Abstract
It is very difficult to study the independent contributions of the afferent and efferent pathways of the subdiaphragmatic vagus to physiology and behavior. Total subdiaphragmatic vagotomy can confound the interpretation of experimental results because it destroys both afferent and efferent vagal fibers. One approach to address this problem involves producing a total ablation of afferent (or efferent) vagal fibers while retaining half of the efferent (or afferent) vagal fibers by making a unilateral rhizotomy plus contralateral subdiaphragmatic vagotomy. However, the completeness of this afferent (or efferent) lesion is based on the assumption that there are no cross-over pathways within the thoracic cavity between the vagal trunks of the rat. To directly test for the presence of vagal cross-over pathways in the rat, we recorded the compound action potentials from the ventral and dorsal trunks of the subdiaphragmatic vagus following electrical stimulation of the left or right cervical vagi. C-fiber cross-over pathways comprised an average of 9% of the total nerve responses (range was 0 to 29%, n = 20). Direct application of the anesthetic bupivacaine to the vagus completely blocked the recorded signals. The vagal cross-over pathways were also demonstrated using capsaicin as a stimulus. These results indicate the presence of thoracic cross-over pathways between vagal trunks in the rat and demonstrate that for most animals it is not possible to produce a “complete” ablation of afferent (or efferent) components of the subdiaphragmatic vagus using unilateral rhizotomy combined with contralateral subdiaphragmatic vagotomy.
Keywords: Vagus, Compound action potential, Electrophysiology, Capsaicin, Rat, Deafferentation
1. Introduction
The vagus is a mixed nerve containing afferent and efferent components that connect the brain to almost all of the internal organs (for review of the vagal afferent system see [2]). Considerable focus has been directed toward understanding the sensory functions of the subdiaphragmatic vagus because it is likely to play important roles in providing information to the brain for nutrition, immune signaling, and detection of toxins. Total subdiaphragmatic vagotomy is commonly used to investigate the role of the subdiaphragmatic vagus in physiology and behavior in the rat. However, results from studies using this lesion method are difficult to interpret because both the vagal afferent and efferent nerve fibers are sectioned. A primary side effect of total subdiaphragmatic vagotomy is gastrointestinal stasis and dysfunction that results from cutting vagal efferent fibers [23]. Total subdiaphragmatic vagotomized animals typically have bloated stomachs and must be fed a liquid diet to return body weight and food intake from reduced to normal levels [17].
Three techniques have been developed to selectively lesion the afferent fibers of the rat vagus. First, kainic acid injections into the nodose ganglia result in destruction of sensory cell bodies and appear not to damage efferent fibers of passage [20]; but, perhaps because of the relative difficulty of surgically isolating and injecting the nodose ganglia, this technique has not been widely used. Second, the neurotoxin capsaicin injected systemically or applied directly to the vagus results in destruction of vagal afferent fibers (e.g., [5,31]). Unfortunately, many vagal afferent fibers are capsaicin-resistant and are not destroyed by this treatment [3]. A third technique uses a unilateral rhizotomy (cutting the afferent vagal rootlets as they exit the cranium before entering the nodose ganglion) combined with a contralateral subdiaphragmatic vagotomy [19,24,26,28,34–37,39]. The result of this method is a bilateral ablation of vagal afferent fibers and a unilateral lesion of vagal efferent fibers. This method can also be used to investigate the efferent system by selectively cutting the efferent vagal rootlets [39]. Unilateral rhizotomy plus contralateral subdiaphragmatic vagotomy is an improvement over the total subdiaphragmatic vagotomy because it does not produce noticeable gastrointestinal motor impairments and animals have normal levels of food intake and body weight shortly after surgery [26,37,39]. This methodology for selective ablation of the afferent (or efferent) vagal fibers relies on the assumption that there are no cross-over pathways between vagal trunks within the thoracic cavity of the rat. However, vagal cross-over pathways are well established for a another species, i.e., the thoracic communicating branch in the ferret [8,9].
Although it is frequently stated that unilateral afferent rootlet rhizotomy combined with contralateral subdiaphragmatic vagotomy is a “complete” or “total” deafferentation of the subdiaphragmatic vagal system, there is some evidence from electrophysiology and tracttracing studies for vagal cross-over pathways in the rat. Sauter and colleagues reported that electrical stimulation of either the left or right cervical vagus evoked compound action potentials from both the dorsal and ventral subdiaphragmatic vagal trunks [33]. Of the six rats tested, five were reported to show a minor left cross-over pathway and four a minor right cross-over pathway [33] (see Fig. 1 for a schematic of the minor and major pathways of the vagal system). Sauter et al. indicate that the minor vagal pathways accounted for approximately 20% of the total responses in those animals that showed minor pathway responses [33].
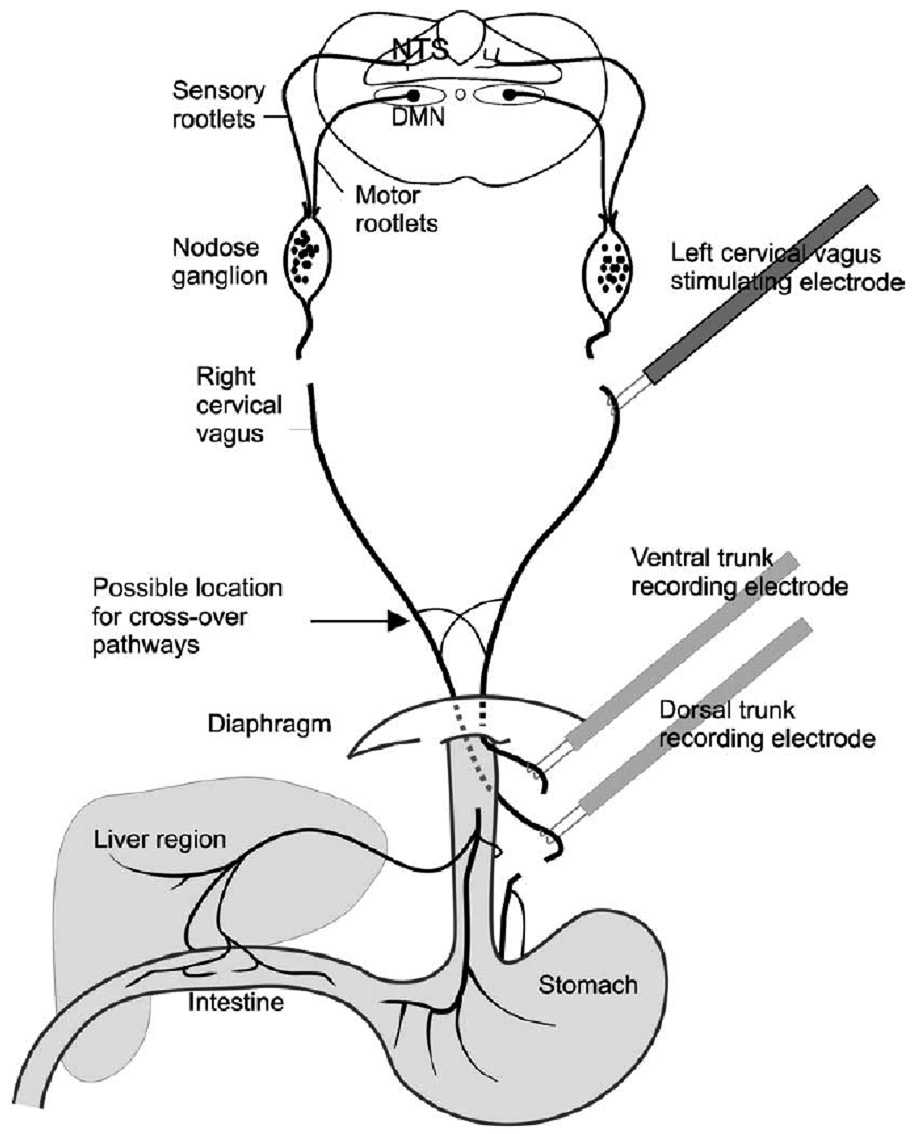
Fig. 1.
