Abstract
Although mounting evidence has demonstrated an important role of Wnt/β-catenin signaling in the development and progression of cancer, the therapeutic potential of small molecules that target this pathway for prostate cancer remains largely unknown. We reported herein that the highly invasive androgen-independent PC-3 and DU145 human prostate cancer cells exhibited higher levels of Wnt/β-catenin signaling than the androgen-dependent LNCaP prostate cancer cells and non-cancerous PZ-HPV-7 and PWR-1E prostate cells, and that exogenous Wnt3A treatment exaggerated the difference of the Wnt/β-catenin signaling levels among these prostate cells. Furthermore, we demonstrated that the non-steroidal anti-inflammatory drug, sulindac sulfide, the cyclooxygenase-2 (COX-2) selective inhibitor, celecoxib, and the nitric oxide-donating aspirin derivative, NO-ASA, blocked Wnt/β-catenin signaling in PC-3 and DU145 cells. These effects occurred at concentrations comparable to those required to inhibit cell proliferation, indicating that the inhibitory effect of these drugs on prostate cancer cell proliferation may involve the suppression of Wnt/β-catenin signaling. Finally, we showed that a novel small molecule inhibitor of Wnt/β-catenin signaling, PKF118–310, inhibited Wnt/β-catenin signaling and proliferation in prostate cancer cells within the same concentration range. Together, these results suggest that small molecules that inhibit Wnt/β-catenin signaling have therapeutic potential for the prevention or treatment of prostate cancer.
Index Words: prostate cancer, Wnt signaling, β-catenin, NSAID, small molecule inhibitor
1. Introduction
At the heart of the canonical Wnt pathway is the stabilization of cytosolic β-catenin, which enters the nucleus to activate Wnt target genes by binding to transcription factors of the T-cell factor/lymphoid enhancing factor (TCF/LEF) family (He et al., 2004; Moon et al., 2004; Dihlmann and von Knebel Doeberitz, 2005; Barker and Clevers, 2006). In the absence of Wnt ligands, β-catenin is phosphorylated by a multi-protein complex that marks it for ubiquitination and degradation by the proteasome. This β-catenin degradation complex contains the adenomatous polyposis coli (APC) tumor suppressor, the scaffold protein Axin, the glycogen synthase kinase 3β (GSK3β), and casein kinase 1. The action of this complex is inhibited upon binding of Wnt to its cell-surface receptors of the Frizzled (Fz) and the low density lipoprotein receptor-related protein (LRP) families. Through several cytoplasmic relay components, the signal is transduced to β-catenin, which then enters the nucleus to form a complex with TCF to activate transcription of Wnt target genes (He et al., 2004; Moon et al., 2004; Dihlmann and von Knebel Doeberitz, 2005; Barker and Clevers, 2006). A variety of Wnt/β-catenin target genes have been identified, which include those that regulate cell proliferation and apoptosis, thus mediating tumor promotion and progression. Deregulation of the Wnt/β-catenin signaling pathway is frequently observed in many types of cancer and is suggested to be an early event in tumorigenesis (He et al., 2004; Moon et al., 2004; Dihlmann and von Knebel Doeberitz, 2005; Barker and Clevers, 2006).
Although genetic mutations of APC or β-catenin are rarely observed in human prostate cancer, compelling evidence has indicated an important role for the Wnt/β-catenin pathway in the development and progression of this type of cancer (Yardy and Brewster, 2005; Verras and Sun, 2006). Up-regulation of Wnt1 expression has been demonstrated in several prostate cancer cell lines, as well as prostate cancer tissues, where it is particularly expressed in lymph node and skeletal metastases of prostate cancer patients (Chen et al., 2004). The examination of β-catenin expression by immunohistochemical analysis has revealed aberrant cytoplasmic/nuclear β-catenin localization in prostate cancer specimens (Chesire et al., 2002; de la Taille et al., 2003; Chen et al., 2004). In addition, specific expression of activated β-catenin in the prostate of transgenic mice induced prostatic hyperplasia and squamous metaplasia (Gounari et al., 2002; Bierie et al., 2003). Other studies have reported a conditional knockout of the APC gene in prostate epithelial cells resulted in the development of prostatic adenocarcinoma, which positively correlated with increased cytoplasmic and nuclear levels of β-catenin (Bruxvoort et al., 2007). Furthermore, it was shown that treatment of prostate cancer cells with Wnt3A significantly enhanced cell growth (Verras et al., 2004).
Despite the accumulating data identifying the importance of Wnt/β-catenin signaling in the development and progression of human prostate cancer, the therapeutic potential of small molecules targeting this signaling pathway for prostate cancer remains largely unknown. In the present study, we characterized Wnt/β-catenin signaling in prostate cancer cells, and tested the effects of the nonsteroidal anti-inflammatory drug (NSAID), sulindac sulfide (Goluboff et al., 1999 & 2001; Narayanan et al., 2004; Han et al., 2008), the COX-2 selective inhibitor, celecoxib (Steinbach et al., 2000; Gupta et al., 2004; Narayanan et al., 2004; Pruthi et al., 2004), the nitric oxide-donating aspirin derivative, NO-ASA (Kashfi et al., 2002; Rigas, 2007), and a novel small molecule inhibitor of Wnt/β-catenin signaling, PKF118–310 (Lepourcelet et al., 2004), on prostate cancer cell Wnt/β-catenin signaling and proliferation. Our results suggest that suppression of the Wnt/β-catenin signaling pathway is a potential target for prostate cancer chemoprevention or chemotherapy.
2. Materials and Methods
2.1. Materials
NO-ASA [NCX4040; 2-(acetyloxy)benzoic acid 4-(nitrooxy-methyl)phenyl ester] was a generous gift from Dr. Basil Rigas (SUNY at Stony Brook, NY). Celecoxib was kindly provided by Dr. Martin Johnson (University of Alabama at Birmingham, AL). Sulindac sulfide was purchased from Sigma. PKF118–310 was purchased from Asinex. Plasmid Glutathione S-transferase (GST)-E-cadherin was kindly provided by Dr. Gail Johnson (University of Rochester, NY). The TOPFLASH TCF luciferase construct was from Upstate Biotechnology. A β-galactosidase-expressing vector was from Promega. Monoclonal anti-β-catenin was from BD Biosciences. Monoclonal anti-Axin2 and anti-Cyclin D1 were from Cell Signaling. Polyclonal rabbit anti-Cyclin D1 was from Chemicon International. Monoclonal anti-actin was from Sigma. Peroxidase labeled anti-mouse antibody and ECL system were purchased from Amersham Life Science. The dual luciferase, β-galactosidase assay and Cell Titer Glo assay systems were from Promega. RMPI-1640 medium, serum, and plastic-ware were obtained from Life Technologies, Inc. Keratinocyte Medium from Lonza. Immobilon-P transfer membrane was purchased from Millipore. Rainbow molecular weight markers were purchased from GE Healthcare. Proteinase inhibitor cocktail Complete™ was obtained from Boehringer Mannheim.
