ABSTRACT
Ecchordosis physaliphora is a rare hamartomatous lesion of notochord origin that is usually asymptomatic. In this case report, such a lesion arose at the clivus, with extension through to the sphenoid sinus. As a result, it presented with cerebrospinal fluid rhinorhea, making the case particularly unusual. We describe the treatment for this rare situation and discuss its differentiation from chordoma, its malignant counterpart.
Keywords: Ecchordosis physaliphora, chordoma, intradural chordoma, notochord, cerebrospinal fluid
The notochord is the primitive skeleton of vertebrates. In the human embryo, it emerges in the third week of life, and, as the axial skeleton develops, it is incorporated into the vertebral column and persists as the nucleus pulposus of the intervertebral disc.1,2 Ectopic nests of notochord cells can be found outside the intervertebral discs anywhere between the two poles of the axial skeleton from the coccyx to the dorsum sella and, most commonly, at the two poles themselves.1
Chordomas are rare, malignant neoplasms of notochord origin that account for some 2 to 4% of primary bone tumors. They are believed to arise specifically from ectopic nests of notochord cells and, as such, are most common in the sacrococcygeal and clival regions.1 Intracranially, the notochord takes a sigmoid course through the clivus and reaches its dorsal aspect at three points.1 A similarly convoluted course is taken in the sacrococcygeal region. This is thought to explain the prevalence of ectopic notochord cells and, subsequently, chordomas within the clivus and sacrum.1 Chordomas are primarily extradural and cause malignant bony infiltration and destruction. Cranial chordomas typically manifest with headache and multiple cranial nerve palsies. The prognosis is poor, with rapid progression to death despite surgical debulking and radiotherapy.1
Ecchordosis physaliphora (EP) is a smaller, hamartomatous lesion of gelatinous tissue, considered to be further evidence of ectopic nests of notochord. It is also found on the dorsum of the clivus and, more rarely, the sacrum.1,3,4 Luschka first described the observation of pathological, ectopic notochord tissue at the posterior clivus in 1856.5 In 1857, Virchow gave the first microscopic description and named the lesion ecchondrosis physaliphora because of his theory that it derived from cartilage of the spheno-occipital synchondrosis.6 In 1858, Müller suggested a notochordal origin that was confirmed by the work of Ribbert who, in 1894, coined the term ecchordosis physaliphora, which continues to be used today.7,8
EP, unlike chordoma, is typically asymptomatic, being found incidentally in ~2% of autopsies, having caused no significant problem during life.1,8 It is most commonly intracranial and, unlike chordoma, intradural. It is usually attached to the dorsal wall of the clivus by a small pedicle that may be associated with a bony defect. It ranges in size from a few millimeters to 2 cm in diameter, lying mostly within the subarachnoid and subdural spaces. Small samples of EP and chordoma are indistinguishable through histological, immunochemical, and ultrastructural study apart from the infiltrative growth of chordoma.3 This further confirms their common origin as does the histological and ultrastructural similarity of fetal notochord with both chordoma and EP.3,9 There are a few rare cases of extraosseus, intradural chordoma, which have a better prognosis than classical chordoma. As such, considering their common origin, there has been some discussion as to whether intradural chordoma and EP are entirely separate entities.10 However, it is not clear whether EP can be a precursor to chordoma.
CASE REPORT
Clinical History and Presentation
A usually fit and well 52-year-old female telecoms operator was referred with an 8-week history of clear, watery, left-sided rhinorrhea exacerbated by leaning forward. There was no other relevant history and examination revealed only the discharge.
Investigations
Glucose levels and a positive β-2-transferrin confirmed the fluid to be cerebrospinal fluid (CSF). A computed tomography (CT) scan of the skull base revealed a 10-mm focal dissolution of the posterosuperior wall of the sphenoid sinus/clivus with a small air-fluid level within the sinus (Fig. 1). There was little suggestion of a soft tissue component on the CT scan. However, a contrast-enhanced magnetic resonance imaging (MRI) scan revealed a 13-mm lobulated mass within the clivus/posterior wall of the sphenoid sinus corresponding to the area of bony dissolution on CT (Fig. 2A and 2B). The lesion extended to the subarachnoid space just anterior to the brain stem and basilar artery. The mass displayed signal characteristics equivalent to CSF on both T1- and T2-weighted MRI sequences and did not enhance.
