Abstract
Recent evidence has suggested that compounds affecting GABAergic transmission may provide useful pharmacological tools for the treatment of cocaine addiction. Using a rat model of self-administration, the present study examined the effects of GABA agonists and antagonists injected directly into the ventral tegmental area (VTA) on cocaine intake in rats trained to self-administer cocaine (0, 125, 250 and 500 µg/infusion) under an FR5 schedule of reinforcement. Separate groups of rats received bilateral intra-VTA injections of the GABA-A antagonist picrotoxin (34 ng/side, n=7; 68 ng/side, n=8), GABA-A agonist muscimol (14 ng/side, n=8), GABA-B agonist baclofen (56 ng/side, n=7; 100 ng/side, n=6), picrotoxin (68 ng/side) co-injected with the GABA-B antagonist 2-hydroxysaclofen (100 ng/side, n=7; 2 µg/side, n=8) or artificial cerebrospinal fluid (aCSF, n=6) to assess the effects of the various compounds on the cocaine self-administration dose-response curve. Both picrotoxin and baclofen reduced responding maintained by cocaine, whereas muscimol had no effect on responding. In contrast, neither picrotoxin (n=6) nor baclofen (n=8) affected responding maintained by food. Interestingly, 2-hydroxysaclofen effectively blocked the suppression of responding produced by picrotoxin, suggesting that both picrotoxin and baclofen exert their effects via activation of GABA-B receptors. Additionally, these effects appear to be specific to cocaine reinforcement, supporting current investigation of baclofen as a treatment for cocaine addiction.
While cocaine addiction persists as an economic, social and psychological problem in the United States and beyond, a reliable and effective treatment for cocaine addiction has remained elusive. The development of pharmacological interventions for cocaine dependence has focused primarily on compounds that directly modulate dopaminergic function. The mesolimbic dopamine system, which originates in the ventral tegmental area (VTA) and projects to several forebrain regions including the nucleus accumbens (NAc), is a critical substrate for the reinforcing effects of drugs of abuse (Wise & Bozarth, 1987; Wise, 1998). Administration of abused drugs activates this pathway and stimulates dopamine neurotransmission in the nucleus accumbens (NAc) in humans, nonhuman primates, and rodents (Porrino, 1993; Lyons et al., 1996) (Volkow et al., 1997), an effect associated with the abuse liability of these substances (Koob & Bloom, 1988). Unfortunately, the compounds that directly antagonize dopamine receptors have proven to have limited clinical efficacy (Johnson, 2005; Johnson et al., 2005; Johnson, 2006).
Indirect modulation of mesolimbic dopamine function provides an alternative strategy. VTA dopaminergic neurons are regulated by both intrinsic and projection GABAergic neurons (Johnson & North, 1992). GABA exerts its effects via ionotropic GABA-A and metabotropic GABA-B receptors GABA exerts its effects via ionotropic GABA-A and metabotropic GABA-B receptor (Sieghart, 1995; Sieghart et al., 1999; Whiting et al., 1999). Dopaminergic cells in the VTA express both GABA receptor subtypes (Kalivas et al., 1990; Xi & Stein, 1998; Backes & Hemby, 2003) and receive tonic GABAergic inhibition . Thus, long-term alterations stemming from cocaine use (as in Backes & Hemby, 2003) or short-term pharmacological alterations of GABAergic transmission could profoundly affect the function of dopaminergic cells in this region and, therefore, the ability of cocaine to act as a reinforcer.
To this end, previous studies have demonstrated the ability of both GABA receptor agonists and antagonists injected into the VTA to alter dopamine release in the nucleus accumbens . Additionally, GABA-A receptor agonists and antagonists are both readily self-administered directly into the VTA, indicating a possible involvement in reinforcement mechanisms (Ikemoto et al., 1997). However, some evidence suggests that the GABA-A agonist muscimol does not affect cocaine self-administration . If drugs acting on GABA-A receptors have a demonstrated ability to affect VTA dopaminergic transmission and to act as reinforcers on their own, it is important to further examine how these compounds may affect cocaine reinforcement to both enhance our understanding of cocaine reinforcement and aid future endeavors to treat cocaine addiction. In addition, an understanding of GABA-A receptor involvement in cocaine reinforcement may help to dissect important differences between GABA-B receptor agonists such as baclofen versus non-specific enhancers of GABA transmission such as vigabatrin in the treatment of cocaine addiction. With these principles in mind, the present study sought to clarify the role of GABA receptors in the VTA during cocaine self-administration.
Methods
Subjects and Surgical Procedures
Male Sprague-Dawley rats (60–90 days; 225–275 g; Charles River, Wilmington, MA) were randomly assigned to groups. Animals were housed in pairs under a reverse 12 h light/dark cycle (lights on: 7:00 pm) and provided food and water ad libitum prior to surgery.