2.2. Cell culture and conditioned media
All cell lines were obtained from ATCC and grown under standard cell culture conditions at 37°C in a humidified atmosphere with 5% CO2. Noncancerous prostate cell lines PZ-HPV-7 and PWR-1E cells were cultured in Keratinocyte Growth Medium from Lonza, while the prostate cancer cell lines PC-3, LNCaP and DU145 were cultured in RPMI-1640 medium containing 10% fetal bovine serum, 2 mM of L-glutamine, 100 units/ml of penicillin, and 100 µg/ml of streptomycin. Wnt3A-secreting L cells and control L cells were cultured in Dulbecco's minimum essential medium containing 10% fetal bovine serum, 2 mM of L-glutamine, 100 units/ml of penicillin, 100 µg/ml of streptomycin and 350 µg/ml of G418. Wnt3A-conditioned medium (CM) and L cell control CM were prepared according to manufacturer’s specifications.
2.3. Luciferase reporter assay
Prostate cells were plated into 12-well plates. After overnight culture, the cells were transiently transfected with 0.2 µg of the TOPFLASH TCF luciferase construct, and 0.2 µg of β-galactosidase-expressing vector in each well. Cells were then lysed 48 h later and both luciferase and β-galactosidase activities were determined. The luciferase activity was normalized to the β-galactosidase activity.
2.4. Western blotting
Cells in 6-well plates were lysed in 0.5 ml of lysis buffer (phosphate-buffered saline containing 1% Triton X-100 and 1 mM PMSF) at 4°C for 30 min. Equal quantities of protein were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions. Following transfer to immobilon-P transfer membrane, successive incubations with anti-β-catenin, anti-Axin2, anti-Cyclin D1, or anti-actin antibody, and horseradish peroxidase-conjugated secondary antibody were carried out for 60–120 min at room temperature. The immunoreactive proteins were then detected using the ECL system.
2.5. GST-E-cadherin binding assay for cytosolic free β-catenin
The GST-E-cadherin binding assay was carried out as previously described in (Lu et al., 2008). Uncomplexed cytosolic free β-catenin present in 100 µg of total cell lysate was subjected to SDS-PAGE and detected using the monoclonal antibody to β-catenin.
2.6. Cell proliferation assay
Cells were seeded into 96-well tissue culture treated microtiter plates at a density of 5000 cells/well. RPMI-1640 containing 1.5% fetal bovine serum was used as assay media for PC-3, DU-145 and LNCaP cells, and Keratinocyte growth medium containing 1.5% fetal bovine serum was used for PWR-1E and PZ-HPV-7 cells. After 24h incubation, the cells were treated with various compounds for 72 h. Cell viability was measured by the Cell Titer Glo Assay, which is a luminescent assay that is an indicator of live cells as a function of metabolic activity and ATP content.
3. Results
3.1. Wnt/β-catenin signaling in prostate cells
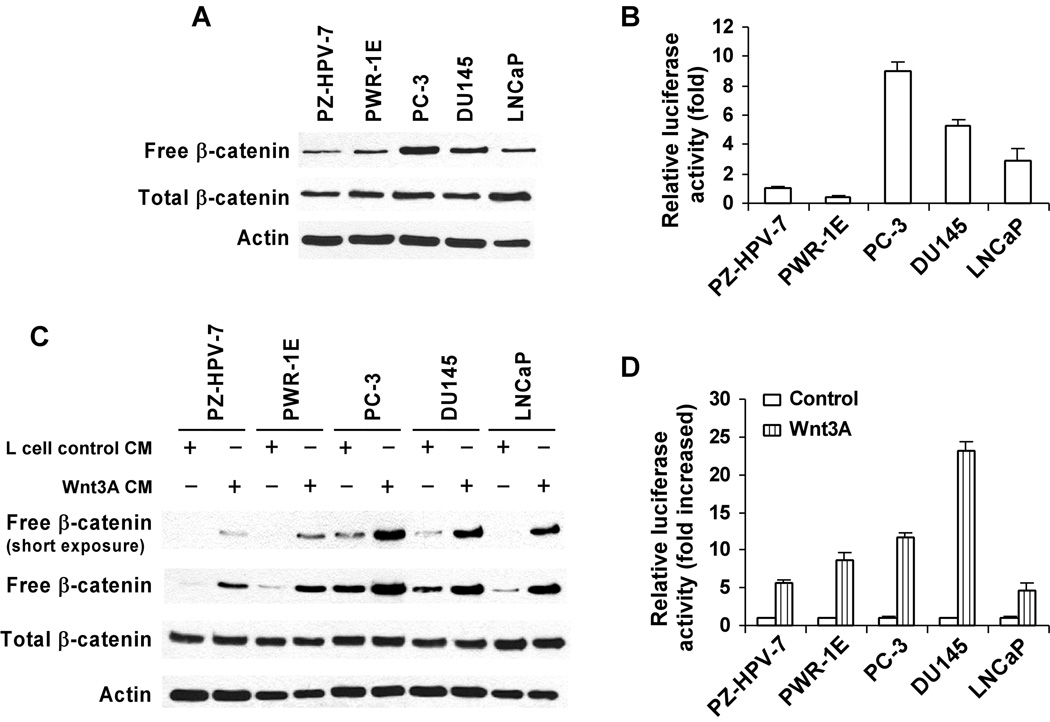
Uncomplexed cytosolic β-catenin (free β-catenin) is the active form of β-catenin that is translocated to the cell nucleus to activate transcription factors of the TCF/LEF family, leading to the transcription of Wnt target genes (Bafico et al., 1998 & 2004; Mi and Johnson 2005; Lu et al., 2008). We examined the levels of Wnt/β-catenin signaling in five human prostate cell lines by determining the levels of free β-catenin present in the cytosol of these cells. As shown in Fig. 1A, the two non-cancerous prostate cell lines, PZ-HPV-7 and PWR-1E, and one noninvasive androgen-dependent prostate cancer cell line, LNCaP, exhibited relatively low levels of cytosolic free β-catenin. By contrast, the invasive androgen-independent prostate tumor cell lines, PC-3 and DU145, displayed appreciably higher levels of cytosolic free β-catenin. To further characterize Wnt/β-catenin signaling in prostate cells, prostate cells were transiently transfected with the Wnt/β-catenin signaling reporter construct TOPFLASH. As expected, it was found that the levels of TOPFLASH luciferase activity were significantly higher in prostate cancer cells than non-cancerous prostate cells (Fig. 1B).
