Abstract
The goal of this research was to determine the effects of different growth factors on the survival and differentiation of murine embryonic stem cell derived neural progenitor cells (ESNPCs) seeded inside of fibrin scaffolds. Embryoid bodies (EBs) were cultured for 8 days in suspension, retinoic acid was applied for the final 4 days to induce ESNPC formation, and then the EBs were seeded inside of 3 dimensional (3D) fibrin scaffolds. Scaffolds were cultured in the presence of media containing different doses of the following growth factors: neurotrophin-3 (NT-3), basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF-AA), ciliary neurotrophic factor (CNTF), and sonic hedgehog (Shh). The cell phenotypes were characterized using fluorescence activated cell sorting (FACS) and immunohistochemistry after 14 days of culture. Cell viability was also assessed at this time point. Shh (10 ng/mL) and NT-3 (25 ng/mL) produced the largest fractions of neurons and oligodendrocytes while PDGF (2 and 10 ng/mL) and bFGF (10 ng/mL) produced an increase in cell viability after 14 days of culture. Combinations of growth factors were tested based on the results of the individual growth factor studies to determine their effect on cell differentiation. The incorporation of ESNPCs and growth factors into fibrin scaffolds may serve as potential treatment for spinal cord injury (SCI).
Keywords: three dimensional culture, hydrogel, neural tissue engineering, lineage restricted precursor cells
Introduction
The potential of stem cells to create engineered tissues as a replacement for those lost to injury has been well documented [1, 2]. Due to their pluripotency, mouse embryonic stem (ES) cells have the capacity to produce the cell types found in all three germ layers, including those found in the central nervous system (CNS) [3, 4]. Thus, ES cells provide a potential means of repopulating cells lost due to spinal cord injury (SCI). Culturing mouse ES cells in suspension as embryoid bodies (EBs) using the 4−/4+ retinoic acid treatment protocol developed by Bain et al. produces embryonic stem cell derived neural progenitor cells (ESNPCs). These cells can differentiate to resemble normal neurons both morphologically and physiologically after additional culture [5, 6]. Cells formed using the 4−/4+ protocol were injected into a chronic SCI model, and demonstrated the ability to promote a modest increase in functional recovery [7]. However, the percentage of cells that survived was low (~10%) and few of the surviving cells differentiated into neurons.
To address these issues, three dimensional (3D) biomaterial scaffolds could be used to increase the viability of transplanted cells by providing a permissive environment for growth and proliferation as well as to promote the differentiation of ES cells into specific cell types based on the properties of the scaffold [8–14]. Fibrin scaffolds have been characterized for use in tissue engineering applications involving stem cells because they promote cell adhesion and migration and are approved for clinical use as surgical sealants [11, 15–20]. Recent work has determined the optimal conditions for seeding ESNPCs into 3D fibrin scaffolds [21]. Additionally, fibrin scaffolds containing controlled release systems for growth factor delivery show promise as a potential treatment for SCI and could potentially be used as scaffolds for cell transplantation [22–26].
Many different growth factors can be used to influence ESNPCs to differentiate into one of three mature neural phenotypes of the CNS (neurons, oligodendrocytes, and astrocytes). For this particular study, growth factors were selected for study based on two criteria: their ability to promote ES cell differentiation and survival and their potential as a therapeutic for SCI. Neurotrophins, such as neurotrophin-3 (NT-3), have been shown to increase ES cell survival and promote differentiation into neural structures in 3D culture [10, 27, 28]. NT-3 delivery from biomaterial scaffolds promotes neural fiber sprouting and in some cases, increases in functional recovery after SCI [22, 23, 29]. Other growth factors, such as platelet derived growth factor (PDGF), ciliary neurotrophic factor (CNTF), and sonic hedgehog (Shh) have been shown to play important roles in stem cell survival and differentiation into specific neural lineages [28, 30–34]. PDGF plays an important role in oligodendrocyte precursor proliferation and promotes differentiation of human ES cells into oligodendrocytes [35, 36]. Treatment of SCI with PDGF results in increased angiogenesis to the wound site, which can be beneficial [37–39]. CNTF promotes differentiation of ES cells into astrocytes as well as the survival of mature neurons [28, 34, 40, 41]. The experimental data on the efficacy of CNTF as a treatment for SCI has been conflicting, with some studies suggesting that it can promote increased migration of neurons and astrocytes into the injury site while another study suggests that neutralization of CNTF results in a lessening of the glial scar [42, 43]. Shh can stimulate differentiation of ES cells into motor neurons when used in conjunction with retinoic acid [32, 33, 44]. Injection of Shh into the site of SCI has been shown to promote proliferation of neural precursors and an increase in oligodendrocyte progenitors cells [31]. Further work showed that implanting oligodendrocyte precursor cells along with Shh into a contusion model of SCI resulted in the sparing of white matter along with functional recovery [45]. Basic fibroblast growth factor (bFGF) can promote ES and neural stem cell proliferation [46–48]. Additionally, bFGF plays many roles in the injured spinal cord, including promoting neural progenitor proliferation, neuronal survival, and enhancing functional recovery [49–52]. Other growth factors, such as epidermal growth factor (EGF) and bone morphogenetic protein (BMP), were considered due their ability to affect stem cell differentiation [53–56]. However, when used a treatment for SCI, EGF did not promote functional recovery and was not included in the present study [57, 58]. BMP promoted astrocyte formation in vivo when used as a treatment for SCI, making it undesirable for further study [59].
This work investigated the response of mouse ESNPCs seeded inside of fibrin scaffolds to these five different growth factors (NT-3, bFGF, CNTF, PDGF, and Shh). The influence of these growth factors was studied over a range of concentrations to determine an appropriate dose for each growth factor and certain growth factors were tested in combination to determine the effect on ESNPC differentiation. For treatment of SCI, it is important to generate neurons and oligodendrocytes from ESNPCs to restore those cells lost to injury while minimizing the amount of astrocytes, which can contribute to the glial scar. The effect of growth factors on cell viability was also assessed to determine what growth factors could help increase cell viability after implantation. This study provides insight into which growth factor combinations promote the differentiation of ESNPCs into neural tissue consisting of neurons and oligodendrocytes to be used as treatment for SCI.
Materials and Methods
Embryonic Stem Cell Culture and Embryoid Body Formation
RW4 mouse ES cells obtained from D. Gottlieb were cultured in T25 flasks (Fisher, Pittsburgh, PA, http://www.fishersci.com) coated with a 0.1% gelatin solution (Sigma, Saint Louis, MO, http://www.sigmaaldrich) in the presence of 1000 U/mL leukemia inhibitory factor (LIF; Chemicon, Temecula, CA, http://www.chemicon.com) and 10−4 M β-mercaptoethanol (BME; Invitrogen, Grand Island, NY, http://www.invitrogen.com) to maintain their undifferentiated state. These cells were grown in complete media consisting of Dubecco’s modified eagle media (DMEM) (Invitrogen) supplemented with 10% newborn calf serum (NBCS; Invitrogen), 10% fetal bovine serum (FBS, Invitrogen), and 0.3 M of each of the following nucleosides: adenosine, guanosine, cytosine, thymidine, and uridine (Sigma) and passaged at a ratio of 1:5 every two days.
Undifferentiated ES cells were induced to form embryoid bodies (EBs) containing ESNPCs using the 4−/4+ retinoic acid treatment protocol [5]. ES cells were cultured in 100 mm Petri dishes (Fisher) coated with a 0.1% agar solution (MidSci, Saint Louis, MO, http://midsci.com/) in complete media in the absence of LIF and BME for 4 days. 500 nM retinoic acid (Sigma) was then added to the complete media for the final 4 days of culture. The media was changed every other day during this eight day process.
RNA preparation and Reverse Transcription Polymerase Chain Reaction (RT-PCR) to Determine Receptor Expression in 4−/4+ Embryoid Bodies
Using an RNeasy Mini kit, RNA was isolated from 4−/4+ EBs homogenized by Qiashredder (both from Qiagen, Valencia, CA, www.qiagen.com) according to manufacturer’s instructions. RT-PCR was performed using a One Step RT-PCR kit (Qiagen) which contains both reverse transcriptase to synthesize cDNA from the RNA isolated and DNA polymerase for the PCR. RT-PCR conditions consisted of a 30 min step at 50°C to allow the reverse transcriptase activity followed by 15 min at 95°C to deactivate the reverse transcriptase and activate the Taq polymerase present in the enzyme mixture. The PCR process consisted of 6 s at 95°C (denaturing step), 30 s at the annealing temperature (55°C for growth factors and 60°C for the receptors), and a 45 s step at 72°C for extension with all steps being repeated for 30 cycles. A final extension step lasted 10 min at 72°C.
