Abstract
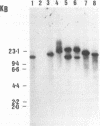
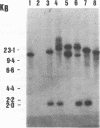
Chromosomal DNA restriction enzyme analysis and Southern blot hybridization were used to characterize Serpulina hyodysenteriae strains. When chromosomal DNAs from selected strains (reference serotypes) of S. hyodysenteriae were digested with the restriction endonuclease Sau3A and hybridized with a 1.1-kb S. hyodysenteriae-specific DNA probe, a common 3-kb band was always detected in S. hyodysenteriae strains but was absent from Serpulina innocens strains. When the chromosomal DNA was digested with the restriction endonuclease Asp 700 and hybridized with two S. hyodysenteriae-specific DNA probes (0.75 and 1.1 kb of DNA), distinct hybridization patterns for each S. hyodysenteriae reference strain and the Australian isolate S. hyodysenteriae 5380 were detected. Neither the 1.1-kb nor the 0.75-kb DNA probe hybridized with Asp 700- or Sau3A-digested S. innocens chromosomal DNA. The presence of the 3-kb Sau3A DNA fragment in S. hyodysenteriae reference strains from diverse geographical locations shows that this fragment is conserved among S. hyodysenteriae strains and can be used as a species-specific marker. Restriction endonuclease analysis and Southern blot hybridization with these well-defined DNA probes are reliable and accurate methods for species-specific and strain-specific identification of S. hyodysenteriae.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderton M. R., Smith S. C., Coloe P. J. Identification of a novel group of Serpulina hyodysenteriae isolates by using a lipopolysaccharide-specific monoclonal antibody. J Clin Microbiol. 1993 May;31(5):1326–1328. doi: 10.1128/jcm.31.5.1326-1328.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum D. H., Joens L. A. Serotypes of beta-hemolytic Treponema hyodysenteriae. Infect Immun. 1979 Sep;25(3):792–796. doi: 10.1128/iai.25.3.792-796.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carles-Nurit M. J., Christophle B., Broche S., Gouby A., Bouziges N., Ramuz M. DNA polymorphisms in methicillin-susceptible and methicillin-resistant strains of Staphylococcus aureus. J Clin Microbiol. 1992 Aug;30(8):2092–2096. doi: 10.1128/jcm.30.8.2092-2096.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D. M., Ross D. E. Restriction endonuclease analysis of Campylobacter strains with particular reference to Campylobacter fetus ss. fetus. J Med Microbiol. 1984 Aug;18(1):117–124. doi: 10.1099/00222615-18-1-117. [DOI] [PubMed] [Google Scholar]
- Combs B., Hampson D. J., Mhoma J. R., Buddle J. R. Typing of Treponema hyodysenteriae by restriction endonuclease analysis. Vet Microbiol. 1989 Apr;19(4):351–359. doi: 10.1016/0378-1135(89)90100-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garaizar J., Kaufmann M. E., Pitt T. L. Comparison of ribotyping with conventional methods for the type identification of Enterobacter cloacae. J Clin Microbiol. 1991 Jul;29(7):1303–1307. doi: 10.1128/jcm.29.7.1303-1307.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D. J., Mhoma J. R., Combs B. Analysis of lipopolysaccharide antigens of Treponema hyodysenteriae. Epidemiol Infect. 1989 Oct;103(2):275–284. doi: 10.1017/s0950268800030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson D. J., Mhoma J. R., Combs B., Buddle J. R. Proposed revisions to the serological typing system for Treponema hyodysenteriae. Epidemiol Infect. 1989 Feb;102(1):75–84. doi: 10.1017/s0950268800029708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. L. Current status of research on swine dysentery. J Am Vet Med Assoc. 1974 Apr 15;164(8):809–812. [PubMed] [Google Scholar]
- Harris D. L., Glock R. D., Christensen C. R., Kinyon J. M. Inoculation of pigs with Treponema hyodysenteriae (new species) and reproduction f the disease. Vet Med Small Anim Clin. 1972 Jan;67(1):61–64. [PubMed] [Google Scholar]
- Joens L. A., Glock R. D., Kinyon J. M. Differentiation of Treponema hyodysenteriae from T innocens by enteropathogenicity testing in the CF1 mouse. Vet Rec. 1980 Dec 6;107(23):527–529. [PubMed] [Google Scholar]
- Kristiansen B. E., Lind K. W., Mevold K., Sørensen B., Frøholm L. O., Bryn K., Tjade T., Bøvre K. Meningococcal phenotypic and genotypic characteristics and human antibody levels. J Clin Microbiol. 1988 Oct;26(10):1988–1992. doi: 10.1128/jcm.26.10.1988-1992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanser J. A., Anargyros P. A. Detection of Escherichia coli adhesins with DNA probes. J Clin Microbiol. 1985 Sep;22(3):425–427. doi: 10.1128/jcm.22.3.425-427.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeFebvre R. B., Perng G. C., Johnson R. C. Characterization of Borrelia burgdorferi isolates by restriction endonuclease analysis and DNA hybridization. J Clin Microbiol. 1989 Apr;27(4):636–639. doi: 10.1128/jcm.27.4.636-639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. S., Bélanger M., Jacques M. Serotyping of Canadian isolates of Treponema hyodysenteriae and description of two new serotypes. J Clin Microbiol. 1991 Dec;29(12):2794–2797. doi: 10.1128/jcm.29.12.2794-2797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapother M. E., Joens L. A. New serotypes of Treponema hyodysenteriae. J Clin Microbiol. 