Abstract
Previous studies demonstrated that the development of auditory conditioned stimulus (CS) input to the cerebellum may be a neural mechanism underlying the ontogenetic emergence of eyeblink conditioning in rats. The current study investigated the role of developmental changes in the projections of the cochlear nucleus (CN) in the ontogeny of eyeblink conditioning using electrical stimulation of the CN as a CS. Rat pups were implanted with a bipolar stimulating electrode in the CN and given six 100-trial training sessions with a 300 ms stimulation train in the CN paired with a 10 ms periorbital shock unconditioned stimulus (US) on postnatal days (P) 17–18 or 24–25. Control groups were given unpaired presentations of the CS and US. Rats in both age groups that received paired training showed significant increases in eyeblink conditioned responses across training relative to the unpaired groups. The rats trained on P24–25, however, showed stronger conditioning relative to the group trained on P17–18. Rats with missed electrodes in the inferior cerebellar peduncle or in the cerebellar cortex did not show conditioning. The findings suggest that developmental changes in the CN projections to the pons, inferior colliculus, or medial auditory thalamus may be a neural mechanism underlying the ontogeny of auditory eyeblink conditioning.
Keywords: learning, memory, cerebellum, auditory, eyelid conditioning
INTRODUCTION
The ontogenetic emergence of associative learning depends in part on the development of sensory systems (Rudy, 1992). Olfactory and gustatory Pavlovian conditioning have been demonstrated in fetal and newborn rats (Smotherman, 1982; Smotherman & Robinson, 1985). In contrast, Pavlovian conditioning with an auditory conditioned stimulus (CS) is not observed until postnatal day (P) 14 (Hyson & Rudy, 1984) and conditioning with a visual CS is not observed until P17 (Moye & Rudy, 1985). The age at which conditioning is first observed also depends on the behavioral response that is being measured (Hunt&Campbell, 1997; Sananes, Gaddy, & Campbell, 1988). For example, conditioned freezing with auditory or visual CSs is seen earlier than potentiated startle or eyeblink conditioning (Hunt & Campbell, 1997; Stanton, 2000; Stanton, Freeman, & Skelton, 1992). The findings of these behavioral analyses indicate that neurobiological approaches to the ontogeny of learning must take into account developmental changes in the sensory modality of the CS and the particular conditioned response that is measured.
Eyeblink conditioning is amenable to a developmental neurobiological approach in that the neural mechanisms underlying the conditioned and unconditioned blink are well characterized in adult animals and infant rats (Freeman & Nicholson, 2004; Nicholson & Freeman, 2004; Thompson, 2005). The cerebellum is essential for acquisition and retention of delay eyeblink conditioning (Thompson, 2005). Auditory CS information is conveyed to the cerebellum through the mossy fiber projection from the contralateral pontine nuclei (Hesslow, Svensson, & Ivarsson, 1999; Steinmetz, Lavond, & Thompson, 1989; Steinmetz et al., 1987; Steinmetz, Rosen, Chapman, Lavond,&Thompson, 1986; Steinmetz & Sengelaub, 1992; Tracy, Thompson, Krupa, & Thompson, 1998). The pontine nuclei receive auditory input from the cochlear nucleus, inferior colliculus, medial auditory thalamus, and auditory cortex (Campolattaro, Halverson, & Freeman, 2007). The auditory cortex is not necessary for acquisition or retention of eyeblink conditioning (Mauk & Thompson, 1987; Oakley & Russell, 1972, 1977). However, the cochlear nucleus, inferior colliculus, and medial auditory thalamus have been shown to be necessary for acquisition of auditory eyeblink conditioning (Campolattaro et al., 2007; Freeman, Halverson, & Hubbard, 2007; Halverson & Freeman, 2006).
Findings of recent neurobiological studies of the ontogeny of eyeblink conditioning demonstrate developmental changes in sensory inputs to the pontine nuclei. Microstimulation of the pontine nuclei as a CS results in robust learning in rats trained on P12–13 or P17–18 (Campolattaro & Freeman, 2007; Freeman, Rabinak, & Campolattaro, 2005b). The conditioning observed in rats trained on P17–18 does not differ from the conditioning seen at P24–25, suggesting that the mossy fiber projection to the cerebellum is capable of supporting conditioning with external stimuli but does not receive sufficient input from sensory nuclei. Moreover, neurons in the pontine nuclei show a substantial developmental change in the magnitude of tone-evoked activity (Freeman & Muckler, 2003). The findings of these neurobiological studies of developmental changes in cerebellar learning suggest that ontogenetic changes in auditory input to the pontine nuclei may be a key factor underlying the ontogeny of eyeblink conditioning.
The current study is the first step in a neurobiological analysis of the development of particular auditory inputs to the pontine nuclei. Demonstrations of auditory conditioning in rats as young as P14 indicate that the CN and some of its efferent targets are fully functional before eyeblink conditioning emerges between P20 and P24 (Hyson & Rudy, 1984; Stanton, Fox,&Carter, 1998). Developmental changes in the CN projection to the pontine nuclei and medial auditory thalamus may, however, develop later than P14 and play a role in the ontogeny of auditory eyeblink conditioning.
