Abstract
The combinatorial effects of TGF-β1 and hydrostatic pressure (HP) were investigated on meniscus cell-seeded PLLA constructs using a two-phase sequential study. The objective was to identify potentially synergistic effects of these stimuli toward enhancing the biomechanical and compositional characteristics of the engineered constructs. In Phase I, the effects of TGF-β1 were examined on the ability of meniscus cells to produce ECM. In Phase II, meniscus cell-seeded PLLA constructs were cultured for 4 wks with a combination of TGF-β1 and HP (10 MPa, 0 Hz or 10 MPa, 0.1 Hz). TGF-β1 was found to increase collagen and GAG deposition in the scaffolds 15-fold and 8-fold, respectively, in Phase I. In Phase II, the combination of TGF-β1 and 10 MPa, 0 Hz HP resulted in 4-fold higher collagen deposition (additive increase), 3-fold higher GAG deposition and enhanced compressive properties (additive and synergistic increases), when compared to the unpressurized no growth factor culture control. Though significant correlations were observed between the compressive properties (moduli and viscosity), and the GAG and collagen content of the constructs, the correlations were stronger with collagen. This study provides robust evidence that growth factors and HP can be used successfully in combination to enhance the functional properties of in vitro engineered knee meniscus constructs.
Keywords: Knee meniscus, hydrostatic pressure, tissue engineering, PLLA, TGF-β1
Introduction
The knee meniscus is a fibrocartilaginous structure that cushions the tibia and the femur [1]. Damage to the meniscus can lead to significant pain around the knee joint and result in osteoarthritis. Current arthroscopic meniscal treatments, such as partial meniscectomy, provide short term relief; however, longer term studies have shown that meniscectomy quickens the onset of osteoarthritis [2]. Thus, other treatment modalities, such as tissue engineering, are widely researched including the use of growth factors and mechanical stimuli, as reviewed elsewhere [1].
A wide range of growth factors including transforming growth factor beta-1 (TGF-β1), insulin-like growth factor-1 (IGF-1), basic fibroblast growth factor (bFGF), platelet-derived growth factor-AB (PDGF-AB), epidermal growth factor (EGF), and hepatocyte growth factor (HGF) have been studied for their potential use in exogenously stimulating meniscus cells to increase production of extracellular matrix (ECM) [3–8]. Of these, TGF-β1 has shown the most promise in terms of production of glycosaminoglycans (GAGs) and collagen in alginate and PGA scaffold studies with meniscus cells [5, 7]. TGF-β1 is also found in varying quantities (2.7 ± 0.37 to 20.9 ± 2.2 ng/ml) in fetal bovine serum (FBS), a vital component of cell culture medium involved in aiding cell proliferation and ECM formation [9]. To examine effects of exogenous TGF-β1 on meniscus cells would, therefore, require removal of FBS from the medium. Pilot studies in the laboratory, however, have shown that cell proliferation decreases and subsequent cell death ensues in meniscal cells grown in basal culture medium devoid of FBS. To overcome this, one may consider the use of FBS, but perhaps at a lower concentration (1%), when compared to the current standard (10%). Lowering of FBS concentration may also be advantageous as it increases clinical translatability by reducing the risk of disease transmission as a result of serum [10].
In addition to growth factors, the application of mechanical stimuli, such as hydrostatic pressure (HP) or direct compression (DC), has been shown to induce positive changes in gene expression and ECM synthesis in meniscal explants and cells [11–15]. During every day activity, the knee joint is exposed to 7–10 MPa HP with a normal observed frequency ranging from 0 to 1.1 Hz [16, 17]. HP is also of particular interest as it results in no macro-scale deformation to the construct while the stimulus is applied. Experiments in the literature focusing on articular chondrocytes and temporomandibular joint (TMJ) disc fibrochondrocytes have shown benefits for both static and intermittent HP regimens [18–20]. For example, static HP of 10 MPa applied for 1 wk for 4 hr using a 2 day on, 1 day off regimen was shown to upregulate collagen synthesis on PGA scaffolds seeded with porcine TMJ disc fibrochondrocytes [18]. Intermittent HP of 6.87 MPa (5 s pressurized, 15 s unpressurized) has been shown to increase collagen and sulfated GAGs on PGA scaffolds seeded with equine articular chondrocytes [21]. Recent growth factor-HP combination studies have shown that TGF-β3 can enhance expression of relevant cartilaginous proteins in human mesenchymal stem cells grown in pellet culture and the addition of intermittent HP of 10 MPa, 1 Hz can further increase gene expression of these proteins [22, 23].
The goal of this experiment was two-fold. In Phase I, we investigated the effects of FBS concentration and TGF-β1 on the ability of meniscal cells to proliferate and produce ECM on poly-l-lactic acid (PLLA). It was hypothesized that TGF-β1 would increase ECM production on the constructs and that lowering FBS concentration would not be detrimental to ECM formation. In Phase II, we examined whether the combination of HP (static or intermittent) and TGF-β1 would enhance the biochemical and biomechanical properties of tissue-engineered constructs. It was hypothesized that an additive or synergistic effect would occur between the HP treatment and the growth factor.
Materials and Methods
Cell harvesting, culture and passage (Phase I and II)
Lateral and medial meniscus cells from ten skeletally mature New Zealand white rabbits were harvested using aseptic technique. All procedures used were in strict accordance with the National Institutes of Health Guidelines on the Care and Use of Laboratory Animals. Ethics approval was obtained from Rice University before commencement of the study. The tissue was minced and digested overnight with 0.2% collagenase (Worthington, Lakewood, NJ). The isolated cells were then counted using a hemocytometer, pooled and stored at −80 °C. At the start of the experiment, meniscus cells were thawed, and the cell viability was calculated to be over 90%. The cells were plated on T-225 flasks at 25% confluence and allowed to expand in culture medium containing 50:50 Dulbecco’s modified Eagle’s medium (DMEM)- Ham’s F12 (Gibco, Grand Island, NY), 10% FBS (Biowhittaker, Walkersville, MD), 1% non-essential amino acids (NEAA) (Invitrogen, Grand Island, NY), 25 µg of l-ascorbic acid (Sigma, St Louis, MO) and 1% penicillin-streptomycin-fungizone (PSF) (Sigma, St Louis, MO). At approximately 90% confluence, the cells were passaged using trypsin/EDTA (Sigma, St Louis, MO) and counted with a hemocytometer. All cells used in the experiment were passaged once.
Scaffold, spinner flask preparation and cell seeding (Phase I and II)
For phase I and II of the experiment, non-woven PLLA (Biomedical Structures, Warwick, RI) scaffold sheets of molecular weight 100 kDa were cut into cylinders 2 mm in thickness and 3 mm in diameter using a 3 mm dermal punch. The scaffolds were sterilized by treatment with ethylene oxide. They were then pre-wetted with 100% ethyl alcohol, washed twice with phosphate buffered saline (PBS) and seeded into a spinner flask filled with medium + 10% FBS using a technique described elsewhere [24]. Briefly, 30 scaffolds were cultured in each spinner flask containing 400 ml of media. A seeding density of 1 million cells/ml was utilized. Stirrer bars at the bottom of the spinner flask were rotated at 60 RPM post-seeding for 3 days. The scaffolds were left in the spinner flask for an additional 4 days to allow cells to adhere to the polymer. After 1 wk, the constructs were transferred to agarose-coated six-well plates. The coatings were prepared by adding 1.5 ml of 2% sterile molten agarose to each well.
Tissue culture and experimental groups (Phase I)
Each agarose coated well plate contained 5 ml of culture medium. The culture medium was supplemented with 1% or 10% FBS in the presence or absence of 10 ng/ml TGF-β1 (PeproTech Inc., Rocky Hill, NJ) (n = 8 per group). Constructs (five per well) were housed in static culture and medium was changed once every three days for a period of four wks. At t = 4 wks, constructs were collected for histological and biochemical analysis.