1985 Aug;22(2):161–164. doi: 10.1128/jcm.22.2.161-164.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. B., Winter P. J., Yanagawa R. Restriction endonuclease DNA analysis of Leptospira interrogans serovars icterohaemorrhagiae and hebdomadis. J Clin Microbiol. 1984 Oct;20(4):808–810. doi: 10.1128/jcm.20.4.808-810.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogollon J. D., Pijoan C., Murtaugh M. P., Collins J. E., Cleary P. P. Identification of epidemic strains of Streptococcus suis by genomic fingerprinting. J Clin Microbiol. 1991 Apr;29(4):782–787. doi: 10.1128/jcm.29.4.782-787.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocera D., Bannerman E., Rocourt J., Jaton-Ogay K., Bille J. Characterization by DNA restriction endonuclease analysis of Listeria monocytogenes strains related to the Swiss epidemic of listeriosis. J Clin Microbiol. 1990 Oct;28(10):2259–2263. doi: 10.1128/jcm.28.10.2259-2263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otal I., Martín C., Vincent-Lévy-Frebault V., Thierry D., Gicquel B. Restriction fragment length polymorphism analysis using IS6110 as an epidemiological marker in tuberculosis. J Clin Microbiol. 1991 Jun;29(6):1252–1254. doi: 10.1128/jcm.29.6.1252-1254.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacciarini M. L., Savio M. L., Tagliabue S., Rossi C. Repetitive sequences cloned from Leptospira interrogans serovar hardjo genotype hardjoprajitno and their application to serovar identification. J Clin Microbiol. 1992 May;30(5):1243–1249. doi: 10.1128/jcm.30.5.1243-1249.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. E., Patterson T. F., Farrel P., Hierholzer W. J., Jr, Zervos M. J. Evaluation of restriction endonuclease analysis as an epidemiologic typing system for Branhamella catarrhalis. J Clin Microbiol. 1989 May;27(5):944–946. doi: 10.1128/jcm.27.5.944-946.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud F., Etienne J., Bertrand A., Brun Y., Greenland T. B., Freney J., Fleurette J. Molecular epidemiology of Staphylococcus haemolyticus strains isolated in an Albanian hospital. J Clin Microbiol. 1991 Jul;29(7):1493–1497. doi: 10.1128/jcm.29.7.1493-1497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud F., Freney J., Etienne J., Bes M., Brun Y., Barsotti O., Andre S., Fleurette J. Restriction endonuclease analysis of Staphylococcus epidermidis DNA may be a useful epidemiological marker. J Clin Microbiol. 1988 Sep;26(9):1729–1734. doi: 10.1128/jcm.26.9.1729-1734.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson A. J., Ramadass P., Lee A., Marshall R. B. Differentiation of subtypes within Leptospira interrogans serovars Hardjo, Balcanica and Tarassovi, by bacterial restriction-endonuclease DNA analysis (BRENDA). J Med Microbiol. 1982 Aug;15(3):331–338. doi: 10.1099/00222615-15-3-331. [DOI] [PubMed] [Google Scholar]
- Schwan T. G., Simpson W. J., Schrumpf M. E., Karstens R. H. Identification of Borrelia burgdorferi and B. hermsii using DNA hybridization probes. J Clin Microbiol. 1989 Aug;27(8):1734–1738. doi: 10.1128/jcm.27.8.1734-1738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirois M., Lemire E. G., Levesque R. C. Construction of a DNA probe and detection of Actinobacillus pleuropneumoniae by using polymerase chain reaction. J Clin Microbiol. 1991 Jun;29(6):1183–1187. doi: 10.1128/jcm.29.6.1183-1187.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. C., Roddick F., Ling S., Gerraty N. L., Coloe P. J. Biochemical and immunochemical characterisation of strains of Treponema hyodysenteriae. Vet Microbiol. 1990 Jul;24(1):29–41. doi: 10.1016/0378-1135(90)90048-z. [DOI] [PubMed] [Google Scholar]
- Sotiropoulos C., Smith S. C., Coloe P. J. Characterization of two DNA probes specific for Serpulina hyodysenteriae. J Clin Microbiol. 1993 Jul;31(7):1746–1752. doi: 10.1128/jcm.31.7.1746-1752.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanton T. B., Jensen N. S., Casey T. A., Tordoff L. A., Dewhirst F. E., Paster B. J. Reclassification of Treponema hyodysenteriae and Treponema innocens in a new genus, Serpula gen. nov., as Serpula hyodysenteriae comb. nov. and Serpula innocens comb. nov. Int J Syst Bacteriol. 1991 Jan;41(1):50–58. doi: 10.1099/00207713-41-1-50. [DOI] [PubMed] [Google Scholar]
- Taylor D. J., Alexander T. J. The production of dysentery in swine by feeding cultures containing a spirochaete. Br Vet J. 1971 Nov;127(11):58–61. doi: 10.1016/s0007-1935(17)37282-2. [DOI] [PubMed] [Google Scholar]
- Tenover F. C. Diagnostic deoxyribonucleic acid probes for infectious diseases. Clin Microbiol Rev. 1988 Jan;1(1):82–101. doi: 10.1128/cmr.1.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins L. S., Troup N. J., Woods T., Bibb W., McKinney R. M. Molecular epidemiology of Legionella species by restriction endonuclease and alloenzyme analysis. J Clin Microbiol. 1987 Oct;25(10):1875–1880. doi: 10.1128/jcm.25.10.1875-1880.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tveten Y., Kristiansen B. E., Ask E., Jenkins A., Hofstad T. DNA fingerprinting of isolates of Staphylococcus aureus from newborns and their contacts. J Clin Microbiol. 1991 Jun;29(6):1100–1105. doi: 10.1128/jcm.29.6.1100-1105.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]