Abstract
Introduction
Systemically administered cannabinoids can reduce intraocular pressure (IOP), but produce undesirable cardiovascular and central nervous system effects. In a chronic model of ocular hypertension, we examined the efficacy of acute topical administration of WIN55212-2 (WIN) in a novel commercially available vehicle and in combination with timolol.
Methods
IOP was chronically elevated by the surgical ligature of vortex veins in Sprague Dawley rats. IOP was measured by using Goldmann applanation tonometry. IOP, blood pressure (BP), and heart rate (HR) were measured at baseline and 30, 60, 90, and 120 min after the topical administration of WIN 1.0%, 0.25%, 0.06%, or 0.015%, the commercially available vehicle, timolol 0.5%, or a combination of WIN and timolol. SR141716 (CB1 antagonist) or SR144528 (CB2 antagonist) was administered topically 30 min before WIN to determine receptor specificity. To determine ocular and systemic penetration, 3H WIN 55212-2 was administered topically and tissues were collected at 60 and 120 min. Ocular irritation was evaluated by slit-lamp examination (SLE) at baseline and 120 min.
Results
WIN significantly decreased IOP in the hypertensive eye, with no BP or HR effects. SR141716 pretreatment significantly inhibited the IOP effects of WIN 1.0% in a dose-dependent manner, while SR 144528 was not as effective. No significant additive effects were observed by combining WIN (0.5% or 1.0%) with timolol 0.5%. WIN was retained in ocular tissue with a t½ of 80–100 min. SLE at 120 min revealed no solvent or drug-related toxic effects.
Conclusions
In a chronic ocular hypertensive rat model, topically applied WIN is an effective, nontoxic ocular hypotensive agent with no hemodynamic side-effects. This effect was predominantly CB1 receptor mediated, but some CB2 contribution could not be ruled out.
INTRODUCTION
Intraocular pressure (IOP) is the most important risk factor for the development of glaucoma, a leading cause of blindness worldwide.1,2 Consequently, the reduction of IOP is recognized as the main treatment to reduce visual loss in glaucoma patients. Several multicenter, clinical trials have demonstrated that lowering IOP decreases the progressive visual field damage in patients with primary open-angle glaucoma or normal tension glaucoma.3,4 Currently available medications to reduce IOP include prostaglandin analogs, beta-adrenergic blockers, alpha-2 adrenergic agonists, carbonic anhydrase inhibitors, and cholinergic agonists. Since the IOP-lowering effects of marijuana smoking were first described more than 30 years ago,5 there has been a strong interest in developing cannabinoid agents.
A variety of naturally occurring, synthetic cannabinoids have been evaluated for their ability to reduce IOP in humans and laboratory animals. 6–11 These cannabinoids bind to two different cannabinoid receptors, the CB1 receptors, which are predominantly localized in the central nervous system (CNS),12,13 and the CB2 receptors identified in the periphery and associated with immune function.14 It has been suggested that the IOP-lowering effects of cannabinoid agonists are related to their actions on the CB1 receptor, whose presence has been demonstrated in the ciliary body, iris, trabecular meshwork, and retina.15–18 Stimulation of these CB1 receptors is hypothesized to result in a decreased IOP and, consequently, may retard the progression of glaucoma. Recently, CB2 receptors in the trabecular meshwork have also been implicated in cannabinoid-mediated IOP reduction.19
The therapeutic usefulness of systemic cannabinoids is limited by their well-described psychomimetic effects; however, topical drug delivery may reduce these problems. The primary purpose of the present study is to determine whether acute topical administration of WIN55212-2, a high efficacy aminoalkylindole cannabinoid analog, obviates undesired systemic and ocular effects while maintaining efficacy. The efficacy of WIN55212-2 alone or in combination with timolol, a standard for clinical management for glaucoma, was evaluated in rats following the surgical occlusion of three vortex veins. As an additional measure, receptor (CB1 or CB2) dependency of the IOP effect in a rat chronic ocular hypertensive model was studied by using selective antagonists, SR 141716A or SR 144528, for each respective cannabinoid receptor.
METHODS
Subjects
Experiments were conducted using male Sprague Dawley rats, each weighing approximately 200 gm. To induce ocular hypertension, rats underwent a vortex vein ligation. IOP was measured under sedation with a custom-machined Goldmann applanation tonometer (Haag-Streit, Mason, OH). All studies were conducted in accordance with the Principles of Laboratory Animal Care (NIH Publication No. 85-23; revised 1985), the OPRR Public Health Service Policy on the Humane Care and Use of Laboratory Animals (revised 1986), the U.S. Animal Welfare Act, as amended, as well as the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Protocols were approved by the Eastern Virginia Medical School (Norfolk, VA) Institutional Animal Care and Use Committee.
Rat ocular hypertensive model
The rat glaucoma model used in these experiments required vortex vein ligation. Sprague Dawley rats were anesthetized by using an intraperitoneal injection of acepromazine (12 mg/kg) and ketamine (80 mg/kg), followed by topical proparacaine (0.5%). Access to the vortex veins in the right eye (OD) was obtained by carefully dissecting through the conjunctiva overlying the veins with minimum disruption of surrounding tissue. A single ligation was performed on 3 of 4 vortex veins with 9.0 silk sutures. Due to ease of access, the superior and temporal veins were ligated. Vein distention proximal to the ligation site indicated a complete ligation. At the end of the procedure, a drop of dexamethasone 0.1% and to-bramycin 0.3% was instilled prior to applying a thin ribbon of erythromycin 0.5% ointment. Buprenorphine (0.05 mg/kg) was administered intraperitoneally immediately postoperatively and b.i.d. postligation. Rats were observed for postoperative inflammation, wound dehiscence, bleeding, or anterior segment abnormalities.
By 3 weeks postoperatively, IOP was greater than baseline in the operated eye. Rats with a consistent difference of >5 mmHg between the operated eye (OD) and the contralateral control eye (OS) were considered hypertensive and remained in the study. A total of 12 rats were included in the study. Rats were allowed a minimum of a 1-week wash-out period between experiments.
