Abstract
Our understanding of important stages in the pathogenesis of the human polyomavirus BK virus (BKV) and JC virus (JCV) infections is limited. In this context, nasopharyngeal aspirates from 201 children with respiratory diseases and saliva from 60 human immunodeficiency virus type 1-infected adults and 10 healthy adult controls were collected and analyzed for the presence of BKV and JCV DNA by PCR. Neither BKV nor JCV DNA was detected in the saliva specimens. We demonstrated BKV DNA, but no infectious BKV, in 2 of 201 nasopharyngeal aspirates. Each sample contained one unique rearranged noncoding control region variant of BKV. The results indicate that (i) BKV and JCV are not regularly associated with respiratory infections in children requiring hospitalization, (ii) nasopharyngeal cells are not an important site for primary replication of human polyomavirus BKV and JCV, and (iii) the salivary glands and oropharyngeal cells seem not to be involved in BKV and JCV persistence. We propose that for the polyomaviruses BKV and JCV the alimentary tract should be considered as a portal of entrance to the human organism.
Full text
PDF

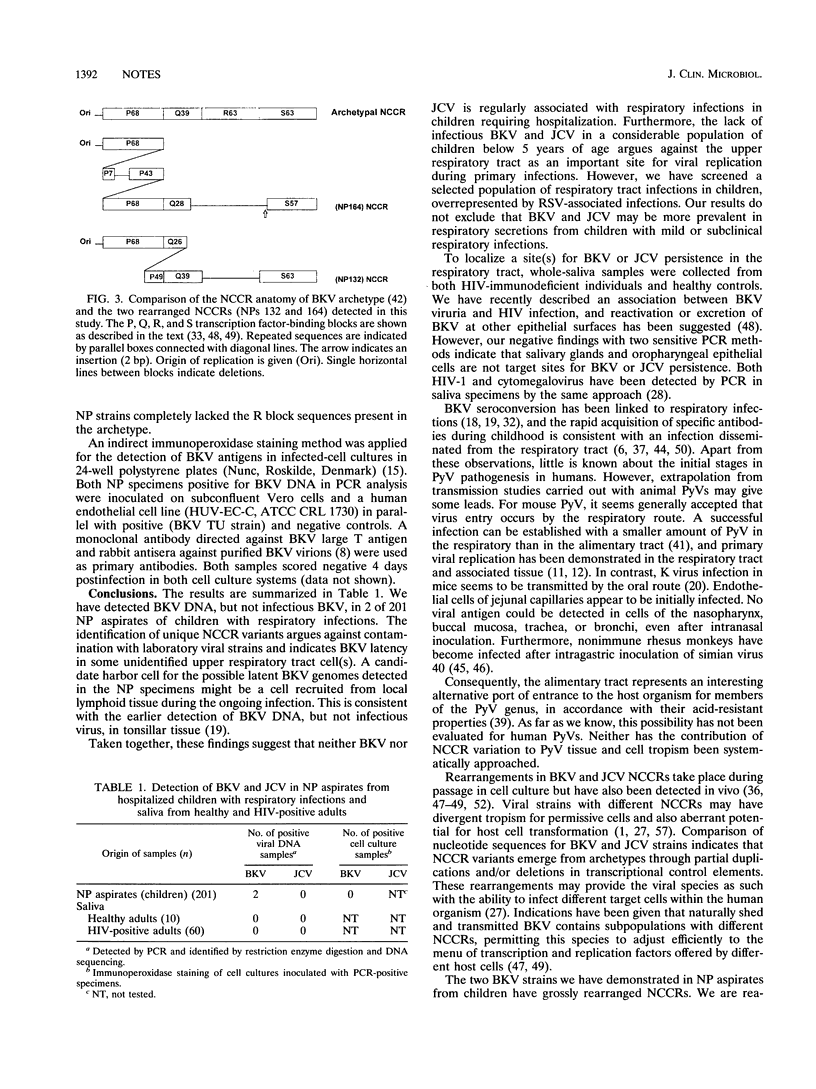


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amalfitano A., Martin L. G., Fluck M. M. Different roles for two enhancer domains in the organ- and age-specific pattern of polyomavirus replication in the mouse. Mol Cell Biol. 1992 Aug;12(8):3628–3635. doi: 10.1128/mcb.12.8.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur R. R., Dagostin S., Shah K. V. Detection of BK virus and JC virus in urine and brain tissue by the polymerase chain reaction. J Clin Microbiol. 1989 Jun;27(6):1174–1179. doi: 10.1128/jcm.27.6.1174-1179.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur R. R., Shah K. V., Charache P., Saral R. BK and JC virus infections in recipients of bone marrow transplants. J Infect Dis. 1988 Sep;158(3):563–569. doi: 10.1093/infdis/158.3.563. [DOI] [PubMed] [Google Scholar]
- Arthur R. R., Shah K. V. Occurrence and significance of papovaviruses BK and JC in the urine. Prog Med Virol. 1989;36:42–61. [PubMed] [Google Scholar]
- Bergsagel D. J., Finegold M. J., Butel J. S., Kupsky W. J., Garcea R. L. DNA sequences similar to those of simian virus 40 in ependymomas and choroid plexus tumors of childhood. N Engl J Med. 1992 Apr 9;326(15):988–993. doi: 10.1056/NEJM199204093261504. [DOI] [PubMed] [Google Scholar]
- Brown P., Tsai T., Gajdusek D. C. Seroepidemiology of human papovaviruses. Discovery of virgin populations and some unusual patterns of antibody prevalence among remote peoples of the world. Am J Epidemiol. 1975 Oct;102(4):331–340. doi: 10.1093/oxfordjournals.aje.a112169. [DOI] [PubMed] [Google Scholar]
- Christie K. E., Flaegstad T., Traavik T. Characterization of BK virus-specific antibodies in human sera by Western immunoblotting: use of a zwitterionic detergent for restoring the antibody-binding capacity of electroblotted proteins. J Med Virol. 1988 Feb;24(2):183–190. doi: 10.1002/jmv.1890240207. [DOI] [PubMed] [Google Scholar]