Rats were anesthetized under pentobarbital anesthesia (1 mg/kg; i.p.) and implanted with chronic indwelling venous catheters, as described previously (Hemby et al., 1995b; Hemby et al., 1996b; Hemby et al., 1997b; Hemby et al., 1999b; Backes & Hemby, 2003; Hemby, 2004; Tang et al., 2004; Hemby et al., 2005). Catheters were inserted into the right jugular vein, terminating just outside the right atrium and anchored to muscle near the point of entry into the vein. The distal end of the catheter was guided subcutaneously to exit above the scapulae through a Teflon shoulder harness. The harness provided a point of attachment for a spring leash connected to a single channel swivel at the opposing end. The catheter was threaded through the leash for attachment to the swivel where the fixed end of the swivel was connected to a syringe by polyethylene tubing. Infusions were administered by a motor driven syringe pump (Med Associates, Inc., East Farfield, VT) controlled by a computer. Infusions of thiopental (0.3 mg/kg; i.v.) were administered as needed to assess catheter patency. All rats were implanted with bilateral 26 ga steel guide cannulae aimed one mm above the VTA (AP −5.2, ML ±0.5, DV −6.0 relative to bregma; Plastics One, Inc., Roanoke, VA) as described previously (Hemby et al., 1995a; Hemby et al., 1997a). Guide cannulae were secured with skull screws and dental acrylic cement and obturators (28 ga; Plastics One), cut flush with the bottom of the cannulae, were inserted to prevent blockage. All experimental procedures were conducted according to the guidelines issued by Institutional Animal Care and Use Committee and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health.
Cocaine Self-Administration
Following surgery, subjects were placed in standard operant conditioning chambers (24.5 × 23.5 × 21 cm; Med Associates, Inc.) and were monitored for signs of discomfort during recovery. The chambers contained a retractable lever and a stimulus light mounted directly above the lever. Rats received infusions of heparinized 0.9% bacteriostatic saline (1.7 U/ml; 200 µl/30 min) via the jugular catheters for 72 h following surgery. On the following day, the self-administration procedure began. The chambers were enclosed in sound-attenuating boxes containing an exhaust fan, a house light, a tone source, and a water bottle. The exhaust fan masked extraneous noise. IBM compatible computers were used for session programming and data collection.
Rats were allowed to self-administer cocaine (250 µg /infusion) during 8 h self-administration session under a fixed ratio-1 (FR1), time-out 20 s (FR1:20 s TO) schedule of reinforcement. Upon completion of the response requirement, a cocaine infusion was delivered and a 20 s time-out was in effect. During the time-out, the lever light was extinguished, the house light illuminated, and a tone was generated. The end of the time-out was signaled by illumination of the lever light and extinguishing of the house light and tone. During the time-out period, responses on levers were recorded but had no scheduled consequence. The response ratio was gradually increased to FR5 (the terminal ratio) over 7 days. Once rats exhibited stable self-administration behavior at the terminal ratio (defined by less than 10% variation in responses over three consecutive days), saline was substituted for cocaine until self-administration behavior was extinguished.
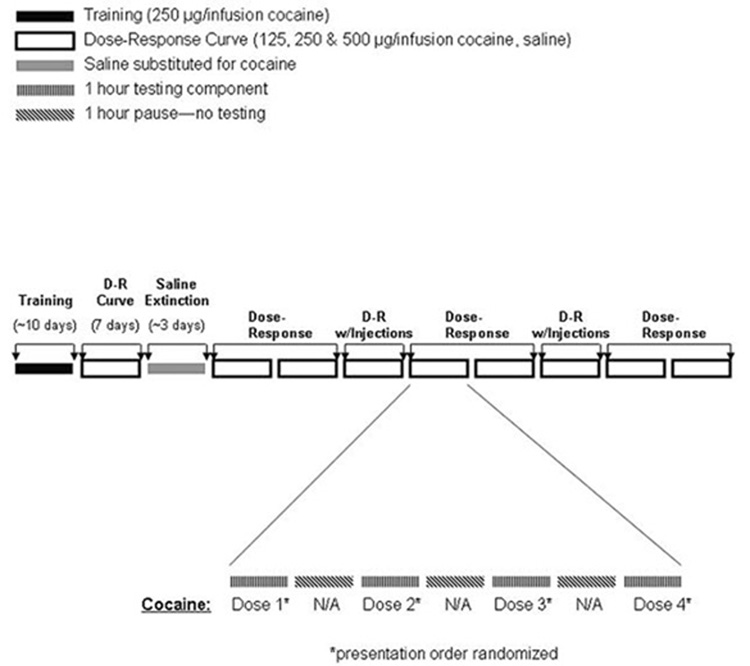
Following the attainment of the terminal ratio and stable responding during saline extinction, the self-administration session was changed to four 1h components with a one hr time out between each component and the ratio was increased to five (FR5). Subjects were allowed to self-administer cocaine i.v. (125, 250 and 500 µg/infusion) and saline. Each dose was available during a different component and the doses were presented in random order. After establishing the dose-response function baseline, testing days ensued in which intra-VTA drug injections were delivered prior to each of the four components of the session. The same dose of drug was infused prior to each component on the test day. Prior to drug administration, the obturators were removed and 33 ga injection cannulae were inserted through the guide cannulae to extend one mm beyond the tip of the guide cannulae into the VTA. Bilateral intracranial injections (700nl/side) were delivered over 60 s using a dual-syringe pump (KD Scientific, Inc., Holliston, MA; Model 200)and injection cannulae remained in place for an additional 30 s (see Table 2). Following, injection cannulae were removed, obturators were inserted into the cannulae, rats were returned to the operant chamber and the next self-administration component of the session was initiated. To assess any lingering effects of drug injection of self-administration, a minimum of two days following each injection was allowed to re-establish the cocaine dose-response baseline responding (Figure 1).
