Abstract
Study Objectives:
Insomnia has been identified as a risk factor for tension-type headache, although the pathogenesis of sleep disturbance in this population is unclear. The present study examined pain-related self-management strategies in a nonclinical, young-adult sample for preliminary evidence to support a novel hypothesis for the development of insomnia in this population.
Methods:
Self-report data on triggers of headache, pain interference with sleep, and pain-related self-management strategies were analyzed for 32 women with tension-type headache and 33 women with minimal pain who served as controls.
Results:
The results revealed that a significantly greater proportion of the headache group relative to the control group reported sleep problems as a trigger of headaches, stress as a trigger of headache, and going to sleep as a coping strategy for pain. The headache group also reported significantly higher ratings of pain interference with sleep. Going to sleep was the most commonly used self-management strategy (81%) by headache sufferers and also rated as the most effective strategy (5.5 out of 7.0).
Conclusions:
These findings suggest that a bidirectional relationship between sleep disturbance and headache is present in this young-adult sample. Furthermore, the frequent use of sleep as a self-management strategy for pain is consistent with the hypothesis that sleep-seeking behavior might be a mediating factor in the development of insomnia among people with tension-type headache. This hypothesis fits within the most widely accepted conceptual model of chronic insomnia and should be further investigated in individuals with both tension-type headache and insomnia.
Citation:
Ong JC; Stepanski EJ; Gramling SE. Pain coping strategies for tension-type headache: Possible implications for insomnia? J Clin Sleep Med 2009;5(1):52-56.
Keywords: Insomnia, tension headache, behavioral risk factor
Tension-type headache (TTH) is a widely prevalent problem among adults that can develop into a chronic condition with significant psychological, social, and economic distress. Muscle tension related to psychosocial stress has been implicated in the etiology of TTH, but the pathophysiologic mechanisms remain unclear, especially with regard to the development of chronic TTH. Sleep disturbance (ie, difficulty falling or staying asleep) has been identified as a risk factor for developing chronic headaches.1 Insomnia symptoms are a frequent complaint among both TTH and migraine sufferers.2–4 Moreover, longitudinal studies have revealed that the presence of insomnia symptoms increases the risk of developing a headache over a 1-year period,5 as well as an increased risk of developing chronic TTH over a 12-year follow-up period.6 In addition, Calhoun and Ford3 found preliminary evidence that a behavioral sleep intervention emphasizing sleep hygiene for individuals with chronic migraines can decrease the frequency and intensity of headaches. Collectively, these findings provide indications that insomnia is a modifiable risk factor for chronic headaches, although the pathogenesis of sleep disturbance in this group remains unclear.
Currently, the predominant etiologic model for insomnia is a diathesis-stress-response model that posits that there are predisposing, precipitating, and perpetuating factors contributing to the development and maintenance of chronic insomnia.7 According to this model, an episode of insomnia is triggered when certain predisposing factors are coupled with a precipitating factor that leads to sleep disturbance. Over time, perpetuating factors, such as increased time in bed or daytime napping, can diminish the homeostatic drive for sleep, thus exacerbating the nocturnal symptoms of insomnia and maintaining the sleep disturbance, even if the original trigger, or precipitating factor, has resolved. Given the complex relationship between insomnia and TTH, it is unclear if there are unique conditions or factors for the development of insomnia in this context. One possible hypothesis involves behavioral coping strategies used to manage pain. For example, it has been reported that sleep alleviates migraine headaches.8,9 Studies using clinical outpatients have reported that sleeping or lying down are used as behavioral strategies to relieve headache pain by more than 60% of people with either TTH or migraine.10 In the context of the model of chronic insomnia, these coping strategies for pain can have a negative impact on nighttime sleep continuity, thus leading to sleep disturbance. To our knowledge, this hypothesis has not been explored and could provide insights into an underlying link between headache pain and sleep disturbance.
The goal of this study was to generate hypotheses for the development of sleep disturbance in TTH by examining the pattern of self-management strategies for headache pain among people with TTH. Although previous studies have examined clinical samples, we examined a sample of young adult women because female sex is a risk factor for both TTH and insomnia and this cohort provides an opportunity to investigate potential risk factors in a nonclinical sample. This report focused on 2 specific research questions: (1) Are there differences between TTH and minimal-pain controls with regard to triggers of headache and pain interference with sleep? (2) What self-management strategies for headache are utilized by people with TTH that could impact sleep patterns?
