Abstract
Angiotensin (Ang) II-infused hypertensive rats exhibit increases in renal angiotensinogen mRNA and protein, as well as urinary angiotensinogen excretion in association with increased intrarenal Ang II content. The present study was performed to determine if the augmentation of intrarenal angiotensinogen requires activation of Ang II type 1 (AT1) receptors. Male Sprague-Dawley rats (200 to 220 g) were divided into 3 groups: sham surgery (n=10), subcutaneous infusion of Ang II (80 ng/min, n=11), and Ang II infusion plus AT1 blocker (ARB), olmesartan (5 mg/d, n=12). Ang II infusion progressively increased systolic blood pressure (SBP) compared with sham (178±8 mm Hg versus119±4 at day 11). ARB treatment prevented hypertension (113±6 at day 11). Twenty-four-hour urine collections were taken at day 12, and plasma and tissue samples were harvested at day 13. The Ang II+ARB group had a significant increase in plasma Ang II compared with Ang II and sham groups (365±46 fmol/mL versus 76±9 and 45±14, respectively). Nevertheless, ARB treatment markedly limited the enhancement of kidney Ang II by Ang II infusion (65±17 fmol/g in sham, 606±147 in Ang II group, and 288±28 in Ang II+ARB group). Ang II infusion significantly increased kidney angiotensinogen compared with sham (1.69±0.21 densitometric units versus 1.00±0.17). This change was reflected by increased angiotensinogen immunostaining in proximal tubules. ARB treatment prevented this increase (1.14±0.12). Urinary angiotensinogen excretion rates were enhanced 4.7× in Ang II group (4.67±0.41 densitometric units versus 1.00±0.21) but ARB treatment prevented the augmentation of urinary angiotensinogen (0.96±0.23). These data demonstrate that augmentation of intrarenal angiotensinogen in Ang II-infused rats is AT1-dependent and provide further evidence that urinary angiotensinogen is closely linked to intrarenal Ang II in Ang II-dependent hypertension.
Keywords: angiotensin II, angiotensinogen, receptors, angiotensin II, rats, kidney
Angiotensin II (Ang II) plays an important role in proximal tubular reabsorptive function primarily via activation of Ang II type 1 (AT1) receptor at both basolateral and luminal membranes.1 Although some Ang II type 2 receptors have been confirmed on proximal tubules,2 most functional studies suggest that the major effects of Ang II on proximal tubules are via AT1 receptors.3 Several studies have suggested that Ang II also plays an important role in the regulation of distal tubular reabsorption rate.4 This effect was blocked by either saralasin or losartan, indicating that this stimulation involves AT1 receptor activation.4 These findings, together with the demonstration that AT1 receptor is present on the luminal membranes of distal nephron segments,5,6 suggest that luminal Ang II plays an important role in the regulation not only of proximal reabsorption rate but also of distal tubular reabsorptive function.7 Studies in isolated proximal tubular cells showed that Ang II stimulates Na+/H+ exchanger via AT1 receptor.8 Peti-Peterdi et al9 demonstrated that Ang II directly stimulates epithelial sodium channel activity in the cortical collecting duct via AT1 receptors. Furthermore, epithelial sodium channel gene expression in the cortical collecting duct is also upregulated by Ang II.10 Thus, Ang II regulates tubular transport in distal as well as in proximal tubular segments via its action on basolateral and luminal receptors.1
Ang II-infused hypertensive rats exhibit increases in renal angiotensinogen (AGT) mRNA11 and protein,12 and an enhancement of urinary excretion rate of AGT.13 Furthermore, chronic Ang II infusions to normal rats significantly increase urinary excretion rates of AGT in a time- and dose-dependent manner, and the urinary AGT excretion rates are positively related to the elevation in kidney Ang II levels.14 In contrast, hypertension elicited with a combination of high-salt diet and deoxycorticosterone acetate salt pellet is not associated with increased urinary AGT excretion rates even though this treatment leads to an increase in urinary excretion of total protein.14 It has also been shown that treatment with AT1 receptor blockers (ARBs) prevents the increases in intrarenal15,16 and endosomal 17 Ang II concentrations caused by chronic Ang II infusions.
The relationship between urinary AGT excretion rates and intrarenal Ang II levels has suggested a direct AT1 receptor-mediated enhancement of intrarenal AGT and urinary AGT excretion rates. However, this has not yet been demonstrated directly. Accordingly, further experiments were performed to determine if the augmentation of intrarenal AGT in Ang II-dependent hypertension is dependent on the activation of AT1 receptors. Such a link between AT1 receptor activation and stimulation of intrarenal AGT would provide a critical amplification mechanism by which modest increases in circulating Ang II would further augment intrarenal AGT and intrarenal Ang II formation.