Figure 1.
Schematic diagram of self-administration procedures and intra-VTA injections.
Food maintained responding
Following cannulation, a separate group of rats were housed in pairs and maintained at 85% of their free-feeding body weight with water available ad-libitum. For training and testing, rats were placed in conditioning chambers similar to those described above but equipped with a food hopper to deliver food pellets (45 mg Noyes pellets) upon completion of the appropriate response ratio. Twice-daily sessions lasted 2 h each and were separated by 2 h. Rats were initially trained to press a lever for food pellet delivery on a FR1 schedule, and the schedule was gradually increased to FR5 over the course of 10 sessions. Coinciding with the delivery of each food pellet, a 20 s tone sounded and a 6 min time-out period ensued to equate the inter-reinforcement interval to that of the 250 µg /infusion dose of cocaine. During the time-out, the lever retracted and the lever light was extinguished.
After 10 sessions at the terminal ratio (FR5), the effects of bilateral intra-VTA injections (700nl/side) of picrotoxin or baclofen on food-maintained responding were tested prior to two separate sessions (Table 2). To re-establish baseline measurements and assess any potential damage to the VTA stemming from the injections, rats received no injections for two sessions following each testing session.
Histology
After completion of the experimental procedures, rats were sacrificed using CO2 and transcardially perfused with 0.1 M PBS followed by 4% paraformaldehyde in PBS. Once removed, brains were placed in a rat coronal brain matrix (ASI Instruments, Inc., Warren, MI; Model RBM-4000C) and 3 mm coronal blocks were taken from the rostral to the caudal portion of the brain. Blocks were post-fixed in 4% paraformaldehyde for one hr, transferred to 30% sucrose overnight or until saturated, then frozen at −80 °C until sectioning. Brain blocks containing the VTA were sectioned to 20 µm with a cryostat, hydrated in descending concentration of ethanol followed by water (2X 100%, 95%, 80%, 70% and ddH20, one min each), stained with 1% Cresyl violet for one min, differentiated in 95% EtOH until background was low, dehydrated in 100% EtOH for 30 s, and cleared in xylene (2 × 5 min) prior to coverslipping.
Drugs
Picrotoxin, muscimol, baclofen and 2-hydroxysaclofen were obtained from Sigma-Aldrich (St. Louis, MO). Cocaine HCl was kindly provided by the National Institute on Drug Abuse. All drugs for intracranial injection were dissolved in artificial cerebrospinal fluid (145 mM NaCl, 1.2 mM CaCl2, 2.8 mM KCl, 1.2 mM MgCl2, 5.4 mM D-glucose, 1.25 mM NaH2PO4). Cocaine HCl was dissolved in bacteriostatic heparinized 0.9% NaCl (McKesson, Suwanee, GA).
Statistical Analysis
Cocaine self-administration data were analyzed by two-way analysis of variance (repeated measures for cocaine dose) with number of infusions as the dependent measure. Dunnett’s test was used for post-hoc analysis. The effects of drugs on food maintained responding were analyzed using a Student’s t-test. Null hypotheses were rejected when P < 0.05.
Results
Intra-VTA microinjections sites
Histological reconstructions of cannula tip locations in the VTA are provided for each experiment (Figure 2 – Figure 6). Examination of Nissl stained sections revealed evidence of a localized lesion and gliosis at the site of injection; however, surrounding tissue remained largely intact.
