Abstract
Objectives
We previously demonstrated that there is a learning curve for open radical prostatectomy. We sought to determine whether the effects of the learning curve are modified by pathologic stage.
Methods
The study included 7765 eligible prostate cancer patients treated with open radical prostatectomy by one of 72 surgeons. Surgeon experience was coded as the total number of radical prostatectomies conducted by the surgeon prior to a patient’s surgery. Multivariable regression models of survival time were used to evaluate the association between surgeon experience and biochemical recurrence, with adjustment for PSA, stage, and grade. Analyses were conducted separately for patients with organ-confined and locally advanced disease.
Results
Five-year recurrence-free probability for patients with organ-confined disease approached 100% for the most experienced surgeons. Conversely, the learning curve for patients with locally advanced disease reached a plateau at approximately 70%, suggesting that about a third of these patients cannot be cured by surgery alone.
Conclusions
Excellent rates of cancer control for patients with organ-confined disease treated by the most experienced surgeons suggest that the primary reason such patients recur is inadequate surgical technique.
Keywords: Prostate cancer, Surgical learning curve, Decision analysis
1. Introduction
There is accumulating evidence that the results of many types of cancer surgery are associated with characteristics of the operating surgeon. Surgeons with a higher yearly volume of cases have been shown to have lower mortality rates for several procedures, including resection for cancer of the stomach, lung, oesophagus, colon, and pancreas [1]. The absolute difference in survival rates between different surgeons (eg, about 5% for gastrectomy), are comparable to those typically sought for adjuvant chemotherapy. Several studies have also shown that the effects of surgeon on outcome are not restricted to immediate postoperative complications. For example, surgeon volume has been found to be associated with overall survival after rectal cancer resection, even though 30-day mortality did not differ according to surgeon volume [2].
Radical prostatectomy is an especially complex procedure, and it is reasonable to suppose that outcomes are particularly sensitive to surgical technique. Begg et al. demonstrated a lower risk of postoperative complications in patients treated by surgeons with a higher yearly case volume [3]. Medicare claims data were analyzed for 11,522 patients undergoing radical prostatectomy, and postoperative complications were found to be significantly higher among surgeons in the lowest quartile of yearly caseload compared to the surgeons with the highest caseloads (32% vs. 26%, p < 0.001). Moreover, the variation in complication rates among surgeons with similar caseloads was much higher than would be expected by chance: 8% of high-volume surgeons had complication rates above the 99th percentile predicted from a statistical distribution. Similar findings were reported by Hu et al. [4], who reported lower complication rates (odds ratio: 0.53) and shorter lengths of stay (4.1 vs. 5.2 days) in patients treatment by high-volume compared to low-volume surgeons. Other studies on the association between volume and outcome have been reviewed by van Poppel [5].
In a previous publication [6], we reported the learning curve for cancer control after radical prostatectomy. Instead of examining a surgeon’s yearly caseload, we investigated the number of prior radical prostatectomies conducted by a surgeon at the time of the incident case. After adjustment for case mix, we found a strong association between biochemical recurrence and surgeon experience (p < 0.001). This association was robust to a wide variety of sensitivity analyses. For a typical patient, we estimated a 5-yr probability of biochemical recurrence of 17.9% if seen by an inexperienced surgeon (10 prior cases) compared to 10.7% if seen by a more experienced surgeon (250 prior cases). On the basis of these findings, we recommended that patients seek more experienced surgeons, and we repeated calls for regionalization of cancer surgery at specialized centres [7].
The learning curve we presented in our original publication is an average across different risk groups. It is plausible that the shape of the learning curve might differ depending on patient risk. In particular, we were interested in how pathologic stage would affect the learning curve. We stratified patients into two risk groups, depending on whether the tumour was organ confined or whether there was evidence of locally advanced disease (extracapsular extension, seminal vesicle invasion, or positive lymph nodes) on pathological analysis of the radical prostatectomy specimen. We then calculated the learning curve separately for each group. It was our hypothesis that any differences in the learning curve between organ-confined and locally advanced disease would be informative as to cancer biology.