METHODS
Participants and Procedures
In the present study, secondary analyses were conducted on self-report data from participants who completed a psychophysiologic assessment investigating differences between TTH and controls on the pattern of physiologic, affective, and behavioral responses to a picture-viewing task.11 The primary study employed a stress-reactivity paradigm that consisted of 3 phases (adaptation, scheduled-viewing, recovery). During the scheduled-viewing phase, participants were presented with 24 pictures designed to elicit positive, neutral, and negative affect and varying levels of arousal with a 30-second waiting period between each picture. The primary measures of this study were frontalis, corrugator, and zygomatic electromyographic (EMG) activity, self-reported affect, and self-reported oral motor behaviors. In addition, within-subject concordance between self-reported measures and EMG activity were examined as evidence of proprioceptive awareness. For additional details of the main study, please see Ong et al.11
All participants were recruited from undergraduate psychology courses at a large urban university in the southeastern United States. Students were offered an opportunity to participate in a research study in exchange for course credit by first completing a research questionnaire packet. A total of 336 female participants completed the initial questionnaire packet, which contained a headache screening questionnaire. Those who met the International Headache Society criteria for TTH12 were then contacted via telephone to complete a semistructured interview conducted by a graduate student trained with the International Headache Society system to verify the diagnosis. In addition, minimal-pain controls were recruited using the following criteria: (1) failed to meet diagnosis of TTH, migraine, or temporomandibular joint disorder; (2) reported no reported history of chronic pain (headache disorder, temporomandibular joint disorder, or arthritis) or previous treatment for these chronic pain conditions; and (3) were not taking medication for pain at the time of assessment. Out of the 336 women, 90 participants (25.7%) met criteria for TTH and 100 participants (29.8%) met criteria for control and were subsequently recruited to participate in the primary study. A total of 65 women (32 headache, 33 controls) were subsequently brought to the laboratory and participated in the psychophysiologic assessment. Prior to the picture-viewing task, participants completed a questionnaire packet that included measures pertaining to pain history and pain coping strategies (see below).
Measures
Data analyses were conducted on several items pertaining to sleep and pain coping strategies in the questionnaire packets. The Headache and Facial Pain Screening Questionnaire was administered during the initial screening packet and includes information about headache history as well as separate items for migraine and TTH symptoms based on the International Headache Society classification system12 to allow for independent diagnosis of each headache type. Specific items used for diagnosis include frequency of headache episodes (past month and year), duration of typical episode, location of pain, quality of pain, intensity of pain, nausea/vomiting, photophobia, and phonophobia. Two items from the Headache and Facial Pain Screening Questionnaire pertaining to headache triggers were also analyzed: (1) Is your headache triggered by stress? (2) Is your headache triggered by sleep problems?
Several items from the pain-history questionnaire packet administered in the laboratory prior to the picture-viewing task were analyzed. One item from the Multidimensional Pain Inventory (To what extent does pain interfere with your ability to get enough sleep?) rated on a 7-point Likert scale was analyzed as a measure of pain interference with sleep. Eight items that inquire about various self-management strategies used to cope with headache pain (hot compress, cold compress, relaxation, go to sleep, have a drink, distract self, exercise) were also analyzed. For each item, participants indicated if they have used the intervention (yes/no), the frequency of use, and the effectiveness of the intervention on a 7-point Likert scale. Finally, participants were asked to list any medications used to treat headache pain, the frequency of use, and the effectiveness of the medication on a 7-point Likert scale.
Data Analysis
The main analyses consisted of between-group comparisons between the headache and control groups and within-group comparisons for the headache group. Nonparametric analyses were used for data collected using nominal scales.13 χ2 analyses were conducted to examine differences between the headache and control groups on 4 dependent variables of interest: (1) sleep disturbance as a trigger for headache (yes, no, unsure); (2) stress as a trigger for headache (yes, no, unsure); (3) go to sleep as a coping strategy (yes or no); and (4) use of medication (yes or no). In addition, an independent samples t-test was conducted to examine differences between the groups on pain interference with sleep. For the within-group analysis, a Wilcoxon Signed Rank Test was used to compare the different self-management strategies used by the headache group. In addition, Spearman rho correlations were conducted to examine the relationships among headache frequency, headache intensity, self-management strategies, and medication use for the headache group.