HP preparation, application and experimental groups (Phase II)
Post-seeding, phase II constructs were placed in agarose coated wells containing culture medium supplemented with 1% FBS for an additional week to increase cell-scaffold adhesion and improve cell migration into the scaffold. The scaffolds were then randomly assigned into HP or control groups and housed in sterile customized histology cartridges capable of holding up to nine constructs (n = 8 per group). Four different HP groups were tested. Constructs were pressurized at 10 MPa, 0 Hz or 10 MPa, 0.1 Hz in the presence or absence of 10 ng/ml TGF-β1 once every 3 days for 1 hr. The growth factor dose and stimulation regimens were chosen based on previous studies that highlighted their benefit.[7, 15] Four control groups were utilized in the experiment. Constructs in the presence or absence of TGF-β1 were housed in the incubator (culture controls) for the experiment duration or manipulated for HP stimulation (HP controls) without receiving any HP stimulus.
Prior to HP stimulation, the cartridges with cell-seeded scaffolds were transferred to individual heat sealable sterile bags (Ampac, Cincinnati, OH) containing 30 ml of culture medium supplemented with 10 mM Hepes (Fisher Scientific, Pittsburg, PA). The bag was tapped lightly to remove air bubbles from the medium and from the cartridges, and heat-sealed. The HP set-up used in this experiment has been described previously [18]. Briefly, control specimens were placed in an open unpressurized chamber while the pressurized specimens were placed in a water filled stainless steel chamber that connected to an Instron 8871. Post-stimulation, the bags were transferred back to the culture hood and unsealed. The cartridges were then placed back into six-well plates with fresh medium. At t = 4 wks, samples were collected for histological, immunohistological, biochemical and biomechanical analyses.
Histology (Phase I and II)
At t = 4 wks, one sample from each group was frozen using HistoPrep (Fisher Scientific, Pittsburg, PA) and then sectioned at 14 µm. Safranin-O and fast grain stains were performed to examine the distribution of GAGs in the section [25]. Picrosirius red was used to determine the presence of collagen [26].
Immunohistochemistry (IHC) (Phase II)
Collagen I and collagen II presence was determined using IHC, as described previously [27]. Briefly, the sectioned samples were fixed in chilled acetone at 4 °C for 20 min and then rinsed with IHC buffer. They were then quenched of peroxidase activity with hydrogen peroxide/methanol for 30 min and blocked with horse serum (Vectastain ABC kit). Each slide was then exposed to mouse anti-COL 1 (1:1000 dilution) (Accurate Chemicals, Westbury, NY) mouse or anti-COL 2 (1:1000 dilution) (Chondrex, Redmond, WA) antibodies for 1 hr. After incubation with the primary antibodies, a secondary mouse IgG antibody (Vectastain ABC kit) was added for 30 min at the dilution specified by the kit protocol and color was developed using the Vectastain ABC reagent and DAB (Vector Labs, Burlingame, CA) for 8 min.
Biochemistry (Phase I and II)
Biochemical tests were performed on samples at t = 0 and t = 4 wks for both phases. The 4 wk samples were obtained post-biomechanical testing for phase II of the experiment. All samples were digested at 65 °C overnight with 125 µg/ml papain (Sigma, St Louis, MO) in 50 mM phosphate buffer (pH = 6.5) containing 2 mM N-acetyl cysteine and 2 mM EDTA. A picogreen cell proliferation assay kit (Molecular Probes) was used to determine total DNA content in each sample. Total GAG was quantified using the Blyscan GAG Assay kit [28]. A modified chloramine-T hydroxyproline assay was used to determine total collagen in the construct [29].
Biomechanics (Phase II)
The viscoelastic compressive properties of samples from each group in Phase II were obtained at t = 4 wks post-stimulation using an Instron 5565 set-up described previously [30]. Incremental stress relaxation curves were obtained at 10%, 20%, and 30% strain. Data were fitted using MATLAB to an incremental stepwise viscoelastic stress relaxation solution for a standard linear solid [31]. Fitted parameters obtained were converted to instantaneous modulus (Ei), relaxation modulus (Er) and coefficient of viscosity (μ).
Statistical analyses
Quantitative biochemical and biomechanical data were compared using analysis of variance (ANOVA). If significant differences were observed, a Tukey’s post hoc test was performed to determine specific differences among groups. For phase II, univariate regression analysis was conducted to determine whether biochemical data correlated with biomechanical data. The presence of synergy between TGF-β1 and HP in our system was examined using the interaction term obtained from the 2-way ANOVA between the culture control (with and without TGF-β1) and each individual HP group (with and without TGF-β1) [32]. A significance level of 95% with a p value of 0.05 was used in all statistical tests performed. All values are reported as mean ± standard deviation. JMP IN statistical software was used for all statistical analyses.
Results
Phase I – Effect of serum and TGF-β1 on PLLA scaffolds
Gross morphology and histology (n = 3)
Figure 1A shows histology sections of constructs at t = 4 wks. Morphologically, white translucent tissue was observed in all groups. Groups treated with TGF-β1 exhibited a higher density of ECM when compared to the untreated samples, at both 1% and 10% FBS concentrations. Collagen and GAG staining was positive in all groups, with more intense staining observed in the TGF-β1 treated groups.
Figure 1.
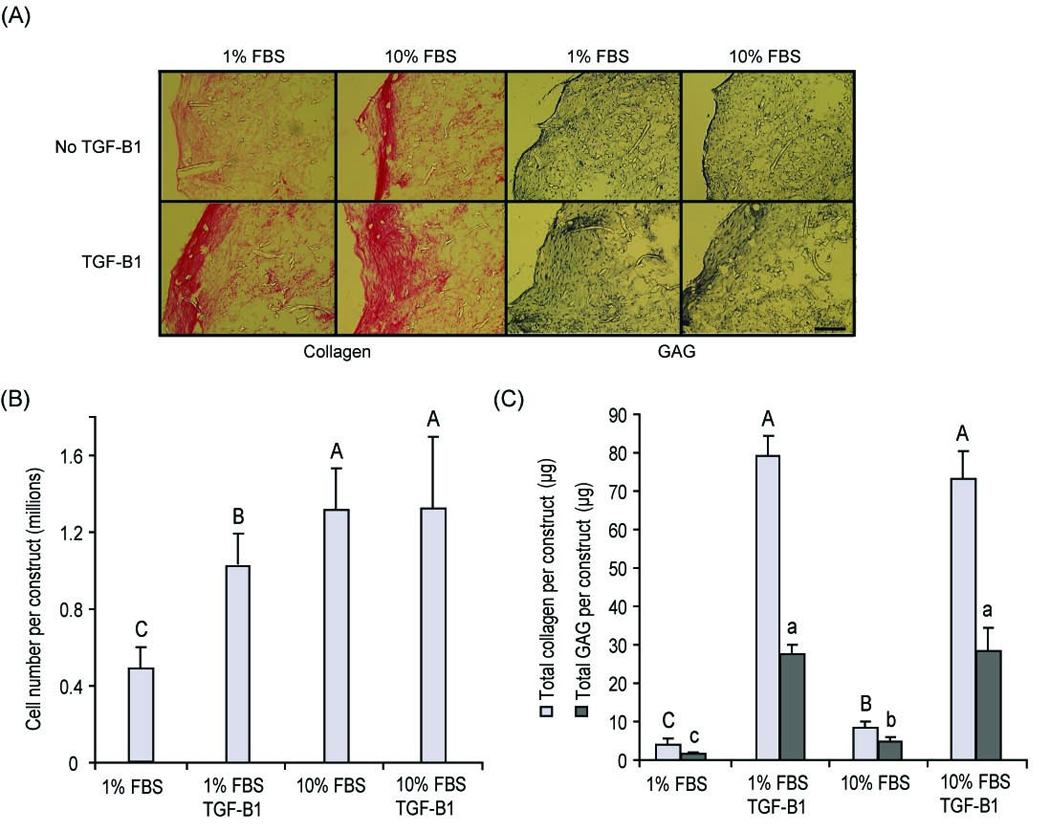
Phase I data at t = 4 wks (A) Histological sections of scaffolds. Collagen and GAG presence is observed, especially on the periphery of the scaffolds. Scale bar: 200 µm. (B) Cell number/construct. Both 10% FBS and 10 ng/ml of TGF-β1 were responsible in increasing cell number on the scaffold. (C) GAG and collagen/construct. The inclusion of TGF-β1 significantly increased collagen and GAG deposition on the construct in both the 1% FBS and 10% FBS groups. 1-way ANOVAs were performed followed by a Tukey’s post hoc analysis to determine significant differences between groups for collagen/construct and GAG/construct. Groups with the same letter (capital or small) are not significantly different from each other. All values are reported as mean ± SD. An n = 5 was used for all biochemical tests.
