Abstract
A lipid transfer protein that facilitates the transfer of glycolipids between donor and acceptor membranes has been investigated using a fluorescence resonance energy transfer assay. The glycolipid transfer protein (23-24 kDa, pI 9.0) catalyzes the high specificity transfer of lipids that have sugars β-linked to either a ceramide or a diacylglycerol backbone, such as simple glycolipids and gangliosides, but not the transfer of phospholipids, cholesterol, or cholesterol esters. In this study, we examined the effect of different charged lipids on the rate of transfer of anthrylvinyl-labeled galactosylceramide (1 mol %) from a donor to acceptor vesicle population at neutral pH. Compared to neutral donor vesicle membranes, introduction of negatively charged lipid at 5 or 10 mol % into the donor vesicles significantly decreased the transfer rate. Introduction of the same amount of negative charge into the acceptor vesicle membrane did not impede the transfer rate as effectively. Also, positive charge in the donor vesicle membrane was not as effective at slowing the transfer rate as was negative charge in the donor vesicle. Increasing the ionic strength of the buffer with NaCl significantly reversed the charge effects. At neutral pH, the transfer protein (pI ≅ 9.0) is expected to be positively charged, which may promote association with the negatively charged donor membrane. Based on these and other experiments, we conclude that the transfer process follows first-order kinetics and that the off-rate of the transfer protein from the donor vesicle surface is the rate-limiting step in the transfer process.
Glycosphingolipids (GSLs)1 are amphipathic molecules that together with phospholipids and cholesterol constitute the basic lipid core structure of biomembranes. Except for their presence at relatively high amounts in the plasma membranes of neural tissues and in the apical membranes of epithelial cells (about 25-30% of total lipids in both membrane types), GSLs are usually minor components in plasma membranes of eukaryotic cells (about 5%) (1, 2). The prevailing view has been that newly synthesized GSLs are localized predominantly in the outer leaflet of the eukaryotic plasma membrane. This location is consistent with their roles as cell surface markers and as modulators of membrane protein function. Also, certain GSLs function as the surface binding sites for certain bacteria, their toxins, and envelope viruses. For instance, sulfated galactosylceramide (sulfatide), but not galactosylceramide or ganglioside GM1, reportedly functions as the binding site for the envelope glycoprotein gp120 of the human immunodeficiency virus, HIV-1, in cells lacking the CD4 receptor (3). It has also been suggested that the simple monohexosyl sphingolipid glucosylceramide has mitogenic properties that stimulate cell growth, differentiation, and DNA synthesis (4). Moreover, the tendency of GSLs to organize into lateral membrane domains is thought to be a key feature, not only in their own intracellular sorting and trafficking but also in the sorting and trafficking of proteins, such as glycosylphosphatidylinositol (GPI)-anchored proteins (5, 6). Given their important roles in various cellular processes, it is clear that the transport and expression of glycolipids within cells must be effectively coordinated and controlled.
Glycolipid transfer proteins (GLTPs) have been identified in a wide variety of cell and tissue types, including mammalian brain, liver, kidney, and spleen, as well as in spinach chloroplasts (for review, see refs 7 and 8). These proteins catalyze the in vitro transfer of glycosphingolipids and glycoglycerolipids between donor and acceptor membranes. GLTPs appear to be cytosolic and transfer any glycolipid with a β-glucosyl or β-galactosyl sugar attached to a hydrophobic ceramide or diglyceride backbone (9). Two other classes of soluble proteins with glycolipid intermembrane transfer activity have been described: (1) glycosidase activator proteins, and (2) nonspecific lipid transfer proteins. Glycosidase activator proteins are lysosomal, and their main function is to serve as nonenzymatic cofactors required for the degradation of glycosphingolipids by the acidic glycosidases (10). In the absence of the degrading enzymes, certain activator proteins display in vitro glycolipid transfer activity (11). As a result, secreted forms of certain activator proteins have been proposed to serve as intercellular transporters of glycosphingolipids. A second class of soluble proteins with glycolipid transfer activity is the nonspecific lipid transfer proteins (nsLTPs). Bloj and Zilversmit (12) reported that different neutral glycosphingolipids as well as ganglioside GM1 were transferred by bovine liver nsLTP. Indeed, several nsLTPs identified in both animal and plant sources have been shown to catalyze the in vitro transfer of a wide range of lipids, including glycolipids (13).
GLTPs have been purified to apparent homogeneity from porcine and bovine brain, and characterization reveals many shared properties (14, 15). Like porcine brain GLTP, the bovine brain GLTP used in the present study is specific for various glycolipids including neutral glycosphingolipids and gangliosides, but does not stimulate phospholipid or neutral lipid intermembrane transfer (16, 17). Sequencing of the porcine GLTP via Edman degradation revealed 208 amino acids and 1 disulfide bond (18, 19). The bovine GLTP is of similar size with a molecular mass of 23-24 kDa and an isoelectric point near pH 9.0 (15). Several characteristics of bovine and porcine brain GLTPs suggest that these proteins are different from other known lipid transfer proteins. Nearly all of the lipid transfer proteins that show specificity for phosphatidylinositol and/or phosphatidylcholine have molecular masses between 25 and 35 kDa, and the isoelectric points are between 4.6 and 5.9. Most of the nonspecific lipid transfer proteins, on the other hand, have basic isoelectric points, but their molecular masses are in the range of 12-14.5 kDa. The amino acid compositions of other lipid transfer proteins also differ from that of GLTP. To date, the only protein known to resemble GLTP is the fungal protein HET-C2. This protein is expressed in Podospora anserina where it helps regulate cell compatibility interactions during heterokaryon fusion. Fusion of compatible cells leads to ascospore production, whereas fusion of incompatible cells triggers a process analogous to apoptosis. Cloning and expression of HET-C2 has revealed that this protein is 208 amino acids long, and that there is a 30% identity and a 37% similarity between the amino acid sequences (20).
To further our understanding of the role that membrane properties play in regulating the lipid transfer properties of the GLTP, we have investigated the effect of surface charge on the initial transfer rates of GalCer between bilayer vesicles. To accomplish this, we used a previously developed fluorescence resonance energy transfer (FRET) assay (21). Here we find that GLTP-mediated glycolipid transfer is significantly inhibited by negatively charged lipids. Also, the kinetic data are consistent with a transfer process in which the off-rate of the GLTP from vesicles containing the glycosphingolipid controls the overall rate of the transfer event.
EXPERIMENTAL PROCEDURES
Lipids
1-Palmitoyl-2-oleoylphosphatidylcholine (POPC), 1-palmitoyl-2-oleoylphosphatidylserine (POPS), 1-palmitoyl-2-oleoylphosphatidylethanolamine (POPE), 1-palmitoyl-2-oleoylphosphatidylglycerol (POPG), 1,2-dipalmitoylphosphatidic acid (DPPA), 1,2-dioleoylphosphatidic acid (DOPA), bovine liver phosphatidylinositol (blPI, 1,2-diacyl-sn-glycero-3-phospho-[1-D-myo-inositol]) containing mostly stearic acid (41%) and arachidonic acid (17.5%), and 1,2-dioleoyl-3-trimethylammonium propane (DOTAP) were all purchased from Avanti Polar Lipids (Alabaster, AL). Dihexadecyl phosphate (dicetyl phosphate, DCP) was purchased from Sigma Chemical Co. (St. Louis, MO). All lipids gave a single spot when analyzed by thin-layer chromatography. The fluorescent probes, N-[(11E)-12-(9-anthryl)-11-dodecenoyl]-1-O-β-galactosylsphingosine (AV-GalCer) and rac-1,2-dioleoyl-3-[9-(3-perylenoyl)nonanoyl]glycerol (Per-TG), were prepared as described earlier (22, 23). Tritiated bovine brain galactosylceramide was prepared using a method described previously (24, 25). Briefly, the alcohol group at the sixth carbon of the galactose sugar was oxidized using galactose oxidase (Sigma Chemicals). The resulting aldehyde group was reduced back to the original carbinol configuration using tritiated sodium borohydride. Labeled [3H]galactosylceramide was purified using an aminopropyl-bonded phase cartridge (Burdick & Jackson Laboratories, Muskegon, MI) as described previously (26).
