ABSTRACT
Objectives: Endoscopic technology is allowing larger resections of the anterior and middle skull base with resultant dural defects. A pedicled nasal septal flap (NSF) based on the posterior nasal septal artery has recently been developed for closure of these defects. We describe our initial experience with the NSF for vascularized coverage of skull base defects. Design: Retrospective review. Setting: Tertiary care skull base center. Participants: Patients undergoing endoscopic harvest of vascularized pedicled flap for skull base reconstruction. Results: Twenty-eight patients had 32 NSFs raised over 14 months for benign (7) or malignant (21) lesions. Surgical defects (mean, 4.95 cm2) were intracranial (25) and intradural (20, average defect 1.86 cm2) in the anterior (10) and central skull base (6), infratemporal fossa (4), orbit (1), or a combination of sites (9). There were no cases of meningitis or cerebrospinal fluid leak (median follow-up, 8.3 months). Two NSFs were injured intraoperatively and two necrosed postoperatively, both in patients with a prior history of radiation to the nasopharynx (p = 0.013). Conclusions: Prior radiation is a risk factor for necrosis. The NSF is easily harvested endonasally, reliably covers a range of skull base defects, and should be considered the first line closure after expanded endonasal skull base resections.
Keywords: Nasal septal flap, skull base, endoscopic approach
Surgery of the anterior skull base and infratemporal fossa (ITF) has evolved over the past 40 years to incorporate a variety of open approaches, and more recently, endoscopic approaches. Cerebrospinal fluid (CSF) leaks can occur in the anterior and central skull base as a result of these surgeries or due to spontaneous, iatrogenic, or traumatic causes. Closure of these skull base defects is performed to separate the cranial cavity from the sinonasal cavity to prevent infection, pneumocephalus, and a CSF leak.1 Only a few techniques of closure exist due to the limited types of vascularized local flaps. The choice of closure depends on the nature of the diagnosis and the dural defect. Transnasal endoscopic closure of small, spontaneous CSF leaks is a well-established approach that is successful using a variety of techniques.2 Spontaneous or iatrogenic leaks after endoscopic sinus surgery tend to be small in size, less than 5 mm. These leaks are amenable to closure using some combination of inlayed bone, overlayed fascia, and a free mucosal graft.3 After open craniotomy, dural defects are closed with a fascial or xenogenic graft, and a vascularized pericranial flap is laid extradurally along the floor of the anterior cranial fossa immediately over the bony defect.4 When the subcranial approach is used, the pericranial flap can be wrapped around the frontal bone to prevent necrosis, and devascularized fascia lata is used to reconstruct the dura.5 Thus, even with large defects spanning several centimeters, an adequate closure is possible without vascularized tissue along the anterior skull base.
Recently, advances in surgical technique, instrumentation, and intraoperative image guidance are making possible larger endoscopic tumor removals with larger dural defects.6,7,8 Closure after endoscopic tumor resection of lesions of the cribiform9 and ventral skull base10 with devascularized techniques have been described. Closure of CSF leak after trans-sphenoidal pituitary surgery can also be achieved successfully with fat or lyophilized dura graft.11 However, the initial experience after endoscopic resection of skull base tumors using inlayed fascia and a fat graft revealed a 3% culture positive bacterial meningitis rate12 and a CSF leak up to 20%.10 Vascularized septal mucosal flaps have been previously described for closure of small CSF leaks of the anterior skull base13 and pedicled turbinate flaps have been described for the closure of septal perforation14,15,16 and sella floor reconstruction.17 To reduce the high rate of CSF leaks, a broad vascularized nasal septal flap (NSF) based on the posterior nasoseptal artery, a branch of the posterior septal artery, has been formally described for closure of skull base defects.18 The addition of the pedicle originating near the sphenopalatine foramen clearly defines the arterial supply, allows harvest of large flaps composed of nearly the entire septal mucosa, and allows rotation of the flap in the posterior, superior, inferior, or lateral planes. In a series of 43 patients, Fortes et al reported a 5% CSF leak using the Hadad–Bassagasteguy NSF, which is similar to the rate after open craniotomy.18 The same group subsequently described their approach using a pedicled inferior turbinate flap as an alternate flap.19
Since 2006, we have adapted the use of the vascularized, pedicled NSF into our practice for closure of skull base defects after tumor resection. We have found in selected cases that the NSF also appears useful for closure of non-tumor-related CSF leaks in some cases. In some situations, when the NSF is unavailable or inappropriate, an alternate middle turbinate flap can be used. Since the initial description of the NSF, few data have been published regarding the availability, ease of harvest, defect selection, and outcomes of the NSF. We therefore reviewed our initial experience at a skull base surgery center using the NSF or vascularized turbinate flap for closure of skull base defects.