Biochemistry (n = 5)
At t = 4 wks, the total number of cells per construct increased by 100% in the TGF-β1 + 1% FBS group when compared to the 1% FBS control, while no significant difference was observed between the TGF-β1 + 10% FBS and the 10% FBS group (p = 0.02) (Fig. 1B). A 15-fold and 8-fold increase was observed in total collagen and total GAG/construct, respectively, in TGF-β1 treated samples when compared to their non-treated controls for the 1% and 10% FBS groups (p < 0.001) (Fig. 1C). No significant difference was observed, however, between the 1% FBS + TGF-β1 and 10% FBS + TGF-β1 groups for collagen or GAG content. Thus, for phase II of the experiment, a concentration of 1% FBS was utilized in the culture medium.
Phase II – Effect of TGF-β1 + HP on PLLA scaffolds
Gross morphology, histology and IHC (n = 3)
On gross examination, all constructs showed presence of ECM. However, constructs cultured with TGF-β1 appeared denser than the constructs cultured without TGF-β1, HP application notwithstanding. At t = 4 wks, construct thickness and diameter ranged from 1.8 ± 0.4 mm to 2.2 ± 0.1 mm and 2.8 ± 0.2 mm to 3.2 ± 0.1 mm, respectively; however, no significant difference was observed between any of the scaffolds for culturing condition (p = 0.43, p = 0.63) or for growth factor treatment (p = 0.09, p = 0.07) (Table 1). Weak staining for collagen and GAG was observed for constructs cultured without TGF-β1 using picrosirius red and safranin-o/fast green stains (Fig. 2). The staining was more intense in TGF-β1 treated groups, especially around the periphery of the scaffolds. IHC staining showed presence of collagen I in all groups, with weak staining for collagen II (images not shown).
Table 1.
Wet weight, dry weight, thickness and diameter of constructs in Phase II. Growth factor application was found to significantly increase the wet and dry weight of the constructs. No significant differences were observed among groups for the wet weight, dry weight, thickness and diameter of the constructs.
| Wet weight | Dry weight | Thickness | Diameter | |||||
|---|---|---|---|---|---|---|---|---|
| Groups | No TGF-β | TGF-β1 | No TGF-β1 | TGF-β1 | No TGF-β1 | TGF-β1 | No TGF-β1 | TGF-β1 |
| (mg) | (mg) | (mg) | (mg) | (mm) | (mm) | (mm) | (mm) | |
| Culture | ||||||||
| 11.3 ± 2.5 | 13.1 ± 1.0 | 1.8 ± 0.1 | 2.0 ± 0.1 | 1.9 ± 0.2 | 2.2 ± 0.1 | 2.8 ± 0.2 | 3.0 ± 0.2 | |
| control | ||||||||
| HP | ||||||||
| 11.2 ± 2.9 | 13.0 ± 2.1 | 1.9 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.3 | 2.9 ± 0.2 | 3.1 ± 0.3 | |
| control | ||||||||
| HP | ||||||||
| 13.2 ± 2.5 | 15.3 ± 3.3 | 1.8 ± 0.3 | 2.1 ± 0.1 | 1.8 ± 0.4 | 2.2 ± 0.1 | 2.8 ± 0.4 | 3.2 ± 0.1 | |
| 10 MPa, 0 Hz | ||||||||
| HP | ||||||||
| 12.7 ± 1.6 | 15.9 ± 1.4 | 1.8 ± 0.2 | 2.0 ± 0.1 | 2.0 ± 0.1 | 2.1 ± 0.2 | 3.0 ± 0.1 | 2.9 ± 0.5 | |
| 10 MPa, 0.1 Hz | ||||||||
| p value – | ||||||||
| 0.004 | 0.03 | 0.07 | 0.09 | |||||
| growth factor | ||||||||
| p value – | ||||||||
| 0.35 | 0.06 | 0.43 | 0.63 | |||||
| culture condition | ||||||||
Figure 2.
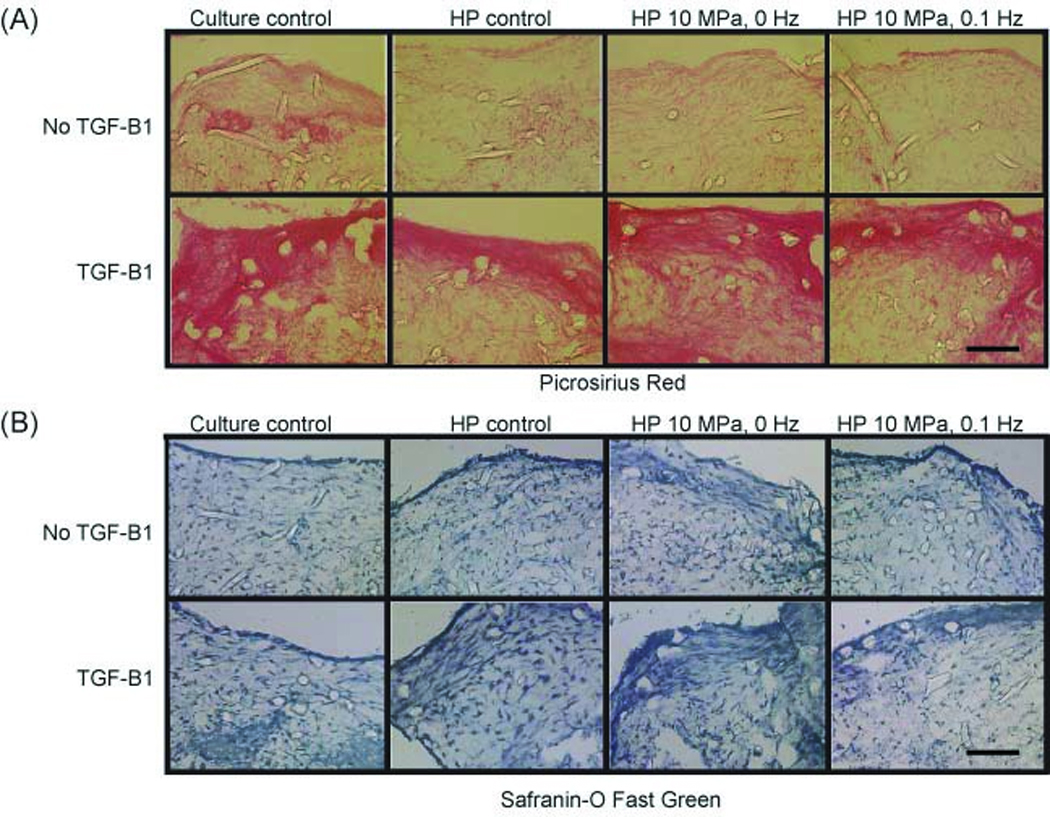
Phase II - histological sections of scaffolds from Phase II at t = 4 wks. (A) Picrosirus Red. (B) Safranin-O Fast Green. TGF-β1 was found to increase intensity in collagen staining in all groups. In the 10 MPa, 0 Hz + TGF-β1 group, strong staining for collagen and GAG was observed throughout the construct. Scale bar: 200 µm
Biochemistry (n = 5)
Wet weight of the constructs ranged from 11.3 ± 2.5 mg to 15.9 ± 1.4 mg (Table 1). The growth factor treatment was found to be a significant factor (p = 0.004) using a 2-way ANOVA while the culturing condition was not (p = 0.35). Dry weights of the constructs ranged from 1.8 ± 0.1 mg to 2.1 ± 0.1 mg, with a significant difference observed in samples treated with TGF-β1 over the untreated groups, (p = 0.03) but not when comparing different culturing conditions (p = 0.06) (Table 1).