Glycolipid Transfer Protein (GLTP)
Glycolipid transfer protein from bovine brain was purified to homogeneity as described earlier (15).
Preparation of Phospholipid Vesicles
Donor vesicles consisting of POPC and the fluorescent probes AV-GalCer and Per-TG, with or without the charged lipids, were prepared by the rapid ethanol injection technique (27, 28). Briefly, POPC was mixed with 1% AV-GalCer and 1.5% Per-TG from stock solutions [hexane/ethanol, 95:5 (Burdick & Jackson Laboratories)] and dried under nitrogen and redissolved immediately before use in absolute ethanol. The mixture (5 μL, 40 nmol) was rapidly injected with a 25 μL Hamilton syringe into a 10 mM sodium phosphate buffer (pH 7.4, containing 1 mM dithiothreitol, 1 mM EDTA, and 0.02% sodium azide) under rapid stirring at 37 °C. The final concentration of the donor vesicles in the assay was 13 μM, and the ethanol concentration was less than 0.2%. The final concentration of AV-GalCer in each assay was 0.13 μM. Previous studies have shown that monohexosylceramides are accommodated up to about 25 mol % within PC vesicles without affecting vesicle stability (29). In our case, only 1 mol % of AV-GalCer was generally used in the donor vesicles. With respect to the critical micelle concentration (CMC) of AV-GalCer, GalCers with long acyl chains are known to self-aggregate to form bilayer structures from X-ray studies (30, 31). It is likely that AV-GalCer exhibits similar behavior because the nonpolar AV group is linked to the carbonyl group via a 12-carbon chain. The CMC of nonfluorescent ganglioside GD1a is estimated to be 10-6 M (32, 33). Previously, we determined the CMC of AV-labeled GD1a to be about 10-8 M, which is 2 orders of magnitude lower than the nonfluorescent GD1a. The CMC of nonfluorescent GalCer is estimated to be between 10-8 and 10-9 M (33). Hence, we estimate that the CMC for the AV-labeled GalCer to be at least 10-10-10-11 M, or 2 orders of magnitude lower than the nonfluorescent GalCer.
The acceptor vesicles were prepared by sonication (34). Briefly, POPC was dried in a vacuum and suspended by vortexing in a sodium phosphate buffer (pH 7.4) to a concentration of 50 mM. The suspension was sonicated with a Heat Systems-Ultrasonics W-225 sonifier on ice, under nitrogen and was then centrifuged for 90 min at 100000g to remove titanium probe particles and multilamellar vesicles and undispersed lipid. The size of the vesicle populations reportedly averages about 25 nm in diameter (27).
[3H]GalCer/Charged Vesicle Transfer Assay
In this well-established assay, donor vesicles are constructed so that they contain a negatively charged phospholipid and can be separated from neutral acceptor vesicles by ion exchange chromatography (35). Sonicated POPC donor vesicles contained 1 mol % [3H]GalCer, 5 or 10 mol % negatively charged phospholipid, and a trace amount of nonexchangeable [14C]tripalmitate. Acceptor vesicles were sonicated POPC small unilamellar vesicles. Each assay time point contained 40 nmol of donors, 400 nmol of acceptors, and transfer protein in a total volume of 3.0 mL of a sodium phosphate buffer, pH 7.4. At desired time intervals, acceptor vesicles were separated from donor vesicles by rapid elution over DEAE Sephacel minicolumns (1.8 mL). Control experiments indicated typical acceptor recoveries between 70 and 80%.
Fluorescence Measurements
All fluorescence measurements were carried out using a SPEX Fluoromax instrument (Instruments S. A., Inc., Edina, NJ). The excitation and emission band-passes were 5 nm, and the sample cuvette holder was temperature-controlled to 37 ± 0.1 °C (Neslab, RTE-111, Neslab Instruments, Portsmouth, NH).
Kinetic Analysis
The fluorescence resonance energy transfer method used for measuring the rate of glycolipid transfer between two bilayer vesicle populations has been described previously (21) and is summarized in Figure 1. The excitation spectrum of AV-GalCer in POPC occurs between 330 and 410 nm with peaks near 370 and 390 nm, whereas the emission spectrum occurs between 390 and 485 nm with peaks near 415 and 430 nm. The excitation spectrum of Per-TG in POPC overlaps the emission of AV-GalCer, also occurring between 390 and 485 nm with a broad maximum peak near 450 nm, whereas the emission spectrum occurs between 470 and 590 nm with a broad maximum peak near 515 nm (21). As a result, when excited at 370 nm, nearly complete quenching of AV-GalCer occurs in donor vesicles containing nontransferable Per-TG (Figure 1A, solid line). Addition of POPC acceptor vesicles and GLTP results in increasing AV-GalCer fluorescence and diminishing Per-TG fluorescence as quenching relief occurs due to transfer of AV-GalCer to the acceptor vesicles (Figure 1A, dotted line). Because of this spectral response, the kinetics of AV-GalCer intermembrane transfer can be determined by continuously monitoring the increase in AV fluorescence at 425 nm as shown in Figure 1B. Quantitation of the kinetic response was achieved by recording the increase in fluorescence (Frec at 425 nm) as a function of time relative to the quenched signal level (Fquenched) observed prior to GLTP addition. A plot of
was linear for at least 5 half-times (Figure 1C), consistent with first-order exponential behavior. The slope of ln [F∞/(F∞-Frec)] was determined by a least-squares procedure. The time point for GLTP addition was taken as the zero time point. The experimental first-order rate constant, kobs, and the half-time, t1/2, for the transfer rate were determined from the relationships:
FIGURE 1.
Resonance energy transfer assay used to measure the transfer rate of AV-labeled galactosylceramide between small unilamellar donor and acceptor vesicles. (A) Emission spectra of POPC donors vesicles containing 1 mol % AV-GalCer, 1.5 mol % Per-TG, and 10-fold excess of POPC acceptor vesicles (solid line). The dotted line shows the same vesicles after addition of GLTP and after the transfer of AV-GalCer to acceptor vesicles reached equilibrium. The arrow indicates the wavelength (425 nm) where the emission was measured. (B) POPC donor vesicles (40 nmol in 3.0 mL) were added to a sodium phosphate buffer (pH 7.4) and allowed to equilibrate for 5 min at 37 °C. Acceptor vesicles were added (400 nmol), and the system was allowed to equilibrate for another 5 min. Next GLTP was added, 2 μg (84.4 pmol), and the change in fluorescence (ΔFrec) was recorded. (C) Semilogarithmic plot of ln [F∞/(F∞ - Frec)] versus time for the trace shown in panel B. The slope of this plot was used to calculate the transfer half-time.