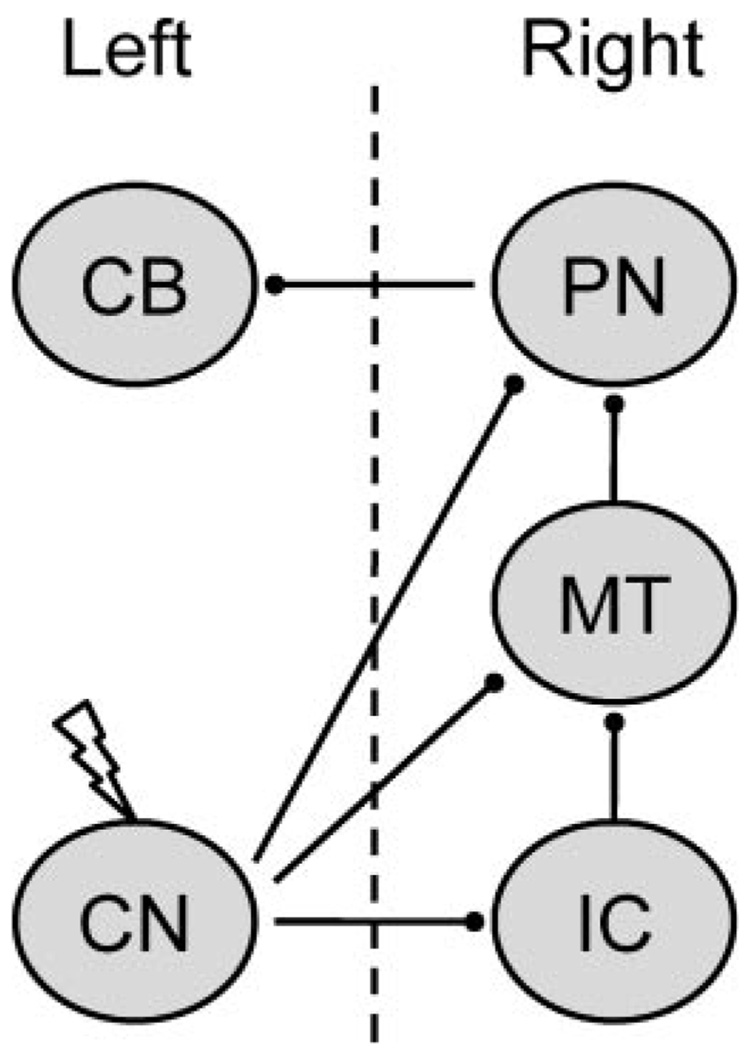
Microstimulation of the CN was used as a CS in the current study to assess developmental changes in projections to other auditory nuclei or the pontine nuclei. Rat pups were given paired or unpaired presentations of CN stimulation as the CS with a shock US in the left periorbital area on P17–18 or P24–25. The stimulation CS was applied to the left CN, which projects to the right pontine nuclei, right inferior colliculus (directly and indirectly), and right medial auditory thalamus (Fig. 1). Inferior collicular and medial auditory thalamic neurons send ipsilateral projections to the pontine nuclei. The pontine nuclei then project back across the midline to the left cerebellum, which controls the left eyeblink CR (Fig. 1). Conditioned response percentage, amplitude, and peak latency were measured during six 100-trial training sessions.
FIGURE 1.
Schematic diagram of the experimental design. Electrical stimulation was delivered to the left cochlear nucleus (CN), which projects to the right inferior colliculus (IC), medial auditory thalamus (MT), and basilar pontine nuclei (PN). The right PN then projects to the left cerebellum. The left cerebellum is necessary for conditioning of the left eye (Freeman, Halverson, & Poremba, 2005a). The dotted line depicts the midline.
METHODS
Subjects
The subjects were 60 Long-Evans rat pups. Twenty-seven pups had accurately placed electrodes and were trained on P17–18 (paired n = 11, unpaired n = 5) or P24–25 (paired n = 7, unpaired n = 4). Pups from the four experimental groups were drawn from 18 litters. The rats were housed in the animal colony in Spence Laboratories of Psychology at the University of Iowa. The rats were maintained on a 12/12-hr light/dark cycle with light onset at 7 a.m. Training sessions occurred between 7 a.m. and 7 p.m.
