Abstract
Study Objectives:
To evaluate the effect of ramelteon on middle-of-the-night balance, mobility, and memory in older insomniacs.
Methods:
Thirty-three older adults (age ≥ 65 years) with insomnia were enrolled in a single-dose, 3-way crossover study of balance after bedtime administration of ramelteon, 8 mg; zolpidem, 10 mg (positive control); or placebo. Subjects were administered study medication 30 minutes before bedtime and were awakened 2 hours after dosing to evaluate balance (Sensory Organization Test), turning speed and stability, memory (immediate and delayed word recall), and adverse events. There was a 4- to 10-day washout between treatments.
Results:
Ramelteon or zolpidem (positive control) was compared with placebo. There were no differences between placebo and ramelteon on the Sensory Organization Test (p = 0.837), turn time (p = 0.776), or turn sway (p = 0.982). The positive control (zolpidem) did reveal significant impairments on the Sensory Organization Test, turn time, and turn sway (p < 0.001, all). Immediate and delayed memory recall were not significantly different with ramelteon (p = 0.683 and p = 0.650, respectively). Immediate recall declined significantly with zolpidem (p = 0.002). Adverse events were infrequent (ramelteon, n = 7; placebo, n = 7; zolpidem, n = 13); none were serious.
Conclusion:
In older adults, ramelteon did not impair middle-of-the night balance, mobility, or memory relative to placebo.
Citation:
Zammit G; Wang-Weigand S; Rosenthal M; Peng X. Effect of ramelteon on middle-of-the-night balance in older adults with chronic insomnia. J Clin Sleep Med 2009;5(1):34–40.
Keywords: Balance, stability, memory, elderly, melatonin receptor agonist, zolpidem
Insomnia is a common complaint in adults and becomes more prevalent with age. In the US, more than 50% of older adults report at least 1 chronic sleep complaint, and nearly half (30%-40%) report difficulty initiating or maintaining sleep.1,2 Remarkably, 1 epidemiologic study (n = 9000) reported that only 12% of older adults reported no sleep complaints.2
Insomnia can lead to a number of exceptionally serious adverse consequences for older adults. Among the most significant of these is an increased risk of falls.3 Injurious falls are the leading cause of death from injury in the home; 86% of such injuries occur in individuals older than 65 years of age.4 Other risks associated with insomnia include attention difficulties, slowed response times, and concentration impairments, which may also increase the risk of falls.5 Additionally, these cognitive effects could be erroneously associated with age-related symptoms of dementia or cognitive impairment.3,5 Overall, insomnia decreases quality of life,6 results in higher rates of institutionalization,7 and increases the risk of mortality.5
Medications commonly prescribed for insomnia have the potential to produce impairments in memory and psychomotor functioning, the latter of which may increase the risk of falling. This may be a consequence of the mechanism of action of these medications. Benzodiazepine receptor agonists, including older benzodiazepines (eg, triazolam, temazepam) and newer benzodiazepine receptor agonists (eg, zolpidem, zaleplon, eszopiclone), achieve their effect through action at the GABAA receptor (specifically the α1 subunit). This mechanism of action has a clear association with anterograde amnesia8–11 and has been shown to impair cognition and psychomotor function,8,10,12 which has the potential to cause injurious falls. Several studies have linked hip-fracture–related falls with benzodiazepine receptor agonist use.13–15 It is recommended that benzodiazepine receptor agonists be administered to older adults at the lowest possible dose due to impaired motor and/or cognitive performance, as well as reduced clearance and increased sensitivity to sedative effects.16–18 Ramelteon provides a distinctly different mechanistic approach to insomnia treatment for older adults. This medication is a highly selective and potent MT1/MT2 melatonin receptor agonist with negligible affinity for MT3 or other neuronal binding sites, including the GABA-receptor complex.19 By exerting its effect at MT1 and MT2 receptors, which are acted upon by melatonin during natural sleep to control circadian rhythms,20 ramelteon produces a sleep-promoting effect. In older adults, ramelteon is rapidly absorbed after oral administration, reaches peak serum concentrations within 1 to 2 hours, and is not associated with adverse pharmacodynamic effects (eg, psychomotor or memory impairment) even at twice the therapeutic dose.21 Thus, unlike most pharmacologic treatments for insomnia, the recommended 8-mg dose of ramelteon does not need to be modified for older adults. The efficacy of ramelteon in older adults has been reported in double-blind, placebo-controlled studies, which have demonstrated significant improvements on objective and subjective sleep parameters and no rebound insomnia, withdrawal effects, or psychomotor or memory impairment.22,23
Two independent pilot studies have evaluated the balance and stability of healthy older adults when awakened in the middle of the night using computerized dynamic posturography (CDP).24 In the first drug-free trial, there was no difference in balance and stability before bedtime and after middle-of-the-night awakening. In the second trial, healthy older adults were administered zolpidem or placebo before going to bed. The zolpidem 10-mg group had significantly impaired balance compared with placebo after middle-of-the-night awakening.