RESULTS
GLTP-Mediated Transfer of AV-GalCer Follows First-Order Kinetics
Because investigations into the kinetics of the GLTP-mediated transfer process can provide insights into the protein's mechanism of action, the following experiments were carried out. First, the effect of GLTP concentration on the GalCer intermembrane transfer rate was determined under conditions of the fluorescence resonance energy transfer (FRET) assay. As shown in Figure 2, GLTP concentrations between 0.12 and 2.0 μM produced progressive increases in the AV-GalCer initial transfer rate when the donor to acceptor vesicle ratio was maintained at 1:10. The relative fluorescence intensity was converted to picomoles, assuming that AV-GalCer is mass-distributed, similar to the POPC matrix in the outer and inner leaflets of the small and highly curved donor vesicles. Then approximately one-third of the AV-GalCer originally present (400 pmol) would be expected to remain in the inner membrane leaflet, be quenched by the Per-TG, and be inaccessible to GLTP. At equilibrium and with a donor to acceptor vesicle ratio of 1:10, the GLTP would transfer 10/11ths (90.9%) of the AV-GalCer on the outer bilayer leaflet to the acceptor vesicles. Considering the preceding, approximately 60-65% recovery of the AV-GalCer emission signal would be expected when intervesicular transfer equilibrium is achieved. This is what we observed for the higher amounts of GLTP (Figure 2, traces a and b). The lower amounts of GLTP ultimately reach the same level after sufficient time. The increase in the transfer half-times at different GLTP concentrations are also shown in Table 1 (left two columns).
FIGURE 2.
Effect of GLTP concentration increase on AV-GalCer transfer from donor to acceptor vesicles. The fluorescence intensity was converted to picomoles by assuming that the AV-GalCer probe is mass-distributed (like POPC) in the outer and inner leaflets of the small and highly curved donor vesicles. Higher concentrations of GLTP reach the equilibrium level after about 15 min, whereas the lower concentrations ultimately reached the same level. See also Table 1, left two columns.
Table 1.
Protein-Mediated AV-GalCer Transfer Half-Times under Different Conditions, Using Different GLTP Concentrations (Columns 1 and 2), Different AV-GalCer Concentrations (Columns 3 and 4), and Altered Donor to Acceptor Vesicle Ratios (Columns 5 and 6)
| [GLTP] (μg) | transfer half-time (s) |
[AV-GalCer] (mol %) |
transfer half-timeb (s) |
donor:acceptor vesicle ratioc |
transfer half-time (s) |
|---|---|---|---|---|---|
| 2.0 | 128 | 0.5 | 236 ± 29 | 1:5 | 258 ± 5 |
| 0.94 | 191 | 1.0 | 198 ± 40 | 1:10 | 161 ± 7 |
| 0.47 | 300 | 1.5 | 236 ± 37 | 1:20 | 170 ± 11 |
| 0.23 | 351 | 2.0 | 216 ± 30 | ||
| 0.12 | 478 | 5.0 | 190 ± 11 | ||
| denatured | —a |
The half-time for the denatured glycolipid transfer protein is very slow and close to the rate for spontaneous transfer, which has been reported to be >20 h (56).
0.94 μg of GLTP.
2.0 μg of GLTP.
Next the concentration of GLTP and the donor to acceptor ratio were held constant, while the concentrations of AV-GalCer and Per-TG were varied within the PC matrix. However, the AV-GalCer:Per-TG molar ratio was kept at 1:1.5 in all experiments, even at higher AV-GalCer mole percents. Because the Per-TG quencher might phase-separate in a fluid phase bilayer at higher concentrations (36), 5 mol % AV-GalCer was the highest concentration examined. The results in Table 1 (center two columns) show that the transfer half-time did not significantly change when the concentration of the transfer protein was kept constant (0.94 μg) and the mole percent of AV-GalCer in the donor vesicle population was varied from 0.5 to 5.0 mol %. The results are consistent with the transfer process following first-order kinetics.
Finally, the donor vesicle concentration and the GLTP concentration were kept constant, while the acceptor vesicle concentration was systematically varied. Decreasing the acceptor vesicle concentration to only a 5-fold excess did slow the transfer rate slightly, whereas increasing the acceptor vesicle concentration to a 20-fold excess did not significantly affect the transfer rate relative to that observed at a 1:10 (donor/acceptor) ratio (Table 1, right two columns). The results suggest that the transfer from the donor surface is the rate-limiting step when there is a sufficient excess of acceptor vesicles.
As pointed out earlier in our FRET experiments (21), the presence of acceptor vesicles is necessary for the transfer process to occur. When catalytic amounts of GLTP (84.4 pmol) were mixed with donor vesicles alone, no increase in the anthrylvinyl emission intensity was detected for time periods extending up to 30 min. Yet, subsequent addition of acceptor vesicles to the mixture resulted in immediate and rapid transfer of AV-GalCer (data not shown). Also, if other proteins of similar size and isoelectric point (e.g., ribonuclease) or with a general affinity for lipids (e.g., bovine serum albumin) were substituted for GLTP, then no change in the AV-GalCer signal response was observed regardless of whether acceptor vesicles were present.
Negative Surface Charge Slows Down the GLTP-Mediated Intervesicular Transfer
Because the pI of bovine brain GLTP is near 9.0 as determined by FLPC chromatofocusing studies (15), we expected that membrane surface charge might play a role in regulating the rate of the GLTP-mediated transfer of glycolipids between membranes. In previous work, testing of this idea was complicated by the radiolabeled transfer assay in use, because negatively charged lipid was included in the donor vesicles to achieve separation of donor and acceptor vesicles by elution over DEAE minicolumns (15, 35). If the charged lipid concentration in the donor vesicles was too low, then separation of donor and acceptor vesicles was not possible, and accurate lipid transfer kinetics could not be achieved. However, the FRET assay provided a means to monitor glycolipid transfer kinetics without having to separate the donor and acceptor vesicles and thus to assess the effect of surface charge on the GLTP-mediated transfer of glycolipids (21).
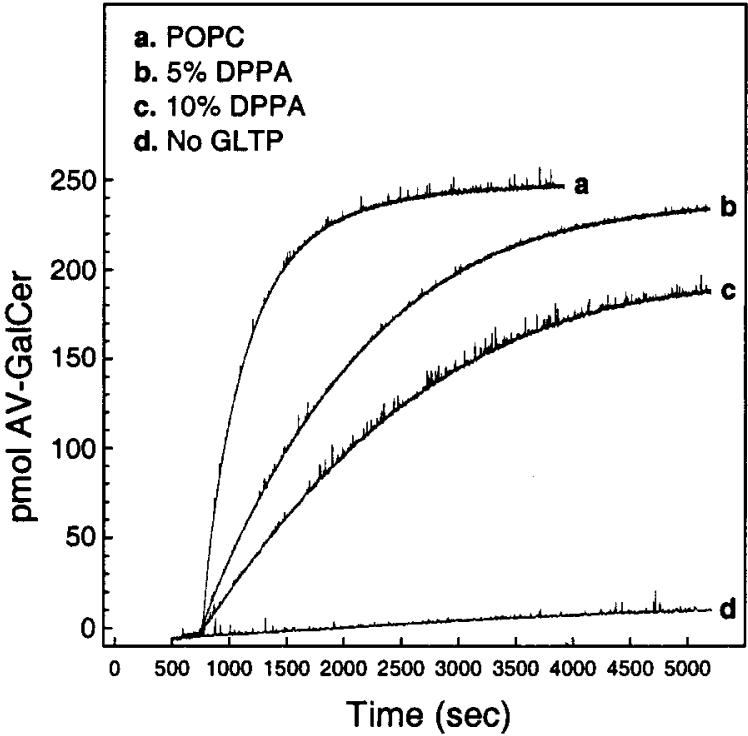
Initially, we investigated the effect of having 5 or 10 mol % dipalmitoylphosphatidic acid (DPPA) in the donor vesicles. Figure 3 shows that increasing DPPA levels in donor vesicles decreased the kinetic rate of AV-GalCer transfer mediated by GLTP. Analysis of the fluorescence data (see Experimental Procedures) permitted calculation of the first-order rate constants and associated transfer half-times for the different DPPA membrane concentrations. As shown in Figure 4A, the transfer half-time is increased over 4-fold when 5 mol % DPPA is included in the POPC donor vesicles compared to when DPPA is absent. Increasing the DPPA concentration to 10 mol % produced a further increase in the transfer half-time.