METHODS AND MATERIALS
A retrospective chart review was performed of patients who underwent endoscopic skull base surgery during a 14-month period from November of 2006 through 2007. Patients undergoing surgery for routine pituitary gland tumor surgery were excluded unless an expanded endonasal approach was used, or there was a revision surgery due to persistent CSF leak that was treated with a vascularized septal flap. At our institution, over the time of the study period, wounds for routine pituitary gland tumor resection were closed with devascularized tissue (free tissue fat graft and/or Duragen [Integra Life Sciences, Plainsboro, NJ]). Four patients undergoing endoscopic pituitary surgery who had an endoscopically harvested vascularized flap raised for closure of the skull base defect were identified and included in the study. Patient demographics, pathology, indication for surgery, goal of surgery (curative resection, planned subtotal resection, palliative resection, or closure of CSF) were recorded. A history of prior radiation to the nasopharynx or paranasal sinuses was recorded. The operative reports were reviewed along with surgical videos when available to determine the number of successfully raised flaps, defect characteristics, and technique of wound closure. Defect size of the wound was estimated based on the site of tumor resection (unilateral or bilateral ethmoid, sphenoid sella, sphenoid planum, clivus, pterygoid fossa, nasopharynx) and confirmed radiographically. Dural defect size was estimated by the primary attending surgeon based on the operative report and operative video when available. The time from surgery to radiation was recorded for patients prescribed radiation therapy. Time until mucosa of the flap was visible in clinic setting and flap survival were recorded.
The NSF is harvested as a mucoperichondrial flap based on the posterior nasoseptal artery (Fig. 1). In general, an incision is made along the floor of the nasal cavity from the choanae to 1.5 cm posterior to the caudal septum with needle-point electrocautery. The anterior incision is then made in a superior direction ~1.5 cm posterior to the caudal septum, but the length can be tailored to the defect size. The superior incision is made with microscissors and carried back to the sphenoid face ~1.0 to 1.5 cm below the skull base. The natural ostium of the sphenoid is identified. The mucosa is dissected off the anterior sphenoid wall creating a pedicle of mucosa from the sphenoid ostia to choanae and taken back to the level of the sphenopalatine foramen. This pedicled NSF is then placed in the nasopharynx or the maxillary antrum for the duration of the case. The defect is closed with a layer of collagen matrix (Duragen) inserted deep to the dura. A fat graft is then applied and held in place with a fibrin sealant (Confluent Surgical, Inc., Waltham, MA), and then the NSF is laid over it and secured with DuraSeal (Confluent Surgical Inc.). Septal splints are placed over denuded septal cartilage and bone and left in place for 3 to 4 weeks. The wound is then covered with a thick layer consisting of pieces of gel foam (Pharmacia & Upjohn, Kalamazoo, MI) and then a Foley catheter balloon is used to support the flap and is inflated with 10 cc of saline. Two merocel sponges (Medtronic Xomed, Jacksonville, FL) are often used underneath the balloon. A lumbar drain may be left in place for 3 to 5 days draining 10 to 20 cc/hr depending on the size and nature of the dural defect. The balloon is removed at 3 to 5 days and the merocel sponges, when present, are removed at 10 days. The sponges are also used in our practice to place direct posterior pressure on sphenoclival defects. In addition, when the wound is in the ITF, the sponges act as a layer upon which the Foley balloon can exert pressure.
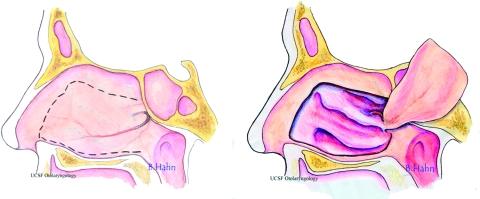
Figure 1.