Methods
Preparation of Animals
The experimental protocol was approved by the Tulane Animal Care and Use Committee. Male Sprague-Dawley rats (200 to 220 g, n=33; Charles River, Wilmington, Mass) were housed in metabolic cages and maintained in a temperature-controlled room regulated on a 12-hour light/dark cycle with free access to water. Rats were maintained on a commercially available rat chow containing normal salt (0.3% sodium chloride, Harlan Teklad, #170950) for 2 weeks. Rats were anesthetized with sodium pentobarbital (50 mg/kg, IP) and were selected at random to either be subjected to sham surgery (n=10) or to receive a subcutaneous infusion of Ang II (80 ng/min, n=23) via an osmotic minipump (Alza) implanted subcutaneously at the dorsum of the neck on day 0. One group of Ang II-infused rats (n=12) also received an ARB, olmesartan (5 mg/d in food, Sankyo) during the period of Ang II infusion. Systolic blood pressure (SBP) was measured in conscious rats using tail-cuff plethysmography at days −1, 3, 7, and 11 as previously described.11–14,18
Sample Collection and Evaluation
Twenty-four-hour urine samples were collected on day 12 in centrifuge tubes containing a protease inhibitor, 4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride (1 mmol/L in final concentration, Sigma). Urine samples were centrifuged and supernatant was separated and stored at −20°C until assayed for total protein, creatinine, N-acetyl-β-d-glucosaminidase (NAG), and AGT. Urinary concentrations of total protein, creatinine, and NAG were measured by colorimetric assays using commercially available kits (Bio-Rad, Oxford Biomedical Research, Boehringer Mannheim Biochemica, respectively). Urinary AGT was evaluated by Western blot analysis using the collected urine samples (0.05% of 24-hour collection) in a standard protocol as described previously.13,14
Blood and kidney samples were harvested on day 13. After decapitation, trunk blood was collected into two chilled tubes containing EDTA (5 mmol/L) for plasma renin activity or EDTA (5 mmol/L), enalaprilat (20 µmol/L), pepstatin A (10 µmol/L), and 1,10-phenanthroline (1.25 mmol/L) for plasma Ang II. Plasma was separated and stored at −20°C until assayed for plasma renin activity and Ang II as previously described.11–14,18 Immediately after removal, one kidney was homogenized in cold methanol and renal Ang II was measured as previously described.11–14,18 The contralateral kidneys were separated into two pieces and snap frozen in liquid nitrogen for Western blot analysis or immersed in zinc-saturated formalin for immunohistochemistry.
Western blot analysis of the collected kidney samples (10 µg of cortex protein) against rat AGT, renin, and β-actin was done with a standard protocol as described previously.11–14,18
Kidney AGT immunohistochemistry was done as previously described11,19 and was analyzed. A description of the procedure can be found in an online supplement available at http://www.hypertensionaha.org. The results were expressed in arbitrary units of the average of the AGT immunostaining of each group.
Statistical Analysis
Statistical analysis was performed using a 1-way factorial or a 2-way repeated ANOVA with post hoc Scheffe F test. All data are presented as mean±SEM. P<0.05 was considered significant.
Results
Systolic BP
Systolic BP (Figure 1) values were similar among the groups before the treatments. Ang II infusion progressively increased systolic BP up to an average of 178±8 mm Hg at day 11 of infusion, whereas the sham group maintained their systolic BP at control levels (119±4 at day 11). ARB treatment with olmesartan prevented the development of hypertension (113±6 at day 11).
Figure 1. Temporal profile of systolic BP of each group.
Systolic BP was similar among the groups before the treatments. Ang II infusion progressively increased systolic BP compared with sham. ARB treatment prevented the hypertension. Ang II indicates angiotensin II; and ARB, angiotensin II type1 receptor blocker, olmesartan. *P<0.05 compared with the corresponding day −1 group; †P<0.05 compared with the corresponding sham group at that time period.
Plasma Renin and Ang II
Plasma renin activity (Figure 2A) was significantly suppressed by Ang II infusion (0.2±0.1 ng/mL per hour versus 5.0±0.8); however, ARB treatment led to marked stimulation of plasma renin activity.
Figure 2. Plasma renin activity and Ang II concentrations in these groups.