FIGURE 3.
Increasing DPPA levels in donor vesicles show a decrease in the kinetic rate of AV-GalCer transfer mediated by GLTP. Trace a represents GLTP-mediated AV-GalCer transfer from POPC donor vesicles, and trace d represents the spontaneous transfer of AV-GalCer from POPC donor vesicles with no addition of GLTP. The amount of GLTP in the a, b, and c experiments was 2.0 μg.
FIGURE 4.
Effect of negatively charged donor vesicles on the GLTP-mediated AV-GalCer transfer rate. Panel A shows the transfer half-times for 5 and 10 mol % charged donors, and panel B shows the half-times at high sodium chloride concentrations (0.5 M). The amount of GLTP in each experiment was 2.0 μg. Values are averages ± SD of at least 3 different experiments.
To establish whether surface electrostatics could account for the diminished transfer rates induced by DPPA, two strategies were employed. First, negative charge was imparted to the donor surface using lipids with different polar headgroups. Second, the effect of increased ionic strength on transfer kinetics was determined. As shown in Figure 4A, replacing DPPA with other negatively charged lipids, such as POPS, DCP, bovine liver PI, POPG, or DOPA, gave the same effect, i.e., dramatically increased the transfer half-time of the GLTP-mediated glycolipid transfer process. Similar transfer half-times resulted regardless of which negatively charged lipid was used at 5 mol % (Figure 4A, solid bars). At 10 mol % negative charge (Figure 4A, gray bars), DCP, bovine liver PI, and DOPA increased the transfer half-time similarly and to a greater extent than POPS, POPG, or DPPA. Replacing the negatively charged lipids with 5 or 10 mol % zwitterionic POPE increased the transfer half-times only slightly.
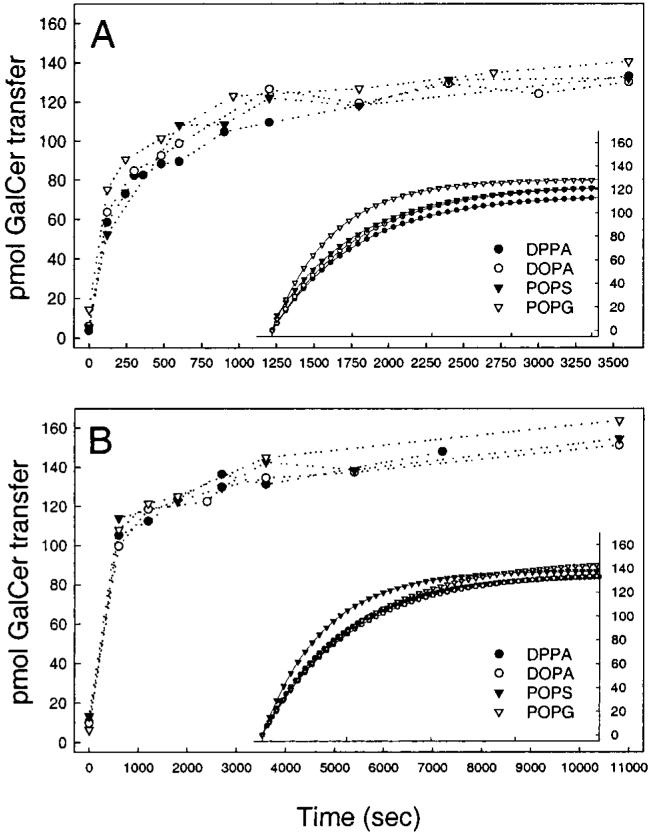
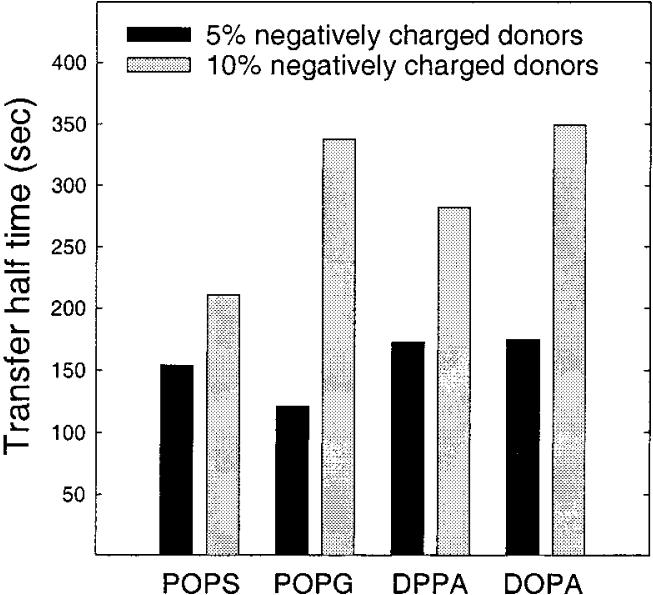
To show that the negative charge effect on GLTP-mediated transfer was not an artifact of using GalCer with a fluorescent reporter group, additional experiments were performed using the [3H]GalCer/charged vesicle assay (see Experimental Procedures). However, because this assay relies on differences in donor and acceptor charge to achieve the vesicle separation needed for accurate assessment of transfer kinetics, only the relative effect of increasing charge could be determined. Using this assay, it was not possible to determine the [3H]GalCer intermembrane transfer rate in the absence of negative charge. Figure 5 shows the GLTP-mediated [3H]-GalCer transfer resulting when 5 mol % (panel A) or 10 mol % (panel B) DPPA, DOPA, POPS, or POPG are present in the donor vesicles. Fitting of the kinetic curves to single exponential behavior and determination of the transfer rate constants revealed that the transfer half-time was significantly greater for the donors containing 10 mol % negative charge (Figure 6, gray bars) compared to those with 5 mol % negative charge (Figure 6, solid bars). Yet, DPPA, DOPA, POPS, and POPG all had similar effects on the transfer rates when present at identical concentrations.
FIGURE 5.
Time course of GLTP-mediated transfer of 1 mol % [3H]GalCer from POPC small unilamellar donor vesicles containing 5 mol % (A) and 10 mol % (B) negatively charged phospholipids to neutral small unilamellar POPC acceptor vesicles. The amount of GLTP in each experiment was 2.0 μg, and the inset shows fitted regression curves.
FIGURE 6.
Transfer half-times for different negatively charged lipids analyzed by a least-squares procedure from the fitted curves in Figure 5, insets. The amount of GLTP in each experiment was 2.0 μg.