2. Methods
2.1. Patients and outcomes
The study cohort and endpoints were described previously [6]. In brief, our cohort consisted of 9376 patients with clinically localized prostate cancer who were treated by open radical retropubic prostatectomy between January 1987 and December 2003 at one of four participating institutions: Memorial Sloan-Kettering Cancer Center (New York, NY, USA), Baylor College of Medicine (Houston, TX, USA), Wayne State University (Detroit, MI, USA), and the Cleveland Clinic (Cleveland, OH, USA). Patients receiving neoadjuvant therapy (n = 1316), adjuvant therapy (n = 85), or who had missing data for either surgeon (n = 144) or PSA (n = 66) were excluded, leaving a total of 7765 patients eligible for analysis. All information was obtained with appropriate institutional review board waivers from the respective institutions, and data were deidentified prior to analysis. Patients were treated by one of 72 surgeons, all of whom treated patients only at the study institutions while on staff. Surgeons who conducted their initial radical prostatectomies at an institution that was not part of the study were asked to provide their prior caseloads. Cancer recurrence was defined as a serum PSA of more than 0.4 ng/ml that was corroborated by a subsequent higher PSA level (ie, biochemical recurrence) [8]. In rare cases (eg, <1% in the Memorial Sloan-Kettering Cancer Center data set), secondary treatment was initiated for patients who did not meet the criteria for recurrence; such treatment was counted as an event.
2.2. Statistical analyses
Our research question is whether more experienced surgeons have better results irrespective of pathologic stage, or whether the association between experience and outcome depends on whether a patient had organ-confined or locally advanced disease on pathological analysis of the prostatectomy specimen. Locally advanced disease was defined as the presence of extracapsular extension, seminal vesical invasion, or lymph node involvement.
For each patient, surgeon experience was coded as the number of radical prostatectomies conducted by the surgeon prior to the patient’s operation. This number reflects total prior experience, including operations conducted at former institutions, and those for patients ineligible for analysis, but not those at which a surgeon assisted, such as during residency or fellowship training. We first conducted exploratory analyses to see if there were differences in surgical experience by pathologic stage. For these analyses, surgeon experience was entered as a continuous variable in a logistic regression model to predict locally advanced disease, with clustering by surgeon.
Our main analysis was to evaluate the association between surgeon experience and recurrence after radical prostatectomy separately for organ-confined and locally advanced disease. To do so, we fitted a multivariable, parametric survival-time regression model. We used a log-logistic survival distribution to model hazard over time because length of follow-up is not independent of surgeon experience. Surgeon experience was entered as a continuous variable. Because the relationship between experience and outcome may be nonlinear, we used restricted cubic splines with knots at the quartiles. To adjust for differences in case mix, we included as covariates preoperative PSA level, Gleason grade in the radical prostatectomy specimen, and presence or absence of extracapsular extension, seminal vesicle invasion, and lymph node involvement. These parameters have been consistently associated with cancer recurrence after radical prostatectomy [9]. We adjusted for within-surgeon clustering using a generalized estimating equations approach [10] by specifying the “cluster” option in Stata 9.2 (Stata Corp., College Station, TX). As described previously [6], few patients died before experiencing recurrence (5-yr overall survival probability of 95%). Therefore, we did not adjust for competing risk and censored patients at the date of death.
We originally intended to use year of surgery as a covariate. However, when first fitting our statistical model to predict recurrence by surgical experience, we observed some implausible results among patients with locally advanced disease: the learning curve increased up to approximately 500 prior cases and then started to decrease, such that very highly experienced surgeons appeared to have comparable results to surgeons treating their first case. For example, the 5-yr probabilities of recurrence for a patient with locally advanced disease treated by a surgeon who had completed 10, 500, and 1800 prior surgeries were estimated at 43%, 31%, and 41%, respectively. On further analysis, this appeared to be due to our inclusion of year of surgery as a covariate. The learning curve did not decline if we removed year of surgery as a covariate or if we restricted analysis to patients treated after 1995, when stage migration in this cohort appeared to be complete [6]. Accordingly, we believe that the apparent decline in the learning curve is a statistical artefact caused by the high correlation between year of surgery and surgeon experience, coupled with the limited number of patients with locally advanced cancer who were treated by the most experienced surgeons. The learning curves for patients with organ-confined disease were unaffected by the inclusion or exclusion of year of surgery as a covariate, whether or not the sample was restricted to patients treated after 1995. Therefore, all results presented hereafter are without adjustment for year of surgery.
