Abstract
Objectives
Commonly used definitions for high-risk prostate cancer identify men at increased risk of PSA relapse after radical prostatectomy (RP). We assessed how accurately these definitions identify patients likely to receive secondary cancer therapy, experience metastatic progression, or die of prostate cancer.
Materials and methods
Among 5960 men with clinically localized or locally advanced prostate cancer who underwent RP, we identified eight different high-risk subsets, each comprising 4[en]40% of the study population. Estimates of freedom from radiation therapy, hormonal therapy, and metastatic progression after surgery were generated for each high-risk cohort with the Kaplan-Meier method, and hazard ratios (HR) were calculated with a Cox proportional hazards regression. The cumulative incidence and HR for prostate cancer[en]specific mortality (PCSM) were estimated with competing risk analysis.
Results
Each of the studied high-risk criteria was associated with increased hazard of secondary cancer therapy (HR = 1.3[en]5.2, p < 0.05) and metastatic progression (HR = 2.1[en]6.9, p < 0.05). However, depending on the definition, the probability of freedom from additional therapy 10 yr after surgery ranged from 35% to 76%. The 10-yr cumulative incidence of PCSM in high-risk patients ranged from 3% to 11% (HR = 3.2[en]10.4, p < 0.0005).
Conclusions
Commonly used definitions for high-risk prostate cancer identify men at increased risk of secondary cancer therapy, metastatic progression, and PCSM following RP. However, a substantial proportion of high-risk patients remain free from additional therapy or metastatic disease many years after surgery. The risk of PCSM within 10 yr of treatment is remarkably low, even for patients at the highest risk of recurrent disease.
Keywords: Radical prostatectomy, Risk assessment, Metastatic progression, Radiation therapy, Hormonal therapy, Prostate cancer[en]specific mortality
1. Introduction
For many years radical prostatectomy (RP) has been considered ill advised for men whose cancer may not be pathologically confined to the prostate (clinical stage T3 or, historically, stage C). These men were presumed to harbor clinically occult metastases at the time of diagnosis and consequently deemed incurable by surgery alone. Subjecting them to the daunting morbidities associated with surgery, namely urinary incontinence and erectile dysfunction, was considered unjustified. This philosophy still relegates patients with large-volume, high-grade tumors to radiation therapy [1] or, if local therapy is considered superfluous, to primary androgen-deprivation therapy [2].
To be clinically useful, criteria defining high-risk prostate cancer should reliably distinguish patients whose cancer is amenable to cure with local therapy alone from those who may require additional systemic therapy. While there is no consensus on what constitutes optimal treatment for the latter, the former can be safely offered surgery with curative intent. However, in light of the diversity of criteria defining high-risk prostate cancer [3], the historical tendency of discouraging these men from having surgical management, and the lack of randomized trials comparing the various local definitive therapies, treatment of men with high-risk prostate cancer is largely driven by physicians' experience and biases.
We previously reported on pathological characteristics and prostate-specific antigen (PSA) outcomes for patients with high-risk prostate cancer treated with RP alone [4]. To identify high-risk cohorts we examined eight different definitions commonly used in the medical literature. Depending on the definition used, clinically high-risk patients had cancers that were organ-confined in 22[en]63% of cases and 5- yr probability of freedom from PSA relapse ranging from 49% to 80%. Moreover, of the high-risk patients who had disease recurrence, 25% (across all definitions) relapsed more than 2 yr after surgery, and in 26[en]39% the PSA doubling time at recurrence was ≥ 10 mo, both considered surrogates for a more protracted clinical course following biochemical recurrence (BCR) [5]. Yet, while a rising PSA level after surgery may herald clinical disease progression, nearly a third of men with BCR never experience further PSA elevations or require additional therapy [6]. Moreover, because of competing mortality risks, the probability of dying from prostate cancer within 15 yr of BCR is similar to the likelihood of dying from other causes [7]. The latter is further accentuated by recent data indicating that long-term androgen-deprivation therapy may predispose patients to metabolic syndrome, myocardial infarction, and sudden cardiac death [8,9]. Thus, in an extension of our previous work, we sought to analyze patterns of secondary cancer therapy and risk of metastatic progression (MP) and cancer-specific mortality in patients with clinically high-risk prostate cancer treated with RP.
2. Methods
After obtaining institutional review board approval, we queried our multidisciplinary prostate cancer registry and identified 6421 patients with clinically localized or locally advanced prostate cancer (cT1[en]T3 N0 M0) treated with RP between 1985 and 2005 at Memorial Sloan-Kettering (n = 5256) or Baylor College of Medicine (n = 1165). Because our study end points were MP and overall and cancer-specific survival, and evidence from randomized trials showed no difference in PSA outcomes for patients treated with androgen deprivation before RP [10,11], we included 815 patients (13%) who received a short course (median, 3.1 mo (interquartile range [IQR], 2.1[en]4.2)) of hormonal therapy before surgery. Most of these patients were treated prior to 2002 when the role of neoadjuvant hormonal therapy in patients undergoing radical prostatectomy was under investigation. We excluded 461 patients (7.1%) for whom preoperative data were missing because their preoperative risk stratification was unattainable. Complete information on PSA levels, primary and secondary biopsy Gleason grades, and 1992 TNM clinical stage was available for 5960 patients (Table 1). On the basis of preoperative characteristics, we used eight different definitions proposed to classify patients as high risk (Table 2) [3,12,13]. PSA velocity before surgery was calculated for the 3177 patients (53%) who had sufficient data available by a linear regression using all PSA values within 1 yr before surgery or before initiation of neoadjuvant androgen-deprivation therapy; the median interval between the last of these PSA values and surgery was 1 mo (IQR, 0.4, 2).
Table 1.