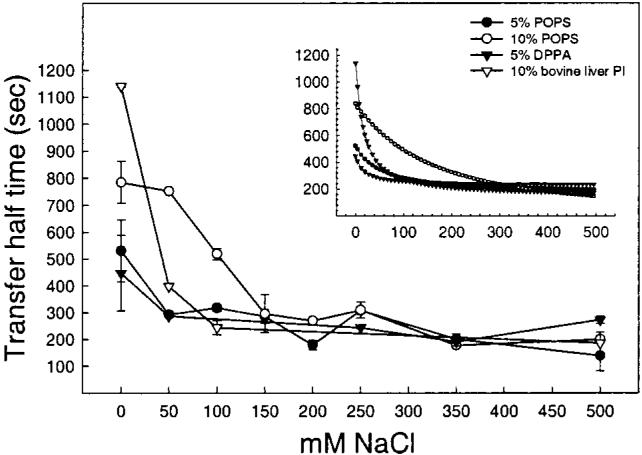
If the increased transfer half-times observed when negatively charged lipids are present in the donor membranes can be attributed to surface electrostatics, then increased ionic strength of the buffer would be expected to reverse the slowdown in transfer rate. With the FRET transfer assay, it was possible to examine the effect of ionic strength on transfer kinetics. Figure 4B shows the effect of 0.5 M NaCl on the transfer half-times when the different negatively charged lipids are present in the donor vesicles at 5 mol % (solid bars) and 10 mol % (gray bars). High ionic strength clearly abolished the diminishing effects that both 5 and 10 mol % negatively charged lipids had on the AV-GalCer transfer rates. Controls with POPC donor vesicles containing no charged lipid revealed only small effects of the high salt concentration on transfer activity. To determine the effectiveness of NaCl at overcoming the inhibition produced by negative charge, we used donor vesicles containing POPS, DPPA, or bovine liver PI to determine the NaCl concentration dependence. As shown in Figure 7, reversal of the negative charge effect occurred at 50 mM NaCl if only 5 mol % POPS or DPPA was present. Interestingly, however, 50 mM NaCl had no effect, reversing the inhibitory effect of donor vesicles containing 10 mol % bovine liver PI. Instead, 150 mM NaCl was necessary to completely reverse that inhibitory effect of the PI.
FIGURE 7.
Effect of increasing concentration of sodium chloride on the transfer rate of AV-GalCer from negatively charged donor vesicles. The inset shows fitted regression curves. Values are averages ± SD of at least 3 different experiments.
Positively Charged Surfaces Are Less Effective at Diminishing GLTP-Mediated Transfer
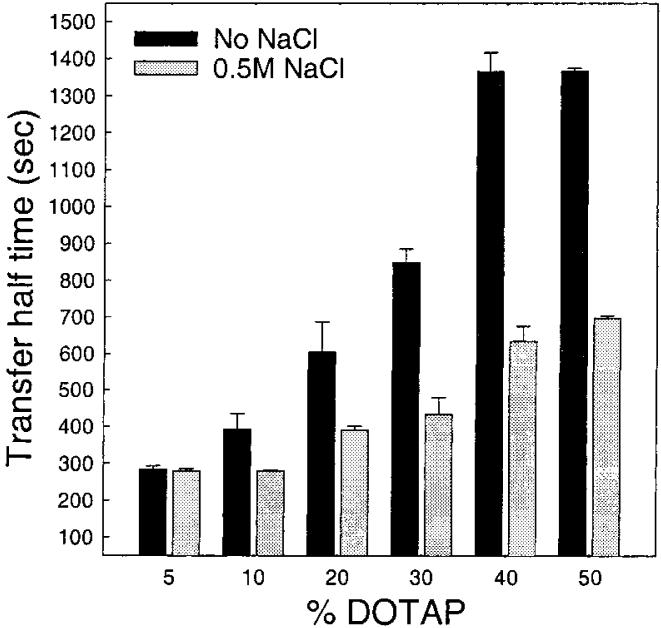
Though cationic lipids are not common in biological membranes, they can occur transiently in response to certain signaling events (e.g., sphingosine) and also have been used as transfection agents. To determine the effect that a positively charged surface has on GLTP-mediated transfer of AV-GalCer, we investigated the effect of the widely used lipid transfection agent dioleoyltrimethylammonium propane (DOTAP). As shown in Figure 8 (solid bars), addition of 5-50 mol % DOTAP did impede glycolipid transfer. However, the amount of positive charge in the donor vesicle surfaces had to be much higher in order to achieve the same inhibitory effect on glycolipid transfer compared to negative charge. Also, 0.5 M NaCl was not as effective at reversing the inhibitory effect on transfer caused by the positive membrane charge (Figure 8, gray bars).
FIGURE 8.
Effect of positively charged donor vesicles on the GLTP-mediated AV-GalCer transfer rate. The solid bars show the transfer half-times for 5-50 mol % charged donors without sodium chloride, and the gray bars show the half-times at high (0.5 M) sodium chloride concentration. The amount of GLTP in each experiment was 2.0 μg. Values are averages ± SD of at least 3 different experiments.
Location of Charged Lipid to Donor versus Acceptor Vesicles Affects the Transfer Kinetics
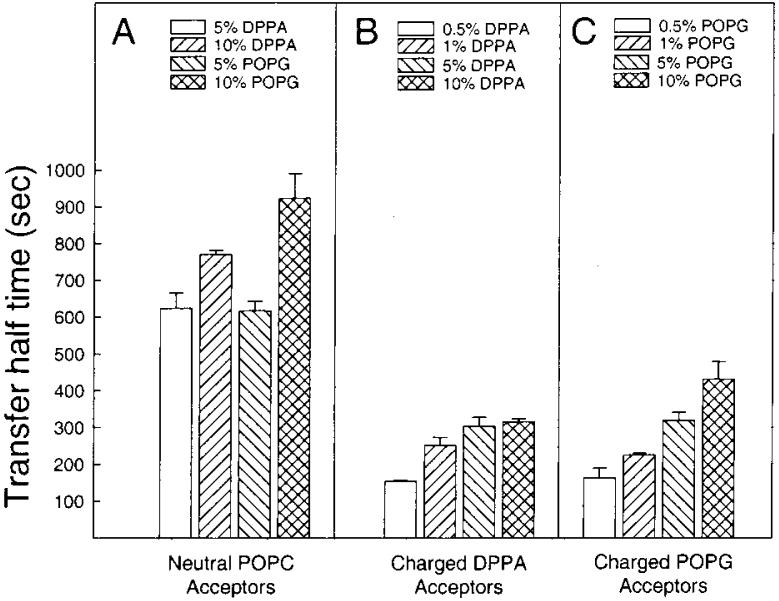
As pointed out earlier, compared to the 10-fold excess of acceptor vesicles routinely utilized in these investigations, increasing the acceptor vesicle concentration to a 20-fold excess did not significantly affect the transfer rate (Table 1, right two columns). This result, along with inhibition of transfer caused by negative charge in the donor surface, suggested that the off-rate of the transfer protein from the donor vesicle membrane is the limiting step in the transfer process. If so, then the location of the charged lipid (donor versus acceptor vesicles) would be expected to impact significantly on the observed transfer kinetics. To test this idea, negative charge was incorporated into the acceptor vesicles, and the impact on the transfer kinetics was assessed. As shown in Figure 9B,C, increasing the charge in the acceptor vesicle population from 0.5 to 10 mol % with either DPPA or POPG gradually slowed the transfer rate, but not to the same extent as when the charge was located in the donor vesicle surface (Figure 9A).
FIGURE 9.
Transfer half-times for different acceptor vesicle compositions. Panel A shows the transfer rate for negatively charged donors and neutral acceptors, whereas panels B and C show the transfer rate for neutral donors with negatively charged acceptors. The amount of GLTP in each experiment was 2.0 μg. Values are averages ± SD of at least 3 different experiments.