Fig. 1. Activation of Wnt/β-catenin signaling in prostate cancer cells.
(A) Non-cancerous prostate PZ-HPV-7 and PWR-1E cells and cancerous prostate PC-3, DU145 and LNCaP cells were cultured in 6-well plates, and the levels of cytosolic free β-catenin and total cellular β-catenin were examined as describe under "Materials and Methods". (B) Prostate cells in 12-well plates were transiently transfected with 0.2 µg of the TOPFLASH luciferase construct and 0.2 µg of the β-galactosidase-expressing vector in each well. The luciferase activity was then measured 48 h later as described under "Materials and Methods". Values are the average of triple determinations with the s.d. indicated by error bars. (C) Prostate cells in 6-well plates were incubated with 25% of Wnt3A CM or L cell control CM for 3 h, and the levels of cytosolic free β-catenin and total cellular β-catenin were examined. All the samples for Western blotting of total cellular β-catenin were also probed with anti-actin antibody to verify equal loading. (D) Prostate cells in 12-well plates were transiently transfected with 0.2 µg of the TOPFLASH luciferase construct and 0.2 µg of the β-galactosidase-expressing vector in each well. After 24 h incubation, cells were treated with 20% Wnt3A CM. The luciferase activity was then measured 24 h later. Values are the average of triple determinations with the s.d. indicated by error bars.
3.2. Effects of Exogenous Wnt3A treatment on Wnt/β-catenin signaling levels in prostate cells
Previous studies have shown that Wnt3A is a canonical Wnt ligand that binds to Wnt co-receptor LRP and Fz and activates Wnt/β-catenin signaling (Liu et al., 2003). Prostate cancer cells have been shown to express Wnt3A (Hall et al., 2005), although differences between normal and tumor cells have not been reported. To further characterize Wnt/β-catenin signaling in prostate cancer cells, we treated the cells with the Wnt3A CM for 3 h prior to measuring the levels of cytosolic free β-catenin and total cellular β-catenin. As shown in Fig. 1C, exogenous Wnt3A stimulation exaggerates the difference of cytosolic free β-catenin levels among these prostate cells. Furthermore, when prostate cells were transiently transfected with the Wnt/β-catenin signaling reporter TOPFLASH, it was found that the Wnt3A treatment resulted in ~12–23 fold increases in the levels of TOPFLASH luciferase activity in the invasive androgen-independent PC3 and DU145 prostate cancer cells, while only ~5–8 fold increases in the non-cancerous PZ-HPV-7 and PWR-1E prostate cells and noninvasive androgen-dependent LNCaP prostate cancer cells (Fig. 1D).
3.3. NASIDs block Wnt/β-catenin signaling in PC-3 and DU145 cells and inhibit cell proliferation
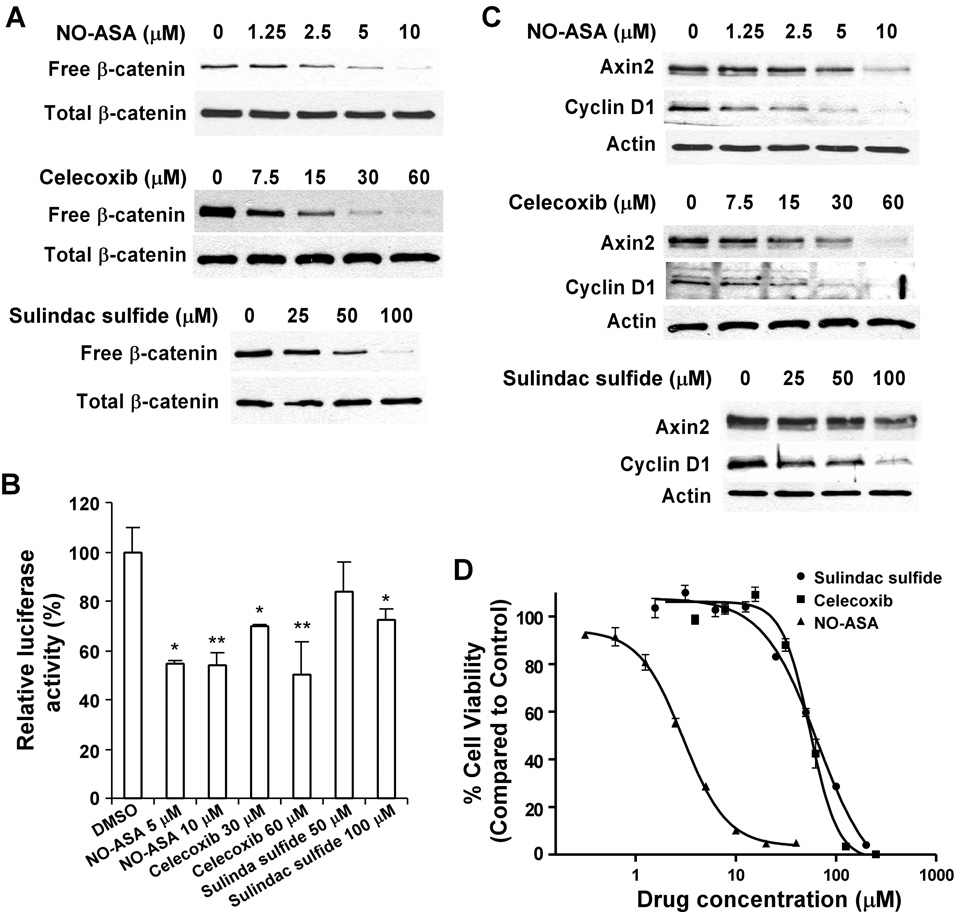
Although experimental studies suggest that NSAIDs directly or indirectly inhibit the Wnt/β-catenin signaling pathway in colon tumor cells (Dihlmann and von Knebel Doeberitz, 2005; Barker and Clevers, 2006), their effect on Wnt/β-catenin signaling in prostate cancer cells has not been well studied. Thus, we treated PC-3 cells with the non-selective COX inhibitor, sulindac sulfide (Goluboff et al., 1999 & 2001; Narayanan et al., 2004; Han et al., 2008), the COX-2 selective inhibitor, celecoxib (Steinbach et al., 2000; Gupta et al., 2004; Narayanan et al., 2004; Pruthi et al., 2004), and a nitric oxide donating derivative of aspirin, NO-ASA (Kashfi et al., 2002; Rigas, 2007). Fig. 2A demonstrates that after 24 h of treatment, all tested NSAIDs significantly reduced cytosolic free β-catenin levels in PC-3 cells. In addition, the Wnt/β-catenin signaling TOPFLASH reporter assay demonstrated that all tested NSAIDs greatly reduced the TOPFlash activity in PC-3 cells after 24 h treatment (Fig. 2B).