[en] Preoperative and pathological characteristics of 5960 patients treated with radical prostatectomy for clinically localized prostate cancer
| Characteristic | No. of patients (%) |
|---|---|
| Median age at surgery, yr (IQR) | 61 (56, 66) |
| Median preoperative PSA, ng/ml (IQR) | 6.34 (4.47, 9.60) |
| Median preoperative PSA velocity, ng/ml/yr (IQR)* | 1.10 ([en]1.19, 3.83) |
| Biopsy Gleason sum | |
| 2[en]6 | 3917 (66) |
| 7 (3+4) | 1175 (20) |
| 7 (4+3) | 467 (8) |
| 8[en]10 | 401 (6) |
| 1992 TNM clinical stage | |
| T1AB | 122 (2) |
| T1C | 2678 (45) |
| T2A | 1260 (21) |
| T2B | 1094 (18) |
| T2C | 563 (10) |
| T3 | 243 (4) |
| Prostatectomy Gleason sum | |
| 2[en]6 | 2125 (36) |
| 7 (3+4) | 1835 (31) |
| 7 (4+3) | 575 (9) |
| 8[en]10 | 650 (11) |
| Not available** | 775 (13) |
| Organ confined | 3280 (70) |
| Extracapsular extension | 1688 (27) |
| Positive surgical margins | 1193 (20) |
| Seminal vesicle invasion | 526 (9) |
| Lymph node involvement | 254 (4) |
IQR, interquartile range; PSA, prostate-specific antigen.
Available for 3177 patients.
Hormonal treatment effect.
Table 2.
[en] Description of the eight high-risk prostate cancer definitions based on preoperative variables.
| High-risk definition | Description and references | No. (% of cohort) | |
|---|---|---|---|
| 1 | bGS 8[en]10 | Poorly differentiated cancer [3] | 401 (6.7) |
| 2 | Preoperative PSA ≥ 20 ng/ml | [3] | 441 (7.4) |
| 3 | 1992 TNM cT3 | Locally advanced cancer [3] | 243 (4.0) |
| 4 | PSA ≥ 20 or 1992 TNM ≥ cT2C or bGS 8[en]10 | D’Amico’s high-risk criteria [15] | 1359 (22.8) |
| 5 | Nomogram 5-yr PFP ≤ 50% | Preoperative nomogram [20]; a ≤ 60% PFP is an entry criterion for CALGB Trial 90203 | 606 (10.2) |
| 6 | PSA ≥ 20 or 1992 ≥ TNM cT3 or bGS 8[en]10 | NCCN guidelines for treatment and classification of clinically localized high-risk cancer [13] | 938 (15.7) |
| 7 | PSA ≥ 15 or 1992 TNM ≥ cT2B or bGS 8[en]10 | Entry criteria into neoadjuvant chemotherapy trials for high-risk patients [3] | 2384 (40.0) |
| 8 | Preoperative PSA velocity > 2 ng/ml/yr | Increased risk of prostate cancer[en]specific mortality after RP or radiotherapy [12] | 1209 (32.4)* |
bGS, biopsy Gleason score; PSA, prostate-specific antigen; PFP, progression-free probability; CALGB, Cancer and Leukemia Group B; NCCN, National Comprehensive Cancer Network; RP, radical prostatectomy.
Percentage of the 3177 patients with calculable preoperative PSA velocity
2.1. Follow-up
In general, patients were followed for recurrence with rectal examinations and serum PSA determinations every 3 mo for the first 3 yr, semiannually during years 4 and 5, and annually thereafter. Median follow-up from surgery was 5.5 yr (IQR, 2.3, 8). There were 2859 patients (48%) followed more than 5 yr and 914 (15%) more than 10 yr.
Secondary cancer treatment was instituted at the discretion of the treating physician. Postoperative radiation was administered to 410 patients, in 379 cases as salvage therapy and in 31 cases as adjuvant therapy because of adverse pathological features. Androgen-deprivation therapy (surgical or chemical castration with luteinizing hormone-releasing hormone agonists, antiandrogens, or a combination thereof) was given to 587 patients, all treated in a salvage setting following BCR. Metastatic disease (unequivocal bony, nodal, or visceral involvement by imaging or biopsy) was documented in 236 patients, 151 (64%) of whom were treated with hormonal therapy only after the first radiographic evidence of metastatic progression. Patients with a rising PSA who deferred hormonal therapy until MP were generally monitored carefully with diagnostic imaging studies and serial PSA measurements at intervals of 3[en]6 mo. At last follow-up 445 patients had died. Death certificateswere used to ascertain or confirm the cause of each death. In 102 cases, death was attributed to widespread progressive castrate metastatic disease and was classified as death from prostate cancer.
2.2. Statistical methods
The Kaplan-Meier method was used to generate estimates of freedom from secondary therapies and freedom from MP for high-risk and non[en]high-risk patients. For these end points, patients were censored if they died of causes other than prostate cancer or were lost to follow-up. The log-rank test was used to compare estimates between high-risk and non[en]high-risk subsets. Univariate Cox proportional hazards regression was used for each of the tested definitions to estimate hazard ratios with 95% confidence intervals for secondary cancer treatment and MP in high-risk versus non[en]high-risk cohorts.
Because of the protracted clinical course of prostate cancer and relatively low number of patients who died from prostate cancer versus other causes, we used competing risk analyses to generate estimates of the cumulative incidence of death from prostate cancer in high-risk and non[en]high-risk subsets. In this method, death from causes other than prostate cancer is considered a competing risk event and is censored in an informative manner (as opposed to censoring due to short follow-up). The association between each high-risk definition and prostate cancer[en]specific death was evaluated with the use of a proportional hazards regression model with adjustment for competing risk [14]. All statistical analyses were two-sided and performed with Stata, version 8.2 (Stata Corp, College Station, TX, USA) and R (R Foundation for Statistical Computing, www.r-project.org) with the cmprsk package, with p < 0.05 considered significant.