The vagal system and electrophysiological recording and stimulating sites. The left and right cervical vagi and ventral and dorsal trunks were transected. Although only the left cervical vagus is stimulated in this configuration, in separate trials, the stimulating electrode was repositioned to stimulate the right cervical vagus. The major vagal pathways are left cervical vagus to ventral trunk and right cervical vagus to dorsal trunk. The minor vagal pathways, containing the cross-over pathways, are left cervical vagus to dorsal trunk and right cervical vagus to ventral trunk. The sensory or motor rootlets are the targets for rhizotomy (not performed in the current study). NTS = nucleus of the solitary tract, DMN = dorsal motor nucleus.
Anatomical tracing also suggests that there might be thoracic cross-over pathways between the rat vagal trunks. In contrast to the heavy staining occurring in the ipsilateral nodose ganglion, there were a few cells observed in the contralateral nodose ganglion when HRP (horseradish peroxidase) was applied to a specific subdiaphragmatic vagal trunk [27]. However, evidence suggests that HRP is a less sensitive method for tract tracing and could potentially underestimate the magnitude of a neural pathway (e.g., [11,38]). More recently, a viral tract-tracing study from the stomach to the brainstem suggests that not all of the vagal afferent fibers are destroyed when rats receive a unilateral afferent rhizotomy combined with contralateral subdiaphragmatic vagotomy [32].
The current experiments were conducted to make a more complete assessment of the vagal cross-over pathways in the rat using electrophysiological techniques. Our experiments were designed to address five issues: (1) use a large group of animals to show descriptive statistics for any cross-over pathways, (2) eliminate sources of signal artifacts, potentially produced by animal movements and neural feedback, by paralyzing animals with pancuronium bromide and cutting the central and distal connections of the vagi, (3) greatly reduce the stimulator produced artifact, probably caused by capacitance discharge through electrode cables, by using biphasic current pulses (see [25]) and offline data processing to remove a slow artifact signal, using a 0.05 Hz high pass filter, (4) use a local anesthetic, bupivacaine, to determine if the electrically evoked compound action potential is neurally mediated, and (5) demonstrate cross-over pathways using a different stimulus, namely direct application of capsaicin to the vagus. This last issue is particularly important since electrical stimulation can produce electromyographic artifacts that can confound interpretations of compound action potential recordings from the vagus nerve (see [33]), but this should not be a problem when using a chemical stimulus, such as capsaicin. Capsaicin was used as a stimulus because it is known to activate a large portion of afferent fiber population of the vagus.
2. Materials and methods
2.1. Subjects
Experiments were conducted on male Sprague–Dawley rats (350–500 g; Charles River, Kingston, NY, USA). Surgery and anesthesia is based on established methodology [13,14]. Animals were euthanized at the conclusion of an experiment by jugular vein infusion of sodium pentobarbital (60 mg). All experiments conformed to established standards of the NIH and the Monell Center IACUC.
2.2. Surgery and electrophysiological recordings
Rats were initially anesthetized with sodium pentobarbital (i.p.; 50 mg/kg) followed by a continuous infusion of sodium methohexital or sodium pentobarbital (25 Al/h) into the left femoral vein. A longitudinal incision was made on the ventral surface of the neck to install a tracheal tube for artificial respiration and to expose the left and right cervical vagi for electrical or chemical stimulation. A surgical retractor was used to expand the opening in the neck and the cavity was filled with warm mineral oil (37 -C). Animals were paralyzed with pancuronium bromide (i.v., 0.4 mg/h) and artificially respirated (95% O2/5% CO2) at 50 cycles/min. Body temperature was kept at 37 -C by a regulated heating pad. Heart rate and expired CO2 were continuously monitored to insure the physiological stability of the preparations.
An incision from the top of the xiphoid process to the lower abdomen was made to expose the abdominal viscera. The edges of the wound were retracted and elevated to create a large ovoid cavity. The stomach was retracted caudally and the liver was displaced to the right of the animal, and the body cavity was filled with warm (37 -C) mineral oil. Silk sutures were tied around the ventral and dorsal vagal trunks along the esophagus and each trunk was cut ¨5 mm below the diaphragm and slightly distal to the sutures. These cuts were done to eliminate neural signals that arise from terminal field connections when cervical vagi are stimulated. At this point, sutures were also tied around the left and right cervical vagi. The nerves were cut just above the sutures to leave ¨5 mm of each cervical vagus free for manipulation. The sutures on the cervical and subdiaphragmatic vagi were used to manipulate the nerves for placement on recording or stimulating electrodes, which eliminated the need to directly handle the nerves with forceps.
The subdiaphragmatic ventral and dorsal trunks were placed on separate silver bipolar hook electrodes (FHC, Bowdoinham, ME) (see Fig. 1). Nerve signals were amplified by 50K using differential AC amplifiers (Grass P511; Astromed) with low (100 Hz) and high (3 kHz) frequency cutoffs. Nerve activity from the ventral and dorsal vagal trunks was recorded simultaneously. Neural activity, body temperature, heart rate, and expired CO2 data were saved to computer hard disk using data acquisition hardware and software (DataWave Tech., Longmont, CO). Nerve signals were digitized at 32 kHz. The distance between recording and stimulating electrodes (see below) averaged 6 cm (see Fig. 1).