Fig. 2. Inhibitory effects of NSAIDs celecoxib, sulindac sulfide and NO-ASA on Wnt/β-catenin signaling in PC-3 cells.
(A) PC-3 cells in 6-well plates were treated with NSAIDs celecoxib, sulindac sulfide and NO-ASA at indicated concentrations for 24 h. The levels of cytosolic free β-catenin and total cellular β-catenin were examined. (B) PC-3 cells in 12-well plates were transiently transfected with 0.2 µg of the TOPFLASH luciferase construct and 0.2 µg of the β-galactosidase-expressing vector in each well. After 24 h incubation, cells were treated with NSAIDs celecoxib, sulindac sulfide and NO-ASA at indicated concentrations. The luciferase activity was then measured 24 h later. Values are the average of triple determinations with the s.d. indicated by error bars. *P<0.05 verse cells treated with treated with DMSO. **p < 0.01 verse cells treated with DMSO. (C) Experimental setup was the same as described in panel A. The total cellular levels of Axin2 and Cyclin D1 were examined. All the samples for each Western blotting were also probed with anti-actin antibody to verify equal loading. (D) PC3 cells in 96-well plates were treated with NSAIDs celecoxib, sulindac sulfide and NO-ASA for 72 h, and cell viability was measured by the Cell Titer Glo Assay system. All the values are the average of triple determinations with the s.d. indicated by error bars.
Axin2 (Yan et al., 2001; Jho et al., 2002; Leung et al., 2002; Lustig et al., 2002) is a specific transcriptional target of the Wnt/β-catenin signaling pathway. It is well recognized that the expression level of Axin2 is the signature of the activation of Wnt/β-catenin signaling. In addition, cyclin D1 has been identified as a key transcriptional target of the Wnt/β-catenin pathway, which is critical for tumor cell proliferation and survival (Shtutman et a., 1999; Tetsu and McCormick, 1999). To confirm the effects of the NSAIDs on Wnt/β-catenin signaling, we examined the expression of Axin2 and Cyclin D1 in PC-3 cells. As shown in Fig. 2C, all tested NSAIDs greatly reduced the expression of Axin2 and Cyclin D1 in PC-3 cells after 24 h treatment.
We then investigated the effects of the NSAIDs on the proliferation of PC-3 cells. Fig. 2D shows that celecoxib, sulindac sulfide and NO-ASA inhibited PC-3 cell proliferation with IC50 values of 57, 55, and 3 µM, respectively. The IC50 values are comparable to those shown to reduce the level of cytosolic free β-catenin and block the expression of Axin2 and Cyclin D1.
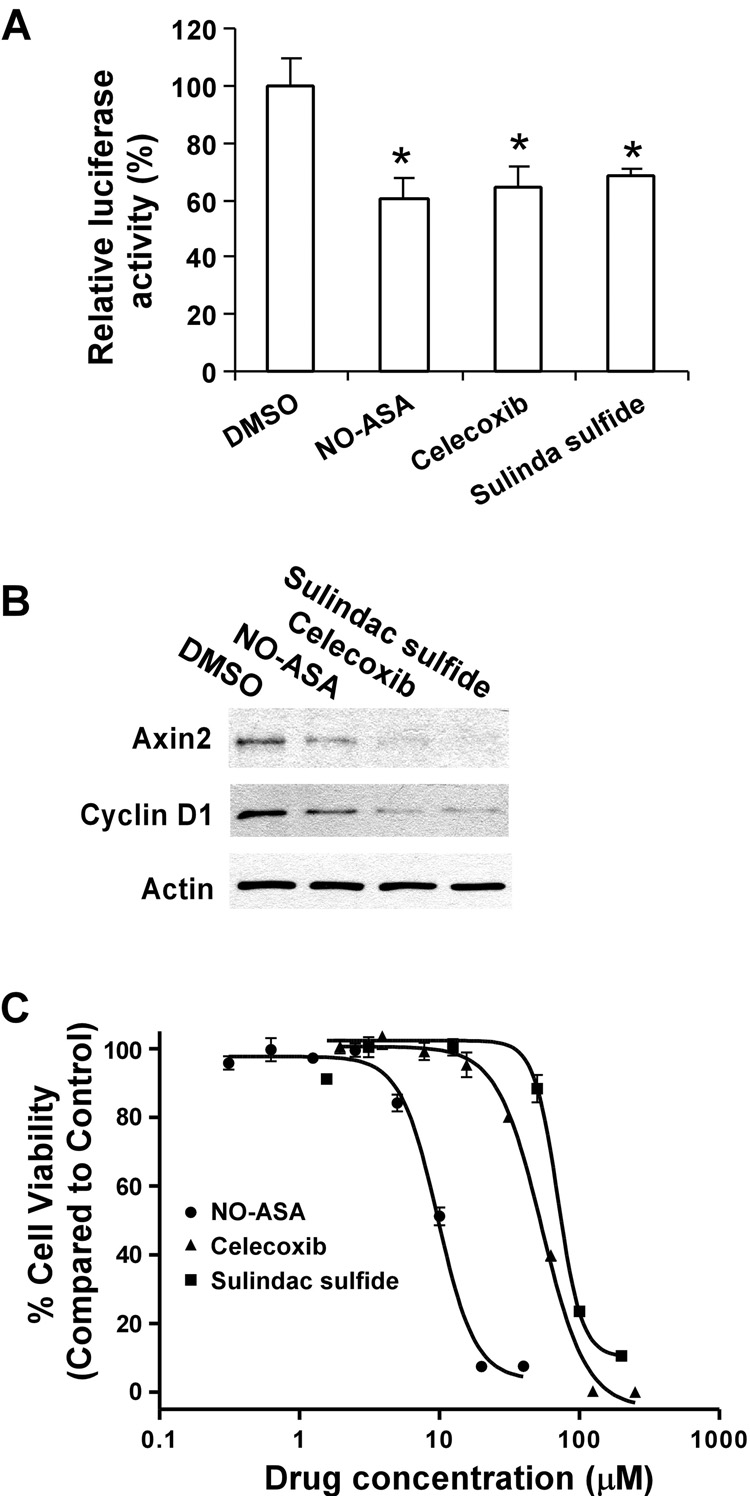
To confirm the effects of the NSAIDs on Wnt/β-catenin signaling in prostate cancer cells, we repeated the experiments with another invasive androgen-independent prostate tumor cell line DU145. As expected, celecoxib, sulindac sulfide and NO-ASA significantly suppressed the level of Wnt/β-catenin signaling reporter TOPFLASH activity in DU145 cells (Fig. 3A), reduced the expression of Axin2 and Cyclin D1 in DU145 cells (Fig. 3B), and inhibited DU145 cell proliferation with IC50 values of 54, 70, and 9.7 µM, respectively (Fig.3C). All together, these results suggest that the inhibitory effects of celecoxib, sulindac sulfide and NO-ASA on prostate cancer cells may involve the suppression of Wnt/β-catenin signaling.