At t = 0, the cell seeding density was found to be approximately 0.61 ± 0.1 million cells/scaffold (shown by the dotted line in Fig 3A). By t = 4 wks, the cell number/scaffold increased to 0.71 ± 0.1 million cells/scaffold in the culture control group. A drop in cell number (approximately 20%) was observed in all HP treated groups when compared to the culture control; however, the cell numbers were not significantly different from the seeding density at t = 0. The addition of TGF-β1 significantly increased cell number in all groups when compared to their respective no-GF groups by at least 15%.
Figure 3.
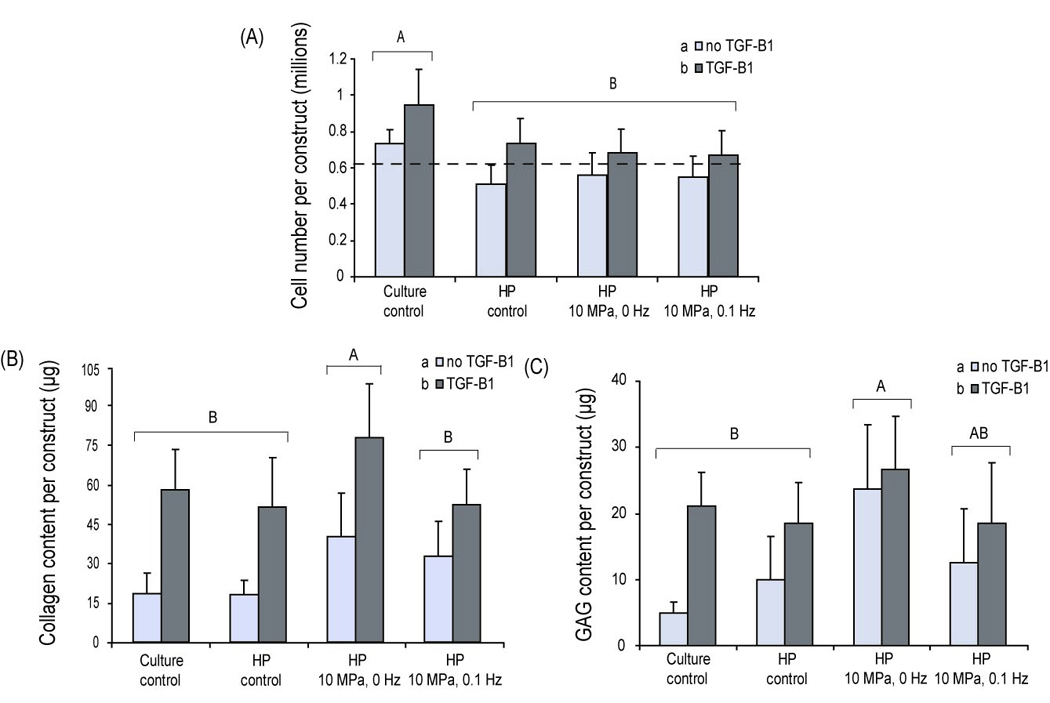
Phase II – Biochemical analysis at t = 4 wks: A) Cell number/scaffold. The static culture control was found to contain more cell/construct than all other groups at t = 4 wks. Dotted line signifies original seeding density of 0.61 million cells/scaffold. B) Collagen/scaffold. A significant increase in collagen deposition is observed in constructs exposed to 10 MPa and 0 Hz hydrostatic pressure. In addition, a significant increase is also observed in all groups treated with growth factor over the untreated groups C) GAG/scaffold. A significant increase in GAG deposition is observed in constructs exposed to 10 MPa and 0 Hz hydrostatic pressure. In addition, a significant increase is also observed in all groups treated with growth factor over the untreated groups. Statistics were conducted using a 2-way ANOVA. Groups with the same letter (capital or small) are not significantly different from each other. All values are reported in mean ± SD. An n=5 was used for all biochemical tests.
In terms of total collagen/scaffold, at t = 4 wks both the type of stimulus applied and growth factor treatment were found to be significant factors (p = 0.01, and p = 0.004, respectively) (Fig. 3B). Specifically, samples stimulated with 10 MPa, 0 Hz induced a two-fold increase in collagen content/construct when compared to the culture and HP control. If normalized to cell number, this effect was even higher with a three-fold increase observed in collagen/cell number. TGF-β1 alone was found to significantly increase collagen deposition on the scaffold up to 4-fold when compared to respective no-GF groups. TGF-β1 was found to further enhance collagen deposition on constructs exposed to HP (10 MPa, 0 Hz) by approximately 1.6 fold. This resulted in constructs with the highest overall collagen/construct with 76 ± 22 µg. If normalized to cell number, this effect was even higher with a 2-fold increase in collagen deposition over the culture control + GF control. Overall, an additive effect was observed in constructs exposed to TGF-β1 and HP, 10 MPa, 0 Hz when compared to the culture control group suggesting a beneficial interplay between the applied chemical and mechanical stimuli.
Both treatment type and growth factor application were found to be significant factors when analyzing total GAG/scaffold (p = 0.03 and p = 0.01, respectively) (Fig. 3C). Without TGF-β1 treatment, constructs in the 10 MPa, 0 Hz group contained at least 50% more GAG than when compared to other groups. If normalized to cell number, a 7-fold increase in GAG content was observed in the 10 MPa, 0 Hz group when compared to the culture control. In the presence of TGF-β1, GAG production increased 4-fold in the culture control group when compared to its no-GF group. A significant increase in GAG was not observed, however, in the 10 MPa, 0 Hz group when compared to its no GF group. Despite this, the highest GAG content was observed in the 10 MPa, 0 Hz + TGF-β1 group (28 ± 6 µg). These results suggest that although the individual application of TGF-β1 and HP enhanced the GAG formation on constructs, their combination did not result in an additive or synergistic effect.
Biomechanics (n = 5)
At all strain levels (10, 20 and 30%), the type of stimulus and growth factor were found to be significant factors for all three tested parameters. TGF-β1 was found to increase the compressive properties of all unpressurized and pressurized constructs. Its effect was most pronounced for the coefficient of viscosity where a 2.3 fold increase was measured in constructs exposed to the combination of TGF-β1 and 10 MPa of HP when compared to the HP group alone. At 10% strain, the 10 MPa, 0 Hz + TGF-β1 group exhibited an instantaneous modulus, relaxation modulus , and coefficient of viscosity of 104 ± 16 kPa, 40 ± 5 kPa, and 1403 ± 193 Pa-s, respectively; these values were statistically higher than the other tested groups (Fig. 4 A, B and C). An additive effect of TGF-β1 and HP 10 MPa, 0 Hz group was observed for the instantaneous modulus. A synergistic increase in the relaxation modulus and coefficient of viscosity was observed in the 10 MPa, 0 Hz group when compared to the culture control group.