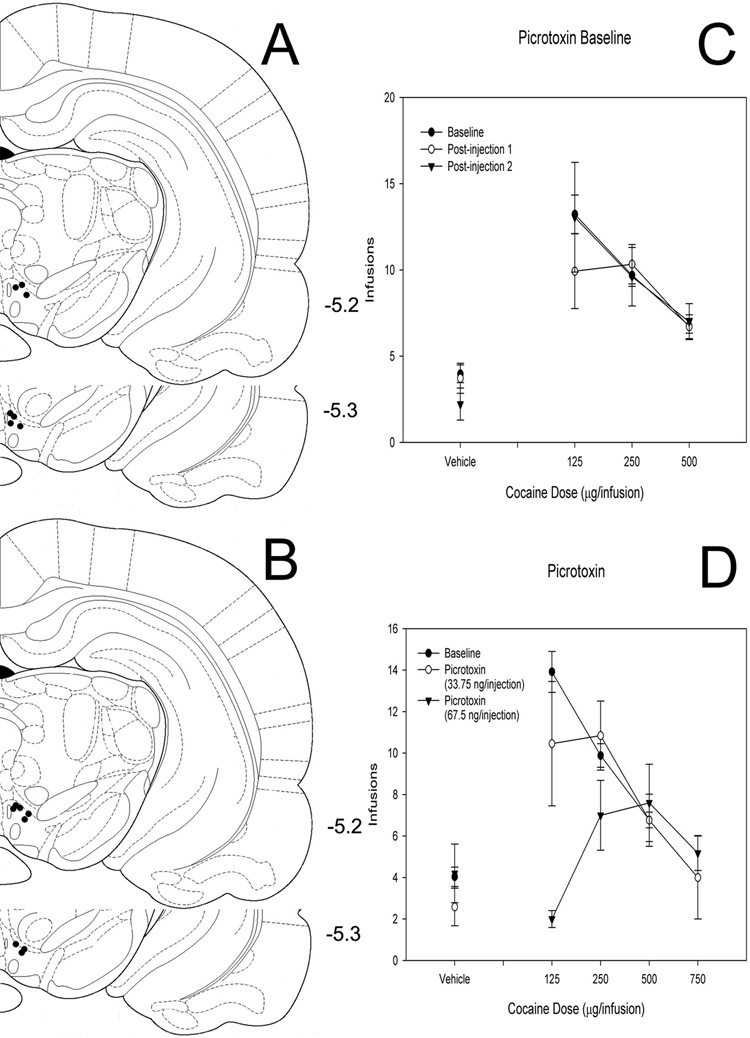
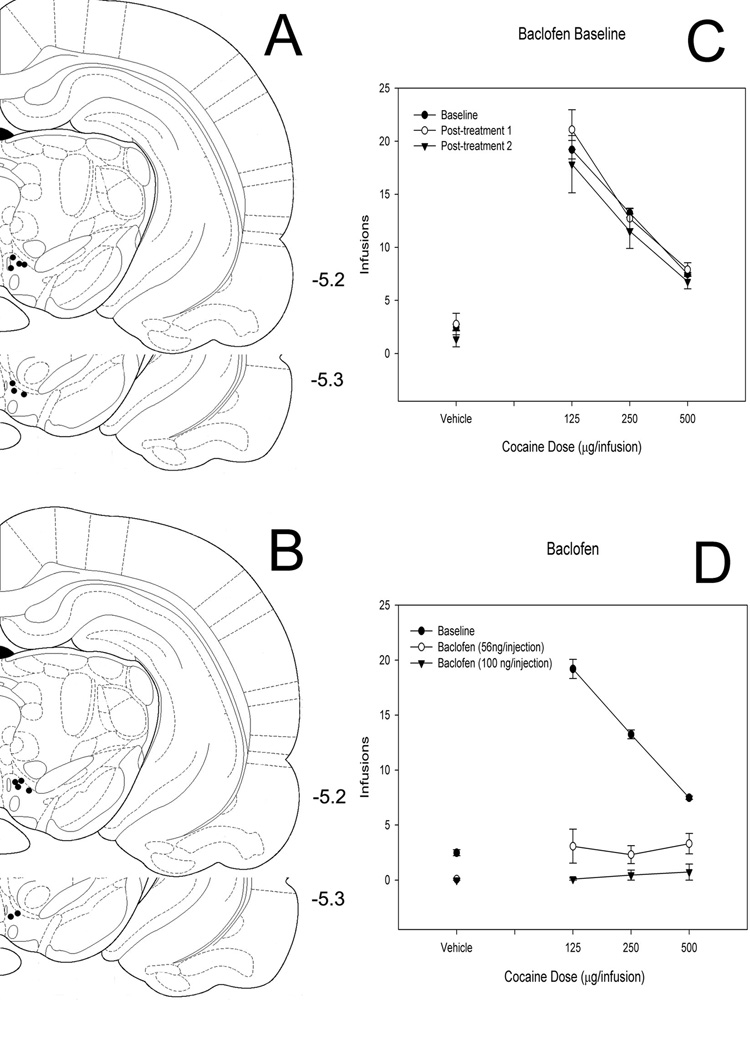
Figure 2. Effects of intra-VTA picrotoxin on cocaine self-administration.
(A, B) Histological reconstruction showing cannulae tip placement for 34 (A) and 68 (B) ng picrotoxin. Numbers indicate coordinates relative to bregma (Paxinos & Watson, 1998). (C) Baseline dose-response functions demonstrating cocaine self-administration on days before and after intra-VTA picrotoxin injections. (D) Dose-response functions demonstrating cocaine self-administration baseline and cocaine self-administration following bilateral intra-VTA injection of two doses of picrotoxin.