The nasal septal flap is a mucoperichondrial pedicled flap based on the nasoseptal artery, a branch of the posterior septal artery. The arterial supply is derived from the sphenopalatine artery emanating at the sphenopalatine foramen on the medial nasal cavity wall. The artery then traverses across the anterior wall of the sphenoid sinus below the sphenoid ostium to the nasal septum. Horizontal incisions (dotted line) are made approximately 1.0 to 1.5 cm inferior to the skull base and along the floor of the nasal cavity and joined with a vertical incision made 1.5 to 2 cm posterior to the caudal edge of the septum. (Artwork by Belinda Hahn.)
Demucosalizing the lateral surface of the turbinate creates middle turbinate flaps. The turbinate is fractured and displaced laterally to cover wounds of the ethmoid sinus roof and held in place with DuraSeal and nasal packing. In this series, the ethmoid is first packed with fat grafts.
Data were stored in a Microsoft Excel 2003 file for descriptive statistical evaluation. Statistical analysis of NSF outcomes was performed through the UCSF Department of Biostatistics using the Fisher's exact test. Approval from the Committee on Human Research was obtained prior to initiation of the study.
RESULTS
Thirty patients having a nasally based vascularized flap for closure of a skull base defect were identified. The average age was 52 years (range, 18 to 86 years) with 15 men and 15 women. Median follow-up time was 8.35 months (standard deviation [SD], 4.2; n = 30). Twenty-nine patients had a purely transnasal endoscopic approach while one patient had an endoscopically harvested NSF after orbital exenteration with dural defect. Twenty-five of the cases were performed using the two-surgeon technique with concomitant dissection by the otolaryngology and neurosurgical team. All surgeries except for two (CSF leak repair) were performed by the same otolaryngologic surgeon (IE). All surgeries except for one (pituitary surgery) were performed by one of two neurosurgeons (A.P., M.M.).
Four of 30 patients had surgery for primary pituitary surgery (macroadenoma). One of these patients had a massive tumor extending to the ITF bilaterally, one patient required a transplanum approach, one patient had a macroadenoma in conjunction with a nasopharyngeal squamous cell carcinoma, and the final patient required resection of the clivus along with a sella dissection. The remaining patients in this series were treated for disease of the anterior, central, or lateral skull base or for a CSF leak (Fig. 2).
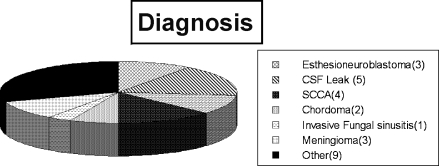
Figure 2.
Diagnosis. Other pathologies were rhabdomyosarcoma, ossifying fibroma, fibrous dysplasia, pancreatic perivascular epithelioid cell tumor, adenoid cystic carcinoma, synchronous nasopharyngeal carcinoma and pituitary adenoma, Wegner's granulomatosous, adenocarcinoma, and schwannomma. CSF, cerebrospinal fluid; SCCA, squamous cell carcinoma.
Indications for surgery included benign tumors (8), malignant tumors (21), and invasive fungal sinusitis (1). The goal of surgery was complete resection of tumor for cure (12); resection for local control, nerve decompression, subtotal resection of tumor prior to radiation (12); and closure of CSF leak (6). Seven patients had resection of tumor from the ITF in conjunction with central or anterior skull base dissection.
Thirty patients were identified with a total of 35 vascularized flaps raised. Thirty-two flaps were vascularized NSFs based on the posterior nasal artery. One NSF was a posteriorly based random flap for a small CSF leak, and three flaps were middle turbinate flaps used to close the fovea ethmoidalis. Of the patients with middle turbinate flaps, one patient had a large septal perforation after prior trans-sphenoidal surgery. The patient had bilateral fovea ethmoidalis skull base fractures with pneumocephalus after a history of open craniotomy. The septum and turbinates were intact and therefore the fovea ethmoidalis was denuded of mucosa and the ethmoids were obliterated with free fat grafts that were covered with a middle turbinate flap.
In four cases, bilateral NSFs were raised. In these cases, the first NSF was raised in the described manner with the caudal incision made 1.5 to 2 cm posterior to the caudal septum. After raising the first flap, the posterior septum is normally transected for access to the central skull base. This portion of mucosa can be saved as a second flap by vertically incising 2 cm anterior to the sphenoid rostrum and elevating the mucosa posteriorly. Alternatively, a complete second flap may be elevated.