DISCUSSION
Previous studies have shown that membrane surface charge has significant effects on lipid transfer protein catalyzed transfer of various lipids. For instance, negatively charged donor and acceptor membranes reportedly inhibit the activity of the intracellular phosphatidylcholine transfer protein and nsLTP (37-39). The phosphatidylcholine transfer activity of a yeast phosphatidylinositol/phosphatidylcholine transfer protein also is inhibited by negatively charged phospholipids and fatty acids (40). The C-terminus of phosphatidylinositol transfer protein has recently been shown to modulate membrane interactions and transfer activity but not phospholipid binding (41, 42). Plasma-derived phospholipid transfer protein and cholesteryl ester transfer protein that facilitate the transfer of phospholipids and cholesterol esters, respectively, between plasma lipoproteins are also sensitive to the lipoprotein surface charge. This has recently been shown with different modifications of lipoprotein particles that altered their surface properties such as charge (43, 44).
In contrast to these inhibitory effects, Sandhoff and colleagues recently reported that negatively charged lipids stimulate the sphingolipid activator protein, SAP-C (45). In this study, model vesicles were used to mimic the surfaces of intralysosomal vesicles, and the lysosomal hydrolysis of glycolipids by glucocerebrosidase and sphingolipid activator protein C, SAP-C. The hydrolysis of glucosylceramide was stimulated 20-30-fold by the presence of SAP-C and anionic lipids such as dolicol phosphate and phosphatidylinositol at up to 30 mol %. They further concluded that the presence of negatively charged groups, such as phosphate, seemed to be essential for the activation of the enzymes involved in the glycolipid hydrolysis. Because certain sphingolipid activator proteins have the capability to function as glycolipid transfer proteins, it was of interest to determine the impact of surface charge on the activity of the glycolipid transfer protein. An especially suitable way to address this issue was by using a fluorescence resonance transfer assay that we recently developed for determining the kinetics of protein-mediated lipid transfer between donor and acceptor membranes (21). The fluorescent reporter group, anthrylvinyl, serves as the resonance energy donor and is linked to GalCer, whereas the resonance energy acceptor is a perylenoyl-labeled triglyceride. In general, FRET assays are non-destructive and can provide real-time analysis into different processes in biological systems. The anthrylvinyl/perylenoyl FRET pair provides distinct benefits over the NBD/lissamine rhodamine FRET pair, which often has been used to monitor protein-mediated lipid transfer and related processes (21). Because lipid transfer proteins often are specific for select lipids or lipid classes, fluorescent labels such as NBD or anthrylvinyl are usually attached to one of the acyl chains in order to avoid altering the lipid polar headgroup. Apolar probes linked to an acyl chain mimic more closely the behavior of natural lipids since the headgroup resembles and retains the same structure and molecular shape as the natural lipid. In the case of the anthrylvinyl and perylenoyl probes, both fluorophores localize to the hydrophobic region of the bilayer and thus cause minimal disturbance of the bilayer interfacial/polar region.
Comparison of the transfer rates obtained using the FRET assay and the radiolabeled GalCer assay reveals that GLTP transfers the AV-GalCer slower than the 3H-labeled GalCer. This behavior could reflect differences either in the relative affinity of GLTP for the two GalCer derivatives or in the packing of each derivative within the donor bilayer vesicles. The glycolipid interaction site on GLTP might not accommodate the bulky AV group as well as a nonfunctionalized acyl chain. Alternatively, embedding of the apolar anthrylvinyl group in the membrane bilayer might be facilitated by the C-12 acyl chain used to attach the AV group to GalCer. In this regard, the AV is quite different from a polar fluorophore like NBD, that probably tends to ‘loop out’ of the bilayer interior to the interfacial region (46, 47). Stronger anchoring of the AV-GalCer into the donor vesicle might make transfer by GLTP more difficult than the radiolabeled GalCer. A decrease in the transfer efficiency of BODIPY-cholesteryl ester compared to its radiolabeled counterpart by the cholesteryl ester transfer protein also has been noted (48). Nonetheless, it is clear the fluorescent assays provide the advantage of continuous and more accurate determination of transfer kinetics. In fact, earlier studies of partially purified bovine GLTP involving pyrene-labeled glucosylceramide showed the dramatic influence that bilayer matrix phase state has on GLTP activity (16, 49).
Using the FRET assay, we observed that if the donor and acceptor vesicle membrane surfaces are neutral and carry no net charge, then GLTP-mediated transfer of AV-GalCer is fast and quickly reaches equilibrium. It appears that, at this equilibrium level, GLTP has transferred nearly all the accessible glycosphingolipids in the outer surfaces of the donor vesicles down the concentration gradient to the acceptors (21). Since the transbilayer distribution of glycosphingolipids in highly curved phospholipid vesicle membranes has not been established by other methods, we assume that the AV-GalCer is uniformly distributed between the inner and the outer layer. The size of the ethanol-injected vesicles averages 25 nm in diameter. Therefore, due to curvature constraints, about two-thirds of the AV-GalCer presumably localizes to the outer leaflet and is accessible to the transfer protein. The changes that we observe in AV-GalCer emission intensity from time zero to equilibrium for both the neutral and the charged vesicle systems are consistent with AV-GalCer being mass-distributed in the donor vesicles. We also found that increasing the amount of AV-GalCer from 1 to 5 mol % did not significantly change the transfer half-time, which would suggest that the transfer process follows first-order kinetics. Increasing the amount of transfer protein did indeed result in a faster transfer rate and a shorter transfer half-time.
In the present study, all experiments were carried out with GalCer initially present only in the donor vesicles. We did not reexamine the impact of having GalCer in both donor and acceptor vesicles upon initiation of GLTP action. Previously, this was reported to have virtually no effect on GLTP-mediated transfer kinetics (14, 16).
Because the isoelectric point for the bovine brain GLTP is near 9.0 (15), we expected that membrane surface charge might play an important role in regulating the rate of the GLTP-mediated transfer of glycolipids between membranes at physiological pH. Our experimental observations support this thinking and suggest the following scenario. When the donor vesicle surface carries a negative charge, an increased residence time of the net positively charged GLTP occurs due to the electrostatic forces that increase the attraction to the donor vesicle surface. The GLTP off-rate from the donor surface slows down, resulting in a diminished rate of GLTP-mediated transfer. The preceding scenario is consistent with several experimental observations. First, a variety of different negatively charged lipids (PA, PS, PI, DCP) all slow GLTP-mediated transfer of AV-GalCer nearly equally well when present in donors at only 5 mol %. Increasing the negatively charged lipid content to 10 mol % slows transfer even further. The variation in the efficiency of the different negatively charged lipids at 10 mol % observed in the FRET assay might be due to differing charge density distributions on the donor surfaces and will require further study. In any event, increasing the ionic strength of the buffer by raising the NaCl concentration prevents the negatively charged lipids from slowing the GLTP-mediated transfer rate. It is clear that the increased ionic strength does not denature the GLTP.
Second, when the donor vesicle surface carries a positive charge, a slowing of the transfer rate by GLTP occurs. Yet, much higher mole fractions of positively charged lipid are needed to slow the transfer rate to levels observed with lower mole fractions of negatively charged lipids. This finding is consistent with repulsion of the positively charged GLTP from the vesicle surface resulting in a diminished GLTP on-rate and a slowing of the transfer process.