Fig. 3. Inhibitory effects of NSAIDs celecoxib, sulindac sulfide and NO-ASA on Wnt/β-catenin signaling in DU145 cells cells.
(A) DU145 cells in 12-well plates were transiently transfected with 0.2 µg of the TOPFLASH luciferase construct and 0.2 µg of the β-galactosidase-expressing vector in each well. After 24 h incubation, cells were treated with NSAIDs celecoxib (30 µM), sulindac sulfide (100 µM) and NO-ASA (5 µM). The luciferase activity was then measured 24 h later. Values are the average of triple determinations with the s.d. indicated by error bars. *P<0.05 verse cells treated with treated with DMSO. (B) DU145 cells in 6-well plates were treated with NSAIDs celecoxib (60 µM), sulindac sulfide (200 µM) and NO-ASA (10 µM) for 24 h. The total cellular levels of Axin2 and Cyclin D1 were examined. All the samples for each Western blotting were also probed with anti-actin antibody to verify equal loading. (C) DU145 cells in 96-plates were treated with NSAIDs celecoxib, sulindac sulfide and NO-ASA for 72 h, and cell viability was measured by the Cell Titer Glo Assay system. All the values are the average of triple determinations with the s.d. indicated by error bars.
3.4. Specific Wnt/β-catenin signaling inhibitor PKF118–310 blocks Wnt/β-catenin signaling in prostate cancer cells and inhibits cell proliferation
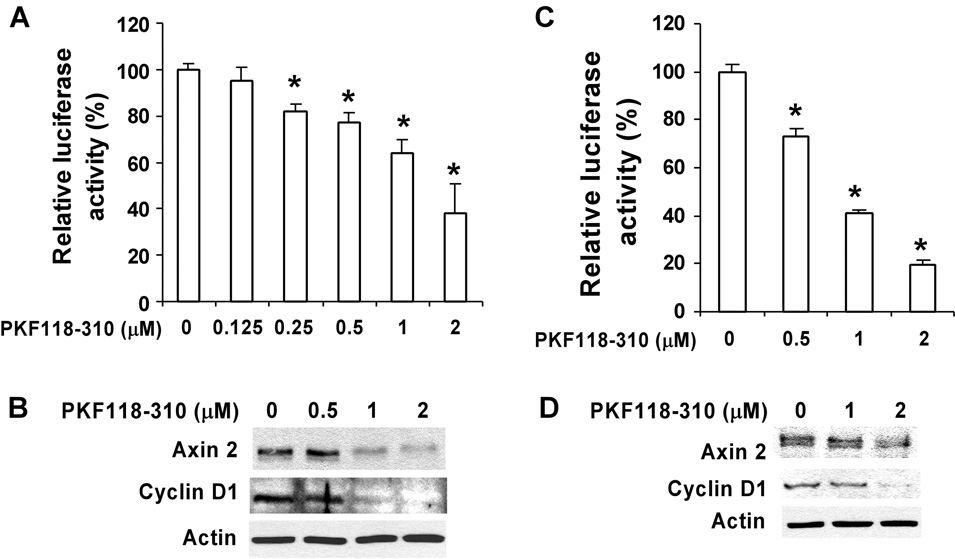
By screening libraries of small molecules in a high-throughput assay, Lepourcelet et al. identified several novel compounds that disrupted TCF/β-catenin complexes and antagonize cellular effects of β-catenin-dependent activities (Lepourcelet et al., 2004). PKF118–310 is one of these compounds (Lepourcelet et al., 2004). We used PKF118–310 as a molecular probe of the Wnt/β-catenin signaling pathway to further study its involvement in prostate cancer cell proliferation. As shown in Fig. 4A, treatment of PC-3 cells with PKF118–310 at a concentration range of 0.5 – 2 µM for 16 h significantly decreased TOPFLASH luciferase activity. In addition, it was found that PKF118–310 significantly suppressed the expression of Wnt/β-catenin signaling targets Axin2 and Cyclin D1 in PC-3 cells within the same concentration range (Fig. 4B).
Fig. 4. Effects of PKF118–310 on Wnt/β-catenin signaling in prostate cancer cells.
(A & C) PC-3 (A) and DU145 (C) cells in 12-well plates were transiently transfected with 0.2 µg of the TOPFLASH luciferase construct and 0.2 µg of the β-galactosidase-expressing vector in each well. After 24 h incubation, cells were treated with PKF118–310 at indicated concentrations. The luciferase activity was then measured 24 h later. Values are the average of triple determinations with the s.d. indicated by error bars. (B & D) PC-3 (B) and DU145 (D) cells in 6-well plates were treated with PKF118–310 at indicated concentrations for 24 h. The total cellular levels of Axin2 and Cyclin D1 were then examined. All the samples were also probed with anti-actin antibody to verify equal loading.