Two of 32 NSFs (6%) were injured during the flap harvest (1) or during the course of surgery (1). The first flap was injured due to a significant 2-cm septal spur over the posterior septum. The mucosa was thin at the level of the deflection and the NSF split into two pieces longitudinally with the vascular pedicle on one side. The second injured flap was devitalized during the course of surgery. The contralateral side was subsequently raised and successfully used in both cases. In two of the four patients, bilateral complete flaps (estimated 4.5 × 2 cm2) were raised due to a prior history of radiation exposure. In the other two patients, the posterior septum was raised. In one of these two patients, the smaller flap was used to close the wound while the larger flap was replaced on the septum. In the other patient, the larger flap was used.
Estimated mean defect size was 4.95 cm2 (range, 0.5 to 24 cm2). Twenty-five patients had intracranial defect through the skull base bone, with 20 having a dural defect with mean size of 1.86 cm2 (range, 0.2 to 6.2 cm2). See Figs. 3 and 4.
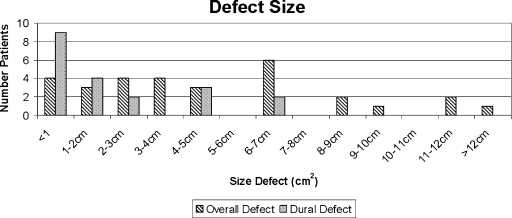
Figure 3.
Distribution of defect size. Estimated mean overall defect size was 4.95 cm2 (n = 30; range, 0.5 to 24 cm2). Twenty-five patients had an intracranial defect through the skull base bone, and 20 had a dural defect with a mean size of 1.86 cm2 (range, 0.2 to 6.2 cm2). One patient had extensive macroadenoma of the central skull base and infratemporal fossa. The resultant defect was estimated to be 24 cm2, completely extradural.
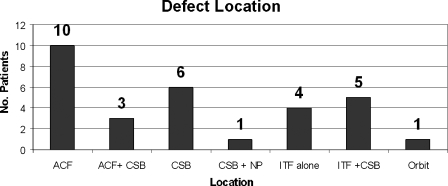
Figure 4.
Defect location. ACF, anterior cranial fossa including ethmoids and sphenoid planum; CSB, sphenoid planum, sphenoid sella, clivus; NP, nasopharynx; ITF, infratemporal fossa including pterygoid plates, pterygoid fossa fat and contents, pterygoid muscles, bone of greater sphenoid wing, to foramen rontundum or ovale, and eustachian tube.
The wound was closed with the NSF alone in 10 cases without a dural defect. In the remaining 20 cases with a dural defect, the wound was closed with (1) Duragen or fascia and the NSF (4); (2) Duragen or fascia, then free fat, then the NSF (8); (3) fat and NSF (4); (4) inlay bone, fat, and middle turbinate flap (3); or (5) inlay bone and NSF (1).
Inlayed bone was only used in cases of CSF leak repair and not after tumor resection. DuraSeal is sprayed onto the flap as a tissue adhesive and then the wound is packed with Gelfoam and secured with Foley balloon catheter for 3 to 5 days. Eleven of 20 patients (55%) with a CSF leak had a lumbar drain placed at time of surgery and postoperatively for 3 to 5 days. The median hospital stay was 4.5 days (SD, 3.6 days).
Twenty-five patients had uncomplicated surgery. One patient had troponin leak consistent with myocardial ischemia postoperatively, but no long-term sequelae. One patient with a history of cervical spine disease had worsening of her symptoms due to cervical disc herniation. This patient is the only patient in the series to have the head repositioned intraoperatively to transition between endoscopic dissection and transnasal dissection under the microscope. One patient had postoperative periorbital edema that did not require intervention. One patient undergoing subtotal resection of a pituitary adenoma with suprasellar extension via a transplanum approach developed postoperative hematoma requiring a return to the operating room for evacuation and hemostasis. In this case, the NSF was successfully elevated off the wound site and used again at the end of the case to close the wound. One patient with extensive chordoma had worsening of dysphagia with dysfunction of cranial nerve XII that was present preoperatively. This occurred presumably due to edema of the tumor at its lateral extent where no dissection was performed.
Fourteen patients had radiation after surgery. The median time to radiation was 7 weeks (SD, 3.05 days). No patients were delayed due to wound healing after surgery. The NSF was visible at a median of 7 weeks after surgery (SD, 3.05 days; n = 22). None of the 25 patients with intracranial defect or 20 patients with intraoperative dural defect had clinical evidence of postoperative CSF leak after surgery (n = 20).