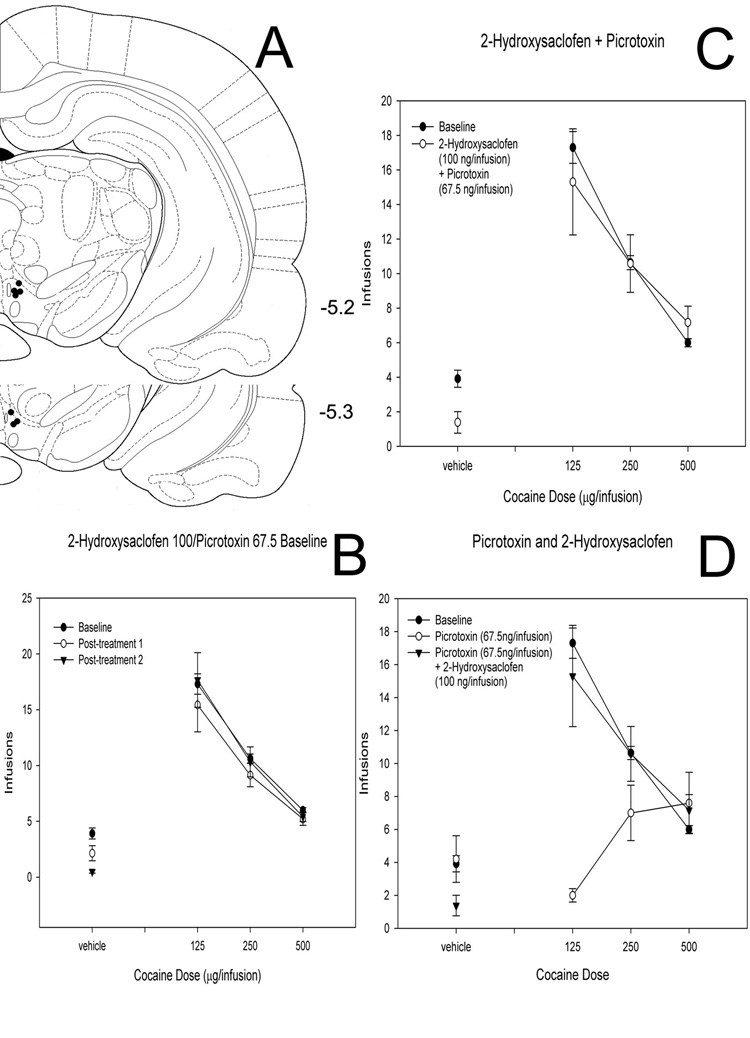
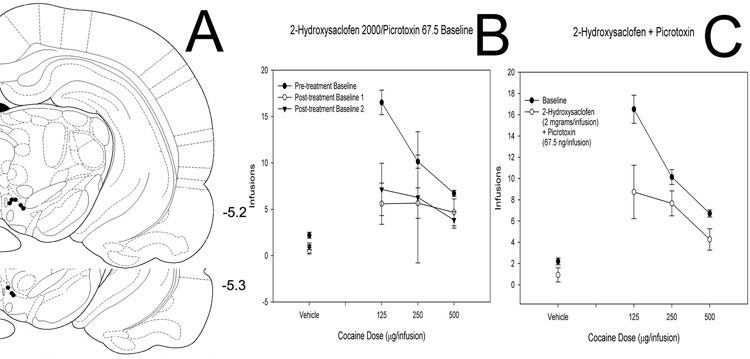
Figure 6. Effects of picrotoxin and a low dose of 2-hydroxysaclofen co-injected into the VTA on cocaine self-administration.
(A) Histological reconstruction showing cannulae tip placement for co-injection of picrotoxin (68 ng) and 2-hydroxysaclofen (100 ng). Numbers indicate coordinates relative to bregma (Paxinos & Watson, 1998). (B) Baseline dose-response functions demonstrating cocaine self-administration on days before and after intra-VTA picrotoxin/2-hydroxysaclofen. (C) Dose-response functions demonstrating cocaine self-administration baseline and cocaine self-administration following bilateral intra-VTA co-injection of picrotoxin and 2-hydroxysaclofen. (D) Data from animals injected with 68 ng picrotoxin alone (also shown in Figure 2D) superimposed on data from animals co-injected with 68 ng picrotoxin and 100 ng 2- hydroxysaclofen (also shown in Panel C, this figure).
Baselines/Vehicle Injections
Other than the 2 µg/injection dose of 2-hydroxysaclofen co-injected with picrotoxin (mentioned above), none of the treatments created a post-injection baseline that differed significantly from the pre-injection baseline. Furthermore, bilateral intra- VTA injections of vehicle (n = 6) showed no effect on cocaine self-administration (main effect of cocaine [F = 22.103, P <0 .001], no main effect of vehicle [F = 0.0193, P = 0.890] and no interaction [F = 0.494, P = 0.687]).
Responding maintained by cocaine and food
The selected doses of cocaine engendered and maintained rates and patterns of responding typically observed under FR schedules of reinforcement (Hemby et al., 1995a; Hemby et al., 1996a; Hemby et al., 1999a). For all groups, cocaine produced a significant main effect (see below) and the number of infusions was inversely proportional to the dose self-administered. There was no statistically significant effect of self-administration session on number of infusions, and group baselines were not significantly different from one another. Similarly, rats responding for food demonstrated no statistically significant effect of session on the number of food pellets obtained.