RESULTS
Group Characteristics
The headache group consisted of 32 participants with an average age of 21.9 years (SD = 7.5) and an ethnic composition of 72% Caucasian, 16% African American, and 9% Asian (1 participant provided no response). The average time since first headache of any type was 9.4 years (SD = 6.8), with an average of 8.11 headache days per month. Participants in this group reported an average of 12.2 TTH over the past year and 2.1 TTH in the past month, with the median duration of these headache lasting 2.0 hours (mean = 6.9 hours, SD = 12.4). The average TTH intensity rating using a 0-to-10 rating scale was 5.6 (SD=1.5). In addition to having TTH, 6 participants (18.8%) in the headache group also met criteria for a migraine disorder. Twenty-four participants in the headache group (75%) reported using any medication (prescription or nonprescription) to relieve headaches.
The control group consisted of 33 participants with an average age of 18.9 years (SD = 2.28) and an ethnic composition of 49% African American, 39% Caucasian, and 12% other. The average time since first headache of any type was 5.7 years (SD = 5.0), with an average of 4.4 headache days per month. Only 6 out of the 33 participants in the control group (18%) reported experiencing TTH, with an average of 2.2 TTH attacks over the past year and 0.2 TTH in the past month. The median duration of these TTH was 0.6 hours (mean = 4.7 hours, SD = 9.5), with an average TTH intensity rating of 5.7 (SD=0.8). Although no participants in this group met criteria for a migraine disorder, 1 participant reported experiencing 2 migraines in the past month. Twenty participants in the control group (61%) reported using any medication to relieve headaches.
Given the disparity in ethnic composition between the headache and control groups, preliminary analyses were conducted to examine the potential impact of ethnicity. Due to the small number of Asians and other minorities, data from these ethnic groups were combined with that of African Americans in the analyses. First, a series of logistic regressions were conducted with headache status (headache, control), ethnicity (Caucasian, non-Caucasian), and the headache x ethnicity interaction term as independent variables and the 4 dependent variables of interest (sleep disturbance as a trigger for headache, stress as a trigger for headache, go to sleep as a coping strategy, use of medication). For the analyses on sleep disturbance, stress, and go to sleep as a coping strategy, the overall model was significant (p < 0.05), but none of the independent variables were significant predictors. For the analysis on use of medication, the overall model was not significant, and none of the independent variables were significant predictors. Finally, a 2 × 2 (headache status × ethnicity) between-groups analysis of variance was conducted on the pain-interference-with-sleep item. The results revealed a significant effect for headache status, F1,61 = 8.26, p < .01, but no significant findings on ethnicity or the group x ethnicity interaction. Since ethnicity was not an aim of this study, all subsequent analyses were collapsed across this variable.
Between-Group Analyses
Comparisons were made between the headache and control groups on triggers of headache, coping strategies for headache pain, and pain interference with sleep (see Table 1). First, a significantly greater proportion of the headache group, compared with the control group, reported sleep problems as a trigger of headaches, χ2 (2) = 11.43, p < .01. Among the headache group, 18 out of 32 subjects (56%) reported that their headaches were triggered by sleep problems, 8 out of 32 (25%) did not believe their headaches were triggered by sleep problems, and 5 out of 32 (16%) were unsure. One participant did not provide a response. In contrast, only 6 out of 33 control subjects (18%) reported that their headaches were triggered by sleep problems, whereas 20 out of 33 (61%) reported that their headaches were not triggered by sleep problems, and 7 out of 33 (21%) were unsure. A second χ2 test revealed that a significantly greater proportion of the headache group reported stress as a trigger of headache, compared with the control group, χ2 (2) = 8.87, p < .05. Nearly all participants in the headache group, 30 out of 32 (94%) reported that their headaches were triggered by stress, with 1 individual reporting unsure and 1 participant with incomplete data. Among the control group, 23 out of 33 (70%) reported that their headaches were triggered by stress, 7 out of 33 (21%) reported that their headaches were not triggered by stress, and 4 out of 33 (9%) were unsure. The third comparison revealed that a significantly greater proportion of the headache group reported “go to sleep” as a coping strategy for pain, χ2 (1) = 4.45, p < .05. Among the headache group, 26 out of 32 participants (81%) reported using sleep as a coping strategy, whereas 18 out of 33 (55%) participants in the control group reported using sleep as a coping strategy. No significant differences were found between the groups on use of medication. Finally, an independent samples t-test conducted on pain interference with sleep revealed that the headache group (mean = 2.09, SD = 1.75) reported significantly more interference with sleep, compared with the control group (mean = 0.76, SD = 1.32), t63 = 3.48, p < .01.