Overall, four patients had a history of radiation to the nasopharynx or ethmoid sinuses, and two of these patients had NSF necrosis detected with visual inspection by 8 weeks after surgery (p = 0. 013). One patient lost 100% of one of two flaps. This patient had a prior history of radiation for nasopharynx cancer and was reirradiated to the nasopharynx after surgery without developing a CSF leak. The second patient, with a current ethmoid squamous cell carcinoma and a history of radiation for nasopharynx carcinoma, lost ~50% of the distal end of a flap within 6 weeks after surgery. This patient subsequently had proton beam therapy without further loss of the remaining NSF.
In long-term follow-up, no complications related to the septectomy or healing of the residual denuded septal cartilage have been observed, including no loss of nasal structural support and no erosion or infection from prolonged septal splint use. Patients commonly complain of nasal crusting which has been managed successfully with nasal saline irrigations in all cases.
DISCUSSION
This series reviews our initial experience with the NSF at an academic center where both the otolaryngologists and neurosurgeons are trained endoscopic surgeons. All cases are done by otolaryngologist and neurosurgeons working together. The NSF is harvested at the onset of the case to preserve its blood supply prior to the septectomy. In general, for a right-handed surgeon, the NSF is raised on the right side. The middle turbinate on the right is resected along with the ethmoids and the posterior septum is then taken down to create a corridor that allows instruments to be passed on the left side and to create room for two surgeons to work in tandem. A left-sided NSF is used when the right side of the NSF or its blood supply is threatened by tumor extension or preoperative embolization.
We examined factors associated with the NSF selection for use, successful harvest, application, and outcome. A total of 28 patients had 32 NSFs harvested. Two additional patients had a total of 3 middle turbinate flaps. Of this group, 25 patients had an intracranial defect, and 20 had a dural defect. Of the two patients who had a middle turbinate flap, one patient was unsuitable for an NSF due to an absent septum. The other patient had focal defects in the fovea ethmoidalis bilaterally that would have required significant tissue resection to prepare the skull base for an NSF. Therefore, due to its proximity and the limited size of the defect, the middle turbinate flap was a simple alternative with minimum morbidity. Thirty NSFs (94%) were successfully harvested, while 2 (6%) NSFs were lost intraoperatively. One NSF was split during harvest due to a large longitudinal septal spur. The septal spur was associated with thinning and friability of the flap mucosa. Several patients in the series had a history of prior nasal septal surgery, but these data were not retrievable in the chart review. While the author was able to elevate NSFs in this situation, the procedure is more difficult.
Overall, none of the 20 patients had a postoperative CSF leak in our series, supporting the initial literature indicating a CSF leak rate of 5%. Prior nasopharyngeal radiation is found to be a risk factor for NSF necrosis (p = 0.013). Only 2 of 32 flaps (6%) necrosed postoperatively, and yet no CSF leak occurred in either case. One of the two cases developed a partial necrosis, while the second case had a complete necrosis. Of note, both of these patients went on to postoperative irradiation, one with intensity-modulated radiation therapy and the other with proton beam therapy to an area outside the prior radiation field. A previous report of 43 patients having closure with the NSF found a CSF leak rate of 5%.18 It is likely that as our experience increases with the NSF, and larger defects are approached, our CSF leak rate will increase; however, these data support the use of NSF closure for smaller dural defects, up to 4 to 5 cm2. Our experience with these smaller defects is that the CSF leak rate is comparable to that of open procedures. A few large dural defects, up to 6.2 cm2, were also successfully closed, indicating that the NSF is also useful for large defects of the anterior and central skull base.
A 0% leak rate prevents further analysis of factors associated with CSF leak. In the early postoperative period, many patients likely have a CSF leak since the wound is not closed in a watertight fashion and the wound requires time to mucosalize. Further, most patients are noted to have nasal drainage while the merocel packs are in (even those without CSF leak noted during surgery). However, no patients had detectable rhinorrhea or CSF at the time of merocel packing removal on postoperative day 10. Most patients in this series did not frequently complain of associated symptoms of CSF leak, such as headache or meningismus, in the first week after surgery. The follow-up time of 8.3 months should be sufficient to identify CSF leaks due to failure of wound closure. As initially described by Hadad and colleagues,18 a fat graft can be used in conjunction with an NSF or it can be lined directly on the dural reconstruction. Our technique differs in that we routinely place extracranial fat between the Duragen and the flap. This may explain why the two cases with NSF failure healed the defect.