A, Plasma renin activity was significantly suppressed by Ang II infusion; however, it was increased by ARB treatment. B, Ang II+ARB group had a significant increase in plasma Ang II levels compared with Ang II and sham groups. Ang II indicates angiotensin II; ARB, angiotensin II type1 receptor blocker, olmesartan; PRA, plasma renin activity. *P<0.05 compared with the sham group.
As previously noted, the Ang II infusions led to modest increases in plasma Ang II concentrations (Figure 2B).However, treatment with the ARB led to large increases in plasma Ang II levels compared with Ang II and sham groups (365±46 fmol/mL versus 76±9 and 45±14, respectively).
Kidney Ang II, AGT, and Renin
Profiles of kidney parameters of each group are depicted in Figure 3A through 3D.
Figure 3. Profiles of kidney parameters of each group.
A, Kidney Ang II levels increased in Ang II-infused rats and ARB treatment markedly limited the enhancement of kidney Ang II levels by Ang II infusion. B, Ang II infusion significantly increased kidney AGT levels compared with sham. ARB treatment prevented this increase. C, Kidney renin levels were significantly suppressed by Ang II infusion; however, they were increased by ARB. D, Densitometric values for kidney β-actin among the groups showing similar values in all three groups. Ang II indicates angiotensin II; ARB, angiotensin II type1 receptor blocker, olmesartan; AGT, angiotensinogen. *P<0.05 compared with the sham group.
As previously described, Ang II infusions elicit increases in intrarenal Ang II levels (Figure 3A). ARB treatment markedly limited the enhancement of kidney Ang II levels by Ang II infusion (65±17 fmol/g in sham, 606±147 in Ang II group, and 288±28 in Ang II+ARB group).
Ang II infusion also significantly increased kidney AGT levels (1.69±0.21 densitometric units [DU], n=7), measured by Western blot analysis, compared with sham (1.00±0.17, n=7, Figure 3B). As shown in Figure 3B, the Ang II-infused rats treated with an ARB did not have a significant increase in kidney AGT levels (1.14±0.12, n=10). Likewise, kidney AGT immunostaining (Figure 4A–4D), performed with an automatic robot system (Dako autostainer) and measured using an automatic quantification system (Image-Pro Plus software, Media Cybernetics), showed a significant enhancement in Ang II group (20424±1744 arbitrary units, Figure 4B) compared with sham group (3825±400, Figure 4A). ARB treatment prevented this augmentation (3871±238,Figure 4C).
Figure 4. A–C, Representative immunohistochemical analysis of rat kidney AGT from each group.
The immunoreactive areas were restricted only to proximal tubular cells. Vascular structures were negative. D, Kidney AGT immunostaining showed a significant enhancement in Ang II group (B) compared with sham group (A). ARB treatment prevented this augmentation (C). Kidney AGT immunohistochemistry was performed as previously described11,19 using an automatic robotic system (Dako autostainer) to apply the exactly same condition on all slides. Ang II indicates angiotensin II; ARB, angiotensin II type1 receptor blocker, olmesartan; AGT, angiotensinogen; AU, arbitrary units. *P<0.05 compared with the sham group.
Kidney cortex renin levels measured by Western blot analysis were significantly suppressed by Ang II infusion (0.59±0.11 DU versus 1.00±0.09, n=7 each, Figure 3C); however, they were increased by ARB (1.31±0.10, n=10).
To verify equal loading, the membranes of kidney samples were reprobed with β-actin antibody and the densitometric values were similar (1.00±0.01 DU in sham, n=7; 1.03±0.02 in Ang II group, n=7; and 0.97±0.02 in Ang II+ARB group, n=10, Figure 3D).