Table 1.
Triggers of Headache and Sleep Disturbance and Pain-Coping Strategies
| Variable | Headache (n = 32) | Control (n = 33) |
|---|---|---|
| Headache triggered by sleep problemsa | 56.3 | 18.2 |
| Headache triggered by stressa | 93.8 | 69.7 |
| Pain interferes with sleepa | 2.09 | 0.76 |
| Hot compress | 31.3 | 15.2 |
| Cold compress | 43.8 | 27.3 |
| Biofeedback | 0 | 0 |
| Relaxation | 50 | 12.1 |
| Go to sleep | 81.3 | 54.5 |
| Any medication | 75 | 60.6 |
Note. Data are presented as percentage of participants responding yes, except the item for pain interferes with sleep, which was rated on a 0-to-7 scale, with higher numbers reflecting greater sleep interference.
Denotes a significant between-group difference (p < 0.05).
Headache Subgroup Analyses
Within-group analyses were conducted to examine the self-management strategies of the headache group. “Go to sleep” was the most-utilized self-management intervention, with 26 out of 32 subjects in the TTH group (81%) using this strategy (see Table 1). A Wilcoxon Signed Rank Test revealed that “go to sleep” was utilized significantly more (p < .05) than any other intervention. Out of the 21 participants who reported frequency of this strategy, 13 reported using sleep “every time” or “frequently,” 7 reported using it at least 2 times per month, and 1 reported “seldom.” Furthermore, sleep was rated as the most effective intervention for those who endorsed this strategy, with an average rating of 5.5 out of 7.0. Other than “go to sleep,” the most widely used strategies were “distract self” (53%) and “relaxation” (50%). Only relaxation (4.8 out of 7.0) received an effectiveness rating above 4.0 out of 7.0. Spearman rho correlations among headache frequency, headache intensity, self-management strategies, and medication use revealed no significant correlations at the p < .05 level. Thus, headache frequency and headache intensity do not appear to be related to the use of any specific self-management strategy or medication use among women with TTH.
DISCUSSION
The overall findings provide preliminary evidence into a behavioral link between headache and sleep disturbance. First, the group comparisons on triggers of headache indicate that a high proportion of both the headache and control groups (97% and 70%, respectively) reported stress as a trigger of headache, but a significantly greater proportion of the headache group relative to the control group (58% and 18%, respectively) reported sleep problems as a trigger of headaches. This pattern suggests that, although stress is a common trigger of tension headaches, sleep problems appear to be a more frequent trigger among individuals who meet criteria for a TTH diagnosis, as compared with controls. In addition, women with headache reported significantly higher ratings of pain interfering with sleep, compared with the control group. These findings are consistent with previous reports in the literature of the co-occurrence of TTH and insomnia symptoms in clinical samples.2–4 Moreover, it suggests that the bidirectional relationship between headache pain and sleep disturbance is present even among young adults with TTH who are not seeking treatment.
The results from the self-management strategies for headache provide further insights into a possible pathway of this reciprocal relationship. More participants in the TTH group relative to the control group reported going to sleep as a coping strategy for headache while the use of medication was not significantly different between the groups. Furthermore, going to sleep was the most frequently used coping strategy by women with TTH, with 81% of the group endorsing this strategy. The findings in this study are similar to previous findings from a clinical population that reported that sleeping was used by 89% of patients with migraine and 67% of patients with TTH.10 In addition, going to sleep received the highest effectiveness ratings in this study, supporting the strong reinforcing properties to use this strategy. The accumulating evidence suggests that going to sleep is a frequently used coping strategy for headache pain across both nonclinical and clinical samples with headache, and it is perceived as a highly effective strategy.