The current study evaluated the effects of ramelteon and placebo on balance, mobility, and memory impairment after middle-of-the-night awakening in older adults with chronic insomnia. Zolpidem was used as a positive control and was selected as a reference compound based on the findings of the above-mentioned pilot studies in healthy older adults.
METHODS
Design
This double-blind, randomized, placebo-controlled, 3-way crossover study was conducted at 5 sleep research centers in the US and was designed to evaluate the effects of ramelteon, 8 mg, versus placebo on middle-of-the-night balance, mobility, and memory in older adults (age ≥ 65 years) with self-reported chronic insomnia. Zolpidem, 10 mg, was used as a positive control. Each subject was randomly assigned to 1 of 6 sequences. Balance and mobility were measured by EquiTest™ CDP (NeuroCom® International, Inc., Clakamas, OR), a technique that quantifies standing postural control in static or dynamic conditions. The validity of EquiTest™ CDP is well documented in clinical literature.25,26 The primary endpoint of this study was the Sensory Organization Test (SOT) composite score (described below). Secondary endpoints included equilibrium scores (ES) on SOT conditions 5 and 6, SOT ratios, step/quick turn test (SQTT) scores, and memory tests (described below).
Population
Eligible subjects were at least 65 years of age and had a self-reported sleep latency of at least 30 minutes on a minimum of 3 nights per week for 3 months, a body mass index between 18 and 34 kg/m2 (inclusive), and a habitual bedtime between 21:00 and 01:00. Subjects were excluded if they had a history of sleep or balance disorders (other than insomnia). Other reasons for exclusion were a history of stroke, degenerative neurologic disease, fibromyalgia, diabetic neuropathy, thyroid dysfunction, hypotension, severe arthritis or musculoskeletal disorder; cancer not in remission for at least 5 years; psychiatric disorder ( ≤ 6 months); drug or alcohol abuse ( ≤ 12 months); a significant neurologic, hepatic, renal, endocrine, cardiovascular, gastrointestinal, pulmonary, hematologic, or metabolic disease; an acute illness (≤ 2 weeks) or hospitalization (≤ 4 weeks); known hypersensitivity to ramelteon or related compounds; any condition or use of an agent that affected the sleep-wake function, prohibited the subject from study completion, or was not in the best interest of the subject; or participation in an investigational study (≤ 30 days). Subjects performing outside normal limits on the screening EquiTest CDP were also excluded.
Procedures
All subjects completed a screening visit 7 to 14 days before randomization, at which time informed consent was obtained, medical history and vital signs were collected, and physical examination and clinical laboratory tests were conducted. Subjects also completed posturography and memory assessments to confirm the absence of impairment of balance, mobility, and memory. On the randomization night, subjects meeting enrollment criteria were admitted to the sleep laboratory and given baseline EquiTest SOT, EquiTest SQTT, and immediate recall testing. Subjects then received 8 mg of ramelteon, 10 mg of zolpidem, or placebo for 1 night each in random sequence 30 minutes before their habitual bedtime. Two hours after study-drug administration, subjects were awakened and asked to perform the SOT, SQTT, and delayed and immediate memory recall tests and were monitored for adverse events (AEs). Upon awakening, subjects were asked to sit up in bed for 2 minutes before being escorted into the testing room to ensure that subjects were completely awake for the testing. Subjects returned to bed immediately after testing. In the morning, clinical laboratory tests and AEs were recorded. The 3 treatment conditions were separated by a 4- to 10-day washout period to ensure that ramelteon and zolpidem were eliminated before dosing with the next study drug.
This study was conducted in accordance with applicable US FDA Code of Federal Regulations, the World Medical Association Declaration of Helsinki (1989), and the International Conference on Harmonisation Harmonised Tripartite Guideline for Good Clinical Practice. Each site's institutional review board approved the protocol. Subjects were recruited using a centralized advertising campaign and were paid for participation.
Measurements
The SOT
The SOT objectively identifies abnormalities in postural stability and assesses fall risk. Subjects are placed into a safety harness to prevent injury during testing and are asked to stand on a force plate inside a partially enclosed, vertical surround. The force plate has rotation and translation capabilities to measure the vertical forces exerted by the subject's feet.27
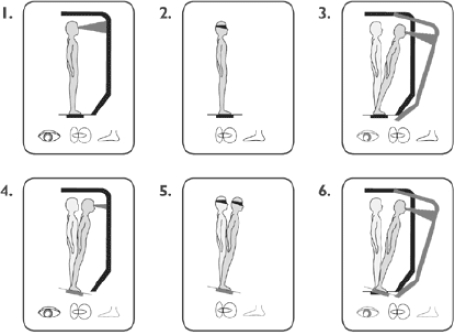
During the SOT, useful information delivered to the subject's eyes, feet, and joints is removed by the movement of the support surface and/or the visual surround, which is calibrated to tilt to directly follow the subject's anterior-posterior body sway (Figure 1). By controlling visual and proprioceptive sensory information, vestibular balance control is isolated and an adaptive response is provoked. Thus, subjects may display an inability to effectively use a sensory system or an inappropriate adaptive response. The SOT comprises 6 sensory conditions (each assessed 3 times), testing different aspects of balance (Figure 1). In condition 1, the subject has normal vision, and postural stability is assessed while the subject stands in a fixed structure. In condition 2, the subject's eyes are closed, and the structure is fixed. In condition 3, the subject's eyes are open, and the walls tilt. In condition 4, the subject's eyes are open, and the floor tilts. In condition 5, the subject's eyes are closed, and the floor tilts. In condition 6, the subject's eyes are open, the floor tilts, and the walls tilt.