Primer sequences were as follows with the expected product length given in parentheses: NT-3, sense 5’ TGC AAC GGA CAC AGA GCT AC 3’, anitsense 5’CGG ACA GTT TGC GAA GT 3’ (420 bp); TrkC receptor (NT-3), sense 5’ TGC CTG ATG TGG ACT GGA TA 3’, antisense 5’ GTG GGC TTT TTG AAG AGC AG 3’ (394 bp, [60]); bFGF, sense 5’ACC AGG CCA CAA GGA C 3’, antisense 5’TCA GCT CTT AGC AGA CAT TGG A 3’ (403 bp); Flg receptor (bFGF), sense 5’ CAT GTG TTC CCC TTG GAC TT 3’, antisense 5’ GAG GGA AGG CGT TAT CAA CA 3’, (701 bp); PDGF-AA, sense 5’CCC TGA GGG ATG GTA CTA AA 3’, antisense 5’AAA TGA CCG TCC TGG TCT TG 3’ (408 bp); PDGF-AA receptor, sense 5’GGG GAG AGT GAA GTG AGC TG 3’, antisense 5’GAA GCC TTT CTC GTG GAC AG 3’, (792 bp); CNTF, sense 5’ GGT GAC TTC CAT CAG GCA AT 3’, antisense 5’GGG TCA ACC CTA CTT GAC GA 3’. (423 bp); CNTFα receptor, sense 5’ CAC AAC ACT ACG GCC ATC AC3’, antisense 5’TAG CTG CAT GGT CCT CCT CT 3’ (784 bp); Shh, sense 5’AGA GAC TGC GAA ATA AGG AA 3’, antisense 5’GCA TAG CAG GAG AGG AAT GC 3’, (458 bp); Patched receptor (Shh), sense 5’ ACT GTC CAG CTA CCC CAA TG 3’, antisense 5’ CTG TGC TTC GTA TTG CCT GA 3’ (701 bp), and β-actin, sense 5’TGT GAT GGT GGG AAT GGG TCA G 3’, antisense 5’ TTT GAT GTC ACG CAC GAT TTC C 3’ (514 bp, [60]). The appropriate size of the RT-PCR products was confirmed by gel electrophoresis.
Fibrin Scaffold Preparation and Cell Seeding in Two and Three Dimensional Culture
For seeding cells in 2D, 24 well plates were coated with a 0.1% gelatin solution and individual EBs were selected using a pipette and placed in each well. For 3D cultue, fibrinogen solutions were prepared as previously described [61] from plasminogen free fibrinogen collected from human pooled plasma. Fibrin scaffolds were polymerized by combining the following components: 10 mg/mL fibrinogen, 2.5 mM CaCl2, and 2 NIH units/mL thrombin (all from Sigma). 300 µL of scaffold was added into each well of a 24 well plate (Corning, Corning, NY, http://www.corning.com) and allowed to polymerize for one hour at 37°C. Individual EBs were selected using a pipette and added to each well. A second layer of scaffold (100 µL) was then added on top of the EB to create a 3D scaffold. After an additional hour of incubation at 37° C, one mL of complete media as described above was added to each well. After 3 days, the media was changed to neural basal media (Invitrogen) containing B27 supplement (Invitrogen) diluted 1:50. The media was not changed for the rest of the experiment. These long term culture conditions had been determined previously to allow the cells to infiltrate the scaffold without bulk degradation occurring [21]. Growth factors were added at the indicated concentration to both complete and neural basal media used in these studies. The concentrations tested for each individual growth factor were 2, 10, and 25 ng/mL. Based the results of the individual studies, six different growth factor combinations listed in Table One were tested. The following growth factors were used in the studies: NT-3 (Peprotech, Rocky Hill, N.J., http://www.peprotech.com), bFGF (Roche, Indianapolis, IN, http://www.roche.com), PDGF-AA (R&D Systems, Minneapolis, MN, http://www.rndsystems.com), CNTF (Sigma), and Shh (R&D Systems).
Fluorescence Activated Cell Sorting
Preparation of Cells for Sorting
To prepare undifferentiated RW4 cells for staining, 1 mL of trypsin-EDTA (0.25%, Invitrogen) was added to a confluent flask of cells and allowed to incubate at 37°C for 5 min. The reaction was then quenched with 4 mL of complete media. The cells were centrifuged for 5 min at 1015 × g (all resulting spins were performed at this speed), the media aspirated and the cells resuspended in phosphate buffered saline (PBS, 7.4). For analysis of EBs, the liquid EB culture was allowed to settle in a 15 mL conical tube and the media aspirated. 5 mL of trypsin-EDTA was added to the cells and the cells were incubated on a shaker at 37°C for 20 min. The reaction was then quenched with 5 mL of complete media. The cells were then centrifuged and the supernatant was aspirated. The cells were triturated using a 1 mL pipette and passed through a 25 gauge needle to obtain single cells. The dissociated cells were resuspended in PBS. For analysis of EBs cultured in 2D on gelatin coated plates or inside of fibrin scaffolds for 14 days, the media was aspirated from each well followed by a wash with 0.5 mL of PBS. 250 µL of trypsin-EDTA was added to each well and incubated at 37°C for 20 min. 250 µL of complete medium was used to stop the reaction. The cells were triturated, centrifuged and resuspended in PBS. All of the staining after this point was performed at 4°C.
Cell Phenotype Analysis
When staining for cell surface antigens, the cells were blocked in PBS containing 5% normal goat serum (NGS, Invitrogen) for 20 min. Primary antibody diluted in PBS containing 2% NGS was applied to the cells for 1 hour. The cells were washed two times with PBS and secondary antibody also diluted in PBS containing 2% NGS was applied for 1 hour. After this incubation, the cells were washed with PBS twice and sorted. The stage specific embryonic antigen 1 marker (SSEA-1, 1:25; Chemicon, Temecula, CA, http://www.chemicon.com) for undifferentiated ES cells and the O4 marker (1:100; Chemicon) for oligodendrocytes were detected using an AlexaFluor 488 goat anti-mouse IgM (1:200; Invitrogen) secondary antibody in this manner.
When staining for intracellular markers, the cells were fixed in PBS containing 1% formaldehyde (Sigma) for 20 min. Following fixing, the cells were permeabilized using 0.5% saponin (Sigma) diluted in PBS for 20 min. The cells were blocked in PBS containing 0.1% saponin and 5% NGS for 20 min. Primary antibody diluted in PBS containing 0.1% saponin and 2% NGS was applied to the cells for 30 min. The cells were washed with PFN (PBS, 2% fetal calf serum, 0.1% sodium azide) twice and secondary antibody diluted in PBS containing 0.1% saponin and 2% NGS applied for 30 min. After this incubation, the cells were washed with PFN twice and sorted. The following markers were stained in this manner: nestin (neural precursors, 1:100; Chemicon), β-tubulin III (Tuj1, early neuronal, 1:1000; Covance, Berkeley, CA, http://store.crpinc.com), glial fibrilary acidic protein (GFAP, astrocytes, 1:40; Immunostar, Hudson, WI, http://www.immunostar.com). The nestin and Tuj1 markers were detected using AlexaFluor 488 goat anti-mouse IgG (1:200; Invitrogen) secondary antibody and GFAP staining was detected using AlexaFluor 488 goat anti-rabbit IgG (1:200; Invitrogen).
Cellular fluorescence was detected using a FACS Calibur flow cytometer (Becton Dickinson, http://www.bd.com) equipped with an argon laser emission of 488 nm. A 530 band pass filter was used to detect fluorescence. Plots of forward scatter versus side scatter were generated and used to select the desired cell populations by excluding debris and clumps. A second histogram plotting fluorescence was used to determine the number of cells staining positive for each marker. As a control, cells stained with only the same secondary antibody were used to eliminate non specific background staining. All analysis was performed using CellQuest software (Becton Dickinson).
Cell Viability Analysis
A Live/Dead Viability/Cytotoxicity kit (Invitrogen), consisting of calcein AM and ethidium homodimer-1 (EthD-1), was used to assess cell viability both qualitatively and quantitatively. The intracellular esterase present in live cells converts calcein AM, a cell permeable dye, to calcein, resulting in a bright green fluorescence. EthD-1 can penetrate damaged membranes of dead cells where it binds to nucleic acids producing intense red fluorescence.
For qualitative analysis, the media was removed from each well and each scaffold was washed once with PBS. The cells were then incubated for 30 min in 1 mL of solution containing 2 µM calcein AM and 4 µM EthD-1 dissolved in PBS. Fluorescence images were then taken to qualitatively assess cell viability. These calcein AM and EthD-1 concentrations were previously used to assay CNS stem and neural progenitor cell viability when cultured inside of collage scaffolds [62].
For quantitative analysis of cell viability, cells were isolated from fibrin scaffolds as described above and incubated with 0.5 mL of solution containing 0.1 µM calcein AM and 16 µM EthD-1 diluted in PBS for 20 min. The cells were analyzed on the flow cytometer using compensation to separate the live and dead cells into two distinct populations. Gating was used to quantify the percentage of live and dead cells present.