Figure 4.
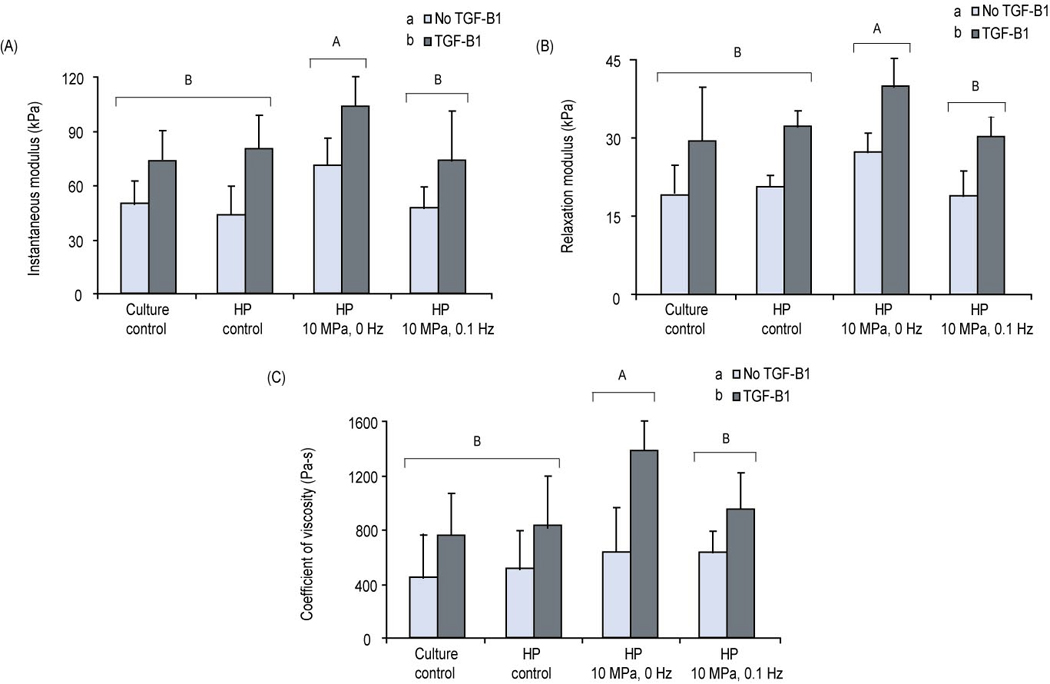
Phase II - compressive properties of the constructs (10% strain) at t = 4 wks: (A) Instantaneous modulus (B) Relaxation modulus (C) Coefficient of viscosity. Statistics were conducted using a 2-way ANOVA. The instantaneous modulus, relaxation modulus and coefficient of viscosity of constructs stimulated at 10 MPa, 0 Hz was significantly high than constructs in other groups. Growth factor treatment was significant for all measurements Groups with the same letter (capital or small) are not significantly different from each other. All values are reported in mean ± SD. An n=5 was used for all biomechanical tests.
Correlation between biochemical and biomechanical data
The three compressive properties obtained from incremental stress relaxation curves were correlated with the two measured ECM components, GAG and collagen at each strain level strain. At 10% strain, univariate regression analysis showed a significant correlation between instantaneous modulus and GAG/construct (r2 = 0.42, p < 0.001), and collagen/construct (r2 = 0.56, p < 0.001) (Fig. 5 A, B). Similar results were obtained when correlating the relaxation modulus and GAG/construct (r2 = 0.24, p = 0.002), and collagen/construct (r2 = 0.45, p < 0.001) (Fig 5. C, D). Further, the coefficient of viscosity correlated significantly with GAG/construct (r2 = 0.16, p = 0.01), and collagen/construct (r2 = 0.52 p < 0.001) (Fig. 5 E, F) as well. Similar significant correlations were obtained at 20 and 30% strain levels (data not shown).
Figure 5.
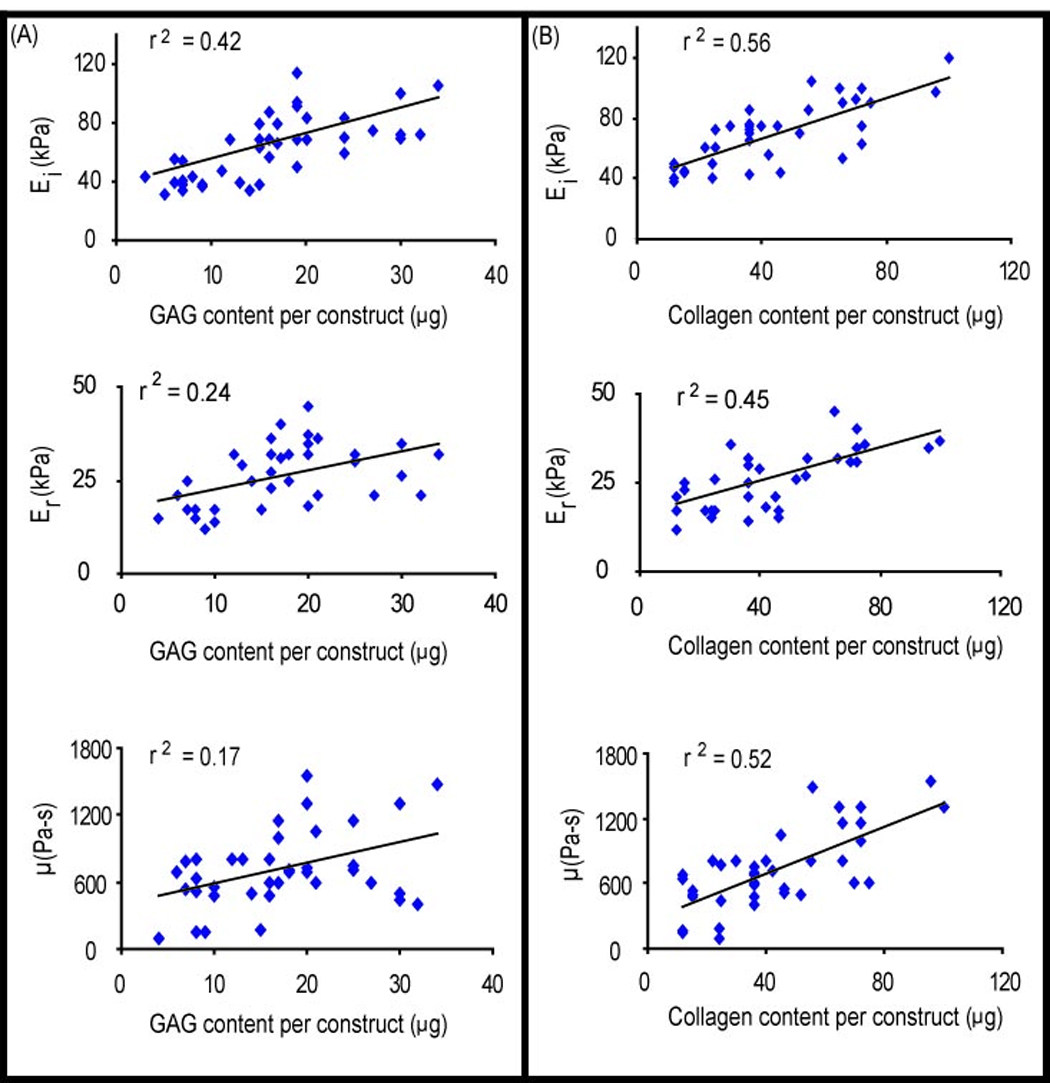
Correlation analysis between compressive properties (10% strain) and biochemical properties at t = 4 wks. (A) Correlation to GAG content/construct (B) Correlation to collagen content/construct. Significant correlations were observed for all parameters between the compressive properties and the two ECM components. 40 data points from TGF-β1 and no-TGF-β1 treated groups were used in each comparison.