To confirm the effects of PKF118–310 on Wnt/β-catenin signaling in prostate cancer cells, we repeated the experiments with another invasive androgen-independent prostate tumor cell line DU145. As expected, PKF118–310 significantly suppressed the level of Wnt/β-catenin signaling reporter TOPFLASH activity, and Axin2 and Cyclin D1 expression in DU145 cells (Fig. 4C & 4D).
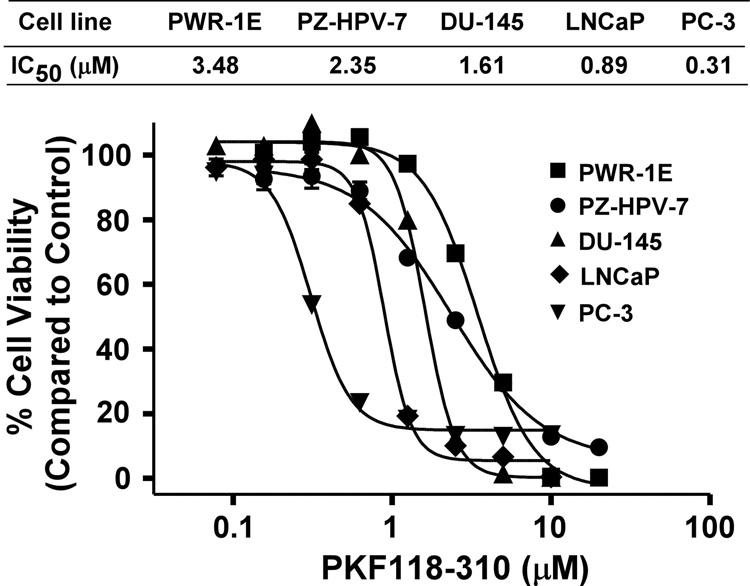
Finally, we examined the effects of PKF118–310 on cell proliferation of three prostate cancer cell lines, PC-3, DU-145 and LNCaP, and the two non-cancerous prostate cell lines, PZ-HPV-7 and PWR-1E. Fig. 5 shows that PKF118–310 inhibited the proliferation of all five cell lines, although the cancer cell lines tended to be more sensitive. Interestingly, PC-3 cells, which exhibit the highest steady-state level of Wnt/β-catenin signaling, displayed the lowest IC50 value at 0.31 µM.
Fig. 5. Effects of PKF118–310 on prostate cell proliferation.
Cells in 96-well plates were treated with PKF118–310 for 72 h, and cell viability was measured by the Cell Titer Glo Assay system. All the values are the average of triple determinations with the s.d. indicated by error bars.
4. Discussion
Deregulation of the Wnt/β-catenin signaling pathway is common in many types of cancer and activation of this pathway may occur early in carcinogenesis (He et al., 2004; Moon et al., 2004; Dihlmann and von Knebel Doeberitz, 2005; Barker and Clevers, 2006). In the present study, we demonstrated that the highly invasive androgen-independent PC-3 and DU145 prostate cancer cells exhibited the higher levels of Wnt/β-catenin signaling compared with the non-cancerous PZ-HPV-7 and PWR-1E prostate cells and androgen-dependent LNCaP prostate cancer cells. We also found that exogenous Wnt3A treatment exaggerated the difference of Wnt/β-catenin signaling levels among these prostate cells. Many Wnt proteins including Wnt3A are expressed in prostate cancer cells (Hall et al., 2005). Our results support the hypothesis that Wnt/β-catenin signaling plays an important role in the development and progression of prostate cancer (Yardy and Brewster, 2005; Verras and Sun, 2006).
Since the aberrant activation of the Wnt signaling pathway was first linked to colon cancer and other cancers in the late 1990s, there has been intense interest in the pharmaceutical and biotechnology sectors in developing specific inhibitors for Wnt signaling. Indeed, experimental studies have shown that NSAIDs inhibit carcinogenesis of various cancer types, which may directly or indirectly inhibit the Wnt-β-catenin signaling pathway (Dihlmann and von Knebel Doeberitz, 2005; Barker and Clevers, 2006). Celecoxib, for example, is a selective cyclooxygenase-2 inhibitor that has been shown to cause adenoma regression in patients with familial adenomatous polyposis, an inherited syndrome that predisposes individuals to colon cancer (Steinbach et al., 2000). Recent studies have demonstrated that celecoxib suppressed prostate carcinogenesis and caused regression of prostatic intraepithelial neoplasia in a transgenic mouse model of prostate cancer (Gupta et al., 2004; Narayanan et al., 2004), and may prevent or delay disease progression in prostate cancer patients (Pruthi et al., 2004). Sulindac has also been shown to inhibit tumorigenesis in various experimental models including prostate (Goluboff et al., 1999 & 2001; Narayanan et al., 2004). Nitric oxide-donating NSAIDs are an emerging class of compounds with promising efficacy as cancer chemopreventive agents (Rigas, 2007). It has been reported that NO-ASA effectively inhibited the growth of prostate cancer LNCaP cells (Kashfi et al., 2002). To our knowledge, the present study is the first report demonstrating that celecoxib, sulindac sulfide, and NO-ASA were able to block Wnt/β-catenin signaling in prostate cancer cells. More importantly, we found that their effects on Wnt/β-catenin signaling occurred at concentrations comparable to those required for inhibiting prostate cancer cell proliferation. Our results suggest that the inhibitory effects of celecoxib, sulindac sulfide and NO-ASA on prostate cancer cell proliferation may involve the suppression of Wnt/β-catenin signaling.
