: 10.1288/00005537-199507000-00006. [DOI] [PubMed] [Google Scholar]
- Olivero W C, Lister J R, Elwood P W. The natural history and growth rate of asymptomatic meningiomas: a review of 60 patients. J Neurosurg. 1995;83:222–224. doi: 10.3171/jns.1995.83.2.0222. [DOI] [PubMed] [Google Scholar]
- Wazen J, Silverstein H, Norrell H, Besse B. Preoperative and postoperative growth rates in acoustic neuromas documented with CT scanning. Otolaryngol Head Neck Surg. 1985;93:151–155. doi: 10.1177/019459988509300204. [DOI] [PubMed] [Google Scholar]
- Silverstein H, McDaniel A, Norrell H, Wazen J. Conservative management of acoustic neuroma in the elderly patient. Laryngoscope. 1985;95:766–770. [PubMed] [Google Scholar]
- Gardner G, Moretz W H, Robertson J H, Clark C, Shea J J., Jr Non-surgical management of small and intracanalicular acoustic tumors. Otolaryngol Head Neck Surg. 1986;94:328–333. doi: 10.1177/019459988609400312. [DOI] [PubMed] [Google Scholar]
- Nedzelski J M, Canter R J, Kassel E E, Rowed D W, Tator C H. Is no treatment good treatment in the management of acoustic neuromas in the elderly? Laryngoscope. 1986;96:825–829. doi: 10.1002/lary.1986.96.8.825. [DOI] [PubMed] [Google Scholar]
- Nedzelski J M, Schessel D A, Pfleidera A, Kassel E E, Rowed D W. Conservative management of acoustic neuromas. Otolaryngol Clin North Am. 1992;25:691–704. [PubMed] [Google Scholar]
- Lesser T HJ, Janzer R C, Kleihues P, Fisch U. Clinical growth rate of acoustic schwannomas: correlation with the growth fraction as defined by the monoclonal antibody Ki-67. Skull Base Surg. 1991;1:11–15. doi: 10.1055/s-2008-1056973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasnick B, Glasscock M E, Haynes D, McMenomey S O, Minor L B. The natural history of untreated acoustic neuromas. Laryngoscope. 1994;104:1115–1119. doi: 10.1288/00005537-199409000-00011. [DOI] [PubMed] [Google Scholar]
- Charabi S, Klinken L, Tos M, Thomsen J. Histopathology and growth pattern of cystic neuromas. Laryngoscope. 1994;104:1348–1352. doi: 10.1288/00005537-199411000-00006. [DOI] [PubMed] [Google Scholar]
- Charabi S, Mantoni M, Tos M, Thomsen J. Cystic vestibular schwannomas: neuroimaging and growth rate. J Laryngol Otol. 1994;108:375–379. doi: 10.1017/s0022215100126854. [DOI] [PubMed] [Google Scholar]
- McGuire W L. Steroid receptors in breast cancer treatment strategy. Recent Prog Horm Res. 1980;36:135–156. doi: 10.1016/b978-0-12-571136-4.50010-3. [DOI] [PubMed] [Google Scholar]
- Valavaara R, Kangas L. The significance of estrogen receptors in tamoxifen and toremifene therapy. Ann Clin Res. 1988;20:380–388. [PubMed] [Google Scholar]
- Rausing A, Ybo W, Stenflo J. Intracranial meningioma: a population study of ten years. Acta Neurol Scand. 1970;46:102–110. doi: 10.1111/j.1600-0404.1970.tb05608.x. [DOI] [PubMed] [Google Scholar]
- Bickerstaff E R, Small J M, Guest I A. The relapsing course of certain meningiomas in relation to pregnancy and menstruation. J Neurol Neurosurg Psychiatry. 1958;21:89–91. doi: 10.1136/jnnp.21.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasantikul V, Netsky M G, Glassock M E, III, Hays J W. Acoustic neurolemmoma: clinicoanatomical study of 103 patients. J Neurosurg. 1980;52:28–35. doi: 10.3171/jns.1980.52.1.0028. [DOI] [PubMed] [Google Scholar]
- Cayé-Thomasen P, Baandrup L, Jacobsen G, Thomsen J, Stangerup S E. Immunohistochemical demonstration of vascular endothelial growth factor in vestibular schwannomas correlates to tumor growth rate. Laryngoscope. 2003;113:2129–2134. doi: 10.1097/00005537-200312000-00014. [DOI] [PubMed] [Google Scholar]
- Cayé-Thomasen P, Werther K, Nalla A, et al. VEGF and VEGF receptor-1 concentration in vestibular schwannoma homogenates correlates to tumour growth rate. Otol Neurotol. 2005;26:98–101. doi: 10.1097/00129492-200501000-00017. [DOI] [PubMed] [Google Scholar]
- Labit-Bouvier C, Crebassa B, Bouvier C, Andrac-Meyer L, Magnan J, Charpin C. Clinicopathologic growth factors in vestibular schwannomas: a morphological and immunohistochemical study of 69 tumours. Acta Otolaryngol. 2000;120:950–954. doi: 10.1080/00016480050218681. [DOI] [PubMed] [Google Scholar]
- Charabi S. Acoustic neuroma/vestibular schwannoma in vivo and in vitro growth models. A clinical and experimental study. Acta Otolaryngol Suppl. 1997;530:1–27. [PubMed] [Google Scholar]