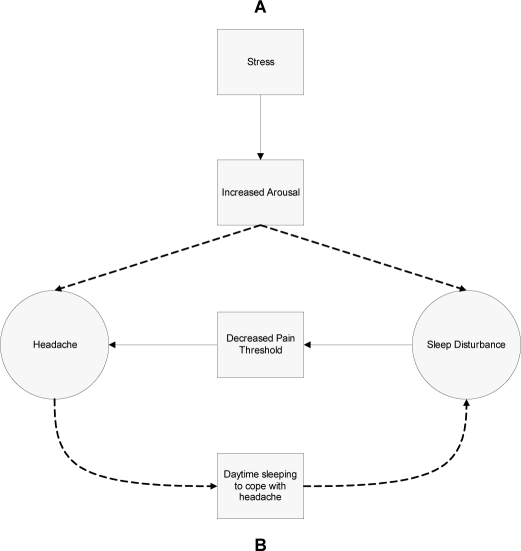
The present findings can be used to guide future hypothesis-testing research. These data are consistent with the hypothesis that stress is a common precipitating factor for both headache and sleep disturbance (see Figure 1, Hypothesis A). Given that stress has been associated with both TTH14 and insomnia,15 this hypothesis suggests that stress could lead to increased arousal (eg, autonomic activation), which accounts for the development of both TTH and insomnia. Further testing of this hypothesis should incorporate biologic or physiologic measures (eg, heart rate variability, cortisol) and might involve interventions targeting a general stress response, such as relaxation training or biofeedback. In addition, the present findings are consistent with the hypothesis that going to sleep as a self-management strategy for headache pain might serve as a mediator of the relationship between headache and sleep disturbance (see Figure 1, Hypothesis B). In this hypothesis, people with headache face a unique dilemma: the efforts to manage headache pain by going to sleep might serve as a behavioral risk factor for developing insomnia. In the context of Spielman’s model of insomnia,7 accumulation of sleep during the day in an attempt to improve a headache could have the unwanted effect of decreasing homeostatic drive for sleep at night and displacing sleep time at night, leading to reduced ability to initiate and maintain sleep during the usual nocturnal sleep period. In other words, the use of sleep or napping behaviors to relieve a headache attack could result in poor sleep hygiene, thus, precipitating an insomnia episode. Given the pain-relieving reinforcement of sleep reported in this sample and previous studies, use of this strategy over time could also serve as a perpetuating factor for chronic insomnia. To test this hypothesis, future studies should be conducted that measure sleep-wake patterns (eg, sleep diaries or actigraphy), headache episodes, and behavioral coping strategies for pain using a longitudinal design. If evidence is found to support this hypothesis, behavioral treatment studies might examine alternative coping strategies for pain that do not involve sleep (eg, acceptance-based coping interventions). It should be noted that the 2 hypotheses are not mutually exclusive and could account for both psychophysiologic and behavioral pathways. In addition to these hypothesized pathways, there are indications from the literature that sleep disturbances can decrease pain threshold,16 leading to a bidirectional relationship between headache and sleep disturbance. The model proposed in Figure 1 could aid in understanding the complex link between sleep and pain.
Figure 1.
Figure 1 depicts 2 hypothesized mechanisms for the relationship between headache and sleep disturbance. Hypothesis A posits that stress leads to increased arousal, which triggers both headache and sleep disturbance. Hypothesis B posits that the use of sleep as a coping strategy for pain might serve to promote poor sleep hygiene, thus precipitating an insomnia episode. Maintenance of this strategy over time could serve as a perpetuating factor for chronic insomnia. Finally, the bidirectional relationship is depicted by the sleep disturbance decreasing pain threshold, thus leading to more headaches.
Given that the present study involved secondary analyses from a primary study examining psychophysiological correlates of people with headache, several limitations must be acknowledged. First, data were unavailable for sleep-wake patterns or the quality of sleep. Thus, the extent of using sleep to cope with pain (ie, timing or length of naps) or the extent of nocturnal sleep disruption is not known, and it is unclear if these individuals also suffer from an insomnia disorder. Since this study employed a case-control design using subjects with minimal pain, it is unclear how these behavioral strategies would generalize to other types of headache disorders (eg, migraine), and the temporal relationship of headaches and sleep disturbance is not known. Longitudinal studies should be conducted to clarify the direction of the relationship, to identify interactions between the 2 disorders, and to examine the impact of behavioral coping strategies on sleep in other headache and pain disorders. Although no statistically significant effects were found in the preliminary analyses on ethnicity, further investigation into ethnic differences in the perception and reporting of headache pain should be conducted.