Surgery
The pups (P16 and P23) were given i.p. injections of ketamine (100 mg/kg), xylazine (5 mg/kg), and atropine (0.8 mg/kg). The pup’s head was positioned and held securely in an infant stereotaxic apparatus and the skull surface was aligned. Differential electromyographic (EMG) electrodes were implanted in the left upper eyelid and a ground electrode was connected to one of two skull hooks. The electrode and ground wires were soldered to gold pins in a plastic connector, which was secured to the skull by a skull hook and dental acrylic. The second skull hook was secured to the skull slightly anterior to lambda. A bipolar stimulating electrode was then implanted into or just dorsal to the left cochlear nucleus. The electrode consisted of two insulated stainless steel wires (50 µm) in a plastic connector. The stereotaxic coordinates for the CN were taken from lambda (P16/P23: −1.1/−1.1 anterior, +3.0/+3.4 medial-lateral, −6.0/ −6.0 dorsal-ventral). Once the electrode was in place it was cemented with dental acrylic covering the entire length of the electrode above the skull surface including the plastic connector. A bipolar stimulating electrode used for delivering the US was implanted subdermally, immediately caudal to the left eye. This bipolar electrode was also encased by a plastic connector, which was secured by dental acrylic. Sutures closed the surgical site on both sides of the head stage. Ketofen (5 mg/kg), an analgesic, was administered at the end of surgery.
Conditioning Apparatus
The conditioning apparatus consisted of a small-animal sound attenuation chamber (BRS/LVE, Laurel, MD) with a small-animal operant chamber (BRS/LVE) contained inside. The rats were kept in the operant chamber during conditioning. Lightweight cables with connectors for the EMG, US, and CS electrodes were attached to a commutator. The back wall of the sound-attenuation chamber was equipped with a small house light that stayed on during conditioning sessions. The electrode leads from the rat’s head stage were connected to peripheral equipment and a desktop computer. Computer software controlled the delivery of stimuli and the recording of eyelid EMG activity (JSA Designs, Raleigh, NC). The US was delivered through a stimulus isolator (model number 365A; World Precision Instruments, Sarasota, FL). EMG activity was recorded differentially, filtered (500–5,000 Hz), amplified (2,000 X), and integrated (time constant = 20 ms). Cochlear nucleus stimulation was triggered through a programmable stimulator, (Master 8, A.M.P.I., Jerusalem, Israel), which controlled signal input to a stimulus isolator (model number 365A; World Precision Instruments), which delivered the electrical stimulation.
Cochlear Nucleus Microstimulation
Electrical stimulation of the cochlear nucleus functioned as the CS, which was administered in a 200 Hz train of 0.1 ms biphasic pulses for 300 ms. A stimulation threshold for the CS was found before training by setting the stimulating current to 50 µA, and either increasing or decreasing the current in 5 µA increments, until a slight movement was detected (Freeman et al., 2005b; Tracy et al., 1998). Observable movements included, but were not limited to, pinna movement, eyeblinks, head turns, and startle-like responses. The level of stimulation during training was set to half the threshold intensity.
Eyeblink Conditioning
During paired training rat pups in both age groups were given six training sessions, three sessions per day. The paired training sessions consisted of 100 trials, each with 90 trials of the stimulation CS paired with the shock US (10 ms, 3.0 mA) and 10 stimulation CS-alone trials, occurring on every tenth trial. The CS-alone trials were included to assess CR amplitude and latency uncontaminated by URs. The interstimulus interval for paired trials was 290 ms. Trials were separated by a variable inter-trial interval (ITI) that averaged 30 s. During unpaired training rat pups in both age groups were given 100 presentations of the stimulation CS and 90 presentations of the US separated by a variable ITI that averaged 15 s. All other aspects of this procedure were the same as paired training.
Behavioral data were examined from computer records of EMG responses during a 1 s trial epoch. Eyelid EMG activity that exceeded a threshold of 0.4 arbitrary units (integrated EMG activity) above the mean of the pre-CS activity was scored as a response. Responses that occurred during the first 80 ms of the CS were scored as alpha (startle) responses. During CS-US trials, responses that occurred between the end of the alpha period and the onset of the US were scored as CRs. During CS-alone trials, responses that occurred between the end of the alpha period and the end of the CS were scored as CRs. Responses that occurred after onset of the US were scored as URs. Conditioned response amplitude and latency were measured from CS-alone test trials in which a CR occurred.
Histology
After training was completed, rats were euthanized with a lethal injection of sodium pentobarbital (120 mg/kg) and transcardially perfused with ~100 ml of physiological saline, followed by ~300 ml of 3% formalin. The brains were post-fixed in formalin for 2 days and then put in a solution of 10% sucrose in PBS before sectioning. The brains were sectioned at 50 µm with a sliding microtome. Sections were then stained with thionin. The electrode locations were determined by examining serial sections.