Experimental design
Sprague Dawley rats were randomly assigned to receive 20 µL of either WIN55212-2 (1.0%, 0.25%, 0.06%, or 0.015%), timolol (0.5%), or vehicle applied topically to the right eye. WIN 55212-2 1.0% equals 20 mM (molecular weight = 522.61). The vehicle served as the negative control, while timolol was a positive control. The left eye (OS) served as the untreated control. A positive (timolol treated) and negative control (vehicle only) were included as part of the daily experiment. For each experiment, 2 animals were assigned to each WIN55212-2 treatment (1.0%, 0.25%, 0.06%, or 0.015%), with 1 animal each for vehicle control and timolol treatment. Experiments were performed over 5 times to obtain an n = 10 for WIN55212-2 treatment groups and an n = 5 each for the vehicle and timolol treatments. All animals were allowed a minimum wash-out period of 1 week between subsequent experiments. At the beginning of each experiment, animals were sedated (ketamine 40 mg/kg and acepromazine 6 mg/kg) and baseline IOP, heart rate (HR), and blood pressure (BP) measurements (t = 0) were obtained. IOP measurements were repeated at 30, 60, 90, and 120 min after drug administration in the treated eye (OD) as well as in the contralateral untreated eye (OS). HR and BP were also measured at 30, 60, 90, and 120 min after drug administration. Before and at the conclusion of each experiment, all eyes were examined and graded by slit lamp for signs of ocular irritation.
Experiments using CB1 and CB2 antagonists were performed to examine the receptor specificity of WIN in this model. Cannabinoid receptor antagonists were applied topically 30 min prior to the instillation of WIN55212-2 (n = 3 per group). The CB1 antagonist was SR141716 (Ki [CB1] = 5.6 nM, Ki [CB2] >1000 nM)20 and the CB2 antagonist was SR 144528 (Ki [CB2] = 0.6 nM, Ki [CB1] = 437 nM).21 Testing for selectivity and crossover effects was performed by the topical application of antagonists (2, 0.04, or 0.02 mM) 30 min prior to topical application of WIN55212-2 (n = 3 per group). In these experiments, an additional IOP, HR, and BP measurement was taken prior to instillation of the antagonist and recorded as t = −30. Another experiment was performed to examine the confounding effects of sedation on IOP.
A final set of experiments was conducted to examine whether the effects of WIN55212-2 and timolol, a β-adrenergic antagonist, are additive. In these experiments, timolol 0.5% (20 µL) was applied OD at t = −30, followed by the administration of either 1.0 or 0.5% WIN55212-2 (20 µL) at t = 0 (n = 3 per group). IOP, BP, and HR were measure prior to timolol administration at t = −30, and then prior to the WIN55212-2 administration at t = 0, followed by serial readings at 30, 60, 90, and 120 min.
Measurement of IOP
IOP measurements were performed under mild sedation (ketamine 40 mg/kg and acepromazine 6 mg/kg), with a custom-machined Goldmann applanation tonometer. Animals were sedated to minimize IOP variability that may result from a Valsalva-like response. One drop of Fluorbenox (fluorescein sodium and benoxinate hydrochloride; Wilson Ophthalmic, Mustang, OK) was instilled into each eye prior to each set of IOP measurements. Two readings per eye were taken at each time interval and averaged.
Measurement of ocular and systemic penetration of WIN 55212-2
In a separate experiment, a single 20-µL dose of an admixture of 1% WIN 55212-2 containing 1 uCi/20 µL of 3H WIN 55212-2 (DuPont, Boston, MA) was applied to both eyes of normal sedated Sprague Dawley rats (n = 3 per group). After 60 or 120 min, the rats were euthanized with FatalPlus (1 mL/kg; Vortech, Dearborn, MI). Eyes and eyelids were removed, eyelids weighed, and both eyes and eyelids rinsed five times in five separate 5-mL volumes of cold saline. Corneas and retina/sclera were dissected out and vitreous humor removed from the eyes. In addition, liver, urine, and blood samples were collected. All samples were weighed and dissolved overnight in 1 mL of Protosol (DuPont, Boston, MA). After the addition of Scintisafe (Fisher Scientific, Pittsburg, PA) scintillation cocktail, the amount of 3H WIN 55212-2 in each sample was counted in a Beckman 6500 scintillation counter (Beckman Coulter Inc., Fullerton, CA).
Measurement of HR and BP
A standard tail cuff apparatus was used to measure HR and blood pressure BP. A pulse amplifier (Model 29; IITC, Woodland Hills, CA) was placed in line for digital data conversion. Data was processed with Dasy Lab (Version 6.0; Dasytech, Amherst, NH), which generated the analysis module.
Assessment for ocular irritation
Slit-lamp examination was performed by an independent knowledgeable observer, using a semiquantitative scale for ocular irritation.22 The exam was conducted at the beginning and end of each experiment to determine if topical application of the agents that were used caused ocular irritation. Each eye was assessed for signs of inflammation, conjunctival chemosis/swelling, conjunctival discharge, aqueous fibrin/flare, loss of the corneal light reflex, obscuration of iris structures, and corneal opacity/vascularization/staining. They were rated on a 4-point scale, where 0 represented normal.
Statistical analysis
Results are reported as the mean ± standard error of the mean. Data were analyzed by a paired t test or analysis of variance, as appropriate. Differences were considered significant at P < 0.05.
Materials
WIN55212-2 (1.0%, 0.25%, 0.06%, and 0.015%) was directly dissolved in Tocrisolve™, a proprietary 1:4 soya oil/water emulsion (vehicle), as was the CB1 antagonist, SR141716, and the CB2 antagonist, SR144528. Tocrisolve™ was obtained from Tocris Cookson, Inc. (Bristol, UK). Sutures were obtained from Sharpoint (Reading, PA). WIN55212-2 was purchased from Sigma-Aldrich (St. Louis, MO). Timolol was purchased from Falcon (Napa, CA). SR141716 and SR144528 were generously supplied by the National Institutes of Health (Bethesda, MD).