Picrotoxin
Two groups of rats received bilateral intra-VTA infusions of the GABA-A antagonist picrotoxin (68 ng picrotoxin, n = 8; 34 ng picrotoxin n = 7) to assess its effects on responding for cocaine (Figure 2). After an initial graphical analysis of the first six rats tested, 750 µg/infusion of cocaine was substituted for 125 µg/infusion of cocaine on testing days for the remaining 2 rats to clearly define the descending limb of the dose-response curve in the rats receiving 68 ng infusions of picrotoxin. As a result of these rats receiving only the 750 µg/infusion dose in the presence of picrotoxin, statistical analysis of the data did not include this dose. ANOVA revealed a significant main effect of cocaine dose (F = 5.649, P > 0.001) and picrotoxin dose (F = 3.482, P = 0.031). The interaction between cocaine and picrotoxin did not reach significance (F = 1.829, P = 0.091). Post-hoc analysis using Dunnett’s test revealed that all doses of cocaine differed significantly from control and that the 68 ng dose of picrotoxin was significantly different from baseline.
To examine whether picrotoxin’s effects are specific to responding for cocaine, an additional group of rats whose responding was maintained by food presentation also received bilateral intra-VTA infusions of picrotoxin (68 ng/injection; n=6). Picrotoxin had no effect on responding maintained by food (P = 0.779).
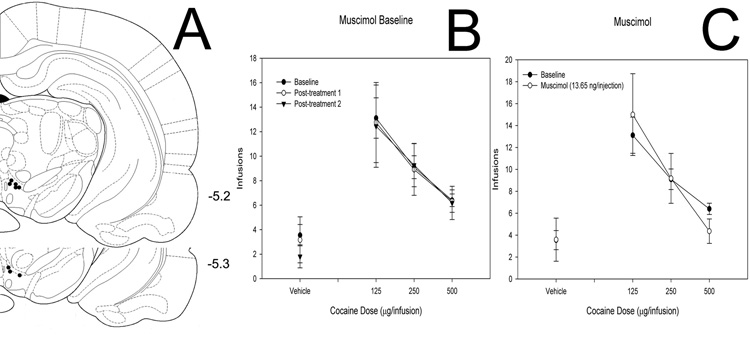
Muscimol
Rats tested with intra-VTA injections of the GABA-A agonist muscimol (14 ng/injection, n = 8) showed a main effect of cocaine (F = 12.181, P < 0.001) but no effect of muscimol (F = 0.0000494, P = 0.994) and no interaction (F = 0.353, P = 0.787; Figure 3).
Figure 3. Effects of intra-VTA muscimol on cocaine self-administration.
(A) Histological reconstruction showing cannulae tip placement. Numbers indicate coordinates relative to bregma (Paxinos & Watson, 1998). (B) Baseline dose-response functions demonstrating cocaine self-administration before and after intra-VTA muscimol injections. (C) Dose-response functions demonstrating cocaine self-administration baseline and cocaine self-administration following bilateral intra-VTA injection of muscimol.
Baclofen
Since previous studies have also shown that the GABA-B agonist baclofen delivered into the VTA suppresses cocaine self-administration on slightly different behavioral schedules, we tested two groups of rats (100 ng/injection, n = 6; 56 ng/injection, n = 7) to assess the effects of bilaterally infused intra-VTA baclofen on responding for cocaine under a FR5 schedule (Figure 4). ANOVA results reveal a main effect of cocaine (F = 13.735, P < 0.001), a main effect of baclofen (F = 122.081, P < 0.001) and an interaction effect (F = 16.078, P < 0.001). Similar to previous studies, baclofen (100 ng/injection, n = 8) did not affect responding maintained by food pellets (P = 0.996)
Figure 4. Effects of intra-VTA baclofen on cocaine self-administration.
(A, B) Histological reconstruction showing cannulae tip placement for 56 (A) and 100 (B) ng baclofen. Numbers indicate coordinates relative to bregma (Paxinos & Watson, 1998). (C) Baseline dose-response functions demonstrating cocaine self-administration on days before and after intra-VTA baclofen injections. (D) Dose-response functions demonstrating cocaine self-administration baseline and cocaine self-administration following bilateral intra-VTA injection of two doses of baclofen.
Picrotoxin/2-hydroxysaclofen
To determine whether picrotoxin and baclofen may act through a common mechanism to reduce cocaine self-administration, we co-injected picrotoxin (68 ng/injection) with the GABA-B antagonist 2-hydroxysaclofen (2 µg/injection, n = 8; 100 ng/injection, n = 7; Figure 5 & Figure 6). The higher dose of 2-hydroxysaclofen co-injected with picrotoxin yielded a main effect of cocaine (F = 23.209, P < 0.001) and a main effect of the picrotoxin/2-hydroxysaclofen combination (F = 12.658, P < 0.001) but no interaction (F = 2.212, P = 0.086). However the post-injection baselines were also affected (main effect of cocaine [F = 16.436, P < 0.001], main effect of baseline number [F = 18.490, P < 0.001] and interaction effect [F = 3.490, P = 0.002]), suggesting pronounced damage to the region as a result of this drug combination.
Figure 5. Effects of picrotoxin and a high dose of 2-hydroxysaclofen co-injected into the VTA on cocaine self-administration.