- Dubensky T. W., Murphy F. A., Villarreal L. P. Detection of DNA and RNA virus genomes in organ systems of whole mice: patterns of mouse organ infection by polyomavirus. J Virol. 1984 Jun;50(3):779–783. doi: 10.1128/jvi.50.3.779-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubensky T. W., Villarreal L. P. The primary site of replication alters the eventual site of persistent infection by polyomavirus in mice. J Virol. 1984 May;50(2):541–546. doi: 10.1128/jvi.50.2.541-546.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dörries K., ter Meulen V. Progressive multifocal leucoencephalopathy: detection of papovavirus JC in kidney tissue. J Med Virol. 1983;11(4):307–317. doi: 10.1002/jmv.1890110406. [DOI] [PubMed] [Google Scholar]
- Elsner C., Dörries K. Evidence of human polyomavirus BK and JC infection in normal brain tissue. Virology. 1992 Nov;191(1):72–80. doi: 10.1016/0042-6822(92)90167-n. [DOI] [PubMed] [Google Scholar]
- Flaegstad T., Sundsfjord A., Arthur R. R., Pedersen M., Traavik T., Subramani S. Amplification and sequencing of the control regions of BK and JC virus from human urine by polymerase chain reaction. Virology. 1991 Feb;180(2):553–560. doi: 10.1016/0042-6822(91)90069-n. [DOI] [PubMed] [Google Scholar]
- Flaegstad T., Traavik T. BK virus in cell culture: infectivity quantitation and sequential expression of antigens detected by immunoperoxidase staining. J Virol Methods. 1987 May;16(1-2):139–146. doi: 10.1016/0166-0934(87)90038-3. [DOI] [PubMed] [Google Scholar]
- Flaegstad T., Traavik T., Kristiansen B. E. Age-dependent prevalence of BK virus IgG and IgM antibodies measured by enzyme-linked immunosorbent assays (ELISA). J Hyg (Lond) 1986 Jun;96(3):523–528. doi: 10.1017/s0022172400066328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Baak M. L., Sleterus K. W., Van der Noordaa J. Human papovavirus isolated from urine of a child with acute tonsillitis. Br Med J (Clin Res Ed) 1981 Nov 21;283(6303):1363–1364. doi: 10.1136/bmj.283.6303.1363-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Wertheim-van Dillen P., van Strien A., van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10(2):91–99. doi: 10.1002/jmv.1890100203. [DOI] [PubMed] [Google Scholar]
- Greenlee J. E. Pathogenesis of K virus infection in mice. Prog Clin Biol Res. 1983;105:325–342. [PubMed] [Google Scholar]
- Kitamura T., Aso Y., Kuniyoshi N., Hara K., Yogo Y. High incidence of urinary JC virus excretion in nonimmunosuppressed older patients. J Infect Dis. 1990 Jun;161(6):1128–1133. doi: 10.1093/infdis/161.6.1128. [DOI] [PubMed] [Google Scholar]
- Knepper J. E., diMayorca G. Cloning and characterization of BK virus-related DNA sequences from normal and neoplastic human tissues. J Med Virol. 1987 Mar;21(3):289–299. doi: 10.1002/jmv.1890210313. [DOI] [PubMed] [Google Scholar]
- Korppi M., Heiskanen-Kosma T., Leinonen M., Halonen P. Antigen and antibody assays in the aetiological diagnosis of respiratory infection in children. Acta Paediatr. 1993 Feb;82(2):137–141. doi: 10.1111/j.1651-2227.1993.tb12624.x. [DOI] [PubMed] [Google Scholar]