Discussion
This study investigated the effects of TGF-β1 and HP at low serum concentrations on the ability of meniscus cells to produce relevant ECM and enhance construct biomechanical functionality. TGF-β1 was found to significantly increase collagen and GAG deposition in PLLA scaffolds in phase I of the study. In addition, no significant differences were observed in the biochemical composition of PLLA constructs cultured with TGF-β1 at 1% or 10% FBS. In phase II, an HP regimen of 10 MPa, 0 Hz was found to significantly increase collagen and GAG deposition on the scaffold over the controls and the 10 MPa, 0.1 Hz group. ECM formation and compressive properties were further enhanced when TGF-β1 was added in conjunction with 10 MPa, 0 Hz HP, with additive increases in collagen deposition on the scaffold and the instantaneous modulus. Synergistic increases in the relaxation modulus and coefficient of viscosity were observed in this group as well over the culture control.
In traditional cell culture, a high concentration of FBS (~10%) is used in the medium to enhance cell attachment and proliferation. Unfortunately, this introduces the risk of disease transmission in clinical settings [10]. The ability to use a defined culture medium in lieu of FBS increases the safety of engineered meniscus constructs and may help to reduce construct variability due to changes in serum components originating from different animals. Defined culture medium like knock-out serum replacement (KOSR) or Nutridoma, have been successfully used in articular cartilage engineering while serum-free medium with bFGF has been reported effective in self-assembling meniscus cells and co-cultures of meniscus cells and articular chondrocytes [33–35]. However, for scaffold culture, elimination of serum during meniscus cell culture on scaffolds significantly lowers the ability of cells to proliferate and produce ECM (data not reported). In this experiment, it was found that lowering FBS concentration from 10% to 1% resulted in a significant drop (~60%) in cell number per scaffold, as well as ~50% less collagen and GAG deposition on the scaffold. However, when both groups were supplemented with 10 ng/ml of TGF-β1 the effect of FBS concentration was overshadowed, resulting in increased cell proliferation and a 15- fold increase in collagen and a 8-fold increase in GAG over the no-growth factor controls. This suggests that lowering FBS concentration to 1% does not have a detrimental effect on the cells’ ability to proliferate on PLLA scaffolds and produce ECM, so long that the medium is supplemented with 10 ng/ml of exogenous TGF-β1.
Our experiment showed a dramatic effect of TGF-β1 on ECM formation by adult meniscal cells in vitro that has not been shown before. Several studies have investigated the effects of TGF-β1 in monolayer culture and on scaffolds using fibroblasts, articular chondrocytes and immature meniscus cells.[7, 36–39] In fibroblasts, TGF-β1 has been shown to increase fibronectin, thrombospondin and collagen mRNA levels [40]. In chondrocytes, TGF-β1 has been implicated in increasing cell proliferation, and collagen II and sulfated GAG synthesis [40–43]. TGF-β1 may directly influence GAG synthesis by accelerating glucose transport in human articular chondrocytes via protein kinase C and extracellular signal-regulated kinase-dependent signaling pathways [44]. A study with human chondrocytes showed that TIMP mRNA was upregulated in the presence of TGF-β1 suggesting that the growth factor also played a protective role once ECM was formed [39]. In all biochemical and biomechanical tests conducted in this study, the growth factor was found to be significant. In Phase I, for example, a dramatic increase was observed in collagen (15-fold) and GAG (8-fold) deposition when TGF-β1 was added. In addition, TGF-β1 was also responsible in increasing cell proliferation on the PLLA scaffolds as determined from picogreen assays. Thus, the results support the hypothesis that TGF-β1 is a powerful modulator of ECM synthesis.
This study showed benefits for both static and intermittent hydrostatic pressure, although static hydrostatic pressure resulted in a greater enhancement of construct biochemical and biomechanical properties. In the absence of TGF-β1, constructs stimulated with static HP at 10 MPa were found to have the highest collagen and GAG content when compared to control and intermittent HP groups. This result is in agreement with previous studies applying static HP in 3-D where increases in protein production were observed for important cartilaginous markers [45, 46]. If the total collagen and GAG production per construct were normalized to cell number, however, both static and intermittent HP regimens were found to significantly enhance collagen and GAG production per cell when compared to the controls. This is consistent with prior studies conducted in monolayer culture with TMJ disc fibrochondrocytes and articular chondrocytes where enhances in collagen and aggrecan gene expression were observed under static and cyclic HP conditions [18, 20, 47, 48].
The hypothesis that the combination of HP and TGF-β1 would result in additive or synergistic effects in the biochemical properties was confirmed. In a previous study, we highlighted the benefit of static and intermittent HP for meniscus tissue engineering purposes [15]. In the current study, we examined the effect of adding a growth factor, TGF-β1 in concert with the HP stimulus on the biochemical and biomechanical properties of the construct. We found that the combination of 10 ng/ml of TGF-β1 and 10 MPa, 0 Hz of HP was responsible in generating PLLA constructs with the highest overall biochemical and biomechanical properties. Additive increases were observed in collagen deposition/construct and the instantaneous modulus, while synergistic increases were observed in the relaxation modulus and coefficient of viscosity for our 10 MPa, 0 Hz constructs exposed to TGF-β1.
The combination of HP (static or intermittent) and TGF-β1 may stimulate similar down-stream pathways leading to adjunctive, additive or synergistic effects. For example, both HP and TGF-β1 have been shown to increase TIMP expression in meniscus cells and chondrocytes [12, 39]. HP is known to alter conformations of cell membrane ion transport pathways resulting in altered intracellular ion flux, thereby influencing other downstream processes [49]. A recent study has shown that intracellular Ca2+ mobilization can significantly affect the cells’ response to the TGF-β1 by controlling the Smad-TGF-β pathway [50]. Certainly, we may expect several different cell-signaling pathways with feedback loops either complementing or antagonizing the final down-stream effect. Though the exact mechanism is yet to be elucidated, we can conclude that for the pressure and dosage combination we chose, i.e., 10 MPa, 0 Hz and 10 ng/ml of TGF-β1, there was a positive effect. It is possible that other HP regimens or TGF-β1 doses may foster alternative responses. For example, a recent study from our laboratory using TGF-β1 (30 ng/nl) and 10 MPa static HP showed synergistic increases in collagen/wet weight and additive increases in aggregate and Young’s modulus in self-assembled articular cartilage constructs [51]. Interestingly, the same result could not be reproduced when static HP of 5 MPa was applied in the presence of TGF-β1. This suggests that cells respond differently to varying HP regimens in 3-D environments, possibly by stimulating different downstream pathways that can affect eventual gene expression and protein production levels. This might explain why additive and synergistic increases in construct biochemical and biomechanical properties under static culturing conditions could not be achieved under the particular intermittent conditions that we examined (10 MPa, 0.1 Hz).
Our compressive biomechanical data (instantaneous modulus, instantaneous modulus and coefficient of viscosity) showed significant correlations with GAG/construct (p < 0.05) and collagen/construct (p < 0.05) data. Traditionally, compressive properties are linked with GAG production since GAGs sequester water molecules which provide the bulk of the resistance to compressive forces [52]. These results suggest, however, that interplay between GAG molecules and collagen fibers might be responsible for the overall compressive biomechanical properties of the constructs, possibly via smaller proteoglycans such as decorin and biglycan which are known to affect collagen diameter and alignment [53]. A detailed analysis on the composition of the proteoglycans, as well as collagen, needs to be performed in the future.