Immunohistochemistry
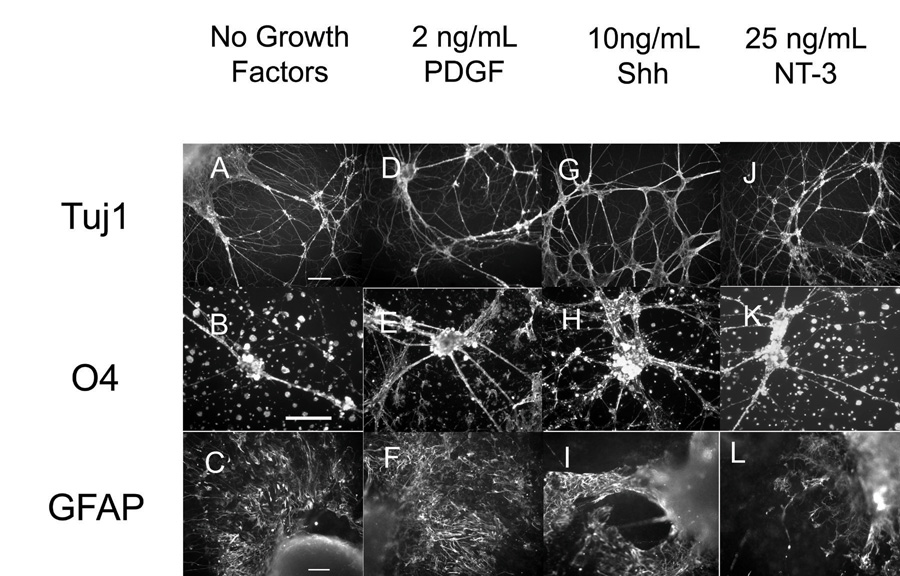
Immunohistochemistry was performed to confirm the FACS analysis and visualize the spatial distribution of cells inside of the scaffolds. After 14 days of EB culture inside of fibrin scaffolds, each well was washed with 1 mL of PBS and then fixed for 1 hour inside of the scaffold with 600 µL of 3.7% formaldehyde (Fisher). When necessary, the cells were permeabilized using 600 µL of 0.1% Triton-X (Sigma) in PBS for 45 min. The cells were blocked with 600 µL of 5% NGS in PBS for 2 hours. The cells were incubated with primary antibody overnight at 4°C. The following primary antibody dilutions were used to characterize the mature cells found in cultures: Tuj1 (1:500), O4 (1:100, Chemicon), and GFAP (1:4). The next day, each well was washed 3 times with PBS for 15 min each. Appropriate secondary antibodies (1:200 dilution) were applied for 4 hours at room temperature. Each well was then washed with PBS and imaged. Fluorescent images of the cell cultured in fibrin scaffolds were taken using a Qicam Fast cooled mono 12 bit camera (Q Imaging, Burnaby, BC, www.qimaging.com) attached to an Olympus IX70 microscope with QCapture 2.90.1. In Figure 2, the 10x objective was used to take photos of the Tuj and GFAP staining and the 20x objective was used to take photos of the O4 staining. In Supplementary Figure 2, the 2x objective was used. The images were processed using Image Pro Express (Media Cybernetics, Silver Spring, MD, www.mediacy.com).
Figure 2. Immunohistochemistry performed on EBs after 14 days inside of fibrin scaffolds for mature cell markers including Tuj1 (neurons), O4 (oligodendrocytes) and GFAP (astrocytes).
A–C) In scaffold staining of EB culture when no growth factors were present. D–F) In scaffold staining of EB culture when 2 ng/mL of PDGF was present. G–I) In scaffold staining of EB culture when 10 ng/mL of Shh was present. J–L) In scaffold staining when 25 ng/mL of NT-3 was present. Scale bar for Tuj staining are 100 µm. Scale bars for O4 and GFAP staining is 50 µm.
Statistical Analysis
For cell phenotype analysis, 2 or more sets of 24 well plates containing fibrin scaffolds with EB cultures were analyzed for each condition tested. This number provided enough cells (5000 per sort) to perform FACS and statistical analysis on the outcomes. For cell viability analysis, 2 of more sets of 6 well replicates were used. Statistical analysis was performed using Statistica (version 5.5, Statsoft, Tulsa, OK). Comparative analyses were completed using the Scheffe’s F post-hoc test by analysis of variance at a 95% confidence level. Mean values and standard deviation are reported.
Results
RT-PCR analysis of Receptor Expression
To determine what growth factors could potentially influence the differentiation state of 4−/4+ EBs seeded inside of fibrin scaffolds, RT-PCR was used to examine the expression of five different growth factors and their corresponding receptors, including NT-3, bFGF, PDGF-AA, CNTFα, and Shh. The receptors included TrkC (NT-3 receptor), Flg (bFGF recepetor), PDGF-AA receptor, CNTFα receptor, and Patched (Shh receptor). Supplementary Figure 1A and B shows the results of the RT-PCR analysis. Single bands of the expected length were observed for each reaction, indicating that the 4−/4+ EBs expressed the mRNA for the five growth factors studied and their corresponding receptors, making them appropriate targets for further investigation.
Analysis of Control Cultures
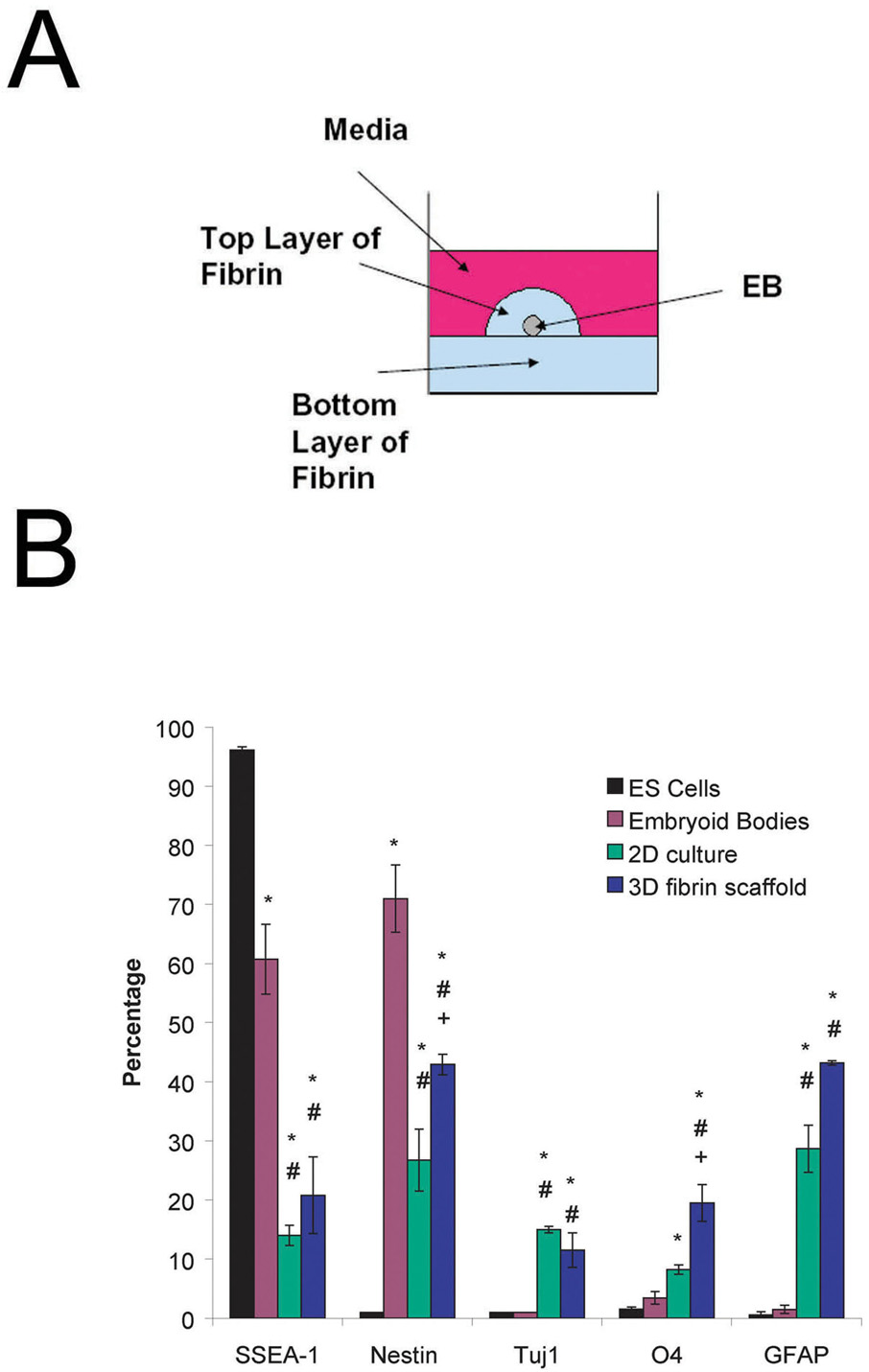
Undifferentiated ES cells were induced to form EBs containing ESNPCs using the 4−/4+ retinoic acid treatment protocol. Single EBs were seeded on 2D plates and inside of 3D fibrin scaffolds as seen in Figure 1A. To characterize cell differentiation, FACS was used to identify immature and mature cells types present after 14 days of culture. Additionally, the undifferentiated cells and the 4−/4+ EBs were also analyzed in the same manner. Figure 1B shows the results of these experiments. Undifferentiated ES cells stained 96.1 ± 0.6% positive for the SSEA-1 marker. Staining for the SSEA-1 marker, found on the cell surface of undifferentiated mouse ES cells, decreased to 60.8 ± 5.9% after the 4−/4+ treatment protocol, indicating that the cells are undergoing differentiation. The percentage of cells staining positive for SSEA-1 further decreased to 14.0 ± 1.7 % and 20.8 ± 6.5% after 14 days of culture on 2D plates and inside of fibrin scaffolds, respectively, when no growth factors are present. The undifferentiated ES cells do not stain positive for nestin, which is expressed by neural progenitors. After the 4−/4+ treatment, the cells stained 71.0 ± 5.7% for nestin and after 14 d in culture, the fraction of cells staining positive dropped to 28.73 ± 5.23% (2D culture) and 42.9 ± 1.7% (3D fibrin scaffold). The 3D cultures produced a larger fraction of cells staining positive for nestin compared to the 2D cultures.