One criticism of the NSF is that it may not cover the wound completely and that it may shrink by 20 to 40% over time. In our experience, when fat was not used, the NSF was applied directly on the defect and covered it entirely. When fat was used, the fat covered the entire defect, but the NSF did not always cover the entire fat graft. The fact that we had no cases of CSF leak in either situation implies that the wounds have healed. Intuitively it would seem that NSF provides a base of healthy mucosa that is expected to remucosalize over the nonvascularized graft (fat or Duragen) through epithelial cellular migration. Once a healthy base of mucosalized epithelium is established, it is unlikely that flap shrinkage would subsequently result in a CSF leak. Further, given this paradigm, it seems likely that if a small CSF leak persisted after the first closure attempt, a subsequent repair with fat graft or free mucosa could be attempted. Flap shrinkage has been noted postoperatively when the pedicle is free-floating in the air, and it is recommended that the pedicle of the flap be lined on bone along its length to reduce shrinkage. There have been no cases of late CSF leak in our series to suggest the NSF can shrink enough to pull off the wound. In our series, the flap was not routinely sutured in place, and watertight closures were not achieved at the time of surgery. The 100% success rate indicates this is an acceptable approach.
Fourteen patients required radiation after surgery. For malignant lesions of paranasal sinuses, patients are recommended to start radiation within 6 weeks of surgery. In the event of the two patients with NSF failure but no CSF leak identified, we still recommended proceeding with radiation in a timely manner. The average time to radiation in this series was 7.5 (SD, 3.4) weeks. Five patients were delayed longer than 6 weeks. Delays were attributable to patient delays (1), need for proton therapy out of the area for chordoma (2), and need for radiation to a pituitary adenoma (2) (where the 6-week rule is not followed). Wound healing issues were not related to the delay to proceed with radiation.
The NSF can be used to close extracranial wounds without a CSF leak as well. If the NSF is being considered for use, it must be raised at the beginning of the case to preserve it. We therefore considered if lining wounds without CSF leaks would provide benefit to the patient. Five patients had an intracranial defect without CSF leak, and five patients had a flap elevated without breach of the skull base bone. Four of these patients with extracranial defect had extensive resection from the pterygoid and ITF. The other patient had a sphenoclival lesion respected. Two of the patients with ITF lesions had had prior radiation and the flap was used to prevent radionecrosis. One of the patients with an ITF lesion had nasopharyngeal extension and had an exposed carotid artery after transcervical exposure of the carotid and dissection of tumor off the carotid artery. The carotid artery was lined with the NSF to prevent carotid infection and leakage of saliva into the neck wound. The other wounds were lined with the NSF to decrease healing time by encouraging earlier mucosalization and decreased crusting. Furthermore, the NSF is still available if a revision surgery is needed.
While inlay bone is used for some cases of CSF leak repair not associated with tumor resection, rigid materials and fixation are avoided after tumor resections. The technique described for endoscopic closure of skull base defects relies on a soft tissue closure without rigid fixation.12 After open anterior craniofacial resection, some surgeons will use rigid material to support the anterior cranial fossa in conjunction with a pericranial flap to prevent brain herniation in large defects.20 However the exact definition of a “large lesion” is not clear in the literature. In our experience at UCSF over the past 20 years, we have routinely repaired defects involving the bilateral ethmoids and sphenoid planum without rigid fixation and have not witnessed brain herniation.21 Furthermore, there is concern that rigid material may eventually extrude, leading to a late CSF leak, or act as a sequestrum promoting an infectious complication. Therefore, the current approach described for endoscopic closure does not deviate significantly from standard techniques used in open craniotomy. Kassam and associates describe using an inlay graft of Duragen deep to the dura, and a second layer of acellular dermis (Alloderm; LifeCell Corporation, Branchburg, NJ) external to the dura.12 This is followed by a fat graft and the NSF. Kassam et al currently avoid the use of an intervening layer of fat and lay the NSF directly on the wound (verbal communication). Our technique differs in that we do not apply a second layer of acellular dermis external to the dura. Further, in this series, we have used DuraSeal, a polyethylene glycol hydrogel sealant, intervening between the fat graft and NSF. The wound is left relatively undisturbed for up to 8 weeks and may account for the tissue ingrowth and vascularization of the fat graft. However, in principle, when the fat graft can be maintained in position prior to placement of the NSF, it seems intuitively reasonable to avoid intervening tissue sealants to accelerate vascularization of the free tissue fat graft and we currently try to avoid intervening material between the NSF and the next layer of tissue. We do not routinely suture in any layer of the reconstruction except when the flap is placed in a manner that is susceptible to retraction (orbit, ITF).