Urine Samples
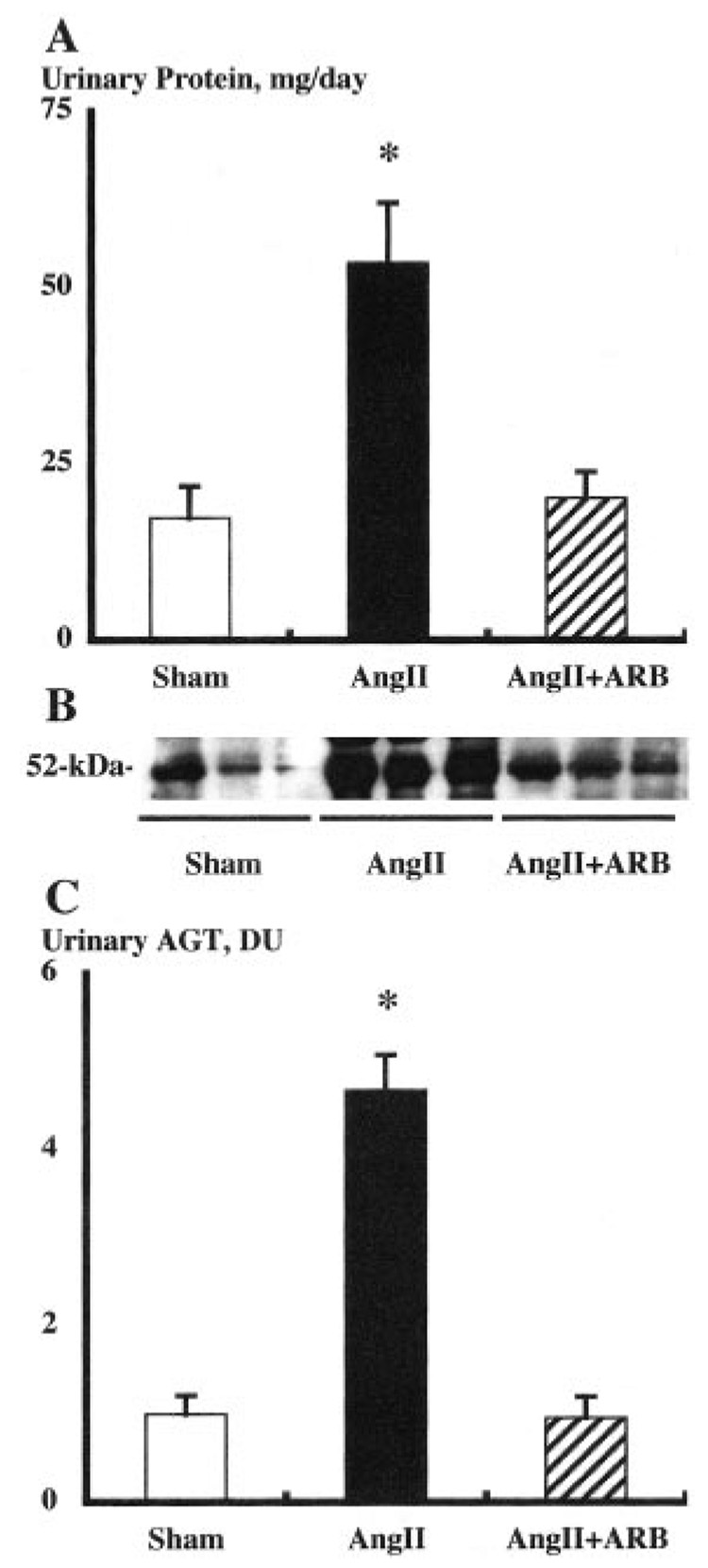
Urinary excretion rates of total protein (Figure 5A) were enhanced in Ang II-infused animals (54±8 mg/d versus 17±5). ARB treatment completely prevented this augmentation (20±4 mg/d).
Figure 5. Urinary protein and AGT excretion rates of each group.
A, Urinary excretion rates of total protein were enhanced in Ang II-infused animals. ARB treatment prevented this augmentation. B, Representative Western blot analysis of urinary AGT levels among groups showing the stimulation in Ang II-infused group. C, Urinary excretion rates of AGT were enhanced 4.7× in Ang II-infused animals. ARB treatment prevented this augmentation. Ang II indicates angiotensin II; ARB, angiotensin II type1 receptor blocker, olmesartan; AGT, angiotensinogen. *P<0.05 compared with the sham group.
Furthermore, urinary excretion rates of AGT, measured by Western blot analysis (Figure 5B), were enhanced by 4.7 times in Ang II-infused animals (4.67±0.41 DU versus 1.00±0.21, Figure 5C). The critical finding of the present study is that AT1-receptor blockade completely prevented this augmentation of urinary AGT excretion rate (0.96±0.23) as well as kidney AGT levels.
To rule out the possibility that this urinary AGT enhancement by Ang II infusion is a consequence of renal damage, urinary NAG excretion, a marker of renal injury, was also determined. Ratios of NAG excretion to creatinine excretion were not altered among the groups (37±9 U/g in sham, 44±9in Ang II group, and 38±9 in Ang II+ARB group).