To produce a learning curve for each subgroup of patients, we calculated the 5-yr recurrence-free probability predicted by the model for each level of surgical experience, using the mean value for covariates in that subgroup. Confidence intervals for the difference in 5-yr recurrence rates for 10 versus 250 prior cases were determined using bootstrap methods with 1000 replications. For a prespecified sensitivity analysis, we repeated all analyses in the subgroup of patients treated after 1995, after which stage migration seemed to be largely complete in this cohort [6].
3. Results
Clinical and pathological patient characteristics are listed in Table 1. There were 5342 (69%) patients with organ-confined disease and 2423 (31%) patients with locally advanced disease. We found a moderate but statistically significant negative association between pathologic stage and surgeon experience (odds ratio for locally advanced disease: 0.97 per 100 cases; 95% CI: 0.95, 0.98; p < 0.001). This appeared to be due to stage migration, as there was no important association between surgeon experience and organ-confined status (odds ratio: 0.99; 95% CI: 0.98, 1.00; p = 0.16) when analysis was restricted to patients treated after 1995.
Table 1.
Clinical and pathological characteristics for patients with and without organ-confined disease
| Organ Confined
|
||
|---|---|---|
| Yes | No | |
| N = 5342 | N = 2423 | |
| Age at surgery (yr) | 61 (56[en]65) | 63 (57[en]67) |
| Total PSA (ng/ml) | 6.3 (4.7[en]8.8) | 8.2 (5.6[en]13.9) |
| Clinical stage* | ||
| T1 | 2894 (54%) | 777 (32%) |
| T2a | 1498 (28%) | 673 (28%) |
| T2b | 370 (7%) | 414 (17%) |
| T2c/T3/T4 | 521 (10%) | 536 (22%) |
| Biopsy Gleason score | ||
| ≤6 | 4116 (77%) | 1207 (50%) |
| 7 | 1074 (20%) | 951 (39%) |
| ≥8 | 152 (3%) | 265 (11%) |
| Pathology Gleason score | ||
| ≤5 | 375 (7%) | 55 (2%) |
| 6 | 2569 (48%) | 466 (19%) |
| 7 | 2251 (42%) | 1549 (64%) |
| 8 | 119 (2%) | 228 (9%) |
| ≥9 | 28 (1%) | 125 (5%) |
| Extracapsular extension | 0 (0%) | 2261 (93%) |
| Seminal vesicle invasion | 0 (0%) | 695 (29%) |
| Lymph node metastasis | 0 (0%) | 291 (12%) |
| Surgeon experience | ||
| 0[en]49 | 944 (18%) | 458 (19%) |
| 50[en]99 | 480 (9%) | 216 (9%) |
| 100[en]249 | 1039 (19%) | 536 (22%) |
| 250[en]999 | 2000 (37%) | 940 (39%) |
| ≥1000 | 879 (16%) | 273 (11%) |
| Positive surgical margins | 1066 (20%) | 993 (41%) |
Clinical stage was unknown for 59 patients with and 23 patients without organ-confined disease.
In total, there were 1256 recurrences. The median follow-up for recurrence-free patients was 3.9 yr. Surgeon experience was associated with outcome irrespective of whether patients had organ-confined or locally advanced disease (both p < 0.001; Table 2). The learning curves for cancer control after radical prostatectomy according to pathologic stage are shown in Figure 1. Of particular interest, the learning curve for locally advanced cancer reached a plateau at approximately a 30% probability of recurrence at 5 yr, whereas for organ-confined disease the learning curve continued to rise towards a 100% recurrence-free probability.
Table 2.
Effects of surgeon experience on outcome, according to pathologic stage
| Analysis | Adjusted P value for surgeon experience | Adjusted 5-yr probability of recurrence | 10 vs. 250 prior cases | ||
|---|---|---|---|---|---|
| 10 prior cases | 250 prior cases | Absolute difference | Relative difference | ||
| All patients (n = 7765) | |||||
| Organ confined | <0.001 | 14.2% | 5.8% | 8.3% (5.5%, 11.7%) | 2.4 (1.9, 3.2) |
| Locally advanced | <0.001 | 46.4% | 35.5% | 10.9% (4.8%, 17.0%) | 1.3 (1.1, 1.5) |
| Sensitivity analysis | |||||
| Patients treated after 1995 (n = 5107) | |||||
| Organ confined | <0.001 | 13.6% | 3.7% | 9.9% (3.0%, 14.3%) | 3.7 (2.2, 6.3) |
| Locally advanced | <0.001 | 45.1% | 22.0% | 23.1% (12.7%, 33.5%) | 2.0 (1.5, 2.9) |
Probabilities are for a patient with typical cancer severity within each group (mean PSA, pathological stage, and grade) treated at the midpoint of the series. The 95% confidence intervals for the absolute and relative differences are given in parentheses. The sensitivity analyses include only patients treated after 1995, when stage migration was largely complete.