Mounting evidence has indicated an important role for the Wnt/β-catenin pathway in the development and progression of prostate cancer (Yardy and Brewster, 2005; Verras and Sun, 2006), however, the expression of β-catenin in this type of cancer remains controversial. For example, aberrant cytoplasmic/nuclear β-catenin localization and β-catenin upregulation have been demonstrated in prostate cancer specimens (Chesire et al., 2002; de la Taille et al., 2003; Chen et al., 2004; Saha et al., 2008), while β-catenin downregulation has been reported in several other studies too (Kallakury et al., 2001; Horvath et al., 2005; Junior et al., 2008; Whitaker et al., 2008). The reason for this discrepancy is unclear, but Whitaker et al. (2008) suggested that variation in immunohistochemical protocols may explain variations in the reported literature. Uncomplexed cytosolic β-catenin (free β-catenin) is the active form of β-catenin, which accumulates in the cytoplasm, subsequently translocates to the nucleus where it binds and activates transcription factors of the TCF/LEF family, which modulate expression of Wnt/β-catenin signaling target genes (Bafico et al., 1998 & 2004; Mi and Johnson 2005; Lu et al., 2008). In the present study, we found that the highly invasive androgen-independent PC-3 and DU145 human prostate cancer cell lines exhibited higher levels of cytosolic free β-catenin than the androgen-dependent LNCaP line and non-cancerous PZ-HPV-7 and PWR-1E prostate cell lines, and that exogenous Wnt3A treatment greatly enhanced the levels of cytosolic free β-catenin in prostate cancer cells. Moreover, we demonstrated that celecoxib, sulindac sulfide, and NO-ASA significantly reduced cytosolic free β-catenin level in PC-3 cells. Our results support the notion that Wnt/β-catenin signaling is activated in prostate cancer cells.
PKF118–310 is a recently identified small molecule that disrupts TCF/β-catenin complexes and antagonizes cellular effects of β-catenin-dependent activities (Lepourcelet et al., 2004). In the present study, we showed that PKF118–310 blocked Wnt/β-catenin signaling in PC-3 and DU145 cells, and inhibited prostate cancer cell proliferation, although more potent and selective inhibitors may have greater clinical potential. PC-3 cells, which exhibit the highest steady-state level of Wnt/β-catenin signaling, are most sensitive to PKF118–310 treatment among the five tested prostate cell lines. However, we also found that LNCaP cells, which exhibit a lower level of Wnt/β-catenin signaling, are more sensitive to PKF118–310 treatment than DU145 cells. β-catenin has been shown to bind to and act as a coactivator of the androgen receptor (Truica et al., 2000; Song et al., 2003). In the future, we should test whether androgen receptor is involved in the action of PKF118–310.
In summary, we have demonstrated that blocking Wnt/β-catenin signaling in prostate cancer cells either by NSAIDs or the novel Wnt/β-catenin signaling inhibitor PKF118–310 results in inhibition of prostate cancer cell proliferation. Our results suggest that disruption of the Wnt/β-catenin pathway represents an opportunity for rational drug design for prostate cancer prevention or treatment.
Acknowledgments
We are grateful to Dr. Gail Johnson (University of Rochester) for providing GST-E Cadherin cDNA, Dr. Basil Rigas (SUNY, Stony Brook) for providing NO-ASA and Dr. Martin Johnson (University of Alabama at Birmingham) for providing celecoxib. This work was supported in part by a grant from the National Institutes of Health to Y.L. (RO1CA124531).
References
- Bafico A, Gazit A, Wu-Morgan SS, Yaniv A, Aaronson SA. Characterization of Wnt-1 and Wnt-2 induced growth alterations and signaling pathways in NIH3T3 fibroblasts. Oncogene. 1998;16:2819–2825. doi: 10.1038/sj.onc.1201797. [DOI] [PubMed] [Google Scholar]
- Bafico A, Liu G, Goldin L, Harris V, Aaronson SA. An autocrine mechanism for constitutive Wnt pathway activation in human cancer cells. Cancer Cell. 2004;6:497–506. doi: 10.1016/j.ccr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Barker N, Clevers H. Mining the Wnt pathway for cancer therapeutics. Nat. Rev. Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, Taketo MM, Cardiff RD, Miyoshi K, Wagner KU, Robinson GW, Hennighausen L. Activation of β-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22:3875–3887. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- Bruxvoort KJ, Charbonneau HM, Giambernardi TA, Goolsby JC, Qian CN, Zylstra CR, Robinson DR, Roy-Burman P, Shaw AK, Buckner-Berghuis BD, Sigler RE, Resau JH, Sullivan R, Bushman W, Williams BO. Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res. 2007;67:2490–2496. doi: 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, Rabbani SA. Up-regulation of Wnt-1 and β-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–1356. doi: 10.1002/cncr.20518. [DOI] [PubMed] [Google Scholar]
- Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear β-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21:2679–2694. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, Buttyan R, Chopin D. β-Catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin. Cancer Res. 2003;9:1801–1807. [PubMed] [Google Scholar]
- Dihlmann S, von Knebel Doeberitz M. Wnt/β-catenin-pathway as a molecular target for future anti-cancer therapeutics. Int. J. Cancer. 2005;113:515–524. doi: 10.1002/ijc.20609. [DOI] [PubMed] [Google Scholar]
- Goluboff ET, Shabsigh A, Saidi JA. Exisulind (sulindac sulfone) suppresses growth of human prostate cancer in a nude mouse xenograft model by increasing apoptosis. Urology. 1999;53:440–444. doi: 10.1016/s0090-4295(98)00513-5. [DOI] [PubMed] [Google Scholar]
- Goluboff ET, Prager D, Rukstalis D. Safety and efficacy of exisulind in the treatment of recurrent prostate cancer following radical prostatectomy. J. Urol. 2001;166:882–886. [PubMed] [Google Scholar]
- Gounari F, Signoretti S, Bronson R, Klein L, Sellers WR, Kum J, Siermann A, Taketo MM, von Boehmer H, Khazaie K. Stabilization of β-catenin induces lesions reminiscent of prostatic intraepithelial neoplasia, but terminal squamous transdifferentiation of other secretory epithelium. Oncogene. 2002;21:4099–4107. doi: 10.1038/sj.onc.1205562. [DOI] [PubMed] [Google Scholar]
- Gupta S, Adhami VM, Subbarayan M, MacLennan GT, Lewin JS, Hafeli UO, Fu P, Mukhtar H. Suppression of prostate carcinogenesis by dietary supplementation of celecoxib in transgenic adenocarcinoma of the mouse prostate model. Cancer Res. 2004;64:3334–3343. doi: 10.1158/0008-5472.can-03-2422. [DOI] [PubMed] [Google Scholar]