RESULTS
Electrode Placement
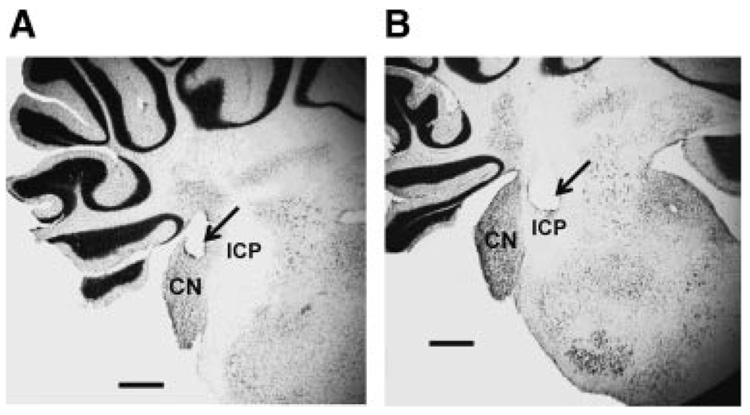
Accurate placement of the stimulation electrodes was difficult due to the small size of the cochlear nucleus in developing rats. The “hit rate” for placing electrodes completely within the cochlear nucleus was 45%. Figure 2 shows a representative image of an electrode placement that was an accurate placement (left, A) and an image of a typical missed electrode placement (right, B). Most of the missed electrodes were either in the inferior cerebellar peduncle (Fig. 2B) or just outside of the cerebellar cortex. Electrodes that were partially in the inferior cerebellar peduncle and partially in the cochlear nucleus were classified as missed placements.
FIGURE 2.
Coronal sections of the rat cochlear nucleus (CN) showing an accurate (A) and misplaced (B) electrode placement (arrow). ICP, inferior cerebellar peduncle. The scale bar indicated 0.5 mm.
Conditioning
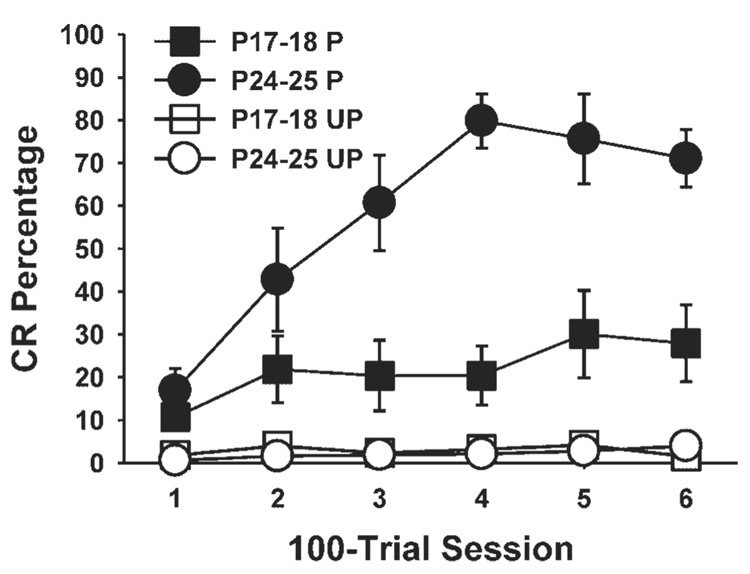
Microstimulation of the cochlear nucleus was an effective CS for eyeblink conditioning in rats trained on P24–25. Pups that received paired presentations of the cochlear nucleus CS paired with the US on P24–25 showed an increase in the percentage of conditioned responses across training sessions relative to the unpaired control group (Fig. 3). The pups that received paired training on P17–18 showed a more modest increase in CR percentage relative to the group trained on P24–25, but their performance differed significantly from the unpaired group (Fig. 3). Both age groups, therefore, showed associative learning with cochlear nucleus microstimulation as the CS.
FIGURE 3.
Mean (±standard error) conditioned response (CR) percentage for rat pups given paired (black symbols) or unpaired (white symbols) presentations of cochlear nucleus stimulation as the conditioned stimulus and the unconditioned stimulus on postnatal days (P) 17–18 (squares) or P24–25 (circles). The amount of learning in each paired group is determined by the increase in responding across training sessions and greater responding than the unpaired control groups.
A repeated measures ANOVA confirmed these observations with a significant interaction of the Age (P17–18 vs. P24–25) and Condition (paired vs. unpaired) variables, F(1,23) = 7.95, p = 0.01. There was also a marginal interaction of the Age, Condition, and Session variables, F(5, 115) = 2.18, p = 0.06. Post hoc tests of this interaction indicated that the group given paired training on P24–25 had a higher CR percentage than the group given paired training on P17–18 during sessions 3–5 (p<0.05). The group given paired training on P24–25 differed from their unpaired control group during sessions 2–6 (p<0.05). The group given paired training on P17– 18 differed from their unpaired controls only during sessions 5 and 6 (p<0.05).