This series of NSF reports on our experience starting with our first NSF at an academic center. The NSF was found to be easily harvested. Snyderman et al examined surgical skill acquisition and recommend a staged approach for learning endoscopic dissection techniques.22 We lost one NSF to a novice surgeon due to tissue handling and surgeon skill should be considered. Overall though, the NSF appears easy to harvest and manipulate. Factors that may predict difficulty raising the NSF include deviated septum, septal spurs, an existing perforation, and prior septal surgery. Other factors that should be considered in the patient history include cocaine use, vasculitic disease, or a predisposition to poor wound healing such as chronic steroid therapy or hypothyroidism.
This series also illustrates when the NSF flap does not appear appropriate or is unavailable. In one case, the NSF was not available due to septal perforation that resulted from the prior trans-sphenoidal surgery. In a second patient, harvest of the flap and preparation of the skull base for the flap to cover the roof of both ethmoids would have required extensive dissection. Use of the middle turbinate bilaterally sufficed in this case.
Potential morbidity exists with use of the NSF. If the flap is taken too high along the skull base, olfactory fibers can be injured which can result in anosmia. In cases where the olfactory bulb is intact, if the flap is elevated on one side, then patients should have functioning olfactory fibers on the contralateral side, but potential for complete anosmia exists. These data were not captured in our retrospective review at this time, but several patients have complained of decreased olfaction after surgery. Loss of olfaction can also occur due to injury from the tumor or resection of the tumor. Nasal crusting is pronounced for several weeks after surgery and may persist indefinitely. However, all patients in our experience are manageable with home saline irrigations and infrequent debridement after the initial postoperative period. Antibiotics are not routinely used except for cases of frank purulent discharge.
CONCLUSION
The pedicled NSF based on the posterior nasoseptal artery is robust and easily harvested endoscopically, and the procedure requires minimal extra training for endoscopic surgeons. It is versatile, able to seal large dural defects of the anterior, midline, and lateral skull base. Prior radiation to the nasopharynx or paranasal sinuses is a risk factor for flap necrosis, while septal spurs and iatrogenic injury can lead to intraoperative injury of the flap. Bilateral NSF should be used in patients with prior history of radiation when possible. Further, there may be a role for use of the NSF without CSF leaks present. The NSF should be considered as the first line of closure after transnasal endoscopic skull base resections.
REFERENCES
- El-Sayed I H, Saleh H. In: Eisele DW, Smith RV, editor. Complications of Otolaryngology—Head and Neck Surgery. 2nd ed. Philadelphia, PA: Elsevier; 2008. Neurosurgical Complications in Otolaryngology MWM. pp. 110–132.