RESULTS
Effects of topically applied WIN55212-2
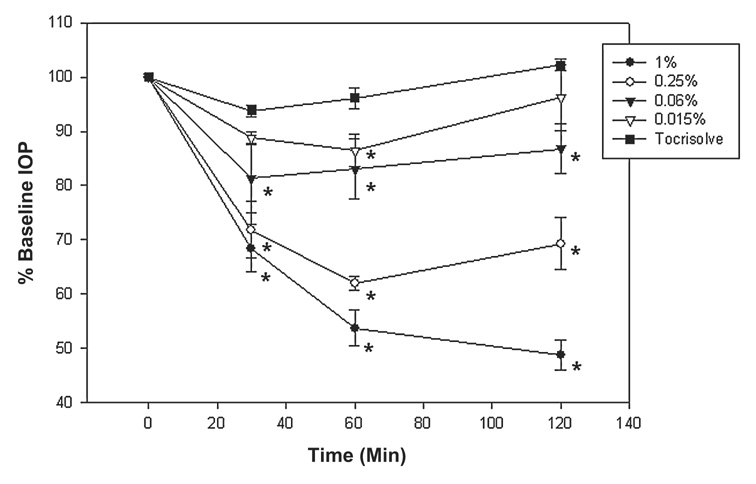
At baseline (t = 0), the IOP for the operated eye (OD) was 16.0 ± 0.9 mmHg and for the contralateral (nonoperated) eye was 10.1 ± 0.5 mmHg. Following a single topical application of WIN55212-2 (1.0%, 0.25%, 0.06%, or 0.015%) or vehicle alone to the operated eye, the change in IOP (mmHg) from baseline was measured for up to 120 min (Fig. 1; n = 10 per group). After 30 min, all concentrations of WIN55212-2 significantly decreased IOP (P < 0.001) from 30 to 120 min, except for WIN55212-2 0.015% (P = 0.59; n = 10). WIN55212-2 1.0%, 0.25%, and 0.06% significantly reduced IOP, compared to the 0.015% concentration, 30 min after administration (P = 0.03). Maximal effect on IOP was observed 60 min after the administration of WIN55212-2. At this time period, both 1.0% and 0.25% elicited a significantly greater IOP reduction than 0.06% and 0.015% concentrations (P < 0.001). At the end of the study period (120 min), only a 1.0% treatment continued to maintain IOP reduction, while 0.25% and 0.06% concentrations had already started to return toward baseline. The magnitude and duration of effect was dose dependent. Tocirsolve ™ (vehicle) alone had no significant effect on IOP (P > 0.08).
FIG. 1.
Dose response to topically applied WIN55212-2 in surgically hypertensive eyes. The baseline IOP (t = 0) was 16.0 ± 0.9 mm Hg. N = 10 rats/group. Data are presented as the mean ± standard error of the mean. *Significantly reduced when compared to baseline (P < 0.05).
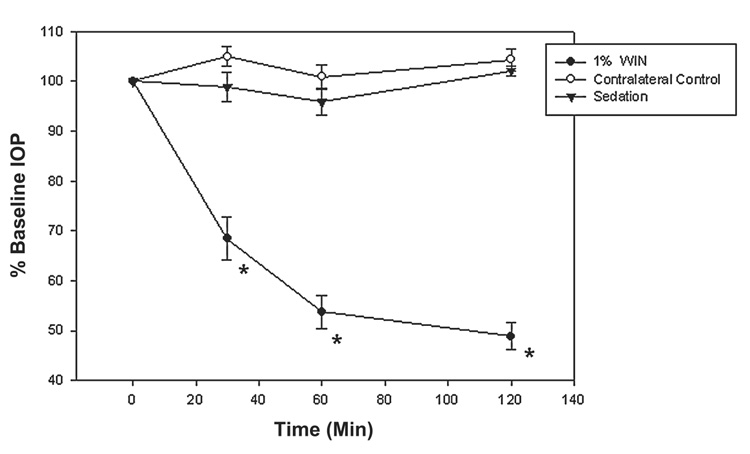
Effect on contralateral eye
Rats treated with WIN (1.0%, 0.25%, 0.06%, or 0.015%) or vehicle were followed for IOP changes in the contralateral control eye (OS) (Fig. 2; n = 10). There was no crossover effect in the contralateral control eye, even at the highest concentration of WIN55212-2 (1.0%). For example, IOP was reduced from 16.2 ± 0.9 mmHg at baseline (t = 0) to 9.0 ± 0.5 mmHg at 60 min in the treated eye. There was no concomitant change in the untreated eye (P = 0.59); that is, IOP was 10.1 ± 0.5 mmHg at baseline and 10.4 ± 0.4 mmHg after 60 min (n = 10). Sedation alone did not significantly alter IOP in either the hypertensive or the normotensive (contralateral) eye during the experiment.
FIG. 2.
Topical administration of WIN55212-2 in the operated eye significantly reduced intraocular pressure, but had no effect in the contralateral eye. Data are presented as the mean ± standard error of the mean. *Significantly reduced when compared to baseline (t = 0; P < 0.001).
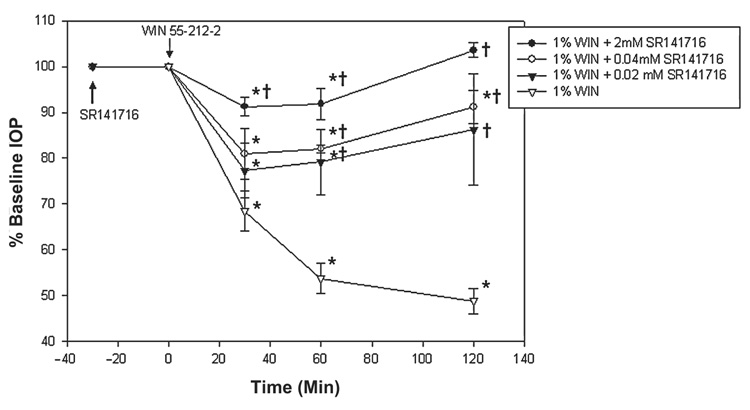
Effect of CB1 receptor antagonism
Topical application of WIN55212-2 1.0% alone reduced IOP by 32% after 30 min and 52% by 120 min. After pretreatment with the CB1 antagonist, SR141716 (2 mM), the maximal reduction of only 8.0% ± 2.1% occurred at 30 min (n = 3). This reduction was significantly less than WIN55212-2 alone (P = 0.009) but significantly lower than baseline (P = 0.03, n = 3; Fig. 3). IOP recovered to baseline by 120 min, indicating a significant antagonism of WIN55212-2 (P < 0.001, compared to WIN55212-2 alone; n = 3).
FIG. 3.
Effect on intraocular pressure of CB1 antagonist, SR141716, followed by WIN55212-2. CB1 antagonist, SR141716, was applied topically 30 min (see arrow) prior to the administration of WIN55212-2 (t = 0 min). Data are presented as mean ± standard error of the mean. *Significantly reduced from baseline (t = 0), P < 0.05. †Significantly greater than 1.0% WIN55212-2 alone treatment (P < 0.05).
As the concentration of SR141716 decreased, a greater effect of WIN55212-2 was noted. After decreasing the concentration of SR141716 fiftyfold (0.04 mM), WIN55212-2 decreased IOP by 20% at 30 min and by 10% at 120 min (P < 0.15, and P = 0.001, respectively, when compared to WIN55212-2 alone; P = 0.02 and P = 0.05, respectively, when compared to baseline; n = 3). After a one hundredfold decrease (0.02 mM), WIN55212-2 maximally reduced IOP by 23% at 30 min and by 14% at 120 min (P = 0.29 and P = 0.04, respectively, when compared to WIN55212-2 alone; P = 0.02 and P = 0.06, respectively, when compared to baseline; n = 3). Topical administration of SR141716 (2 to 0.02 mM) alone had no effect on IOP (data not shown).