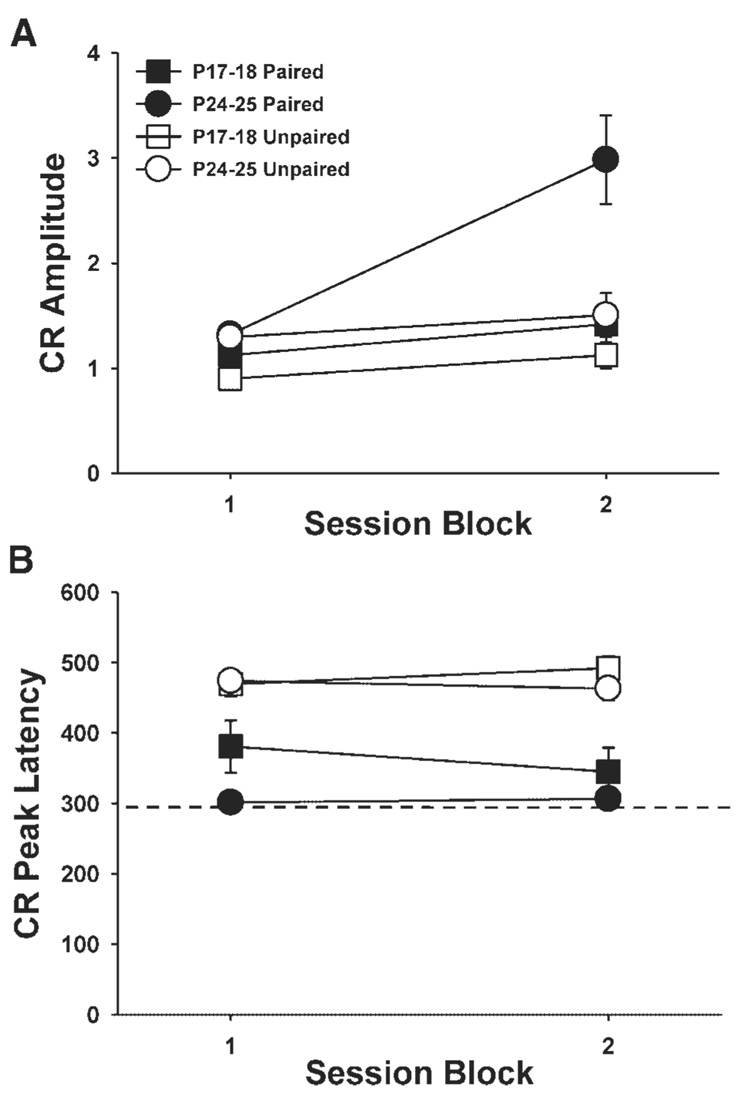
The CR amplitude data were collapsed across sessions 1–3 and 4–6 because some of the pups did not produce CRs during CS-alone test trials in the groups given paired training. We were able to include CR amplitude data for all subjects by collapsing data into three-session blocks. An age-related increase in CR amplitude was observed in the groups given paired training. The pups given paired training on P24–25 showed higher amplitude CRs across sessions relative to the pups given paired training on P17–18, which did not differ from their unpaired controls (Fig. 4A). An ANOVA revealed an interaction of the Age, Condition, and Session (session 1–3 vs. sessions 4–6) variables, F(1,23) = 6.61, p = 0.02. Post hoc tests indicated that the group given paired training on P24–25 differed significantly from the group given paired training on P17–18 and the unpaired groups on sessions 4–6 (p<0.05).
FIGURE 4.
(A) Mean (±standard error) conditioned response (CR) amplitude for rat pups given paired (black symbols) or unpaired (white symbols) presentations of cochlear nucleus stimulation as the conditioned stimulus (CS) and the unconditioned stimulus (US) on postnatal days (P) 17–18 (squares) or P24–25 (circles). (B) Mean (± standard error) CR peak latency for the same groups as described above for A. The dashed line indicates the onset time of the US. The data are displayed in blocks of three sessions (sessions 1–3 and 4–6). Response amplitudes and latencies were measured from CRs that occurred during CS-alone test trials.
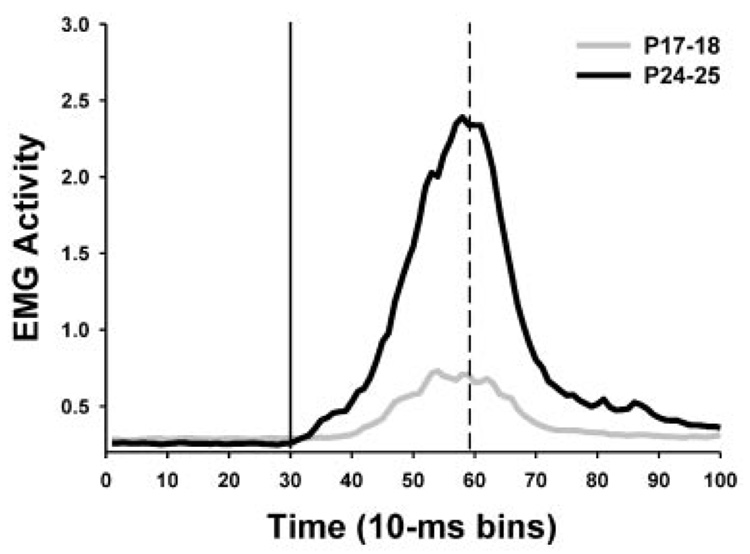
The peak latency of the CR is a standard measure of timing. In adults, the peak latency of the CR typically occurs at the onset time of the US. Conditioned response latency data were collapsed across sessions 1–3 and 4–6 as described above for the CR amplitude data (Fig. 4B). Pups in the paired groups showed shorter latency CRs than pups in the unpaired group, F(1, 23) = 49.99, p<0.001. When the paired groups were compared without the unpaired groups, pups trained on P17–18 showed longer latency CRs relative to the group trained on P24–25, which was reflected in a significant main effect of the Age variable, F(1,16) = 5.06, p = 0.04. As in adult rats, the peak latency of the CR in pups trained on P24–25 occurred within 10 ms of the US onset (mean peak latency for sessions 1–3 and 4–6 were 301.3 and 306.4 ms, respectively). The peak CR latency for the pup trained on P17–18 occurred after US onset (mean peak latency for sessions 1–3 and 4–6 were 380.8 and 345.0 ms, respectively). Figure 5 shows average eyelid EMG responses for pups given paired training on P17–18 and P24–25 during the entire trial epoch for CS-alone test trials.