Figure 1. Schematic of 3 dimensional culture system and results of preliminary characterization of ES cell cultures using FACS.
A) Side view of an individual well of a 24 well plate showing the placement of the EB and surrounding fibrin scaffold. B) Results of FACS analysis performed on undifferentiated mouse ES cells, 4−/4+ embryoid bodies, 2D culture and EBs seeded into fibrin scaffolds after 14 d of culture when no growth factors are present. The markers examined are as follows with the phenotype description given in parentheses: SSEA-1 (undifferentiated mouse ES cells), nestin (neural precursors), Tuj1 (neurons), O4 (oligodendrocytes), and GFAP (astrocytes). * indicates p < 0.05 for that marker compared to undifferentiated ES cells. # indicates p <0.05 for that marker compared to embryoid bodies. + indicates p < 0.05 for that marker compared to the 2D culture.
To determine what mature cell types were present after 14 d in culture, staining was performed using 3 different markers: β-tubulin-III (Tuj1, early neurons), O4 (oligodendrocytes), and glial acidic fibrilary protein (GFAP, astrocytes). Cells cultured inside of fibrin scaffolds for 14 d showed increased staining for all 3 mature cell markers when compared to the results for undifferentiated ES cells and 4−/4+ EBs while the 2D cultures showed increased staining for Tuj and GFAP when compared to the ES cells and EBs, but the O4 staining was only significantly increased when compared to the ES cells. Additionally, cells cultured in the 3D fibrin scaffolds showed increased staining for oligodendrocytes compared to the 2D culture. When cultured on 2D plates and inside of scaffolds with no growth factors present for 14 days, the largest fraction of ESNPCs differentiates into astrocytes (~30–40%) with smaller numbers of cells differentiating into neurons and oligodendrocytes. Immunohistochemistry confirming the presence of the mature markers expressed by cells seeded into 3D fibrin scaffolds can be seen in Figure 2A–C.
Growth Factor Dose Response Studies
Based on previous literature, five different growth factors were selected for study based their ability to promote ES cell differentiation and survival. Each growth factor was tested at three different doses (2, 10, and 25 ng/mL) to determine an appropriate concentration for maximizing the fractions of neurons and oligodendrocytes produced while minimizing the fraction of astrocytes produced. For each dose tested, the resulting cell phenotypes were characterized and cell viability was determined quantitatively using FACS.
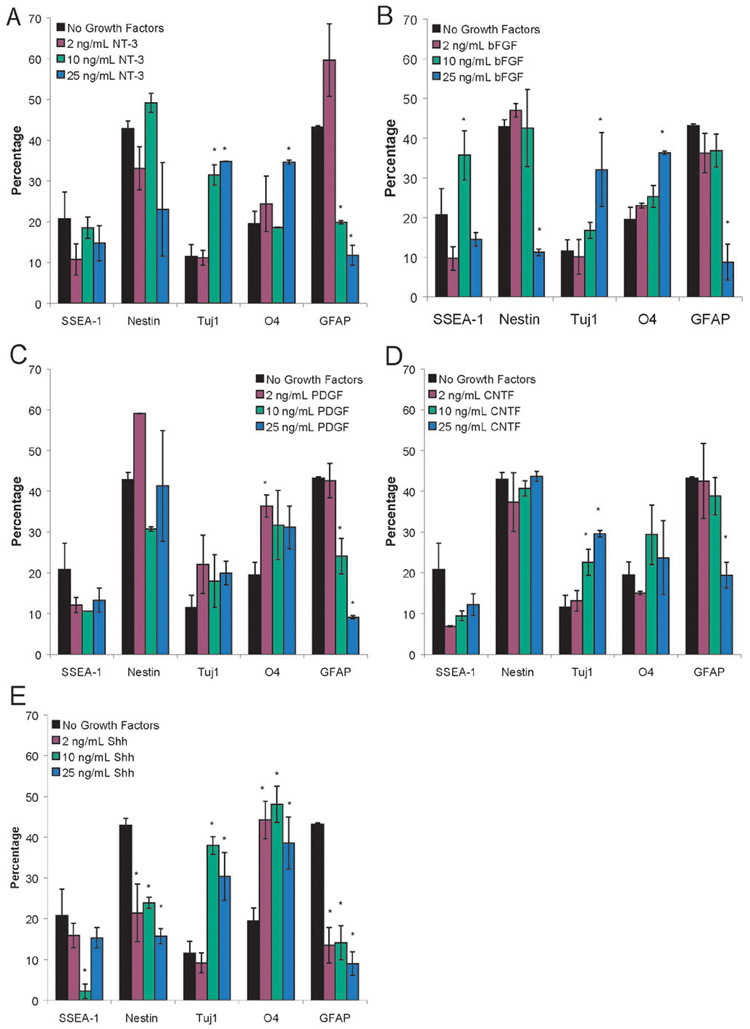
Figure 3A–E show the cell phenotypes that result at each of the doses tested for the individual growth factors compared to ESNPCs cultured in fibrin scaffolds when no growth factors are present Figure 3A shows the dose response of the ESNPCs to NT-3 when seeded in 3D culture. At 10 and 25 ng/mL of NT-3, an increase in the fraction of neurons, as indicated by the Tuj1 staining, along with a decrease in the GFAP staining (astrocytes), was observed. At 25 ng/mL, an increase in the fraction of oligodendrocytes produced was also observed, making this dose appropriate for the growth factor combination studies. As seen in Figure 3B, treatment with 2 ng/mL of bFGF did not change the resulting cell phenotypes when compared to cultures with no growth factors added. For the 10 ng/mL dose of bFGF, an increase in the fraction of undifferentiated ES cells, as indicated by SSEA-1 staining, was observed. At 25 ng/mL of bFGF, many differences were observed including a decrease in nestin and GFAP staining and an increase in the Tuj1 and O4 staining. For all doses of PDGF, no differences were observed in the fraction of cells staining positive for either immature cell marker (SSEA-1 and nestin) when compared to the no growth factor control group as seen in Figure 3C. However, the group treated with 2 ng/mL of PDGF showed increased nestin staining when compared to the group treated with 10 ng/mL. For 2 ng/mL of PDGF, an increase in oligodendrocytes was observed. At higher doses of PDGF, lower fractions of cells staining positive for GFAP was observed. Both 10 and 25 ng/mL of CNTF produced an increase in the fraction of neurons present (Figure 3D). Additionally, 25 ng/mL of CNTF reduced the number of astrocytes present after 14 d of culture inside of fibrin scaffolds. Figure 2E shows the response of ESNPCs to different doses of Shh. All three doses of Shh resulted in decreases in the fraction of cells staining positive for nestin and GFAP, while producing an increase in the fraction of O4 staining. At the two higher doses, increases in the Tuj1 staining were also observed. At 10 ng/mL of Shh, a decrease in SSEA-1 staining observed, making this dose the most appropriate for testing in combination with other growth factors. Immunohistochemistry was used to confirm these results and representative photos for 2 ng/mL PDGF, 10 ng/mL Shh, and 25 ng/mL NT-3 can be seen in Figure 2D–L.
Figure 3. Results of FACS analysis on the dose response studies of EBs cultured in fibrin scaffolds for 14 days in the presence of individual growth factors at the following concentrations: 2, 10, and 25 ng/mL.
A) EBs seeded into fibrin scaffolds after 14 d of culture in the presence of NT-3. B) EBs seeded into fibrin scaffolds after 14 d of culture in the presence of bFGF. C) EBs seeded into fibrin scaffolds after 14 d of culture in the presence of PDGF. D) Results of FACS analysis performed on EBs seeded into fibrin scaffolds after 14 d of culture in the presence of CNTF. E) Results of FACS analysis performed on EBs seeded into fibrin scaffolds after 14 d of culture in the presence of Shh. The markers examined were SSEA-1 (undifferentiated mouse ES cells), nestin (neural precursors), Tuj1 (neurons), O4 (oligodendrocytes), and GFAP (astrocytes). * indicated p < 0.05 for that marker compared to EBs cultured in fibrin for 14 days with no growth factors present.