2.3. Electrical or chemical stimulation of the vagus
2.3.1. Experiment 1
In 20 animals, a silver bipolar hook electrode was used to electrically stimulate the cervical vagi. Only one cervical vagal trunk, left or right, was stimulated at a time. An isolated stimulator (Model 2100; AM Systems) was used to produce stimulus pulses that were 0.5 to 1 mA and 1 ms in duration (biphasic current). Test runs using lower current levels did not produce consistent results. Experiments were conducted using a computer controlled sequence of 10 stimulus pulses, each separated by 1 s. Ventral and dorsal trunk nerve responses were recorded for 500 ms after each stimulus pulse and the average response of these 10 trials was used for analysis.
2.3.2. Experiment 2
Ten animals from the first experiment were also used to determine whether a local anesthetic would block the electrically evoked compound action potentials. For these experiments, following the initial test of the nerve responses to electrical stimulation, a second set of trials was conducted after placing a small droplet of bupivacaine (0.5%, Abbott Laboratories) on the subdiaphragmatic vagi ¨3 mm above the ventral or dorsal trunk recording sites.
2.3.3. Experiment 3
Eight animals from the first experiment (not used in Experiment 2) and two new animals were used to determine the vagal responses to capsaicin treatment. Five of these animals showed a dorsal trunk minor pathway and five showed a ventral trunk minor pathway (see Fig. 1 for description of minor pathways). The mineral oil from the neck cavity was removed and 100 Al of vehicle (10% Tween 80 in olive oil) was applied to stimulate the minor pathway. After 5 min, 100 Al of capsaicin (10 mg/ml in vehicle) was applied to the same location. For this experiment, neural activity was recorded continuously from the ventral and dorsal trunks of the vagus.
2.4. Data analysis
2.4.1. Experiment 1 and 2
Microvolt levels of neural activity were averaged over 1 s time bins and those data bins from 1 to 150 ms were analyzed using ANOVA and Least Significant difference (LSD) tests were used to compare means (Statistica; Statsoft). Minor pathways were analyzed by comparing the evoked responses to a baseline level, an average AV value from 400–500 ms poststimulus (a time period with no evoked response). A criterion of P < 0.05 was used to indicate statistical significance.
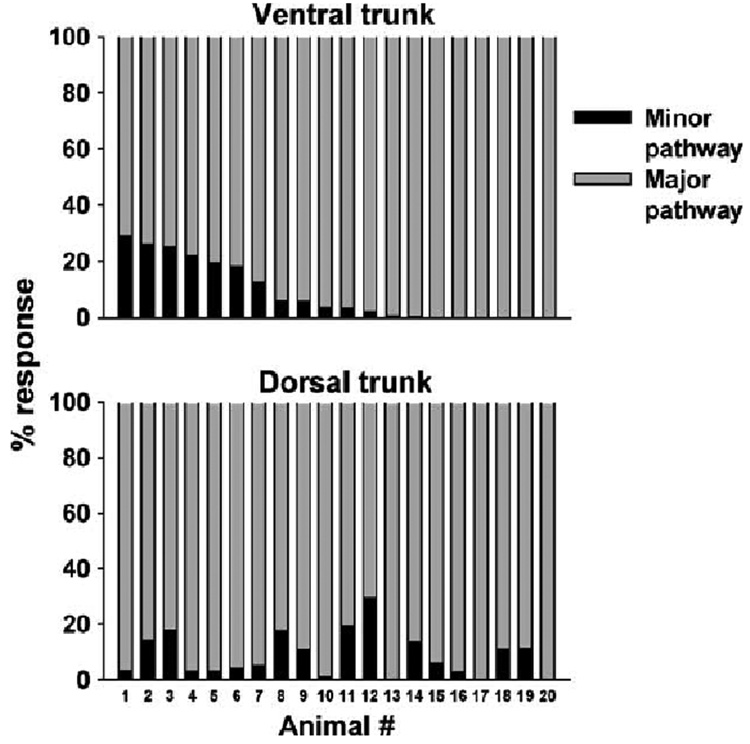
The percentage of response in each pathway (see Fig. 6.) was computed using the formula [(PATHWAY RESPONSE / TOTAL RESPONSE) × 100]. The PATHWAY RESPONSE was the rectified response, from 25 to 135 ms after the stimulus, for each pathway, e.g., right vagus/ ventral trunk, minus the total rectified baseline level, from 400 to 500 ms after the stimulus. The TOTAL RESPONSE was either the sum of ventral trunk minor and major pathways or dorsal trunk minor and major pathways.
Fig. 6.
Percent of compound action potential response in each vagal pathway in 20 animals.
2.4.2. Experiment 3
Voltage response levels were averaged over 1 s time bins, but because of differences in baseline levels when signals were rectified, each value was converted to a percentage relative to baseline, which was the average for the 10 s period prior to vehicle or capsaicin treatment. These percentage values from 10 s prior to and 30 s after vehicle or capsaicin treatment were analyzed by ANOVA and LSD-tests were used to compare means (Statistica; Statsoft). A criterion of P < 0.05 was used to indicate statistical significance.
3. Results
3.1. Experiment 1
Fig. 2 shows the raw data from a representative recording. A stimulus of 1 mA was used for all analyses because this produced the most reliable responses. Electrical stimulation of the cervical vagi evoked responses from the ventral and dorsal trunks that were dominated by two distinct phases of activity. A statistical analysis of the signals from 1 to 150 ms revealed that electrical stimulation produced statistically significant differences in responses from the major pathways compared to the minor pathways [overall ANOVA, interaction effect, F(447,8493) = 8.81, P < 0.0001, 4 × 150 factorial, pathway by time].