FIGURE 5.
Averaged eyelid EMG activity for pups given paired training on P17–18 (gray line plot) and P24–25 (black line plot) during the entire trial epoch for all CS-alone test trials for the last training session. The solid vertical line indicates the onset of the conditioned stimulus. The dashed line indicates the onset of the unconditioned stimulus.
DISCUSSION
Rats that were given CN stimulation as a conditioned stimulus paired with a periorbital shock unconditioned stimulus showed rapid acquisition of eyeblink conditioning on P24–25. Rats trained on P17–18 showed less conditioning than the group trained on P24–25. However, both age groups given paired training showed significantly higher levels of conditioned responses than their respective unpaired control groups, indicating that associative learning was established at P17–18 and P24–25. An age-related increase in conditioned response amplitude and a decrease in CR latency were also observed in the pups given paired training.
The rate of acquisition in pups trained on P24–25 was very similar to the rate of conditioning seen with a tone conditioned stimulus (Nicholson & Freeman, 2004). Conditioning in adult rats, however, is faster when using cochlear nucleus stimulation relative to a tone conditioned stimulus (Halverson & Freeman, 2008, unpublished work). Adult rats typically reach asymptotic conditioning within the first 100-trial training session, whereas the pups trained on P24–25 in the current study did not reach asymptotic conditioning until the fourth session. The difference in relative rate of conditioning in adult rats and pups trained on P24–25 suggests that the cochlear nucleus projections to the pontine nucleus, inferior colliculus, and thalamus may continue to develop past P25.
The ontogenetic change in learning using cochlear nucleus stimulation as the conditioned stimulus indicates that developmental changes in efferent targets of the cochlear nucleus may play a role in the ontogeny of eyeblink conditioning. A developmental change in the efficacy of the monosynaptic projection from the cochlear nucleus to the pons could be a factor in the ontogeny of eyeblink conditioning by influencing auditory input to the cerebellum. As mentioned earlier, however, recent studies indicate that the auditory conditioned stimulus pathway in eyeblink conditioning includes the medial auditory thalamus and its projections to the pontine nuclei. Developmental changes in medial auditory thalamic input to the pontine nuclei might therefore also be a factor in the ontogeny of auditory eyeblink conditioning. In support of this hypothesis, we recently found an age-related increase in the efficacy of medial auditory thalamic stimulation as a conditioned stimulus in rats (Campolattaro & Freeman, 2006). The findings of this study suggest that the development of the thalamic projection to the pons may be an ontogenetic bottleneck for the flow of auditory input to the cerebellum during eyeblink conditioning. It is important to note, however, that the findings of the thalamic stimulation study do not rule out the influence of developmental changes in the projection from the cochlear nucleus to the pons, direct and indirect projections from the cochlear nucleus to the inferior colliculus, or the inferior colliculus projections to the thalamus.
Not all of the multisynaptic efferent pathways from the cochlear nucleus are incapable of supporting associative learning at P17. Conditioned freezing with an auditory CS has been demonstrated as early as P16 (Hunt & Campbell, 1997), suggesting that the cochlear nucleus projections that ultimately reach the amygdala are functional at this age. However, conditioned changes in heart rate do not emerge until P21 and fear potentiated startle does not emerge until P23 (Hunt & Campbell, 1997; Richardson, Wang,&Campbell, 1995), which may indicate that neural pathways efferent to the amygdala that are necessary for producing different fear responses continue to develop after P16. On the other hand, retrieval of fear conditioning is seen only in response systems that were available to the pups at the time of learning (Barnet & Hunt, 2006; Richardson & Fan, 2002; Richardson, Paxinos, & Lee, 2000; Yap, Stapinski, & Richardson, 2005). This finding suggests that the ontogeny of different conditioned fear responses depends, in part, on the development of neural plasticity mechanisms that are efferent to the amygdala (Richardson et al., 2000; Weber & Richardson, 2004). Thus, unlike eyeblink conditioning, the ontogeny of fear conditioning is not due to a developmental bottleneck in sensory input from the auditory CS pathway.