Discussion
In the present study, chronic Ang II infusion led to progressive increases in systolic BP and resulted in the augmentation of kidney cortex AGT protein levels and kidney Ang II levels, as well as increases in urinary excretion rates of AGT. These results are consistent with previous data demonstrating amplification of the intrarenal AGT and Ang II levels in Ang II-infused hypertension.11–14,20 Furthermore, the increases in kidney Ang II levels were substantially attenuated by concomitant treatment with an ARB even under conditions in which the plasma Ang II levels increased markedly. As previously reported, urinary excretion rates of AGT were closely correlated with kidney Ang II content (R=0.73), but not with plasma Ang II concentration (R=0.12), thus supporting the hypothesis that urinary AGT reflects intrarenal Ang II content. The novel issue addressed in the present study is whether the augmentation of intrarenal AGT and urinary excretion rates of AGT in Ang II-dependent hypertension is dependent on activation of AT1 receptors. Accordingly, Ang II-infused rats were treated with an ARB. The data demonstrate that renal AGT and urinary AGT excretion rate were not increased in the rats treated with an ARB, thus supporting the hypothesis that AT1-receptor activation is the critical link in the mechanism by which modest increases in circulating Ang II stimulate renal AGT formation and release it into the tubular network.
It is generally recognized that AGT is synthesized in the liver and is secreted into plasma. Therefore, it is possible that urinary AGT could be derived from circulating AGT. However, previous studies have indicated that AGT excreted into urine reflects the intrarenal production of this protein rather than filtered AGT.21 AGT is poorly filtered across the glomerular membrane because of its large size (50- to 65-kDa).7,22,23 Moreover, a recent study provided evidence that urinary AGT is of renal origin.14 Human AGT was administered intravenously and both rat and human AGT were assessed in plasma and urine. Rat AGT was readily detected in plasma and urine both before and after an acute injection of exogenous human AGT; however, human AGT was detected only in the plasma collected after the acute administration of human AGT, but was not detected in the urine in either Ang II-infused hypertensive or sham-operated normotensive rats.14 The failure to detect human AGT in the urine supports limited glomerular permeability and/or tubular degradation. These data indicate that urinary AGT originates from the kidneys and not from the plasma.
Because intrarenal AGT protein i s predominantly localized to proximal tubular cells11,24–30 and there is extensive expression of AT1 receptors on proximal tubular cells,2,5,28,31,32 it seems likely that, in Ang II-infused rats, the increases in Ang II, either in the filtrate or intersti tial fluid, activate the Ang II-AT1 receptor complex leading to enhancement of AGT expression in proximal tubular cells and subsequent increased AGT secretion. In the present study, the immunohistochemical analysis demonstrates the localization of AGT almost exclusively to proximal tubular cells and shows an increased intensity of staining but not altered localization in Ang II-infused rats. Subsequent to its stimulation, increased AGT secretion from proximal tubular cells into the lumen is then responsible for the increased urinary excretion rates of AGT. These increased levels of AGT in the urine indicate that, in Ang II-dependent hypertension, greater amounts of AGT traverse the distal nephron segments and may result in increased formation of Ang II in distal as well as in proximal tubular segments. The increased tubular Ang II formation may be partially responsible for the elevated intrarenal Ang II content and the enhanced fractional sodium reabsorption found in this model.33 Recent studies support these interpretations. Peti-Peterdi et al9 reported that Ang II directly stimulates the amiloride-sensitive epithelial sodium channel activity in the cortical collecting duct via activation of AT1 receptor. This group also demonstrated that angiotensin-converting enzyme is present, allowing Ang II to be formed intraluminally in the collecting duct.34 Moreover, Beutler et al10 showed that chronic Ang II infusions stimulate the mRNA expression of the α subunit of the epithelial sodium channel in collecting duct cells of rats and that treatment with a chronic ARB prevents this augmentation. The present study demonstrates further that stimulation of intrarenal AGT by the AT1 receptor creates an amplification mechanism for the generation of intratubular AGT ultimately leading to increased intratubular formation of Ang I and Ang II. Thus, once Ang II production is increased in the kidney, this mechanism further propagates the production of intratubular Ang II. This is a novel concept that Ang II itself regulates this amplification mechanism via activation of AT1 receptors.