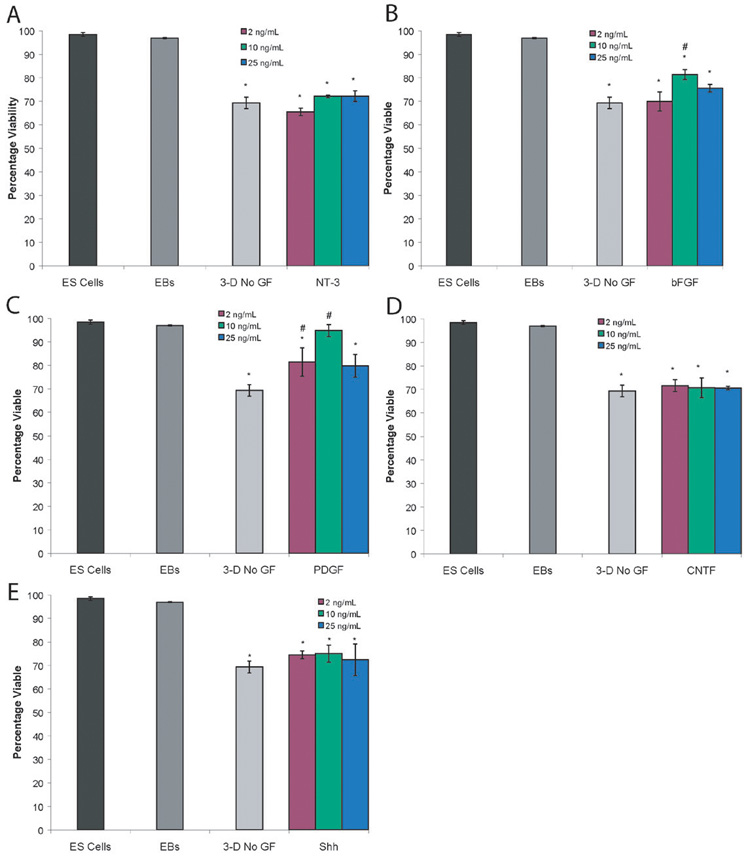
Through the use of Live/Dead staining and FACS, the effect of growth factors on cell viability was quantified. The undifferentiated ES cell culture and EBs showed high levels of viability (98.1 ± 0.8% and 96.9 ± 0.2% respectively). After two weeks of cultures inside of 3D fibrin scaffolds, the viability decreases to 69.4 ± 2.5%. This level is comparable to EBs seeded onto gelatinized plates in 2D culture under the same conditions, which were 66.8 ± 5.5% viable. Figure 4 shows the results of cell viability analysis at different growth factor doses and Supplementary Figure 2 shows representative photos of Live/Dead staining. For NT-3, CNTF, and Shh, all doses showed decreased viability compared to ES cells and EBs while showing similar levels of viability to the cultures with no growth factors added, as shown in Figures 4A, 4D, and 4E. As seen in Figure 4B, 10 ng/mL of bFGF increased viability compared to cultures with no growth factors added. Two different doses of PDGF (2 ng/mL and 10 ng/mL) produced an increase in cell viability compared to cultures with no growth factors added and 10 ng/mL of PDGF produced similar viability to ES cells and EBs. Thus, these two growth factors could potentially increase the cell survival rate after transplantation and were tested as part of the growth factor combinations. 2 ng/mL of PDGF was chosen for use in combinations due to its ability to promote an increase in oligodendrocytes.
Figure 4. Results of Live/Dead analysis of dose response studies of EBs cultured in fibrin scaffolds for 14 days in the presence of individual growth factors. Graph showed percentage of viable cells for each group tested.
A) Cell viability after treatment with NT-3. B) Cell viability after treatment with bFGF. C) Cell viability after treatment with PDGF. D) Cell viability after treatment with CNTF. E) Cell viability after treatment with Shh. * indicated p<0.05 compared to the cell viability for ES cells and EBs. # indicated p<0.05 compared to cell viability EBs cultured inside of fibrin scaffolds for 14 days with no growth factors present.
Growth Factor Combination Studies
To determine if additional effects would be observed when multiple growth factors were present, six different growth factor combinations (shown in Table 1) were tested to determine their effect on the resulting cell phenotypes. The resulting cell phenotype data is shown in Figure 5.
Table 1.
List of Growth Factor Combinations Tested
| 1 | 25 ng/mL NT-3 and 10 ng/mL Shh |
| 2 | 25 ng/mL NT-3 and 2 ng/mL PDGF |
| 3 | 10 ng/mL Shh and 2 ng/mL PDGF |
| 4 | 25 ng/mL NT-3, 10 ng/mL bFGF and 2 ng/mL PDGF |
| 5 | 10 ng/mL Shh, 10 ng/mL bFGF and 2 ng/mL PDGF |
| 6 | 25 ng/mL NT-3, 10 ng/mL Shh, 10 ng/mL bFGF, 25 ng/mL CNTF, and 2 ng/mL PDGF |
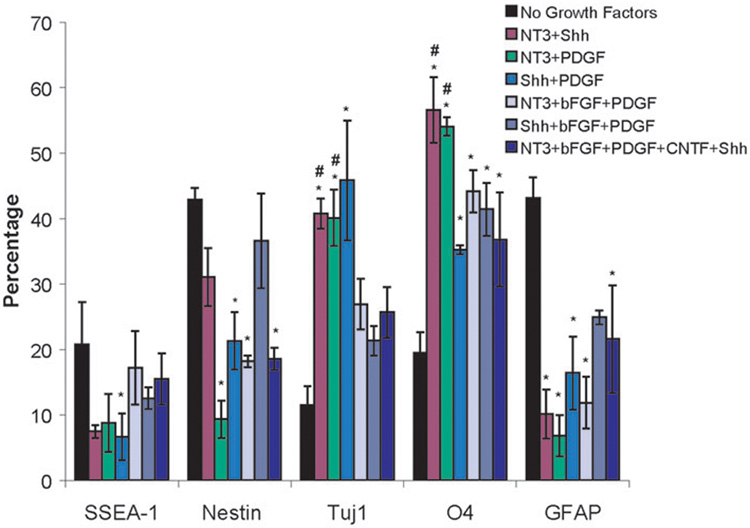
Figure 5. Results of FACS analysis on EBs cultured in fibrin scaffolds for 14 days in the presence of different combinations of growth factors.
The markers examined were SSEA-1 (undifferentiated mouse ES cells), nestin (neural precursors), Tuj1 (neurons), O4 (oligodendrocytes), and GFAP (astrocytes). * indicated p < 0.05 for that marker compared to EBs cultured in fibrin for 14 days with no growth factors present. # indicated p < 0.05 for that marker compared to EBs cultured in fibrin for 14 days with the optimal dose of each of the five growth factors present.
All of the two growth factor combinations produced an increase in the fraction of oligodendrocytes and neurons produced while reducing the fraction of astrocytes present compared to cultures with no growth factors added to the media. Additionally, the combinations of NT-3 and Shh as well as NT-3 and PDGF were able to increase the fraction of neurons and oligodendrocytes produced compared to the groups treated with all five growth factors at their optimal concentrations.
The three growth factor combinations (NT-3, PDGF, and bFGF and Shh, PDGF, and bFGF) were able to increase the fraction of oligodendrocytes present compared to cultures with no growth factors added, but not the fraction of neurons. Treatment with the combination of Shh, PDGF, and bFGF resulted in similar levels of undifferentiated ES cells, neural precursors, neurons and astrocytes as the groups not treated with group factors. The other three growth factor combination (NT-3, PDGF, and bFGF) produced decreased levels of nestin and GFAP staining compared to cultures with no growth factors added. Treatment of EBs with the combination of the optimal doses of all five growth factors resulted in a decrease in neural progenitors and astrocytes and an increase in oligodendrocytes, but not neurons.
Discussion
Preliminary characterization of ESNPCs cultured inside of 3D fibrin scaffolds showed significant differences when compared to traditional 2D culture in terms of the effect on differentiation. When cells were cultured in 3D fibrin scaffolds, increases in the fraction of neural precurors and oligodendrocytes were observed. Both of these populations of cells are desirable for treatment of SCI. Neural precursors have the potential to differentiate into mature neural cells and the addition of growth factors could help promote further differentiation of this population of cells. Oligodendrocytes play an important role in the CNS by myelinating neurons and more recently the use of oligodendrocytes to treat the demyelination that occurs after SCI has become a popular regeneration strategy [63]. The use of fibrin scaffolds may help the oligodendrocytes to interact with neurons by allowing them to form 3D architecture similar to an in vivo setting.
Based on RT-PCR studies, it was hypothesized that ESNPCs seeded inside of fibrin scaffolds would respond to the five growth factors selected from the literature and that treatment of ESNPCs with these growth factors would promote differentiation into a greater percentage of neurons and oligodendrocytes while reducing the percentage of undifferentiated ES cells and astrocytes present after 14 d of culture. Based on calculations using previously published diffusion coefficients for growth factors inside of fibrin scaffolds [64–66], it was determined that it takes 1–2 hours for the concentration of growth factor present in the media to reach the cells seeded inside the scaffolds. Thus these doses can be used in other 2D and 3D culture systems if such diffusion calculations are performed to ensure that the cells receive a similar concentration of growth factor.