The findings of the current study suggest that developmental changes in projections from the cochlear nucleus to other auditory nuclei or the pontine nuclei play a role in the ontogenetic emergence of auditory eyeblink conditioning. The developmental increase in auditory input to the pontine nuclei causes an increase in the strength of CS-related excitatory input to the cerebellar cortex and nuclei during eyeblink conditioning. Weaker auditory input to the pontine nuclei in younger rats results in weaker input to the cerebellum (Nicholson & Freeman, 2004). As a result, induction of activity-dependent synaptic plasticity within the cerebellum during eyeblink conditioning is limited by the development of auditory input to the pontine nuclei, which limits the rate and magnitude of conditioning (Freeman & Nicholson, 2004). Current studies are examining connectivity between various auditory nuclei and the basilar pons in developing rats to identify sites of ontogenetic change in auditory input to the cerebellum. Other studies are beginning to extend the developmental analysis of the neural mechanisms underlying eyeblink conditioning to visual and somatosensory CS pathways.
Acknowledgments
Contract grant sponsor: National Institute for Neurological Disorders and Stroke
Contract grant number: NS38890
Footnotes
Published online in Wiley InterScience (www.interscience.wiley.com).
REFERENCES
- Barnet RC, Hunt PS. The expression of fear-potentiated startle during development: Integration of learning and response systems. Behavioral Neuroscience. 2006;120:861–872. doi: 10.1037/0735-7044.120.4.861. [DOI] [PubMed] [Google Scholar]
- Campolattaro MM, Freeman JH. Development of eyeblink conditioning: Stimulation of the medial auditory thalamus as a conditioned stimulus. Developmental Psychobiology. 2006;48:607. [Google Scholar]
- Campolattaro MM, Freeman JH. Pontine stimulation is a sufficient CS for eyeblink conditioning in 12-day-old rats. Developmental Psychobiology. 2007;49:724. [Google Scholar]
- Campolattaro MM, Halverson HE, Freeman JH. Medial auditory thalamic stimulation as a conditioned stimulus for eyeblink conditioning in rats. Learning and Memory. 2007;14:152–159. doi: 10.1101/lm.465507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Halverson HE, Hubbard EM. Inferior colliculus lesions impair eyeblink conditioning in rats. Learning and Memory. 2007;14:842–846. doi: 10.1101/lm.716107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Halverson HE, Poremba A. Differential effects of cerebellar inactivation on eyeblink conditioned excitation and inhibition. Journal of Neuroscience. 2005a;25:889–895. doi: 10.1523/JNEUROSCI.4534-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Muckler AS. Developmental changes in eyeblink conditioning and neuronal activity in the pontine nuclei. Learning and Memory. 2003;10:337–345. doi: 10.1101/lm.63703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Nicholson DA. Developmental changes in the neural mechanisms of eyeblink conditioning. Behavioral and Cognitive Neuroscience Reviews. 2004;3:3–13. doi: 10.1177/1534582304265865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman JH, Jr, Rabinak CA, Campolattaro MM. Pontine stimulation overcomes developmental limitations in the neural mechanisms of eyeblink conditioning. Learning and Memory. 2005b;12:255–259. doi: 10.1101/lm.91105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson HE, Freeman JH. Medial auditory thalamic nuclei are necessary for eyeblink conditioning. Behavioral Neuroscience. 2006;120:880–887. doi: 10.1037/0735-7044.120.4.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesslow G, Svensson P, Ivarsson M. Learned movements elicited by direct stimulation of cerebellar mossy fiber afferents. Neuron. 1999;24:179–185. doi: 10.1016/s0896-6273(00)80831-4. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Campbell BA. Developmental dissociation of the components of conditioned fear. In: Bouton ME, Fanselow MS, editors. Learning, motivation, and cognition: The functional behaviorism of Robert C. Bolles. Washington, DC: American Psychological Association; 1997. pp. 53–74. [Google Scholar]
- Hyson RL, Rudy JW. Ontogenesis of learning: II. Variation in the rat's reflexive and learned responses to acoustic stimulation. Developmental Psychobiology. 1984;17:263–283. doi: 10.1002/dev.420170307. [DOI] [PubMed] [Google Scholar]
- Mauk MD, Thompson RF. Retention of classically conditioned eyelid responses following acute decerebration. Brain Research. 1987;403:89–95. doi: 10.1016/0006-8993(87)90126-0. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of learning: VI. Learned and unlearned responses to visual stimulation in the infant hooded rat. Developmental Psychobiology. 1985;18:395–409. doi: 10.1002/dev.420180505. [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Freeman JH., Jr Developmental changes in eyeblink conditioning and simple spike activity in the cerebellar cortex. Developmental Psychobiology. 2004;44:45–57. doi: 10.1002/dev.10149. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Russell IS. Neocortical lesions and Pavlovian conditioning. Physiology and Behavior. 1972;8:915–926. doi: 10.1016/0031-9384(72)90305-8. [DOI] [PubMed] [Google Scholar]