- Dutton J EM, Ramsden R T, Lye R H, et al. Acoustic neuroma (schwannoma) surgery. J Laryngol Otol. 1991;105:165–173. doi: 10.1017/s0022215100115270. [DOI] [PubMed] [Google Scholar]
- Markwalder T M, Seiler R W, Zava D T. Antiestrogen therapy of meningioma—a pilot study. Surg Neurol. 1985;24:245–249. doi: 10.1016/0090-3019(85)90030-8. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen J, Laasonen E, Karkkainen J, Haltia M, Troupp H. Hormone treatment of meningioma. Lack of response to medroxyprogesterone acetate (MPA) Acta Neurochir (Wien) 1986;80:35–41. doi: 10.1007/BF01809555. [DOI] [PubMed] [Google Scholar]
- Beatty C W, Scheithauer B W, Katzmann J A, Roche P C, Kjeldahl K S, Ebersold M J. Acoustic schwannoma and pregnancy: a DNA flow cytometric, steroid hormone receptor, and proliferation marker study. Laryngoscope. 1995;105:693–700. doi: 10.1288/00005537-199507000-00005. [DOI] [PubMed] [Google Scholar]
- Chen J M, Houle S, Ang L C, et al. A study of vestibular schwannomas using positron emission tomography and monoclonal antibody Ki-67. Am J Otol. 1998;19:840–845. [PubMed] [Google Scholar]
- Diensthuber M, Brandis A, Lenarz T, Stöver T. Co-expression of transforming growth factor β-1 and glial cell line-derived neurotrophic factor in vestibular schwannoma. Otol Neurotol. 2004;25:359–365. doi: 10.1097/00129492-200405000-00026. [DOI] [PubMed] [Google Scholar]
- O'Reilly B F, Kishore A, Crowther J A, Smith C. Correlation of growth factor receptor expression with clinical growth in vestibular schwannomas. Otol Neurotol. 2004;25:791–796. doi: 10.1097/00129492-200409000-00024. [DOI] [PubMed] [Google Scholar]
- Niemczyk K, Vaneecloo F M, Lecomte M H, et al. Correlation between Ki-67 index and some clinical aspects of acoustic neuromas (vestibular schwannomas) Otolaryngol Head Neck Surg. 2000;123:779–783. doi: 10.1067/mhn.2000.111356. [DOI] [PubMed] [Google Scholar]
- Szeremeta W, Monsell E M, Rock J P, Caccamo D V. Proliferation indices of vestibular schwannomas by Ki-67 and proliferating cell nuclear antigen. Am J Otol. 1995;16:616–619. [PubMed] [Google Scholar]
- Kasantikul V, Brown W J. Estrogen receptors in acoustic neurilemmoma. Surg Neurol. 1981;15:105–109. doi: 10.1016/0090-3019(81)90023-9. [DOI] [PubMed] [Google Scholar]
- Martuza R L, MacLaughlin D T, Ojemann R G. Specific estradiol binding in schwannomas, meningiomas, and neurofibromas. Neurosurgery. 1981;9:665–671. doi: 10.1227/00006123-198112000-00009. [DOI] [PubMed] [Google Scholar]
- Wittliff J L. Steroid-hormone receptors in breast cancer. Cancer. 1984;53:630–643. doi: 10.1002/1097-0142(19840201)53:3+<630::aid-cncr2820531308>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Monsell E M, Wiet R J. Estrogen and progesterone binding by acoustic neuroma tissue. Otolaryngol Head Neck Surg. 1990;103:377–379. doi: 10.1177/019459989010300307. [DOI] [PubMed] [Google Scholar]
- Whittle I R, Hawkins R A, Miller J D. Sex hormone receptors in intracranial tumours and normal brain. Eur J Surg Oncol. 1987;13:303–307. [PubMed] [Google Scholar]
- Siglock T J, Rosenblatt S S, Finck F, House W F, Hitselberger W E. Sex hormone receptors in acoustic neuromas. Am J Otol. 1990;11:237–239. [PubMed] [Google Scholar]
- Klinken L, Thomsen J, Rasmussen B B, Wiet R, Tos M. Estrogen and progesterone receptors in acoustic neuromas. Arch Otolaryngol Head Neck Surg. 1990;116:202–204. doi: 10.1001/archotol.1990.01870020078020. [DOI] [PubMed] [Google Scholar]
- Curley J WA, Ramsden R T, Howell A, Healy K, Lye R H. Oestrogen and progesterone receptors in acoustic neuroma. J Laryngol Otol. 1990;104:865–867. doi: 10.1017/s0022215100114197. [DOI] [PubMed] [Google Scholar]
- Brieger J, Bedavanija A, Jans-Anton L, Maurer J, Mann W. Expression of angiogenic growth factors in acoustic neuroma. Acta Otolaryngol. 2003;123:1040–1045. doi: 10.1080/00016480310005101. [DOI] [PubMed] [Google Scholar]
- Kim K J, Li B, Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumor growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Neff B A, Welling D B, Akhamametyeva E, Chang L S. The molecular biology of vestibular schwannomas: dissecting the pathogenic process at the molecular level. Otol Neurotol. 2006;27:197–208. doi: 10.1097/01.mao.0000180484.24242.54. [DOI] [PubMed] [Google Scholar]