Each of the growth factors exhibited dose dependent effects on the differentiation state of the ESNPCs. Higher doses of NT-3 and Shh induced formation of neurons, which is consistent with previous literature [10, 28, 33, 60]. Shh also increased the percentage of oligodendrocytes present at all doses while only at 25 ng/mL NT-3, an increase in oligodendrocytes was observed. The ability of Shh to promote differentiation of ESNPCs into neurons and oligodendrocytes is consistent with its role during development [67]. 10 ng/mL of bFGF promotes proliferation of undifferentiated ES cells as evidenced by an increase in SSEA-1 staining compared to cultures with no growth factors added. This dose of bFGF is often used to culture neural stem cells [62, 68]. At 25 ng/mL of bFGF, this effect is not observed while increases in neurons and oligodendrocytes are observed, suggesting higher doses may promote differentiation of ES cells into mature cells types, which is consistent with previous literature [69–71]. Similarly, at higher doses of CNTF, increases in the fraction of neurons present are observed could be due to the ability of CNTF to promote neuron survival [72]. Low doses of PDGF promote oligodendrocyte differentiation, which is consistent with its development role [36], while higher doses reduce astrocyte formation. These results, along with the cell viability data, suggested that PDGF would be more useful in combinations with other growth factors that promote neuronal differentiation than by itself.
The results of the growth factor combination studies suggest that crosstalk between different signaling pathways occurs and influences the differentiation of ESNPCs into mature cell phenotypes. Of all the growth factor combinations tested, Shh combined with NT-3 and NT-3 combined with PDGF produced the most neurons and oligodendrocytes compared to both the no growth factor control and the five growth factor combination (NT-3, bFGF, PDGF, CNTF, and Shh) while reducing the percentage of astrocytes present. The first combination, NT-3 and Shh, had been previously shown to work synergististically to promote motorneuron formation [73] and thus the crosstalk that occurs between these two signaling pathways appeared to be beneficial. Similar results are observed when NT-3 is combined with PDGF. Previously published work indicated that combining these two growth factors led to remyelination in model of multiple sclerosis [74] and this current work suggests that combining these two growth factors promoted increased neuronal and oligodendrocyte differentiation. The other two growth factor combination (Shh and PDGF) only increased the amount of neurons and oligodendrocytes present compared the no growth factor control. A recent study showed a peptide inhibitor anatogonized the ability of these two growth factors to promote oligodendrocyte precursor proliferation [75]. Combined with the results from this study, it suggests that combining these growth factors may be saturating the pathways that promote oligodendrocyte differentiation, leading to decreased levels of O4 staining compared to the other two growth factor combinations.
When bFGF was added in combination with other growth factors, the fraction of neurons produced was similar to the levels produced when no growth factors are present. One reason for this reduction compared to the two growth factor combinations may be that the bFGF could be inhibiting signaling pathways that promote differentiation by promoting cell proliferation instead. Recent work out of the Welsher-Reya lab showed that the addition of bFGF blocks Shh signaling in neuronal precursors [76]. The inhibition of Shh signaling by bFGF and PDGF could help explain the lack of increase neuronal staining observed in the three growth factor combination studies. bFGF may also have a similar effect on NT-3 signaling since the other three growth factor combination (NT-3, bFGF and Shh) also did not show increased staining for neurons. Finally, the five growth factor combination showed increased oligodendrocyte staining compared to the no growth factor control group, but not increased neuronal staining. The fraction of oligodendrocytes produced was also less than what was observed for the combinations of NT-3 plus Shh and NT-3 plus PDGF. CNTF has been shown to reduce the effects of Shh induced oligodendrocyte formation [77] and there is some evidence that CNTF can also interefere with NT-3 signaling [78]. Overall, the combinations that contained the least number of growth factors were the most effective at promoting differentiation of ESNPCs into neurons and oligodendrocytes.
This work establishes a foundation for further investigation of combining ES cells, growth factors and biomaterial scaffolds as a potential treatment for SCI. Future work includes translating these optimal doses of growth factors for use with a controlled drug delivery system [22, 23, 26] and in vivo testing of these scaffolds in a model of SCI. This data also can be extended to other drug delivery systems and cell types for engineering neural tissue.
Conclusion
This work has quantitatively determined the appropriate doses and combinations of five different growth factors (NT-3, bFGF, PDGF, CNTF, and Shh) for producing neurons and oligodendrocytes from 4−/4+ EBs cultured inside of 3D fibrin scaffolds. 25 ng/mL of NT-3 and 10 ng/mL of Shh were the most effective for producing both neurons and oligodendrocytes. Combining 25 ng/mL of NT-3 and 10 ng/mL of Shh as well as 25 ng/mL of NT-3 with 2 ng/mL of PDGF resulted in largest fractions and neurons and oligodendrocytes produced. The combination of ESNPCs, growth factors and fibrin scaffolds provides a potential platform for treatment of SCI.
Supplementary Material
Resulting products of the RT-PCR ran out a 2% agarose gel. A) Lane 1 – 100 bp molecular marker. Lane 2 - β actin control (expected size: 514 bp). Lane 3 - Shh (expected size: 458 bp). Lane 4 - CNTF (expected size: 423 bp). Lane 5 – PDGF-AA (expected size: 408 bp). Lane 6 - bFGF (expected size: 403 bp). Lane 7 - NT-3 (expected size: 420 bp). Lane 8 – 100 bp molecular marker. B) Lane 1– 100 bp molecular marker. Lane 2 - β actin control (expected size: 514 bp). Lane 3 - Patched receptor for Shh (expected size: 701 bp). Lane 4 - CNTFα receptor (expected size: 784 bp). Lane 5 - PDGFα receptor (expected size: 792 bp). Lane 6 - Flg receptor for bFGF (expected size: 701 bp). Lane 7 - TrkC receptor for NT-3 (expected size: 394 bp). Lane 8 – 100 bp molecular marker.
Live/Dead staining performed on EBs after 14 days inside of fibrin scaffolds. A) In scaffold staining of EB culture when no growth factors were present. B) In scaffold staining of EB culture when 10 ng/mL of PDGF was present. Green indicated live cells and red indicated dead cells. Areas where both live and dead cells were present appear yellow. Scale bar is 1 mm.
Acknowledgements
This study was supported by NIH R01 NS051454. The authors would also like to thank Amy Boyet and Megan Kaneda for technical assistance with the FACSCalibur.
References
- 1.Elisseeff JH. Embryonic stem cells: potential for more impact. Trends Biotechnol. 2004;22(4):155–156. doi: 10.1016/j.tibtech.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Polak JM, Bishop AE. Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci. 2006;1068:352–366. doi: 10.1196/annals.1346.001. [DOI] [PubMed] [Google Scholar]
- 3.Wobus AM, Holzhausen H, Jakel P, Schoneich J. Characterization of a pluripotent stem cell line derived from a mouse embryo. Exp Cell Res. 1984;152(1):212–219. doi: 10.1016/0014-4827(84)90246-5. [DOI] [PubMed] [Google Scholar]
- 4.Gossler A, Joyner AL, Rossant J, Skarnes WC. Mouse embryonic stem cells and reporter constructs to detect developmentally regulated genes. Science. 1989;244(4903):463–465. doi: 10.1126/science.2497519. [DOI] [PubMed] [Google Scholar]
- 5.Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev Biol. 1995;168(2):342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- 6.Qu Y, Vadivelu S, Choi L, Liu S, Lu A, Lewis B, Girgis R, Lee CS, Snider BJ, Gottlieb DI, McDonald JW. Neurons derived from embryonic stem (ES) cells resemble normal neurons in their vulnerability to excitotoxic death. Exp Neurol. 2003;184(1):326–336. doi: 10.1016/j.expneurol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 7.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5(12):1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 8.Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A. 2003;100(22):12741–12746. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang NS, Kim MS, Sampattavanich S, Baek JH, Zhang Z, Elisseeff J. Effects of three-dimensional culture and growth factors on the chondrogenic differentiation of murine embryonic stem cells. Stem Cells. 2006;24(2):284–291. doi: 10.1634/stemcells.2005-0024. [DOI] [PubMed] [Google Scholar]