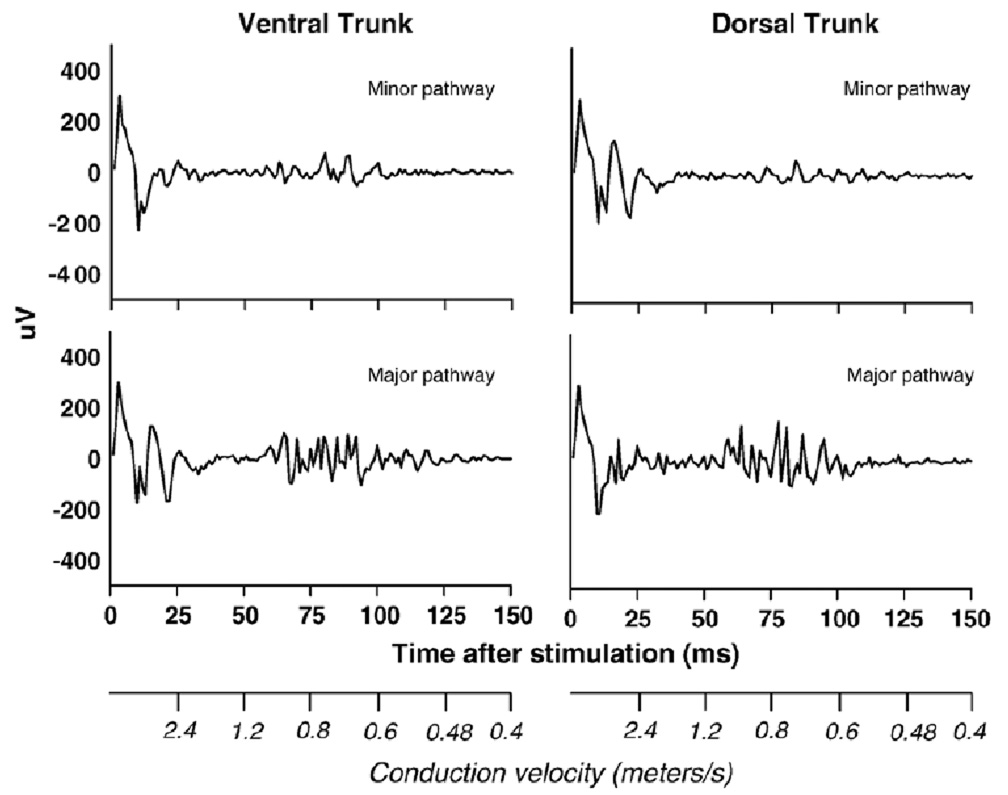
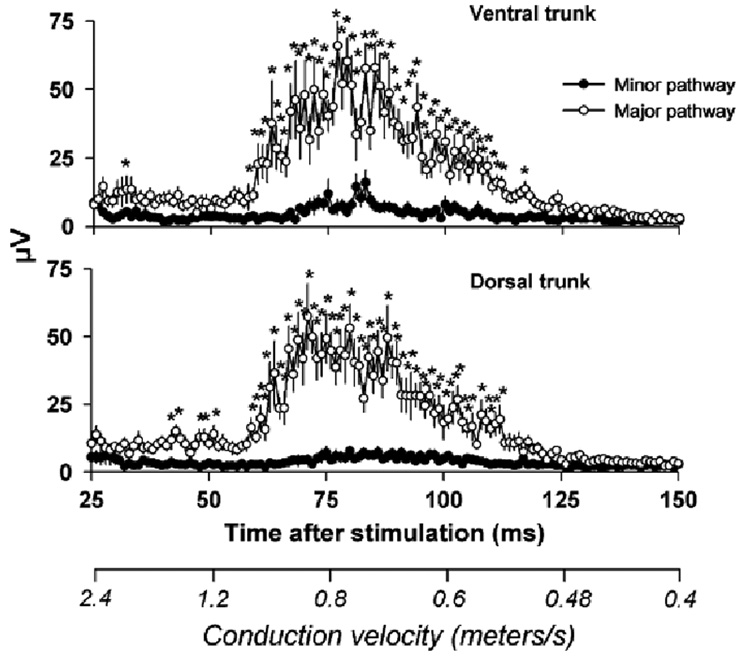
Fig. 2.
A representative set of compound action potentials recorded from the vagi of a rat. The four figures show the minor and major pathways contained within the ventral and dorsal trunks of the subdiaphragmatic vagus after electrical stimulation of the cervical vagus. Scales showing the conduction velocities for each time period are presented on the x-axes.
The early phase from 0 to 25 ms contained a large stimulus artifact that is a common feature of compound action potential recordings (see Fig. 3). The size of this artifact was greatly reduced by using an isolated stimulator, a biphasic pulse, and offline processing of data to remove a slow signal (0.05 Hz high pass filter), perhaps the result of capacitance discharge from stimulator cables. Because it was not possible to determine the actual size of the stimulus artifact, it was therefore not feasible to measure the contribution of the A-fiber component that is embedded within the early phase of this recording (see Fig. 3). Although there are a few significant differences between the minor and major pathways within the first 25 ms, the magnitudes of the signals were very similar (see Fig. 3). Because the major pathways would very likely contain many more A-fibers than the minor pathways, these results suggest that these responses are primarily stimulus artifacts.
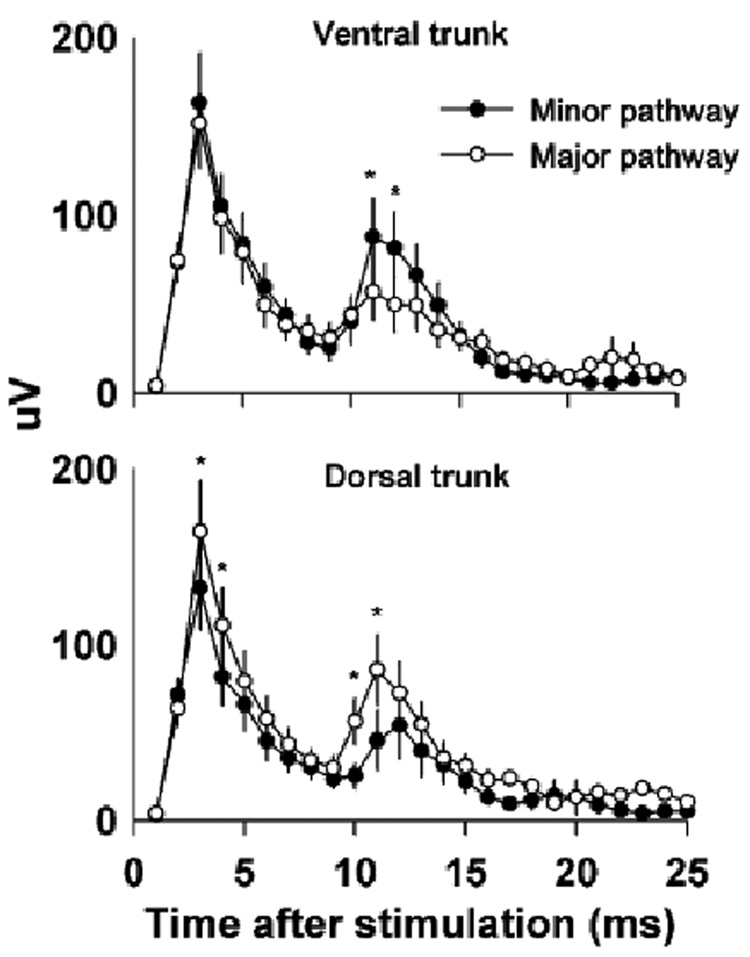
Fig. 3.