After 4 wks in culture, the instantaneous moduli in the 10 MPa, 0 Hz group was approximately 105 kPa. This result is exciting since as it approaches native values; for example, the aggregate modulus of the tibial posterior portion of the medial leporine meniscus is 130 ± 30 kPa [54]. One needs to be cautious, however, in directly comparing results from the biphasic model to derive the aggregate modulus to a viscoelastic model to derive the instantaneous modulus. A comparison between the two techniques shows that values for instantaneous moduli are generally slightly lower than the aggregate moduli [55]. In a prior study, an HP regimen of 10 MPa, 0 Hz significantly increased construct compressive properties [15]. The inclusion of TGF-β1 in this study in conjunction with the HP stimulus resulted in additive and synergistic increases to the instantaneous modulus, relaxation modulus and the coefficient of viscosity. This effect was observed at all tested strain levels (10, 20 and 30% - data not shown for 20 and 30% strain). In addition, to ensure that the mechanical properties obtained were not those of the PLLA scaffold, separate biomechanical tests were performed on unseeded constructs at t = 0 and t = 4 wks in regular culture medium with an obtained instantaneous modulus ranging from 10–15 kPa.
Conclusions
It was found that 90% of the FBS content in the standard tissue culture medium could be successfully replaced with addition of 10 ng/ml TGF-β1 in knee meniscus tissue engineering, yielding significant increases in ECM production and cell proliferation. Furthermore, a combination of HP and TGF-β1 led to additive and synergistic increases in the biochemical and compressive properties of tissue engineered meniscal constructs. To produce constructs mimicking native tissue, additional combinatorial stimulation regimens will need to be tested in future experiments to maximize the effect of the applied biochemical and biomechanical stimuli.
Acknowledgments
The authors would like to thank Dr. Jerry Hu for aiding with the revision of the manuscript. In addition, the authors would like to acknowledge NIAMS RO1 AR 47839-2 for funding this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sweigart MA, Athanasiou KA. Toward tissue engineering of the knee meniscus. Tissue Eng. 2001;7(2):111–129. doi: 10.1089/107632701300062697. [DOI] [PubMed] [Google Scholar]
- 2.Maletius W, Messner K. The effect of partial meniscectomy on the long-term prognosis of knees with localized, severe chondral damage. A twelve- to fifteen-year followup. Am J Sports Med. 1996;24(3):258–262. doi: 10.1177/036354659602400302. [DOI] [PubMed] [Google Scholar]
- 3.Webber RJ, Harris MG, Hough AJ., Jr Cell culture of rabbit meniscal fibrochondrocytes: proliferative and synthetic response to growth factors and ascorbate. J Orthop Res. 1985;3(1):36–42. doi: 10.1002/jor.1100030104. [DOI] [PubMed] [Google Scholar]
- 4.Bhargava MM, Attia ET, Murrell GA, Dolan MM, Warren RF, Hannafin JA. The effect of cytokines on the proliferation and migration of bovine meniscal cells. Am J Sports Med. 1999;27(5):636–643. doi: 10.1177/03635465990270051601. [DOI] [PubMed] [Google Scholar]
- 5.Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis Cartilage. 1995;3(2):127–138. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 6.Tumia NS, Johnstone AJ. Promoting the proliferative and synthetic activity of knee meniscal fibrochondrocytes using basic fibroblast growth factor in vitro. Am J Sports Med. 2004;32(4):915–920. doi: 10.1177/0363546503261710. [DOI] [PubMed] [Google Scholar]
- 7.Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res. 2005;23(5):1184–1190. doi: 10.1016/j.orthres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Adesida AB, Grady LM, Khan WS, Hardingham TE. The matrix-forming phenotype of cultured human meniscus cells is enhanced after culture with fibroblast growth factor 2 and is further stimulated by hypoxia. Arthritis Res Ther. 2006;8(3):R61. doi: 10.1186/ar1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright M. TGF-B1 in bovine serum. Art to Science. 2001;19(3):1–3. [Google Scholar]
- 10.Mandl EW, van der Veen SW, Verhaar JA, van Osch GJ. Serum-free medium supplemented with high-concentration FGF2 for cell expansion culture of human ear chondrocytes promotes redifferentiation capacity. Tissue Eng. 2002;8(4):573–580. doi: 10.1089/107632702760240490. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki T, Toyoda T, Suzuki H, Hisamori N, Matsumoto H, Toyama Y. Hydrostatic pressure modulates mRNA expressions for matrix proteins in human meniscal cells. Biorheology. 2006;43(5):611–622. [PubMed] [Google Scholar]
- 12.Natsu-Ume T, Majima T, Reno C, Shrive NG, Frank CB, Hart DA. Menisci of the rabbit knee require mechanical loading to maintain homeostasis: cyclic hydrostatic compression in vitro prevents derepression of catabolic genes. J Orthop Sci. 2005;10(4):396–405. doi: 10.1007/s00776-005-0912-x. [DOI] [PubMed] [Google Scholar]
- 13.Fink C, Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Guilak F. The effect of dynamic mechanical compression on nitric oxide production in the meniscus. Osteoarthritis Cartilage. 2001;9(5):481–487. doi: 10.1053/joca.2001.0415. [DOI] [PubMed] [Google Scholar]
- 14.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12(9):736–744. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Gunja NJ, Athanasiou KA. Effects of hydrostatic pressure on leporine meniscus cell-seeded PLLA scaffolds. Tissue Eng. 2008 doi: 10.1002/jbm.a.32451. Submitted. [DOI] [PubMed] [Google Scholar]
- 16.Waters RL, Lunsford BR, Perry J, Byrd R. Energy-speed relationship of walking: standard tables. J Orthop Res. 1988;6(2):215–222. doi: 10.1002/jor.1100060208. [DOI] [PubMed] [Google Scholar]
- 17.Huberti HH, Hayes WC. Patellofemoral contact pressures. The influence of q-angle and tendofemoral contact. J Bone Joint Surg Am. 1984;66(5):715–724. [PubMed] [Google Scholar]
- 18.Almarza AJ, Athanasiou KA. Effects of hydrostatic pressure on TMJ disc cells. Tissue Eng. 2006;12(5):1285–1294. doi: 10.1089/ten.2006.12.1285. [DOI] [PubMed] [Google Scholar]
- 19.Hansen U, Schunke M, Domm C, Ioannidis N, Hassenpflug J, Gehrke T, et al. Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech. 2001;34(7):941–949. doi: 10.1016/s0021-9290(01)00050-1. [DOI] [PubMed] [Google Scholar]
- 20.Smith RL, Lin J, Trindade MC, Shida J, Kajiyama G, Vu T, et al. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev. 2000;37(2):153–161. [PubMed] [Google Scholar]
- 21.Carver SE, Heath CA. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotechnol Bioeng. 1999;62(2):166–174. [PubMed] [Google Scholar]
- 22.Miyanishi K, Trindade MC, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, et al. Dose- and time-dependent effects of cyclic hydrostatic pressure on transforming growth factor-beta3-induced chondrogenesis by adult human mesenchymal stem cells in vitro. Tissue Eng. 2006;12(8):2253–2262. doi: 10.1089/ten.2006.12.2253. [DOI] [PubMed] [Google Scholar]
- 23.Miyanishi K, Trindade MC, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, et al. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng. 2006;12(6):1419–1428. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- 24.Allen KD, Athanasiou KA. Scaffold and growth factor selection in temporomandibular joint disc engineering. J Dent Res. 2008;87(2):180–185. doi: 10.1177/154405910808700205. [DOI] [PubMed] [Google Scholar]