Figure 1.
Sensory Organization Test: 6 conditions to test balance, used courtesy of NeuroCom® International, Inc.
One battery of tests consists of 18 trials (3 trials per condition). For each trial, an ES was calculated by comparing anterior-posterior sway during each trial to maximal theoretical sway limits (8.5° anterior, 4° posterior). The angular difference between the maximum center of gravity displacement and the theoretical maximum sway is expressed as a percentage. Thus, a score of 100 represents no sway, whereas a score of 0 indicates that the sway exceeds the limit of stability, resulting in a fall. For each condition, the average score was calculated over 3 trials and reported as ES1 to ES6. The SOT composite score (CES) was then calculated as a weighted average of all 6 conditions with each of the first 2 conditions carrying 1/14th of the weight and each of the last 4 conditions carrying 3/14ths of the weight. CES was the primary endpoint because it summarizes overall performance on the balance test.
The fifth and sixth conditions (ES5 and ES6) are the most challenging of the 6 tests and were secondary endpoints. Additional secondary endpoints included somatosensory ratio (condition 2/condition 1): ability to use somatosensory input to maintain balance; visual ratio (condition 4/condition 1): ability to use visual input to maintain balance; vestibular ratio (condition 5/condition 1): ability to use vestibular input to maintain balance; and preference ratio (condition 3+6/condition 2+5): degree to which visual information is relied upon to maintain balance, even when the information is incorrect.
The SQTT
The SQTT assesses fall risk and mobility through turn performance. The subject takes 2 steps forward on a pressure-sensitive surface, quickly turns 180°, and returns to the starting point. Time to execute the turn is recorded as turn time (in seconds), and postural stability (average center of gravity sway velocity in degrees/second) during the turn is recorded as turn sway.
The Immediate Memory Recall Test
The Immediate Memory Recall Test assesses anterograde amnesia. Subjects are presented 16 words and asked to recall the list 2 minutes later. The list used for the posttreatment recall test is different than those used for the pretreatment recall test.
The Delayed Memory Recall Test
The Delayed Memory Recall Test assesses retrograde amnesia. Approximately 2 hours after dosing, the subjects are asked to recall the list of 16 words used for the predosing immediate memory recall test. The same list is used to improve efficiency of study conduct.
Safety
Safety was measured by incidence of AEs, recorded during the middle-of-the-night awakening (2 hours after treatment) and the following morning. AEs were recorded from spontaneous reports by the subject and investigator observations. Vital sign assessment, clinical laboratory tests, and electrocardiograms were also administered the morning after treatment. Follow-up clinical laboratory tests and AE monitoring were conducted 7 days after final treatment.
Statistical Analysis
All statistical analyses were performed using SAS® 8.02. SOT for each treatment phase was summarized using descriptive statistics. Least-squares means, standard error (SE), and 97.5% confidence intervals of the differences between ramelteon, zolpidem, and placebo were calculated. Least-squares means and standard errors for comparisons of ramelteon (or zolpidem) versus placebo were obtained by a mixed-model analysis of covariance with treatment, period, sequence, and study center as fixed class effects; subject as random effect; and baseline values as covariates. Baseline was the last observation before administration of double-blind medication at each treatment period. Treatment effects were tested at the 0.025 significance level as justified by the Bonferroni correction to ensure the family-wise error rate was controlled at the 0.05 level. All secondary endpoints were analyzed in the same manner as the primary endpoint.
RESULTS
Disposition, Demographics, and Baseline Characteristics
Thirty-three subjects met screening criteria and were randomly assigned to study groups. All enrolled subjects completed the study. There were no relevant differences in baseline characteristics of subjects among the 3 treatment sequences. Twenty women and 13 men with a mean age of 71.0 (5.07 SD) years participated. Overall, the mean habitual bedtime was 22:54, mean self-reported sleep latency at screening was 50.9 minutes, and the mean self-reported total sleep time was 329.1 minutes.