Responses recorded from the minor and major pathways contained within the ventral and dorsal trunks of the subdiaphragmatic vagus up to 25 ms after electrical stimulation of the cervical vagus. Note that the recorded signals have been rectified to facilitate analysis. Values represent means ± SEMs (n = 20). *P < 0.05, LSD-test, minor versus major pathway. Large stimulus artifacts and perhaps some fast-conducting A-fiber responses are contained within this time frame.
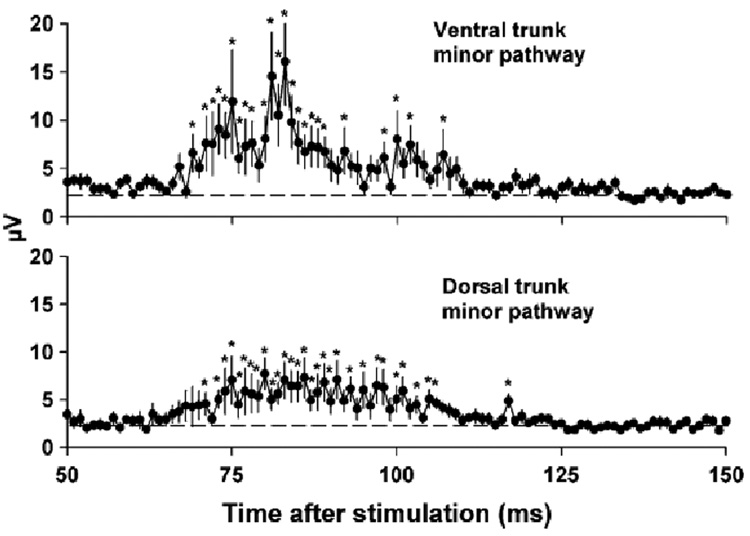
The second phase of the recording, 25 to 150 ms, contained the responses of slow conducting small diameter unmyelinated C-fibers (1.2 to 0.48 m/s). A statistical analysis of the C-fiber response revealed that electrical stimulation produced a significantly greater response in the major pathways compared to the minor pathways (see Fig. 4 for LSD-tests). An analysis of the minor pathways also showed that compound action potentials were significantly greater than baseline [see Fig. 5 for LSD-tests; one-way ANOVAs, interaction effects, ventral trunk minor pathway, F(100, 1900) = 3.8, P < 0.0001, dorsal trunk minor pathway, F(100, 1900) = 2.7, P < 0.0001]. Fig. 6 shows the percent of the response in each pathway for each of the 20 animals (animals were arranged in order from highest to lowest ventral trunk minor pathway response). There was no significant correlation between the response percentage of the ventral trunk and dorsal trunk minor pathways (Pearson’s r = 0.02).
Fig. 4.
C-fiber responses in the compound action potentials recorded from the minor and major pathways contained within the ventral and dorsal trunks of the subdiaphragmatic vagus from 25 to 150 ms after electrical stimulation of the cervical vagus. Values represent means ± SEMs (n = 20). *P < 0.05, LSD-test, minor versus major pathway. A scale showing the conduction velocities for each time period is presented on the x-axis.
Fig. 5.
C-fiber responses from the minor pathways from 50 to 150 ms from Fig. 4. Values represent means ± SEMs (n = 20). *P < 0.05, LSD-test, data at each time point versus baseline (average voltage level from 400– 500 ms post-stimulus).
3.2. Experiment 2
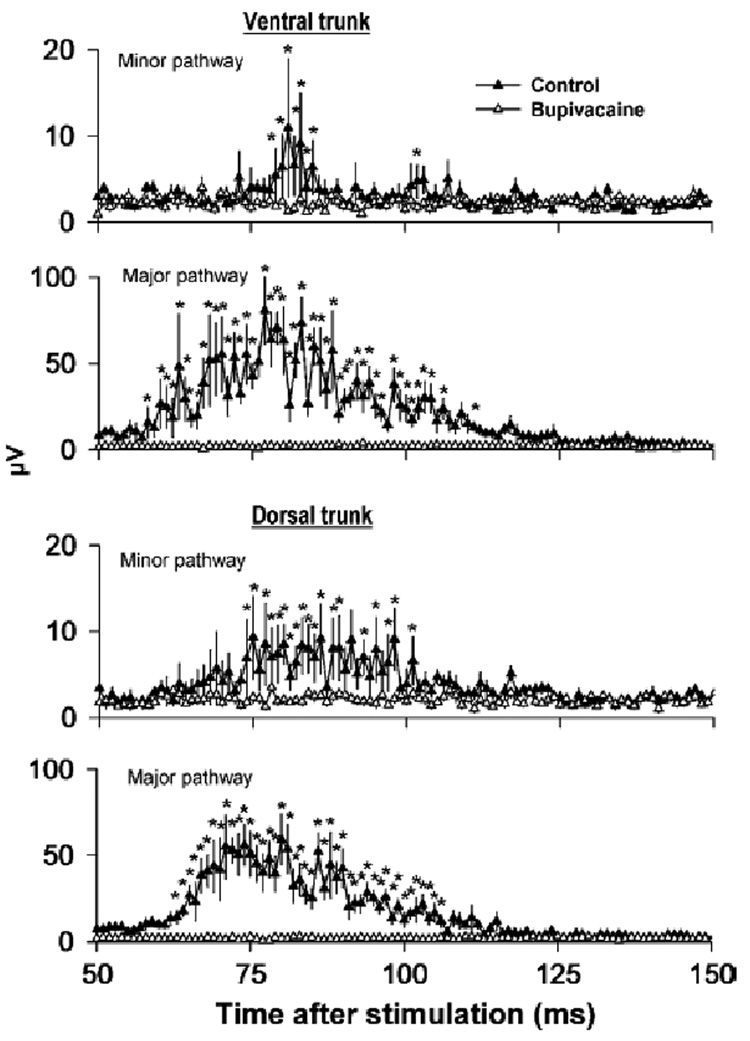
Vagal treatment with bupivacaine greatly reduced the responses produced by electrical stimulation [see Fig. 7 for LSD-tests; two-way ANOVAs, 2 × 100 factorial, treatment by time interaction effects, ventral trunk minor pathway, F(99,891) = 1.3, P = 0.02, ventral trunk major pathway, F(99,891) = 5.1, P = 0.0001, dorsal trunk minor pathway, F(99,891) = 1.5, P = 0.002, dorsal trunk major pathway, F(99,891) = 6.9, P < 0.0001].