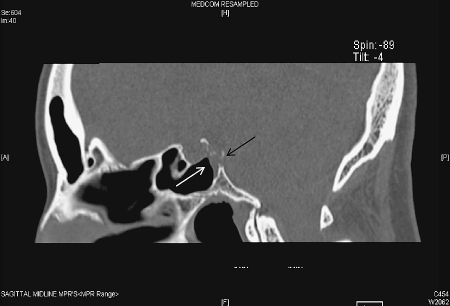
Figure 1.
A sagittal reconstruction of an axial, unenhanced computed tomography scan demonstrates bony dissolution of the posterior wall of the sphenoid sinus/clivus (black arrow) and an air-fluid level within the sphenoid sinus (white arrow).
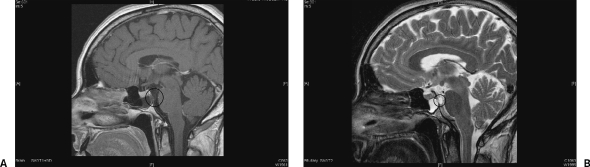
Figure 2.
(A) A T1-weighted, postcontrast magnetic resonance image demonstrates the hypointense mass (circle) spanning a clival defect that corresponds to that seen on the initial computed tomography scan and extends into the sphenoid sinus and into the posterior cranial fossa as well as a fluid level within the sphenoid sinus. (B) On a corresponding T2-weighted image, the mass is hyperintense (circle).
Management
While awaiting a surgical opinion, the patient developed an episode of bacterial meningitis for which she was successfully treated. Following this, she had no further rhinorrhea for 9 months at which time an MRI was repeated. This still demonstrated fluid within the sphenoid sinus, however, and therefore could not exclude a continued CSF leak. Consultation was made with the neurosurgeons and an intracranial approach to the defect was ruled out. The choice lay between an endoscopic intranasal approach with potentially limited access and an open approach to the sphenoid via a midfacial degloving. The former option was chosen as a more conservative initial approach. CSF leakage was observed at the time of the procedure, and the defect was repaired with a septal mucosal graft and Tisseel tissue glue. A soft, gray mass was noted just below the pituitary bulge but was not biopsied for fear of creating further complication and given that there had been no growth demonstrated in the 12-month period between MRI scans. There was therefore no sample with which a histological diagnosis could be made and, in light of this, follow-up imaging (contrast MRI) was arranged. Six months postsurgery, there was no change to the size of the lesion. A further scan for 2 years' time was arranged, but the patient represented just prior to this with her first recurrence of rhinorrhea since her episode of meningitis, which was confirmed again to be CSF. Another MRI scan again confirmed no change to the lesion. Referral was made to a neurosurgeon with experience in pituitary surgery for a transsphenoidal repair of the clival defect. The anterior wall of the sphenoid was removed. The sphenoid septum was found to be orientated in part coronally and required removal to access the clivus where the defect was found to be ~1 cm2 and in close proximity to the basilar artery. For this reason, the defect was not opened to place fat intradurally. Instead, a fascia lata graft was laid over the defect and glued into place with DuraSeal (Confluent Surgical, Waltham, MA, USA), a hydrogel tissue sealant. Further fascia lata was laid over the sealant, held in place with a fat graft within the sphenoid sinus, more glue, and a final layer of spongistan. A nasal pack was in place for 24 hours and a lumbar drain (10 mL/h) for 5 days. To date, this procedure has proved effective in controlling the CSF leak.
DISCUSSION
It is the complex course of the notochord within the clivus and sacrum that is responsible for the prevalence of EP and chordomas in these regions. The notochord may perforate through the dorsal wall of the clivus, forming intradural EP.4 It may also perforate into the paranasal sinuses.11 The subarachnoid or subdural EP may be attached to cells within the clivus by a thin stalk or may simply be isolated intradurally. There is, therefore, a potential continuum of cells between the extradural, subdural, and subarachnoid spaces, which can explain the existence of lesions like that observed in our patient where the pathological tissue was in all three spaces and extended into the sphenoid sinus.