- 25.Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53(1):69–82. [PubMed] [Google Scholar]
- 26.Battlehner CN, Carneiro Filho M, Ferreira Junior JM, Saldiva PH, Montes GS. Histochemical and ultrastructural study of the extracellular matrix fibers in patellar tendon donor site scars and normal controls. J Submicrosc Cytol Pathol. 1996;28(2):175–186. [PubMed] [Google Scholar]
- 27.Elder BD, Athanasiou KA. Effects of confinement on the mechanical properties of self-assembled articular cartilage constructs in the direction orthogonal to the confinement surface. J Orthop Res. 2008;26(2):238–246. doi: 10.1002/jor.20480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pietila K, Kantomaa T, Pirttiniemi P, Poikela A. Comparison of amounts and properties of collagen and proteoglycans in condylar, costal and nasal cartilages. Cells Tissues Organs. 1999;164(1):30–36. doi: 10.1159/000016640. [DOI] [PubMed] [Google Scholar]
- 29.Woessner JF. The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 30.Allen KD, Athanasiou KA. Viscoelastic characterization of the porcine temporomandibular joint disc under unconfined compression. J Biomech. 2006;39(2):312–322. doi: 10.1016/j.jbiomech.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Allen KD, Athanasiou KA. A surface-regional and freeze-thaw characterization of the porcine temporomandibular joint disc. Ann Biomed Eng. 2005;33(7):951–962. doi: 10.1007/s10439-005-3872-6. [DOI] [PubMed] [Google Scholar]
- 32.Slinker BK. The statistics of synergism. J Mol Cell Cardiol. 1998;30(4):723–731. doi: 10.1006/jmcc.1998.0655. [DOI] [PubMed] [Google Scholar]
- 33.Taha MF, Valojerdi MR. Effect of bone morphogenetic protein-4 on cardiac differentiation from mouse embryonic stem cells in serum-free and low-serum media. Int J Cardiol. 2007 doi: 10.1016/j.ijcard.2007.04.173. [DOI] [PubMed] [Google Scholar]
- 34.Yates KE, Allemann F, Glowacki J. Phenotypic analysis of bovine chondrocytes cultured in 3D collagen sponges: effect of serum substitutes. Cell Tissue Bank. 2005;6(1):45–54. doi: 10.1007/s10561-005-5810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoben GM, Athanasiou KA. Creating a spectrum of fibrocartilages through different cell sources and biochemical stimuli. Biotechnol Bioeng. 2007 doi: 10.1002/bit.21768. [DOI] [PubMed] [Google Scholar]
- 36.Ye C, Deng Z, Li B. Effect of three growth factors on proliferation and cell phenotype of human fetal meniscal cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2007;21(10):1137–1141. [PubMed] [Google Scholar]
- 37.Marsano A, Millward-Sadler SJ, Salter DM, Adesida A, Hardingham T, Tognana E, et al. Differential cartilaginous tissue formation by human synovial membrane, fat pad, meniscus cells and articular chondrocytes. Osteoarthritis Cartilage. 2007;15(1):48–58. doi: 10.1016/j.joca.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Fortier LA, Nixon AJ, Mohammed HO, Lust G. Altered biological activity of equine chondrocytes cultured in a three-dimensional fibrin matrix and supplemented with transforming growth factor beta-1. Am J Vet Res. 1997;58(1):66–70. [PubMed] [Google Scholar]
- 39.Gunther M, Haubeck HD, van de Leur E, Blaser J, Bender S, Gutgemann I, et al. Transforming growth factor beta 1 regulates tissue inhibitor of metalloproteinases-1 expression in differentiated human articular chondrocytes. Arthritis Rheum. 1994;37(3):395–405. doi: 10.1002/art.1780370314. [DOI] [PubMed] [Google Scholar]
- 40.Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Kraan P, Vitters E, van den Berg W. Differential effect of transforming growth factor beta on freshly isolated and cultured articular chondrocytes. J Rheumatol. 1992;19(1):140–145. [PubMed] [Google Scholar]
- 42.Rosen DM, Stempien SA, Thompson AY, Seyedin SM. Transforming growth factor-beta modulates the expression of osteoblast and chondroblast phenotypes in vitro. J Cell Physiol. 1988;134(3):337–346. doi: 10.1002/jcp.1041340304. [DOI] [PubMed] [Google Scholar]
- 43.de Haart M, Marijnissen WJ, van Osch GJ, Verhaar JA. Optimization of chondrocyte expansion in culture. Effect of TGF beta-2, bFGF and L-ascorbic acid on bovine articular chondrocytes. Acta Orthop Scand. 1999;70(1):55–61. doi: 10.3109/17453679909000959. [DOI] [PubMed] [Google Scholar]
- 44.Shikhman AR, Brinson DC, Lotz MK. Distinct pathways regulate facilitated glucose transport in human articular chondrocytes during anabolic and catabolic responses. Am J Physiol Endocrinol Metab. 2004;286(6):E980–E985. doi: 10.1152/ajpendo.00243.2003. [DOI] [PubMed] [Google Scholar]
- 45.Mizuno S, Tateishi T, Ushida T, Glowacki J. Hydrostatic fluid pressure enhances matrix synthesis and accumulation by bovine chondrocytes in three-dimensional culture. J Cell Physiol. 2002;193(3):319–327. doi: 10.1002/jcp.10180. [DOI] [PubMed] [Google Scholar]
- 46.Toyoda T, Seedhom BB, Yao JQ, Kirkham J, Brookes S, Bonass WA. Hydrostatic pressure modulates proteoglycan metabolism in chondrocytes seeded in agarose. Arthritis Rheum. 2003;48(10):2865–2872. doi: 10.1002/art.11250. [DOI] [PubMed] [Google Scholar]
- 47.Ikenoue T, Trindade MC, Lee MS, Lin EY, Schurman DJ, Goodman SB, et al. Mechanoregulation of human articular chondrocyte aggrecan and type II collagen expression by intermittent hydrostatic pressure in vitro. J Orthop Res. 2003;21(1):110–116. doi: 10.1016/S0736-0266(02)00091-8. [DOI] [PubMed] [Google Scholar]
- 48.Smith RL, Rusk SF, Ellison BE, Wessells P, Tsuchiya K, Carter DR, et al. In vitro stimulation of articular chondrocyte mRNA and extracellular matrix synthesis by hydrostatic pressure. J Orthop Res. 1996;14(1):53–60. doi: 10.1002/jor.1100140110. [DOI] [PubMed] [Google Scholar]
- 49.Hall AC. Differential effects of hydrostatic pressure on cation transport pathways of isolated articular chondrocytes. J Cell Physiol. 1999;178(2):197–204. doi: 10.1002/(SICI)1097-4652(199902)178:2<197::AID-JCP9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 50.Wicks SJ, Lui S, Abdel-Wahab N, Mason RM, Chantry A. Inactivation of smad-transforming growth factor beta signaling by Ca(2+)-calmodulin-dependent protein kinase II. Mol Cell Biol. 2000;20(21):8103–8111. doi: 10.1128/mcb.20.21.8103-8111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factor application on engineered articular cartilage constructs; ASME Summer Bioengineering Conference; Marco Island, FL. 2008. [Google Scholar]
- 52.Almarza AJ, Athanasiou KA. Design characteristics for the tissue engineering of cartilaginous tissues. Ann Biomed Eng. 2004;32(1):2–17. doi: 10.1023/b:abme.0000007786.37957.65. [DOI] [PubMed] [Google Scholar]
- 53.Hasler EM, Herzog W, Wu JZ, Muller W, Wyss U. Articular cartilage biomechanics: theoretical models, material properties, and biosynthetic response. Crit Rev Biomed Eng. 1999;27(6):415–488. [PubMed] [Google Scholar]
- 54.Sweigart MA, Athanasiou KA. Biomechanical characteristics of the normal medial and lateral porcine knee menisci. Proc Inst Mech Eng [H] 2005;219(1):53–62. doi: 10.1243/095441105X9174. [DOI] [PubMed] [Google Scholar]
- 55.Leipzig ND, Athanasiou KA. Unconfined creep compression of chondrocytes. J Biomech. 2005;38(1):77–85. doi: 10.1016/j.jbiomech.2004.03.013. [DOI] [PubMed] [Google Scholar]