3. Results
Depending on the definition used, high-risk cohorts comprised 4[en]40% of the entire study population (Table 2). The 10-yr probability of postoperative radiation therapy in high-risk patients ranged from 14% (preoperative PSA velocity > 2 ng/ml/yr) to 26% (Gleason score ≥ 8) (Fig. 1 A). Similarly, the probability of receiving androgen-deprivation therapy within 10 yr after surgery ranged from 18% (preoperative PSA velocity > 2 ng/ml/yr) to 59% (cT3) (Fig. 1 B). Combined estimates of secondary cancer therapy (radiation, hormones, or both, whichever occurred first) are listed in Table 3. Compared with the non[en]high-risk patients, those at high-risk had a 1.3-fold (95%CI, 1.1, 1.5) to 5.2-fold (95%CI, 4.5, 6.0) increased hazard of being treated with secondary therapy. However, 35% (95%CI, 27, 44) to 76% (95%CI, 71, 80) of the high-risk patients were free from any type of secondary cancer treatment 10 yr after surgery.
Fig. 1.
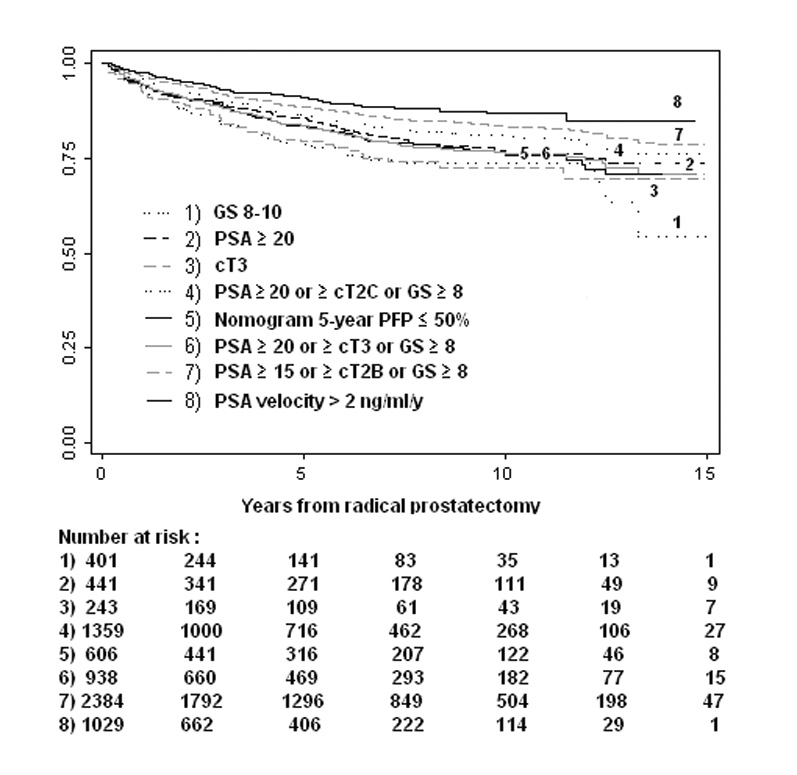
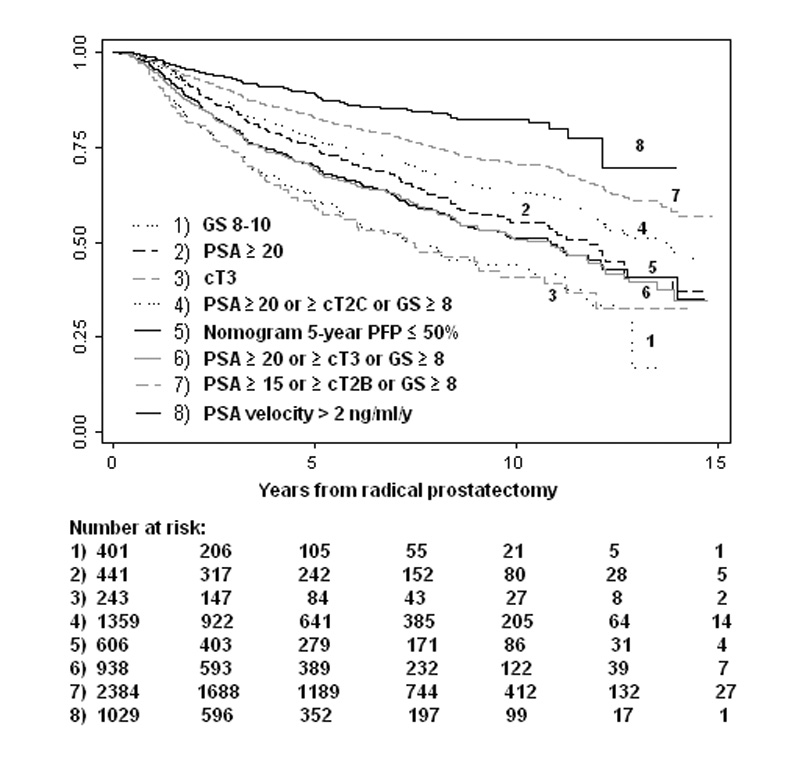
[en](A) Radiation therapy[en]free survival in high-risk prostate cancer patients treated with radical prostatectomy. (B) Hormonal therapy[en]free survival in high-risk prostate cancer patients treated with radical prostatectomy.
Table 3.