Histological and structural examination reveals striking similarities between fetal notochord, EP, and chordomas.12 They are all composed of physaliphorous cells with copious intracytoplasmic vacuoles and extracellular pools of mucin. These features are consistent with the hypointensity and poor enhancement of EP on T1-weighted MRI scans and its hyperintense appearance on T2-weighted MRI (Figs. 1 and 2). However, EP shows no contrast enhancement, unlike chordomas, and, in most cases, the signal intensity extends via the stalklike projection to the posterior wall of the clivus and in some cases through it.13 Furthermore, chordomas exhibit bony infiltration and tumor calcification but EP does not. Additionally, although chordomas have a degree of cellular pleomorphism, hypercellularity, and atypia that corresponds to their malignant clinical course, EP is rarely symptomatic primarily due to its very slow growth pattern. In this case, there was no evidence of change or growth at all over 2 years of follow-up, which would be extremely unusual for chordoma but typical of EP. Problems may arise, however, when trying to distinguish EP from intradural chordomas, which have a more benign clinical picture and better prognosis than their extradural counterparts.
Symptomatic cases of EP are extremely rare, with only seven other reports identified in the literature.2,4,14,15 In two of these, the patients had fatal subarachnoid hemorrhages.4,14 Mapstone et al described a large prepontine lesion seen on CT and MRI scans in a patient with cranial nerve palsies caused by mass effect,15 and Wolfe and Scheithauer described two patients exhibiting focal neurological deficit caused by expansile lesions found to be compressing brain tissue at pathological examination.2 Ling et al reported on the case of a 45-year-old man presenting with sudden-onset sensorineural hearing loss found to be caused by mass effect from a giant EP, which also caused local bone remodeling.10 The latter four cases exhibited features (size of the lesion, bony destruction, and cranial nerve palsy due to mass effect) more in keeping with a diagnosis of chordoma.
There was only one other case of CSF leak and rhinorrhea secondary to EP described previously in the literature. Macdonald et al described a 61-year-old female patient with a 2-year history of clear right nostril rhinorrhea who was found to have a lesion almost identical to that observed in our patient.12 They described how the mass was visualized with CT and MRI scans and excised via a sublabial, midline, rhinoseptal, transsphenoidal approach. Their subsequent histological and immunochemical investigations in conjunction with the benign history led the authors to conclude that the lesion was indeed EP, which, in the light of their evidence, is a sound conclusion.
For a lesion of this size and in this position, one alternate diagnosis that should be considered is intracranial neurenteric cysts, although these are extremely rare (only ~60 in the literature16). Furthermore, it is a reasonable contention that the very presence of a bony defect causing CSF rhinorrhea may put the diagnosis of EP in our case into doubt as bone destruction is typically a characteristic of chordoma. However, the imaging obtained in our case revealed bony “dissolution” as opposed to aggressive bony invasion and destruction as might be associated with a malignant process. In addition, as discussed, the complex path of the notochord in the clival region makes the existence of a continuum of cell nests that spans the clivus and dura to reach the subarachnoid space entirely feasible. The subsequent hamartomatous expansion of this cellular “bridge” thus results in a clival defect. In cases of extradural chordoma, rapid progression and neurological involvement with worsening headache would be expected.1 Another alternative would be a purely intradural chordoma; however, not only are these exquisitely rare but by definition they do not breach the dura so cannot invade bone.2 Furthermore, a series of 42 cases by Crockard et al17 demonstrated a mean volume for chordoma of 40.8 cm3 (range 2.3 to 129 cm3), and the range of diameter for EP runs from a few millimeters to 2 cm. Therefore, the stable size of 13 mm over several years and the benign behavior of the lesion in our case are more suggestive of EP. It has been suggested by several authors18 that a spectrum exists among notochord derivatives such that hamartomatous remnants like EP may undergo transformation to chordoma. However, this is pure speculation at present, and there is no evidence of this occurring during the management of this case.
The MRI characteristics of the lesion we describe exactly match the MRI profile of retroclival EP already outlined above, as determined by Mehnert et al in their review of the available literature.13 Despite a lack of histological confirmation, we are confident that our case represents a further incidence of symptomatic EP, confirming this lesion as a cause of CSF rhinorrhea that can be complicated by meningitis. The case highlights the benefit of serial scanning of supposedly benign lesions, an accepted practice for other such skull base lesions (such as acoustic neuromas).
In cases of symptomatic retroclival EP, where complete excision of the mass (allowing for histological examination) poses a high risk, more conservative surgical options for symptom control are possible. A reasonable working clinical diagnosis can be made based on a consistent history and appropriate MRI profile alone.
ACKNOWLEDGMENTS
We would like to thank Dr Colin Archibald, Consultant Radiologist, Royal Berkshire Hospital, Reading for his assistance with image selection for this report.