Fig. 1.
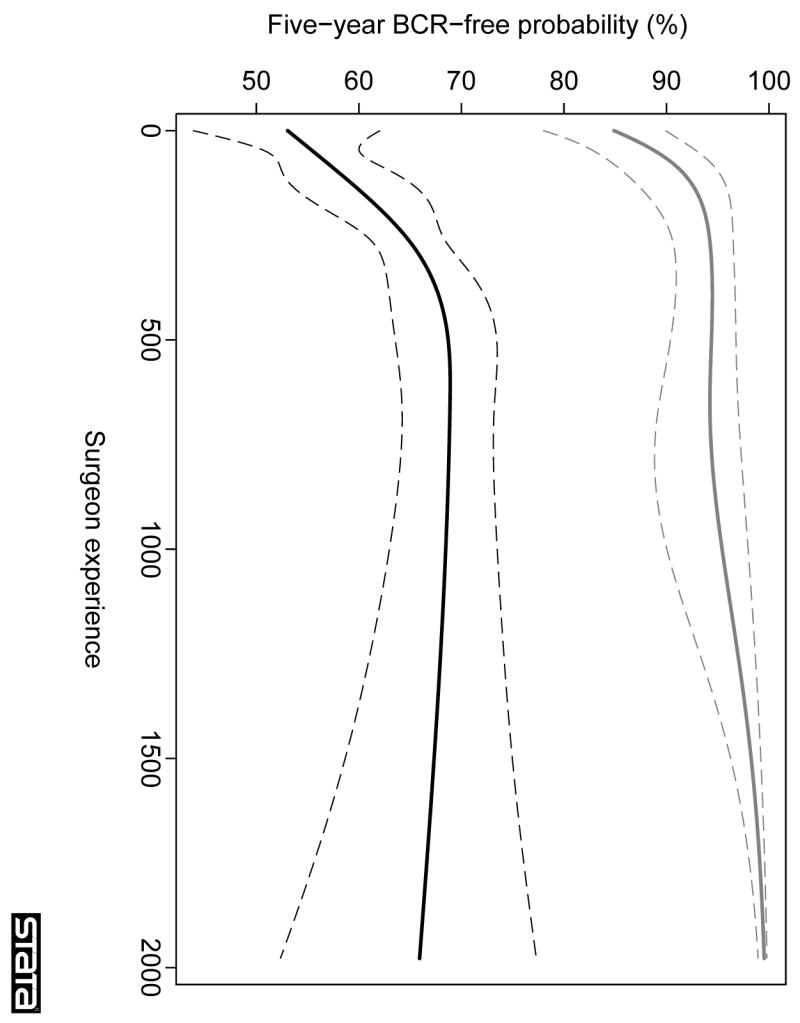
The learning curve for cancer control after radical prostatectomy, stratified by presence of organ-confined disease. The graph illustrates the predicted probability of freedom of biochemical recurrence (BCR) at 5 yr with increasing surgeon experience. Probabilities are for a patient with typical cancer severity (mean PSA, pathological stage, and grade) within each group. Grey lines, organ-confined disease; black lines, locally advanced disease; dashed lines, 95% CIs.