[en] Estimates of freedom from secondary cancer therapy and hazard ratios in high-risk versus non[en]high-risk patients treated with RP
| Patient | High-risk definition | Freedom from 2nd cancer therapy (95%CI) |
||
|---|---|---|---|---|
| 5-year | 10-year | HR (95%CI) | ||
| 1 | Biopsy Gleason score 8[en]10 | |||
| Yes | 53 (47, 59) | 40 (33, 47) | 4.9 (4.1, 5.8) | |
| No | 88 (87, 89) | 78 (76, 80) | ||
| 2 | Preoperative PSA ≥ 20 | |||
| Yes | 67 (62, 72) | 47 (41, 53) | 3.2 (2.7, 3.8) | |
| No | 87 (86, 88) | 80 (78, 81) | ||
| 3 | 1992 TNM cT3 | |||
| Yes | 50 (43, 57) | 35 (27, 44) | 4.6 (3.8, 5.6) | |
| No | 87 (86, 88) | 78 (76, 80) | ||
| 4 | PSA ≥ 20 or 1992 TNM ≥ cT2C or bGS 8[en]10 | |||
| Yes | 70 (67, 72) | 56 (53, 60) | 3.7 (3.2, 4.3) | |
| No | 91 (90, 92) | 84 (81, 86) | ||
| 5 | Nomogram 5-yr PFP ≤ 50% | |||
| Yes | 61 (57, 65) | 43 (38, 48) | 4.1 (3.6, 4.8) | |
| No | 89 (88, 90) | 81 (79, 83) | ||
| 6 | PSA ≥ 20 or 1992 TNM ≥ cT3 or bGS 8[en]10 | |||
| Yes | 61 (57, 64) | 43 (39, 48) | 5.2 (4.5, 6.0) | |
| No | 91 (90, 92) | 84 (83, 86) | ||
| 7 | PSA ≥ 15 or 1992 TNM ≥ cT2B or bGS 8[en]10 | |||
| Yes | 76 (74, 77) | 63 (61, 66) | 4.5 (3.8, 5.4) | |
| No | 94 (93, 95) | 89 (86, 91) | ||
| 8 | Preoperative PSA velocity > 2 ng/ml/yr | |||
| Yes | 82 (79, 85) | 76 (71, 80) | 1.3 (1.1, 1.5) | |
| No | 86 (85, 87) | 76 (74, 78) | ||
RP, radical prostatectomy; CI, confidence interval; HR, hazard ratio; PSA, prostate-specific antigen; bGS, biopsy Gleason score; PFP, progression free probability.
High risk patients were significantly more likely to receive secondary therapy, irrespective of definition.
Estimates of MP 5 and 10 yr after surgery are shown in Table 4. As indicated in the table, each of the studied high-risk criteria was associated with significantly higher rates of MP after surgery (hazard ratios ranging from 2.1 [95%CI, 1.3, 5.1] to 6.9 [4.7, 10.2], p < 0.05). Because the various high-risk subsets in our study are not mutually exclusive (ie, they contain, at least in part, some of the same patients), estimates of MP could not be compared between cohorts in a statistically valid manner. In general, however, definitions in which PSA level above 15 or 20 ng/ml was a sufficient criterion for a high-risk classification (definitions 2, 4, 6, and 7) were associated with lower rates of MP compared with those based solely on biopsy Gleason score 8[en]10 (definition 1) or clinical stage T3 (definition 3). It should also be noted that use of hormonal therapy for BCR before the first radiographic evidence of metastatic disease (in 47% of the patients with documented BCR in this series) may have skewed our estimates by virtue of delaying the progression to event.
Table 4.
[en] Metastasis-free survival and hazard ratios for metastatic progression in high-risk versus non[en]high-risk patients treated with RP
| –– | High-risk definition | No. of events/no. of pts | Metastasis-free survival (95%CI) |
||
|---|---|---|---|---|---|
| 5-year | 10-year | HR (95%CI) | |||
| 1 | Biopsy Gleason score 8[en]10 | ||||
| Yes | 62/401 | 85 (80, 89) | 76 (69, 82) | 6.1 (4.6, 8.2) | |
| No | 174/5559 | 98 (97, 98) | 94 (93, 95) | ||
| 2 | Preoperative PSA ≥ 20 | ||||
| Yes | 65/441 | 89 (86, 92) | 82 (77, 86) | 3.2 (2.4, 4.3) | |
| No | 171/5519 | 97 (97, 98) | 94 (93, 95) | ||
| 3 | 1992 TNM cT3 | ||||
| Yes | 47/243 | 85 (79,89) | 72 (63, 79) | 5.4 (3.9, 7.4) | |
| No | 189/5717 | 97 (97, 98) | 94 (93, 95) | ||
| 4 | PSA ≥ 20 or 1992 TNM ≥ cT2C or bGS 8[en]10 | ||||
| Yes | 146/1359 | 92 (90, 93) | 85 (83, 88) | 4.2 (3.2, 5.4) | |
| No | 90/4601 | 99 (98, 99) | 95 (94, 96) | ||
| 5 | Nomogram 5-yr PFP ≤ 50% | ||||
| Yes | 78/606 | 89 (86, 92) | 82 (78, 86) | 3.4 (2.6, 4.5) | |
| No | 158/5354 | 98 (97, 98) | 94 (93, 95) | ||
| 6 | PSA ≥ 20 or 1992 TNM ≥ cT3 or bGS 8[en]10 | ||||
| Yes | 140/938 | 88 (85, 90) | 78 (75, 82) | 6.5 (5.0, 8.5) | |
| No | 96/5022 | 99 (98, 99) | 96 (95, 97) | ||
| 7 | PSA ≥ 15 or 1992 TNM ≥ cT2B or bGS 8[en]10 | ||||
| Yes | 206/2178 | 94 (93, 95) | 88 (86, 90) | 6.9 (4.7, 10.2) | |
| No | 30/3546 | 99 (98, 100) | 98 (97, 99) | ||
| 8 | Preoperative PSA velocity > 2 ng/ml/yr | ||||
| Yes | 32 / 1029 | 98 (97, 99) | 91 (87, 93) | 2.1 (1.3, 5.1) | |
| No | 45 / 2148 | 97 (95, 98) | 94 (91, 96) | ||
RP, radical prostatectomy; pts, patients; CI, confidence interval; HR, hazard ratio; PSA, prostate-specific antigen; bGS, biopsy Gleason score; PFP, progression free probability.
High risk patients were significantly more likely to experience metastatic progression, irrespective of definition.