Despite these limitations, the hypotheses generated by these findings highlight potential future directions in behavioral sleep medicine for examining the relationship between pain and sleep disturbance. The assessment of daytime napping behaviors among individuals who report insomnia and headaches might be particularly important for behavioral sleep interventions, and clinicians should be sensitive to the dilemma of managing pain and sleep disturbance. Uncovering the mechanisms of the reciprocal relationship between headache and sleep remains challenging, but identifying specific behavioral risk factors could provide targets for improving treatment in this comorbid population.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This study was funded in part by a Southern Regional Education Board Dissertation Year Fellowship awarded to the first author. Portions of this study were presented at the annual meeting of the Associated Professional Sleep Societies, Philadelphia, PA, June 2004.
REFERENCES
- 1.Rains JC. Chronic headache and potentially modifiable risk factors: screening and behavioral management of sleep disorders. Headache. 2008;48:32–9. doi: 10.1111/j.1526-4610.2007.00972.x. [DOI] [PubMed] [Google Scholar]
- 2.Kelman L, Rains JC. Headache and sleep: examination of sleep patterns and complaints in a large clinical sample of migraineurs. Headache. 2005;45:904–10. doi: 10.1111/j.1526-4610.2005.05159.x. [DOI] [PubMed] [Google Scholar]
- 3.Calhoun AH, Ford S. Behavioral Sleep modification may revert transformed migraine to episodic migraine. Headache. 2007;47:1178–83. doi: 10.1111/j.1526-4610.2007.00780.x. [DOI] [PubMed] [Google Scholar]
- 4.Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53:65–72. doi: 10.1016/0304-3959(93)90057-V. [DOI] [PubMed] [Google Scholar]
- 5.Boardman HF, Thomas E, Millson DS, Croft PR. The natural history of headache: predictors of onset and recovery. Cephalalgia. 2006;26:1080–8. doi: 10.1111/j.1468-2982.2006.01166.x. [DOI] [PubMed] [Google Scholar]
- 6.Lyngberg AC, Rasmussen BK, Jorgensen T, Jensen R. Prognosis of migraine and tension-type headache: a population-based follow-up study. Neurology. 2005;65:580–5. doi: 10.1212/01.wnl.0000172918.74999.8a. [DOI] [PubMed] [Google Scholar]
- 7.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10:541–53. [PubMed] [Google Scholar]
- 8.Blau JN. Resolution of migraine attacks: sleep and the recovery phase. J Neurol Neurosurg Psychiatry. 1982;45:223–6. doi: 10.1136/jnnp.45.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson M, Williams K, Leyton M. Observations on the treatment of an acute attack of migraine. Res Clin Stud Headache. 1978;6:141–6. [PubMed] [Google Scholar]
- 10.Bag B, Karabulut N. Pain-relieving factors in migraine and tension-type headache. Int J Clin Pract. 2005;59:760–3. doi: 10.1111/j.1368-5031.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 11.Ong JC, Gramling SE, Vrana SR, Nicholson RA, Buenaver LF. Psychophysiological responses of female headache sufferers and controls using a picture-viewing paradigm. Appl Psychophysiol Biofeedback. 2006;31:295–313. doi: 10.1007/s10484-006-9026-2. [DOI] [PubMed] [Google Scholar]
- 12.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(Suppl 7):1–96. [PubMed] [Google Scholar]
- 13.Howell DC. 3rd. Belmont, CA: Wadsworth Publishing Company; 1995. Fundamental Statistics for the Behavioral Sciences. [Google Scholar]
- 14.Holm JE, Holroyd LA, Hursey KG, Penzien DB. The role of stress in recurrent tension headache. Headache. 1986;26:160–7. doi: 10.1111/j.1526-4610.1986.hed2604160.x. [DOI] [PubMed] [Google Scholar]
- 15.Morin CM, Rodrigue S, Ivers H. Role of stress, arousal, and coping skills in primary insomnia. Psychosom Med. 2003;65:259–67. doi: 10.1097/01.psy.0000030391.09558.a3. [DOI] [PubMed] [Google Scholar]
- 16.Smith MT, Haythornthwaite JA. How do sleep disturbance and chronic pain inter-relate? Insights from the longitudinal and cognitive-behavioral clinical trials literature. Sleep Med Rev. 2004;8:119–32. doi: 10.1016/S1087-0792(03)00044-3. [DOI] [PubMed] [Google Scholar]