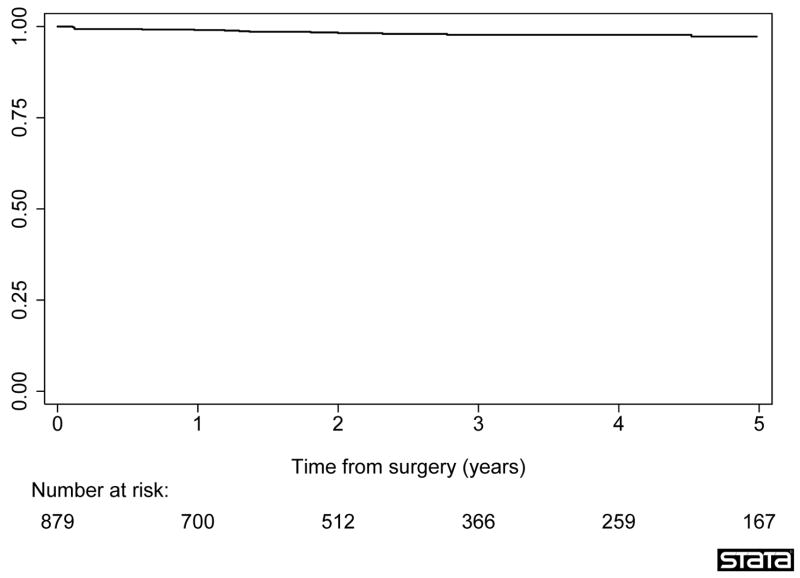
To explore this result further, we conducted a Kaplan-Meier analysis examining only those patients with organ-confined disease whose surgeons had conducted at least 1000 prior radical prostatectomies. There were 879 patients in this cohort, of whom 16 had recurrence. Median follow-up for recurrence-free patients was 2.5 yr. The 5-yr recurrence-free probability was 97% (95% CI: 95%, 98%; Fig. 2).
Fig. 2.
Biochemical recurrence after radical prostatectomy. Analysis was restricted to patients with organ-confined disease treated by surgeons with at least 1000 prior surgeries.
Figure 3 shows the learning curve for the sensitivity analysis, restricted to patients treated after 1995, when stage migration in our cohort appeared largely complete. These results confirm our main findings, in particular, the 5-yr recurrence-free probability was greater than 99% for a patient with organ-confined disease treated by a surgeon with the greatest level of experience. For locally advanced disease, the learning curve was initially steeper (eg, the adjusted 5-yr probability of recurrence for a surgeon with 250 prior cases was 22% after 1995 compared to 36% in the entire series; Table 2) but then, similar to the main analysis, reached a plateau at approximately a 30% probability of recurrence at 5 yr.
Fig. 3.
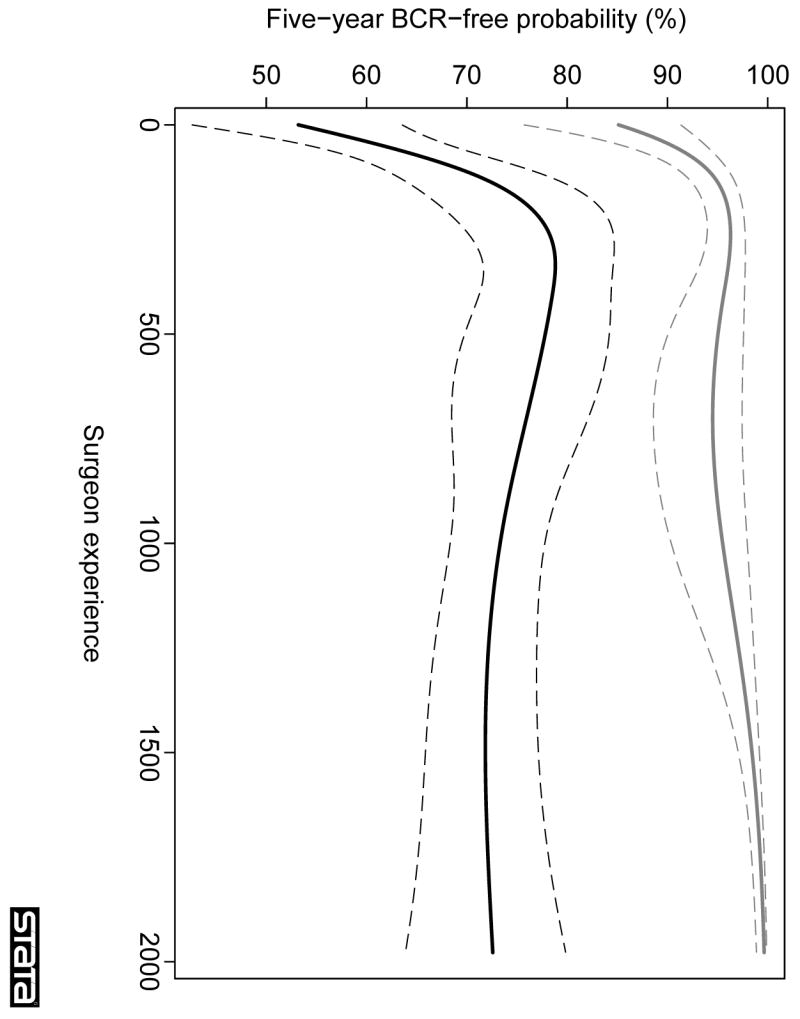
Learning curve for cancer control after radical prostatectomy, stratified by presence of organ-confined disease, in patients treated after 1995 (sensitivity analysis). The graph illustrates the predicted probability of freedom of biochemical recurrence (BCR) at 5 yr with increasing surgeon experience. Probabilities are for a patient with typical cancer severity (mean PSA, pathological stage, and grade) within each group. Grey lines, organ-confined disease; black lines: locally advanced disease; dashed lines, 95% CIs.