- Hall CL, Bafico A, Dai J, Aaronson SA, Keller ET. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65:7554–7560. doi: 10.1158/0008-5472.CAN-05-1317. [DOI] [PubMed] [Google Scholar]
- Han A, Song Z, Tong C, Hu D, Bi X, Augenlicht LH, Yang W. Sulindac suppresses β-catenin expression in human cancer cells. Eur. J. Pharmacol. 2008;583:26–31. doi: 10.1016/j.ejphar.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Horvath LG, Henshall SM, Lee CS, Kench JG, Golovsky D, Brenner PC, O'Neill GF, Kooner R, Stricker PD, Grygiel JJ, Sutherland RL. Lower levels of nuclear β-catenin predict for a poorer prognosis in localized prostate cancer. Int. J/ Cancer. 2005;113:415–422. doi: 10.1002/ijc.20599. [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/β-catenin/ Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol. Cell. Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junior JP, Srougi M, Borra PM, Dall’ Oglio MF, Ribeiro-Filho LA, Leite KR. E-cadherin and β-catenin loss of expression related to bone metastasis in prostate cancer. Appl. Immunohistochem. Mol. Morphol. 2008 doi: 10.1097/PAI.0b013e3181640bca. In press. [DOI] [PubMed] [Google Scholar]
- Kallakury BV, Sheehan CE, Winn-Deen E, Oliver J, Fisher HA, Kaufman RP, Jr, Ross JS. Decreased expression of catenins (α and β), p120 CTN, and E-cadherin cell adhesion proteins and E-cadherin gene promoter methylation in prostatic adenocarcinomas. Cancer. 2001;92:2786–2795. doi: 10.1002/1097-0142(20011201)92:11<2786::aid-cncr10128>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kashfi K, Ryan Y, Qiao LL, Williams JL, Chen J, Del Soldato P, Traganos F, Rigas B. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: evidence of a tissue type-independent effect. J. Pharmacol. Exp. Ther. 2002;303:1273–1282. doi: 10.1124/jpet.102.042754. [DOI] [PubMed] [Google Scholar]
- Lepourcelet M, Chen YN, France DS, Wang H, Crews P, Petersen F, Bruseo C, Wood AW, Shivdasani RA. Small-molecule antagonists of the oncogenic Tcf/β-catenin protein complex. Cancer Cell. 2004;5:91–102. doi: 10.1016/s1535-6108(03)00334-9. [DOI] [PubMed] [Google Scholar]
- Liu G, Bafico A, Harris VK, Aaronson SA. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol. Cell. Biol. 2003;23:5825–2835. doi: 10.1128/MCB.23.16.5825-5835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Activation of AXIN2 expression by β-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling. J. Biol. Chem. 2002;277:21657–21665. doi: 10.1074/jbc.M200139200. [DOI] [PubMed] [Google Scholar]
- Lu W, Kim KA, Liu J, Abo A, Feng X, Cao X, Li Y. R-spondin1 Synergizes with Wnt3A in Inducing Osteoblast Differentiation and Osteoprotegerin Expression. FEBS Lett. 2008;582:643–650. doi: 10.1016/j.febslet.2008.01.035. [DOI] [PubMed] [Google Scholar]
- Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, van de Wetering M, Clevers H, Schlag PM, Birchmeier W, Behrens J. Negative feedback loop of Wnt signaling through upregulation of conductin/axin2 in colorectal and liver tumors. Mol. Cell. Biol. 2002;22:1184–1193. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi K, Johnson GV. Role of the intracellular domains of LRP5 and LRP6 in activating the Wnt canonical pathway. J. Cell. Biochem. 2005;95:328–338. doi: 10.1002/jcb.20400. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and β-catenin signalling: diseases and therapies. Nat. Rev. Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Narayanan BA, Narayanan NK, Pittman B, Reddy BS. Regression of mouse prostatic intraepithelial neoplasia by nonsteroidal anti-inflammatory drugs in the transgenic adenocarcinoma mouse prostate model. Clin. Cancer Res. 2004;10:7727–7737. doi: 10.1158/1078-0432.CCR-04-0732. [DOI] [PubMed] [Google Scholar]
- Pruthi RS, Derksen JE, Moore D. A pilot study of use of the cyclooxygenase-2 inhibitor celecoxib in recurrent prostate cancer after definitive radiation therapy or radical prostatectomy. BJU Int. 2004;93:275–278. doi: 10.1111/j.1464-410x.2004.04601.x. [DOI] [PubMed] [Google Scholar]
- Rigas B. The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr. Opin. Gastroenterol. 2007;23:55–59. doi: 10.1097/MOG.0b013e32801145b0. [DOI] [PubMed] [Google Scholar]
- Saha B, Arase A, Imam SS, Tsao-Wei D, Naritoku WY, Groshen S, Jones LW, Imam SA. Overexpression of E-cadherin and β-catenin proteins in metastatic prostate cancer cells in bone. Prostate. 2008;68:78–84. doi: 10.1002/pros.20670. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, Ben-Ze'ev A. The cyclin D1 gene is a target of the β-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LN, Herrell R, Byers S, Shah S, Wilson EM, Gelmann EP. β-Catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol. Cell. Biol. 2003;23:1674–1687. doi: 10.1128/MCB.23.5.1674-1687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, Wakabayashi N, Saunders B, Shen Y, Fujimura T, Su LK, Levin B. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N. Engl. J. Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;39:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Truica CI, Byers S, Gelmann EP. β-Catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000;60:4709–4713. [PubMed] [Google Scholar]
- Verras M, Brown J, Li X, Nusse R, Sun Z. Wnt3a growth factor induces androgen receptor-mediated transcription and enhances cell growth in human prostate cancer cells. Cancer Res. 2004;64:8860–8866. doi: 10.1158/0008-5472.CAN-04-2370. [DOI] [PubMed] [Google Scholar]
- Verras M, Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Whitaker HC, Girling J, Warren AY, Leung H, Mills IG, Neal DE. Alterations in β-catenin expression and localization in prostate cancer. Prostate. 2008;68:1196–1205. doi: 10.1002/pros.20780. [DOI] [PubMed] [Google Scholar]
- Yan D, Wiesmann M, Rohan M, Chan V, Jefferson AB, Guo L, Sakamoto D, Caothien RH, Fuller JH, Reinhard C, Garcia PD, Randazzo FM, Escobedo J, Fantl WJ, Williams LT. Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/β-catenin signaling is activated in human colon tumors. Proc. Natl. Acad. Sci. USA. 2001;98:14973–14978. doi: 10.1073/pnas.261574498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardy GW, Brewster SF. Wnt signaling and prostate cancer. Prostate Cancer Prostatic. Dis. 2005;8:119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]