Competing risk analyses were performed to assess whether each of the high-risk definitions was associated with increased likelihood of death from prostate cancer (Table 5, Fig. 2). Depending on the definition used, the 10-year cumulative incidence of prostate cancer[en]specific mortality in high-risk patients ranged from 3% (PSA velocity > 2 ng/ml/yr; 95%CI, 2, 6) to 11% (cT3; 95%CI, 7, 19). While estimates of dying from other causes did not differ significantly between high-risk and non[en]high-risk subsets (data not shown), those classified as high risk by seven of the eight definitions had a significantly increased risk of dying from prostate cancer (adjusted hazard ratios ranging from 3.2 [95%CI, 2.1, 4.8] to 10.5 [95%CI, 4.8, 22.9], p < 0.05). Noteworthy is that even among the high-risk patients the likelihood of dying from other causes was generally 2- to 3-fold higher than the likelihood of dying from prostate cancer (Fig. 2).
Table 5.
[en] Cumulative incidence estimates of prostate cancer[en]specific mortality and hazards ratios in high-risk versus non[en]highrisk patients treated with RP
| Estimated prostate cancer[en]specific mortality | ||||||
|---|---|---|---|---|---|---|
| High-risk definition | No. dead of disease /pts | No. dead of other cause/pts | 5-year (95%CI) | 10-year (95%CI) | HR (95%CI) | |
| 1 | Biopsy Gleason score 8[en]10 | |||||
| Yes | 23/401 | 47/401 | 4.2 (2.3, 7.8) | 12 (7, 19) | 5.1 | |
| No | 79/5559 | 398/5559 | 0.4 (0.3, 0.7) | 3 (2, 4) | (3.2, 8.1) | |
| 2 | Preoperative PSA ≥ 20 | |||||
| Yes | 35/441 | 89/441 | 2.7 (1.5, 5.1) | 9 (6, 13) | 3.5 | |
| No | 67/5519 | 356/5519 | 0.5 (0.3, 0.8) | 3 (2, 4) | (2.3, 5.4) | |
| 3 | 1992 TNM cT3 | |||||
| Yes | 20/243 | 36/243 | 4.3 (2.1, 9) | 11 (7, 19) | 4.6 | |
| No | 82/5717 | 409/5717 | 0.5 (0.3, 0.8) | 3 (2, 4) | (2.8, 7.5) | |
| 4 | PSA ≥ 20 or ≥1992 TNM cT2C or bGS 8[en]10 | |||||
| Yes | 36/1359 | 195/1359 | 2.3 (1.5, 3.4) | 7 (5, 9) | 3.9 | |
| No | 66/4601 | 250/4601 | 0.1 (0.0, 0.3) | 2 (1, 3) | (2.6, 6.0) | |
| 5 | Nomogram 5-yr PFP ≤ 50% | |||||
| Yes | 37/606 | 98/606 | 2.4 (1.3, 4.3) | 8 (6, 12) | 3.2 | |
| No | 65/5354 | 347/5354 | 0.4 (0.3, 0.7) | 2 (2, 3) | (2. 1, 4.8) | |
| 6 | PSA ≥ 20 or ≥ 1992 TNM cT3 or bGS 8[en]10 | |||||
| Yes | 59/938 | 145/938 | 2.8 (1.8, 4.4) | 8 (6, 11) | 5.0 | |
| No | 43/5022 | 300/5022 | 0.2 (0.1, 0.4) | 2 (1, 3) | (3.4, 7.5) | |
| 7 | PSA ≥ 15 or ≥ 1992 TNM cT2B or bGS 8[en]10 | |||||
| Yes | 95/2384 | 137/2384 | 1.4 (1, 2.1) | 5 (4, 7) | 10.4 | |
| No | 7/3576 | 308/3576 | 0 | 1 (0, 2) | (4.8, 22.7) | |
| 8 | Preoperative PSA velocity > 2 ng/ml/yr | |||||
| Yes | 13/1029 | 56/1029 | 0.1 (0.0, 0.7) | 3 (2, 6) | 2.5 | |
| No | 10/2148 | 96/2148 | 0.2 (0.0, 0.9) | 1 (1, 3) | (1.0, 5.7) | |
Pts, patients; CI, confidence interval; HR, hazard ratio; PSA, prostate-specific antigen; bGS, biopsy Gleason score; PFP, progression free probability; RP, radical prostatectomy.
All hazard ratios (except for definition 8) were significant at the 0.0005 level.
Fig. 2.