- 10.Levenberg S, Burdick JA, Kraehenbuehl T, Langer R. Neurotrophin-induced differentiation of human embryonic stem cells on three-dimensional polymeric scaffolds. Tissue Eng. 2005;11(3–4):506–512. doi: 10.1089/ten.2005.11.506. [DOI] [PubMed] [Google Scholar]
- 11.Bensaid W, Triffitt JT, Blanchat C, Oudina K, Sedel L, Petite H. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24(14):2497–2502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- 12.Teng YD, Lavik EB, Qu X, Park KI, Ourednik J, Zurakowski D, Langer R, Snyder EY. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci U S A. 2002;99(5):3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yim EK, Leong KW. Proliferation and differentiation of human embryonic germ cell derivatives in bioactive polymeric fibrous scaffold. J Biomater Sci Polym Ed. 2005;16(10):1193–1217. doi: 10.1163/156856205774269485. [DOI] [PubMed] [Google Scholar]
- 14.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 15.Catelas I, Sese N, Wu BM, Dunn JC, Helgerson S, Tawil B. Human Mesenchymal Stem Cell Proliferation and Osteogenic Differentiation in Fibrin Gels in Vitro. Tissue Eng. 2006 doi: 10.1089/ten.2006.12.2385. [DOI] [PubMed] [Google Scholar]
- 16.Ho W, Tawil B, Dunn JC, Wu BM. The Behavior of Human Mesenchymal Stem Cells in 3D Fibrin Clots: Dependence on Fibrinogen Concentration and Clot Structure. Tissue Eng. 2006;12(6):1587–1595. doi: 10.1089/ten.2006.12.1587. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, Collins SF, Suggs LJ. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006 doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 18.Sreerekha PR, Divya P, Krishnan LK. Adult stem cell homing and differentiation in vitro on composite fibrin matrix. Cell Prolif. 2006;39(4):301–312. doi: 10.1111/j.1365-2184.2006.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G, Wang X, Wang Z, Zhang J, Suggs L. A PEGylated fibrin patch for mesenchymal stem cell delivery. Tissue Eng. 2006;12(1):9–19. doi: 10.1089/ten.2006.12.9. [DOI] [PubMed] [Google Scholar]
- 20.Albala DM. Fibrin sealants in clinical practice. Cardiovasc Surg. 2003;11 Suppl 1:5–11. doi: 10.1016/S0967-2109(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 21.Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27(36):5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor SJ, McDonald JW, 3rd, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 from fibrin gels for spinal cord injury. J Control Release. 2004;98(2):281–294. doi: 10.1016/j.jconrel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Taylor SJ. Rosenzweig ES, McDonald JW, 3rd, Sakiyama-Elbert SE. Delivery of neurotrophin-3 from fibrin enhances neuronal fiber sprouting after spinal cord injury. J Control Release. 2006;113(3):226–235. doi: 10.1016/j.jconrel.2006.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273(5274):510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- 25.Iwaya K, Mizoi K, Tessler A, Itoh Y. Neurotrophic agents in fibrin glue mediate adult dorsal root regeneration into spinal cord. Neurosurgery. 1999;44(3):589–595. doi: 10.1097/00006123-199903000-00085. discussion 595-6. [DOI] [PubMed] [Google Scholar]
- 26.Taylor SJ, Sakiyama-Elbert SE. Effects of controlled delivery of neurotrophin-3 from fibrin on spinal cord injury in a long term model. J Control Release. 2006 doi: 10.1016/j.jconrel.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyle AD, Lock LF, Donovan PJ. Neurotrophins mediate human embryonic stem cell survival. Nat Biotechnol. 2006;24(3):344–350. doi: 10.1038/nbt1189. [DOI] [PubMed] [Google Scholar]
- 28.Lachyankar MB, Condon PJ, Quesenberry PJ, Litofsky NS, Recht LD, Ross AH. Embryonic precursor cells that express Trk receptors: induction of different cell fates by NGF, BDNF, NT-3, and CNTF. Exp Neurol. 1997;144(2):350–360. doi: 10.1006/exnr.1997.6434. [DOI] [PubMed] [Google Scholar]
- 29.Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol. 2006;201(2):359–367. doi: 10.1016/j.expneurol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 30.Caldwell MA, He X, Wilkie N, Pollack S, Marshall G, Wafford KA, Svendsen CN. Growth factors regulate the survival and fate of cells derived from human neurospheres. Nat Biotechnol. 2001;19(5):475–479. doi: 10.1038/88158. [DOI] [PubMed] [Google Scholar]
- 31.Bambakidis NC, Wang RZ, Franic L, Miller RH. Sonic hedgehog-induced neural precursor proliferation after adult rodent spinal cord injury. J Neurosurg. 2003;99(1 Suppl):70–75. doi: 10.3171/spi.2003.99.1.0070. [DOI] [PubMed] [Google Scholar]
- 32.Li XJ, Du ZW, Zarnowska ED, Pankratz M, Hansen LO, Pearce RA, Zhang SC. Specification of motoneurons from human embryonic stem cells. Nat Biotechnol. 2005;23(2):215–221. doi: 10.1038/nbt1063. [DOI] [PubMed] [Google Scholar]
- 33.Kondo T, Johnson SA, Yoder MC, Romand R, Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc Natl Acad Sci U S A. 2005;102(13):4789–4794. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hughes SM, Lillien LE, Raff MC, Rohrer H, Sendtner M. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature. 1988;335(6185):70–73. doi: 10.1038/335070a0. [DOI] [PubMed] [Google Scholar]
- 35.Kang SM, Cho MS, Seo H, Yoon CJ, Oh SK, Choi YM, Kim DW. Efficient induction of oligodendrocytes from human embryonic stem cells. Stem Cells. 2007;25(2):419–424. doi: 10.1634/stemcells.2005-0482. [DOI] [PubMed] [Google Scholar]
- 36.Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, Betsholtz C, Richardson WD. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20(5):869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- 37.Hiraizumi Y, Fujimaki E, Transfeldt EE, Kawahara N, Fiegel VD, Knighton D, Sung JH. The effect of the platelet derived wound healing formula and the nerve growth factor on the experimentally injured spinal cord. Spinal Cord. 1996;34(7):394–402. doi: 10.1038/sc.1996.71. [DOI] [PubMed] [Google Scholar]
- 38.Hiraizumi Y, Transfeldt EE, Kawahara N, Fiegel VD, Knighton D, Sung JH. The effect of growth factor formula (platelet derived wound healing formula) in experimental spinal cord injuries. J Am Paraplegia Soc. 1992;15(1):7–13. doi: 10.1080/01952307.1992.11735856. [DOI] [PubMed] [Google Scholar]
- 39.Hiraizumi Y, Transfeldt EE, Kawahara N, Sung JH, Knighton D, Fiegel VD. In vivo angiogenesis by platelet-derived wound-healing formula in injured spinal cord. Brain Res Bull. 1993;30(3–4):353–357. doi: 10.1016/0361-9230(93)90264-c. [DOI] [PubMed] [Google Scholar]
- 40.Lillien LE, Sendtner M, Rohrer H, Hughes SM, Raff MC. Type-2 astrocyte development in rat brain cultures is initiated by a CNTF-like protein produced by type-1 astrocytes. Neuron. 1988;1(6):485–494. doi: 10.1016/0896-6273(88)90179-1. [DOI] [PubMed] [Google Scholar]
- 41.van Adel BA, Arnold JM, Phipps J, Doering LC, Ball AK. Ciliary neurotrophic factor protects retinal ganglion cells from axotomy-induced apoptosis via modulation of retinal glia in vivo. J Neurobiol. 2005;63(3):215–234. doi: 10.1002/neu.20117. [DOI] [PubMed] [Google Scholar]
- 42.Ye J, Cao L, Cui R, Huang A, Yan Z, Lu C, He C. The effects of ciliary neurotrophic factor on neurological function and glial activity following contusive spinal cord injury in the rats. Brain Res. 2004;997(1):30–39. doi: 10.1016/j.brainres.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 43.Ishii K, Nakamura M, Dai H, Finn TP, Okano H, Toyama Y, Bregman BS. Neutralization of ciliary neurotrophic factor reduces astrocyte production from transplanted neural stem cells and promotes regeneration of corticospinal tract fibers in spinal cord injury. J Neurosci Res. 2006 doi: 10.1002/jnr.21079. [DOI] [PubMed] [Google Scholar]
- 44.Miles GB, Yohn DC, Wichterle H, Jessell TM, Rafuse VF, Brownstone RM. Functional properties of motoneurons derived from mouse embryonic stem cells. J Neurosci. 2004;24(36):7848–7858. doi: 10.1523/JNEUROSCI.1972-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bambakidis NC, Miller RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 2004;4(1):16–26. doi: 10.1016/j.spinee.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 46.Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11(5):951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- 47.Murtie JC, Zhou YX, Le TQ, Vana AC, Armstrong RC. PDGF and FGF2 pathways regulate distinct oligodendrocyte lineage responses in experimental demyelination with spontaneous remyelination. Neurobiol Dis. 2005;19(1–2):171–182. doi: 10.1016/j.nbd.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 48.Ludwig TE, Levenstein ME, Jones JM, Berggren WT, Mitchen ER, Frane JL, Crandall LJ, Daigh CA, Conard KR, Piekarczyk MS, Llanas RA, Thomson JA. Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol. 2006;24(2):185–187. doi: 10.1038/nbt1177. [DOI] [PubMed] [Google Scholar]