4. Discussion
Our findings indicate that cancer control after radical prostatectomy improves with increasing surgeon experience irrespective of pathologic stage. The learning curve for patients with organ-confined disease approaches 100% recurrence-free probability at 5 yr with increasing surgeon experience. Conversely, the learning curve for locally advanced disease flattens at approximately 70%, suggesting that about a third of these patients cannot be cured by surgery alone. These findings have implications for both clinical care and for our understanding of prostate cancer biology.
With respect to clinical practice, if a sufficiently experienced surgeon is able to cure all or nearly all patients with organ-confined disease, the obvious corollary is that recurrence in these patients is primarily a matter of surgical technique. This suggests that regionalization of cancer care should be encouraged, so that fewer surgeons conduct a larger number of operations. Although regionalization would no doubt incur some additional costs, Ramirez et al. reported that total hospital charges are lower for high-volume surgeons, possibly due to reduced complication rates [11].
With respect to research, it is currently unclear how exactly the most experienced surgeons avoid recurrence, or what it is that less experienced surgeons are doing that leads to recurrence in organ-confined disease. Systematic research is required to identify the critical aspects of radical prostatectomy that are associated with cancer control. Our results also reinforce the need to expand opportunities for training in surgical technique for surgeons in the early years after residency training. Novel educational methods could be explored, such as the use of surgical simulation, having senior surgeons scrub-in with newly trained surgeons, or interactive video training. Our findings also show that it is critical to measure and provide feedback on a surgeon’s outcomes, not merely immediate complications, but also surgical margins, long-term function, and cancer control. Van Poppel reported a pilot attempt at a quality-control program of this nature [12].
With respect to cancer biology, considerable research has focused on predicting recurrence after radical prostatectomy and numerous prognostic markers have been studied. For example, in a Medline search for “immunohistochemistry prostatectomy recurrence” in June 2007, 15 of the first 20 papers retrieved reported empirical data, and more than 20 markers were investigated in these 15 studies. In a typical study, levels of the molecular marker in patients who recurred were compared to levels in patients without recurrence. Our data, however, suggest that surgery is a major confounder: a patient might well have a tumour with an aggressive phenotype, as evidenced by strong expression of a molecular marker, but this has little clinical relevance if he has organ-confined disease and a sufficiently experienced surgeon. Accordingly, investigators studying molecular markers that predict biochemical recurrence or clinical progression after surgery might consider studying only patients of very inexperienced surgeons or only patients of highly experienced surgeons with locally advanced disease.
We discussed several limitations of our data set in our previous paper [6]. Briefly, given the observational nature of our study, we cannot rule out residual confounding by differences in case mix. However, results were very similar if the data set was restricted to patients treated after 1995, in whom we found no associations between surgeon experience and tumour characteristics. Moreover, it is difficult to imagine what unmeasured patient factors might account for effects as large as 10[en]20% differences in absolute risk between more and less experienced surgeons. In addition, biochemical recurrence is arguably only a surrogate endpoint for clinical outcomes such as metastasis and prostate-cancer-specific mortality. Yet such endpoints are inevitably preceded by biochemical recurrence. In addition, biochemical recurrence does impact patient quality of life, as treatments for recurrence are associated with important toxic effects. A third limitation of our study is that the model is based on patients treated at major academic centres. It is not clear that our results pertain to surgeons practicing in other settings. For example, surgeons in our cohort may have steeper learning curves than those in private practice in the community because they have protected research time, work in a competitive environment that promotes criticism and self-evaluation, and are constantly exposed to new ideas and techniques.