REFERENCES
- Sassin J F. In: Vinken PJ, Bruyn GW, editor. Handbook of Clinical Neurology. Vol. 18. Amsterdam: North Holland; 1975. Intracranial chordoma. pp. 151–164.
- Wolfe J T, Scheithauer B W. “Intradural chordoma” or “giant ecchordosis physaliphora”? Report of two cases. Clin Neuropathol. 1987;6:98–103. [PubMed] [Google Scholar]
- Ho K L. Ecchordosis physaliphora and chordoma: a comparative ultrastructural study. Clin Neuropathol. 1985;4:77–86. [PubMed] [Google Scholar]
- Stam F C, Kamphorst W. Ecchordosis physaliphora as a cause of fatal pontine haemorrhage. Eur Neurol. 1982;21:90–93. doi: 10.1159/000115460. [DOI] [PubMed] [Google Scholar]
- Luschka H. Die Altersveranderungen der Zwischenwirbelknorpel. Virchows Arch A. 1856;9:311–327. [Google Scholar]
- Virchow R. Untersuschungen über die Entwickelung des Schädelgrundes im Gesunden und Krankhaften zur Tande und über den Einfluss Derselbe auf Schädelform, Gesichtsbildung und Gehirnbau. Berlin: G Reimer; 1857. p. 47.
- Müller H. Über das Vorkommen von Resten der Chorda Dorsalis bei Menschen nach der Geburt und über ihr Verhältnis zu den Gallertgeschwulsten am Clivus. Z Rat Med. 1858;2:202–229. [Google Scholar]
- Ribbert H. Über die Ecchondrosis Physalifora Sphenooccipitalis. Zentralbl Allg Path. 1894;5:457–461. [Google Scholar]
- Rutherfoord G S, Davies A G. Chordomas—ultrastructure and immunohistochemistry: a report based on the examination of six cases. Histopathology. 1987;11:775–787. doi: 10.1111/j.1365-2559.1987.tb01882.x. [DOI] [PubMed] [Google Scholar]
- Ling S S, Sader C, Robbins P, Rajan G P. A case of giant ecchordosis physaliphora: a case report and literature review. Otol Neurotol. 2007;28:931–933. doi: 10.1097/MAO.0b013e318068b2c8. [DOI] [PubMed] [Google Scholar]
- Wright D. Nasopharyngeal and cervical chordoma—some aspects of their development and treatment. J Laryngol Otol. 1967;81:1337–1355. doi: 10.1017/s0022215100068341. [DOI] [PubMed] [Google Scholar]
- Macdonald R L, Cusimano M D, Deck J, Gullane P J, Dolan E J. Cerebrospinal fluid fistula secondary to ecchordosis physaliphora. Neurosurgery. 1990;26:515–519. doi: 10.1097/00006123-199003000-00022. [DOI] [PubMed] [Google Scholar]
- Mehnert F, Beschorner R, Küker W, Hah U, Nägele T. Retroclival ecchordosis physaliphora: MR imaging and review of the literature. AJNR Am J Neuroradiol. 2004;25:1851–1855. [PMC free article] [PubMed] [Google Scholar]
- Bartolini G. Il cordoma del clivus quale causa di emorragia cerebrale: discussione anatomo-clinica su di una osservazione autopica. Boll Soc Ital Biol Sper. 1974;50:912–918. [PubMed] [Google Scholar]
- Mapstone T B, Kaufman B, Ratcheson R A. Intradural chordoma without bone involvement: nuclear magnetic resonance (NMR) appearance. Case report. J Neurosurg. 1983;59:535–537. doi: 10.3171/jns.1983.59.3.0535. [DOI] [PubMed] [Google Scholar]
- Preece M T, Osborn A G, Chin S S, Smirniotopoulos J G. Intracranial neurenteric cysts: imaging and pathology spectrum. AJNR Am J Neuroradiol. 2006;27:1211–1216. [PMC free article] [PubMed] [Google Scholar]
- Crockard H A, et al. A multidisciplinary team approach to skull base chordomas. J Neurosurg. 2001;95:175–183. doi: 10.3171/jns.2001.95.2.0175. [DOI] [PubMed] [Google Scholar]
- Chang S W, Gore P A, Nakaji P, Rekate H L. Juvenile intradural chordoma: case report. Neurosurgery. 2008;62:E525–E526. discussion E527. doi: 10.1227/01.neu.0000316022.74162.00. [DOI] [PubMed] [Google Scholar]