Balance
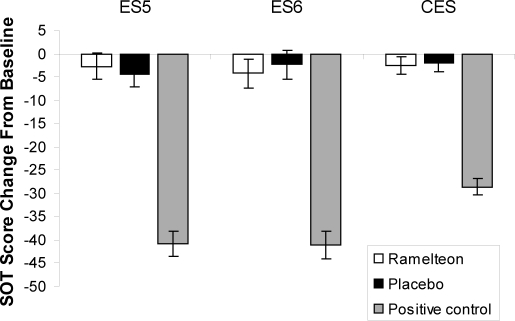
Baseline CES for each group (placebo: 77.61, ramelteon: 78.70, zolpidem: 78.36) were well within the generally accepted normal range of an age-matched group.25 Compared with placebo (-2.02), the CES, ES5, and ES6 did not change significantly from baseline with ramelteon (p = 0.837, p = 0.650, and p = 0.670, respectively) (Table 1 and Figure 2). Significant reductions in CES, ES5, and ES6 were detected with the zolpidem positive control (p < 0.001, all) (Table 1 and Figure 2).
Table 1.
Primary and Secondary Efficacy Variables
| SOT Variable | Placebo n = 33 |
Ramelteon n = 33 |
Positive Control n = 33 |
|---|---|---|---|
| ES5 | |||
| Baseline | 67.76 (7.247) | 67.82 (9.385) | 67.77 (8.147) |
| Change from baseline | −4.34 (2.834) | −2.70(2.829) | −40.87 (2.781) |
| p Value | — | 0.650 | < 0.001 |
| ES6 | |||
| Baseline | 61.06 (13.544) | 66.48 (10.102) | 64.96 (12.973) |
| Change from Baseline | −2.35 (3.143) | −4.23 (3.128) | −41.08 (3.048) |
| p Value | — | 0.670 | < 0.001 |
| CES | |||
| Baseline | 77.61 (5.539) | 78.70 (5.040) | 78.36 (4.904) |
| Change from baseline | −2.02 (1.843) | −2.54 (1.835) | −28.60 (1.799) |
| p Value | — | 0.837 | < 0.001 |
| Somatosensory Ratio | |||
| Baseline | 0.97 (0.030) | 0.97 (0.027) | 0.97 (0.032) |
| Change from Baseline | 0.00 (0.041) | 0.00 (0.041) | 0.03 (0.041) |
| p Value | — | 0.960 | 0.567 |
| Visual Ratio | |||
| Baseline | 0.89 (0.057) | 0.88 (0.070) | 0.87 (0.068) |
| Change from baseline | −0.03 (0.029) | 0.00 (0.029) | −0.26 (0.028) |
| p Value | — | 0.439 | < 0.001 |
| Vestibular Ratio | |||
| Baseline | 0.73 (0.074) | 0.73 (0.096) | 0.73 (0.083) |
| Change from baseline | −0.04 (0.032) | −0.02 (0.032) | −0.42 (0.032) |
| p Value | — | 0.531 | < 0.001 |
| Preference Ratio | |||
| Baseline | 0.96 (0.065) | 0.99 (0.065) | 0.99 (0.083) |
| Change from baseline | 0.01 (0.023) | 0.00 (0.023) | 0.02 (0.023) |
| p Value | — | 0.820 | 0.777 |
Baseline values are reported as mean (SD). Change from baseline values are reported as mean (SEM); p values are based on comparisons of ramelteon or positive control (zolpidem, 10 mg) vs placebo. SOT refers to Sensory Organization Test; ES5, equilibrium score for condition 5; ES6, equilibrium score for condition 6; CES, composite equilibrium score for all 6 conditions.
Figure 2.
Sensory Organization Test scores: Least-squares mean change from baseline to posttreatment for ramelteon, 8 mg; placebo; and positive control (zolpidem, 10 mg). ES5 refers to equilibrium score for condition 5; ES6, equilibrium score for condition 6; CES, composite equilibrium score for all 6 conditions. *p < 0.001 compared with placebo.
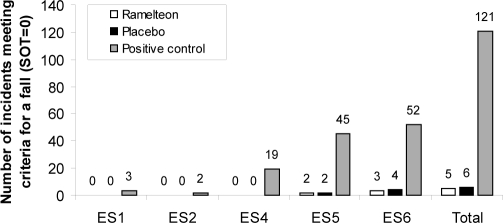
In a posthoc analysis, the number of incidents meeting criteria for a fall during each of the 6 SOT conditions was calculated. In this experimental model, a fall was defined as a score of 0, as measured by EquiTest CDP. Because subjects stood in a safety harness to prevent injury during testing, no actual falls occurred during this test. However, a score of 0 indicated that a fall would have occurred without the harness. Subjects performed each condition 3 times (33 subjects × 6 conditions × 3 trials/condition); there were 594 fall “opportunities” with each treatment. No falls measured by EquiTest CDP were recorded for subjects in the placebo and ramelteon groups under SOT conditions 1 through 4. Under the more difficult conditions, ES5 and ES6, the number of falls measured by EquiTest CDP was relatively low in the ramelteon (n = 2 and n = 3, respectively) and placebo groups (n = 2 and n = 4, respectively) (Figure 3). With the zolpidem positive control, falls measured by EquiTest CDP were reported under all SOT conditions except condition 3. The greatest number of falls measured by EquiTest CDP with zolpidem were observed during the ES5 (n = 45) and ES6 (n = 52) conditions out of a total of 99 fall opportunities for each condition.