(A) Histological reconstruction showing cannulae tip placement for co-injection of picrotoxin (68 ng) and 2-hydroxysaclofen (2 µg). Numbers indicate coordinates relative to bregma (Paxinos & Watson, 1998). (B) Baseline dose-response functions demonstrating cocaine self-administration on days before and after intra-VTA picrotoxin/2-hydroxysaclofen. Note the depressed baselines following co-injection of this combination of doses. (C) Dose-response functions demonstrating cocaine self-administration baseline and cocaine self-administration following bilateral intra-VTA co-injection of picrotoxin and 2-hydroxysaclofen.
A lower dose of 2-hydroxysaclofen co-injected with picrotoxin, however, revealed a main effect of cocaine (F = 55.270, P < 0.001), but no effect of the drug combination (F = 1.163, P = 0.282) and no interaction effect (F = 1.183, P = 0.316).
Discussion
Results of the present experiments demonstrate that both the GABA-A antagonist picrotoxin and the GABA-B agonist baclofen administered into the VTA can negatively impact the reinforcing efficacy of cocaine. Although several investigators have already demonstrated similar results with baclofen, this study represents the first evidence of such an effect using a GABA-A antagonist. Importantly, the effects of the GABA-A antagonist picrotoxin can be blocked by the GABA-B antagonist 2-hydroxysaclofen, suggesting that picrotoxin also exerts its net effect through GABA-B receptors.
Modulation of cocaine intake with picrotoxin but not muscimol at first seems a bit puzzling in light of previous neurochemical and intracranial self-administration data using GABA-A compounds. For example, some investigators have encountered an increase in NAc dopamine following intra-VTA injection of the GABA-A antagonists picrotoxin and bicuculline and either no effect or a decrease in NAc dopamine with muscimol. Furthermore, rats will self-administer picrotoxin and bicuculline directly into the VTA (Ikemoto et al., 1997). Since cocaine reinforcement involves an increase in NAc dopamine, one might anticipate compounds that also increase NAc dopamine and can act as reinforcing stimuli in their own right would either enhance or have no effect on cocaine self-administration. Similarly, one would expect compounds that decrease NAc dopamine to negatively impact cocaine self-administration. Neither expectation held true in the present study, as picrotoxin decreased cocaine self-administration and muscimol had no effect.
To further complicate the matter, other studies have observed an increase in NAc dopamine in response to intra-VTA muscimol (Kalivas et al., 1990; Xi & Stein, 1998). Ikemoto and colleagues suggest that regional differences within the VTA may underlie the differences among the studies, but data from Xi and Stein (1998) contradicts this idea. The latter study, though, used cyclic voltammetry where all of the other studies mentioned utilized microdialysis. All of the above studies, however, tested GABAergic compounds in the absence of cocaine exposure. A further explanation for the present results, then, appears to be the influence that cocaine exposure exerts on GABAergic function in the VTA.
As Xi and Stein (1998) point out, the net effect of GABAergic compounds will rely on the relative balance of GABA-A receptor expression on dopaminergic versus non-dopaminergic cells, with the former serving to inhibit dopaminergic cell activity and the latter serving to disinhibit those cells. Variations in the balance of expression could explain regional differences in response to GABAergic compounds or differences along the anterior/posterior gradient within the VTA seen in other studies . Given the ability of cocaine to affect gene expression and cellular function (Tang et al., 2004), it is reasonable to consider the possibility that cocaine could perturb the balance of GABA receptor expression found in the drug naïve state.
Indeed, we have previously demonstrated that GABA-A subunit expression in dopaminergic cells of the VTA is altered after even a single 8 h session of cocaine self-administration (Backes & Hemby, 2003). Furthermore, Grubb et al. (2002) have demonstrated alterations in rotational behavior induced by intra-VTA injections of the GABA-A antagonist bicuculline in rats with a cocaine self-administration history. If the changes in subunit expression resulting from cocaine self-administration shift the balance such that GABA-A receptors on non-dopaminergic cells exert a greater influence than on dopaminergic cells in the VTA, one would expect the results seen in the present study. Disinhibition by picrotoxin of GABAergic cells that synapse onto the dopaminergic cells would presumably foster GABA release onto the dopaminergic cells. With picrotoxin similarly blocking GABA-A receptors on the dopaminergic cells (or with a decline in receptor function engendered by cocaine exposure), the GABA released would act primarily through GABA-B receptors, thus mimicking the effects of baclofen. In fact, the present results support this hypothesis in that the GABA-B antagonist 2- hydroxysaclofen completely blocks picrotoxin’s effects in the VTA. Thus, picrotoxin most likely leads to increased GABA release, leading to GABA-B receptor stimulation on dopaminergic cells while GABA-A receptors on dopaminergic cells are blocked by picrotoxin, reduced as a result of cocaine exposure, or both.
The data presented here lend further support to the strategy of using baclofen as a component of treatment for cocaine addiction but also raise the possibility of significant changes in VTA GABAergic transmission through the GABA-A receptor following cocaine exposure. Some of the apparent contradictions between this study and previous studies may be resolved by an examination of the effects of GABA-A agonists and antagonists on NAc dopamine release before and after cocaine self-administration. By examining changes in receptor expression in non-dopaminergic cells in the VTA, future experiments may also clarify the changes cocaine induces in the mesolimbic dopamine system.