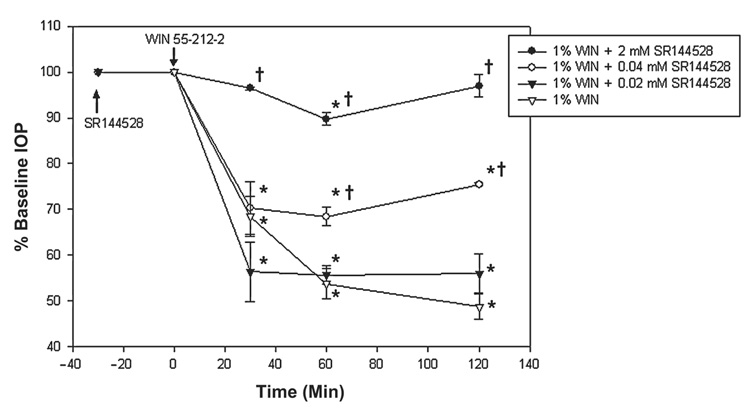
Effect of CB2 receptor antagonism
Pretreatment with CB2 antagonist, SR144528 (0.02 mM) did not significantly inhibit the effects of WIN55212-2 1.0% (Fig. 4; n = 3). Following a SR144528 pretreatment, IOP was reduced from a baseline of 19.5 ± 0.4 to 9.0 ± 0.4 mmHg at 30 min (P = 0.13, when compared to WIN55212-2 alone; P = 0.05, when compared to baseline; n = 3) and 9.0 ± 0.1 mmHg at 120 min (P = 0.18, when compared to WIN55212-2 alone; P = 0.03, when compared to baseline; n = 3).
FIG. 4.
Effect of CB2 antagonist, SR 144528, followed by WIN55212-2 on intraocular pressure and tested for 120 min. CB2 antagonist was applied topically 30 min prior to administration of WIN55212-2. Data are presented as the mean ± standard error of the mean. *Significantly different from baseline (t = 0; P < 0.05). †Significantly greater than 1.0% WIN55212-2 alone treatment (P < 0.05).
When pretreated with higher doses of SR144528 (0.04 or 2 mM), the hypotensive effects of WIN55212-2 1.0% diminished. After doubling the concentration (0.04 mM), WIN55212-2 decreased IOP by 30% at 30 min and by 25% at 120 min (P = 0.80, and P = 0.01, respectively, when compared to WIN55212-2 alone; P = 0.02 and P = 0.05, respectively, when compared to baseline; n = 3). A further SR144528 increase (to 2 mM) blocked the WIN55212-2 mediated IOP change (for 30 and 120 min, P = 0.003 and P = 0.001, respectively when compared to WIN55212-2 alone; P = 0.11 and P = 0.17, respectively, when compared to baseline; n = 3). Topical administration of SR144528 (2 to 0.02 mM) alone did not have any effect on IOP (data not shown).
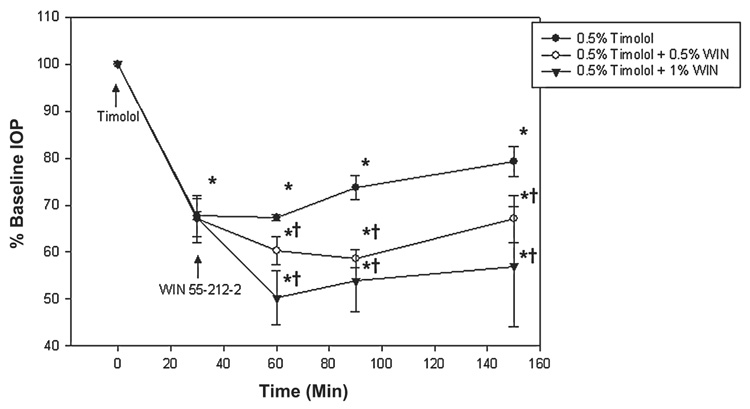
Combination of timolol and WIN55212-2
Timolol and WIN55212-2 were administered successively to evaluate their combined effect on IOP (Fig. 5). At the beginning of the experiment, baseline IOP measurements were obtained and timolol 0.5% applied to all treatment groups (t = 0 min, baseline; n = 3 per group). After 30 min, a single dose of timolol 0.5% reduced IOP from 15.3 ± 1.4 mmHg at baseline to 10.5 ± 1.6 mmHg (P < 0.001; n = 3). At this time point, WIN55212-2 (1.0% or 0.5%) was administered (t = 30 min).
FIG. 5.
Combination of timolol and WIN55212-2. First timolol was applied (see arrow) followed by WIN55212-2 at t = 0. Data are presented as the mean ± standard error of the mean. *Significantly lower than baseline (P < 0.001). †Significantly lower than timolol alone (P < 0.05).
After 60 min, IOP in rats treated with WIN55212-2 (1.0%) + timolol (0.5%) further decreased to 8.0 ± 0.7 mmHg (P < 0.001; n = 3). This decrease was significantly greater than the timolol-alone administration (P = 0.008). In the WIN55212-2 0.5% + timolol 0.5% treated rats, IOP was 8.5 ± 1.4 mmHg after 60 min. This reduction was significantly greater than timolol alone (P < 0.001).
Experiments were terminated 150 min after the timolol 0.5% administration. A final IOP measurement of 11.5 ± 2.2 mmHg was obtained for the timolol 0.5% only treatment, 9.5 ± 1.8 mmHg for the WIN55212-2 1.0% + timolol 0.5% treatment, and 9.5 ± 1.4 mmHg for the WIN55212-2 0.5% + timolol 0.5% treatment. All treatments significantly reduced IOP, compared to baseline (P < 0.001; n = 3 per group). The combined efficacy of WIN55212-2 and timolol was significantly greater than timolol alone (P < 0.05) and similar to that of WIN55212-2 alone (P > 0.74).
Ocular and systemic penetration of WIN 55212-2
Concentration of drug in cornea, vitreous humor, retina/sclera, eyelids, blood, liver, and urine was determined at 60 and 120 min after the administration of a single drop (20 µL) of H3 WIN 55212-2 admixture (1.0%, ~20 mM, n = 3 per group; Table 1). After 60 min, 15% of administered drug was found in the eyelids, 0.5% by the cornea, 0.4% by vitreous humor, and 1.5% by retina/sclera. The apparent t½ in the retina/sclera was 100 min and in the vitreous humor 80 min, indicating WIN 55212-2 was effectively retained in both the anterior and posterior segments. At 120 min, 15% of administered drug was retained by the eyelids, 0.4% by the cornea, 0.5% by the vitreous humor, and 0.8% by the retina/sclera. From 60–120 min, a twentyfold increase in WIN 55212-2 was seen in the urine. WIN 55212-2 concentration in the blood was below detectable limits.