Fig. 7.
Effect of a local anesthetic, bupivacaine, on compound action potentials recorded from minor and major pathways of the ventral or dorsal trunks of the subdiaphragmatic vagus after electrical stimulation of the cervical vagi. Control line represents the response before bupivacaine treatment. Values represent means ± SEMs (n = 10). *P < 0.05, LSD-test, before (control) versus after bupivacaine treatment at each time point.
3.3. Experiment 3
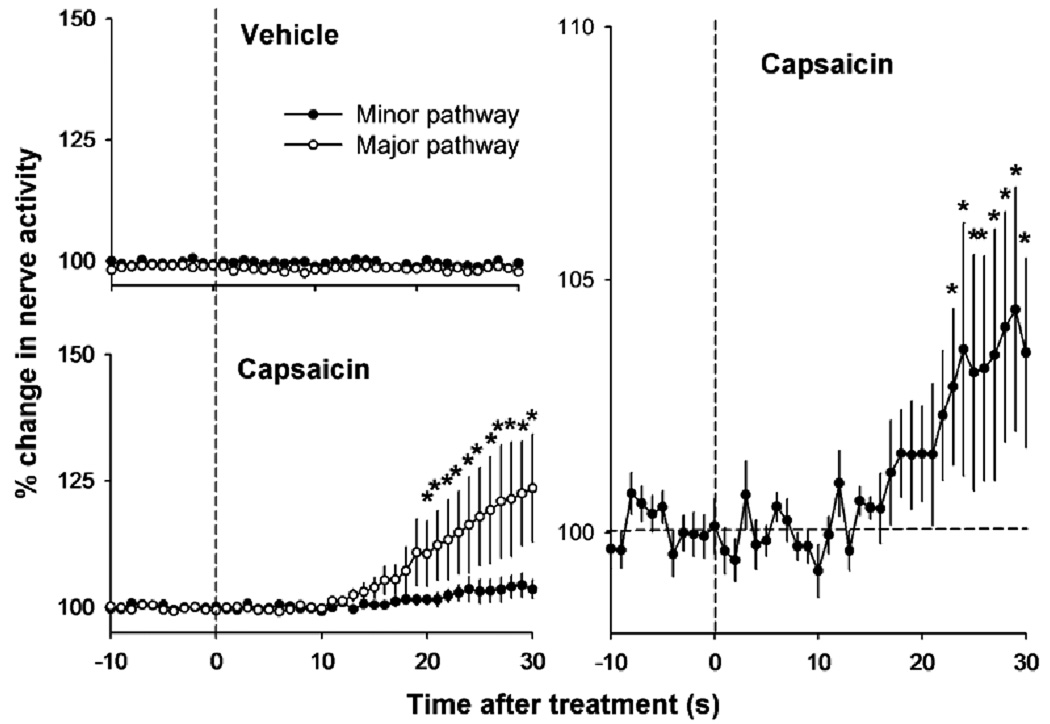
Capsaicin applied to the vagi increased the neural activity in the major and minor pathways [see Fig. 8 for LSD-tests; two-way ANOVA, 2 × 41 factorial, treatment by time, interaction effect, F(40,360) = 3.8, P < 0.0001]. Vehicle treatment had no effect on vagal activity. Capsaicin treatment produced neural activation in the minor pathway that was significantly greater than baseline [see Fig. 8 for LSD-tests; one-way ANOVA, F(41,369) = 2.4, P = 0.00001].
Fig. 8.
Effect of vehicle or capsaicin applied to the cervical vagus on neural activity recorded from the subdiaphragmatic vagal trunks. The right graph shows the same data from the left lower graph for the minor pathway using a different y-axis scale. Values represent means ± SEMs (n = 10). *P < 0.05, LSD-tests, minor pathway versus major pathway at each time point (for the left graphs), and each time point versus the average baseline level, -10 to 0 s (for the right graph).
4. Discussion
The current electrophysiology experiments provide strong evidence for vagal cross-over pathways in the rat. It is likely that these cross-over pathways are within the thoracic cavity like they are in the ferret [8,9]. Electrical stimulation of the left or right cervical vagi revealed C-fiber cross-over pathways that account for an average of 9% of the total response, but there was a great amount of variability (range = 0 to 29%) (see Fig. 6). A neural origin for these responses is indicated by the complete inhibition of the compound action potentials after perineural application of the local anesthetic bupivacaine (Fig. 7). Although the response was smaller than with electrical stimulation, capsaicin applied to the cervical vagi also revealed vagal cross-over pathways in the response of the dorsal and ventral vagal trunks (Fig. 8). The response to capsaicin also indicates the activation of afferent fibers of the vagus and that these pathways are not the result of electromyographic artifacts caused by electrical stimulation [33].
The results extend the findings of Sauter et al. in which they showed cross-over pathways using electrical stimulation [33]. Unlike prior studies, none of the current recordings had stimulus artifacts extending beyond 25 ms and into the range of long latency C-fiber responses. The reduction in size of the stimulus artifact in the current recordings is likely the result of using a paralyzed preparation, biphasic current pulses, and offline filtering of a low-frequency artifact signal (<0.05 Hz). It seems unlikely that esophageal muscle contractions account for the cross-over activation, because pancuronium bromide, a nicotinic receptor antagonist, is a potent inhibitor of esophageal striated muscle contractions in rodents [7,16]. The present electrical stimulation studies indicate that the minor pathway responses are not the result of experimental artifacts from the animal or external equipment, and this is strongly supported by the use of capsaicin to evoke minor pathway responses. Although the average response of 9% for the crossover pathways is lower than the approximate 20% reported by Sauter et al. [33], this difference is probably due to the inclusion of low and non-responding animals in the calculation of the average value in the current study.
The current experiments are likely to underestimate the magnitude of the cross-over pathways of the vagus in the rat. The recordings of the compound action potentials suggest that there are also faster conducting fibers that were not included in the analysis (see responses in Fig. 2 that occur prior to 25 ms). Furthermore, the capsaicin responses are likely to represent much less than a complete afferent response from the vagal pathways since a significant component of this system is capsaicin-resistant [3]. It appears that most of the cross-over pathways are composed of afferent fibers, because most afferent fibers of the rat vagus are C-fibers, and reported to be capsaicin sensitive (e.g., [4]). This is also supported by the lack of labeling in the contralateral dorsal motor nucleus of the brainstem using intraperitoneal injection of retrograde fluorescent tracers [10,30], a technique used to label vagal afferent pathways.