Figure 3.
Number of incidents meeting criteria for a fall for ramelteon, 8 mg; placebo; and positive control (zolpidem, 10 mg) during the posttreatment Sensory Organization Test. n = 33 for each treatment group. The equilibrium score for condition 3 is not shown because no falls were reported for any group. Each subject performed each condition 3 times (33 subjects x 6 conditions x 3 trials/condition). Overall, there were 594 fall “opportunities” with each treatment. ES5 refers to equilibrium score for condition 5; ES6, equilibrium score for condition 6; CES, composite equilibrium score for all 6 conditions
The SOT somatosensory, visual, vestibular, and preference ratios are shown in Table 1. There were no significant differences between ramelteon and placebo on any of the calculated SOT ratios. The visual ratio (the ability to use visual input to maintain balance) and the vestibular ratio (the ability to use vestibular input to maintain balance) were significantly worse with the zolpidem positive control compared with placebo (p ≤ 0.001, each).
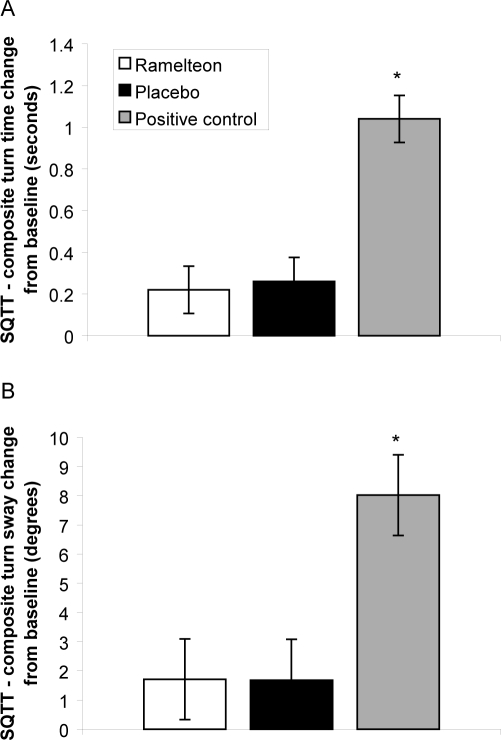
Baseline SQTT turn time (placebo: 1.56, ramelteon: 1.42, zolpidem: 1.38) and turn sway (placebo: 28.21, ramelteon: 27.44, zolpidem: 27.16) scores for each group were within generally accepted age-matched ranges.28 No significant changes in turn time and turn sway measured by SQTT were observed between ramelteon and placebo groups (p = 0.776 and p = 0.982, respectively) (Figure 4). Significant increases in turn time and turn sway measured by SQTT were observed between the zolpidem positive control and placebo groups (p < 0.001, both).
Figure 4.
Mean change in Step-Quick Turn Test (SQTT) composite turn-time score (A) and turn-sway score (B) from baseline for ramelteon, 8 mg; placebo; and positive control (zolpidem, 10 mg). *p < 0.001 compared with placebo.
Immediate and Delayed Memory Recall Tests
At baseline, the number of words recalled on the immediate memory recall test (placebo: 6.15, ramelteon: 6.36, zolpidem: 6.00) and the delayed memory recall test (placebo: 4.16, ramelteon: 4.38, zolpidem: 3.76) were similar across groups. There was no significant change in immediate memory recall with ramelteon compared with placebo (5.56 vs 5.40 words, respectively, p = 0.683). Significantly fewer words were recalled with the zolpidem positive control compared with placebo on the immediate memory recall test (4.10 vs 5.40 words, respectively, p = 0.002). Neither treatment significantly affected delayed recall (ramelteon vs placebo, p = 0.650; zolpidem vs placebo, p = 0.247).
Safety
No subject discontinued participation in the study due to an AE. The same number of subjects reported an AE with placebo and ramelteon treatment (7 subjects each [21.2%]). AEs were reported in 13 subjects (39.4%) during zolpidem treatment. Overall, the AEs that occurred in at least 2 subjects in the placebo, ramelteon, or zolpidem groups were dizziness (n = 2 [6.1%], n = 2 [6.1%], and n = 4 [12.1%], respectively), headache (n = 2 [6.1%], n = 4 [12.1%], and n = 3 [9.1%], respectively), nausea (n = 2 [6.1%], n = 0 [0.0%], and n = 5 [15.2%], respectively), and somnolence (n = 0 [0.0%], n = 2 [6.1%], and n = 2 [6.1%], respectively). No AE occurred in more than 5 patients under each treatment condition. No serious AEs or deaths were reported.