Table 1.
Ocular and systemic penetration of 3H WIN 55212-2 after a single topical dose (20 µL)
| 3H WIN 55212-2 (µM) | |||
|---|---|---|---|
| 60 min | 120 min | N/group | |
| Cornea | 84.7 ± 25.0 | 117.0 ± 19.7 | 4 |
| Vitreous humor | 76.6 ± 26.7 | 47.4 ± 12.5 | 4 |
| Retina/sclera | 95.7 ± 12.9 | 66.3 ± 15.4 | 4 |
| Eyelids | 1169.9 ± 482.8 | 1154.4 ± 792.8 | 4 |
| Blood | BDL | BDL | 2 |
| Liver | BDL | 0.1 ± 0.0 | 2 |
| Urine | 0.2 ± 0.1 | 2.2 ± 0.7 | 2 |
BDL, below detection limit.
Effects of WIN55212-2 on HR and BP
Even at the highest concentration tested, WIN55212-2 (1.0%) had no effect on HR or BP (Table 2). Although HR decreased from 434 ± 12 bpm at baseline to 414 ± 10 bpm at 120 min, this change was not significant (P = 0.70). Likewise, neither systolic nor diastolic BP changed significantly (P = 1.00 and P = 0.67, respectively, compared to baseline).
Table 2.
Topical application of WIN55212-2 fails to affect heart rate or blood pressure (BP) in sprague dawley rats
| Baseline | 30 min | 60 min | 120 min | N | |
|---|---|---|---|---|---|
| Heart | 434 ± 12 | 424 ± 17 | 417 ± 12 | 414 ± 10 | 10 |
| Systolic | 126 ± 3 | 136 ± 5 | 132 ± 4 | 132 ± 7 | 10 |
| Diastolic | 99 ± 2 | 104 ± 5 | 102 ± 1 | 101 ± 3 | 10 |
Note. Data are shown as the mean ± standard error of the mean.
Analysis of ocular irritation
Slit-lamp examination conducted at the end of each experiment revealed no signs of ocular toxicity attributable to the vehicle, WIN55212-2, SR141716, SR144528, or timolol. No signs of inflammation, conjunctival chemosis/swelling, conjunctival discharge, aqueous fibrin/flare, loss of the pupillary light reflex, obscuration of iris structures, or corneal opacity were observed. In all treatment groups, mild central corneal staining was noted consistently. However, similar corneal staining also occurred in the untreated control eyes. This finding was most likely a result of repeated applanation with the Goldmann tonometer, rather than a drug- or vehicle-induced effect.
DISCUSSION
It has been over a decade since a new class of pharmacologic agents was introduced for the clinical management of glaucoma.23 Currently available therapeutic approaches for the treatment of glaucoma include prostaglandin analogs, beta-adrenergic blockers, alpha-2 adrenergic agonists, carbonic anhydrase inhibitors, and cholinergic agonists. Each of these has a well-known profile of clinical response and adverse effects. Up to 50% of patients cannot be maintained on single-drug therapy; most require the use of two or even three drugs to control their IOP.24 Even the first-line agent for the treatment of glaucoma, timolol, as a monotherapy controlled IOP in only 98 of 155 patients (63.2%).25 More recently, in the Ocular Hypertension Treatment Study, 40% of patients randomized to treatment required more than one medication to achieve the 20% reduction goal.26
For over 30 years, cannabinoids have been touted for their potential to decrease IOP.5 Unfortunately, cardiovascular and psychotropic effects complicated systemic administration.27,28 Numerous studies showed that the systemic administration of cannabinoids produced unwanted reductions in systolic and diastolic BP, as well as decreased HR and variable changes in pupil diameter.18,29,30 Moreover, the associated psychotropic effects of these agents have been well documented.31
Topical administration of cannabinoids offers the theoretic advantage of providing desirable local ocular effects with minimal, if any, systemic side-effects. However, poor cannabinoid solubility has hindered the preparation of a suitable topical dosage form, resulting in a diminished effect on IOP.6,32 Vehicles with the potential for dissolving lipophilic cannabinoids include sesame oil,33,34 mineral oil,34,35 polyethylene glycol,6,7 Tween 80,34 and submicron aqueous emulsions.36 While these agents have the ability to dissolve the lipophilic cannabinoids, we found some of these vehicles to have a limited ability to dissolve WIN55212-2 (data not shown). Tocrisolve™ was chosen as the vehicle because of its ability to readily dissolve WIN55212-2 without much effort. This solution maintained its efficacy over a period of 2 months (data not shown).
Tocrisolve™, a proprietary preparation, is a vehicle designed for lipophilic compounds, such as cannabinoids and vanilloids. Tocrisolve™ is composed of 1:4 soya oil and water and is emulsified with the block copolymer Pluronic F68. In the past, we have used 2-hydroxypropyl-β-cyclodextrin as a vehicle to dissolve cannabinoids. Both 2- hydroxypropyl-β-cyclodextrin and Tocrisolve™ are good solvents for WIN55212-2. However, Tocrisolve™ not only dissolves up to 2% in WIN55212-2, compared to 1% in 2-hydroxypropyl-β-cyclodextrin, but also does not require ethanol to promote solubility.37 In this study, the acute application of WIN55212-2 dissolved in Tocrisolve™ and applied topically produced a significant reduction in IOP. Moreover, there was a notable lack of systemic effects (e.g., HR and BP). In a recent study, 4 weeks of daily topical treatment of rats with WIN55212-2 in Tocrisolve™ did not cause local ocular toxicity (i.e., ocular inflammation, conjunctival chemosis/swelling, conjunctival discharge, aqueous flare or fibrin, diminished corneal light reflex, iris or corneal opacity, or corneal vascularization/staining). 37 This excellent tolerability highlights the potential use of these cannabinoid emulsions in the management of glaucoma in humans where long-term treatment is needed.