Moreover, our findings that recurrence rates for organ-confined cancers tend towards zero with increasing surgical experience is based on a very limited number of surgeons: only two surgeons in our series treated more than 1000 cases. We do not believe that this affects our conclusions, however, because even if only one surgeon can achieve near-zero recurrence rates, recurrence must be due to inadequate surgical technique. However, caution is advised in applying these results to other surgeons. It seems plausible that outcome may differ between two surgeons with similar levels of experience and, as such, it may not be the case that all highly experienced surgeons have uniformly excellent results with organ-confined disease. Finally, the right-hand tail of the learning curve for locally advanced disease was sensitive to the method of analysis. We cannot entirely discount the possibility that rates of cancer control in locally advanced disease may actually decrease once a surgeon reaches a certain level of experience. The mechanism for such an effect, however, is unclear.
In conclusion, the surgical learning curve for radical prostatectomy is relevant for all patients, irrespective of pathologic stage. Recurrence rates were close to zero for patients with organ-confined disease treated by the most experienced surgeons in our data set, suggesting that the primary reason such patients recur is inadequate surgical technique.
Take home message
For patients with organ-confined prostate cancer, recurrence after surgery approaches zero for the most experienced surgeons, suggesting that recurrence in such patients relates to inadequate surgical technique. Analysis of learning curves suggests that about 30% of patients with locally advanced disease cannot be cured by surgery alone.
Acknowledgments
This research was funded in part by a P50-CA92629 SPORE grant from the National Cancer Institute and by the Allbritton Fund and the Koch Foundation. The funding bodies had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–20. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 2.Schrag D, Panageas KS, Riedel E, et al. Hospital and surgeon procedure volume as predictors of outcome following rectal cancer resection. Ann Surg. 2002;236:583–92. doi: 10.1097/00000658-200211000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begg CB, Riedel ER, Bach PB, et al. Variations in morbidity after radical prostatectomy. N Engl J Med. 2002;346:1138–44. doi: 10.1056/NEJMsa011788. [DOI] [PubMed] [Google Scholar]
- 4.Hu JC, Gold KF, Pashos CL, Mehta SS, Litwin MS. Role of surgeon volume in radical prostatectomy outcomes. J Clin Oncol. 2003;21:401–5. doi: 10.1200/JCO.2003.05.169. [DOI] [PubMed] [Google Scholar]
- 5.Van Poppel H, Boulanger SF, Joniau S. Quality assurance issues in radical prostatectomy. Eur J Surg Oncol. 2005;31:650–5. doi: 10.1016/j.ejso.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Vickers AJ, Bianco FJ, Serio AM, et al. The surgical learning curve for prostate cancer control after radical prostatectomy. J Natl Cancer Inst. 2007;99:1171–7. doi: 10.1093/jnci/djm060. [DOI] [PubMed] [Google Scholar]
- 7.Lipscomb J. Transcending the volume-outcome relationship in cancer care. J Natl Cancer Inst. 2006;98:151–4. doi: 10.1093/jnci/djj055. [DOI] [PubMed] [Google Scholar]
- 8.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 9.Kattan MW, Wheeler TM, Scardino PT. Postoperative nomogram for disease recurrence after radical prostatectomy for prostate cancer. J Clin Oncol. 1999;17:1499–507. doi: 10.1200/JCO.1999.17.5.1499. [DOI] [PubMed] [Google Scholar]
- 10.Lee EW, ——— WL, Amato D. Cox-type regression analysis for large number of small groups of correlated failure time observations. Surviv Anal. 1992 ———: [Google Scholar]
- 11.Ramirez A, Benayoun S, Briganti A, et al. High radical prostatectomy surgical volume is related to lower radical prostatectomy total hospital charges. Eur Urol. 2006;50:58–62. doi: 10.1016/j.eururo.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 12.Van Poppel H, Collette L, Kirkali Z, et al. Quality control of radical prostatectomy: a feasibility study. Eur J Cancer. 2001;37:884–91. doi: 10.1016/s0959-8049(01)00056-9. [DOI] [PubMed] [Google Scholar]