- Oakley DA, Russell IS. Subcortical storage of Pavlovian conditioning in the rabbit. Physiology and Behavior. 1977;18:931–937. doi: 10.1016/0031-9384(77)90203-7. [DOI] [PubMed] [Google Scholar]
- Richardson R, Fan M. Behavioral expression of learned fear in rats is appropriate to their age at training, not their age at testing. Animal Learning and Behavior. 2002;30:394–404. doi: 10.3758/bf03195964. [DOI] [PubMed] [Google Scholar]
- Richardson R, Paxinos G, Lee J. The ontogeny of conditioned odor potentiation of startle. Behavioral Neuroscience. 2000;114:1167–1173. [PubMed] [Google Scholar]
- Richardson R, Wang P, Campbell BA. Delayed development of conditioned heart rate responses to auditory stimuli in the rat. Developmental Psychobiology. 1995;28:221–238. doi: 10.1002/dev.420280404. [DOI] [PubMed] [Google Scholar]
- Rudy JW. Development of learning: From elemental to configural associative networks. Advances in Infancy Research. 1992;7:247–289. [Google Scholar]
- Sananes CB, Gaddy JR, Campbell BA. Ontogeny of conditioned heart rate to an olfactory stimulus. Developmental Psychobiology. 1988;21:117–133. doi: 10.1002/dev.420210202. [DOI] [PubMed] [Google Scholar]
- Smotherman WP. Odor aversion learning by the rat fetus. Physiology and Behavior. 1982;29:769–771. doi: 10.1016/0031-9384(82)90322-5. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. The rat fetus in its environment: Behavioral adjustments to novel, familiar, aversive, and conditioned stimuli presented in utero. Behavioral Neuroscience. 1985;99:521–530. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- Stanton ME. Multiple memory systems, development and conditioning. Behavioural Brain Research. 2000;110:25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Fox GD, Carter CS. Ontogeny of the conditioned eyeblink response in rats: Acquisition or expression? Neuropharmacology. 1998;37:623–632. doi: 10.1016/s0028-3908(98)00072-0. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Freeman JH, Jr, Skelton RW. Eyeblink conditioning in the developing rat. Behavioral Neuroscience. 1992;106:657–665. doi: 10.1037//0735-7044.106.4.657. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Lavond DG, Thompson RF. Classical conditioning in rabbits using pontine nucleus stimulation as a conditioned stimulus and inferior olive stimulation as an unconditioned stimulus. Synapse. 1989;3:225–233. doi: 10.1002/syn.890030308. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Logan CG, Rosen DJ, Thompson JK, Lavond DG, Thompson RF. Initial localization of the acoustic conditioned stimulus projection system to the cerebellum essential for classical eyelid conditioning. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:3531–3535. doi: 10.1073/pnas.84.10.3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz JE, Rosen DJ, Chapman PF, Lavond DG, Thompson RF. Classical conditioning of the rabbit eyelid response with a mossy-fiber stimulation CS: I. Pontine nuclei and middle cerebellar peduncle stimulation. Behavioral Neuroscience. 1986;100:878–887. doi: 10.1037//0735-7044.100.6.878. [DOI] [PubMed] [Google Scholar]
- Steinmetz JE, Sengelaub DR. Possible conditioned stimulus pathway for classical eyelid conditioning in rabbits. I. Anatomical evidence for direct projections from the pontine nuclei to the cerebellar interpositus nucleus. Behavioral and Neural Biology. 1992;57:103–115. doi: 10.1016/0163-1047(92)90593-s. [DOI] [PubMed] [Google Scholar]
- Thompson RF. In search of memory traces. Annual Review of Psychology. 2005;56:1–23. doi: 10.1146/annurev.psych.56.091103.070239. [DOI] [PubMed] [Google Scholar]
- Tracy JA, Thompson JK, Krupa DJ, Thompson RF. Evidence of plasticity in the pontocerebellar conditioned stimulus pathway during classical conditioning of the eyeblink response in the rabbit. Behavioral Neuroscience. 1998;112:267–285. doi: 10.1037//0735-7044.112.2.267. [DOI] [PubMed] [Google Scholar]
- Weber M, Richardson R. Pretraining inactivation of the caudal pontine reticular nucleus impairs the acquisition of conditioned fear-potentiated startle to an odor, but not a light. Behavioral Neuroscience. 2004;118:965–974. doi: 10.1037/0735-7044.118.5.965. [DOI] [PubMed] [Google Scholar]
- Yap CS, Stapinski L, Richardson R. Behavioral expression of learned fear: Updating of early memories. Behavioral Neuroscience. 2005;119:1467–1476. doi: 10.1037/0735-7044.119.6.1467. [DOI] [PubMed] [Google Scholar]