In this study, the topical application of WIN55212-2 was compared with timolol, a β-adrenergic blocker and first-line agent in glaucoma medical therapy.38–47 WIN55212-2 1.0% decreased IOP by 52% in eyes with sustained ocular hypertension. By contrast, timolol 0.5% attained a mean peak reduction in IOP of 35%. Neither WIN55212-2 nor timolol had an effect on the contralateral normotensive eye. The combined efficacy of WIN55212-2 (0.5% or 1.0%) and timolol (0.5%) was not significantly different from that of WIN55212-2 (0.5% or 1.0%) alone, suggesting a possibility that the combination did not produce additive or synergistic effects. Indeed, there was no significant contribution of timolol to the magnitude of the IOP reduction, suggesting that the CB agonists may, in some fashion, draw on a receptor population that includes those affected by timolol. In this light, it has been noted that CB receptors can reduce beta-adrenergic receptor activity through G protein–modulated effects, thus mimicking the beta-adrenergic blockade.48 Pharmacologic and histologic studies support a direct role for CB receptors in ocular tissues of the human eye, including the ciliary epithelium, trabecular meshwork, Schlemm′s canal, ciliary muscle, ciliary body vessels, and retina.49 Also, Sugrue suggests that cannabinoid receptors in the retina may convey neuroprotection to retinal ganglion cells.50
Evidence from this study, using ocular hypertensive rats, indicates that the effect of WIN55212-2 is mediated primarily by CB1 receptor activation, congruent with the ability of the CB1 antagonist, SR141716, to inhibit the effects of WIN55212-2 (1%). When pretreated with SR141716 (2-0.02 mM), the magnitude and duration of the WIN55212-2-mediated hypotensive effect was severely diminished. In this study, the effect of SR141716 was noted as early as 30 min and lasting for the entire study period. Although IOP was only measured for 2 h, Boyd and Fremming have demonstrated that when given orally, the duration of blockade by SR141716 is 8 h.51
The role of CB2 receptors in IOP control has not been definitively determined. The Ki values of the CB2 antagonist (SR144528, Ki [CB2] = 0.6 nM, and Ki [CB1] = 437 nM) used in this study were determined in Chinese hamster ovary cells, whose membranes expressed CB2 receptors.21 The topical dose of SR144528 used in the current study was considerably higher (2.0-0.02 mM) than its Ki (CB2) of 0.6 nM. Although the data indicate a preferential contribution of CB1 to CB2 mediation in the WIN55212-2 IOP reduction, the differences in receptor selectivity of SR144528 and SR141716 in vivo were much less than expected from the in vitro experiments. This may be related to a number of factors, including the amount of antagonist delivered to the receptors, the residency of the antagonist on the receptor, and the CB1/CB2 profile of WIN55212-2 in this preparation. When administered topically in rabbits, the maximum concentration of timolol in the vitreous humor was one hundred thousandth of that in the drop,52 indicative of the type of reduction in concentration that might be expected in our model. With a difference in in vivo Ki, the blockade of WIN55212-2 observed at the higher concentrations of SR144528 could be due to the administration of this agent in concentrations greatly exceeding its Ki (CB2) and approaching its Ki (CB1). Thus, crossover binding to the CB1 receptor may have blunted the effect of WIN55212-2 in this setting. Although CB1 receptor effects are predominant, WIN55212-2 may also exhibit mixed agonist activity in some tissues, suggesting that there may be some role for CB2 receptors in cannabinoid-mediated IOP control.53
With topical treatment, effective ocular penetration to the site of action is an issue. In this study, effective ocular penetration was achieved as concentrations >40 µM WIN 55212-2 were maintained for at least 120 min after a single topical dose. Corneal penetration of WIN 55212-2 may have been facilitated by pretreatment with Fluorbenox (fluorescein sodium and benoxinate hydrochloride), as ophthalmic anesthetics similar to benoxinate hydrochloride have been shown to enhance the effects of various topical agents.54–56 Interestingly, human glaucoma patients resistant to conventional therapies respond to acute topical WIN 55212-2 at doses of 25 or 50 µg.57 Of particular note is the accumulation of the drug in the eyelids. The majority of the administered drug was absorbed by the eyelids and maintained for at least 120 min, leaning toward a possible role for the eyelids as a sink mechanism for topically applied drugs. This needs to be further investigated. Blood levels of WIN 55212-2 were below detection limits, indicating low systemic bioavailability, and therefore, negating the drug′s ability to elicit unwanted side-effects.
CONCLUSIONS
It is a well-accepted therapeutic principle that when multidrug therapy is needed, each drug must belong to a different pharmacologic class.58 Thus, a new class of medications, such as topically administered cannabinoids, to the current armamentarium offers the clinician more alternatives in the management of glaucoma. In this study, topically applied WIN55212-2 effectively penetrated the ocular structures and decreased IOP in a rat model of sustained ocular hypertension. This effect on IOP was dose dependent and was mediated predominantly through the CB1 receptors. Equally important was a notable lack of systemic effects and local toxicity to ocular structures as well as a low systemic bioavailability.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the superb technical assistance provided by Patty Loose-Thurman.
This study was funded, in part, by the Commonwealth of Virginia Health Research Board and the American Health Assistance Foundation and DA003672 and DA002396 (NIH).