Table 1.
Summary of Drug Administration
| Picrotoxin | Muscimol | Picrotoxin/2-OH saclofen | Baclofen | |
|---|---|---|---|---|
| Cocaine | 34 ng/side (n=7) | 14 ng/side (n=8) | 68 ng/100 ng/side (n=7) | 56 ng/side (n=7) |
| 68 ng/side (n=8) | 68 ng/2 µg/side (n=8) | 100 ng/side (n=6) | ||
| Food | 68 ng/side (n=6) | 100 ng/side (n=8) |
Acknowledgements
The research was funded in part by NIH grants DA012498, DA003628, and DA006634.
References
- Backes E, Hemby SE. Discrete cell gene profiling of ventral tegmental dopamine neurons after acute and chronic cocaine self-administration. J Pharmacol Exp Ther. 2003;307:450–459. doi: 10.1124/jpet.103.054965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb MC, Welch JR, Finn DA, Mark GP. Cocaine self-administration alters the locomotor response to microinjection of bicuculline into the ventral tegmental area of rats. Brain Res. 2002;952:44–51. doi: 10.1016/s0006-8993(02)03192-x. [DOI] [PubMed] [Google Scholar]
- Hemby SE. Morphine-induced alterations in gene expression of calbindin immunopositive neurons in nucleus accumbens shell and core. Neuroscience. 2004;126:689–703. doi: 10.1016/j.neuroscience.2004.01.056. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999a;288:274–280. [PubMed] [Google Scholar]
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine/heroin combinations (Speedball) in rats. Journal of Pharmacology and Experimental Therapeutics. 1999b;288:274–280. [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997a;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997b;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Reboussin D, Davies HM, Dworkin SI, Smith JE. Comparison of a novel tropane analog of cocaine, 2 beta-propanoyl-3 beta-(4-tolyl) tropane with cocaine HCl in rats: nucleus accumbens extracellular dopamine concentration and motor activity. Journal of Pharmacology & Experimental Therapeutics. 1995a;273:656–666. [PubMed] [Google Scholar]
- Hemby SE, Horman B, Tang W. Differential regulation of ionotropic glutamate receptor subunits following cocaine self-administration. Brain Res. 2005;1064:75–82. doi: 10.1016/j.brainres.2005.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. Journal of Pharmacology and Experimental Therapeutics. 1995b;273:591–598. [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. Journal of Pharmacology & Experimental Therapeutics. 1996a;277:1247–1258. [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine/heroin combinations in rats. Journal of Pharmacology and Experimental Therapeutics. 1996b;277:1247–1258. [erratum appears in J Pharmacol Exp Ther 1996 Oct;279(1):442] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABA(A) antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997;111:369–380. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Recent advances in the development of treatments for alcohol and cocaine dependence: focus on topiramate and other modulators of GABA or glutamate function. CNS Drugs. 2005;19:873–896. doi: 10.2165/00023210-200519100-00005. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Pills for speedballing or cocaine dependence. Lancet. 2006;367:1714–1716. doi: 10.1016/S0140-6736(06)68749-7. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Mann K, Willenbring ML, Litten RZ, Swift RM, Lesch OM, Berglund M. Challenges and opportunities for medications development in alcoholism: an international perspective on collaborations between academia and industry. Alcohol Clin Exp Res. 2005;29:1528–1540. doi: 10.1097/01.alc.0000174690.63787.fc. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Research - Brain Research Reviews. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Eberhardt H. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. Journal of Pharmacology & Experimental Therapeutics. 1990;253:858–866. [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci. 1996;16:1230–1238. doi: 10.1523/JNEUROSCI.16-03-01230.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edition. Academic Press; 1998. [Google Scholar]
- Porrino LJ. Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacology (Berl) 1993;112:343–351. doi: 10.1007/BF02244931. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sieghart W, Fuchs K, Tretter V, Ebert V, Jechlinger M, Hoger H, Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS. Imaging studies of cocaine in the human brain and studies of the cocaine addict. Ann N Y Acad Sci. 1997;820:41–54. doi: 10.1111/j.1749-6632.1997.tb46188.x. discussion 54-45. [DOI] [PubMed] [Google Scholar]
- Westerink BH, Enrico P, Feimann J, De Vries JB. The pharmacology of mesocortical dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and prefrontal cortex of the rat brain. J Pharmacol Exp Ther. 1998;285:143–154. [PubMed] [Google Scholar]
- Whiting PJ, Bonnert TP, McKernan RM, Farrar S, Le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Thompson SA, Wafford KA. Molecular and functional diversity of the expanding GABA-A receptor gene family. Ann N Y Acad Sci. 1999;868:645–653. doi: 10.1111/j.1749-6632.1999.tb11341.x. [DOI] [PubMed] [Google Scholar]
- Wise RA. Drug-activation of brain reward pathways. Drug and Alcohol Dependence. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychological Review. 1987;94:469–492. [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain Res. 1998;798:156–165. doi: 10.1016/s0006-8993(98)00406-5. [DOI] [PubMed] [Google Scholar]