- Loeber G., Dörries K. DNA rearrangements in organ-specific variants of polyomavirus JC strain GS. J Virol. 1988 May;62(5):1730–1735. doi: 10.1128/jvi.62.5.1730-1735.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht E., Albert J., Linde A., Xu W., Brytting M., Lundeberg J., Uhlén M., Bratt G., Sandström E., Heimdahl A. Human immunodeficiency virus type 1 and cytomegalovirus in saliva. J Med Virol. 1993 Feb;39(2):156–162. doi: 10.1002/jmv.1890390213. [DOI] [PubMed] [Google Scholar]
- Lucht E., Heimdahl A., Nord C. E. Periodontal disease in HIV-infected patients in relation to lymphocyte subsets and specific micro-organisms. J Clin Periodontol. 1991 Apr;18(4):252–256. doi: 10.1111/j.1600-051x.1991.tb00423.x. [DOI] [PubMed] [Google Scholar]
- Markowitz R. B., Dynan W. S. Binding of cellular proteins to the regulatory region of BK virus DNA. J Virol. 1988 Sep;62(9):3388–3398. doi: 10.1128/jvi.62.9.3388-3398.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz R. B., Thompson H. C., Mueller J. F., Cohen J. A., Dynan W. S. Incidence of BK virus and JC virus viruria in human immunodeficiency virus-infected and -uninfected subjects. J Infect Dis. 1993 Jan;167(1):13–20. doi: 10.1093/infdis/167.1.13. [DOI] [PubMed] [Google Scholar]
- Marshall W. F., Telenti A., Proper J., Aksamit A. J., Smith T. F. Survey of urine from transplant recipients for polyomaviruses JC and BK using the polymerase chain reaction. Mol Cell Probes. 1991 Apr;5(2):125–128. doi: 10.1016/0890-8508(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Myers C., Frisque R. J., Arthur R. R. Direct isolation and characterization of JC virus from urine samples of renal and bone marrow transplant patients. J Virol. 1989 Oct;63(10):4445–4449. doi: 10.1128/jvi.63.10.4445-4449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntyjärvi R. A., Meurman O. H., Vihma L., Berglund B. A human papovavirus (B.K.), biological properties and seroepidemiology. Ann Clin Res. 1973 Oct;5(5):283–287. [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L. Prevalence of antibodies in human sera against JC virus, an isolate from a case of progressive multifocal leukoencephalopathy. J Infect Dis. 1973 Apr;127(4):467–470. doi: 10.1093/infdis/127.4.467. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Quinlivan E. B., Norris M., Bouldin T. W., Suzuki K., Meeker R., Smith M. S., Hall C., Kenney S. Subclinical central nervous system infection with JC virus in patients with AIDS. J Infect Dis. 1992 Jul;166(1):80–85. doi: 10.1093/infdis/166.1.80. [DOI] [PubMed] [Google Scholar]
- ROWE W. P. The epidemiology of mouse polyoma virus infection. Bacteriol Rev. 1961 Mar;25:18–31. doi: 10.1128/br.25.1.18-31.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein R., Pare N., Harley E. H. Structure and function of the transcriptional control region of nonpassaged BK virus. J Virol. 1987 May;61(5):1747–1750. doi: 10.1128/jvi.61.5.1747-1750.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Daniel R. W., Warszawski R. M. High prevalence of antibodies to BK virus, an SV40-related papovavirus, in residents of Maryland. J Infect Dis. 1973 Dec;128(6):784–787. doi: 10.1093/infdis/128.6.784. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Hess D. M. Presence of antibodies to simian virus 40 (SV40) T antigen in rhesus monkeys infected experimentally or naturally with SV40. Proc Soc Exp Biol Med. 1968 Jun;128(2):480–485. doi: 10.3181/00379727-128-33043. [DOI] [PubMed] [Google Scholar]