DISCUSSION
The effect of insomnia, or the treatment of insomnia, on balance poses a risk of falling, especially in older adults who may already have limited mobility. This is an important consideration for any patient who may awaken and need to ambulate in the middle of the night. Older adults who may have to walk around in the middle of the night due to nocturia, due to care for a spouse, or who are in unfamiliar environments (eg, hospital or nursing home) are particularly susceptible to falls. Consequences of falls include severe injury,29 prolonged hospitalization/institutionalization,30 functional decline,31 and even death.32 Health care costs, which are already high among older adults, increase dramatically after a fall resulting in injury.33,34
In this study, the performance effects of ramelteon, 8 mg, were evaluated at approximate maximal plasma concentration (2 hours after dose).21 At near peak plasma concentrations, ramelteon did not impair balance, mobility, or memory and was well tolerated, with an incidence of AEs similar to that of placebo.
The validity of EquiTest CDP is well documented in clinical literature.25,26 A number of studies have demonstrated that impaired postural control on EquiTest CDP, which challenges a subject's sensory and motor skills, is a significant predictor of real-world falls. In a retrospective study25 comparing individuals with a history of falls to those without, individuals with a CES less than 38 were 4 times more likely to fall than were those with higher scores. An 18-point decrease in CES from pretest to posttest was a significant predictor of falling. A separate study demonstrated that the SOT (measured by CDP) was the best predictor of recurrent falls in noninstitutionalized, healthy older adults ( > 65 years).35 It is notable that the subjects enrolled in this study had baseline values that were better than those of age-matched controls,25,26 suggesting that subjects in this trial had good postural stability prior to receiving study drug.
Ramelteon did not impair balance or mobility, as measured by the CES, ES5, and ES6 conditions and the SQTT. In contrast, the positive control drug significantly impeded performance on each of these measures. Consistent with these results, the tests meeting criteria for a fall during the SOT was much higher with the positive control drug (121 out of 594 opportunities) than with placebo (6 out of 594) or ramelteon (5 out of 594).
In this study, ramelteon did not produce impairments in psychomotor function or in immediate or delayed memory recall. This is consistent with the lack of deficits on psychomotor tasks or memory in a number of previous ramelteon studies.22,23,39,40
Ramelteon was well tolerated, with an AE profile similar to that of placebo. Overall, AEs occurred at a relatively low frequency. The most common AEs in this study were dizziness, somnolence, headache, and nausea, which were generally mild or moderate in severity for all groups. These findings are consistent with the safety profile of ramelteon.
When interpreting these findings, several limitations should be considered. No polysomnographic or subjective sleep measures were recorded. Whether subjects were asleep (or in what stage of sleep) before the balance assessment 2 hours postdose was not recorded. However, polysomnographic data from a similar study evaluating balance in subjects taking ramelteon, 8 mg, or placebo, using zopiclone as a positive control, showed that mean latency to persistent sleep was similar for subjects in the ramelteon and zopiclone groups on Nights 1 and 2 (30.84 minutes and 34.45 minutes, respectively). Another limitation is that a previous study has shown that the mean peak plasma concentration of ramelteon in older adults is approximately 1.5 hours and is highly variable.21 Peak plasma concentrations are similar with zolpidem (1.6 hours).17 Thus, it is possible that subjects were at lower than peak plasma concentrations 2 hours postdose during active treatment. Also, the recommended dose of zolpidem in older adults is 5 mg; however, the 10-mg dose was used for this study to maximize possible balance deficits and because a pilot study showed that zolpidem, 10 mg, was capable of showing deficits on the SOT assessment. The purpose of this study was not to evaluate zolpidem or to compare its effects on balance with the effects of ramelteon but, rather, to use zolpidem as a positive control for the balance tests.
In conclusion, in older adults with chronic insomnia, ramelteon did not impair balance, mobility, or memory in the middle of the night. Ramelteon was also well tolerated, with an AE profile similar to that of placebo.
DISCLOSURE STATEMENT
This was a multicenter study conducted at 5 sleep research centers in the US. This study was funded by Takeda Pharmaceutical Company Limited. Dr. Zammit has received research support from Ancile Pharmaceuticals, Arena, Aventis, Cephalon, Elan, Epix, Evotec, Forest, GlaxoSmithKline, H. Lundbeck A/S, King Pharmaceuticals, Merck, National Institutes of Health, Neurim, Neurocrine Biosciences, Neurogen, Organon, Orphan Medical, Pfizer, Respironics, Sanofi-Aventis, Sanofi-Synthelabo, Schering-Plough, Sepracor, Somaxon, Takeda, Transcept, UCB Pharma, Predix, Vanda, and Wyeth-Ayerst Research; has been a consultant for Aventis, Boehringer Ingelheim, Cephalon, Elan, GlaxoSmithKline, Jazz, King Pharmaceuticals, Merck, Neurocrine Biosciences, Organon, Pfizer, Renovis, Sanofi-Aventis, Select Comfort, Sepracor, Shire, and Takeda; has participated in speaking engagements for Neurocrine Biosciences, King Pharmaceuticals, McNeil, Sanofi-Aventis, Sanofi-Synthelabo, Sepracor, Takeda, Vela, and Wyeth-Ayerst Research; and has ownership in and is a director of Clinilabs IPA, Inc., and Clinilabs Physicians Services, PC. Dr. Wang-Weigand and Dr. Peng are employees of Takeda. Dr. Rosenthal is Medical Director of a clinical trials company.