On the other hand, introducing negative charge into the donor vesicle membrane slows the protein-mediated transfer to a significantly greater extent than when the negative charge is present in the acceptor membrane. This suggests that the GLTP does not necessarily have to be associated with the acceptor membrane to be able to release the AV-GalCer. If interaction of the positively charged GLTP with the negatively charged acceptor membrane is essential for AV-GalCer to be dissociated from the GLTP, an equally diminished transfer rate would be expected, since the positively charged GLTP would be attracted to the negatively charged acceptor membrane. Therefore, it is plausible to suggest that GLTP facilitates glycosphingolipid release from the donor membrane and that the association of the AV-GalCer with the GLTP is weak. This contrasts the behavior of a classical carrier protein, which interacts with the acceptor membrane to complete the transfer event. Additional evidence supporting this model comes from earlier attempts to isolate a glycosphingolipid-GLTP complex by Abe and co-workers, who reported a stoichiometry of 0.13 to 1 glycosphingolipids to GLTP in solution (50).
All lipids used in this study are negatively charged under our experimental and physiological conditions except for phosphatidylcholine and phosphatidylethanolamine. Among the negatively charged lipids used in our study, phosphatidylserine is the major anionic phospholipid found in the inner leaflet of many cell plasma membranes (about 8-10% of rat hepatocyte total lipids). Phosphatidylinositol, which also has a net negative charge at physiological pH but is not transferred by GLTP, is found in almost equal amounts in the inner leaflet of the plasma membrane of rat hepatocytes. In contrast, the subcellular content of phosphatidylinositol is much higher in the Golgi complex, lysosomes, microsomes, and the nuclear membrane compared to phosphatidylserine. Phosphatidic acid has a plasma membrane content of about 1% and is also found in the ER where it can serve as a precursor for many phospholipids. Phosphatidic acid at a plasma membrane level most likely serves as a signaling molecule derived through hydrolysis by phospholipase D activity (51). Phosphatidylglycerol is found mainly in the mitochondria in relatively high amounts, where it functions as a precursor for the synthesis of the dimeric phospholipid cardiolipin. GLTP is thought to be a cytosolic protein, but no definitive subcellular localization has been established. Therefore, it is difficult to conclude with certainty if negatively charged lipids in different intracellular compartments represent a major regulatory mechanism for glycolipid transfer protein activity in vivo.
Most lipid transport processes in vivo occur through the Golgi vesicular secretory pathways (52, 53). It could be that the function of GLTP is to maintain and control more specific glycosphingolipid intracellular distribution, rather than being a bulk lipid transporter. This is supported by the finding by Warnock and colleagues that observed a continued transport of newly synthesized glucosylceramide in Chinese hamster ovary cells, even though the Golgi vesicular transport pathway was disrupted by Brefeldin A or by incubation at 15 °C (54). GLTP might also exhibit an activator protein-like capability, perhaps in the glycosphingolipid synthesis or homeostasis, since certain glycosphingolipid activator proteins have shown in vitro glycolipid transfer activities (11, 55). In any case, it is clear that physical characteristics of the membrane, such as surface charge, impact strongly on the ability of GLTP to transfer glycolipids between membranes.
ACKNOWLEDGMENT
We thank Dr. Howard Brockman for his helpful comments and stimulating discussions.
We gratefully acknowledge the support of the Academy of Finland, Åbo Akademi Foundation, Magnus Ehrnrooth Foundation, a NAS/NRC COBASE Project Development Grant, Hormel Foundation, and USPHS Grant GM45928.
Footnotes
Abbreviations: GLS, glycosphingolipid; GalCer, galactosylceramide; GLTP, glycolipid transfer protein; FRET, fluorescence resonance energy transfer; AV-GalCer, N-[(11E)-12-(9-anthryl)-11-dodecenoyl]-1-O-β-galactosylsphingosine; Per-TG, rac-1,2-dioleoyl-3-[9-(3-perylenoyl)nonanoyl]glycerol; POPC, 1-hexadecanoyl-2-[cis-9-octadecenoyl]-sn-glycero-3-phosphocholine; POPE, 1-hexadecanoyl-2-[cis-9-octadecenoyl]-sn-glycero-3-phosphoethanolamine; POPS, 1-hexadecanoyl-2-[cis-9-octadecenoyl]-sn-glycero-3-phospho-L-serine; POPG, 1-hexadecanoyl-2-[cis-9-octadecenoyl]-sn-glycero-3-[phospho-rac-(1-glycerol)]; DPPA, 1,2-dihexadecanoyl-sn-glycero-3-phosphate; DOPA, 1,2-di[cis-9-octadecenoyl]-sn-glycero-3-phosphate; blPI, bovine liver phosphatidylinositol; DCP, dihexadecyl phosphate (dicetyl phosphate); DOTAP, 1,2-dioleoyl-3-trimethylammonium propane.
REFERENCES
- 1.Maggio B. Prog. Biophys. Mol. Biol. 1994;62:55–117. doi: 10.1016/0079-6107(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 2.Merrill AH, Schmelz EM, Dillehay DL, Spiegel S, Shayman JA, Schroeder JJ, Riley RT, Voss KA, Wang E. Toxicol. Appl. Pharmacol. 1997;142:208–225. doi: 10.1006/taap.1996.8029. [DOI] [PubMed] [Google Scholar]
- 3.McAlarney T, Apostolski S, Lederman S, Latov N. J. Neurosci. Res. 1994;37:453–460. doi: 10.1002/jnr.490370404. [DOI] [PubMed] [Google Scholar]
- 4.Radin NS, Inokuchi J. Biochem. Pharmacol. 1988;37:2879–2886. doi: 10.1016/0006-2952(88)90271-7. [DOI] [PubMed] [Google Scholar]
- 5.Anderson RGW. Annu. Rev. Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 6.Brown RE. J. Cell Sci. 1998;111:1–9. doi: 10.1242/jcs.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki T. Experientia. 1990;46:611–616. doi: 10.1007/BF01939700. [DOI] [PubMed] [Google Scholar]
- 8.Bankaitis VA, Cartee RT, Fry MR, Kagiwada S. Phospholipid Transfer Proteins: Emerging Roles in Vesicle Trafficking, Signal Transduction, and Metabolic Regulation. R. G. Landers Company and Chapman & Hall; Springer-Verlag, Heidelberg, Germany: 1996. pp. 51–72. [Google Scholar]
- 9.Yamada K, Abe A, Sasaki T. Biochim. Biophys. Acta. 1986;879:345–349. [PubMed] [Google Scholar]