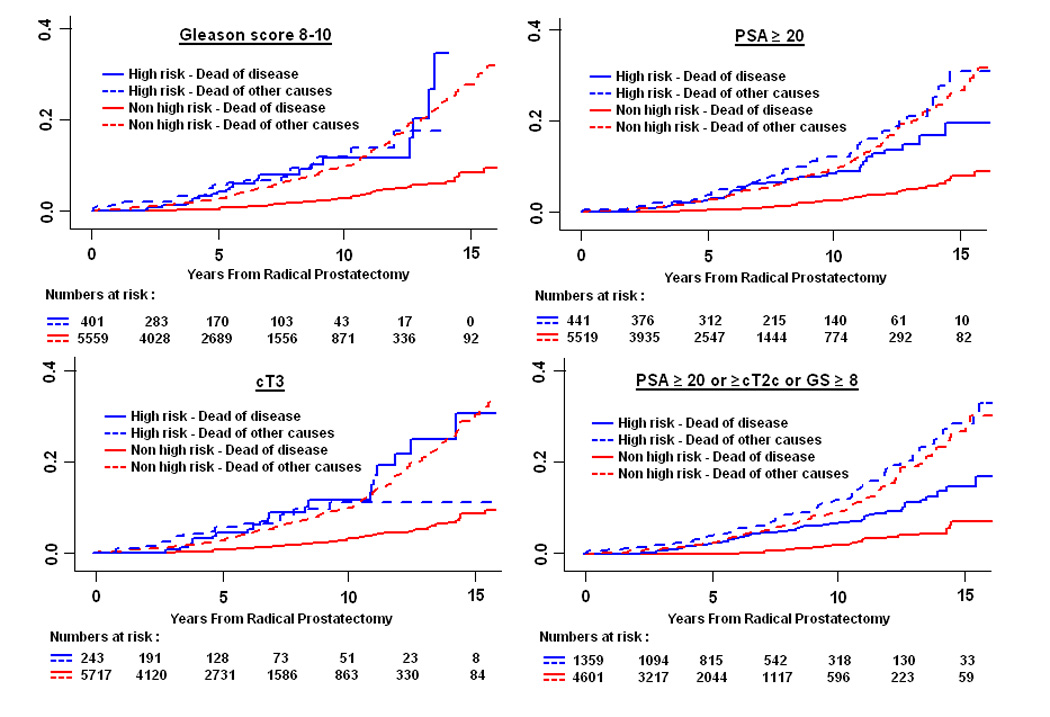
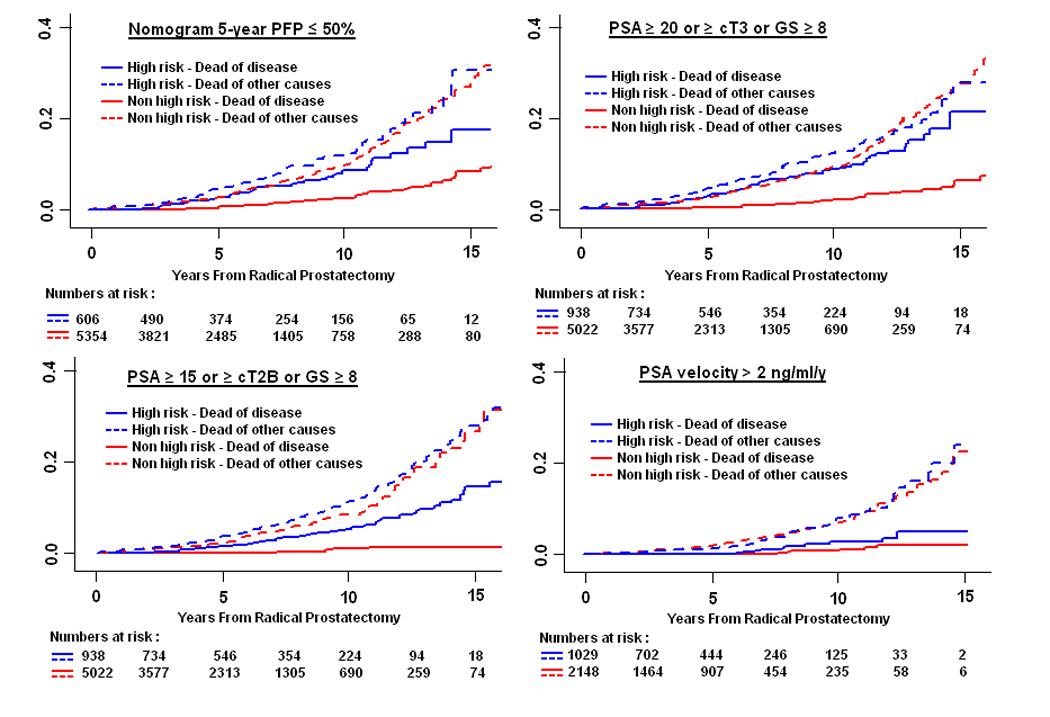
(A[en]B) Prostate cancer[en]specific mortality in high-risk and non[en]high-risk patients treated with radical prostatectomy.
4. Discussion
Criteria that define high-risk prostate cancer are typically based on an increased likelihood of BCR following local definitive therapy [15]. BCR after RP, a cause of angst in both patient and physician, often prompts the initiation of secondary therapies [16]. However, BCR can have a highly variable clinical course that does not inexorably culminate in MP and death from prostate cancer [6]. With the ultimate goal of identifying those who can be cured with surgery alone from those in whom monotherapy is likely to fail, it is imperative that clinical trials enrolling high-risk patients enable assessment of treatment efficacy using clinically meaningful end points. Further, given the range of toxicities associated with neoadjuvant therapies [3] and the substantial morbidity of long-term androgen deprivation [8,9], patients considering enrollment onto these trials must be provided with realistic expectations regarding the success of surgery monotherapy. With this in mind we endeavored to analyze risk of secondary cancer treatment, MP, and death from prostate cancer after RP among patients considered high risk by various definitions.
The current study demonstrates that commonly used definitions of high-risk prostate cancer are associated not only with an increased likelihood of PSA relapse[4] but also with significantly higher rates of secondary cancer therapy, MP, and death from prostate cancer. These results confirm the observations of others indicating that PSA > 20 at diagnosis and biopsy Gleason score 8[en]10 are pretreatment predictors of ultimately receiving additional cancer treatment after RP [17] and dying from prostate cancer [18]. Compared with non[en]high-risk patients, those at high-risk had a 1.3- to 5.2-fold increased hazard of being treated with secondary cancer therapy, a 2.1- to 6.9-fold higher probability of MP, and a 3.2- to 10.4-fold higher risk of dying from their cancer. However, equally important is the fact that 35[en]76% of the high-risk patients were alive and free of additional cancer therapy 10 yr after surgery, highlighting the ability of surgery alone to control the disease in many of these men. Thus, while the currently available risk stratification systems are generally sensitive in determining patients at high risk for secondary cancer therapies, MP, and cancerspecific mortality, their lack of specificity leads to inclusion of many men whose disease is, in fact, amenable to cure with local therapy alone.