ACKNOWLEDGMENTS
This study was supported by the Takeda Pharmaceutical Company Limited. Assistance with preparation of the manuscript was provided by Kelly Guerrettaz, MS, and Sara Sarkey, PhD, employees of Takeda Pharmaceuticals North America.
REFERENCES
- 1.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–32. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 2.Foley D, Ancoli-Israel S, Britz P, Walsh J. Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey. J Psychosom Res. 2004;56:497–502. doi: 10.1016/j.jpsychores.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Hill EL, Cumming RG, Lewis R, Carrington S, Le Couteur DG. Sleep disturbances and falls in older people. J Gerontol A Biol Sci Med Sci. 2007;62:62–6. doi: 10.1093/gerona/62.1.62. [DOI] [PubMed] [Google Scholar]
- 4.National Safety Council. [Last updated 1999];Report on injuries in America. National Safety Council. 1999 http://www.nsc.org/lrs/statinfo/99report.htm.
- 5.Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. J Am Geriatr Soc. 2005;53(7) Suppl:S264–S271. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- 6.Schubert CR, Cruickshanks KJ, Dalton DS, Klein BE, Klein R, Nondahl DM. Prevalence of sleep problems and quality of life in an older population. Sleep. 2002;25:889–93. [PubMed] [Google Scholar]
- 7.Pollock BG, Perel JM, Reynolds CF. Pharmacodynamic issues relevant to geriatric psychopharmacology. J Geriatr Psychiatry Neurol. 1990;3:221–8. doi: 10.1177/089198879000300408. [DOI] [PubMed] [Google Scholar]
- 8.Glass J, Lanctot KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169–73. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barbee JG. Memory, benzodiazepines, and anxiety: integration of theoretical and clinical perspectives. J Clin Psychiatry. 1993;54(Suppl):86–97. [PubMed] [Google Scholar]
- 10.O'Hanlon JF. Residual effects on memory and psychomotor performance of zaleplon and other hypnotic drugs. Prim Care Companion. J Clin Psychiatry. 2002;4(Suppl 1):38–44. [Google Scholar]
- 11.Mintzer MZ, Griffiths RR. Selective effects of zolpidem on human memory functions. J Psychopharmacol. 1999;13:18–31. doi: 10.1177/026988119901300103. [DOI] [PubMed] [Google Scholar]
- 12.Roehrs T, Merlotti L, Zorick F, Roth T. Sedative, memory, and performance effects of hypnotics. Psychopharmacology. 1994;116:130–4. doi: 10.1007/BF02245054. [DOI] [PubMed] [Google Scholar]
- 13.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Hazardous benzodiazepine regimens in the elderly: effects of half-life, dosage, and duration on risk of hip fracture. Am J Psychiatry. 2001;158:892–8. doi: 10.1176/appi.ajp.158.6.892. [DOI] [PubMed] [Google Scholar]
- 14.Wagner AK, Zhang F, Soumerai SB, et al. Benzodiazepine use and hip fractures in the elderly: who is at greatest risk? Arch Intern Med. 2004;164:1567–72. doi: 10.1001/archinte.164.14.1567. [DOI] [PubMed] [Google Scholar]
- 15.Wang PS, Bohn RL, Glynn RJ, Mogun H, Avorn J. Zolpidem use and hip fractures in older people. J Am Geriatr Soc. 2001;49:1685–90. doi: 10.1111/j.1532-5415.2001.49280.x. [DOI] [PubMed] [Google Scholar]
- 16.King Pharmaceuticals Inc. Sonata [package insert] Bristol, TN: 2003. [Google Scholar]
- 17.Sanofi-Synthelabo Inc. Zolpidem [package insert] New York, NY: 2005. [Google Scholar]
- 18.Eszopiclone (Lunesta), a new hypnotic. Med Lett Drugs Ther. 2005;47:17–9. [PubMed] [Google Scholar]
- 19.Kato K, Hirai K, Nishiyama K, et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacol. 2005;48:301–10. doi: 10.1016/j.neuropharm.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 20.Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8:d1093–d1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- 21.Greenblatt DJ, Harmatz JS, Karim A. Age and sex effects on the pharmacokinetics and pharmacodynamics of ramelteon, a hypnotic agent acting via melatonin receptors MT1 and MT2. J Clin Pharmacol. 2007;47:485–96. doi: 10.1177/0091270006298602. [DOI] [PubMed] [Google Scholar]