- 49.Xu Y, Kitada M, Yamaguchi M, Dezawa M, Ide C. Increase in bFGF-responsive neural progenitor population following contusion injury of the adult rodent spinal cord. Neurosci Lett. 2006;397(3):174–179. doi: 10.1016/j.neulet.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 50.Rabchevsky AG, Fugaccia I, Fletcher-Turner A, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances tissue sparing and functional recovery following moderate spinal cord injury. J Neurotrauma. 1999;16(9):817–830. doi: 10.1089/neu.1999.16.817. [DOI] [PubMed] [Google Scholar]
- 51.Rabchevsky AG, Fugaccia I, Turner AF, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol. 2000;164(2):280–291. doi: 10.1006/exnr.2000.7399. [DOI] [PubMed] [Google Scholar]
- 52.Meijs MF, Timmers L, Pearse DD, Tresco PA, Bates ML, Joosten EA, Bunge MB, Oudega M. Basic fibroblast growth factor promotes neuronal survival but not behavioral recovery in the transected and Schwann cell implanted rat thoracic spinal cord. J Neurotrauma. 2004;21(10):1415–1430. doi: 10.1089/neu.2004.21.1415. [DOI] [PubMed] [Google Scholar]
- 53.Moses D, Teper Y, Gantois I, Finkelstein DI, Horne MK, Drago J. Murine embryonic EGF-responsive ventral mesencephalic neurospheres display distinct regional specification and promote survival of dopaminergic neurons. Exp Neurol. 2006;199(1):209–221. doi: 10.1016/j.expneurol.2006.02.120. [DOI] [PubMed] [Google Scholar]
- 54.Nakashima K, Takizawa T, Ochiai W, Yanagisawa M, Hisatsune T, Nakafuku M, Miyazono K, Kishimoto T, Kageyama R, Taga T. BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc Natl Acad Sci U S A. 2001;98(10):5868–5873. doi: 10.1073/pnas.101109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sterneckert JL, Hill CM, Palmer R, Gearhart JD. Bone morphogenetic proteins produced by cells within embryoid bodies inhibit ventral directed differentiation by Sonic Hedgehog. Cloning Stem Cells. 2005;7(1):27–34. doi: 10.1089/clo.2005.7.27. [DOI] [PubMed] [Google Scholar]
- 56.Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17(4):595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 57.Kojima A, Tator CH. Intrathecal administration of epidermal growth factor and fibroblast growth factor 2 promotes ependymal proliferation and functional recovery after spinal cord injury in adult rats. J Neurotrauma. 2002;19(2):223–238. doi: 10.1089/08977150252806974. [DOI] [PubMed] [Google Scholar]
- 58.Jimenez Hamann MC, Tator CH, Shoichet MS. Injectable intrathecal delivery system for localized administration of EGF and FGF-2 to the injured rat spinal cord. Exp Neurol. 2005;194(1):106–119. doi: 10.1016/j.expneurol.2005.01.030. [DOI] [PubMed] [Google Scholar]
- 59.Setoguchi T, Nakashima K, Takizawa T, Yanagisawa M, Ochiai W, Okabe M, Yone K, Komiya S, Taga T. Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Exp Neurol. 2004;189(1):33–44. doi: 10.1016/j.expneurol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Lee CS, Tee LY, Dusenbery S, Takata T, Golden JP, Pierchala BA, Gottlieb DI, Johnson EM, Jr, Choi DW, Snider BJ. Neurotrophin and GDNF family ligands promote survival and alter excitotoxic vulnerability of neurons derived from murine embryonic stem cells. Exp Neurol. 2005;191(1):65–76. doi: 10.1016/j.expneurol.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 61.Schense JC, Hubbell JA. Cross-linking exogenous bifunctional peptides into fibrin gels with factor XIIIa. Bioconjug Chem. 1999;10(1):75–81. doi: 10.1021/bc9800769. [DOI] [PubMed] [Google Scholar]
- 62.Ma W, Fitzgerald W, Liu QY, O'Shaughnessy TJ, Maric D, Lin HJ, Alkon DL, Barker JL. CNS stem and progenitor cell differentiation into functional neuronal circuits in three-dimensional collagen gels. Exp Neurol. 2004;190(2):276–288. doi: 10.1016/j.expneurol.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 63.Faulkner J, Keirstead HS. Human embryonic stem cell-derived oligodendrocyte progenitors for the treatment of spinal cord injury. Transpl Immunol. 2005;15(2):131–142. doi: 10.1016/j.trim.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Maxwell DJ, Hicks BC, Parsons S, Sakiyama-Elbert SE. Development of rationally designed affinity-based drug delivery systems. Acta Biomater. 2005;1(1):101–113. doi: 10.1016/j.actbio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 65.Willerth SM, Johnson PJ, Maxwell DJ, Parsons SR, Doukas ME, Sakiyama-Elbert SE. Rationally designed peptides for controlled release of nerve growth factor from fibrin matrices. J Biomed Mater Res A. 2007;80(1):13–23. doi: 10.1002/jbm.a.30844. [DOI] [PubMed] [Google Scholar]
- 66.Saltzman WM, Radomsky ML, Whaley KJ, Cone RA. Antibody diffusion in human cervical mucus. Biophys J. 1994;66(2 Pt 1):508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuccillo M, Joyner AL, Fishell G. Morphogen to mitogen: the multiple roles of hedgehog signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7(10):772–783. doi: 10.1038/nrn1990. [DOI] [PubMed] [Google Scholar]
- 68.Chen YW, Chiou SH, Wong TT, Ku HH, Lin HT, Chung CF, Yen SH, Kao CL. Using gelatin scaffold with coated basic fibroblast growth factor as a transfer system for transplantation of human neural stem cells. Transplant Proc. 2006;38(5):1616–1617. doi: 10.1016/j.transproceed.2006.02.084. [DOI] [PubMed] [Google Scholar]
- 69.Abematsu M, Kagawa T, Fukuda S, Inoue T, Takebayashi H, Komiya S, Taga T. Basic fibroblast growth factor endows dorsal telencephalic neural progenitors with the ability to differentiate into oligodendrocytes but not gamma-aminobutyric acidergic neurons. J Neurosci Res. 2006;83(5):731–743. doi: 10.1002/jnr.20762. [DOI] [PubMed] [Google Scholar]
- 70.Wang TT, Jing AH, Luo XY, Li M, Kang Y, Zou XL, Chen H, Dong J, Liu S. Neural stem cells: isolation and differentiation into cholinergic neurons. Neuroreport. 2006;17(13):1433–1436. doi: 10.1097/01.wnr.0000227980.06013.31. [DOI] [PubMed] [Google Scholar]
- 71.Benzing C, Segschneider M, Leinhaas A, Itskovitz-Eldor J, Brustle O. Neural conversion of human embryonic stem cell colonies in the presence of fibroblast growth factor-2. Neuroreport. 2006;17(16):1675–1681. doi: 10.1097/01.wnr.0000236861.01210.72. [DOI] [PubMed] [Google Scholar]
- 72.Houle JD, Ye JH. Survival of chronically-injured neurons can be prolonged by treatment with neurotrophic factors. Neuroscience. 1999;94(3):929–936. doi: 10.1016/s0306-4522(99)00359-0. [DOI] [PubMed] [Google Scholar]
- 73.Dutton R, Yamada T, Turnley A, Bartlett PF, Murphy M. Sonic hedgehog promotes neuronal differentiation of murine spinal cord precursors and collaborates with neurotrophin 3 to induce Islet-1. J Neurosci. 1999;19(7):2601–2608. doi: 10.1523/JNEUROSCI.19-07-02601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fressinaud C. Repeated injuries dramatically affect cells of the oligodendrocyte lineage: effects of PDGF and NT-3 in vitro. Glia. 2005;49(4):555–566. doi: 10.1002/glia.20136. [DOI] [PubMed] [Google Scholar]
- 75.Lelievre V, Ghiani CA, Seksenyan A, Gressens P, de Vellis J, Waschek JA. Growth factor-dependent actions of PACAP on oligodendrocyte progenitor proliferation. Regul Pept. 2006;137(1–2):58–66. doi: 10.1016/j.regpep.2006.04.024. [DOI] [PubMed] [Google Scholar]
- 76.Fogarty MP, Emmenegger BA, Grasfeder LL, Oliver TG, Wechsler-Reya RJ. Fibroblast growth factor blocks Sonic hedgehog signaling in neuronal precursors and tumor cells. Proc Natl Acad Sci U S A. 2007 doi: 10.1073/pnas.0605770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Du ZW, Li XJ, Nguyen GD, Zhang SC. Induced expression of Olig2 is sufficient for oligodendrocyte specification but not for motoneuron specification and astrocyte repression. Mol Cell Neurosci. 2006;33(4):371–380. doi: 10.1016/j.mcn.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Hapner SJ, Nielsen KM, Chaverra M, Esper RM, Loeb JA, Lefcort F. NT-3 and CNTF exert dose-dependent, pleiotropic effects on cells in the immature dorsal root ganglion: neuregulin-mediated proliferation of progenitor cells and neuronal differentiation. Dev Biol. 2006;297(1):182–197. doi: 10.1016/j.ydbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Resulting products of the RT-PCR ran out a 2% agarose gel. A) Lane 1 – 100 bp molecular marker. Lane 2 - β actin control (expected size: 514 bp). Lane 3 - Shh (expected size: 458 bp). Lane 4 - CNTF (expected size: 423 bp). Lane 5 – PDGF-AA (expected size: 408 bp). Lane 6 - bFGF (expected size: 403 bp). Lane 7 - NT-3 (expected size: 420 bp). Lane 8 – 100 bp molecular marker. B) Lane 1– 100 bp molecular marker. Lane 2 - β actin control (expected size: 514 bp). Lane 3 - Patched receptor for Shh (expected size: 701 bp). Lane 4 - CNTFα receptor (expected size: 784 bp). Lane 5 - PDGFα receptor (expected size: 792 bp). Lane 6 - Flg receptor for bFGF (expected size: 701 bp). Lane 7 - TrkC receptor for NT-3 (expected size: 394 bp). Lane 8 – 100 bp molecular marker.
Live/Dead staining performed on EBs after 14 days inside of fibrin scaffolds. A) In scaffold staining of EB culture when no growth factors were present. B) In scaffold staining of EB culture when 10 ng/mL of PDGF was present. Green indicated live cells and red indicated dead cells. Areas where both live and dead cells were present appear yellow. Scale bar is 1 mm.