Chronic Ang II-infused hypertension was associated not only with increased urinary excretion rates of AGT but also increased urinary excretion rates of total protein. ARB treatment ameliorated both of these components. Thus, it is possible that the enhanced urinary excretion rates of AGT during Ang II infusions are nonspecific consequences of the hypertension-induced renal injury or of the increases in urinary protein excretion. However, we previously reported that in deoxycorticosterone acetate salt-induced hypertension, which is a common model of volume-dependent hypertension, the urinary protein excretion was significantly higher than in Ang II-infused hypertension, whereas urinary excretion of AGT was significantly lower in deoxycorticosterone acetate salt-induced hypertension than in Ang II-infused hypertension.14 This dissociation between the urinary protein excretion and the urinary AGT excretion rate indicates that the enhanced urinary excretion rate of AGT during Ang II infusions is not simply the consequence of the hypertension or of the hypertension-induced renal injury. Collectively, the results indicate that the enhanced urinary excretion rate of AGT is specifically a consequence of Ang II-induced hypertension and the associated stimulation of intrarenal AGT mRNA and protein levels.
It is interesting to note that chronic Ang II infusion significantly suppressed both plasma renin activity and kidney renin levels, whereas chronic treatment with an ARB significantly increased both of them. Although renin and renin mRNA may be expressed in many extrarenal cells, such as the placental, ovarian, testicular,35 adrenal,36 and pituitary37,38 cells of a number of species, the juxtaglomerular cells of the kidney are thought to be the only source of circulating renin.39 This explains the parallel changes of plasma renin activity and kidney renin levels elicited by chronic Ang II infusion and chronic ARB treatment.
In contrast to renin, Ang II levels in plasma and kidney did not change in parallel. ARB treatment increased plasma Ang II concentrations; however, it markedly limited the enhanced kidney Ang II contents elicited by chronic Ang II infusions. Importantly, although comparisons between plasma concentrations (fmol/mL) and tissue contents (fmol/g) only provide a crude estimate, the fact that the kidney Ang II levels in the Ang II-infused rats were greater than the plasma Ang II levels indicates that the total tissue content is greater than can be explained by passive equilibration. After ARB treatment, however, the plasma levels increased, whereas the kidney contents were lower and thus the plasma levels exceeded the kidney levels. Thus, the failure to reduce the kidney Ang II levels back to control may be partially because of the increased contribution of circulating Ang II under conditions of AT1-receptor blockade. In addition, ARB treatment prevented the Ang II-induced inhibition of renin release leading to enhanced plasma renin activity and intrarenal renin content. This augmented renin in both plasma and kidney led to markedly elevated plasma Ang II levels and probably increased intrarenal Ang II content. These mechanisms help explain why ARB treatment only attenuated the increase in kidney Ang II contents by chronic Ang II infusion, whereas it completely blocked the increases in systolic BP and kidney AGT levels. Previous studies have shown that the augmented kidney Ang II contents caused by chronic Ang II infusions occur by 2 mechanisms, enhanced internalization of Ang II15–17 and stimulation of intrarenal AGT expression.11–14 Both mechanisms require activation of AT1 receptors, suggesting a linkage between them. Our present data extend these findings demonstrating that chronic ARB treatment blocks the increases in kidney cortex AGT protein levels and urinary excretion rates of AGT. Thus, ARB treatment antagonizes both the enhanced internalization of circulating Ang II into kidney cells15–17 and the stimulation of intrarenal AGT expression,11–14 resulting in the amelioration of the enhanced intrarenal Ang II contents seen during chronic Ang II infusion. ARB treatment also blocked the Ang II-mediated inhibition of renin release leading to increased circulating Ang II levels.15–17 Collectively, the data support the hypotheses that the augmentation of intrarenal AGT in Ang IIdependent hypertension is dependent on activation of AT1 receptors and that the enhanced urinary excretion rates of AGT during Ang II infusions are the consequence of the stimulation of AGT production by the kidney. These results provide an innovative mechanism by which ARB treatment contributes to a proportionally greater protective effect on the kidney versus other tissues.
As previously described,11,27,28 renal AGT mRNA and its protein are mainly synthesized in the proximal tubular cells, predominantly on the apical side. Once AGT protein is synthesized, it is easily secreted into the lumen and secreted AGT traverses along nephrons and is finally excreted to urine. Under normal conditions, only a tiny fraction of the secreted AGT spills into the distal nephron and urine. Therefore, the measured urinary AGT is very small. However, when the renal production of AGT is stimulated by intrarenal Ang II so that there is greater spillover into the distal nephron and urine, a small absolute increase in the amount that finally reaches the urine results in a large fractional increase because the basal levels are so low. This interpretation may account for the different magnitudes of increases in kidney AGT and urinary AGT.