These findings can help guide clinical decision making. If, for example, a neoadjuvant trial is planned in which the drug has fairly minimal toxicity, it might be acceptable to use a definition of high-risk disease that is highly sensitive (ie, would encompass most patients in whom local therapy is likely to fail) at the expense of reduced specificity (ie, would also include a number of patients who could otherwise be cured by surgery alone). Definition 7 in our study (PSA ≥ 15 or 1992 TNM ≥ cT2B or biopsy Gleason score 8[en]10) might be applicable in this case. Conversely, in a trial in which the neoadjuvant drug has substantial expected toxicity, more stringent criteria to define high-risk candidates should be employed. One approach to risk assessment, as recently advocated by D'Amico et al [19], is to count the number of equally weighted categorical high-risk features, up to four, and relegate the patient to one of four risk groups (including 1, 2, 3, or all features). Another utilizes nomograms, which incorporate data from all relevant risk factors, both continuous and categorical, and proportionately weigh their relative contribution to the overall probability of treatment failure to allot a risk score. The latter approach is not only more accurate but also more appropriate for assigning risk to an individual patient [20]. While most current models are limited to predicting BCR following local therapy, enhanced nomograms developed on more mature cohorts will allow prediction of more meaningful clinical end points such as MP [21] and cancer-specific mortality. The addition of detailed biopsy data, magnetic resonance imaging findings, and, possibly, emerging molecular markers to the current risk-stratification tools might provide more precise information to guide clinical decision making and enable researchers to better target populations suitable for particular clinical trials. As currently classified, patients with "high-risk" prostate cancer by currently available definitions should not be categorically disqualified from having surgical therapy with curative intent despite their increased risk of treatment failure.
Regardless of definition, the optimal therapy for high-risk prostate cancer remains elusive. No study has provided persuasive evidence favoring one treatment modality over another. Radiation therapy has the advantage of avoiding the immediate morbidity of surgery, particularly since surgery for locally advanced cancers may be associated with a slightly higher complication rate than that for organ-confined cancers [22]. RP, compared with radiation therapy, may minimize late sequelae of local disease progression, although data supporting this conjecture are currently unavailable [23]. In an era of novel drug and multimodal strategies, surgery yields a tissue specimen for histological and molecular analyses. In fact, our observed 3[en]12% cumulative incidence of cancer-specific death at 10 yr after RP, depending on the definition used, is comparable or even superior to published estimates of cancer-specific mortality in high-risk patients treated by radiation with or without use of adjuvant hormonal therapy [24,25]. One may argue that our high-risk subsets represent a selected population of patients treated surgically. However, the proportion of high-risk patients in our study (23% by definition 4, the D'Amico criteria, and 16% by definition 6, the National Comprehensive Cancer Network guidelines) is comparable to contemporary radiation therapy series. Morgan and colleagues [26] classified 390 of their 1833 radiation-treated patients (21%) as high risk on the basis of a PSA ≥ 20 ng/ml, clinical stage T3, or biopsy Gleason score ≥ 8 (definition 6 in our study). Similar criteria were applied by Zelefsky et al [27] who identified 103 of their 561 contemporary patients treated with intensity-modulated radiation therapy (18%) as being high risk. Acknowledging the bias inherent in comparing treatment results across selected retrospective cohorts and understanding that optimal local control for high-risk cancers can only be determined in a well-designed clinical trial, our study clearly supports the notion that surgery is an option for high-risk patients.
Several limitations of our study are worth noting. First, while MP represents a clinically meaningful end point, lack of consistency in timing of androgen-deprivation therapy for PSA relapse may have rendered our estimates of metastasis-free survival somewhat inaccurate. The true risk of metastatic disease following RP failure can be assessed only by clinical trials in which use of secondary cancer therapies has been dictated by a predefined protocol. However, in the absence of uniform guidelines and standardized practice with respect to timing of radiation therapy or hormonal therapy for RP failures, our estimates of secondary therapy and metastatic progression reflect, in fact, a "real-world" practice. Second, given improvements in surgical technique and changes in grading of tumors overtime [28] as well as the lead time bias associated with widespread PSA screening [29], our findings may not be perfectly applicable to a contemporary era, and the actual risk of MP and cancer-specific mortality for high-risk patients treated with RP may be even lower than reported herein. Third, we included patients who received androgen-deprivation therapy prior to RP on the basis of evidence from randomized trials showing that a short course of hormonal therapy before surgery did not impact PSA outcomes [10,11]. However, it has also been suggested that a short course of hormonal therapy early in the course of disease may attenuate the response to salvage androgen-deprivation therapy administered after disease progression [30]. Thus, the true impact of neoadjuvant hormonal therapy prior to RP on the risk of prostate cancer[en]specific mortality requires further investigation. Lastly, because most prostate cancer deaths occur more than 10 yr after initial treatment, further follow-up is required to provide more robust estimates of prostate cancer[en]specific mortality and to confirm the long-term efficacy of surgery for disease control in clinically high-risk patients.
5. Conclusions
Patients classified with high-risk prostate cancer by currently available definitions do not have a uniformly poor prognosis after RP. While high-risk criteria for PSA relapse are also associated with increased risk of secondary cancer therapy, metastatic progression, and death from prostate cancer, most of these men are alive and many remain free of additional therapy or metastatic disease 10 yr after surgery. The risk of prostate cancer[en]specific mortality within 10 yr of treatment is remarkably low, even for patients at the highest risk of recurrent disease on the basis of PSA, clinical stage, and tumor grade.
Acknowledgement
This study is supported in part by funds from National Cancer Institute grant CA 92629-05 SPORE in prostate cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
I hereby certify that all authors have made a substantial contribution to the information or material submitted for publication, and have read and approved the final manuscript. None of the authors has direct or indirect commercial financial incentive associated with publishing the article. The manuscript or portions thereof are not under consideration by another journal or electronic publication and have not been previously published.
Conflict of interests: None.
Disclosures: None.