- 10.Fürst W, Sandhoff K. Biochim. Biophys. Acta. 1992;1126:1–16. doi: 10.1016/0005-2760(92)90210-m. [DOI] [PubMed] [Google Scholar]
- 11.Conzelmann E, Burg J, Stephan G, Sandhoff K. Eur. J. Biochem. 1982;123:455–464. doi: 10.1111/j.1432-1033.1982.tb19789.x. [DOI] [PubMed] [Google Scholar]
- 12.Bloj B, Zilversmit DB. J. Biol. Chem. 1981;256:5988–5991. [PubMed] [Google Scholar]
- 13.Wirtz KWA. Biochem. J. 1997;324:353–360. doi: 10.1042/bj3240353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe A, Sasaki T. J. Biol. Chem. 1985;260:11231–11239. [PubMed] [Google Scholar]
- 15.Brown RE, Jarvis KL, Hyland KJ. Biochim. Biophys. Acta. 1990;1044:77–83. doi: 10.1016/0005-2760(90)90221-i. [DOI] [PubMed] [Google Scholar]
- 16.Brown RE, Stephenson FA, Markello T, Barenholz Y, Thompson TE. Chem. Phys. Lipids. 1985;38:79–93. doi: 10.1016/0009-3084(85)90059-3. [DOI] [PubMed] [Google Scholar]
- 17.Gammon CM, Vaswani KD, Ledeen RW. Biochemistry. 1987;26:6239–6243. doi: 10.1021/bi00393a043. [DOI] [PubMed] [Google Scholar]
- 18.Abe A. J. Biol. Chem. 1990;265:9634–9637. [PubMed] [Google Scholar]
- 19.Abe A, Sasaki T. Biochim. Biophys. Acta. 1989;985:45–50. doi: 10.1016/0005-2736(89)90101-6. [DOI] [PubMed] [Google Scholar]
- 20.Saupe S, Descamps C, Turcq B, Bégueret J. Proc. Natl. Acad. Sci. U.S.A. 1994;91:5927–5931. doi: 10.1073/pnas.91.13.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattjus P, Molotkovsky JG, Smaby JM, Brown RE. Anal. Biochem. 1999;268:297–304. doi: 10.1006/abio.1998.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molotkovsky JG, Imbs AB, Bergelson LD. Bioorg. Khim. (Russia) 1983;9:114. [Google Scholar]
- 23.Molotkovsky JG, Bergelson LD. Bioorg. Khim. (Russ.) 1982;8:1262. [Google Scholar]
- 24.Brown RE, Thompson TE. Biochemistry. 1987;26:5454–5460. doi: 10.1021/bi00391a036. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki Y, Suzuki K. J. Lipid Res. 1972;13:687–690. [PubMed] [Google Scholar]
- 26.Kaluzny MA, Duncan LA, Merrit MV, Epps DE. J. Lipid Res. 1985;26:135–140. [PubMed] [Google Scholar]
- 27.Batzri S, Korn ED. Biochim. Biophys. Acta. 1973;298:1015–1019. doi: 10.1016/0005-2736(73)90408-2. [DOI] [PubMed] [Google Scholar]
- 28.Kremer JMH, Esker M. W. J. v. d., Pathmamanoharan C, Wiersema PH. Biochemistry. 1977;16:3932–3935. doi: 10.1021/bi00636a033. [DOI] [PubMed] [Google Scholar]
- 29.Thompson TE, Tillack TW. Annu. Rev. Biophys. Biophys. Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- 30.Reed RA, Shipley GG. Biophys. J. 1989;55:281–292. doi: 10.1016/S0006-3495(89)82803-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed RA, Shipley GG. Biochim. Biophys. Acta. 1987;896:153–164. doi: 10.1016/0005-2736(87)90175-1. [DOI] [PubMed] [Google Scholar]
- 32.Molotkovsky JG, Mikhalyov II, Imbs AB, Bergelson LD. Chem. Phys. Lipids. 1991;58:199–212. [Google Scholar]
- 33.Ulrich-Bott B, Wiegandt H. J. Lipid Res. 1984;25:1233–1245. [PubMed] [Google Scholar]
- 34.Barenholz Y, Gibbes D, Littman BJ, Goll J, Thompson TE, Carlson FD. Biochemistry. 1977;16:2806–2810. doi: 10.1021/bi00631a035. [DOI] [PubMed] [Google Scholar]
- 35.Wetterau JR, Zilversmit DB. Methods Biochem. Anal. 1984;30:226. doi: 10.1002/9780470110515.ch4. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton JA. Biochemistry. 1989;28:2514–2520. doi: 10.1021/bi00432a025. [DOI] [PubMed] [Google Scholar]
- 37.Machida K, Ohnishi SI. Biochim. Biophys. Acta. 1978;507:156–164. doi: 10.1016/0005-2736(78)90382-6. [DOI] [PubMed] [Google Scholar]
- 38.Dicorleto PE, Fakharzadeh FF, Searles LL, Zilversmit DB. Biochim. Biophys. Acta. 1977;468:296–304. doi: 10.1016/0005-2736(77)90122-5. [DOI] [PubMed] [Google Scholar]
- 39.Somerharju PJ, Brockerhoff H, Wirtz KWA. Biochim. Biophys. Acta. 1981;649:521–528. doi: 10.1016/0005-2736(81)90155-3. [DOI] [PubMed] [Google Scholar]
- 40.Szolderits G, Hermetter A, Paltauf F, Daum G. Biochim. Biophys. Acta. 1989;986:301–309. doi: 10.1016/0005-2736(89)90481-1. [DOI] [PubMed] [Google Scholar]
- 41.Tremblay JM, Helmkamp GM, Yarbrough LR. J. Biol. Chem. 1996;271:21075–21080. doi: 10.1074/jbc.271.35.21075. [DOI] [PubMed] [Google Scholar]
- 42.Tremblay JM, Voziyan PA, Helmkamp GM, Yarbrough LR. Biochim. Biophys. Acta. 1998;1389:91–100. [PubMed] [Google Scholar]
- 43.Huuskonen J, Olkkonen VM, Jauhiainen M, Sareneva T, Somerharju PJ, Ehnholm C. Biochim. Biophys. Acta. 1998;1391:181–192. doi: 10.1016/s0005-2760(98)00008-3. [DOI] [PubMed] [Google Scholar]
- 44.Desrumaux C, Athias A, Masson D, Gambert P, Lallemant C, Lagrost L. J. Lipid Res. 1998;39:131–142. [PubMed] [Google Scholar]
- 45.Wilkening G, Linke T, Sandhoff K. J. Biol. Chem. 1998;273:30271–30278. doi: 10.1074/jbc.273.46.30271. [DOI] [PubMed] [Google Scholar]
- 46.Chattopadhyay A. Chem. Phys. Lipids. 1990;53:1–15. doi: 10.1016/0009-3084(90)90128-e. [DOI] [PubMed] [Google Scholar]
- 47.Chattopadhyay A, London E. Biochim. Biophys. Acta. 1988;938:24–34. doi: 10.1016/0005-2736(88)90118-6. [DOI] [PubMed] [Google Scholar]
- 48.Bisgaier CL, Minton LL, Essenberg AD, White A, Homan R. J. Lipid Res. 1993;34:1625–1634. [PubMed] [Google Scholar]
- 49.Wong M, Brown RE, Barenholz Y, Thompson TE. Biochemistry. 1984;23:6498–6505. doi: 10.1021/bi00321a035. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki T, Abe A, Roerink F. Subcell. Biochem. 1990;16:113–127. doi: 10.1007/978-1-4899-1621-1_5. [DOI] [PubMed] [Google Scholar]
- 51.Liscovitch M, Ben-Av P, Danin M, Faiman G, Eldar H, Livneh E. J. Lipid Mediators. 1993;8:177–182. [PubMed] [Google Scholar]
- 52.Trotter PJ, Voelker DR. Biochim. Biophys. Acta. 1994;1213:241–262. doi: 10.1016/0005-2760(94)00073-5. [DOI] [PubMed] [Google Scholar]
- 53.Voelker DR. Microbiol. Rev. 1991;55:543–560. doi: 10.1128/mr.55.4.543-560.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warnock DE, Lutz MS, Blackburn WA, Young WW, Baenziger JU. Proc. Natl. Acad. Sci. U.S.A. 1994;91:2708–2712. doi: 10.1073/pnas.91.7.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahuran DJ. Biochim. Biophys. Acta. 1998;1393:1–18. doi: 10.1016/s0005-2760(98)00057-5. [DOI] [PubMed] [Google Scholar]
- 56.Jones JD, Almeida PFF, Thompson TE. Biochemistry. 1990;29:3892–3897. doi: 10.1021/bi00468a015. [DOI] [PubMed] [Google Scholar]