Perspectives
The present study demonstrates that chronic Ang II infusions to normal rats significantly increased urinary excretion rates of AGT which were associated with elevations in systolic BP, kidney cortex AGT protein, and kidney Ang II levels. These enhancements were either completely (systolic BP and kidney cortex AGT protein) or partially (kidney Ang II levels) blocked by ARB treatment. These data indicate that the augmentation of intrarenal AGT in Ang II-dependent hypertension is dependent on activation of AT1 receptors and that blockade of these receptors markedly reduces urinary excretion rates of AGT and kidney AGT. These data provide further evidence that urinary excretion rates of AGT provide a specific index of intrarenal Ang II levels in Ang IIdependent hypertension. When this hypothesis is shown to be applicable to human subjects, a diagnostic test to identify those hypertensive patients most likely to respond to reninangiotensin system blockade could provide useful information to allow a mechanistic rationale for selecting an optimized approach to the treatment of hypertensive subjects.
Supplementary Material
Acknowledgments
This work was supported by grants from the Institutional Development Award Program of the National Center for Research Resources (P20RR017659), National Heart, Lung, and Blood Institute (R01HL026371), Health Excellence Fund from Louisiana Board of Regents, and Sankyo Co Ltd. (Tokyo, Japan). The authors acknowledge the excellent technical assistance of My-Linh Rauv and Dale M. Seth (Tulane University).
Footnotes
Hypertension is available at http://www.hypertensionaha.org
References
- 1.Navar LG, Kobori H, Prieto-Carrasquero MC. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep. 2003;5:135–143. doi: 10.1007/s11906-003-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyata N, Park F, Li XF, Cowley AW., Jr Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277:F437–F446. doi: 10.1152/ajprenal.1999.277.3.F437. [DOI] [PubMed] [Google Scholar]
- 3.Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A. Intrarenal angiotensin II levels in normal and hypertensive states. J Renin-Angiotensin-Aldosterone Syst. 2001;2:S176–S184. doi: 10.1177/14703203010020013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 5.Harrison-Bernard LM, Navar LG, Ho MM, Vinson GP, El-Dahr SS. Immunohistochemical localization of ANG II AT1 receptor in adult rat kidney using a monoclonal antibody. Am J Physiol. 1997;273:F170–F177. doi: 10.1152/ajprenal.1997.273.1.F170. [DOI] [PubMed] [Google Scholar]
- 6.Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282:F19–F25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–322. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saccomani G, Mitchell KD, Navar LG. Angiotensin II stimulation of Na(+)-H+ exchange in proximal tubule cells. Am J Physiol. 1990;258:F1188–F1195. doi: 10.1152/ajprenal.1990.258.5.F1188. [DOI] [PubMed] [Google Scholar]
- 9.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 10.Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143–1150. doi: 10.1161/01.HYP.0000066129.12106.E2. [DOI] [PubMed] [Google Scholar]
- 11.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–439. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–1335. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou LX, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin II augmentation in angiotensin II-infused rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. [DOI] [PubMed] [Google Scholar]
- 16.Zou LX, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11:570–578. doi: 10.1016/s0895-7061(97)00410-x. [DOI] [PubMed] [Google Scholar]
- 17.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002;39:116–121. doi: 10.1161/hy0102.100780. [DOI] [PubMed] [Google Scholar]
- 18.Kobori H, Nishiyama A, Abe Y, Navar LG. Enhancement of intrarenal angiotensinogen in Dahl salt-sensitive rats on high salt diet. Hypertension. 2003;41:592–597. doi: 10.1161/01.HYP.0000056768.03657.B4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prieto M, Dipp S, Meleg-Smith S, El-Dahr SS. Ureteric bud derivatives express angiotensinogen and AT1 receptors. Physiol Genomics. 2001;6:29–37. doi: 10.1152/physiolgenomics.2001.6.1.29. [DOI] [PubMed] [Google Scholar]