REFERENCES
- 1.Quigley HA, McKinnon SJ, Zack DJ, et al. Retrograde axonal transport of BDNF in retinal ganglion cells is blocked by acute IOP elevation in rats. Invest. Ophthalmol. Vis. Sci. 2000;41:3460–3466. [PubMed] [Google Scholar]
- 2.Quigley HA. Number of people with glaucoma worldwide. Br. J. Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7 The relationship between control of intraocular pressure and visual field deterioration. Am. J. Ophthalmol. 2000;130:429–440. doi: 10.1016/s0002-9394(00)00538-9. [DOI] [PubMed] [Google Scholar]
- 4.The Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am. J. Ophthalmol. 1998;126:487–497. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 5.Hepler RS, Frank IR. Marihuana smoking and intraocular pressure. JAMA. 1971;217:1392. [PubMed] [Google Scholar]
- 6.Colasanti BK, Powell SR, Craig CR. Intraocular pressure, ocular toxicity, and neurotoxicity after administration of delta 9-tetrahydrocannabinol or cannabichromene. Exp. Eye Res. 1984;38:63–71. doi: 10.1016/0014-4835(84)90139-8. [DOI] [PubMed] [Google Scholar]
- 7.Colasanti BK, Craig CR, Allara RD. Intraocular pressure, ocular toxicity and neurotoxicity after administration of cannabinol or cannabigerol. Exp. Eye Res. 1984;39:251–259. doi: 10.1016/0014-4835(84)90013-7. [DOI] [PubMed] [Google Scholar]
- 8.Colasanti BK, Brown RE, Craig CR. Ocular hypotension, ocular toxicity, and neurotoxicity in response to marihuana extract and cannabidiol. Gen. Pharmacol. 1984;15:479–484. doi: 10.1016/0306-3623(84)90202-7. [DOI] [PubMed] [Google Scholar]
- 9.Colasanti BK. A comparison of the ocular and central effects of delta 9-tetrahydrocannabinol and cannabigerol. J. Ocul. Pharmacol. 1990;6:259–269. doi: 10.1089/jop.1990.6.259. [DOI] [PubMed] [Google Scholar]
- 10.Colasanti BK. Ocular hypotensive effect of marihuana cannabinoids: correlate of central action or separate phenomenon? J. Ocul. Pharmacol. Ther. 1986;2:295–304. doi: 10.1089/jop.1986.2.295. [DOI] [PubMed] [Google Scholar]
- 11.Colasanti BK. Intraocular pressure, ocular toxicity, and neurotoxicity in response to 11-hydroxy-delta 9-tetrahydrocannabinol and 1-nantradol. J. Ocul. Pharmacol. Ther. 1985;1:123–135. doi: 10.1089/jop.1985.1.123. [DOI] [PubMed] [Google Scholar]
- 12.Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 13.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J. Comp. Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 14.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 15.Stamer WD, Golightly SF, Hosohata Y, et al. Cannabinoid CB(1) receptor expression, activation, and detection of endogenous ligand in trabecular meshwork and ciliary process tissues. Eur. J. Pharmacol. 2001;431:277–286. doi: 10.1016/s0014-2999(01)01438-8. [DOI] [PubMed] [Google Scholar]
- 16.Song ZH, Slowey CA. Involvement of cannabinoid receptors in the intraocular pressure-lowering effects of WIN55212-2. J. Pharmacol. Exp. Ther. 2000;292:136–139. [PubMed] [Google Scholar]
- 17.Porcella A, Casellas P, Gessa GL, et al. Cannabinoid receptor CB1 mRNA is highly expressed in the rat ciliary body: Implications for the antiglaucoma properties of marihuana. Brain Res. Mol. Brain Res. 1998;58:240–245. doi: 10.1016/s0169-328x(98)00105-3. [DOI] [PubMed] [Google Scholar]
- 18.Pate DW, Jarvinen K, Urtti A, et al. Effect of the CB1 receptor antagonist, SR141716A, on cannabinoid-induced ocular hypotension in normotensive rabbits. Life Sci. 1998;63:2181–2188. doi: 10.1016/s0024-3205(98)00499-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhong L, Geng L, Njie Y, et al. CB2 cannabinoid receptors in trabecular meshwork cells mediate JWH015-induced enhancement of aqueous humor outflow facility. Invest. Ophthalmol. Vis. Sci. 2005;46:1988–1992. doi: 10.1167/iovs.04-0651. [DOI] [PubMed] [Google Scholar]
- 20.Rinaldi-Carmona M, Barth F, Heaulme M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 21.Rinaldi-Carmona M, Barth F, Millan J, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J. Pharmacol. Exp. Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- 22.Samudre SS, Lattanzio FA, Jr., Williams PB, et al. Comparison of topical steroids for acute anterior uveitis. J. Ocul. Pharmacol. Ther. 2004;20:533–547. doi: 10.1089/jop.2004.20.533. [DOI] [PubMed] [Google Scholar]
- 23.Quaranta L, Gandolfo F, Turano R, et al. Effects of topical hypotensive drugs on circadian IOP, blood pressure, and calculated diastolic ocular perfusion pressure in patients with glaucoma. Invest. Ophthalmol. Vis. Sci. 2006;47:2917–2923. doi: 10.1167/iovs.05-1253. [DOI] [PubMed] [Google Scholar]
- 24.Kobelt-Nguyen G, Gerdtham UG, Alm A. Costs of treating primary open-angle glaucoma and ocular hypertension: A retrospective, observational two-year chart review of newly diagnosed patients in Sweden and the United States. J. Glaucoma. 1998;7:95–104. [PubMed] [Google Scholar]
- 25.Uusitalo RJ, Palkama A. Long-term evaluation of timolol. Acta. Ophthalmol. (Copenh.) 1989;67:573–581. doi: 10.1111/j.1755-3768.1989.tb04110.x. [DOI] [PubMed] [Google Scholar]
- 26.Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch. Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. discussion 829-730. [DOI] [PubMed] [Google Scholar]
- 27.Hodges LC, Reggio PH, Green K. Evidence against cannabinoid receptor involvement in intraocular pressure effects of cannabinoids in rabbits. Ophthalmic Res. 1997;29:1–5. doi: 10.1159/000267984. [DOI] [PubMed] [Google Scholar]
- 28.Naveh N, Weissman C, Muchtar S, et al. A submicron emulsion of HU-211, a synthetic cannabinoid, reduces intraocular pressure in rabbits. Graefe’s Arch. Clin. Exp. Ophthalmol. 2000;238:334–338. doi: 10.1007/s004170050361. [DOI] [PubMed] [Google Scholar]
- 29.Green KE, Kearse CE. Ocular penetration of topical delta9-tetrahydrocannabinol from rabbit corneal or cul-de-sac application site. Curr. Eye Res. 2000;21:566–570. [PubMed] [Google Scholar]
- 30.Kearse EC, Green K. Effect of vehicle upon in vitro transcorneal permeability and intracorneal content of delta9-tetrahydrocannabinol. Curr. Eye Res. 2000;20:496–501. [PubMed] [Google Scholar]
- 31.Juntunen J, Jarvinen T, Niemi R. In-vitro corneal permeation of cannabinoids and their water-soluble phosphate ester prodrugs. J. Pharm. Pharmacol. 2005;57:1153–1157. doi: 10.1211/jpp.57.9.0009. [DOI] [PubMed] [Google Scholar]