The existence of cross-over pathways poses an interpretive problem for studies using unilateral rhizotomy combined with contralateral vagotomy to isolate effects of afferent lesions of the vagus. Nearly all of these prior studies used a left unilateral afferent rootlet rhizotomy combined with a dorsal trunk vagotomy and it is likely that a significant number of animals still maintained a ventral trunk with a minor pathway originating from the right cervical vagus; thus, these surgical preparations were likely not “complete” or “total” vagal subdiaphragmatic deafferentations as suggested [19,24,26,28,34–37]. This does not seem to be a major problem when it is clear that unilateral rhizotomy plus contralateral vagotomy produces a complete deficit in a response, e.g., blocked CCK-induced suppression of food intake and increased meal-size [24,35,37]. However, when no effects of this lesion are observed, despite other evidence indicating vagal involvement, it might be due to the functioning of the still intact vagal cross-over pathway [19,28,34,36]. Although it might seem reasonable to dismiss 9% of the fibers as a trivial amount, this connection might be functionally important. By comparison, the common hepatic branch of the vagus contains a small amount, approximately 12%, of the vagal fibers of the rat subdiaphragmatic trunks [29], but this branch is very important for chemosensation in the small intestine and hepatic portal region (e.g., [6,18]).
An additional concern for vagal lesioning studies is the propensity for plasticity in the peripheral nervous system following vagotomy (see [21]). The effect of vagotomy on the emetic response in ferrets is closely related to the time following ablation. Acute vagotomy produced a complete inhibition of copper sulfate and radiation-induced emesis, but after a week, the responses return, which indicates the formation of alternate pathways, possibly via the spinal nerves [1,22]. Data from the rat indicate that splanchnic nerve fibers begin to take over some of the functions of vagal afferent fibers after vagotomy. The cytotoxin cisplatin produced a response in vagal afferent fibers that is dependent on activation of 5-HT3 (serotonin type 3) receptors [12,15]. Following vagotomy, there is a switch from 5-HT3 mediated activation of vagal afferent fibers to a 5-HT3-independent activation of nerve fibers, probably due to the involvement of spinal afferent fibers [12]. These prior studies and the current experiments indicate that an adequate test for the role of vagal afferent fibers in a response will require a total deafferentation, cutting the sensory rootlets bilaterally, and a test of function very shortly following the ablation.
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK065971 and DK36339). The authors are grateful for the initial instruction in recording compound action potentials from the vagi of the rat and for the excellent comments on the manuscript by Dr. Ralph Norgren, Pennsylvania State College of Medicine. The authors also acknowledge the excellent technical assistance of Mr. Marc Ciucci.
References
- 1.Andrews PL, Davis CJ, Bingham S, Davidson HI, Hawthorn J, Maskell L. The abdominal visceral innervation and the emetic reflex: pathways, pharmacology, and plasticity. Can J. Physiol. Pharmacol. 1990;68:325–345. doi: 10.1139/y90-047. [DOI] [PubMed] [Google Scholar]
- 2.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 3.Berthoud HR, Patterson LM, Willing AE, Mueller K, Neuhuber WL. Capsaicin-resistant vagal afferent fibers in the rat gastrointestinal tract: anatomical identification and functional integrity. Brain Res. 1997;746:195–206. doi: 10.1016/s0006-8993(96)01222-x. [DOI] [PubMed] [Google Scholar]
- 4.Berthoud HR, Lynn PA, Blackshaw LA. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am. J. Physiol., Regul. Integr. Comp Physiol. 2001;280:R1371–R1381. doi: 10.1152/ajpregu.2001.280.5.R1371. [DOI] [PubMed] [Google Scholar]
- 5.Chavez M, Kelly L, York DA, Berthoud HR. Chemical lesion of visceral afferents causes transient overconsumption of unfamiliar highfat diets in rats. Am. J. Physiol. 272;1997:R1657–R1663. doi: 10.1152/ajpregu.1997.272.5.R1657. [DOI] [PubMed] [Google Scholar]
- 6.Cox JE, Kelm GR, Meller ST, Spraggins DS, Randich A. Truncal and hepatic vagotomy reduce suppression of feeding by jejunal lipid infusions. Physiol. Behav. 2004;81:29–36. doi: 10.1016/j.physbeh.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Dondas N, Karatas Y, Dikmen A. Characterization of nicotinic cholinergic receptors. Indian J. Pharmacol. 2004;36:72–75. [Google Scholar]
- 8.Fitzakerley JL, Lucier GE. Connections of a vagal communicating branch in the ferret: I. Pathways and cell body location. Brain Res. Bull. 1988;20:189–196. doi: 10.1016/0361-9230(88)90178-5. [DOI] [PubMed] [Google Scholar]
- 9.Fitzakerley JL, Lucier GE. Connections of a vagal communicating branch in the ferret: II. Central projections. Brain Res. Bull. 1988;20:479–486. doi: 10.1016/0361-9230(88)90138-4. [DOI] [PubMed] [Google Scholar]
- 10.Fox EA, Powley TL. Longitudinal columnar organization within the dorsal motor nucleus represents separate branches of the abdominal vagus. Brain Res. 1985;341:269–282. doi: 10.1016/0006-8993(85)91066-2. [DOI] [PubMed] [Google Scholar]
- 11.Gonatas NK, Harper C, Mizutani T, Gonatas JO. Superior sensitivity of conjugates of horseradish peroxidase with wheat germ agglutinin for studies of retrograde axonal transport. J. Histochem. Cytochem. 1979;27:728–734. doi: 10.1177/27.3.90065. [DOI] [PubMed] [Google Scholar]
- 12.Hillsley K, Grundy D. Plasticity in the mesenteric afferent response to cisplatin following vagotomy in the rat. J. Auton. Nerv. Syst. 1999;76:93–98. doi: 10.1016/s0165-1838(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 13.Horn CC, Friedman MI. Detection of single unit activity from the rat vagus using cluster analysis of principal components. J. Neurosci. Methods. 2003;122:141–147. doi: 10.1016/s0165-0270(02)00304-7. [DOI] [PubMed] [Google Scholar]