- 20.Schunkert H, Ingelfinger JR, Jacob H, Jackson B, Bouyounes B, Dzau VJ. Reciprocal feedback regulation of kidney angiotensinogen and renin mRNA expressions by angiotensin II. Am J Physiol. 1992;263:E863–E869. doi: 10.1152/ajpendo.1992.263.5.E863. [DOI] [PubMed] [Google Scholar]
- 21.Wang E, Yayama K, Takano M, Okamoto H. Sexual dimorphism of urine angiotensinogen excretion in the rat. Jpn J Pharmacol. 1994;64:243–250. doi: 10.1254/jjp.64.243. [DOI] [PubMed] [Google Scholar]
- 22.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G, Thomas J, Xiao S, Ishigami T, Herrmann T, Terreros DA, Ward K, Lalouel JM. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002;39:1007–1014. doi: 10.1161/01.hyp.0000016177.20565.a0. [DOI] [PubMed] [Google Scholar]
- 23.Tewksbury D. Angiotensinogen. In: Fray JCS, editor. The Endocrine System: Endocrine Regulation of Water and Electrolyte Balance: Handbook of Physiology. 1st ed. Oxford, UK: Oxford University Press; 2000. pp. 59–80. [Google Scholar]
- 24.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E, Zhang S, Cheng T, Inagami T, Ward K, Terreros DA, Lalouel JM. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34:1265–1274. doi: 10.1161/01.hyp.34.6.1265. [DOI] [PubMed] [Google Scholar]
- 25.Davisson RL, Ding Y, Stec DE, Catterall JF, Sigmund CD. Novel mechanism of hypertension revealed by cell-specific targeting of human angiotensinogen in transgenic mice. Physiol Genomics. 1999;1:3–9. doi: 10.1152/physiolgenomics.1999.1.1.3. [DOI] [PubMed] [Google Scholar]
- 26.Richoux JP, Cordonnier JL, Bouhnik J, Clauser E, Corvol P, Menard J, Grignon G. Immunocytochemical localization of angiotensinogen in rat liver and kidney. Cell Tissue Res. 1983;233:439–451. doi: 10.1007/BF00238309. [DOI] [PubMed] [Google Scholar]
- 27.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 29.Darby IA, Congiu M, Fernley RT, Sernia C, Coghlan JP. Cellular and ultrastructural location of angiotensinogen in rat and sheep kidney. Kidney Int. 1994;46:1557–1560. doi: 10.1038/ki.1994.445. [DOI] [PubMed] [Google Scholar]
- 30.Darby IA, Sernia C. In situ hybridization and immunohistochemistry of renal angiotensinogen in neonatal and adult rat kidneys. Cell Tissue Res. 1995;281:197–206. doi: 10.1007/BF00583388. [DOI] [PubMed] [Google Scholar]
- 31.Wang ZQ, Millatt LJ, Heiderstadt NT, Siragy HM, Johns RA, Carey RM. Differential regulation of renal angiotensin subtype AT1A and AT2 receptor protein in rats with angiotensin-dependent hypertension. Hypertension. 1999;33:96–101. doi: 10.1161/01.hyp.33.1.96. [DOI] [PubMed] [Google Scholar]
- 32.Bouby N, Hus-Citharel A, Marchetti J, Bankir L, Corvol P, Llorens-Cortes C. Expression of type 1 angiotensin II receptor subtypes and angiotensin II-induced calcium mobilization along the rat nephron. J Am Soc Nephrol. 1997;8:1658–1667. doi: 10.1681/ASN.V8111658. [DOI] [PubMed] [Google Scholar]
- 33.Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319–F325. doi: 10.1152/ajprenal.2000.279.2.F319. [DOI] [PubMed] [Google Scholar]
- 34.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 35.Sealey JE, Glorioso N, Itskovitz J, Laragh JH. Prorenin as a reproductive hormone: new form of the renin system. Am J Med. 1986;81:1041–1046. doi: 10.1016/0002-9343(86)90402-x. [DOI] [PubMed] [Google Scholar]
- 36.Sigmund CD, Gross KW. Structure, expression, and regulation of the murine renin genes. Hypertension. 1991;18:446–457. doi: 10.1161/01.hyp.18.4.446. [DOI] [PubMed] [Google Scholar]
- 37.Deschepper CF, Mellon SH, Cumin F, Baxter JD, Ganong WF. Analysis by immunocytochemistry and in situ hybridization of renin and its mRNA in kidney, testis, adrenal, and pituitary of the rat. Proc Natl Acad Sci U S A. 1986;83:7552–7556. doi: 10.1073/pnas.83.19.7552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naruse K, Takii Y, Inagami T. Immunohistochemical localization of renin in luteinizing hormone-producing cells of rat pituitary. Proc Natl Acad Sci U S A. 1981;78:7579–7583. doi: 10.1073/pnas.78.12.7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, physiology, and molecular biology of renin secretion. Physiol Rev. 1990;70:1067–1116. doi: 10.1152/physrev.1990.70.4.1067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.