Ofer Yossepowitch
Scott E Eggener
Angel M Serio
Brett S Carver
Fernando J Bianco Jr
Peter T Scardino
James A Eastham
Take-home message Patients classified with high-risk prostate cancer by currently available definitions do not have a uniformly poor prognosis after RP. Most of these men are alive and many remain free of additional therapy or metastatic disease long-term after surgery.
References
- 1.Meng MV, Elkin EP, Latini DM, Duchane J, Carroll PR. Treatment of patients with high risk localized prostate cancer: results from cancer of the prostate strategic urological research endeavor (CaPSURE) J Urol. 2005;173:1557[en]–1561. doi: 10.1097/01.ju.0000154610.81916.81. [DOI] [PubMed] [Google Scholar]
- 2.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981[en]–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleave M, Kelly WK. High-risk localized prostate cancer: a case for early chemotherapy. J Clin Oncol. 2005;23:8186[en]–8191. doi: 10.1200/JCO.2005.03.3068. [DOI] [PubMed] [Google Scholar]
- 4.Yossepowitch O, Eggener SE, Bianco FJ, Jr, et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. J Urol. 2007;178:493[en]–499. doi: 10.1016/j.juro.2007.03.105. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294:433[en]–439. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973[en]–3978. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 7.Bianco FJ, Jr, Scardino PT, Eastham JA. Radical prostatectomy: long-term cancer control and recovery of sexual and urinary function ("trifecta") Urology. 2005;66:83[en]–94. doi: 10.1016/j.urology.2005.06.116. [DOI] [PubMed] [Google Scholar]
- 8.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979[en]–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 9.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448[en]–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 10.Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112[en]–116. [PubMed] [Google Scholar]
- 11.Hurtado-coll A, Goldenberg SL, Klotz L, Gleave ME. Preoperative neoadjuvant androgen withdrawal therapy in prostate cancer: the Canadian experience. Urology. 2002;60:45[en]–51. doi: 10.1016/s0090-4295(02)01570-4. discussion. [DOI] [PubMed] [Google Scholar]
- 12.D'Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125[en]–135. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 13.Scardino P. Update: NCCN prostate cancer Clinical Practice Guidelines. J Natl Compr Canc Netw. 2005;3 suppl 1:S29[en]–S33. [PubMed] [Google Scholar]
- 14.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496[en]–509. [Google Scholar]
- 15.D'Amico AV, Whittington R, Malkowicz SB, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969[en]–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 16.Simmons MN, Stephenson AJ, Klein EA. Natural history of biochemical recurrence after radical prostatectomy: risk assessment for secondary therapy. Eur Urol. 2007;51:1175[en]–1184. doi: 10.1016/j.eururo.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Grossfeld GD, Li YP, Lubeck DP, Broering JM, Mehta SS, Carroll PR. Predictors of secondary cancer treatment in patients receiving local therapy for prostate cancer: data from cancer of the prostate strategic urologic research endeavor. J Urol. 2002;168:530[en]–535. [PubMed] [Google Scholar]
- 18.Tsai HK, Chen MH, McLeod DG, Carroll PR, Richie JP, D'Amico AV. Cancer-specific mortality after radiation therapy with short-course hormonal therapy or radical prostatectomy in men with localized, intermediate-risk to high-risk prostate cancer. Cancer. 2006;107:2597[en]–2603. doi: 10.1002/cncr.22279. [DOI] [PubMed] [Google Scholar]
- 19.D'Amico AV, Chen MH, Catalona WJ, Sun L, Roehl KA, Moul JW. Prostate cancer-specific mortality after radical prostatectomy or external beam radiation therapy in men with 1 or more high-risk factors. Cancer. 2007;110:56[en]–61. doi: 10.1002/cncr.22737. [DOI] [PubMed] [Google Scholar]
- 20.Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. J Natl Cancer Inst. 2006;98:715[en]–717. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephenson AJ, Scardino PT, Eastham JA, Bianco FJ, Jr, Kattan MW. Pretreatment nomogram predicting the long-term risk of metastatic progression of prostate cancer after radical prostatectomy. J Clin Oncol. 2005;23:4545. doi: 10.1200/JCO.2005.01.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gontero P, Marchioro G, Pisani R, et al. Is radical prostatectomy feasible in all cases of locally advanced non-bone metastatic prostate cancer? Results of a single-institution study. Eur Urol. 2007;51:922[en]–929. doi: 10.1016/j.eururo.2006.08.050. discussion 9[en]30. [DOI] [PubMed] [Google Scholar]
- 23.Frohmuller HG, Theiss M, Manseck A, Wirth MP. Survival and quality of life of patients with stage D1 (T1-3 pN1-2 M0) prostate cancer. Radical prostatectomy plus androgen deprivation versus androgen deprivation alone. Eur Urol. 1995;27:202[en]–206. doi: 10.1159/000475161. [DOI] [PubMed] [Google Scholar]
- 24.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360:103[en]–106. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 25.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma[em]long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61:1285[en]–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 26.Morgan PB, Hanlon AL, Horwitz EM, Buyyounouski MK, Uzzo RG, Pollack A. Timing of biochemical failure and distant metastatic disease for low-, intermediate-, and high-risk prostate cancer after radiotherapy. Cancer. 2007;110:68[en]–80. doi: 10.1002/cncr.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelefsky MJ, Chan H, Hunt M, Yamada Y, Shippy AM, Amols H. Long-term outcome of high dose intensity modulated radiation therapy for patients with clinically localized prostate cancer. J Urol. 2006;176:1415[en]–1419. doi: 10.1016/j.juro.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248[en]–1253. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
- 29.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868[en]–878. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 30.Pollack A, Grignon DJ, Heydon KH, et al. Prostate cancer DNA ploidy and response to salvage hormone therapy after radiotherapy with or without short- term total androgen blockade: an analysis of RTOG 8610. J Clin Oncol. 2003;21:1238[en]–1248. doi: 10.1200/JCO.2003.02.025. [DOI] [PubMed] [Google Scholar]