- 14.Horn CC, Friedman MI. Separation of hepatic and gastrointestinal signals from the common and “hepati” branch of the vagus. Am. J. Physiol., Regul. Integr. Comp. Physiol. 2004;287:R120–R126. doi: 10.1152/ajpregu.00673.2003. [DOI] [PubMed] [Google Scholar]
- 15.Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton. Neurosci. 2004;115:74–81. doi: 10.1016/j.autneu.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Kerr KP, Stevenson JE, Mitchelson F. Simultaneous comparison of nicotinic receptor antagonists on three nicotinic acetylcholine receptors. J. Pharm. Pharmacol. 1995;47:1002–1006. doi: 10.1111/j.2042-7158.1995.tb03286.x. [DOI] [PubMed] [Google Scholar]
- 17.Kraly FS, Jerome C, Smith GP. Specific postoperative syndromes after total and selective vagotomies in the rat. Appetite 7. 1986;7:1–17. doi: 10.1016/s0195-6663(86)80038-1. [DOI] [PubMed] [Google Scholar]
- 18.Lee KC. Reflex suppression and initiation of gastric contractions by electrical stimulation of the hepatic vagus nerve. Neurosci. Lett. 1985;53:57–62. doi: 10.1016/0304-3940(85)90097-7. [DOI] [PubMed] [Google Scholar]
- 19.Leonhardt M, Hrupka BJ, Langhans W. Subdiaphragmatic vagal deafferentation fails to block the anorectic effect of hydroxycitrate. Physiol. Behav. 2004;82:263–268. doi: 10.1016/j.physbeh.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Lewis SJ, Verberne AJ, Louis CJ, Jarrott B, Beart PM, Louis WJ. Excitotoxin-induced degeneration of rat vagal afferent neurons. Neuroscience. 1990;34:331–339. doi: 10.1016/0306-4522(90)90143-r. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Owyang C. Musings on the wanderer: what’s new in our understanding of vagovagal reflexes? V. Remodeling of vagus and enteric neural circuitry after vagal injury. Am. J. Physiol., Gastrointest. Liver Physiol. 2003;174:G461–G469. doi: 10.1152/ajpgi.00119.2003. [DOI] [PubMed] [Google Scholar]
- 22.Makale MT, King GL. Plasticity of autonomic control of emesis in irradiated ferrets. Am. J. Physiol. 1993;265:R1092–R1099. doi: 10.1152/ajpregu.1993.265.5.R1092. [DOI] [PubMed] [Google Scholar]
- 23.Martin JR, Rogers RC, Novin D. Excessive gastric retention by vagotomized rats and rabbits given a solid diet. Bull. Psychon. Soc. 1977;10:291–294. [Google Scholar]
- 24.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am. J. Physiol. 1997;272:R1245–R1251. doi: 10.1152/ajpregu.1997.272.4.R1245. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson J, Ravits J, Hallett M. Stimulus artifact compensation using biphasic stimulation. Muscle Nerve. 1988;11:597–602. doi: 10.1002/mus.880110612. [DOI] [PubMed] [Google Scholar]
- 26.Norgren R, Smith GP. A method for selective section of vagal afferent or efferent axons in the rat. Am. J. Physiol. 1994;267:R1136–R1141. doi: 10.1152/ajpregu.1994.267.4.R1136. [DOI] [PubMed] [Google Scholar]
- 27.Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J. Comp. Neurol. 1988;273:207–223. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- 28.Porter MH, Hrupka BJ, Langhans W, Schwartz GJ. Vagal and splanchnic afferents are not necessary for the anorexia produced by 160 C.C. Horn, M.I. Friedman / Brain Research 1060 (2005) 153- 161 peripheral IL-1beta, LPS, and MDP. Am. J. Physiol. 1998;275:R384–R389. doi: 10.1152/ajpregu.1998.275.2.R384. [DOI] [PubMed] [Google Scholar]
- 29.Powley TL, Prechtl JC, Fox EA, Berthoud H-R. In: Anatomical considerations for surgery of the rat abdominal vagus: distribution, paraganglia and regeneration. Kral JG, Powley TL, Brooks CM, editors. Vagal Nerve Function: Behavioral and Methodological Considerations, Elsevier; 1983. pp. 79–97. [DOI] [PubMed] [Google Scholar]
- 30.Powley TL, Fox EA, Berthoud H-R. Retrograde tracer technique for assessment of selective and total subdiaphragmatic vagotomies. Am. J. Physiol., Regul. Integr. Comp. Physiol. 1987;253(22):R361–R370. doi: 10.1152/ajpregu.1987.253.2.R361. [DOI] [PubMed] [Google Scholar]
- 31.Raybould HE, Tache Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am. J. Physiol. 1988;255:G242–G246. doi: 10.1152/ajpgi.1988.255.2.G242. [DOI] [PubMed] [Google Scholar]
- 32.Rinaman L, Schwartz G. Anterograde transneuronal viral tracing of central viscerosensory pathways in rats. J. Neurosci. 2004;24:2782–2786. doi: 10.1523/JNEUROSCI.5329-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauter JF, Niijima A, Berthoud HR, Jeanrenaud B. Vagal neurons and pathways to the rat’s lower viscera: an electrophysiological study. Brain Res. Bull. 1983;11:487–491. doi: 10.1016/0361-9230(83)90119-3. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz GJ, Plata-Salaman CR, Langhans W. Subdiaphragmatic vagal deafferentation fails to block feeding-suppressive effects of LPS and IL-1 beta in rats. Am. J. Physiol. 1997;273:R1193–R1198. doi: 10.1152/ajpregu.1997.273.3.R1193. [DOI] [PubMed] [Google Scholar]
- 35.Schwartz GJ, Salorio CF, Skoglund C, Moran TH. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am. J. Physiol. 1999;276:R1623–R1629. doi: 10.1152/ajpregu.1999.276.6.R1623. [DOI] [PubMed] [Google Scholar]
- 36.Sclafani A, Ackroff K, Schwartz GJ. Selective effects of vagal deafferentation and celiac-superior mesenteric ganglionectomy on the reinforcing and satiating action of intestinal nutrients. Physiol. Behav. 2003;78:285–294. doi: 10.1016/s0031-9384(02)00968-x. [DOI] [PubMed] [Google Scholar]
- 37.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am. J. Physiol. 1985;249:R638–R641. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- 38.Trojanowski JQ, Gonatas JO, Gonatas NK. Conjugates of horseradish peroxidase (HRP) with cholera toxin and wheat germ agglutinin are superior to free HRP as orthogradely transported markers. Brain Res. 1981;223:381–385. doi: 10.1016/0006-8993(81)91151-3. [DOI] [PubMed] [Google Scholar]
- 39.Walls EK, Wang FB, Holst MC, Phillips RJ, Voreis JS, Perkins AR, Pollard LE, Powley TL. Selective vagal rhizotomies: a new dorsal surgical approach used for intestinal deafferentations. Am. J. Physiol. 1995;269:R1279–R1288. doi: 10.1152/ajpregu.1995.269.5.R1279. [DOI] [PubMed] [Google Scholar]