- 32.Green K, Roth M. Ocular effects of topical administration of delta9-tetrahydrocannabinol in man. Arch. Ophthalmol. 1982;100:265–267. doi: 10.1001/archopht.1982.01030030267006. [DOI] [PubMed] [Google Scholar]
- 33.Green K, Wynn H, Bowman KA. A comparison of topical cannabinoids on intraocular pressure. Exp. Eye Res. 1978;27:239–246. doi: 10.1016/0014-4835(78)90092-1. [DOI] [PubMed] [Google Scholar]
- 34.Green K, Bigger JF, Kim K, et al. Cannabinoid penetration and chronic effects in the eye. Exp. Eye Res. 1977;24:197–205. doi: 10.1016/0014-4835(77)90260-3. [DOI] [PubMed] [Google Scholar]
- 35.Merritt JC, Perry DD, Russell DN, et al. Topical delta 9-tetrahydrocannabinol and aqueous dynamics in glaucoma. J. Clin. Pharmacol. 1981;21:467S–471S. doi: 10.1002/j.1552-4604.1981.tb02626.x. [DOI] [PubMed] [Google Scholar]
- 36.Muchtar S, Almog S, Torracca MT, et al. A submicron emulsion as ocular vehicle for delta-8-tetrahydrocannabinol: Effect on intraocular pressure in rabbits. Ophthalmic Res. 1992;24:142–149. doi: 10.1159/000267160. [DOI] [PubMed] [Google Scholar]
- 37.Hosseini A, Lattanzio FA, Williams PB, et al. Chronic topical administration of WIN-55-212-2 maintains a reduction in IOP in a rat glaucoma model without adverse effects. Exp. Eye Res. 2006;82:753–759. doi: 10.1016/j.exer.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 38.Ozturk F, Ermis SS, Inan UU, et al. Comparison of the efficacy and safety of dorzolamide 2% when added to brimonidine 0.2% or timolol maleate 0.5% in patients with primary open-angle glaucoma. J. Ocul. Pharmacol. Ther. 2005;21:68–74. doi: 10.1089/jop.2005.21.68. [DOI] [PubMed] [Google Scholar]
- 39.Tomita G, Araie M, Kitazawa Y, et al. A three-year prospective, randomized, and open comparison between latanoprost and timolol in Japanese normal-tension glaucoma patients. Eye. 2004;18:984–989. doi: 10.1038/sj.eye.6701373. [DOI] [PubMed] [Google Scholar]
- 40.Stewart RH, Kimbrough RL, Ward RL. Betaxolol versus timolol. A six-month double-blind comparison. Arch. Ophthalmol. 1986;104:46–48. doi: 10.1001/archopht.1986.01050130056019. [DOI] [PubMed] [Google Scholar]
- 41.Schuman JS, Horwitz B, Choplin NT, et al. The Chronic Brimonidine Study Group. A 1- year study of brimonidine twice-daily in glaucoma and ocular hypertension. A controlled, randomized, multicenter clinical trial. Arch. Ophthalmol. 1997;115:847–852. doi: 10.1001/archopht.1997.01100160017002. [DOI] [PubMed] [Google Scholar]
- 42.Palkama A, Uusitalo H, Raij K, et al. Comparison of the effects of adrenergic agonists and alpha-, beta 1-, beta 2-antagonists on the intraocular pressure and adenylate cyclase activity in the ciliary processes of the rabbit. Acta. Ophthalmol. (Copenh.) 1985;63:9–15. doi: 10.1111/j.1755-3768.1985.tb05206.x. [DOI] [PubMed] [Google Scholar]
- 43.Mishima HK, Masuda K, Kitazawa Y, et al. A comparison of latanoprost and timolol in primary open-angle glaucoma and ocular hypertension. A 12-week study. Arch. Ophthalmol. 1996;114:929–932. doi: 10.1001/archopht.1996.01100140137004. [DOI] [PubMed] [Google Scholar]
- 44.Hass I, Drance SM. Comparison between pilocarpine and timolol on diurnal pressures in open-angle glaucoma. Arch. Ophthalmol. 1980;98:480–481. doi: 10.1001/archopht.1980.01020030476006. [DOI] [PubMed] [Google Scholar]
- 45.Diestelhorst M, Roters S, Krieglstein GK. The effect of latanoprost 0.005% once-daily versus 0.0015% twice-daily on intraocular pressure and aqueous humour protein concentration in glaucoma patients. A randomized, double-masked comparison with timolol 0.5% Graefe’s Arch. Clin. Exp. Ophthalmol. 1997;235:20–26. doi: 10.1007/BF01007833. [DOI] [PubMed] [Google Scholar]
- 46.Aung T, Wong HT, Yip CC, et al. Comparison of the intraocular pressure-lowering effect of latanoprost and timolol in patients with chronic angle closure glaucoma: A preliminary study. Ophthalmology. 2000;107:1178–1183. doi: 10.1016/s0161-6420(00)00073-7. [DOI] [PubMed] [Google Scholar]
- 47.Alm A, Stjernschantz J The Scandinavian Latanoprost Study Group. Effects on intraocular pressure and side-effects of 0.005% latanoprost applied once-daily, evening or morning. A comparison with timolol. Ophthalmology. 1995;102:1743–1752. doi: 10.1016/s0161-6420(95)30798-1. [DOI] [PubMed] [Google Scholar]
- 48.Vasquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J. Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jarvinen T, Pate DW, Laine K. Cannabinoids in the treatment of glaucoma. Pharmacol. Ther. 2002;95:203–220. doi: 10.1016/s0163-7258(02)00259-0. [DOI] [PubMed] [Google Scholar]
- 50.Sugrue MF. New approaches to antiglaucoma therapy. J. Med. Chem. 1997;40:2793–2809. doi: 10.1021/jm9608725. [DOI] [PubMed] [Google Scholar]
- 51.Boyd ST, Fremming BA. Rimonabant—a selective CB1 antagonist. Ann. Pharmacother. 2005;39:684–690. doi: 10.1345/aph.1E499. [DOI] [PubMed] [Google Scholar]
- 52.Maurice DM. Drug delivery to the posterior segment from drops. Surv. Ophthalmol. 2002;47 Suppl. 1:S41–S52. doi: 10.1016/s0039-6257(02)00326-0. [DOI] [PubMed] [Google Scholar]
- 53.Niederhoffer N, Szabo B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br. J. Pharmacol. 1999;126:457–466. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mordi JA, Lyle WM, Mousa GY. Does prior instillation of a topical anesthetic enhance the effect of tropicamide? Am. J. Optom. Physiol. Opt. 1986;63:290–293. doi: 10.1097/00006324-198604000-00010. [DOI] [PubMed] [Google Scholar]
- 55.Apt L, Henrick A. Pupillary dilatation with single eyedrop mydriatic combinations. Am. J. Ophthalmol. 1980;89:553–559. doi: 10.1016/0002-9394(80)90065-3. [DOI] [PubMed] [Google Scholar]
- 56.Lyle WM, Bobier WR. Effects of topical anesthetics on phenylephrine-induced mydriasis. Am. J. Optom. Physiol. Opt. 1977;54:276–281. doi: 10.1097/00006324-197705000-00002. [DOI] [PubMed] [Google Scholar]
- 57.Porcella A, Maxia C, Gessa GL, et al. The synthetic cannabinoid WIN55212-2 decreases the intraocular pressure in human glaucoma resistant to conventional therapies. Eur. J. Neurosci. 2001;13:409–412. doi: 10.1046/j.0953-816x.2000.01401.x. [DOI] [PubMed] [Google Scholar]
- 58.Gamell LS. Glaucoma Medical Therapy: Principles and Management. San Francisco, CA: American Academy of Ophthalmology; 1999. pp. 179–191. [Google Scholar]