- Hegazy H M, Carrau R L, Snyderman C H, Kassam A, Zweig J. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000;110:1166–1172. doi: 10.1097/00005537-200007000-00019. [DOI] [PubMed] [Google Scholar]
- Basu D, Haughey B H, Hartman J M. Determinants of success in endoscopic cerebrospinal fluid leak repair. Otolaryngol Head Neck Surg. 2006;135:769–773. doi: 10.1016/j.otohns.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Gil Z, Abergel A, Leider-Trejo L, et al. A comprehensive algorithm for anterior skull base reconstruction after oncological resections. Skull Base. 2007;17:25–37. doi: 10.1055/s-2006-959333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliss D M, Gil Z, Spektor S, et al. Skull base reconstruction after anterior subcranial tumor resection. Neurosurg Focus. 2002;12:e10. doi: 10.3171/foc.2002.12.5.11. [DOI] [PubMed] [Google Scholar]
- Kassam A B, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005;19:E6. [PubMed] [Google Scholar]
- Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part I. Crista galli to the sella turcica. Neurosurg Focus. 2005;19:E3. [PubMed] [Google Scholar]
- Kassam A, Snyderman C H, Mintz A, Gardner P, Carrau R L. Expanded endonasal approach: the rostrocaudal axis. Part II. Posterior clinoids to the foramen magnum. Neurosurg Focus. 2005;19:E4. [PubMed] [Google Scholar]
- Leong J L, Citardi M J, Batra P S. Reconstruction of skull base defects after minimally invasive endoscopic resection of anterior skull base neoplasms. Am J Rhinol. 2006;20:476–482. doi: 10.2500/ajr.2006.20.2931. [DOI] [PubMed] [Google Scholar]
- Snyderman C H, Kassam A B, Carrau R, Mintz A. Endoscopic reconstruction of cranial base defects following endonasal skull base surgery. Skull Base. 2007;17:73–78. doi: 10.1055/s-2006-959337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sade B, Mohr G, Frenkiel S. Management of intra-operative cerebrospinal fluid leak in transnasal transsphenoidal pituitary microsurgery: use of post-operative lumbar drain and sellar reconstruction without fat packing. Acta Neurochir (Wien) 2006;148:13–18. discussion 18–19. doi: 10.1007/s00701-005-0664-6. [DOI] [PubMed] [Google Scholar]
- Kassam A, Carrau R L, Snyderman C H, Gardner P, Mintz A. Evolution of reconstructive techniques following endoscopic expanded endonasal approaches. Neurosurg Focus. 2005;19:E8. [PubMed] [Google Scholar]
- Goldberg A N, Lanza D C. In: Baily BJ, Calhoun KH, Friedman N, Newlands SD, Vrabec JT, editor. Atlas of Head and Neck Surgery—Otolaryngology. 2nd ed. Philadelphia, PA: Lippincott Williams and Wilkins; 2001. Extracranial closure of cerebrospinal fluid rhinorrhea using a mucoperiosteal flap. 2nd ed. pp. 890–893.
- Friedman M, Ibrahim H, Ramakrishnan V. Inferior turbinate flap for repair of nasal septal perforation. Laryngoscope. 2003;113:1425–1428. doi: 10.1097/00005537-200308000-00031. [DOI] [PubMed] [Google Scholar]
- Berinstein T H, Bernstein P. The turbinate flap for reconstruction of nasal septal mucosal defects. Laryngoscope. 1996;106:1047–1048. doi: 10.1097/00005537-199608000-00027. [DOI] [PubMed] [Google Scholar]
- Schultz-Coulon H J. Experiences with the bridge-flap technique for the repair of large nasal septal perforations. Rhinology. 1994;32:25–33. [PubMed] [Google Scholar]
- El-Banhawy O A, Halaka A N, El-Dien A E, Ayad H. Sellar floor reconstruction with nasal turbinate tissue after endoscopic endonasal transsphenoidal surgery for pituitary adenomas. Minim Invasive Neurosurg. 2003;46:289–292. doi: 10.1055/s-2003-44453. [DOI] [PubMed] [Google Scholar]
- Hadad G, Bassagasteguy L, Carrau R L, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116:1882–1886. doi: 10.1097/01.mlg.0000234933.37779.e4. [DOI] [PubMed] [Google Scholar]
- Fortes F S, Carrau R L, Snyderman C H, et al. The posterior pedicle inferior turbinate flap: a new vascularized flap for skull base reconstruction. Laryngoscope. 2007;117:1329–1332. doi: 10.1097/mlg.0b013e318062111f. [DOI] [PubMed] [Google Scholar]
- Cantu G, Solero C L, Pizzi N, Nardo L, Mattavelli F. Skull base reconstruction after anterior craniofacial resection. J Craniomaxillofac Surg. 1999;27:228–234. doi: 10.1016/s1010-5182(99)80034-1. [DOI] [PubMed] [Google Scholar]
- Deschler D G, Gutin P H, Mamelak A N, McDermott M W, Kaplan M J. Complications of anterior skull base surgery. Skull Base Surg. 1996;6:113–118. doi: 10.1055/s-2008-1058652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman C, Kassam A, Carrau R, et al. Acquisition of surgical skills for endonasal skull base surgery: a training program. Laryngoscope. 2007;117:699–705. doi: 10.1097/MLG.0b013e318031c817. [DOI] [PubMed] [Google Scholar]