- 22.Roth T, Seiden D, Sainati S, Wang-Weigand S, Zhang J, Zee P. Effects of ramelteon on patient-reported sleep latency in older adults with chronic insomnia. Sleep Med. 2006;7:312–8. doi: 10.1016/j.sleep.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 23.Roth T, Seiden D, Wang-Weigand S, Zhang J. A 2-night, 3-period, crossover study of ramelteon's efficacy and safety in older adults with chronic insomnia. Curr Med Res Opin. 2007;23:1005–14. doi: 10.1185/030079907x178874. [DOI] [PubMed] [Google Scholar]
- 24.Zammit G, Wang-Weigand S, Peng X. Use of computerized dynamic posturography to assess balance in older adults after nighttime awakenings using zolpidem as a reference. BMC Geriatrics. 2008;8:15. doi: 10.1186/1471-2318-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitney SL, Marchetti GF, Schade AI. The relationship between falls history and computerized dynamic posturography in persons with balance and vestibular disorders. Arch Phys Med Rehabil. 2006;87:402–7. doi: 10.1016/j.apmr.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Wallmann HW. Comparison of elderly nonfallers and fallers on performance measures of functional reach, sensory organization, and limits of stability. J Gerontol A Biol Sci Med Sci. 2001;56:M580–M583. doi: 10.1093/gerona/56.9.m580. [DOI] [PubMed] [Google Scholar]
- 27.NeuroCom International I. Objective Quantification of Balance and Mobility. [Accessed September 26, 2007];NeuroCom International, Inc. 2007 Last updated 2007. http://www.onbalance.com/program/role/cdp/index.aspx.
- 28.NeuroCom International I. Step Quick Turn Protocol. [Accessed September 26, 2007];NeuroCom International, Inc. 2007 Last updated 2007. http://www.onbalance.com/neurocom/protocols/functionalLimitation/sqt.aspx.
- 29.Centers for Disease Control and Prevention. Web-based injury statistics query and reporting system (WISQARS) [Accessed July 6, 2007];2007 Last updated 2006. http://www.cdc.gov/ncipc/wisqars/.
- 30.Magaziner J, Hawkes W, Hebel JR, et al. Recovery from hip fracture in eight areas of function. J Gerontol A Biol Sci Med Sci. 2000;55:M498–M507. doi: 10.1093/gerona/55.9.m498. [DOI] [PubMed] [Google Scholar]
- 31.Tinetti ME, Williams CS. The effect of falls and fall injuries on functioning in community-dwelling older persons. J Gerontol A Biol Sci Med Sci. 1998;53:M112–M119. doi: 10.1093/gerona/53a.2.m112. [DOI] [PubMed] [Google Scholar]
- 32.Popovic JR. 1999 National Hospital Discharge Survey: annual summary with detailed diagnosis and procedure data. Vital Health Stat 13. 2001;151(i-v):1–206. doi: 10.1037/e309042005-001. [DOI] [PubMed] [Google Scholar]
- 33.Roudsari BS, Ebel BE, Corso PS, Molinari NA, Koepsell TD. The acute medical care costs of fall-related injuries among the U.S. older adults. Injury. 2005;36:1316–22. doi: 10.1016/j.injury.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Stevens JA, Corso PS, Finkelstein EA, Miller TR. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006;12:290–5. doi: 10.1136/ip.2005.011015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buatois S, Gueguen R, Gauchard GC, Benetos A, Perrin PP. Posturography and risk of recurrent falls in healthy non-institutionalized persons aged over 65. Gerontology. 2006;52:345–52. doi: 10.1159/000094983. [DOI] [PubMed] [Google Scholar]
- 36.Evans SM, Funderburk FR, Griffiths RR. Zolpidem and triazolam in humans: behavioral and subjective effects and abuse liability. J Pharmacol Exp Ther. 1990;255:1246–55. [PubMed] [Google Scholar]
- 37.Rush CR, Baker RW, Wright K. Acute behavioral effects and abuse potential of trazodone, zolpidem and triazolam in humans. Psychopharmacology. 1999;144:220–33. doi: 10.1007/s002130050997. [DOI] [PubMed] [Google Scholar]
- 38.Avidan AY, Fries B, James M, Szafara K, Wright G, Chervin R. Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc. 2005;53:955–62. doi: 10.1111/j.1532-5415.2005.53304.x. [DOI] [PubMed] [Google Scholar]
- 39.Erman M, Seiden D, Zammit G, Sainati S, Zhang J. An efficacy, safety, and dose-response study of ramelteon in patients with chronic primary insomnia. Sleep Med. 2006;7:17–24. doi: 10.1016/j.sleep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Zammit G, Erman M, Wang-Weigand S, Sainati S, Zhang J, Roth T. Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med. 2007;3:495–504. [PMC free article] [PubMed] [Google Scholar]