- Shah K. V., Willard S., Myers R. E., Hess D. M., DiGiacomo R. Experimental infection of rhesus with simian virus 40 (SV40). Proc Soc Exp Biol Med. 1969 Jan;130(1):196–203. doi: 10.3181/00379727-130-33520. [DOI] [PubMed] [Google Scholar]
- Sugimoto C., Hara K., Taguchi F., Yogo Y. Growth efficiency of naturally occurring BK virus variants in vivo and in vitro. J Virol. 1989 Jul;63(7):3195–3199. doi: 10.1128/jvi.63.7.3195-3199.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundsfjord A., Flaegstad T., Flø R., Spein A. R., Pedersen M., Permin H., Julsrud J., Traavik T. BK and JC viruses in human immunodeficiency virus type 1-infected persons: prevalence, excretion, viremia, and viral regulatory regions. J Infect Dis. 1994 Mar;169(3):485–490. doi: 10.1093/infdis/169.3.485. [DOI] [PubMed] [Google Scholar]
- Sundsfjord A., Johansen T., Flaegstad T., Moens U., Villand P., Subramani S., Traavik T. At least two types of control regions can be found among naturally occurring BK virus strains. J Virol. 1990 Aug;64(8):3864–3871. doi: 10.1128/jvi.64.8.3864-3871.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F., Kajioka J., Miyamura T. Prevalence rate and age of acquisition of antibodies against JC virus and BK virus in human sera. Microbiol Immunol. 1982;26(11):1057–1064. doi: 10.1111/j.1348-0421.1982.tb00254.x. [DOI] [PubMed] [Google Scholar]
- Tominaga T., Yogo Y., Kitamura T., Aso Y. Persistence of archetypal JC virus DNA in normal renal tissue derived from tumor-bearing patients. Virology. 1992 Feb;186(2):736–741. doi: 10.1016/0042-6822(92)90040-v. [DOI] [PubMed] [Google Scholar]
- Tornatore C., Berger J. R., Houff S. A., Curfman B., Meyers K., Winfield D., Major E. O. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol. 1992 Apr;31(4):454–462. doi: 10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- Ulrich P. P., Romeo J. M., Daniel L. J., Vyas G. N. An improved method for the detection of hepatitis C virus RNA in plasma utilizing heminested primers and internal control RNA. PCR Methods Appl. 1993 Feb;2(3):241–249. doi: 10.1101/gr.2.3.241. [DOI] [PubMed] [Google Scholar]
- Vallbracht A., Löhler J., Gossmann J., Glück T., Petersen D., Gerth H. J., Gencic M., Dörries K. Disseminated BK type polyomavirus infection in an AIDS patient associated with central nervous system disease. Am J Pathol. 1993 Jul;143(1):29–39. [PMC free article] [PubMed] [Google Scholar]
- White F. A., 3rd, Ishaq M., Stoner G. L., Frisque R. J. JC virus DNA is present in many human brain samples from patients without progressive multifocal leukoencephalopathy. J Virol. 1992 Oct;66(10):5726–5734. doi: 10.1128/jvi.66.10.5726-5734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Kitamura T., Sugimoto C., Hara K., Iida T., Taguchi F., Tajima A., Kawabe K., Aso Y. Sequence rearrangement in JC virus DNAs molecularly cloned from immunosuppressed renal transplant patients. J Virol. 1991 May;65(5):2422–2428. doi: 10.1128/jvi.65.5.2422-2428.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]





